Key Points
Question
Do patients with acute intracerebral hemorrhage with vs without cerebral microbleeds have different rates of hematoma expansion, 3-month outcomes, or response to intensive blood pressure lowering?
Findings
In this predefined subgroup analysis of a randomized clinical trial investigating optimal blood pressure lowering in 167 patients with intracerebral hemorrhage, the rates of hematoma expansion and 3-month death or disability did not differ between patients with microbleeds and those without. Patients with microbleeds responded similarly to intensive treatment.
Meaning
Patients with intracerebral hemorrhage with vs without microbleeds have similar rates of hematoma expansion and death or disability at 3 months, without apparent differential response to intensive blood pressure lowering.
Abstract
Importance
Response to intensive blood pressure (BP) lowering in acute intracerebral hemorrhage (ICH) might vary with the degree of underlying cerebral small vessel disease.
Objectives
To characterize cerebral microbleeds (CMBs) in acute ICH and to assess the potential for interaction between underlying small vessel disease (as indicated by CMB number and location) and assignment to acute intensive BP targeting for functional outcomes and hematoma expansion.
Design, Setting, and Participants
Preplanned subgroup analyses in the Antihypertensive Treatment of Acute Cerebral Hemorrhage 2 (ATACH-2) trial were performed. The ATACH-2 was an open-label international randomized clinical trial that investigated optimal acute BP lowering in 1000 patients with acute ICH. Analyses followed the intent-to-treat paradigm. Participants were enrolled between May 2011 and September 2015 and followed up for 3 months. Eligible participants were aged at least 18 years with ICH volumes less than 60 mL on computed tomography (CT) and a Glasgow Coma Scale score of at least 5 on initial assessment, in whom study drug could be initiated within 4.5 hours of symptom onset. Eight hundred thirty-three participants were excluded, leaving 167 who had an interpretable axial T2*-weighted gradient-recalled echo sequence on magnetic resonance imaging to assess CMBs for inclusion in these subgroup analyses.
Main Outcomes and Measures
The primary outcome of interest was death or disability (modified Ranking Scale score, 4-6) at 3 months. The secondary outcome of interest was hematoma volume expansion of at least 33% on a CT scan obtained 24 hours after randomization compared with the entry scan.
Results
A total of 167 patients were included; their mean (SD) age was 61.9 (13.2) years, and 98 (58.7%) were male. Cerebral microbleeds were present in 120 patients. Forty-six of 157 (29.3%) patients had poor outcome (modified Ranking Scale score, ≥4), and hematoma expansion was observed in 29 of 144 (20.1%) patients. Risk of poor outcome was similar for those assigned to intensive vs standard acute BP lowering among patients with CMBs (relative risk, 1.19; 95% CI, 0.61-2.33; P = .61) and those without CMBs (relative risk, 1.42; 95% CI, 0.43-4.70; P = .57), and no significant interaction was observed (interaction coefficient, 0.18; 95% CI, −1.20 to 1.55; P = .80). Risk of hematoma expansion was also similar, and no significant interaction between treatment and CMBs was observed (interaction coefficient, 0.62; 95% CI, −1.08 to 2.31; P = .48).
Conclusions and Relevance
Cerebral microbleeds are highly prevalent among patients with ICH but do not seem to influence response to acute intensive BP treatment.
Trial Registration
ClinicalTrials.gov Identifier: NCT01176565
This predefined subgroup analysis of a randomized clinical trial characterizes cerebral microbleeds in acute intracerebral hemorrhage and assesses the potential for interaction between underlying small vessel disease and assignment to acute intensive blood pressure targeting for functional outcomes and hematoma expansion.
Introduction
Cerebral microbleeds (CMBs) are remnants of prior cerebral microhemorrhages at the level of arterioles and capillaries visualized on blood-sensitive magnetic resonance imaging (MRI) sequences.1 Among patients with intracerebral hemorrhage (ICH), CMBs are highly prevalent and have evolved as radiological markers of underlying cerebral small vessel disease (CSVD), representing most notably hypertensive arteriopathy (arteriolosclerosis) (deep CMBs) or cerebral amyloid angiopathy (CAA) (strictly lobar CMBs).2 In both CSVD subtypes, advanced disease (marked by increasing CMB counts) is characterized histopathologically by thickened vessel walls.
