This study describes the development and assessment of 3-dimensionally printed sinus and skull base models for use in endoscopic skull base surgery.
Key Points
Question
Is it feasible to create patient-specific, 3-dimensionally printed sinus and skull base models that are anatomically accurate and provide realistic haptic feedback comparable to cadaveric models?
Findings
In this study, 7 otolaryngology residents and 2 attending physicians evaluated the haptic feedback of a patient-specific, 3-dimensionally printed model and confirmed its anatomical accuracy with computed tomography and intraoperative navigation. The model scored high on the Likert scale for haptic accuracy with intranasal instruments.
Meaning
Three-dimensionally printed sinus and skull base models can be generated with anatomical and haptic accuracy and are potentially useful in surgical planning and as a supplemental or alternative simulation or training platform to cadaveric dissection.
Abstract
Importance
Three-dimensional (3D) printing is an emerging tool in the creation of anatomical models for simulation and preoperative planning. Its use in sinus and skull base surgery has been limited because of difficulty in replicating the details of sinus anatomy.
Objective
To describe the development of 3D-printed sinus and skull base models for use in endoscopic skull base surgery.
Design, Setting, and Participants
In this single-center study performed from April 1, 2017, through June 1, 2017, a total of 7 otolaryngology residents and 2 attending physicians at a tertiary academic center were recruited to evaluate the procedural anatomical accuracy and haptic feedback of the printed model.
Interventions
A 3D model of sinus and skull base anatomy with high-resolution, 3D printed material (VeroWhite) was printed using a 3D printer. Anatomical accuracy was assessed by comparing a computed tomogram of the original patient with that of the 3D model across set anatomical landmarks (eg, depth of cribriform plate). Image-guided navigation was also used to evaluate accuracy of 13 surgical landmarks. Likert scale questionnaires (1 indicating strongly disagree; 2, disagree; 3, neutral; 4, agree; and 5, strongly agree) were administered to 9 study participants who each performed sinus and skull base dissections on the 3D-printed model to evaluate anatomical accuracy and haptic feedback.
Main Outcomes and Measures
Main outcomes of the study include objective anatomical accuracy through imaging and navigation and haptic evaluation by the study participants.
Results
Seven otolaryngology residents (3 postgraduate year [PGY]-5 residents, 2 PGY-4 residents, 1 PGY-3 resident, and 1 PGY-2 resident) and 2 attending physicians evaluated the haptic feedback of the 3D model. Computed tomographic comparison demonstrated a less than 5% difference between patient and 3D model measurements. Image-guided navigation confirmed accuracy of 13 landmarks to within 1 mm. Likert scores were a mean (SD) of 4.00 (0.71) for overall procedural anatomical accuracy and 4.67 (0.5) for haptic feedback.
Conclusions and Relevance
This study shows that high-resolution, 3D-printed sinus and skull base models can be generated with anatomical and haptic accuracy. This technology has the potential to be useful in surgical training and preoperative planning and as a supplemental or alternative simulation or training platform to cadaveric dissection.
Introduction
Use of 3-dimensional (3D) printing in the creation of anatomical models for resident training and operative planning is gaining traction in otolaryngology. Although current otolaryngology resident training relies primarily on the use of cadavers and direct clinical experience, the Accreditation Council for Graduate Medical Education is now requiring simulation-based training for general surgery training programs.1,2 As a result of this mandate, the development of new technology that emphasizes the optimization of visual, tactile, and kinesthetic model qualities is vital for the accurate reproduction of the direct clinical experience. The use of 3D-printed models in surgical education is one such technology that is emerging as a valuable tool in numerous surgical subspecialties.3,4
Endoscopic sinus and skull base operations involve an area of complex anatomy and require the ability to maneuver within a narrow operative field. The use of 3D printing in sinus surgery has been limited because of difficulty in printing the intricate details of sinus anatomy that would allow for accurate representation of cadaveric models or direct clinical experiences. The ability to manufacture a model for resident simulation-based training may allow for improved teaching, increased trainee autonomy in technically challenging cases, and potentially improved patient safety.
