Abstract
Importance
The introduction and evaluation of a novel technique to create nasal prostheses with 3-dimensional (3-D) imaging software may circumvent the need for an anaplastologist.
Objectives
To describe a novel computer algorithm for the creation of a 3-D model of a nose and to evaluate the similarity of appearance of the nasal prosthesis with that of the individual’s nose.
Design, Setting, and Participants
A prospective pilot study with a cross-sectional survey was conducted from August 1 to October 31, 2016, at a tertiary care academic center. Five volunteers were used for creation of the nasal prostheses, and 36 survey respondents with a medical background were involved in evaluating the nasal prostheses.
Exposures
A computer algorithm using a 3-D animation software (Blender; Blender Foundation) and Adobe Photoshop CS6 (Adobe Systems) were used to create a 3-D model of a nose. Photographs of 5 volunteers were processed with the computer algorithm. The model was then printed using a desktop 3-D printer. Attending physicians, residents, and medical students completed a survey and were asked to rate the similarity between the individuals’ photographs and their 3-D printed nose on a Likert-type scale.
Main Outcomes and Measures
The similarity between 3-D printed nasal models and photographs of the volunteers’ noses based on survey data.
Results
Thirty-six survey respondents evaluated 4 views for each of the 5 modeled noses (from 4 women and 1 man; mean [SD] age, 26.6 [5.7] years). The mean (SD) score for the overall similarity between the photographs and the 3-D models was 8.42 (1.34). The mean scores for each nasal comparison ranged from 7.97 to 8.62. According to the survey, respondents were able to match the correct 3-D nose to the corresponding volunteers’ photographs in 171 of 175 photographs (97.7%). All surveyed clinicians indicated that they would consider using this tool to create a temporary prosthesis instead of referring to a prosthodontist.
Conclusions and Relevance
This algorithm can be used to model and print a 3-D prosthesis of a human nose. The printed models closely depicted the photographs of each volunteer’s nose and can potentially be used to create a temporary prosthesis to fill external nasal defects. The appropriate clinical application of this technique is yet to be determined.
This pilot study describes a novel computer algorithm for the creation of a 3-dimensional model of a nose and uses a survey to evaluate the similarity of appearance of the nasal prosthesis with that of the individual’s nose.
Key Points
Question
Can the novel technique developed for 3-dimensional construction of nasal prostheses create nasal prostheses that are similar to nasal appearance?
Findings
In this pilot study, a novel technique to construct nasal prostheses using 3-dimensional printing technology was evaluated. Survey respondents indicated that the created nasal prostheses appeared to closely resemble actual nasal photographs.
Meaning
The study presents a novel technique to construct nasal prostheses using 3-dimensional imaging software; however, the appropriate clinical application of this technique is yet to be determined.
Introduction
Nasal cutaneous defects result from Mohs surgery for the treatment of malignant neoplasms such as basal cell carcinoma, squamous cell carcinoma, and melanoma. Nasal defects caused by trauma are less commonly seen and are caused most often by amputations due to dog bites or crush injuries.1 These defects are conspicuous and may cause social marginalization and psychological stress for patients. The nose is perceived by the eye as a series of topographic visual subunits that are outlined by shadows and natural contours.2 Subtle changes in these shadows are viewed by the common observer as a deformity because we are trained to find symmetric facial features as more aesthetically pleasing.2 The complexity of the reconstruction of nasal defects depends on the size of the defect and whether it involves the scaffolding structures of the nose, nasal septum, or ala. Often, complex multistep procedures are required for the reconstruction of many nasal defects.
When patients present with nasal defects, comprehensive counseling and open communication with the reconstructive surgeon help the patient to understand the reconstructive process. Prosthetics are commonly used in maxillofacial rehabilitation to offset the burden of complex reconstructive surgeries.3 Conventional nasal prosthetics require technically skilled individuals, such as anaplastologists or maxillofacial prosthodontists, to create a hand-sculpted impression made of wax, which is subsequently cast in medical-grade silicon rubber. This process can take up to 5 to 7 hospital visits and almost 10 weeks to complete the customization. The cost of prosthetics can range from $10 000 to $15 000 per device and may not be covered by all health insurance plans.3 In addition, there is a barrier to access these services because there are very few specialists trained in this field, and patients may have to travel to seek care.