While many analyses have examined whether CSVD subtypes influence risk of developing incident ICH (or recurrent ICH), one intriguing possibility is that their presence can be used clinically during acute ICH to mark those at highest risk of ongoing bleeding and hematoma expansion. Observational data characterizing the association between CMBs and hematoma expansion have been conflicting.3,4,5 On the one hand, it might be thought that more severe CSVD marks more fragile vessels with higher risk of continued bleeding after ICH.4,5 On the other hand, the thickened vessel walls associated with high CMB counts6 may be more resistant to secondary vessel rupture from perihematomal mechanical shear stress during hematoma expansion, limiting hematoma growth.3,6 Finally, CMBs may have additional important clinical implications as predictors of stroke-related outcome and mortality.7,8,9,10
The results of the Antihypertensive Treatment of Acute Cerebral Hemorrhage 2 (ATACH-2) trial offer a powerful opportunity to examine the association of CMBs, blood pressure (BP) management, hematoma expansion, and outcome in ICH. To explore the role of CSVD in ICH, we performed a preplanned secondary analysis of MRI images obtained during the ATACH-2 trial of intensive BP reduction in ICH. We hypothesized that the likelihood of hematoma expansion, clinical deterioration, and response to intensive BP lowering might vary with the underlying CSVD state, as inferred by CMB number and location.
Methods
Study Design
The rationale, design, and main results of the ATACH-2 international randomized clinical trial have been reported elsewhere.11,12 Spot Sign Score in Restricting ICH Growth (SCORE-IT) is a prospective observational study nested within the ATACH-2 trial with preplanned subgroup analysis of CMBs in trial participants who underwent a clinical brain MRI during their initial hospitalization.13
Standard Protocol Approvals, Registrations, and Patient Consents
The ATACH-2 trial protocol and consent forms were approved by the institutional review board or equivalent ethics committee at each participating site (eAppendix in the Supplement). All participants or their legally authorized representative provided written informed consent.
Study Participants
In brief, ATACH-2 was an open-label international randomized clinical trial that investigated optimal acute BP target in 1000 patients with acute ICH (eFigure in the Supplement). Eligible participants were patients aged at least 18 years with ICH volumes less than 60 mL on computed tomography (CT) and a Glasgow Coma Scale (GCS) score of at least 5 on initial assessment, in whom study drug could be initiated within 4.5 hours of symptom onset. At least one systolic BP reading exceeding 179 mm Hg from the time of symptom onset was required for eligibility.
The ATACH-2 trial participants were eligible for the present subgroup analyses if they had a clinical brain MRI as part of their initial hospitalization. The MRI had to have an interpretable axial T2*-weighted gradient-recalled echo (GRE) sequence allowing for CMB detection.
Intervention
Eligible participants were randomly assigned (1:1) to a systolic BP target of 110 to 139 mm Hg (intensive treatment) vs a target of 140 to 179 mm Hg (standard treatment) with the use of intravenous nicardipine hydrochloride. The infusion was started within 4.5 hours of symptom onset.
Data Collection
Demographic information and vascular risk factors were prospectively recorded at the time of study enrollment. This has been described previously for the ATACH-2 trial.11,12
Imaging Acquisition and Analysis
Computed tomography and MRI images were reviewed centrally by the ATACH-2 and SCORE-IT teams. Intracerebral hemorrhage topography, volume, and associated intraventricular hemorrhage (IVH) were rated on CTs obtained at entry. Intrahematomal contrast extravasation (or spot sign) was rated on CT angiography as previously described.13
In addition to CMBs, MRI markers of interest included diffusion-weighted imaging hyperintense lesions (DWIHLs), defined as regions of intraparenchymal hyperintensity on diffusion-weighted imaging, with associated hypointensity or isointensity on apparent diffusion coefficients that is distinct from the primary ICH and perihematomal edema.14,15 White matter hyperintensity (WMH) was evaluated visually on fluid-attenuated inversion recovery images using the Fazekas scale.15
Cerebral microbleeds were rated according to criteria proposed by the Microbleed Study Group.1 The CMB count severity was coded a priori as absent (0 CMBs), mild (1-2 CMBs), moderate (3-10 CMBs), or severe (>10 CMBs),16 and CMB topography was coded as strictly lobar (with or without concurrent cerebellar CMBs), strictly deep (deep and/or cerebellar CMBs), or mixed (concurrent lobar and deep CMBs).