In the additive manufacturing process, digital images from computed tomography (CT) or magnetic resonance imaging (MRI) are converted into 3D printouts by layering heated plastics to form a physical model. These models can then be used in resident training and simulations as well as operative planning. Within otolaryngology, multiple 3D-printed trainers have been reported.1,5,6,7,8,9,10,11
The current simulators available for training include commercially available generic sinus trainers, such as the S.I.M.O.N.T. sinus models (Global Technologies) or nonanatomical trainers.12,13,14 Although these simulators can be valuable to practicing instrumentation, they lack variety and patient specificity and are often dissimilar in haptics to human anatomy.
In this study, the feasibility of developing patient-specific, 3D-printed sinus and skull base models for use in endoscopic skull base approaches is described. The time needed for fabrication, anatomical accuracy, and haptic feedback of the 3D-printed models were evaluated.
Methods
Model Creation
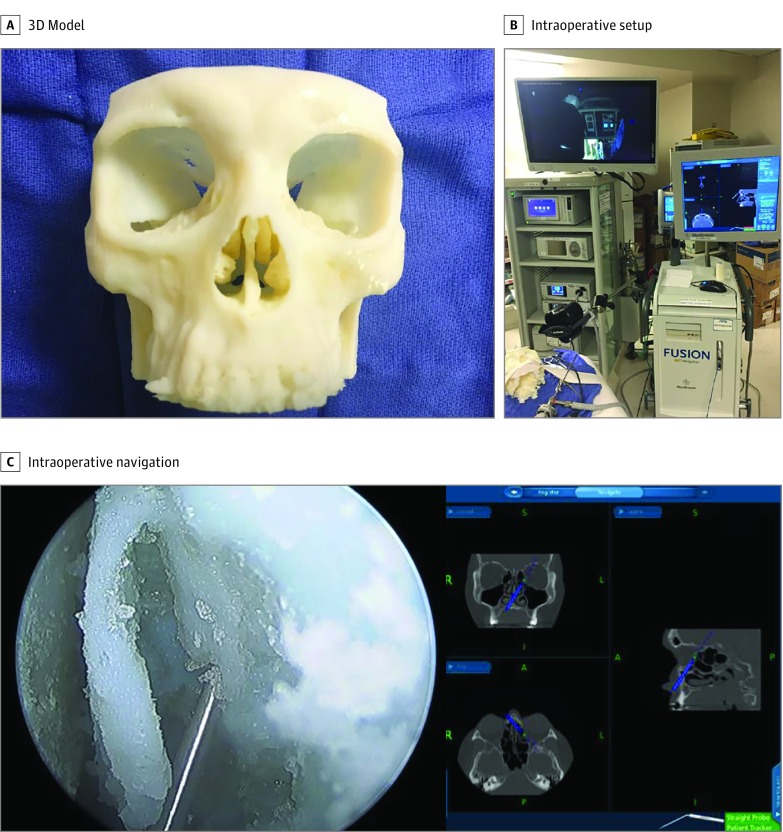
Open source software (3D Slicer, version 4.6 and Meshmixer, version 3.2) was used to convert an anonymized high-resolution CT of the paranasal sinuses and skull base into printable Standard Tessellation Language (STL) code by segmenting relevant structures. The CT was from a deidentified patient with absence of pathologic findings and well-pneumatized paranasal sinuses. To ensure that relevant sinonasal anatomy was incorporated into the 3D-printed model, we performed the software planning to ensure that bony lamella, sinus ostia, and anatomical boundaries were accurately captured. The model was then printed with high-resolution, 3D-printed material (VeroWhite) using a 3D printer (Stratasys Connex Polyjet 3D printer; Stratasys Direct Inc) (Figure 1). The high-resolution, 3D-printed material was chosen because it is a common 3D-print material with rigid quality that is capable of simulating human bone. It has previously been used to simulate bone in other 3D-printed models.15 A water-soluble support material was used to create the model to prevent alterations during postprocessing. Data on software formatting time, print time, and postprocessing time of the model were collected to determine the feasibility of using these 3D-printed models in practice. The University of California, Davis institutional review board reviewed the study and granted a waiver of informed consent.
Figure 1. Three-Dimensionally (3D) Printed Model and Setup.
Anatomical Accuracy
To assess anatomical accuracy, the 3D-printed model was imaged using cone-beam volumetric CT (MiniCat, Xoran Technologies LLC). The locations of 7 anatomical landmarks were identified on the original patient CT and the 3D-printed model CT (Table 1). Measurements among these landmarks were taken and then compared between the original and 3D model CTs.