Three-dimensional (3-D) printing has been widely used in manufacturing for decades and has increasingly been used in medicine. The simple concept of converting a 2-dimensional (2-D) digital image into a 3-D model by printing successive layers of synthetic polymers has expanded this application to the creation of human organs.4 Three-dimensional printed models are currently used in preoperative surgical planning of high-risk procedures and for medical education and training.5
Advances in computer technology have allowed the mapping of patient anatomy using a computed tomographic (CT) scan, which is then converted into a 3-D virtual model using computer-aided design and manufacturing. These advanced techniques can create anatomically accurate and sophisticated models that can be used in lieu of referral to a prosthodontist. In addition, the models created via computer-aided design and manufacturing may be more accurate than hand-carved prostheses.6 However, many of these techniques rely on expensive imaging technology and require a substantial investment in 3-D modeling software. This financial burden may not be feasible for many institutions, especially in regions of the world with emerging economies. Furthermore, use of 3-D modeling software and 3-D printing often require technical knowledge of computer science and graphic design.
Most recently, 3-D printing has revolutionized the field of facial prosthetics by allowing the manufacturing of patient-tailored products in a few hours and for a fraction of the cost of traditional facial prosthetics.7 He et al6 described a 3-D printing technique using patient-specific CT scans to create a silicone soft-tissue prosthetic ear for approximately $30 in addition to the cost of the CT scan and 3-D modeling software used. This method, while anatomically accurate and promising for the future of prosthetics, still requires skilled technicians to craft the product and relies on expensive computer software and scanning techniques. Also, an additional CT scan exposes the patient to potentially harmful radiation.
Although creating 3-D models from CT scans and by using computer-aided design and manufacturing technology may be cumbersome for the reconstructive surgeon, all surgeons have the ability to create preoperative 2-D photographs of patients. Previous studies have used this fact in an attempt to create realistic 3-D images. Oliveira-Santos et al8 previously described the application of 2-D photography to produce 3-D photographic renderings to be used for patient counseling for aesthetic procedures. These renderings, however, were not used to create prostheses or models. In an attempt to create realistic models, Salazar-Gamarra et al9 described the use of a mobile device application using photogrammetry and a web-based platform. The programs that were used for this technique are no longer available.
In this study, we describe an algorithm to model the patient’s nose using 2-D images to model and print a 3-D model using a desktop 3-D printer. We propose a user-friendly and inexpensive technique to model external anatomical features of a nose using 2 commercially available software packages. Our hypothesis is that the algorithm can produce a 3-D model of a nose that is similar to the patient’s actual anatomy. To test this hypothesis, we used the algorithm to create 5 individual noses based on 2-D photographs. We then surveyed practicing physicians, residents, and medical students regarding the similarity of the 3-D printed nasal prostheses to the 2-D photographs. The objective of this study was to determine the feasibility of creating nasal prostheses by this method and to evaluate the similarity of these prostheses to patient images. Furthermore, we examined whether this computer technique could be used by individuals who do not have an extensive background in graphic design.
Methods
Computer Algorithm
This prospective pilot study was conducted from August 1 to October 31, 2016. The algorithm created uses 2 commercially available software packages to convert 2-D photographs into 3-D nasal prostheses. The first program, Adobe Photoshop CS6 (Adobe Systems) was used to calibrate and size the photographs to scale. The second program is an animation software program, Blender (Blender Foundation [https://www.blender.org]), which was used to create a 3-D model of a desired structure based on the previously resized 2-D images. This study was approved by the University of Maryland School of Medicine institutional review board, and all volunteers provided verbal consent to participate in the study.
Prior to computer processing, frontal, right lateral, left lateral, and basal views were obtained from each of 5 volunteers with a high-resolution camera (Canon EOS Rebel T4i; Canon). These images were taken in front of a blue background as is standard in our practice. The images were then imported into Adobe Photoshop CS6. The free transform tool within the program was then used to resize the images such that the size of the printed version matched that of the actual patient. These images were then used to complete the virtual reconstruction.