All MRI images were independently rated by one primary rater (A.S.) and by one secondary rater (A.M., J.O.-F., or F.S.). The primary rater (A.S.) has previously demonstrated excellent intrarater reliability (n = 55, κ = 0.82, 91% agreement)16 and interrater reliability (A.S. and J.O.-F.) (n = 40; intraclass correlation coefficient, 0.99)17 for CMB detection in separate cohorts. The raters were masked to baseline features and outcomes. Any disagreement between the primary rater and secondary rater was reviewed and resolved according to consensus between the primary rater and whichever secondary rater had rated the particular image in question.
Outcomes
The primary outcome of interest in the ATACH-2 trial was death or disability (defined as a modified Ranking Scale score of 4-6) at 3 months. The secondary outcome of interest was hematoma volume expansion of at least 33% on a CT scan obtained 24 hours after randomization compared with the entry scan.
Statistical Analysis
Patient demographic and clinical characteristics were compared between groups in cross-sectional analyses using a χ2 test or Fisher exact test for categorical variables and a t test or Kruskal-Wallis test for continuous variables. The association between CMBs and death or disability was investigated with a multivariable logistic regression analysis and adjusted for assigned treatment group, age, baseline GCS score, the presence or absence of IVH at baseline, and other covariates. The association between CMBs and hematoma expansion was investigated with multivariable logistic regression analysis and adjusted for assigned treatment group, age, WMH score (Fazekas scale total score), and time from onset to baseline CT. These covariates were selected a priori based on the known predictors of these outcomes. Treatment interaction was assessed. Analyses followed the intent-to-treat paradigm, and participants were followed up for 90 days. All tests were 2 sided, and statistical significance was accepted at the .05 level. Analyses were performed with statistical software (SAS, version 9.4; SAS Institute).
A priori power calculations were performed. Assuming that the overall frequency of ICH expansion would be 40%, we projected that 30% of those with CMBs and 50% of those without CMBs will develop expansion. If 500 patients had GRE MRI available, we would have had 91% power to detect this. If 200 patients had these imaging data available, there would need to be a 27% difference in expansion rates to have 80% power to detect this. For the primary outcome of death or disability, if 200 patients had GRE MRI available, we would have 87% power to detect a 40% difference in outcome due to the intervention, and if 500 patients had GRE MRI available, there would need to be a 20% difference in outcome to have 94% power to detect this.
Results
Overall, 167 of 1000 (16.7%) enrolled participants between May 2011 and September 2015 had images available to assess CMBs and were included in these analyses. Of 833 participants who were excluded, 763 (91.6%) did not undergo MRI, 41 (4.9%) did not have an axial GRE sequence available, 15 (1.8%) were enrolled at centers where the institutional review board did not approve central review of MRIs, 12 (1.4%) had uninterpretable (ie, motion degraded) GRE MRI, and 2 (0.2%) were excluded because the MRI suggested that the symptomatic hemorrhage was hemorrhagic infarction rather than the primary ICH. Patients included in this analysis had lower ICH severity as measured by the GCS score and the National Institutes of Health Stroke Scale score compared with the remainder of the ATACH-2 trial participants, manifested higher white blood cell counts, and were less likely to have a history of hypertension, but they did not otherwise differ significantly (eTable in the Supplement). Included participants had a mean (SD) age of 61.9 (13.2) years, and 98 (58.7%) were male. Histories of hypertension (125 of 165 [75.8%]), smoking (71 of 154 [46.1%]), and hyperlipidemia (45 of 162 [27.8%]) were prevalent. Magnetic resonance imaging was performed a median of 1.3 days (interquartile range [IQR], 0.2-5 days) after randomization.
Seventy-two percent (n = 120) of 167 patients had at least one CMB (Table 1). Worse renal function was overrepresented in patients with CMBs compared with those without CMBs. Black and Asian race/ethnicity seemed to be overrepresented in patients with CMBs. Table 2 lists detailed neuroimaging findings in the patients with CMBs. Their median CMB count was 4 (IQR, 1-12). Among patients with at least one CMB, CMBs were strictly lobar in 30 patients (25.0%), strictly deep in 34 patients (28.3%), and mixed in 56 patients (46.7%). Patients with CMBs had greater degrees of WMH on MRI but not DWIHLs. There were no appreciable differences in regard to ICH volume or topography, the presence of IVH, or computed tomographic angiography (CTA) spot sign between patients with and without CMBs.