Table 1. Computed Tomography Comparison Measures.
| Variable | Measurements, mm | Absolute Difference, mm (%) | |
|---|---|---|---|
| Patient | Model | ||
| Right nasal spine to sphenoid face | 63.9 | 67.0 | 3.1 (4.6) |
| Left nasal spine to sphenoid face | 63.8 | 67.0 | 3.2 (4.7) |
| Axilla of middle turbinate to axilla of middle turbinate | 5.0 | 4.8 | 0.2 (4.2) |
| Width of cribriform | 6.6 | 6.8 | 0.2 (3.0) |
| Height of cribriform on the right | 5.3 | 5.2 | 0.1 (1.9) |
| Height of cribriform on the left | 5.7 | 5.7 | 0 |
| Lamina to lamina at nasolacrimal duct | 19.4 | 19.6 | 0.2 (1.0) |
The 3D model was then registered into an intraoperative navigation system (Medtronic) using the patient’s original CT. Image-guided navigation was used to assess the accuracy of 13 important surgical landmarks, including location of the maxillary ostia, posterior wall of the maxillary sinus, lamina papyracea, anterior wall of the sphenoid sinus, posterior wall of the sphenoid sinus, opticocarotid recess, and the skull base (Figure 1).
Participants and Setting
Seven otolaryngology residents and 2 attending physicians were recruited to evaluate the haptic feedback of the 3D model from April 1, 2017, through June 1, 2017. The model was then prepared for various sinus and skull base approaches. Then, 0° and 30° endoscopes (Karl Storz Endoskope) were used with a video tower. The model was secured to the operating table with the nasal cavity pointing superiorly to re-create the operating position. Foam tape was secured over the piriform aperture with two 1.5 × 1.5-cm openings to simulate the opening at the nares and to facilitate camera stability. Sinus and endoscopic skull base surgical instruments, including the curette, backbiter, through-cutting instruments, and high-speed self-irrigating drill with a diamond burr, were used (Midus Rex; Medtronic).
Tasks
Study participants were then asked to perform 1 or several key steps in each of the following procedures: modified endoscopic Lothrop, endoscopic anterior craniofacial resection, transpterygoid, and transclival approaches. During the procedure sessions, participants transitioned from simple to more advanced techniques to encompass the most relevant aspects of endoscopic sinus and skull base surgery.
Model Rating
Likert scale questionnaires were administered to evaluate the anatomical and haptic accuracy of the 3D model using the different instruments of dissection. The questionnaire used a 1- to 5-point rating scale and a predefined value question regarding the anatomical and haptic accuracy (1 indicating strongly disagree; 2, disagree; 3, neutral; 4, agree; and 5, strongly agree). The anatomical accuracy was assessed as follows: “The 3D-printed sinonasal model demonstrated anatomical accuracy during the indicated portion of the dissection.” Participants were then instructed to provide a Likert scale rating for anatomical accuracy for each procedure performed on the 3D model. The haptic accuracy was assessed as follows: “The haptic feedback was similar to that of bone when using the (instrument).” Participants were asked to rate each instrument separately (cutting burr, through-cutting instruments, curette, and backbiter). Survey results were stored and statistical analysis performed using Microsoft Excel (Microsoft Inc).
Results
Seven otolaryngology residents and 2 attending physicians evaluated the haptic feedback of the 3D model. Three postgraduate year (PGY)-5 residents, 2 PGY-4 residents, 1 PGY-3 resident, and 1 PGY-2 resident participated in the study.
Model Creation
The conversion and formatting of Digital Imaging and Communications in Medicine (DICOM) images to a 3D-printable STL file required 52 minutes. Printing time was 18 hours. Postprocessing time was 45 minutes. During postprocessing, the water-soluble support material was removed from the 3D-printed model by placing the model in warm water. The model was then gently brushed and dried to remove the residual support material. Total fabrication time was 19 hours 37 minutes. The approximate cost of the model was $800.
Anatomical Accuracy
Comparisons between the original patient CT and the 3D-model CT demonstrated a less than 5% difference between the images (Table 1 and Figure 2). The absolute difference shows the greatest discrepancy between measurements from the nasal spine to the sphenoid face of approximately 3 mm. All other differences were 0.2 mm or less. Image-guided navigation using the Medtronic Fusion system confirmed accuracy of 13 important surgical landmarks to within 1 mm based on direct visualization.
