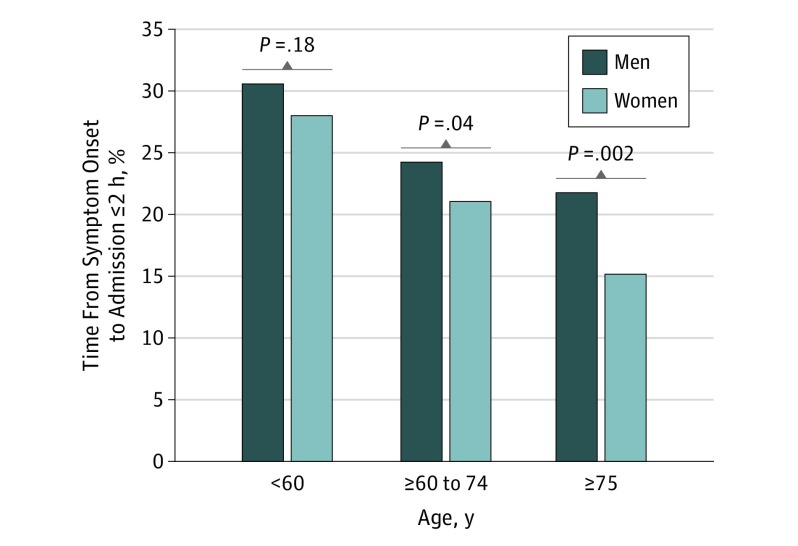Key Points
Question
Can sex differences in outcomes in young patients after STEMI be explained only by sex disparities in the quality and timing of care?
Findings
This observaional study found that a disproportionate burden of coronary risk factors and comorbidities is a clear feature of younger women with STEMI, but does not account for age-dependent difference in mortality. Younger age was associated with higher 30-day mortality rates in women even after adjustment for medications, primary PCI, and other coexisting comorbidities, but this difference declined after age 60 and was no longer observed in oldest women.
Meaning
Sex-related pathophysiological differences may contribute to the higher mortality rates in younger women compared with men of the same age category.
This observational study examines sex differences in outcomes in patients after ST-segment elevation myocardial infarction.
Abstract
Importance
Previous works have shown that women hospitalized with ST-segment elevation myocardial infarction (STEMI) have higher short-term mortality rates than men. However, it is unclear if these differences persist among patients undergoing contemporary primary percutaneous coronary intervention (PCI).
Objective
To investigate whether the risk of 30-day mortality after STEMI is higher in women than men and, if so, to assess the role of age, medications, and primary PCI in this excess of risk.
Design, Setting, and Participants
From January 2010 to January 2016, a total of 8834 patients were hospitalized and received medical treatment for STEMI in 41 hospitals referring data to the International Survey of Acute Coronary Syndromes in Transitional Countries (ISACS-TC) registry (NCT01218776).
Exposures
Demographics, baseline characteristics, clinical profile, and pharmacological treatment within 24 hours and primary PCI.
Main Outcomes and Measures
Adjusted 30-day mortality rates estimated using inverse probability of treatment weighted (IPTW) logistic regression models.
Results
There were 2657 women with a mean (SD) age of 66.1 (11.6) years and 6177 men with a mean (SD) age of 59.9 (11.7) years included in the study. Thirty-day mortality was significantly higher for women than for men (11.6% vs 6.0%, P < .001). The gap in sex-specific mortality narrowed if restricting the analysis to men and women undergoing primary PCI (7.1% vs 3.3%, P < .001). After multivariable adjustment for comorbidities and treatment covariates, women under 60 had higher early mortality risk than men of the same age category (OR, 1.88; 95% CI, 1.04-3.26; P = .02). The risk in the subgroups aged 60 to 74 years and over 75 years was not significantly different between sexes (OR, 1.28; 95% CI, 0.88-1.88; P = .19 and OR, 1.17; 95% CI, 0.80-1.73; P = .40; respectively). After IPTW adjustment for baseline clinical covariates, the relationship among sex, age category, and 30-day mortality was similar (OR, 1.56 [95% CI, 1.05-2.3]; OR, 1.49 [95% CI, 1.15-1.92]; and OR, 1.21 [95% CI, 0.93-1.57]; respectively).
Conclusions and Relevance
Younger age was associated with higher 30-day mortality rates in women with STEMI even after adjustment for medications, primary PCI, and other coexisting comorbidities. This difference declines after age 60 and is no longer observed in oldest women.
Introduction
Previous studies have demonstrated a significant relationship between sex and age after acute myocardial infarction.1,2 Younger women were found to have a higher mortality rate than younger men, but among the older population, the mortality rate for women and men was similar. However, most of these studies that risk stratified on age included patients with and without ST-segment elevation myocardial infarction (STEMI), representing a rather heterogenous population of patients with acute coronary syndrome.3,4,5
Less is known about sex differences in mortality in patients with STEMI. Few studies have addressed this topic.6,7,8,9 None of these studies have adjusted outcomes for treatment. Medications and primary percutaneous coronary interventions (PCI) may produce substantial variability in risk estimates. Further, treatment selection and referral to catheterization are often influenced by delays to hospital presentation, which contribute to variations in clinical decision making,10,11,12 and women often experience longer delays than their male counterparts. As such, it remains unclear whether sex differences in mortality persist in STEMI and which factors may contribute to this gap in mortality, if any.
The main purpose of this study was to investigate whether the risk of 30-day mortality after STEMI is higher in women than men and, if so, to assess the role of age, medications, and primary PCI in this excess of risk.
