Abstract
Importance
Effective treatments for chronic spinal pain are essential to reduce the related high personal and socioeconomic costs.
Objective
To compare pain neuroscience education combined with cognition-targeted motor control training with current best-evidence physiotherapy for reducing pain and improving functionality, gray matter morphologic features, and pain cognitions in individuals with chronic spinal pain.
Design, Setting, and Participants
Multicenter randomized clinical trial conducted from January 1, 2014, to January 30, 2017, among 120 patients with chronic nonspecific spinal pain in 2 outpatient hospitals with follow-up at 3, 6, and 12 months.
Interventions
Participants were randomized into an experimental group (combined pain neuroscience education and cognition-targeted motor control training) and a control group (combining education on back and neck pain and general exercise therapy).
Main Outcomes and Measures
Primary outcomes were pain (pressure pain thresholds, numeric rating scale, and central sensitization inventory) and function (pain disability index and mental health and physical health).
Results
There were 22 men and 38 women in the experimental group (mean [SD] age, 39.9 [12.0] years) and 25 men and 35 women in the control group (mean [SD] age, 40.5 [12.9] years). Participants in the experimental group experienced reduced pain (small to medium effect sizes): higher pressure pain thresholds at primary test site at 3 months (estimated marginal [EM] mean, 0.971; 95% CI, –0.028 to 1.970) and reduced central sensitization inventory scores at 6 months (EM mean, –5.684; 95% CI, –10.589 to –0.780) and 12 months (EM mean, –6.053; 95% CI, –10.781 to –1.324). They also experienced improved function (small to medium effect sizes): significant and clinically relevant reduction of disability at 3 months (EM mean, –5.113; 95% CI, –9.994 to –0.232), 6 months (EM mean, –6.351; 95% CI, –11.153 to –1.550), and 12 months (EM mean, –5.779; 95% CI, –10.340 to –1.217); better mental health at 6 months (EM mean, 36.496; 95% CI, 7.998-64.995); and better physical health at 3 months (EM mean, 39.263; 95% CI, 9.644-66.882), 6 months (EM mean, 53.007; 95% CI, 23.805-82.209), and 12 months (EM mean, 32.208; 95% CI, 2.402-62.014).
Conclusions and Relevance
Pain neuroscience education combined with cognition-targeted motor control training appears to be more effective than current best-evidence physiotherapy for improving pain, symptoms of central sensitization, disability, mental and physical functioning, and pain cognitions in individuals with chronic spinal pain. Significant clinical improvements without detectable changes in brain gray matter morphologic features calls into question the relevance of brain gray matter alterations in this population.
Trial Registration
clinicaltrials.gov Identifier: NCT02098005
This randomized clinical trial compares pain neuroscience education combined with cognition-targeted motor control training with current best-evidence physiotherapy for reducing pain and improving functionality, gray matter morphologic features, and pain cognitions in individuals with chronic spinal pain.
Key Points
Question
Can a program of pain neuroscience education combined with cognition-targeted motor control training reduce pain and improve function, gray matter morphologic features, and pain cognitions in individuals with chronic spinal pain?
Findings
Results from this randomized clinical trial of 120 individuals with chronic spinal pain indicate that pain neuroscience education combined with cognition-targeted motor control training is superior to usual care at reducing pain and improving function and pain cognitions. Gray matter morphologic features did not change in response to treatment.
Meaning
Clinically relevant changes in response to effective treatment without changes in gray matter morphologic features question the relevance of the well-established alterations at brain level in individuals with chronic spinal pain.
Introduction
Nonspecific chronic spinal pain (nCSP) is associated with significant health care use and high rates of disability worldwide.1 Currently, nonsurgical treatments for nCSP, including nonsteroidal anti-inflammatory drugs, joint manipulation, acupuncture, and exercise therapy, seem to have limited benefits (small to moderate effect sizes).2 This finding may be explained by the fact that such treatments do not comply with recent advances in chronic pain research.
These recent advances suggest hyperexcitability of the central nervous system, including malfunctioning of descending nociceptive inhibition, and gray matter morphologic changes in people with nCSP.3,4,5 These brain alterations—with a trend toward decreased gray matter volume—are reported in regions involved in modulatory, emotional-affective, and sensory-discriminative pain processing.4 More important, these morphologic changes are suggested to be reversible in response to successful treatment.6 However, to our knowledge, controlled clinical studies investigating this outcome in people with nCSP are currently lacking.
Individuals with nCSP also tend to have inappropriate pain cognitions associated with poor treatment outcome, such as kinesiophobia, hypervigilance, and pain catastrophizing.7,8,9 As with the changes in gray matter morphology, current treatment of nCSP does not account for inappropriate pain cognitions.2 Therefore, a treatment addressing both gray matter morphologic findings and inappropriate pain cognitions might result in larger effect sizes and clinically relevant changes. Such an approach (ie, combined pain neuroscience education with cognition-targeted exercise therapy) has previously been investigated in a pilot study with 1 year of follow-up, including a small sample of people with chronic low back pain.10 The reductions in pain and disability were high, but that study did not include a comparison with current best-evidence physiotherapy and did not include individuals with chronic neck pain, despite the similarities in underlying pain mechanisms between people with neck pain and those with low back pain.11
Based on the above-described voids, this sufficiently powered, multicenter randomized clinical trial investigated whether pain neuroscience education combined with cognition-targeted motor control training is superior to current best-evidence physiotherapy in reducing pain and improving functionality, gray matter morphologic features, and pain cognitions in individuals with nCSP.
Methods
Design
Data were collected from January 1, 2014, to January 30, 2017, in the University Hospitals of Ghent and Brussels in Belgium. This study was approved by the ethics committees of the University Hospital of Ghent (2013/1133) and Brussels (2013/385). Participants provided written informed consent. A detailed study protocol has been published12 (see also study protocol in Supplement 1).
Blinding
The study participants, statistician, and outcomes assessors were blinded (triple-blind study). Participants did not meet in the waiting rooms (no contamination).
Study Population and Sample Size
Participants were recruited through flyers distributed to the university hospitals and universities, occupational health services, and primary care practices and via social media and advertisements. Inclusion criteria were as follows: native Dutch speaking, 18 to 65 years of age, and having nCSP (≥3 days per week and ≥3months of chronic low back pain, failed back surgery syndrome >3 year prior, chronic whiplash, or chronic nontraumatic neck pain). Participants were not allowed to continue other therapies, except for usual medication. Participants were asked not to start a new therapy or new medication 6 weeks before the trial and during study participation. Exclusion criteria were specific medical conditions (neuropathic pain, neck or back surgery in the prior 3 years, osteoporotic vertebral fractures, or rheumatologic diseases) or chronic widespread pain syndromes (fibromyalgia or chronic fatigue syndrome) and residence more than 50 km away from the hospital (to avoid dropping out of the trial).