Figure 2. Computed Tomography (CT) Comparisons.
Model Rating
Models were rated based on extended anterior skull base resection, transpterygoid skull base approach, and transclival approach. In the extended anterior skull base resection, the mean Likert scores were 4.67 (0.5) for the modified Lothrop procedure, 4.33 (0.5) for the sphenoidotomy, and 4.33 (0.67) for anterior skull base removal (Table 2 and Figure 3). For the transpterygoid skull base approach, the maxillary antrostomy score was 4, the posterior maxillary wall resection score was 3, and the superior pterygoid cuts score was 2.67 (Table 2). For the transclival approach, the removal of the anterior and posterior clival cortices had a mean (SD) score of 3.67 (0.5), and removal of the floor of the sphenoid had a mean (SD) score of 4.67 (0.71) (Table 2).
Table 2. Anatomical Accuracy for Procedures and Instrument Haptics Assessment on Likert Scale.
| Procedure | Likert Score | ||
|---|---|---|---|
| Mean (SD) | Median | Mode | |
| Modified Lothrop procedure | 4.67 (0.5) | 5 | 5 |
| Sphenoidotomy | 4.33 (0.5) | 4 | 4 |
| Removal of anterior cranial base | 4.33 (0.67) | 4 | 4 |
| Removal of floor of sphenoid | 4.67 (0.71) | 5 | 5 |
| Removal of anterior and posterior clival cortices | 3.67 (0.5) | 4 | 4 |
| Maxillary antrostomy | 4.00 (0.87) | 4 | 5 |
| Posterior maxillary wall resection | 3.00 (0.87) | 3 | 4 |
| Superior pterygoid cuts | 2.67 (0.5) | 3 | 3 |
| Overall model anatomical accuracy | 4.00 (0.71) | 4 | 4 |
| Instrument haptics | |||
| Curette | 3.67 (0.71) | 4 | 3 |
| Backbiter | 4.33 (0.83) | 4 | 5 |
| Through-cutting instruments | 4.67 (0.71) | 5 | 5 |
| Drill burr | 5.00 (0) | 5 | 5 |
| Overall manipulation of intranasal instruments | 4.67 (0.5) | 5 | 5 |
Figure 3. Intraoperative Photographs.
Haptic Feedback
When the haptic accuracy of the different instruments was assessed, the mean (SD) Likert scores were 3.67 (0.71) for the curette, 4.33 (0.83) for the backbiter, 4.67 (0.71) for through-cutting instruments, and 5.00 (0) for the drill burr. The mean Likert score for the overall manipulation of intranasal instruments was 4.67 (Table 2).
Discussion
Three-dimensionally printed models are emerging as a valuable tool in surgical training and preoperative planning. Currently, surgical residency training involves primarily cadaveric dissection and clinical training. Although cadaveric specimens have high anatomical and physical validity, they are often challenging to obtain, lack patient-specific pathologic features, and are associated with costs that may be prohibitive to repetitive training. Direct and early supervised clinical experience has been the criterion standard model in which future surgeons are taught. However, surgical time constraints, high costs of operating room time, and work hour restrictions are commonly encountered barriers in today’s academic climate. Simulation-based training with 3D models offers a supplemental approach to provide the additional hands-on experience that is required to master critical surgical skills and improve patient safety, although this strategy has yet to be directly studied.
Although some models have been created for otolaryngology trainees, challenges in printing complex 3D sinus and skull base anatomy is a limitation acknowledged by authors of previous studies.1,14,16 Alrasheed et al16 developed and validated a 3D-printed model of the ostiomeatal complex for endoscopic sinus training. Narayanan et al1 also developed a skull base training model using 3D-printing technology. However, our 3D-printed model is the first surgical trainer, to our knowledge, to incorporate endoscopic sinus surgery procedures and skull base surgery.