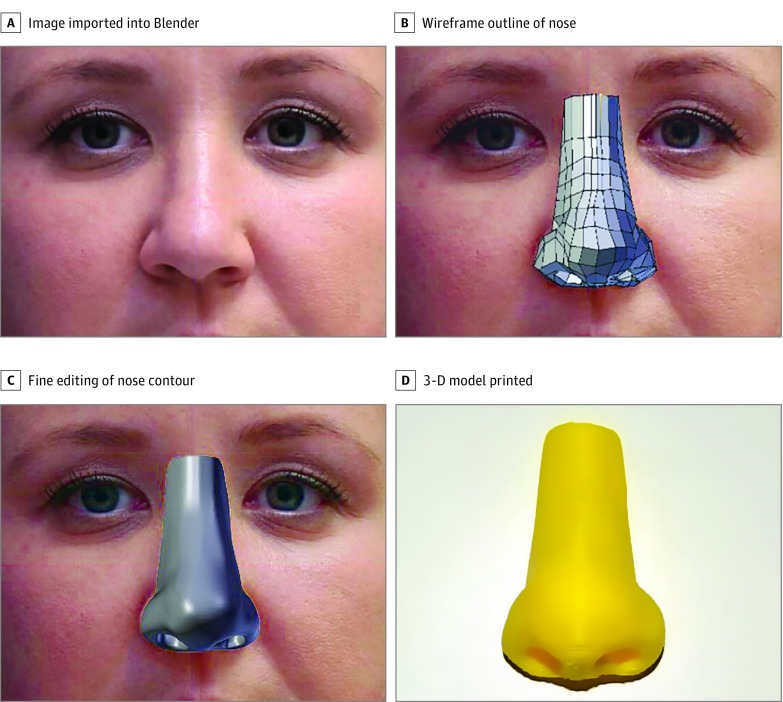
The steps of the algorithm are outlined in Figure 1. The first step of the algorithm starts with importing the resized images into Blender under the “background images” tab. The course structure of the nose was then created using the “wireframe” outline. This operation was performed for all 4 photographic images obtained for each volunteer. Fine editing of the nose contour is done by adding the “subdivision surface” modification tool. Prior to printing, the virtual 3-D model was compared with the photographs obtained. This comparison allowed for the final stage of editing and visualization of errors in modeling. After the shadows and contours of the 3-D model were fine-tuned, the virtual model was exported in a stereolithography file format.
Figure 1. Algorithm Used to Create the 3-Dimensional (3-D) Nose Model.
A, The first step involves importing 4 photographs into Blender. B, The nose is then outlined using the wireframe tool. C, A more fine-tuned edit of the model is performed using the subdivision surface modifier tool. D, The final model was produced using a LulzBot Taz 5 Desktop 3-D printer (Aleph Objects Inc).
All computer design involved in this study was performed by research staff (M.S. and C.J.R.) without prior experience in computer-aided design. This trial was a pilot study to prove the concept of using computer-aided design for printing a 3-D nasal prosthesis. The prostheses created were not used clinically.
3-D Printing
The stereolithography file was uploaded to a computer with 3-D printing capability. The width of the nose from ala to ala and the nose size were scaled to print the nose to real-life size. A LulzBot Taz 5 Desktop 3-D printer (Aleph Objects Inc) was used for the models in this study. High-impact polystyrene (Matter Hackers) was the material used to print the models evaluated in this study.
Cross-sectional Survey
To test our model, we created a survey using a Likert-type scale (from 0 to 10, where 0 is completely different from the photograph and 10 is identical to the photograph). Five volunteers participated in this study. Demographic data for these volunteers was collected and is depicted in the Table. Photographs of each volunteer were taken by the research staff. All images were deidentified prior to processing. The survey asked participants to compare the 3-D printed nasal models with the actual photographs of the volunteers. Individuals surveyed included attending physicians and residents in the otolaryngology, plastic surgery, and oral maxillofacial surgery departments, as well as medical students at the University of Maryland School of Medicine. The surveys were anonymous, but the level of training was recorded. Verbal consent was provided by all survey respondents.