Table 1. Baseline Characteristics and Neuroimaging Findings by Status of Cerebral Microbleeds (CMBs).
| Variable | No CMBs (n = 47) | CMBs (n = 120) |
|---|---|---|
| Demographic and Clinical Characteristics | ||
| Male, No. (%) | 25 (53.2) | 73 (60.8) |
| Age, mean (SD), y | 61.6 (13.4) | 62.0 (13.2) |
| Race/ethnicity, No. (%) | ||
| White | 21 (44.7) | 31 (25.8) |
| Black | 5 (10.6) | 22 (18.3) |
| Asian | 21 (44.7) | 63 (52.5) |
| Other | 0 | 4 (3.3) |
| Smoking, No./total No. (%) | 16/44 (36.4) | 55/110 (50.0) |
| Cocaine use, No./total No. (%) | 2/46 (4.3) | 8/105 (7.6) |
| Hypertension, No./total No. (%) | 33/47 (70.2) | 92/118 (78.0) |
| SBP at initial presentation, mean (SD), mm Hg | 197.2 (27.1) | 198.9 (27.3) |
| DBP at initial presentation, mean (SD), mm Hg | 109.3 (22.2) | 111.5 (19.7)a |
| Prior stroke/transient ischemic attack, No. (%) | 8 (17.0) | 18 (15.0) |
| Congestive heart failure, No. (%) | 2 (4.3) | 5 (4.2) |
| Atrial fibrillation, No. (%) | 0 | 2 (1.7) |
| Diabetes, No./total No. (%) | 8/47 (17.0) | 23/118 (19.5) |
| Ischemic heart disease, No. (%)b | 2 (4.3) | 2 (1.7) |
| Hyperlipidemia, No./total No. (%) | 14/46 (30.4) | 31/116 (26.7) |
| Peripheral vascular disease, No./total No. (%) | 0/46 | 3/120 (2.5) |
| Time from onset to baseline computed tomography, median (IQR), min | 79 (64-108) | 80 (58-121) |
| Laboratory Values | ||
| Total white blood cell count, mean (SD), /μL | 7800 (2700) | 7500 (2400) |
| Platelet count, mean (SD), ×103/μL | 211.4 (47.4) | 215.1 (57.4) |
| Hemoglobin, mean (SD), g/dL | 14.3 (1.4) | 14.3 (1.7) |
| Hematocrit, mean (SD), % | 42.4 (3.7) | 42.2 (4.9) |
| Serum glucose, median (IQR), mg/dL | 125 (106-156) | 119 (105-143) |
| Serum creatinine, median (IQR), mg/dL | 0.8 (0.7-1.1) | 0.9 (0.7-1.2) |
| Creatinine >1.5 mg/dL, No. (%) | 1 (2.1) | 17 (14.2) |
| Neurological Scales | ||
| Baseline Glasgow Coma Scale score, No. (%) | ||
| 3-11 | 5 (10.6) | 12 (10.0) |
| 12-14 | 11 (23.4) | 34 (28.3) |
| 15 | 31 (66.0) | 74 (61.7) |
| Baseline NIHSS score, median (IQR) | 9 (6-16) | 9 (4-15) |
| Assigned Treatment Group, No. (%) | ||
| Intensive treatment | 27 (57.4) | 55 (45.8) |
Abbreviations: DBP, diastolic blood pressure; IQR, interquartile range; NIHSS, National Institutes of Health Stroke Scale; SBP, systolic blood pressure.
SI conversion factors: To convert white blood cell count to ×109/L, multiply by 0.001; platelet count to ×109/L, multiply by 1.0; hemoglobin level to grams per liter, multiply by 10.0; hematocrit to proportion of 1.0, multiply by 0.01; glucose level to millimoles per liter, multiply by 0.0555; creatinine level to micromoles per liter, multiply by 88.4.
Data were missing for 1 patient in the CMB group.
Previous coronary artery bypass grafting, myocardial infarction within 3 months, angina pectoris, or percutaneous transluminal coronary angioplasty.
Table 2. Neuroimaging Findings by Status of Cerebral Microbleeds (CMBs).
| Variable | No CMBs (n = 47) | CMBs (n = 120) |
|---|---|---|
| CT Findings, No./Total No. (%) | ||
| ICH location | ||
| Lobar | 12/46 (26.1) | 26/119 (21.8) |
| Basal ganglia | 22/46 (47.8) | 58/119 (48.7) |
| Thalamus | 12/46 (26.1) | 35/119 (29.4) |
| Intraventricular hemorrhage | 12/46 (26.1) | 20/119 (16.8) |
| ICH volume >30 mL | 6/46 (13.0) | 6/119 (5.0) |
| CTA spot sign | 4/13 (30.8) | 12/28 (42.9) |
| MRI Findings | ||
| Fazekas scale total score, mean (IQR) | 4 (3-5)a | 5 (3-5)b |
| DWIHLs, No./total No. (%) | 11/44 (25.0) | 21/103 (20.4) |
| CMB count, median (IQR) | NA | 4 (1-12) |
| CMB topography, median (IQR) | ||
| Strictly lobar | NA | 1 (1-3) |
| Strictly deep | NA | 2 (1-3) |
| Mixed | NA | 11 (4-24) |
Abbreviations: CT, computed tomography; CTA, computed tomographic angiography; DWIHLs, diffusion-weighted imaging hyperintense lesions; ICH, intracerebral hemorrhage; IQR, interquartile range; MRI, magnetic resonance imaging; NA, not applicable.
