This cohort study evaluates the use of preemptive human leukocyte antigen allele HLA-A*31:01 genetic screening to reduce carbamazepine-induced cutaneous adverse drug reactions in a Japanese population.
Key Points
Question
Does an association exist between genetic screening for the allele HLA-A*31:01 and reduced incidence of carbamazepine-induced cutaneous adverse drug reactions among Japanese patients?
Findings
In this cohort study conducted in Japan, neuropsychiatrists were asked to prescribe carbamazepine for HLA-A*31:01–negative patients and alternative drugs for HLA-A*31:01–positive patients. Of the 1130 patients, 23 (2.0%) had carbamazepine-induced cutaneous adverse drug reactions, a significant 40% decrease compared with the incidence observed in a historical control group.
Meaning
Preemptive HLA-A*31:01 screenings might reduce the rate of carbamazepine-induced cutaneous adverse drug reactions in routine clinical practice, providing additional evidence for implementing individualized medicine.
Abstract
Importance
Carbamazepine, a commonly used antiepileptic drug, is one of the most common causes of cutaneous adverse drug reactions (cADRs) worldwide. The allele HLA-A*31:01 is reportedly associated with carbamazepine-induced cADRs in Japanese and European populations; however, the clinical utility of HLA-A*31:01 has not been evaluated.
Objective
To assess the use of HLA-A*31:01 genetic screening to identify Japanese individuals at risk of carbamazepine-induced cADRs.
Design, Setting, and Participants
This cohort study was conducted across 36 hospitals in Japan from January 2012 to November 2014 among 1202 patients who had been deemed suitable to start treatment with carbamazepine. Preemptive HLA-A*31:01 genetic screening was performed for 1187 participants. Patients who did not start treatment with carbamazepine or alternative drugs were excluded. Participants were interviewed once weekly for 8 weeks to monitor the development of cADRs. Data analysis was performed from June 8, 2015, to December 27, 2016.
Exposures
Neuropsychiatrists were asked to prescribe carbamazepine for patients who tested negative for HLA-A*31:01 and alternative drugs for those who tested positive for HLA-A*31:01.
Main Outcomes and Measures
Incidence of carbamazepine-induced cADRs.
Results
Of the 1130 included patients who were prescribed carbamazepine or alternative drugs, the mean (range) age was 37.4 (0-95) years, 614 (54.3%) were men, and 198 (17.5%) were positive for HLA-A*31:01. Expert dermatologists identified 23 patients (2.0%) who had carbamazepine-induced cADRs, of which 4 patients required hospitalization. Drug-induced hypersensitivity syndrome was observed for 3 patients, maculopapular eruption for 9 patients, erythema multiforme for 5 patients, and an undetermined type of cADR for 6 patients. No patient developed Stevens-Johnson syndrome or toxic epidermal necrolysis. Compared with historical controls, the incidence of carbamazepine-induced cADRs was significantly decreased (for BioBank Japan data: incidence, 3.4%; odds ratio, 0.60; 95% CI, 0.36-1.00; P = .048; for the Japan Medical Data Centre claims database: incidence, 5.1%; odds ratio, 0.39; 95% CI, 0.26-0.59; P < .001).
Conclusions and Relevance
Preemptive HLA-A*31:01 genetic screening significantly decreased the incidence of carbamazepine-induced cADRs among Japanese patients, which suggests that it may be warranted in routine clinical practice.
Introduction
Cutaneous adverse drug reactions (cADRs) are independent of the dose prescribed, are unpredictable, and are sometimes life-threatening.1 Several drugs are known to cause higher incidences of cADRs.1 Although recent advances in pharmacogenomics have shown the importance of genetic variations in cADRs induced by several drugs,2,3,4,5,6 the clinical implementation of such pharmacogenomic findings has been slow, mainly owing to the need to establish the clinical utility of the genetic variations for decisions regarding drug prescriptions.7
Many patients of European ancestry have experienced cADRs after filling carbamazepine prescriptions, with the frequency varying from 3.7% to 13%.8,9,10,11 For carbamazepine-induced cADRs, the allele HLA-B*15:02 was first reported to be associated with Stevens-Johnson syndrome (SJS) and toxic epidermal necrolysis (TEN) in a Han Chinese population.12,13,14 Prospective genetic screening has revealed the clinical utility of HLA-B*15:02.15 The association of HLA-A*31:01 with carbamazepine-induced cADRs has also been reported in Japanese16 and European10 populations: the risk of HLA-A*31:01 was 8.0 times as high as that among tolerant controls in a Japanese population with mild cADRs and 33.9 times as high for SJS/TEN in the same population.16 Similar trends were observed in the European population.10 However, to date, the clinical utility of HLA-A*31:01 has not yet been evaluated.
In Japan, indications for the use of carbamazepine have not changed since 2003, and no description of the utility of genetic testing has been incorporated into the carbamazepine label in contrast to these labels in the United States and Taiwan. Therefore, to examine the clinical utility of HLA-A*31:01, we conducted a genotype-based carbamazepine therapy (GENCAT) study to determine whether screening for HLA-A*31:01 prior to prescribing carbamazepine reduces the incidence of carbamazepine-induced cADRs.