Sample size was calculated using G*Power software (Universität Düsseldorf) based on the effects on pain in the pilot study (partial η2 = 0.02, α = .05, power = 0.80) and accounted for F tests and 30% loss to follow-up after 1 year, resulting in a total sample size of 117 individuals.10
Randomization
Randomization was performed at the Biostatistics Unit (Ghent University) by an independent investigator, using a stratified permuted block allocation (block size of 4). Stratification factors were treatment center, dominant pain location, and sex.13
Outcome Measures
The clinimetric properties and details of the questionnaires (all Dutch versions) can be found in the a priori published protocol.12 Analyses of outcome measures related to motor control and muscle properties will be reported in a subsequent article.
Primary Outcome Measures: Pain and Function
Pain was measured using 2 questionnaires (the Numeric Rating Scale [NRS]14 and the Central Sensitization Inventory [CSI]15) and 2 experimental pain measures (pressure pain thresholds [PPTs] and conditioned pain modulation [CPM]). The NRS measures the mean pain intensity during the last 3 days using an 11-point scale, with a 30% decrease in the NRS score considered clinically important.14 The CSI assesses the presence of hypersensitivity by evaluating 25 symptoms that people with chronic pain might encounter (eg, sensitivity to light or concentration difficulties) on a 5-point Likert-scale (where 0 indicates that this symptom never occurs and 5 indicates that this symptom always occurs).15 Pressure pain thresholds (ie, the point of minimum pressure that induces an unpleasant sensation) were determined using a digital pressure algometer with a 1-cm2 tip (Wagner Instruments) at the most painful side or at the dominant side in case of bilateral pain. Pressure pain thresholds were randomly measured twice at the symptomatic sites (trapezius muscle midway between C7 and the acromion tip and 5 cm lateral of the spinous process of L3) and at the remote sites (quadriceps muscle and the web between the thumb and index finger). The CPM paradigm evaluates the descending nociceptive inhibition efficacy, using a cold-water bath (12°C; Versacool) for 2 minutes’ immersion of the hand contralateral to the PPT measurements. After 30 seconds, PPTs were measured again. Initial PPTs were subtracted from PPTs during the cold pressor test. A negative value indicates an impaired inhibitory response (ie, lower PPT than before the cold pressor test). A positive value indicates an inhibitory response (ie, higher PPT than before the cold pressor test).
Function was measured using the 36-Item Short Form Health Survey (SF36),16 which evaluates health-related quality of life and is analyzed into 2 main domains (the physical and mental component), and the Pain Disability Index (PDI), which assesses the level of perceived disability due to pain during 7 daily-life activities, such as family and home responsibilities, recreation, and social activities. A decrease in PDI scores of 8.5 to 9.5 is considered to be clinically relevant.17
Secondary Outcome Measures
Gray matter morphologic features were evaluated using magnetic resonance imaging (Siemens; 32-channel radiofrequency head coil). Details on the type of scan and the preprocessing analysis in FreeSurfer (Athinoula A. Martinos Center for Biomedical Imaging at Massachusetts General Hospital) can be found in the eAppendix in Supplement 2. The gray matter cortical thickness of 10 a priori selected cortical regions based on the Desikan gyral parcellation18 (caudal middle frontal, inferior parietal, inferior temporal, medial orbitofrontal, parahippocampal, postcentral, precentral, rostral middle frontal, superior parietal, and supramarginal gyri) and the gray matter volumes of 5 a priori selected regions from the FreeSurfer subcortical segmentation (amygdala, caudate, hippocampus, putamen, and thalamus) were derived for further statistical analysis.19 These selected regions of interest have shown alterations after conservative treatments in populations with chronic musculoskeletal pain.6
To evaluate pain cognitions, the following questionnaires were used: the Pain Catastrophizing Scale,20 which evaluates catastrophic thoughts and feelings regarding pain; the Tampa Scale for Kinesiophobia,21 which assesses fear of movement or (re)injury; and the Pain Vigilance and Awareness Questionnaire,22 which measures the participants’ awareness of and attention to pain.
Intervention
Both interventions (duration, 12 weeks) comprised 3 educational sessions (group session, home-based online module, and individual session) and 15 one-on-one exercise sessions. The content of the therapy differed between groups (eTable 1 in Supplement 2).
The experimental intervention combined pain neuroscience education with cognition-targeted motor control training. Pain neuroscience education aims to reconceptualize patients’ beliefs about pain, to increase their knowledge of pain and to decrease its threat. The content included the following topics in nontechnical terms: the neuron, the synapse, descending nociceptive inhibition and facilitation, peripheral sensitization, and central sensitization.23 The exercise program started with sensorimotor control training, adapted to comply with modern pain neuroscience using a time-contingent rather than pain-contingent approach (eTable 1 in Supplement 2) and aiming to change inappropriate beliefs and perceptions into correct ones. Simultaneously, movements that participants feared and avoided were introduced using a graded approach with increasing complexity (ie, progression toward physically, cognitively, and psychosocially demanding situations). The detailed treatment protocol is available elsewhere.24
The control intervention comprised current best-evidence physiotherapy, including traditional back and neck education and general exercises.25,26 The education covered the following topics: mechanical causes of back and neck pain; spine anatomy, physiology, and biomechanics; the importance of self-care and ergonomics; intradiscal pressure and joint forces; lifting techniques; and the value of stretching and strength, endurance, and fitness training. The pain-contingent exercise program focused on treating biomedical dysfunctions of the spine (ie, mobility, muscle strength, muscle endurance, and general fitness exercises), with an evolution toward functional activities and physically demanding tasks while keeping the spine in physiologically neutral positions. When the participant reported pain during or after an exercise, the intensity or duration of the exercise was reduced.
Statistical Analysis
All data were analyzed using SPSS, version 24.0 (SPSS Inc). Measurements of PPT and CPM at the primary body site (lower back and trapezius muscle) were taken together using body site as a covariate in the model. For each variable, the percentage of change compared with baseline was calculated. Effect sizes of the mean group differences were calculated as the Cohen d. To assess the difference between groups in response to treatment, a random-intercept linear mixed models analysis, using Bonferroni post hoc analyses, was applied with an unstructured covariance matrix. The model included treatment, time, and treatment × time as fixed effects together with a random intercept for each patient. P < .05 (2-sided) was considered significant.