Table. Volunteer Demographics.
| Volunteer No. | Age, y | Sex | Race/Ethnicity |
|---|---|---|---|
| 1 | 23 | F | African American |
| 2 | 36 | F | White |
| 3 | 23 | F | Asian |
| 4 | 28 | M | White |
| 5 | 23 | F | White |
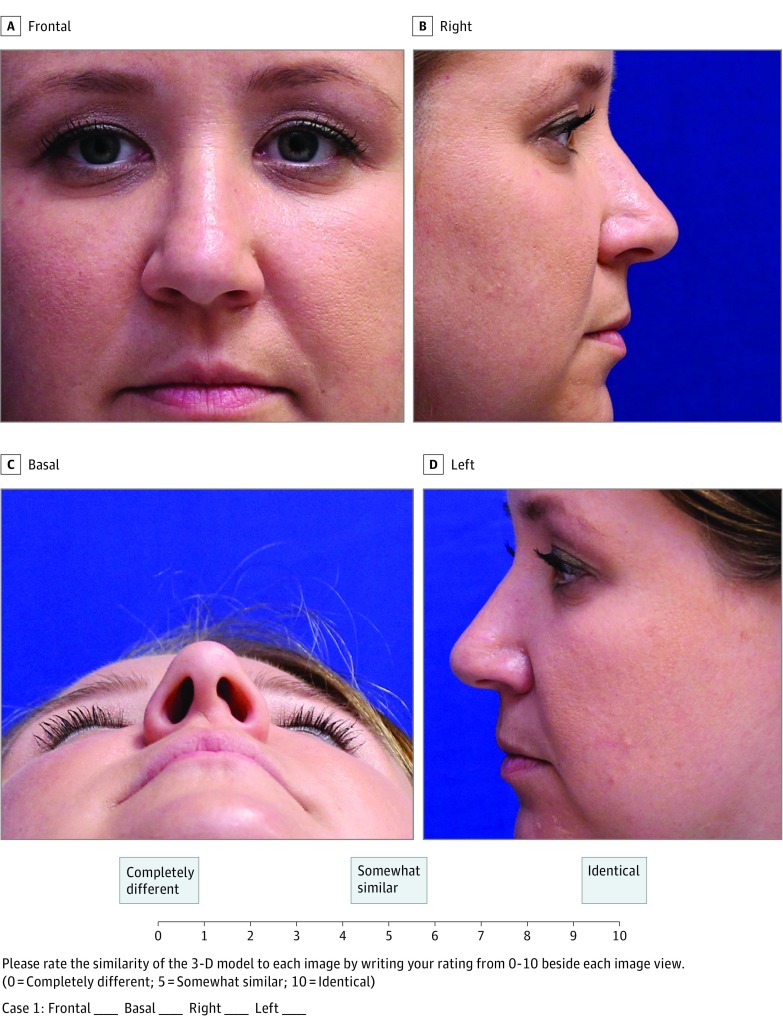
In completing the survey, the respondents were supplied with the 4 photographs obtained of each volunteer, as well as the physical 3-D printed models. Respondents were then asked to match the 3-D models with the corresponding volunteer image series in a blinded fashion. The respondents were asked to critically evaluate the similarity of the 3-D printed models to the photographic images and assign each photograph a score. Separate ratings were obtained for each of the 4 photographs for the 5 models created. An example of a survey is depicted in Figure 2. Finally, the physician respondents were asked if they would consider using an inexpensive tool, such as our proposed algorithm, in lieu of prosthodontic referral. Survey data were then collected and statistical analysis performed.
Figure 2. A Sample Survey Question Demonstrating Volunteer 1 as an Example.
The photographs of each of the 5 volunteers were displayed in 4 different views (frontal, basal, right, and left). The survey respondents were asked to rate each view of the 3-dimensional (3-D) model as it compares to the above images. The scores are graded on a Likert-type scale from 0 to 10, where 0 is completely different from the photograph, and 10 is identical to the photograph.
Statistical Analysis
Categorical variables were compared by percentages, and continuous variables were evaluated as means and SD. Data from the surveys were collected, and mean values of the ratings for each model and each view were calculated. Effect sizes associated with comparisons of the ratings for each model by attending physicians, residents, and medical students were reported using η2 and the precision of the estimate defined by the 95% CI around η2. The following conventions were used for the magnitude of the effect size: 0.01 (small), 0.06 (medium), and 0.14 (large). P < .05 (2-sided) was considered significant.