Methods
Study Design
This study was registered with the University Hospital Medical Information Network Clinical Trials Registry of Japan before enrollment began. We recruited and enrolled patients from 36 cooperating hospitals throughout Japan from January 2012 to November 2014. To ensure that all hospitals complied with regulations and protocol requirements, the study was monitored by Sogo Rinsho Medefi. Patients who fulfilled the following 3 criteria were invited to participate: (1) those deemed suitable to start treatment with carbamazepine based on the decision of neuropsychiatrists at cooperating hospitals, (2) those of Japanese descent of any age who had not received carbamazepine within 1 month of enrollment, and (3) those patients (or guardians) providing written informed consent. We excluded patients with a history of carbamazepine-induced cADRs, patients who were or were planning to become pregnant, and patients with renal failure (serum creatinine level ≥2.5 mg/dL [to convert to micromoles per liter, multiply by 88.4]), liver cirrhosis, or hypoproteinemia (serum albumin level ≤2.5 g/dL [to convert to grams per liter, multiply by 10]). All severe adverse reactions were monitored by an efficacy and safety evaluation committee (T. Furuta, Y. Saito, and N.I.). We performed the study in accordance with the provisions of the Declaration of Helsinki.17 The study protocol was approved by the research ethics committees of RIKEN, all cooperating hospitals, and the Institute of Medical Science, University of Tokyo. All participants or their guardians provided written informed consent.
We obtained a sample (2 mL) of whole blood from each participant. The HLA-A*31:01 screening was performed at participating hospitals using an automated molecular diagnostic system.18,19 The HLA-A*31:01 status was reported within 1.5 hours to the neuropsychiatrists (including Y.T., T. Onuma, T. Kamei, T.H., K.T., K.O., M.O., M.W., K.K., T. Oshima, A.W., S.M., K.S., H.T., Y. Shimo, M.H., S.S., T. Kinoshita, M. Kato, N.Y., N.A., T. Fukuchi, S.I., and S.Y.), who explained the HLA-A*31:01 screening result and the risk of carbamazepine-induced cADRs to participants or guardians. Patients who were negative for HLA-A*31:01 were recommended a prescription of carbamazepine, whereas those who were positive for HLA-A*31:01 were prescribed alternative drugs according to the neuropsychiatrists’ recommendations. Telephone interviews of all participants were conducted once weekly for 8 weeks to monitor for symptoms of cADRs or until the discontinuation of prescribed drugs owing to adverse drug reactions or miscellaneous causes. Other adverse events were graded according to the Common Terminology Criteria for Adverse Events Version 3.0 from the National Cancer Institute. This follow-up length was selected because most carbamazepine-induced cADRs occur within 2 months of the start of treatment with carbamazepine.15
At enrollment, participants were requested to immediately visit cooperating hospitals or the nearest hospital specializing in dermatology for evaluation of any suspected cADR symptoms. We asked the neuropsychiatrists to start appropriate treatment immediately following the initial diagnosis and to provide detailed case reports of the cADRs by completing a standardized case report form that included onset, symptoms, treatment, outcome, cADR photographs, and the initial diagnosis provided by the attending dermatologist. The collected case reports were independently reviewed by 2 expert dermatologists (Y.K. and T.S.) who classified the carbamazepine-induced cADR diagnoses into the following 4 categories: definite, meaning that there was sufficient information to diagnose carbamazepine-induced cADRs; probable, meaning that some information was missing but that the collected information was sufficient to diagnose carbamazepine-induced cADRs; possible, meaning that the reported cADR indicated a possibility of other skin disease or cADR caused by other drugs; and unlikely, meaning that the patients were considered to have developed other skin diseases or cADRs caused by other drugs or that information was insufficient. To determine the cADR culprit drug, we considered a cADR to be caused by another drug if the drug treatment was started or terminated during the carbamazepine treatment and if the cADR onset date was within 1 week of the start of the other drug treatment. Other drug treatments that were started during the carbamazepine treatment and continued even after the cADR resolved were excluded. Detailed classification criteria are given in eTable 1 in the Supplement. In this study, we defined only definite or probable cases as carbamazepine-induced cADRs. The 2 expert dermatologists (Y.K. and T.S.) also classified cADRs into an SJS/TEN, drug-induced hypersensitivity syndrome (DIHS), a drug reaction with eosinophilia and systemic symptoms (DRESS) syndrome, a maculopapular eruption, an erythema multiforme, a fixed drug eruption, and so on. If the collected information was insufficient, the cADR was defined as an undetermined type. If the diagnosis of the 2 expert dermatologists was discordant, they achieved a final diagnosis following discussion.
Genetic Screening for HLA-A*31:01
We previously developed a rapid genotyping method to detect the presence or absence of HLA-A*31:01.19 This method was applied to a DNA chip developed by RIKEN Genesis, which monitored the HLA-A*31:01 screening performed at cooperating hospitals throughout the study.