Data were missing for 1 patient.
Data were missing for 7 patients.
During a mean (SD) follow-up of 92.3 (8.3) days, 46 of 157 (29.3%) patients had poor outcome of death or disability (modified Ranking Scale score, ≥4), including 12 of 41 (29.3%) patients without CMBs and 34 of 116 (29.3%) patients with CMBs (Table 3). Participants with CMBs were not at increased risk of death or disability (adjusted relative risk [aRR], 0.83; 95% CI, 0.40-1.71; P = .61). Hematoma expansion was observed in 29 of 144 (20.1%) patients, including 8 of 40 (20.0%) patients without CMBs and 21 of 104 (20.2%) patients with CMBs. In multivariable analysis, patients with CMBs were not at reduced risk of hematoma expansion (relative risk [RR], 1.00; 95% CI, 0.42-2.39; P = .99). The lack of association between CMBs and the outcomes of interest was consistent across the prespecified CMB severity and topography categories. In post hoc exploratory analyses of 13 of 167 (7.8%) participants fulfilling clinicoradiographic criteria for probable CAA (lobar ICH with strictly lobar CMBs18), we detected a difference in the rates of hematoma expansion at 24 hours between patients with probable CAA (5 of 9 [55.6%]) and patients without CMBs (8 of 40 [20.0%]) (crude RR, 2.78; 95% CI, 1.19-6.51; P = .04). However, this association did not withstand adjusted analyses (aRR, 1.79; 95% CI, 0.44-7.31; P = .42). The lack of association with death or disability was consistent in patients with probable CAA.
Table 3. Primary and Secondary Outcomes.
| Outcome | Death or Disability at 3 mo | Hematoma Expansion at 24 h | ||||
|---|---|---|---|---|---|---|
| No. of Events | Crude RR (95% CI) | Adjusted RR (95% CI)a | No. of Events | Crude RR (95% CI) | Adjusted RR (95% CI)b | |
| CMB Status | ||||||
| No CMBs (n = 47) | 12/41 | 1 [Reference] | 1 [Reference] | 8/40 | 1 [Reference] | 1 [Reference] |
| CMBs (n = 120) | 34/116 | 1.00 (0.52-1.93) | 0.83 (0.40-1.71) | 21/104 | 1.01 (0.45-2.28) | 1.00 (0.42-2.39) |
| CMB Count | ||||||
| None (n = 47) | 12/41 | 1 [Reference] | 1 [Reference] | 8/40 | 1 [Reference] | 1 [Reference] |
| 1-2 (n = 45) | 11/44 | 0.85 (0.38-1.94) | 0.76 (0.33-1.76) | 8/40 | 1.00 (0.38-2.66) | 1.03 (0.37-2.87) |
| 3-10 (n = 42) | 13/42 | 1.06 (0.48-2.32) | 0.90 (0.37-2.24) | 7/38 | 0.92 (0.33-2.54) | 0.89 (0.30-2.64) |
| >10 (n = 33) | 10/30 | 1.14 (0.49-2.64) | 0.90 (0.34-2.42) | 6/26 | 1.15 (0.40-3.33) | 1.11 (0.36-3.44) |
| CMB Topography | ||||||
| None (n = 47) | 12/41 | 1 [Reference] | 1 [Reference] | 8/40 | 1 [Reference] | 1 [Reference] |
| Strictly lobar (n = 30) | 9/30 | 1.03 (0.43-2.43) | 0.90 (0.36-2.28) | 7/26 | 1.35 (0.49-3.71) | 1.15 (0.38-3.48) |
| Strictly deep (n = 34) | 8/34 | 0.80 (0.33-1.97) | 0.74 (0.29-1.91) | 6/31 | 0.97 (0.34-2.79) | 1.15 (0.39-3.45) |
| Mixed (n = 56) | 17/52 | 1.12 (0.53-2.34) | 0.85 (0.34-2.08) | 8/47 | 0.85 (0.32-2.27) | 0.83 (0.29-2.34) |
Abbreviations: CMBs, cerebral microbleeds; RR, relative risk.