Results
Three-dimensional nose models were created based on volunteers who varied in age, sex, and race/ethnicity (Figure 1). Respondents were able to correctly match the volunteer’s photograph with the equivalent 3-D nose model in 171 of 175 photographs (97.7%). All 7 (100%) attending physicians responded that they would use this tool instead of referral to a prosthodontist. Twenty photographs were displayed for a total of 5 cases and were compared with five 3-D nose models corresponding to each of the cases. The survey was administered to 36 respondents including 7 attending physicians, 14 residents, and 15 medical students. Of the residents and attending physicians surveyed, 17 were otolaryngologists, 3 were plastic surgeons, and 4 were oral maxillofacial surgeons.
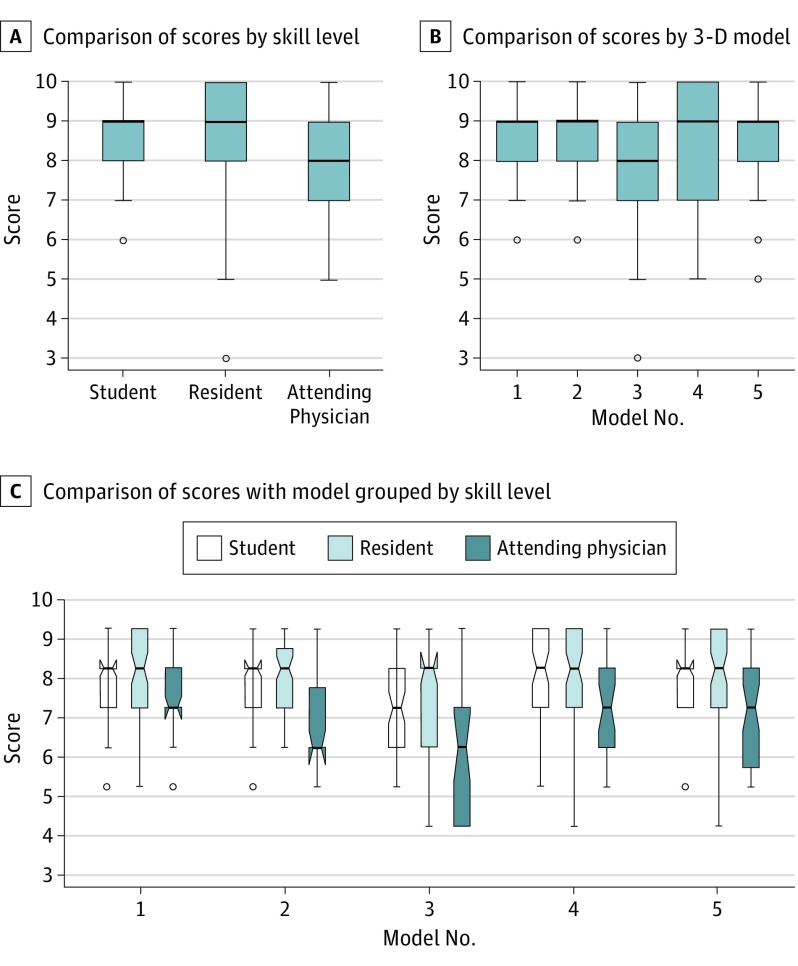
Thirty-six survey respondents evaluated 4 views for each of the 5 modeled noses (from 4 women and 1 man; mean [SD] age, 26.6 [5.7] years). The mean scores for each nasal comparison ranged from 7.97 to 8.62. The mean (SD) score for the overall similarity between the models and the photographs among all 36 respondents was 8.42 (1.34). A total of 613 of the 720 scores (85.1%) were a grade of 8 and above. The mean (SD) score was 7.71 (1.09) for attending physicians, 8.54 (1.01) for resident physicians, and 8.56 (0.74) for medical students. Multiple comparisons performed showed a difference in the mean (SD) attending physician group scores (7.71 [1.09]; F = 31.6; P < .001) compared with the 2 other groups. The η2 was 0.06 (95% CI, 0.04-0.08), supporting a medium effect size for this comparison. However, for comparisons of score grouped by model, the η2 was 0.02 (95% CI, 0.01-0.03), and for comparisons of score grouped by interaction (model grouped by skill), the η2 was 0.01 (95% CI, 0.00-0.02), indicating small effect sizes. Figure 3 depicts the results from the statistical comparisons.