Validation of HLA-A*31:01 Genetic Screening
To examine the accuracy of HLA-A*31:01 screening, we collected an additional sample (5 mL) of whole blood from participants at enrollment. The whole blood was sent to a central laboratory (BML Inc) for DNA extraction. Anonymized DNA samples were transferred to and stored at BioBank Japan.
For analytical validation, we used DNA samples stored at BioBank Japan and genotyped HLA-A alleles using a WAKFlow HLA typing kit (Wakunaga), which is based on a Luminex system. Data analysis was performed with WAKFlow typing software using HLA sequence–specific oligonucleotide probes and a reverse line blot assay (Dynal Biotech). We found that the HLA-A*31:01 genetic screening results were wholly consistent with the results obtained using the Luminex system for all samples analyzed.
Historical Incidence
BioBank Japan is a multi-institutional hospital-based registry that was established in 2003 to collect genomic DNA and clinical information from Japanese patients who received a diagnosis of any of 47 diseases, including epilepsy and drug eruption.20,21 Physicians at cooperating hospitals provide the diagnoses. Clinical information is collected at baseline and then annually after the baseline survey through reviews of medical records using a standardized questionnaire. We searched the BioBank Japan clinical database from April 1, 2003, to December 28, 2010, and found 1274 patients who were prescribed carbamazepine and 44 patients who experienced a carbamazepine-induced cADR. A carbamazepine-induced cADR was identified as a diagnosis of drug eruption with carbamazepine specified as the culprit drug or as an adverse drug reaction, such as intoxication dermatosis or drug eruption, with carbamazepine specified as a culprit drug. Six patients were included in the group with 1274 patients prescribed carbamazepine and in the group of 44 patients who experienced a carbamazepine-induced cADR. Thus, we calculated the incidence of carbamazepine-induced cADRs as 44 to be 1312 carbamazepine users (3.4%) over the course of the study.
We also searched the Japan Medical Data Centre (JMDC) claims database22 from January 1, 2005, to December 31, 2014. Carbamazepine-induced cADRs were defined by the presence of at least 1 of the following International Statistical Classification of Diseases and Related Health Problems, Tenth Revision (ICD-10) codes during the period coincident with a prescription of carbamazepine: drug-induced dermatitis (ICD-10 codes L27.0, L27.1, and L27.9), erythema multiforme, including SJS/TEN (ICD-10 code L51), drug urticaria (ICD-10 code L50.8), carbamazepine intoxication (ICD-10 code T42.1), adverse reaction caused by antiepileptic drugs (ICD-10 code T42.6), and drug hypersensitivity (ICD-10 code T88.7). We found 12 060 patients who were prescribed carbamazepine, 610 of whom experienced a cADR, for an incidence of carbamazepine-induced cADRs of 5.1%.
Statistical Analysis
We analyzed all patients who received carbamazepine or alternative medications after HLA-A*31:01 screening. Analyses were conducted with SAS, version 9.2 (SAS Institute Inc) from June 8, 2015, to December 27, 2016. We used the Fisher exact test to compare the incidence of carbamazepine-induced cADRs with historical incidences. To evaluate the significance of any differences in clinical characteristics, we used 2-tailed, unpaired t tests or 2-tailed χ2 tests as appropriate. All reported P values are 2-sided, and P < .05 was considered statistically significant. On the basis of the incidence of carbamazepine-induced cADRs obtained using BioBank Japan data, we determined that 1059 participants would provide a statistical power of 80% to detect a reduction in the incidence from 3.4% to 1.5% with a 2-sided significance level of .05.
Results
Study Participants
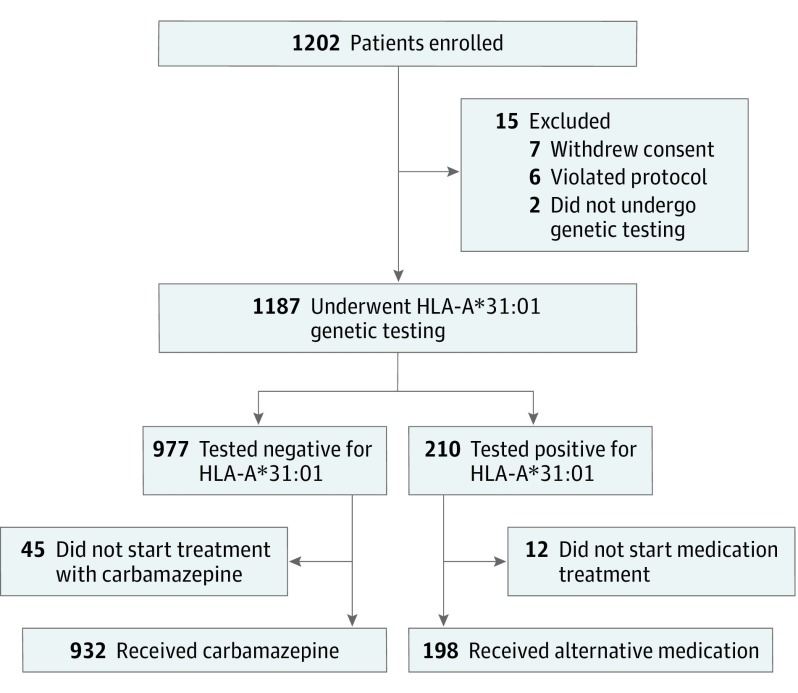
The Figure shows the participant flowchart for this GENCAT study. Of the 1202 enrolled participants, 1187 underwent preemptive HLA-A*31:01 screening. Of the 210 participants positive for HLA-A*31:01, we excluded 12 because the neuropsychiatrists decided not to start any medication treatment. Of the 977 participants negative for HLA-A*31:01, we excluded 45 who did not start treatment with carbamazepine. Thus, 1130 patients were included in the analysis. All 932 patients negative for HLA-A*31:01 started carbamazepine treatment. The 198 patients positive for HLA-A*31:01 were prescribed various alternative drugs (eTable 2 in the Supplement). There were 9 patients who had been prescribed drugs other than carbamazepine before the enrollment; these patients continued using the same drugs without adding alternative drugs because the neuropsychiatrists determined that no other drug was more appropriate. Despite the screening result, 1 patient positive for HLA-A*31:01 started carbamazepine treatment based on the neuropsychiatrist’s decision.