Results
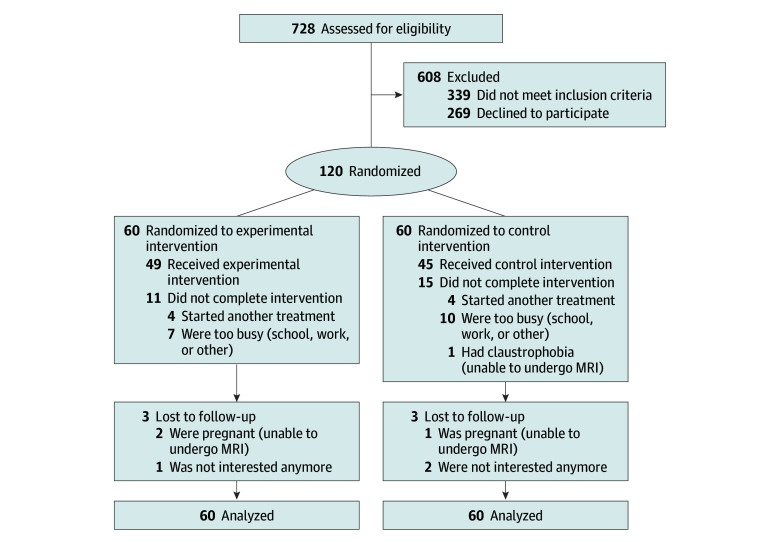
A total of 120 people with nCSP were included in the trial and received pain neuroscience education combined with cognition-targeted motor control training (experimental treatment [n = 60]) or current best-evidence physiotherapy (control treatment [n = 60]) (Figure). The baseline characteristics of these participants are presented in Table 1.
Figure. CONSORT Flow Diagram.
MRI indicates magnetic resonance imaging.
Table 1. Demographic and Baseline Characteristics of the Participants With Chronic Spinal Paina.
| Characteristic | Mean (SD) | Median (IQR) [Range] |
|---|---|---|
| Demographic | ||
| Age, y | ||
| Experimental group | 39.91 (11.95) | 37.50 (24.00) [20-65] |
| Control group | 40.53 (12.88) | 40.00 (22.00) [19-65] |
| Body height, cm | ||
| Experimental group | 172.86 (8.86) | 172.00 (12.00) [157.00-201.00] |
| Control group | 171.52 (10.65) | 170.00 (17.00) [153.00-197.00] |
| Body weight, kg | ||
| Experimental group | 69.68 (14.28) | 66.00 (19.00) [49.00-110.00] |
| Control group | 71.11 (13.78) | 69.50 (18.80) [46.00-108.00] |
| Body mass indexb | ||
| Experimental group | 23.16 (3.30) | 23.31 (4.74) [16.65-32.41] |
| Control group | 24.09 (3.81) | 23.65 (4.69) [18.44-36.11] |
| Pain duration, mo | ||
| Experimental group | 121.55 (100.77) | 97.00 (154.50) [6.00-420.00] |
| Control group | 103.41 (82.88) | 68.75 (105.00) [7.00-348.00] |
| Baseline | ||
| PPT prim, kgfc | ||
| Experimental group | 4.56 (2.40) | 4.99 (3.35) [1.19-13.67] |
| Control group | 4.43 (2.45) | 4.35 (3.35) [0.80-11.57] |
| PPT hand, kgf | ||
| Experimental group | 3.60 (1.88) | 3.84 (2.25) [1.10-10.67] |
| Control group | 3.60 (1.87) | 3.39 (2.04) [1.31-11.63] |
| PPT leg, kgf | ||
| Experimental group | 5.33 (2.57) | 5.71 (3.77) [1.60-16.42] |
| Control group | 5.08 (2.53) | 4.90 (3.76) [1.97-14.58] |
| CMP prim, scorec | ||
| Experimental group | 1.08 (1.38) | 1.02 (1.59) [−2.18 to 5.69] |
| Control group | 1.05 (1.30) | 1.04 (1.79) [−3.43 to 5.96] |
| CPM leg, score | ||
| Experimental group | 1.03 (1.25 | 1.09 (1.75 [−2.17 to 6.08] |
| Control group | 1.01 (1.46) | 0.65 (1.48) [−3.32 to 7.31] |
| NRS score (out of 10) | ||
| Experimental group | 5.15 (1.89) | 5.00 (3.00) [2.00-10.00] |
| Control group | 4.98 (1.90) | 5.00 (4.00) [2.00-8.00] |
| CSI score (out of 100) | ||
| Experimental group | 40.17 (11.43) | 37.00 (13.00) [15.00-72.00] |
| Control group | 39.88 (11.39) | 39.50 (16.00) [12.00-64.00] |
| PDI score (out of 70) | ||
| Experimental group | 21.92 (14.61) | 19.00 (24.00) [4.00-63.00] |
| Control group | 21.58 (13.43) | 18.50 (16.00) [0.00-63.00] |
| SF36 mental health score (out of 400) | ||
| Experimental group | 279.34 (70.63) | 289.00 (89.50) [85.00-372.00] |
| Control group | 280.24 (70.67) | 289.00 (101.96) [105.50-390.00] |
| SF36 physical health score (out of 400) | ||
| Experimental group | 235.75 (66.29) | 237.50 (107.50) [30.00-347.50] |
| Control group | 225.63 (79.30) | 226.25 (130.60) [45.00-360.00] |
| TSK score (out of 68) | ||
| Experimental group | 34.37 (7.09 | 33.00 (11.00) [21.00-51.00] |
| Control group | 36.72 (6.85) | 37.00 (10.00) [26.00-61.00] |
| PVAQ score (out of 80) | ||
| Experimental group | 36.90 (11.92) | 36.00 (19.00) [14.00-69.00] |
| Control group | 35.77 (12.66) | 33.50 (19.00) [4.00-70.00] |
| PCS score (out of 52) | ||
| Experimental group | 16.53 (9.75) | 15.00 (14.00) [0.00-39.00] |
| Control group | 16.85 (10.52) | 14.50 (16.00) [0.00-48.00] |
| Participants, No. (%) | ||
| Dominant pain problem | ||
| Experimental group | ||
| Neck pain | 32 (53) | |
| Low back pain | 28 (47) | |
| Control group | ||
| Neck pain | 32 (53) | |
| Low back pain | 28 (47) | |
| Sex, No. (%) | ||
| Experimental group | ||
| Male | 22 (37) | |
| Female | 38 (63) | |
| Control group | ||
| Male | 25 (42) | |
| Female | 35 (58) | |
| Educational level, No. (%) | ||
| Experimental group | ||
| No degree | 0 | |
| Lower secondary school | 4 (7) | |
| Higher secondary school | 11 (18) | |
| Higher education | 45 (75) | |
| Control group | ||
| No degree | 0 | |
| Lower secondary school | 8 (13) | |
| Higher secondary school | 13 (22) | |
| Higher education | 39 (65) | |
| Handedness, No. (%) | ||
| Experimental group | ||
| Left-handed | 5 (8) | |
| Right-handed | 55 (92) | |
| Control group | ||
| Left-handed | 3 (5) | |
| Right-handed | 57 (95) |
Abbreviations: CPM, conditioned pain modulation; CSI, Central Sensitization Inventory; IQR, interquartile range; kgf, kilogram force; NRS, Numeric Rating Scale; PCS, Total Score of the Pain Catastrophizing Scale; PDI, Pain Disability Index; PPT, pressure pain threshold; prim, primary (pain) test site; PVAQ, Pain Vigilance and Awareness Questionnaire; SF36, 36-Item Short Form Health Survey; TSK, Tampa Scale for Kinesiophobia.