Multivariable analysis adjusting for age, systolic blood pressure at initial presentation, baseline Glasgow Coma Scale score, assigned treatment group, the presence or absence of intraventricular hemorrhage at baseline, Fazekas scale total score, and estimated glomerular filtration rate.
Multivariable analysis adjusting for age, assigned treatment group, Fazekas scale total score, and time from onset to baseline computed tomography.
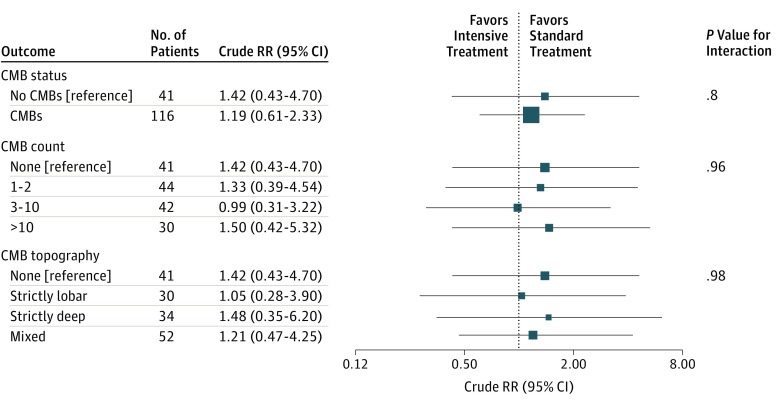
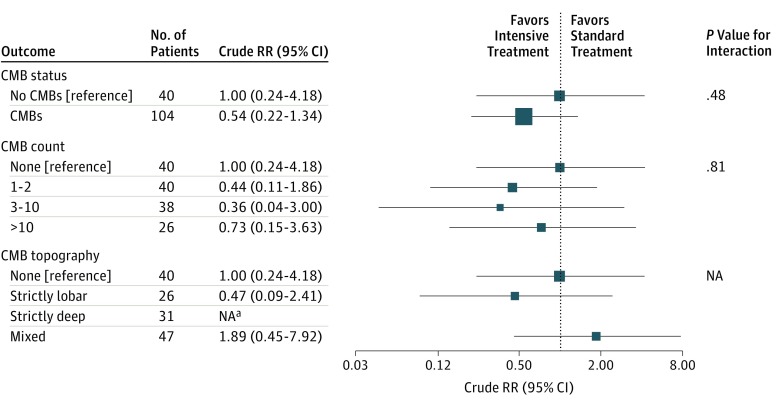
Risk of poor outcome was similar for those assigned to intensive vs standard acute BP lowering among patients with CMBs (crude RR, 1.19; 95% CI, 0.61-2.33; P = .61) and those without CMBs (crude RR, 1.42; 95% CI, 0.43-4.70; P = .57), and no significant interaction was observed (interaction coefficient, 0.18; 95% CI, −1.20 to 1.55; P = .80) (Figure 1). The rates of hematoma expansion at 24 hours were similar with intensive acute BP lowering in patients with CMBs (crude RR, 0.54; 95% CI, 0.22-1.34; P = .18) compared with those without CMBs (crude RR, 1.00; 95% CI, 0.24-4.18; P = 1.00), and no significant interaction between treatment and CMBs was observed (interaction coefficient, 0.62; 95% CI, −1.08 to 2.31; P = .48) (Figure 2). There was no effect modification observed with the prespecified CMB severity and topography categories for either outcome or in exploratory post hoc analyses of patients with probable CAA.
Figure 1. Death or Disability at 3 Months and Response to Treatment Assignment by Status of Cerebral Microbleeds (CMBs).
RR indicates relative risk.
Figure 2. Hematoma Expansion at 24 Hours and Response to Treatment Assignment by Status of Cerebral Microbleeds (CMBs).
RR indicates relative risk; NA, not applicable. aCannot be shown because of small number of events.
Discussion
In this well-characterized cohort of patients with ICH of mild to moderate severity who had an acute hypertensive response (SBP, >179 mm Hg), CMBs were highly frequent and disproportionately associated with renal dysfunction. In addition, CMBs were associated with the presence of WMH. We did not observe greater death or disability at 3 months in patients with CMBs, and the rates of hematoma expansion were similar in patients with CMBs compared with those without CMBs. Moreover, there was no interaction observed between the degree of BP lowering and CMBs for the outcomes of death or disability at 3 months or hematoma expansion at 24 hours.