Figure. Flowchart of Screened Patients.
Table 1 provides the demographic and clinical characteristics of the study participants. The mean age was 37.4 years (range, 0-95 years), and 614 men were included. The indications for carbamazepine included epilepsy, schizophrenia, bipolar disorder, and trigeminal neuralgia. There were no differences in mean age, sex distribution, or the indication for carbamazepine between the patients who were positive and the patient who were negative for HLA-A*31:01.
Table 1. Demographic and Clinical Characteristics of Participants Grouped by HLA-A*31:01 Genetic Screening Results.
| Characteristic | Participants, No. (%) | P Value | |
|---|---|---|---|
| Negative for HLA-A*31:01 (n = 932) | Positive for HLA (n = 198) | ||
| Sex | |||
| Male | 510 (54.7) | 104 (52.5) | .58 |
| Female | 422 (45.3) | 94 (47.5) | |
| Age, mean (range), y | 37.1 (0-95) | 38.5 (0-89) | .44 |
| Indication for carbamazepinea | |||
| Epilepsy | 737 (78.5) | 151 (75.9) | .25 |
| Schizophrenia | 55 (5.9) | 9 (4.5) | |
| Bipolar disorder | 38 (4.0) | 15 (7.5) | |
| Trigeminal neuralgia | 42 (4.5) | 11 (5.5) | |
| Other condition | 67 (7.1) | 13 (6.5) | |
Eight patients had multiple diagnoses.
Adverse Events
During the 8-week follow-up, discontinuation of carbamazepine treatment because of cADRs, other adverse reactions, or other causes of carbamazepine occurred among 153 patients who tested negative for HLA-A*31:01, and discontinuation of alternative drugs occurred among 19 patients who tested positive for HLA-A*31:01. No significant difference was observed in the frequency of carbamazepine or alternative medication discontinuation due to cADRs between patients who tested negative (43 of 932 [4.6%]) and patients who tested positive (4 of 198 [2.0%]) for HLA-A*31:01 (P = .12).
After the expert review, among the 43 patients who were negative for HLA-A*31:01, carbamazepine-induced cADRs were diagnosed as being definite for 11 patients, probable for 12 patients, possible for 9 patients, and unlikely for 11 patients (eTable 3 in the Supplement). Of the 4 HLA-A*31:01–positive patients with cADRs, 3 had cADRs caused by other drugs, and 1 had insufficient information. Although 1 HLA-A*31:01–positive patient was prescribed carbamazepine, this patient did not develop a cADR.
Definite or probable carbamazepine-induced cADRs were observed in 23 patients (2.0%) in this study (Table 2). No patient developed SJS/TEN. Of the 23 patients with a definite or probable carbamazepine-induced cADR, 4 (2 with DIHS or DRESS, 1 with a maculopapular eruption, and 1 with erythema multiforme) required hospitalization for treatment. The 2 patients with DIHS or DRESS and the patient with a maculopapular eruption underwent intravenous steroid pulse therapy, whereas the patient with an erythema multiforme recovered following the discontinuation of carbamazepine treatment.
Table 2. Expert Dermatologist Diagnoses Following Their Review of Cases Initially Reported as Cutaneous Adverse Drug Reactions Irrespective of Causative Drug.
| Adverse Eventa | Patients, No. | Total | |||||
|---|---|---|---|---|---|---|---|
| Positive for HLA-A*31:01b | Negative for HLA-A*31:01 | ||||||
| Possible | Unlikely | Definite | Probable | Possible | Unlikely | ||
| Drug-induced hypersensitivity syndrome | 0 | 0 | 2 | 1 | 0 | 0 | 3 |
| Maculopapular eruption | 0 | 2 | 5 | 4 | 4 | 1 | 16 |
| Erythema multiforme | 0 | 0 | 4 | 1 | 2 | 0 | 7 |
| Other | 0 | 0 | 0 | 0 | 0 | 4 | 4 |
| Unknown | 1 | 1 | 0 | 6 | 3 | 6 | 17 |
| Total | 1 | 3 | 11 | 12 | 9 | 11 | 47 |
No patient developed Stevens-Johnson syndrome, toxic epidermal necrolysis, or fixed drug eruption.