There were 60 participants in the experimental group and 60 participants in the control group.
Calculated as weight in kilograms divided by height in meters squared.
Values of people with neck pain and low back pain measures at the primary test site were analyzed together, using PPT location as confounding factor.
The primary outcome measures included pain and function (Table 2; eFigures 1 and 2 in Supplement 2). Although the NRS did not show significant interaction effects, a significant main effect of time was found. Significant treatment × time interaction effects were found for primary site PPTs, the CSI, the PDI, and the SF36 mental and physical subscale. Post hoc tests showed larger improvements in the experimental group (small to medium effect sizes): lower CSI scores at 6 months (estimated marginal [EM] mean, –5.684; 95% CI, –10.589 to –0.780]) and 12 months (EM mean, –6.053; 95% CI, –10.781 to –1.324); lower PDI scores at 3 months (EM mean, –5.113; 95% CI, –9.994 to –0.232), 6 months (EM mean, –6.351; 95% CI, –11.153 to –1.550), and 12 months (–5.779; 95% CI, –10.340 to –1.217]); higher SF36 mental health scores at 6 months (EM mean, 36.496; 95% CI, 7.998-64.995); and higher SF36 physical health scores at 3 months (EM mean, 39.263; 95% CI, 9.644-66.882), 6 months (53.007; 95% CI, 23.805-82.209), and 12 months of follow-up (32.208; 95% CI, 2.402-62.014]).
Table 2. Primary and Secondary Outcomesa.
| Characteristic | Experimental Treatment | Control Treatment | Group Difference, Mean (95% CI) | Effect Size | Main Effect of Time | Interaction Effect | Bonferroni Post Hoc Tests | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Estimated Marginal Mean (SE) | Change Relative to Baseline, % | Estimated Marginal Mean (SE) | Change Relative to Baseline, % | F Score | P Value | F Score | P Value | Time | Group | |||
| Pain as a Primary Outcome Measure | ||||||||||||
| PPT prim, kgfb | ||||||||||||
| Baseline | 4.558 (0.339) | NA | 4.429 (0.330) | NA | 0.129 (−1.064 to 0.806) | NA | 37.483 | .23 | 4.866 | .03 |
P < .001c; P = .009d |
P = .06c,d |
| At 3 mo | 6.148 (0.353) | 25.86 | 5.177 (0.363) | 14.45 | 0.971 (−0.028 to 1.970) | .46 | ||||||
| PPT hand, kgf | ||||||||||||
| Baseline | 3.598 (0.250) | NA | 3.604 (0.243) | NA | −0.006 (−0.695 to 0.683) | NA | 20.977 | <.001 | 1.738 | .19 | P < .001c,d | NA |
| At 3 mo | 4.413 (0.260) | 18.47 | 4.049 (0.267) | 11.00 | 0.364 (−0.372 to 1.100) | .26 | ||||||
| PPT leg, kgf | ||||||||||||
| Baseline | 5.328 (0.357) | NA | 5.077 (0.348) | NA | 0.250 (−0.736 to 1.237) | NA | 21.168 | <.001 | 2.583 | .11 | P < .001c,d | NA |
| At 3 mo | 6.544 (0.372) | 18.59 | 5.654 (0.382) | 10.21 | 0.889 (−0.164 to 1.942) | .31 | ||||||
| CPM primb | ||||||||||||
| Baseline | 1.075 (0.196) | NA | 1.054 (0.193) | NA | 0.021 (−0.522 to 0.564) | NA | 3.259 | .07 | 2.480 | .12 | NA | NA |
| At 3 mo | 1.511 (0.211) | 28.86 | 1.190 (0.224) | 11.43 | 0.321 (−0.287 to 0.928) | .16 | ||||||
| CPM leg | ||||||||||||
| Baseline | 1.058 (0.205) | NA | 0.904 (0.199) | NA | 0.154 (−0.719 to 0.411) | NA | 1.505 | .22 | 0.019 | .89 | NA | NA |
| At 3 mo | 1.259 (0.221) | 15.97 | 1.156 (0.234) | 21.80 | 0.103 (−0.532 to 0.739) | .09 | ||||||
| NRS score (of 10) | ||||||||||||
| Baseline | 5.153 (0.247) | NA | 4.983 (0.245) | NA | 0.169 (−0.519 to 0.858) | NA | 34.831 | <.001 | 2.172 | .10 | Baseline >3, 6, 12 mo (P < .001c,d) |
NA |
| At 3 mo | 2.462 (0.302) | −52.22 | 3.332 (0.311) | −33.13 | −0.871 (−1.731 to −0.010) | .52 | ||||||
| At 6 mo | 2.948 (0.326) | −42.79 | 3.808 (0.341) | −23.58 | −0.859 (−1.796 to 0.078) | .43 | ||||||
| At 12 mo | 2.655 (0.308) | −48.48 | 3.369 (0.315) | −32.39 | −0.714 (−1.588 to 0.160) | .44 | ||||||
| CSI score (of 100) | ||||||||||||
| Baseline | 40.017 (1.473) | NA | 39.883 (1.473) | NA | 0.133 (−3.992 to 4.259) | NA | 39.254 | <.001 | 7.537 | .003 | Baseline >3, 6, 12 mo (P < .001c; P ≤ .003d) |
6 mo (P = .02); 12 mo (P = .01) |
| At 3 mo | 30.668 (1.690) | −23.36 | 35.235 (1.714) | −11.65 | −4.567 (−9.334 to 0.201) | .68 | ||||||
| At 6 mo | 28.538 (1.732) | −28.69 | 34.222 (1.769) | −14.19 | −5.684 (−10.589 to −0.780) | .73 | ||||||
| At 12 mo | 29.358 (1.671) | −26.64 | 35.410 (1.703) | −11.22 | −6.053 (−10.781 to −1.324) | .74 | ||||||
| Function as a Primary Outcome Measure | ||||||||||||
| PDI score (of 70) | ||||||||||||
| Baseline | 21.800 (1.811) | NA | 21.583 (1.811) | NA | 0.217 (−4.856 to 5.289) | 34.979 | <.001 | 2.999 | .03 | Baseline >3, 6, 12 mo (P < .001c,d) |
3 mo (P = .04); 6 mo (P = .01); 12 mo (P = .01) |
|
| At 3 mo | 9.384 (1.720) | −56.95 | 14.497 (1.762) | −32.83 | −5.113 (−9.994 to −0.232) | .49 | ||||||