The observed 71.9% (120 of 167) prevalence of CMBs in our ICH cohort is higher than that previously reported in Western populations19 and likely reflects the large proportion of Asian participants in the present study. This may also be explained by the fact that the trial’s eligibility criteria mandated an acute hypertensive response with SBP exceeding 179 mm Hg, which would have selected patients having ICH with more advanced CSVD. The association between CMBs and poor renal function is consistent with the premise that both renal dysfunction and CMBs can serve as markers of hypertensive end-organ damage.16,20,21
Contrary to our hypothesis, CMBs were not a predictor of death or disability at 3 months. Accordingly, underlying vascular disease and particularly CSVD may have confounded the previously reported associations with mortality in broader populations.9,10 Fittingly, CMBs were not reported to be a predictor of mortality in patients with lacunar stroke enrolled in the Secondary Prevention of Small Subcortical Strokes (SPS3) trial.16 The lack of an association between CMBs and hematoma expansion or CTA spot sign was unexpected. Prior studies4,5 have demonstrated associations between CMBs and hematoma expansion or CTA spot sign in the setting of CAA-related ICH. Therefore, it is possible that patients with CAA-related ICH, who may typically be seen with lower levels of acute hypertensive response, were underrepresented in the ATACH-2 trial. Indeed, only 7.8% (13 of 167) of participants included in our analyses fulfilled criteria for probable CAA.
Strengths and Limitations
To our knowledge, these reported findings are the first assessing effect modification between CMBs and randomized hyperacute stroke therapies. We did not detect a treatment interaction between treatment assignment and CMBs for the outcomes of death or disability at 3 months or hematoma expansion at 24 hours, although our sample size was lower than expected and may have lacked sufficient power to detect an effect of even moderate size. There was a suggestion for change in the direction of the point effect estimate favoring intensive BP lowering in patients with CMBs for the outcome of hematoma expansion. However, because the treatment interaction was statistically insignificant and our analysis had limited power to confidently detect such an effect, these observations require further exploration in larger samples. Moreover, our results were limited by the unavailability of MRI sequences to allow for CMB assessment in all ATACH-2 trial participants and by the trial’s eligibility criteria, which limit the generalizability of our findings to all ICH. Selection bias was indeed evident, with patients undergoing MRI who were included in our analysis having less neurological deficit than the other ATACH-2 trial participants. The nonstandardization of GRE sequence acquisition parameters and the unavailability of data on these parameters, which were never captured, is a major limitation that may have resulted in heterogeneous CMB detection rates across the various recruitment centers and confounded our results. A final limitation is that our sample was underpowered to appropriately assess risk by CMB burden and topography.
Conclusions
Subgroup analysis of the first randomized clinical trial to date assessing treatment interactions with CMBs in patients with acute ICH demonstrated that CMBs are highly frequent in patients with ICH of mild to moderate severity who are seen with an acute hypertensive response (SBP, >179 mm Hg). We did not find that CMBs influence ICH-related death or disability at 3 months or hematoma expansion at 24 hours. Response to intensive acute BP treatment did not differ in patients having ICH with vs without CMBs.