No HLA-A*31:01–positive patient developed a definite or probably adverse event.
We also monitored patients for other adverse events, including fever, sore throat, fatigue, dizziness, insomnia, and gastrointestinal symptoms. The frequency of these adverse events showed no significant differences between HLA-A*31:01–positive and HLA-A*31:01–negative patients (eTable 4 in the Supplement).
Incidence of Carbamazepine-Induced cADRs
When we compared the incidence of carbamazepine-induced cADRs observed in this study with that determined for the historical controls obtained using data from BioBank Japan, the results of the present study showed a significant decrease in the incidence of carbamazepine-induced cADRs of nearly 40% (odds ratio, 0.60; 95% CI, 0.36-1.00; P = .048) (Table 3). Using the JMDC claims database as an independent historical control, we also found a significant decrease in the incidence of carbamazepine-induced cADRs in the present study (odds ratio, 0.39; 95% CI, 0.26-0.59; P < .001) (Table 3). When we limited the cases to the 932 HLA-A*31:01–negative patients who were exposed to carbamazepine, the incidence of carbamazepine-induced cADRs (2.5%) did not significantly differ from that of the BioBank Japan (odds ratio, 0.73; 95% CI, 0.44-1.22; P = .26) but was significantly lower than that of the JMDC database (odds ratio, 0.48; 95% CI, 0.31-0.72; P < .001).
Table 3. GENCAT Study and Historical Control Incidences of Carbamazepine-Induced Cutaneous Adverse Reactions.
| Adverse Event | Patients, No. | ||
|---|---|---|---|
| GENCAT Study (N = 1130) | Historical Control | ||
| BioBank Japan Data (N = 1312) | JMDC Claims Database (N = 12 060) | ||
| Carbamazepine-induced cutaneous adverse reaction,a No. (%) | 23 (2.0) | 44 (3.4) | 610 (5.1) |
| Stevens-Johnson syndrome or toxic epidermal necrolysis | 0 | 3 | 6 |
| Drug-induced hypersensitivity syndrome | 3 | 1 | NA |
| Maculopapular eruption | 9 | 6 | NA |
| Erythema multiforme | 5 | 15 | NA |
| Fixed drug eruption | 0 | 0 | NA |
| Others | 0 | 8 | NA |
| Unknown | 6 | 11 | NA |
| Statistical analysis, compared with GENCAT study | NA | ||
| P value | .048 | <.001 | |
| Odds ratio (95% CI) | 0.60 (0.36-1.0) | 0.39 (0.26-0.59) | |
Abbreviations: GENCAT, Genotype-Based Carbamazepine Therapy; JMDC, Japan Medical Data Centre; NA, not available, regarding information on specific cutaneous adverse drug reactions.
In the GENCAT study, all 23 patients who developed definite or probable carbamazepine-induced cutaneous adverse reactions were negative for HLA-A*31:01.
Discussion
In this prospective cohort study using HLA-A*31:01 screening prior to treatment, 1130 patients were prescribed carbamazepine or alternative drugs on the basis of the genetic screening results. Among them, 23 patients developed carbamazepine-induced cADRs during the 8-week follow-up. Comparison with a historical control indicated that the preemptive use of HLA-A*31:01 genetic screening in the present study was associated with a 40% reduction in the incidence of carbamazepine-induced cADRs. The frequency of HLA-A*31:01 carriers in the population was high (17.7%) in this study, although previous reports have also indicated a similar expected frequency of HLA-A*31:01 carriers (16.6%-17.5%).23,24 These results suggested that HLA-A*31:01 genetic screening would be useful for the prevention of carbamazepine-induced cADRs among Japanese patients.
For carbamazepine-induced cADRs, the clinical utility of the HLA-B*15:02 genetic test has already been established by preemptive screening,15 and the test is recommended in the Clinical Pharmacogenetics Implementation Consortium guidelines.25 However, the frequency of the HLA-B*15:02 allele is low in Korean, Japanese, African, and European populations.23 In addition, HLA-B*15:02 is specifically associated with SJS/TEN. By contrast, HLA-A*31:01 is associated with carbamazepine-induced cADRs in Japanese,16,26,27 Han Chinese,13 Northern European,10 Korean,28 and Canadian29 populations. The frequency of the HLA-A*31:01 allele is 7% to 9% in Japanese, 5% in Korean, 2% in Chinese, 2% to 3% in European, and 1% in African populations.23 Moreover, HLA-A*31:01 has been associated with a full spectrum of carbamazepine-induced cADRs. Therefore, HLA-A*31:01 genetic screening prior to prescribing carbamazepine would be useful for preventing many types of carbamazepine-induced cADRs in a range of patient populations.