| At 6 mo | 7.884 (1.688) | −63.83 | 14.235 (1.720) | −34.05 | −6.351 (−11.153 to −1.550) | .56 | ||||||
| At 12 mo | 8.138 (1.605) | −62.67 | 13.916 (1.649) | −35.52 | −5.779 (−10.340 to −1.217) | .48 | ||||||
| SF36 mental health component score (of 400) | ||||||||||||
| Baseline | 279.344 (9.121) | NA | 280.242 (9.121) | NA | −0.897 (−26.440 to 24.646) | NA | 7.019 | <.001 | 3.213 | .03 | Baseline <3, 6, 12 mo (P < .001c) |
6 mo (P = .01) |
| At 3 mo | 316.700 (9.427) | 11.80 | 293.030 (9.717) | 4.36 | 23.670 (−3.168 to 50.509) | .42 | ||||||
| At 6 mo | 320.942 (10.002) | 12.96 | 284.446 (10.326 | 1.24 | 36.496 (7.998 to 64.995) | .62 | ||||||
| At 12 mo | 316.204 (12.192) | 11.66 | 291.520 (12.582) | 3.87 | 24.685 (−10.092 to 59.461) | .27 | ||||||
| SF36 physical health component score (of 400) | ||||||||||||
| Baseline | 235.750 (9.540) | NA | 225.625 (9.540) | NA | 10.125 (−16.593 to 36.843) | NA | 45.269 | <.001 | 4.983 | .003 | Baseline <3, 6, 12 mo (P < .001c,d) |
3 mo (P = .009); 6 mo (P < .001); 12 mo (P = .03) |
| At 3 mo | 304.915 (10.036) | 22.68 | 266.652 (10.366) | 15.39 | 39.263 (9.644 to 66.882) | .46 | ||||||
| At 6 mo | 320.130 (10.277) | 26.36 | 267.123 (10.552) | 15.54 | 53.007 (23.805 to 82.209) | .78 | ||||||
| At 12 mo | 314.764 (10.475) | 25.30 | 282.557 (10.769) | 20.15 | 32.208 (2.402 to 62.014) | .30 | ||||||
| Secondary Outcome Measures | ||||||||||||
| TSK score (of 68) | ||||||||||||
| Baseline | 34.367 (0.900) | NA | 36.717 (0.900) | NA | −2.350 (−4.870 to 0.170) | NA | 53.394 | <.001 | 13.165 | <.001 | Baseline >3, 6, 12 mo (P < .001c; P ≤ .007d) |
3 mo (P < .001); 6 mo (P < .001); 12 mo (P < .001) |
| At 3 mo | 24.349 (0.838) | −29.15 | 33.029 (0.714) | −10.04 | −8.680 (−11.067 to −6.294) | 1.11 | ||||||
| At 6 mo | 23.904 (0.871) | −30.44 | 33.714 (0.904) | −8.18 | −9.810 (−12.301 to −7.320) | 1.25 | ||||||
| At 12 mo | 24.020 (0.785) | −30.11 | 32.882 (0.808) | −10.44 | −8.862 (−11.097 to −6.628) | 1.14 | ||||||
| PVAQ score (of 80) | ||||||||||||
| Baseline | 36.900 (1.587) | NA | 35.767 (1.587) | NA | 1.133 (−3.312 to 5.579) | NA | 20.764 | <.001 | 6.374 | .001 | Baseline >3, 6, 12 mo (P < .001c) |
3 mo (P = .001); 6 mo (P = .005); 12 mo (P = .01) |
| At 3 mo | 24.878 (1.648) | −32.58 | 33.142 (1.699) | −7.34 | −8.265 (−12.959 to −3.571) | .88 | ||||||
| At 6 mo | 24.740 (1.762) | −32.95 | 32.008 (1.823) | −10.51 | −7.269 (−12.294 to −2.243) | .79 | ||||||
| At 12 mo | 25.346 (1.673) | −31.31 | 31.662 (1.673) | −11.48 | −6.316 (−11.070 to −1.561) | .73 | ||||||
| PCS total score (of 52) | ||||||||||||
| Baseline | 16.533 (1.310) | NA | 16.850 (1.310) | NA | −0.317 (−3.986 to 3.353) | NA | 41.653 | <.001 | 1.609 | .19 | Baseline >3, 6, 12 mo (P < .001c,d); 3 mo >6, 12 mo (P = .001c,d) |
NA |
| At 3 mo | 8.845 (1.201) | −46.50 | 12.496 (1.233) | −25.84 | −3.652 (−7.065 to −0.239) | .44 | ||||||
| At 6 mo | 6.576 (1.145) | −60.23 | 9.550 (1.183) | −43.32 | −2.974 (−6.239 to 0.291) | .36 | ||||||
| At 12 mo | 6.051 (1.135) | −63.40 | 9.488 (1.171) | −43.69 | −3.437 (−6.673 to −0.201) | .35 | ||||||
Abbreviations: CPM, conditioned pain modulation; CSI, Central Sensitization Inventory; kgf, kilogram force; NA, not applicable; NRS, Numeric Rating Scale; PCS, Pain Catastrophizing Scale, PDI, Pain Disability Index; PPT, pressure pain threshold; prim, primary (pain) test site; PVAQ, Pain Vigilance and Awareness Questionnaire; SF36, 36-Item Short Form Health Survey; TSK, Tampa Scale for Kinesiophobia.
All analyses were performed using linear mixed models. The Cohen d is interpreted as very large (>1.3), large (0.80-1.29), medium (0.50-0.79), small (0.20-0.49), and negligible (<0.20).
Values of people with neck pain and low back pain measures at the primary test site were analyzed together, using PPT location as confounding factor within the linear mixed models analysis.
Results of Bonferroni post hoc tests in the experimental group.
Results of Bonferroni post hoc tests in the control group.
The secondary outcome measures included gray matter morphologic features and pain cognitions (Table 2; eTable 2 and eFigure 3 in Supplement 2). No significant interaction effects or increased gray matter volumes at the subcortical level were found. Regarding the cortical regions of interest, the supramarginal gyrus showed an interaction effect. Bonferroni post hoc analysis showed that the experimental treatment led to a significantly higher supramarginal thickness at 3 months (EM mean, 0.046; 96% CI, 0.000-0.093; P = .049) and 12 months (EM mean, 0.049; 95% CI, 0.005-0.092; P = .03).