Figure 3. Results From Statistical Comparisons Using Box Plots.
A, Comparison of scores assigned by skill level. Analysis of variance demonstrated a statistically significant difference, with the attending physician group showing the lowest mean (SD) scores (7.71 [1.09]; F = 31.6; P < .001) compared with the 2 other groups. B, Comparison of scores by 3-dimensional (3-D) model did not show a significant difference (F = 1.5; P = .22). C, Similar comparison of score with model grouped by skill level of assessment (F = 2.23; P = .13). Effect size is provided in the text. In each comparison, the box plot indicates the 25th to 75th interquartile range within the box centered around the median and spread of data by the whiskers. Circles indicate outliers.
Discussion
In this study, we sought to describe an algorithm that uses inexpensive and easily accessible computer-aided design techniques to model a nose. We photographed 5 volunteers and modeled their noses using 2 commercially available software products. Our results from this pilot study indicate that the algorithm described has the ability to create 3-D models of noses that are rated to be similar in appearance to the actual images of volunteers. Through the algorithm described, we open up new avenues for the use of computer-aided design in modeling a human nose and for future applications in surgical planning and manufacturing of an inexpensive temporary prosthesis.
A recognized advantage of this algorithm is that it can be used to create 3-D models that are highly specific to each patient’s unique nasal appearance. The volunteers were of different ages and had different racial and ethnic backgrounds. The computer algorithm can be applied to noses of individuals of different ethnicities, allowing the user to create 3-D models that have different structures and shapes. The overall mean scores for each training level were similar; however, the attending physicians’ scores were slightly lower, likely owing to their years of expertise making them more critical in grading. As seen in Figure 3B, model 3 was rated lower than the rest of the nasal models. The volunteer corresponding to model 3 is an Asian woman (Table). Her nose had a low radix and dorsum with a very small dorsal hump. This combination of features was difficult to replicate in the final 3-D model, which may explain the lower rating of this model by the survey participants. From a cosmetic standpoint, this technique can also be used to create virtual noses that can be tailored to the patient’s preferences, and the prosthetic can be digitally manipulated prior to printing.
The study was based on a survey that was administered to participants for ease of comparison between the 2-D photographs and the 3-D models. Although the participants were not informed about the details of the study or who created the models, we recognize that there is an intrinsic bias in the rating process. This bias is unavoidable owing to the nature of the study and the need for administering the survey in person to demonstrate the 3-D models. Another limitation of this method of survey administration is the difficulty of comparing 2-D photographs with a plastic model. As shown in Figure 1, the final nose models were printed with yellow polystyrene. Nasal contour is significantly affected by different skin tones, which makes comparing photographs with a yellow model challenging. In future studies, using material in neutral colors, such as cream or beige, may improve the aesthetic appearance of the models. Another concept that was discussed is using makeup for different skin tones to match a specific patient’s preference if this technology is to be used in clinical practice.
The described algorithm is not only inexpensive but also can be learned with relative ease. These programs are generally associated with an acceptable learning curve. A medical student with no prior background in computer-aided manufacturing completed the modeling using the 2 software packages for this pilot study. This algorithm relies solely on 2-D photographs as a template for creating a 3-D model, with a total printing cost of approximately $20 per model. Blender is an open-source software package available free of cost to the public, and Adobe Photoshop CS6 software can be purchased for $20 per month.7 This process is in contrast to previous techniques for nasal prosthetic production that have required CT scans to serve as a template for computer-aided modeling.6 Additional methods have been described using a 3-D laser scanner10; however, this technology is generally not available to the reconstructive surgeon and requires outside referral. The described algorithm has the potential to be used by clinicians to create temporary prostheses for their patients.