Similar to studies evaluating HLA-B*15:02 genetic screening, no patient in the present study developed SJS/TEN. We compared the incidence of SJS/TEN in this study with those of 2 historical controls (BioBank Japan: incidence, 0.23%; P = .25; JMDC database: incidence, 0.05%; P > .99) (Table 3); however, we found no significant differences, likely because the present study was designed to evaluate the association of HLA-A*31:01 with all types of carbamazepine-induced cADRs.
Our study showed that HLA-A*31:01 screening was associated with a significant reduction in the incidence of carbamazepine-induced cADRs; however, a 2.0% incidence of carbamazepine-induced cADRs still remained. This indicates that even patients who tested negative for HLA-A*31:01 should be monitored for cADRs. In our previous study,16 genetic screening of HLA-A*31:01 had a sensitivity of 60.7% and a specificity of 87.5%, which suggested that some HLA-A*31:01–positive individuals will not develop cADRs. In the present study, neuropsychiatrists could not find suitable alternative drugs for 9 patients who tested positive for HLA-A*31:01. Moreover, 1 patient who tested positive for HLA-A*31:01 was prescribed carbamazepine and did not develop cADRs. Thus, although the results of the present study indicate that drug alternatives to carbamazepine are recommended for treating patients with HLA-A*31:01, neuropsychiatrists may prescribe carbamazepine after considering both the patient’s genetic risk of developing a cADR and the risk of withholding the drug. Further research to identify additional genetic factors is needed for a more precise anticipation of carbamazepine-induced cADRs.
In addition to the clinical utility, the cost-effectiveness of HLA-A*31:01 screening should be discussed. The debate on the implementation of genetic testing has arisen in part because of the uncertainty as to whether it is truly cost-effective for the health care system when accounting for the cost of testing, increased cost of alternative treatments, and low incidence of cADRs. A recent cost-effectiveness analysis conducted in a UK health care setting showed that HLA-A*31:01 testing reduced the expected rate of cADRs from 780 to 700 per 10 000 patients with an incremental cost-effective ratio of £12 808 (approximately US $17 700) per quality-adjusted life-years.30 Because the UK National Institute for Health and Care Excellence specifies an incremental cost-effective ratio of £20 000 (approximately US $27 660) as the threshold to represent cost-effective use of resources by the National Health Service in the United Kingdom,31 routine testing for HLA-A*31:01 is likely to be cost-effective. However, because health insurance systems differ for each country, an economic evaluation should be conducted from the perspective of the national health insurance system and the clinical settings of Japan.
Limitations
The primary limitation of this study was its nonrandomized study design. However, the clinical utility of HLA alleles associated with cADRs has been shown by a prospective randomized study32 and single-arm prospective screening studies.15,33 The presence of HLA-A*31:01 has been associated with increased risk of all types of cADRs and has substantially increased the risk of life-threatening SJS/TEN.10,16 Moreover, cADRs occasionally progress after discontinuation of carbamazepine treatment.1 Therefore, ethical considerations disallowed a randomized study design. To help mitigate this limitation, we compared the incidence of carbamazepine-induced cADRs with that of historical controls. Because there was no reliable information available on a Japanese population, we first used data from BioBank Japan. Case ascertainment of a carbamazepine-induced cADR in the present study was equivalent to that of Biobank Japan. However, the incidence of carbamazepine-induced cADR may have been underestimated in this historical control because some mild cases might not have been reported in the patients’ medical records. Therefore, we also accessed the JMDC claims database, the largest epidemiology claims database available in Japan. The definition of a cADR in the JMDC database is broader than that used for BioBank Japan because of the lack of culprit drug information. Hence, we included cADR cases caused by other drugs and unlikely cases due to insufficient information as cADR cases for comparison with those in the JMDC historical control. We found that the incidence of carbamazepine-induced cADRs was still significantly decreased with the use of preemptive HLA-A*31:01 genetic screening in the present study (2.8% vs 5.1%, P<.001). Because the prevalence of carbamazepine-induced cADRs has been reported to be between 3.7% and 13%,8,9,10,11 we believe the results of the present study were not distorted by an overestimation of historical controls.
A second limitation is that we did not include genetic testing for HLA-B*15:02, a well-known genetic predictor of a carbamazepine-induced cADR. However, the carrier frequency of HLA-B*15:02 is estimated to be 0.062%24; thus, the expected number of HLA-B*15:02 carriers in our study would have been less than 1 patient. In addition, the aim of the present study was to evaluate the clinical utility of HLA-A*31:01. Thus, we believe the lack of HLA-B*15:02 genetic testing did not affect our findings. A third limitation is that although we excluded patients who took carbamazepine within 1 month of study entry, we did not exclude patients who had previously been exposed to carbamazepine without a history of a cADR. However, inclusion of those patients in this study would have decreased the incidence of cADRs regardless of genotype.