The Tampa Scale for Kinesiophobia and the Pain Vigilance and Awareness Questionnaire showed significant group × time interaction effects (Table 2). Bonferroni post hoc analysis showed lower scores in the experimental group on the Tampa Scale for Kinesiophobia (medium to large effect size) at 3 months (EM mean, –8.680; 95% CI, –11.067 to –6.294), 6 months (EM mean, –9.810; –12.301 to –7.320), and 12 months of follow-up (EM mean, –8.862; 95% CI, –11.097 to –6.628) and on the Pain Vigilance and Awareness Questionnaire (medium to large effect size) at 3 months (EM mean, –8.265; 95% CI, –12.959 to –3.571), 6 months (EM mean, –7.269; 95% CI, –12.294 to –2.243), and 12 months of follow-up (EM mean, –6.316; –11.070 to –1.561).
Discussion
Despite the inability to change gray matter morphologic features, pain neuroscience education combined with cognition-targeted motor control training improved pressure pain sensitivity, central sensitization symptoms, mental and physical functioning, kinesiophobia, and hypervigilance and reduced disability in patients with nCSP. These effects were of clinical importance (medium to large effect sizes and 50% improvement in pain) and were maintained at long-term follow-up.
Primary Outcomes: Pain and Function
Despite the absence of treatment differences, only the experimental group showed an increase in PPTs of more than 15% (an increase of >1.5 kg/cm2 at the primary test site), which is considered to be clinically relevant.27 Also, although the decrease in NRS pain scores exceeded the minimal clinically important difference (ie, 30%) in both groups,14 percentage changes and effect sizes were considerably higher in the experimental group (42.79%-52.22% reduction) compared with the control group (23.58%-33.13% reduction). Lastly, the experimental group showed significantly lower CSI scores after treatment (medium effect sizes) than the control group.
These significant changes in CSI scores and the clinical interpretation of the PPTs and the NRS (medium to large effect sizes and/or clinically important changes) demonstrate the superiority of pain neuroscience education combined with cognition-targeted motor control training compared with current best-evidence physiotherapy for individuals with nCSP at reducing pain. Although, to our knowledge, no previous studies have evaluated pain neuroscience education combined with cognition-targeted motor control training, studies evaluating pain neuroscience education alone report small effect sizes.28 The larger effect sizes presented here (in the short term and long term) are likely the result of integrating the newly derived pain neuroscience understanding in specific fearful movements and activities, enabling patients to deal with pain in daily life.
Although the statistical and/or clinically relevant changes in these pain parameters support the effectiveness of the experimental intervention, we did not find any effect on the efficacy of CPM. The CPM paradigm evaluates the descending nociceptive inhibitory systems, and previous research has reported less efficacious CPM in people with chronic spinal pain compared with healthy pain-free controls.29,30 Still, there is no association between CPM and the intensity of chronic pain,31 and reduced CPM has been found in healthy individuals prior to the development of chronic neck pain.32 Together with the results of our present study, these observations question the clinical importance of the CPM paradigm for people with nCSP, which could explain why the CPM parameter did not change while other pain parameters improved in response to the experimental treatment.
For perceived pain disability and mental and physical health, the experimental group showed greater improvement compared with current best-evidence physiotherapy (small to medium effect sizes). This outcome is in line with a pilot study for individuals with chronic low back pain.10 Again, these positive effects can be attributed to the content of the experimental treatment as participants learn to put pain into the right perspective, to move regularly, and to be physically active. Consequently, participants probably feel empowered, whereas, previously, they viewed pain was as a life-controlling factor.
Secondary Outcomes: Gray Matter Morphologic Features and Pain Cognitions
Based on previous studies,4,6 we expected to find increases of gray matter volume or thickness in response to treatment. However, no substantial increases (or decreases) were found in any of the evaluated brain areas. This outcome indicates that neither intervention led to a change in gray matter morphometry. Some might argue that this is not a surprising result because the interventions did not include surgery or medication, both elements that are known to affect brain gray matter morphologic features.33,34 However, the absence of changes in gray matter morphologic features in response to treatment is clearly at odds with an uncontrolled study35 reporting improvements in gray matter after cognitive behavioral treatment in people with chronic spinal pain. This study35 compared patients before and after therapy with healthy individuals at baseline, whereas our study is unique in evaluating brain morphologic features using a randomized clinical design with 2 different therapies. Taking into account the significant clinical reduction in pain and improvement in function in response to pain neuroscience education combined with cognition-targeted motor control training, we believe that these findings at the brain level question the previously reported brain changes in response to therapy in uncontrolled studies.
Regarding kinesiophobia, the experimental treatment showed larger reductions than current best-evidence physiotherapy. Whereas both groups showed baseline Tampa Scale for Kinesiophobia scores around the cutoff to indicate kinesiophobia, only the experimental group showed decreases exceeding the minimally important change of 5.5 (large effect sizes).36 Similarly, hypervigilance improved more in the experimental group (medium to large effect sizes) than in the control group. Again, the combination of pain neuroscience education with cognition-targeted exercises reveals its added value, as previous research indicates that pain neuroscience education alone did not significantly reduce hypervigilance.37
No effect was seen for pain catastrophizing, although we expected a greater decrease in the experimental group. Combining pain neuroscience education with cognition-targeted motor control training stimulates people to put pain into the right perspective, to understand the pain problem, to increase functionality, and to use physical activity to positively influence symptoms.10,38 However, our results imply that this approach is not better than current best-evidence physiotherapy to reduce pain catastrophizing. Still, there was a reduction of 46.50% (3 months’ follow-up) to 63.40% (12 months’ follow-up) in the experimental group, whereas the control group showed reductions of 25.84% (3 months’ follow-up) to 43.69% (12 months’ follow-up) (Table 2).
Practical Implications and Recommendations for Research
Our results emphasize the need for a shift from a biomedical approach toward a biopsychosocial approach that combines pain neuroscience education with cognition-targeted motor control training for people with nCSP. Clinicians should integrate modern pain neuroscience into the management of nCSP, rather than focusing on a possible biomedical origin of pain.
However, because this study included people only with nCSP, other populations require study to see if these results can be generalized to a broad population with chronic pain. Further research should investigate if the combination of pain neuroscience education with cognition-targeted exercises is also effective in subgroups, such as individuals with chronic whiplash–associated disorders, and other populations with chronic pain, such as chronic knee osteoarthritis and postcancer pain. In addition, future research should focus on other brain imaging techniques, such as white matter properties, and functional connectivity analyses.
Limitations and Strengths
This study has some limitations. Statistical analyses were not corrected for hormonal influences, which may affect pain and other outcome measures, but it is still unclear what the hormonal effects on pain intensity are.39
This study also has several strengths. To our knowledge, this is the first experimental study examining the possible treatment effects on brain morphologic features in people with nCSP. Likewise, to our knowledge, this is the first triple-blind, adequately controlled randomized clinical trial examining the effectiveness of pain neuroscience education combined with cognition-targeted motor control training for people with nCSP. This multicenter study was carried out using a large sample and exhibited substantial improvements and corresponding effect sizes among measures of pain, function, and psychosocial correlates. The trial compared balanced treatment arms, was sufficiently powered, used current best-evidence physiotherapy as the control intervention, relied on a published trial and treatment protocol,12,24 and applied blinded outcomes assessments up to 1 year after treatment.