The present study revealed that it is feasible to create an anatomically and haptically accurate 3D model of sinus and skull base anatomy using 3D-printed material. The model was created in less than 20 hours. The software manipulation was performed by the surgeons and the printing process performed within the institution. This approach helped streamline the process for rapid acquisition of this high-fidelity model. The anatomical accuracy of the model was assessed with CT comparisons and CT-guided stereotactic navigation. This model was accurate for several important surgical landmarks. Absolute differences were clinically negligible with the exception of the measurement from the anterior nasal spine to the face of the sphenoid sinus (3-mm difference). This difference is potentially explained by inaccuracy in the manipulation of the STL code before model printing. Although 3 mm could have surgical implications, the measurement from the anterior nasal spine to the sphenoid face can vary substantially and is one of the less reliable anatomical landmarks for identifying the sphenoid sinus.17,18 This study examined the accuracy of 3D models compared with original patient CTs and intraoperative navigation and demonstrated that complex sinonasal anatomy is reproducible using a 3D-printed model. Overall, the model demonstrates accurate replication of patient-specific anatomy, which is important for surgical planning and instrumentation.
Likert survey results showed that participants thought the 3D model was overall anatomically accurate during the endoscopic sinus and skull base procedures. For the transpterygoid skull base approach, the mean Likert scores indicated that the models accurately demonstrated the anatomy during the antrostomy portion of the procedure, but responses were neutral as to whether this anatomy was accurately demonstrated during the posterior wall removal and superior pterygoid cuts. In terms of haptic accuracy, the Likert scores indicated that participants typically agreed that these models felt similar to human bone with all of the instruments used with the exception of the curette.
On the basis of this study’s findings, 3D sinonasal printing provides an anatomically accurate model of sinonasal anatomy. Furthermore, 3D-printed material realistically represents haptic feedback. Although not specifically evaluated in this study, we hypothesize that 3D-printed materials will be a safe and effective method for teaching endoscopic sinonasal approaches. Future study will need to verify construct validity of the 3D-printed materials for sinonasal and skull base models. A 3D-printed material model provides a realistic platform on which to practice important skills, such as using endoscopic drills in a small, anatomically complex area without model failure or anatomical distortion.
Limitations
The findings from this study must be interpreted in the context of its limitations, which include lack of soft tissue and blood during the dissection, small study size, variations in surgeon experience, and use of a single print material. One of the most promising aspects of 3D printing is the ability to create patient-specific disease models. For example, one could print a 3D model with a Meckel cave lesion and practice the approach to this lesion in the laboratory. Although resident experience with these types of procedures is relatively low, the 3D model would allow for these rarer procedures to be performed in a practice or laboratory setting. We chose to include the procedures outlined in this study because we view the ability to simulate these more complicated and intricate dissections as a future direction for 3D-printed sinonasal models. We recognize that our analysis is in part based on a subjective Likert scale, which is subject to response bias, especially given our small sample size. However, for the purposes of this feasibility study, the use of a Likert survey to assess the anatomical and haptic accuracy of our 3D-printed material model produced overall positive responses and supports further research into this area. The Likert questions were defined specifically for this study and have not been previously validated. Although we recognize that this factor is a limitation of the current study, Likert questionnaires are commonly used to evaluate simulation models.12,19 Future studies with a larger sample size, including a larger number of surgeons with more skull base surgery experience, are needed to endorse the validity of these results. Studies that compare different print materials should also be investigated.
Conclusions
This study demonstrated that 3D models printed with 3D-printed material on a 3D printer can accurately simulate sinus anatomy, offering an alternative to cadaveric dissection. This method could potentially play a significant role in surgical training and preoperative planning.
References
- 1.Narayanan V, Narayanan P, Rajagopalan R, et al. . Endoscopic skull base training using 3D printed models with pre-existing pathology. Eur Arch Otorhinolaryngol. 2015;272(3):753-757. [DOI] [PubMed] [Google Scholar]
- 2.Accreditation Council for Graduate Medical Education (ACGME) (2011) Common program requirements. http://www.acgme.org/acgmeweb/Portals/0/dh_dutyhoursCommonPR07012007.pdf. Accessed March 21, 2018.