Conclusions
Preemptive HLA-A*31:01 screening prior to dispensing carbamazepine or alternative drug prescriptions was associated with a 40% reduction in the incidence of cADRs in a Japanese population compared with a historical control. Although cost-effectiveness analyses are required, the use of HLA-A*31:01 screening to reduce the rate of carbamazepine-induced cADRs in routine clinical practice appears warranted.
eTable 1. Detailed Classification Criteria of Cutaneous Adverse Drug Reactions (cADRs) Used for the Review by Expert Dermatologists
eTable 2. List of Drugs Prescribed Before and After HLA-A*31:01 Screening of 198 HLA-A*31:01-Positive Participants
eTable 3. Details of the Review by Expert Dermatologists of Cases Initially Diagnosed as Cutaneous Adverse Drug Reactions (cADRs) According to HLA-A*31:01 Screening Results
eTable 4. Comparison of the Incidences of other Adverse Events Between HLA-A*31:0-Positive and HLA-A*31:0-Negative Patients
References
- 1.Shiohara T, Kano Y. A complex interaction between drug allergy and viral infection. Clin Rev Allergy Immunol. 2007;33(1-2):124-133. [DOI] [PubMed] [Google Scholar]
- 2.Mallal S, Nolan D, Witt C, et al. Association between presence of HLA-B*5701, HLA-DR7, and HLA-DQ3 and hypersensitivity to HIV-1 reverse-transcriptase inhibitor abacavir. Lancet. 2002;359(9308):727-732. [DOI] [PubMed] [Google Scholar]
- 3.Hung SI, Chung WH, Liou LB, et al. HLA-B*5801 allele as a genetic marker for severe cutaneous adverse reactions caused by allopurinol. Proc Natl Acad Sci U S A. 2005;102(11):4134-4139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ueta M, Kaniwa N, Sotozono C, et al. Independent strong association of HLA-A*02:06 and HLA-B*44:03 with cold medicine-related Stevens-Johnson syndrome with severe mucosal involvement. Sci Rep. 2014;4:4862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chantarangsu S, Mushiroda T, Mahasirimongkol S, et al. HLA-B*3505 allele is a strong predictor for nevirapine-induced skin adverse drug reactions in HIV-infected Thai patients. Pharmacogenet Genomics. 2009;19(2):139-146. [DOI] [PubMed] [Google Scholar]
- 6.Giacomini KM, Yee SW, Mushiroda T, Weinshilboum RM, Ratain MJ, Kubo M. Genome-wide association studies of drug response and toxicity: an opportunity for genome medicine. Nat Rev Drug Discov. 2017;16(1):1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang L, McLeod HL, Weinshilboum RM. Genomics and drug response. N Engl J Med. 2011;364(12):1144-1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kramlinger KG, Phillips KA, Post RM. Rash complicating carbamazepine treatment. J Clin Psychopharmacol. 1994;14(6):408-413. [PubMed] [Google Scholar]
- 9.Arif H, Buchsbaum R, Weintraub D, et al. Comparison and predictors of rash associated with 15 antiepileptic drugs. Neurology. 2007;68(20):1701-1709. [DOI] [PubMed] [Google Scholar]
- 10.McCormack M, Alfirevic A, Bourgeois S, et al. HLA-A*3101 and carbamazepine-induced hypersensitivity reactions in Europeans. N Engl J Med. 2011;364(12):1134-1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shirzadi M, Alvestad S, Hovdal H, Espeset K, Lydersen S, Brodtkorb E. Comparison of carbamazepine rash in multiple sclerosis and epilepsy. Acta Neurol Scand. 2012;125(1):60-63. [DOI] [PubMed] [Google Scholar]
- 12.Chung WH, Hung SI, Hong HS, et al. Medical genetics: a marker for Stevens-Johnson syndrome. Nature. 2004;428(6982):486. [DOI] [PubMed] [Google Scholar]
- 13.Hung SI, Chung WH, Jee SH, et al. Genetic susceptibility to carbamazepine-induced cutaneous adverse drug reactions. Pharmacogenet Genomics. 2006;16(4):297-306. [DOI] [PubMed] [Google Scholar]
- 14.Yip VL, Marson AG, Jorgensen AL, Pirmohamed M, Alfirevic A. HLA genotype and carbamazepine-induced cutaneous adverse drug reactions: a systematic review. Clin Pharmacol Ther. 2012;92(6):757-765. [DOI] [PubMed] [Google Scholar]
- 15.Chen P, Lin JJ, Lu CS, et al. ; Taiwan SJS Consortium . Carbamazepine-induced toxic effects and HLA-B*1502 screening in Taiwan. N Engl J Med. 2011;364(12):1126-1133. [DOI] [PubMed] [Google Scholar]
- 16.Ozeki T, Mushiroda T, Yowang A, et al. Genome-wide association study identifies HLA-A*3101 allele as a genetic risk factor for carbamazepine-induced cutaneous adverse drug reactions in Japanese population. Hum Mol Genet. 2011;20(5):1034-1041. [DOI] [PubMed] [Google Scholar]
- 17.World Medical Association World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191-2194. doi: 10.1001/jama.2013.281053 [DOI] [PubMed] [Google Scholar]
- 18.Kitano S, Myers J, Nakamura J, et al. A novel fully automated molecular diagnostic system (AMDS) for colorectal cancer mutation detection. PLoS One. 2013;8(5):e62989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Aoki M, Hosono N, Takata S, Nakamura Y, Kamatani N, Kubo M. New pharmacogenetic test for detecting an HLA-A*31:01 allele using the InvaderPlus assay. Pharmacogenet Genomics. 2012;22(6):441-446. [DOI] [PubMed] [Google Scholar]
- 20.Nagai A, Hirata M, Kamatani Y, et al. ; BioBank Japan Cooperative Hospital Group . Overview of the BioBank Japan project: study design and profile. J Epidemiol. 2017;27(3S):S2-S8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hirata M, Kamatani Y, Nagai A, et al. ; BioBank Japan Cooperative Hospital Group . Cross-sectional analysis of BioBank Japan clinical data: a large cohort of 200,000 patients with 47 common diseases. J Epidemiol. 2017;27(3S):S9-S21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Japan Medical Data Center JMDC claims database. https://www.jmdc.co.jp/en/pharma/database.html. Accessed May 1, 2017.