New horizons in 3-D printing of human organs have been introduced recently. Techniques for 3-D printing of human tissue, such as cartilage and skin, have been proposed and are under way. Scientists have successfully printed cartilage using human nasal chondrocytes.4 The manufacturing of tissue-engineered 3-D ears for patients with microtia is currently being investigated.11 Similarly, manufacturing of epidermis and dermis from keratinocytes and fibroblasts using laser-printing technology has been successful.12 This type of tissue printing has the capacity to transform the field of organ transplant and plastic surgery. Unfortunately, the cost of such techniques remains a significant barrier to large-scale availability of these products. One meta-analysis of the literature on 3-D printing of human tissue estimated that the cost of printing an artificial nose using tissue can be up to $40 000.4 The material we used to print the models for this pilot study was high-impact polystyrene, which was used owing to availability of the material at our institution and the associated ease of use, as well as the ability to print multiple drafts rapidly at once. For further clinical application of this technique and possible use as a temporary prosthetic, we plan to use polylactic acid, a biodegradable thermoplastic with widespread applications in both nonmedical and biomedical fields.13,14 It is widely used in food packaging materials and medical implants such as sutures, plates, screws, and rods. Agarwal et al15 reviewed the use of resorbable implants and screws for mandibular fixation. Polylactic acid is not toxic in the solid form and can be used for safe, implantable prosthetics in the future. For example, injectable polylactic acid has been used for restoring facial volume and was approved by the US Food and Drug Administration in 2004 for treatment of facial lipoatrophy associated with HIV and in 2009 for cosmetic purposes.16,17 However, polylactic acid is only for temporary use as the material degrades over time.18
Limitations
Some limitations of this technique include the learning curve needed to first master the technique, which requires a few hours of training but is not prohibitive and requires no prior knowledge of computer-aided design. Also, this method requires a surgeon or resident to customize the prosthetic by drawing the virtual nose, which may require a few hours of their time. The initial training for the animation program took approximately 4 hours to learn keystrokes and shortcuts for drawing the model. Although the first model created took around 2 to 3 hours to complete, the time required for a person experienced with the algorithm to create the final nasal model was approximately 1 hour. This estimate does not include the time to print the model using a 3-D printer, which can take up to 2 hours. Nonetheless, the importance and opportunity cost of the physician’s time required to create these prostheses should not be ignored.
This method may be less convenient than programs such as 3-D Slicer (https://www.slicer.org), which relies on importing readily available radiographic images to create the 3-D model.19,20 When using Blender, we experimented with different tools to create the final product and developed specific techniques to optimize the efficiency of the process and the accuracy of the prosthetic produced. For example, printing the models with the nasal tip facing upward created lower-quality models with some material collecting at the nasal tip, resulting in a bothersome printing artifact in the final product. The solution that gave the best-quality models was to draw a very small upper lip extension from the columella before printing and then printing the model with an upright orientation. The lip extension was later removed and smoothed with a metal file after printing. This method avoided artifacts of the printing material from interfering with the final model design.
Although the results of this pilot study were promising, the applicability of this technique to clinical practice is still undetermined. The algorithm described gives the reconstructive surgeon, who may have limited experience in prosthetic construction, the ability to create a temporary nasal prosthesis that is similar to a patient’s preoperative nasal appearance. The creation of this prosthesis obviates the need for referral to a clinician specifically trained in prosthetic construction. A potential subsequent study could be designed to compare a prosthesis created by this algorithm with a prosthesis created by an anaplastologist, which can be used as control data. However, the cost of models created by a trained professional remains prohibitive at this time. The low cost and high efficiency of this algorithm increase the potential applicability to clinical practice. The prostheses produced by this method would likely be used in the interim between resection and final reconstruction and not as a permanent prosthesis, which would allow time for appropriate confirmation of margin clearance and further analysis of the resultant defect.
Conclusions
This novel algorithm has the ability to produce anatomically accurate, temporary nasal prostheses for patients undergoing total rhinectomy. This low-cost solution obviates the need for referral to an anaplastologist or prosthodontist for creation of a temporary nasal prosthesis while further reconstructive efforts may be planned. More investigations are necessary to definitively determine the applicability of this technique to different nasal subtypes and the ideal printing material to do so.
References
- 1.Fischer H, Gubisch W. Nasal reconstruction: a challenge for plastic surgery. Dtsch Arztebl Int. 2008;105(43):741-746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Park SS. Reconstruction of nasal defects larger than 1.5 centimeters in diameter. Laryngoscope. 2000;110(8):1241-1250. [DOI] [PubMed] [Google Scholar]
- 3.American Academy of Maxillofacial Prosthetics. Facial prostheses. https://www.maxillofacialprosthetics.org/patients/patiented_fp.html#userconsent#. Accessed January 10, 2017.