- 3.Cheung CL, Looi T, Lendvay TS, Drake JM, Farhat WA. Use of 3-dimensional printing technology and silicone modeling in surgical simulation: development and face validation in pediatric laparoscopic pyeloplasty. J Surg Educ. 2014;71(5):762-767. [DOI] [PubMed] [Google Scholar]
- 4.Waran V, Narayanan V, Karuppiah R, et al. . Injecting realism in surgical training-initial simulation experience with custom 3D models. J Surg Educ. 2014;71(2):193-197. [DOI] [PubMed] [Google Scholar]
- 5.Hochman JB, Rhodes C, Wong D, Kraut J, Pisa J, Unger B. Comparison of cadaveric and isomorphic three-dimensional printed models in temporal bone education. Laryngoscope. 2015;125(10):2353-2357. [DOI] [PubMed] [Google Scholar]
- 6.Scawn RL, Foster A, Lee BW, Kikkawa DO, Korn BS. Customised 3D printing: an innovative training tool for the next generation of orbital surgeons. Orbit. 2015;34(4):216-219. [DOI] [PubMed] [Google Scholar]
- 7.Lioufas PA, Quayle MR, Leong JC, McMenamin PG. 3D printed models of cleft palate pathology for surgical education. Plast Reconstr Surg Glob Open. 2016;4(9):e1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Berens AM, Newman S, Bhrany AD, Murakami C, Sie KC, Zopf DA. Computer-aided design and 3D printing to produce a costal cartilage model for simulation of auricular reconstruction. Otolaryngol Head Neck Surg. 2016;155(2):356-359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Waran V, Narayanan V, Karuppiah R, et al. . Neurosurgical endoscopic training via a realistic 3-dimensional model with pathology. Simul Healthc. 2015;10(1):43-48. [DOI] [PubMed] [Google Scholar]
- 10.Daniel M, Watson J, Hoskison E, Sama A. Frontal sinus models and onlay templates in osteoplastic flap surgery. J Laryngol Otol. 2011;125(1):82-85. [DOI] [PubMed] [Google Scholar]
- 11.Hsieh TY, Dedhia R, Cervenka B, Tollefson TT. 3D printing: current use in facial plastic and reconstructive surgery. Curr Opin Otolaryngol Head Neck Surg. 2017;25(4):291-299. [DOI] [PubMed] [Google Scholar]
- 12.Malekzadeh S, Pfisterer MJ, Wilson B, Na H, Steehler MK. A novel low-cost sinus surgery task trainer. Otolaryngol Head Neck Surg. 2011;145(4):530-533. [DOI] [PubMed] [Google Scholar]
- 13.Wais M, Ooi E, Leung RM, Vescan AD, Lee J, Witterick IJ. The effect of low-fidelity endoscopic sinus surgery simulators on surgical skill. Int Forum Allergy Rhinol. 2012;2(1):20-26. [DOI] [PubMed] [Google Scholar]
- 14.Chang DR, Lin RP, Bowe S, et al. . Fabrication and validation of a low-cost, medium-fidelity silicone injection molded endoscopic sinus surgery simulation model. Laryngoscope. 2017;127(4):781-786. [DOI] [PubMed] [Google Scholar]
- 15.Mayer R, Liacouras P, Thomas A, Kang M, Lin L, Simone CB II. 3D printer generated thorax phantom with mobile tumor for radiation dosimetry. Rev Sci Instrum. 2015;86(7):074301. [DOI] [PubMed] [Google Scholar]
- 16.Alrasheed AS, Nguyen LHP, Mongeau L, Funnell WRJ, Tewfik MA. Development and validation of a 3D-printed model of the ostiomeatal complex and frontal sinus for endoscopic sinus surgery training. Int Forum Allergy Rhinol. 2017;7(8):837-841. [DOI] [PubMed] [Google Scholar]
- 17.Anusha B, Baharudin A, Philip R, Harvinder S, Shaffie BM, Ramiza RR. Anatomical variants of surgically important landmarks in the sphenoid sinus: a radiologic study in Southeast Asian patients. Surg Radiol Anat. 2015;37(10):1183-1190. [DOI] [PubMed] [Google Scholar]
- 18.Berjis N, Hashemi SM, Rogha M, Biron MA, Setareh M. Some anatomical variation of paranasal sinuses using sinus endoscopic approach on “cadaver” in Isfahan, Iran. Adv Biomed Res. 2014;3:51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.AlReefi MA, Nguyen LH, Mongeau LG, et al. . Development and validation of a septoplasty training model using 3-dimensional printing technology. Int Forum Allergy Rhinol. 2017;7(4):399-404. [DOI] [PubMed] [Google Scholar]