- 23.Kurose K, Sugiyama E, Saito Y. Population differences in major functional polymorphisms of pharmacokinetics/pharmacodynamics-related genes in Eastern Asians and Europeans: implications in the clinical trials for novel drug development. Drug Metab Pharmacokinet. 2012;27(1):9-54. [DOI] [PubMed] [Google Scholar]
- 24.Ikeda N, Kojima H, Nishikawa M, et al. Determination of HLA-A, -C, -B, -DRB1 allele and haplotype frequency in Japanese population based on family study. Tissue Antigens. 2015;85(4):252-259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Leckband SG, Kelsoe JR, Dunnenberger HM, et al. ; Clinical Pharmacogenetics Implementation Consortium . Clinical Pharmacogenetics Implementation Consortium guidelines for HLA-B genotype and carbamazepine dosing. Clin Pharmacol Ther. 2013;94(3):324-328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Niihara H, Kakamu T, Fujita Y, Kaneko S, Morita E. HLA-A31 strongly associates with carbamazepine-induced adverse drug reactions but not with carbamazepine-induced lymphocyte proliferation in a Japanese population. J Dermatol. 2012;39(7):594-601. [DOI] [PubMed] [Google Scholar]
- 27.Kaniwa N, Saito Y. The risk of cutaneous adverse reactions among patients with the HLA-A*31:01 allele who are given carbamazepine, oxcarbazepine or eslicarbazepine: a perspective review. Ther Adv Drug Saf. 2013;4(6):246-253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim SH, Lee KW, Song WJ, et al. ; Adverse Drug Reaction Research Group in Korea . Carbamazepine-induced severe cutaneous adverse reactions and HLA genotypes in Koreans. Epilepsy Res. 2011;97(1-2):190-197. [DOI] [PubMed] [Google Scholar]
- 29.Amstutz U, Ross CJ, Castro-Pastrana LI, et al. ; CPNDS Consortium . HLA-A*31:01 and HLA-B*15:02 as genetic markers for carbamazepine hypersensitivity in children. Clin Pharmacol Ther. 2013;94(1):142-149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Plumpton CO, Yip VL, Alfirevic A, Marson AG, Pirmohamed M, Hughes DA. Cost-effectiveness of screening for HLA-A*31:01 prior to initiation of carbamazepine in epilepsy. Epilepsia. 2015;56(4):556-563. [DOI] [PubMed] [Google Scholar]
- 31.National Institute for Health and Care Excellence Guide to the methods of technology appraisal 2013. https://www.nice.org.uk/process/pmg9/chapter/foreword. Accessed November 1, 2016. [PubMed]
- 32.Mallal S, Phillips E, Carosi G, et al. ; PREDICT-1 Study Team . HLA-B*5701 screening for hypersensitivity to abacavir. N Engl J Med. 2008;358(6):568-579. [DOI] [PubMed] [Google Scholar]
- 33.Ko TM, Tsai CY, Chen SY, et al. ; Taiwan Allopurinol-SCAR Consortium . Use of HLA-B*58:01 genotyping to prevent allopurinol induced severe cutaneous adverse reactions in Taiwan: national prospective cohort study. BMJ. 2015;351:h4848. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Detailed Classification Criteria of Cutaneous Adverse Drug Reactions (cADRs) Used for the Review by Expert Dermatologists
eTable 2. List of Drugs Prescribed Before and After HLA-A*31:01 Screening of 198 HLA-A*31:01-Positive Participants
eTable 3. Details of the Review by Expert Dermatologists of Cases Initially Diagnosed as Cutaneous Adverse Drug Reactions (cADRs) According to HLA-A*31:01 Screening Results
eTable 4. Comparison of the Incidences of other Adverse Events Between HLA-A*31:0-Positive and HLA-A*31:0-Negative Patients