- 4.Radenkovic D, Solouk A, Seifalian A. Personalized development of human organs using 3D printing technology. Med Hypotheses. 2016;87:30-33. [DOI] [PubMed] [Google Scholar]
- 5.Schmauss D, Haeberle S, Hagl C, Sodian R. Three-dimensional printing in cardiac surgery and interventional cardiology: a single-centre experience. Eur J Cardiothorac Surg. 2015;47(6):1044-1052. [DOI] [PubMed] [Google Scholar]
- 6.He Y, Xue GH, Fu JZ. Fabrication of low cost soft tissue prostheses with the desktop 3D printer. Sci Rep. 2014;4:6973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Adobe. Creative cloud pricing and membership plans. https://www.adobe.com/creativecloud/plans.html. Accessed January 20, 2017.
- 8.Oliveira-Santos T, Baumberger C, Constantinescu M, et al. 3D face reconstruction from 2D pictures: first results of a web-based computer aided system for aesthetic procedures. Ann Biomed Eng. 2013;41(5):952-966. [DOI] [PubMed] [Google Scholar]
- 9.Salazar-Gamarra R, Seelaus R, da Silva JV, da Silva AM, Dib LL. Monoscopic photogrammetry to obtain 3D models by a mobile device: a method for making facial prostheses. J Otolaryngol Head Neck Surg. 2016;45(1):33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chae MP, Lin F, Spychal RT, Hunter-Smith DJ, Rozen WM. 3D-printed haptic “reverse” models for preoperative planning in soft tissue reconstruction: a case report. Microsurgery. 2015;35(2):148-153. [DOI] [PubMed] [Google Scholar]
- 11.Markstedt K, Mantas A, Tournier I, Martínez Ávila H, Hägg D, Gatenholm P. 3D bioprinting human chondrocytes with nanocellulose–alginate bioink for cartilage tissue engineering applications. Biomacromolecules. 2015;16(5):1489-1496. [DOI] [PubMed] [Google Scholar]
- 12.Koch L, Deiwick A, Schlie S, et al. Skin tissue generation by laser cell printing. Biotechnol Bioeng. 2012;109(7):1855-1863. [DOI] [PubMed] [Google Scholar]
- 13.Tan ETW, Ling JM, Dinesh SK. The feasibility of producing patient-specific acrylic cranioplasty implants with a low-cost 3D printer. J Neurosurg. 2016;124(5):1531-1537. [DOI] [PubMed] [Google Scholar]
- 14.Lasprilla AJR, Martinez GAR, Lunelli BH, Jardini AL, Filho RM. Poly-lactic acid synthesis for application in biomedical devices—a review. Biotechnol Adv. 2012;30(1):321-328. [DOI] [PubMed] [Google Scholar]
- 15.Agarwal S, Gupta A, Grevious M, Reid RR. Use of resorbable implants for mandibular fixation: a systematic review. J Craniofac Surg. 2009;20(2):331-339. [DOI] [PubMed] [Google Scholar]
- 16.Bartus C, William Hanke C, Daro-Kaftan E. A decade of experience with injectable poly-l-lactic acid: a focus on safety. Dermatol Surg. 2013;39(5):698-705. [DOI] [PubMed] [Google Scholar]
- 17.Vleggaar D, Fitzgerald R, Lorenc ZP, et al. Consensus recommendations on the use of injectable poly-l-lactic acid for facial and nonfacial volumization. J Drugs Dermatol. 2014;13(4)(suppl):s44-s51. [PubMed] [Google Scholar]
- 18.Creative Mechanisms. Everything you need to know about polylactic acid (PLA). https://www.creativemechanisms.com/blog/learn-about-polylactic-acid-pla-prototypes. Accessed January 16, 2018.
- 19.3D Slicer. Slicer 4.8 released. https://www.slicer.org. Accessed August 23, 2016.
- 20.Fedorov A, Beichel R, Kalpathy-Cramer J, et al. 3D Slicer as an image computing platform for the Quantitative Imaging Network. Magn Reson Imaging. 2012;30(9):1323-1341. [DOI] [PMC free article] [PubMed] [Google Scholar]