Conclusions
Combining pain neuroscience education with cognition-targeted exercises does not affect brain gray matter morphologic features but can reduce pain and disability and improve mental and physical functioning and pain cognitions in people with nCSP (clinically important results, long-term benefits, medium to large effect sizes, and 50% improvement in self-reported pain). The presence of significant clinical improvements without changes at the brain level challenges the clinical relevance of these alterations in gray matter morphologic findings in people with nCSP. This finding emphasizes the need for a shift toward a biopsychosocial focus (ie, cognition and perceptions underlying the pain problem), rather than maintaining a focus toward a purely biomedical origin when treating these patients in clinical practice.
Trial protocol
eAppendix. Detailed Information on MRI Scan and Preprocessing of MRI Data in FreeSurfer
eTable 1. Main Differences Between the Two Treatment Arms
eTable 2. Effect of Physiotherapy Treatment on Gray Matter Cortical Thickness and Subcortical Volumes in People With Chronic Spinal Pain (n=120)
eFigure 1. Pain Neuroscience Education Combined With Cognition-Targeted Motor Control Training Is Effective for Increasing Pain Pressure Thresholds (A) and for Reducing Self-Reported Symptoms of Hypersensitivity for Nonmusculoskeletal Stimuli (B; Central Sensitization Inventory), Compared to Best Evidence Physiotherapy in Patients with Chronic Spinal Pain (n=120)
eFigure 2. Pain Neuroscience Education Combined With Cognition-Targeted Motor Control Training Is Effective for Reducing Disability (A), for Increasing Perceived Mental Health (B), and for Increasing Perceived Physical Health (C), Compared to Best Evidence Physiotherapy in Patients with Chronic Spinal Pain (n=120)
eFigure 3. Pain Neuroscience Education Combined With Cognition-Targeted Motor Control Training Is Effective for Reducing Fear of Movement Pain Pressure Thresholds (A) and for Reducing Pain Vigilance and Awareness (B), Compared to Best Evidence Physiotherapy in Patients with Chronic Spinal Pain (n=120)
References
- 1.Murray CJL, Vos T, Lozano R, et al. Disability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990-2010: a systematic analysis for the Global Burden of Disease Study 2010 [published correction appears in Lancet. 2013;381(9867):628]. Lancet. 2012;380(9859):2197-2223. [DOI] [PubMed] [Google Scholar]
- 2.Keller A, Hayden J, Bombardier C, van Tulder M. Effect sizes of non-surgical treatments of non-specific low-back pain. Eur Spine J. 2007;16(11):1776-1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Roussel NA, Nijs J, Meeus M, Mylius V, Fayt C, Oostendorp R. Central sensitization and altered central pain processing in chronic low back pain: fact or myth? Clin J Pain. 2013;29(7):625-638. [DOI] [PubMed] [Google Scholar]
- 4.Kregel J, Meeus M, Malfliet A, et al. Structural and functional brain abnormalities in chronic low back pain: a systematic review. Semin Arthritis Rheum. 2015;45(2):229-237. [DOI] [PubMed] [Google Scholar]
- 5.Van Oosterwijck J, Nijs J, Meeus M, Paul L. Evidence for central sensitization in chronic whiplash: a systematic literature review. Eur J Pain. 2013;17(3):299-312. [DOI] [PubMed] [Google Scholar]
- 6.Kregel J, Coppieters I, DePauw R, et al. Does conservative treatment change the brain in patients with chronic musculoskeletal pain? a systematic review. Pain Physician. 2017;20(3):139-154. [PubMed] [Google Scholar]
- 7.Crombez G, Vlaeyen JW, Heuts PH, Lysens R. Pain-related fear is more disabling than pain itself: evidence on the role of pain-related fear in chronic back pain disability. Pain. 1999;80(1-2):329-339. [DOI] [PubMed] [Google Scholar]
- 8.Rivest K, Côté JN, Dumas J-P, Sterling M, De Serres SJ. Relationships between pain thresholds, catastrophizing and gender in acute whiplash injury. Man Ther. 2010;15(2):154-159. [DOI] [PubMed] [Google Scholar]
- 9.Picavet HSJ, Vlaeyen JWS, Schouten JSAG. Pain catastrophizing and kinesiophobia: predictors of chronic low back pain. Am J Epidemiol. 2002;156(11):1028-1034. [DOI] [PubMed] [Google Scholar]
- 10.Moseley L. Combined physiotherapy and education is efficacious for chronic low back pain. Aust J Physiother. 2002;48(4):297-302. [DOI] [PubMed] [Google Scholar]
- 11.Nijs J, Meeus M, Cagnie B, et al. A modern neuroscience approach to chronic spinal pain: combining pain neuroscience education with cognition-targeted motor control training. Phys Ther. 2014;94(5):730-738. [DOI] [PubMed] [Google Scholar]
- 12.Dolphens M, Nijs J, Cagnie B, et al. Efficacy of a modern neuroscience approach versus usual care evidence-based physiotherapy on pain, disability and brain characteristics in chronic spinal pain patients: protocol of a randomized clinical trial. BMC Musculoskelet Disord. 2014;15(1):149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kernan WN, Viscoli CM, Makuch RW, Brass LM, Horwitz RI. Stratified randomization for clinical trials. J Clin Epidemiol. 1999;52(1):19-26. [DOI] [PubMed] [Google Scholar]
- 14.Farrar JT, Troxel AB, Stott C, Duncombe P, Jensen MP. Validity, reliability, and clinical importance of change in a 0-10 numeric rating scale measure of spasticity: a post hoc analysis of a randomized, double-blind, placebo-controlled trial. Clin Ther. 2008;30(5):974-985. [DOI] [PubMed] [Google Scholar]
- 15.Neblett R, Cohen H, Choi Y, et al. The Central Sensitization Inventory (CSI): establishing clinically significant values for identifying central sensitivity syndromes in an outpatient chronic pain sample. J Pain. 2013;14(5):438-445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aaronson NK, Muller M, Cohen PD, et al. Translation, validation, and norming of the Dutch language version of the SF-36 Health Survey in community and chronic disease populations. J Clin Epidemiol. 1998;51(11):1055-1068. [DOI] [PubMed] [Google Scholar]
- 17.Soer R, Reneman MF, Vroomen PCAJ, Stegeman P, Coppes MH. Responsiveness and minimal clinically important change of the Pain Disability Index in patients with chronic back pain. Spine (Phila Pa 1976). 2012;37(8):711-715. [DOI] [PubMed] [Google Scholar]