eAppendix. Supplemental Appendix
eFigure. Flow Diagram of the ATACH-II Trial
eTable. Comparison of Baseline Characteristics and Neuroimaging Findings for Subjects Included in CMB Analysis and the Rest of the Subjects From ATACH-2
References
- 1.Greenberg SM, Vernooij MW, Cordonnier C, et al. ; Microbleed Study Group . Cerebral microbleeds: a guide to detection and interpretation. Lancet Neurol. 2009;8(2):165-174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shoamanesh A, Kwok CS, Benavente O. Cerebral microbleeds: histopathological correlation of neuroimaging. Cerebrovasc Dis. 2011;32(6):528-534. [DOI] [PubMed] [Google Scholar]
- 3.Martí-Fàbregas J, Delgado-Mederos R, Granell E, et al. ; RENEVAS Group (Stroke Research Network, RETICS, Instituto de Salud Carlos III) . Microbleed burden and hematoma expansion in acute intracerebral hemorrhage. Eur Neurol. 2013;70(3-4):175-178. [DOI] [PubMed] [Google Scholar]
- 4.Evans A, Demchuk A, Symons SP, et al. The spot sign is more common in the absence of multiple prior microbleeds. Stroke. 2010;41(10):2210-2217. [DOI] [PubMed] [Google Scholar]
- 5.Boulouis G, van Etten ES, Charidimou A, et al. Association of key magnetic resonance imaging markers of cerebral small vessel disease with hematoma volume and expansion in patients with lobar and deep intracerebral hemorrhage. JAMA Neurol. 2016;73(12):1440-1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Greenberg SM, Nandigam RN, Delgado P, et al. Microbleeds versus macrobleeds: evidence for distinct entities. Stroke. 2009;40(7):2382-2386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Altmann-Schneider I, Trompet S, de Craen AJ, et al. Cerebral microbleeds are predictive of mortality in the elderly. Stroke. 2011;42(3):638-644. [DOI] [PubMed] [Google Scholar]
- 8.Song TJ, Kim J, Song D, et al. Association of cerebral microbleeds with mortality in stroke patients having atrial fibrillation. Neurology. 2014;83(15):1308-1315. [DOI] [PubMed] [Google Scholar]
- 9.Akoudad S, Ikram MA, Koudstaal PJ, Hofman A, van der Lugt A, Vernooij MW. Cerebral microbleeds and the risk of mortality in the general population. Eur J Epidemiol. 2013;28(10):815-821. [DOI] [PubMed] [Google Scholar]
- 10.Romero JR, Preis SR, Beiser A, et al. Cerebral microbleeds as predictors of mortality: the Framingham Heart Study. Stroke. 2017;48(3):781-783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Qureshi AI, Palesch YY. Antihypertensive Treatment of Acute Cerebral Hemorrhage (ATACH) II: design, methods, and rationale. Neurocrit Care. 2011;15(3):559-576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Qureshi AI, Palesch YY, Barsan WG, et al. ; ATACH-2 Trial Investigators and the Neurological Emergency Treatment Trials Network . Intensive blood-pressure lowering in patients with acute cerebral hemorrhage. N Engl J Med. 2016;375(11):1033-1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goldstein J, Brouwers H, Romero J, et al. SCORE-IT: the Spot Sign Score in Restricting ICH Growth: an ATACH-II ancillary study. J Vasc Interv Neurol. 2012;5(suppl):20-25. [PMC free article] [PubMed] [Google Scholar]
- 14.Menon RS, Burgess RE, Wing JJ, et al. Predictors of highly prevalent brain ischemia in intracerebral hemorrhage. Ann Neurol. 2012;71(2):199-205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wahlund LO, Barkhof F, Fazekas F, et al. ; European Task Force on Age-Related White Matter Changes . A new rating scale for age-related white matter changes applicable to MRI and CT. Stroke. 2001;32(6):1318-1322. [DOI] [PubMed] [Google Scholar]
- 16.Shoamanesh A, Pearce LA, Bazan C, et al. ; SPS3 Trial Investigators . Microbleeds in the Secondary Prevention of Small Subcortical Strokes trial: stroke, mortality, and treatment interactions. Ann Neurol. 2017;82(2):196-207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shoamanesh A, Martinez-Ramirez S, Oliveira-Filho J, et al. Interrelationship of superficial siderosis and microbleeds in cerebral amyloid angiopathy. Neurology. 2014;83(20):1838-1843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Knudsen KA, Rosand J, Karluk D, Greenberg SM. Clinical diagnosis of cerebral amyloid angiopathy: validation of the Boston criteria. Neurology. 2001;56(4):537-539. [DOI] [PubMed] [Google Scholar]
- 19.Cordonnier C, Al-Shahi Salman R, Wardlaw J. Spontaneous brain microbleeds: systematic review, subgroup analyses and standards for study design and reporting. Brain. 2007;130(pt 8):1988-2003. [DOI] [PubMed] [Google Scholar]
- 20.Shoamanesh A, Catanese L, Romero JR, et al. High prevalence of cerebral microbleeds in inner city young stroke patients. J Stroke Cerebrovasc Dis. 2016;25(4):733-738. [DOI] [PubMed] [Google Scholar]
- 21.Ovbiagele B, Wing JJ, Menon RS, et al. Association of chronic kidney disease with cerebral microbleeds in patients with primary intracerebral hemorrhage. Stroke. 2013;44(9):2409-2413. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eAppendix. Supplemental Appendix
eFigure. Flow Diagram of the ATACH-II Trial
eTable. Comparison of Baseline Characteristics and Neuroimaging Findings for Subjects Included in CMB Analysis and the Rest of the Subjects From ATACH-2