- 18.Desikan RS, Ségonne F, Fischl B, et al. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage. 2006;31(3):968-980. [DOI] [PubMed] [Google Scholar]
- 19.Fischl B, Salat DH, Busa E, et al. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron. 2002;33(3):341-355. [DOI] [PubMed] [Google Scholar]
- 20.Sullivan MJL, Bishop SR, Pivik J. The pain catastrophizing scale: development and validation. Psychol Assess. 1995;7(4):524-532. [Google Scholar]
- 21.Goubert L, Crombez G, Van Damme S, Vlaeyen JW, Bijttebier P, Roelofs J. Confirmatory factor analysis of the Tampa Scale for Kinesiophobia: invariant two-factor model across low back pain patients and fibromyalgia patients. Clin J Pain. 2004;20(2):103-110. [DOI] [PubMed] [Google Scholar]
- 22.Roelofs J, Peters ML, McCracken L, Vlaeyen JW. The pain vigilance and awareness questionnaire (PVAQ): further psychometric evaluation in fibromyalgia and other chronic pain syndromes. Pain. 2003;101(3):299-306. [DOI] [PubMed] [Google Scholar]
- 23.Butler DS, Moseley GL. Explain Pain. Adelaide: Australia: NOI Group Publishing; 2003. [Google Scholar]
- 24.Malfliet A, Kregel J, Meeus M, et al. Applying contemporary neuroscience in exercise interventions for chronic spinal pain: treatment protocol. Braz J Phys Ther. 2017;21(5):378-387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Glomsrød B, Lønn JH, Soukup MG, Bø K, Larsen S. “Active back school”, prophylactic management for low back pain: three-year follow-up of a randomized, controlled trial. J Rehabil Med. 2001;33(1):26-30. [DOI] [PubMed] [Google Scholar]
- 26.Soukup MG, Lönn J, Glomsröd B, Bö K, Larsen S. Exercises and education as secondary prevention for recurrent low back pain. Physiother Res Int. 2001;6(1):27-39. [DOI] [PubMed] [Google Scholar]
- 27.Fischer AA. Application of pressure algometry in manual medicine. J Manual Med. 1990;5(4):145-150. [Google Scholar]
- 28.Louw A, Diener I, Butler DS, Puentedura EJ. The effect of neuroscience education on pain, disability, anxiety, and stress in chronic musculoskeletal pain. Arch Phys Med Rehabil. 2011;92(12):2041-2056. [DOI] [PubMed] [Google Scholar]
- 29.Corrêa JB, Costa LOP, de Oliveira NTB, Sluka KA, Liebano RE. Central sensitization and changes in conditioned pain modulation in people with chronic nonspecific low back pain: a case-control study. Exp Brain Res. 2015;233(8):2391-2399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ng TS, Pedler A, Vicenzino B, Sterling M. Less efficacious conditioned pain modulation and sensory hypersensitivity in chronic whiplash-associated disorders in Singapore. Clin J Pain. 2014;30(5):436-442. [DOI] [PubMed] [Google Scholar]
- 31.Mlekusch S, Schliessbach J, Cámara RJA, Arendt-Nielsen L, Jüni P, Curatolo M. Do central hypersensitivity and altered pain modulation predict the course of chronic low back and neck pain? Clin J Pain. 2013;29(8):673-680. [DOI] [PubMed] [Google Scholar]
- 32.Shahidi B, Maluf KS. Adaptations in evoked pain sensitivity and conditioned pain modulation after development of chronic neck pain. Biomed Res Int. 2017;2017:8985398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Talati A, Pantazatos SP, Hirsch J, Schneier F. A pilot study of gray matter volume changes associated with paroxetine treatment and response in social anxiety disorder. Psychiatry Res. 2015;231(3):279-285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gwilym SE, Filippini N, Douaud G, Carr AJ, Tracey I. Thalamic atrophy associated with painful osteoarthritis of the hip is reversible after arthroplasty: a longitudinal voxel-based morphometric study. Arthritis Rheum. 2010;62(10):2930-2940. [DOI] [PubMed] [Google Scholar]
- 35.Seminowicz DA, Shpaner M, Keaser ML, et al. Cognitive-behavioral therapy increases prefrontal cortex gray matter in patients with chronic pain. J Pain. 2013;14(12):1573-1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Monticone M, Ambrosini E, Rocca B, Foti C, Ferrante S. Responsiveness of the Tampa Scale of Kinesiophobia in Italian subjects with chronic low back pain undergoing motor and cognitive rehabilitation. Eur Spine J. 2016;25(9):2882-2888. [DOI] [PubMed] [Google Scholar]
- 37.Van Oosterwijck J, Meeus M, Paul L, et al. Pain physiology education improves health status and endogenous pain inhibition in fibromyalgia: a double-blind randomized controlled trial. Clin J Pain. 2013;29(10):873-882. [DOI] [PubMed] [Google Scholar]
- 38.Malfliet A, Kregel J, Meeus M, et al. Blended learning pain neuroscience education for people with chronic spinal pain: a randomized-controlled multi-centre trial [published online September 1, 2017]. Phys Ther. doi: 10.1093/ptj/pzx092 [DOI] [PubMed] [Google Scholar]
- 39.Hassan S, Muere A, Einstein G. Ovarian hormones and chronic pain: a comprehensive review. Pain. 2014;155(12):2448-2460. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial protocol
eAppendix. Detailed Information on MRI Scan and Preprocessing of MRI Data in FreeSurfer
eTable 1. Main Differences Between the Two Treatment Arms
eTable 2. Effect of Physiotherapy Treatment on Gray Matter Cortical Thickness and Subcortical Volumes in People With Chronic Spinal Pain (n=120)
eFigure 1. Pain Neuroscience Education Combined With Cognition-Targeted Motor Control Training Is Effective for Increasing Pain Pressure Thresholds (A) and for Reducing Self-Reported Symptoms of Hypersensitivity for Nonmusculoskeletal Stimuli (B; Central Sensitization Inventory), Compared to Best Evidence Physiotherapy in Patients with Chronic Spinal Pain (n=120)
eFigure 2. Pain Neuroscience Education Combined With Cognition-Targeted Motor Control Training Is Effective for Reducing Disability (A), for Increasing Perceived Mental Health (B), and for Increasing Perceived Physical Health (C), Compared to Best Evidence Physiotherapy in Patients with Chronic Spinal Pain (n=120)
eFigure 3. Pain Neuroscience Education Combined With Cognition-Targeted Motor Control Training Is Effective for Reducing Fear of Movement Pain Pressure Thresholds (A) and for Reducing Pain Vigilance and Awareness (B), Compared to Best Evidence Physiotherapy in Patients with Chronic Spinal Pain (n=120)