Methods
Setting and Design
The International Survey of Acute Coronary Syndromes in Transitional Countries (ISACS-TC; NCT01218776) is a large observational and multinational registry.12,13,14,15,16,17 Data were collected from 41 centers in 12 countries: Bosnia and Herzegovina, Croatia, Italy, Kosovo, Lithuania, Macedonia, Hungary, Moldova, Montenegro, Romania, Russian Federation, and Serbia. Among these, there were 22 tertiary health care services providing advanced medical investigation and treatment including PCI and/or cardiac surgery, and 19 secondary health care services providing intensive care in critical coronary care units. The data coordinating center has been established at the University of Bologna. The study was approved by the local research ethics committee from each hospital. Because patient information was collected anonymously, institutional review boards waived the need for individual informed consent.
Patient Population
The study population consisted of 8834 eligible patients with STEMI enrolled between January 2010 and January 2016, which is consistent with estimates of patients enrolled per year and per center of similar registries5 according to policies and procedures described by the Registry of Patient Registries (RoPR).18 Patients included in the analysis were categorized into 3 groups: young patients, younger than 60 years; young old patients, aged 60 to 74 years; and old patients, aged 75 years or older according to recent definitions.19 Multivessel disease was defined as at least 2 main branches of the epicardial coronary artery with 70% or more stenotic lesions or 50% or greater stenosis in the left main coronary artery. The PCI was rated successful if there was a residual stenosis of less than 50% after balloon angioplasty without stenting and less than 20% after PCI with stent application plus normal thrombolysis in myocardial infarction (TIMI) 3 flow.
Outcome Measures
The primary end point was 30-day all-cause mortality. Secondary outcomes were: (1) stroke; and (2) major and minor bleeding at 30 days. We used the definition of bleeding previously reported by the TIMI.20 Decreases in hemoglobin levels of 5 g/dL or more occurring during hospitalization or intracranial hemorrhage classified bleeding as major (to convert hemoglobin to d/L, multiply by 10). The use of medications given at hospital admission was noted, as well as the use of primary PCI.
Statistical Analysis
We compared the baseline characteristics, treatment, and clinical outcomes between women and men. Baseline characteristics were reported percentages for categorical variables and means with standard deviation (SD) for continuous variables. Comparisons between groups were made either by Pearson χ2 test for baseline categorical variables and student t test for continuous variables as appropriate (Table 1). We used inverse probability of treatment weighted (IPTW) logistic-regression models to assess the effect of variables on the associations of interest (eMethods in the Supplement). Logistic regression models were adjusted to covariates significantly different between groups in univariate analysis. We calculated odds ratios (OR) with their 95% CIs from these models. Fixed covariates included demographic information and baseline clinical characteristics (Table 2). Covariates introduced in the secondary analyses as categorical variables were medications given within 24 hours, specifically aspirin, clopidogrel, unfractionated heparin (UFH), and primary PCI. Further analyses were done to compare sex differences in outcomes between countries with higher vs lower public health care expenditures (PHE) values. The rationale to assess country PHE characteristics was the potential for having different sex differences in outcomes depending on the quality of health care services.21 Bosnia and Herzegovina, Croatia, Hungary, Italy, and Serbia have high PHE values, whereas Macedonia, Romania, Lithuania, Russian Federation, Kosovo, Moldova, and Montenegro, have low values. We had complete data on age, sex, and 30-day mortality. Some patients had missing data on other variables. We imputed the missing values of the clinical variables that had a missing rate of less than 10% using IVEWARE software.22 For the clinical features with a missing rate that exceeded 10%, we carried out a Pearson χ2 statistical test for independence between those features and mortality. Only 1 variable, Killip class, had missing rates that exceeded 10%, and was found to be statistically dependent on the endpoint mortality. We therefore did not dismiss this variable from the predictive model of mortality and we kept it as missing using listwise deletion and complete case analysis.23,24 Owing to the considerable amount of missing data for Killip class, we also used k-nearest neighbor (KNN) algorithms as imputation method to treat missing data.25,26,27 Furthermore, to assess significant heterogeneity of outcomes in function of age, we made statistical comparisons across 3 age subgroups (<60 years, 60 to 74 years, and ≥75 years) using a test of interaction for comparison of the estimated quantities, each with its standard error (eMethods in the Supplement).28,29
Table 1. Baseline Characteristics Sorted by Sex.
| Characteristic | Overall Study Population (n = 8834) | Primary PCI Population (n = 5590) | ||||
|---|---|---|---|---|---|---|
| Men (n = 6177) | Women (n = 2657) | P Value | Men (n = 4061) | Women (n = 1529) | P Value | |
| Age, mean (SD), y | 59.9 (11.7) | 66.1 (11.6) | <.001 | 58.8 (11.1) | 64.3 (11.1) | <.001 |
| Diabetes | 21.6 | 30.7 | <.001 | 19.6 | 28.1 | <.001 |
| Hypertension | 60.7 | 73.7 | <.001 | 61.8 | 74.1 | <.001 |
| History of hypercholesterolemia | 39.1 | 40.3 | .34 | 41.2 | 43.5 | .14 |
| Current smoking | 46.3 | 29.5 | <.001 | 49.2 | 35.4 | <.001 |
| Prior angina pectoris | 13.4 | 17.0 | <.001 | 13.0 | 17.3 | <.001 |
| Prior MI | 13.1 | 11.0 | .006 | 11.8 | 9.2 | .005 |
| Prior PCI | 12.0 | 11.0 | .17 | 13.5 | 13.2 | .80 |
| Prior CABG | 1.5 | 0.9 | .05 | 0.8 | 0.4 | .10 |
| Prior heart failure | 3.6 | 4.7 | .01 | 3.0 | 3.5 | .32 |
| Prior stroke | 3.8 | 5.4 | .001 | 2.9 | 4.2 | .01 |
| Prior PAD | 2.2 | 2.3 | .85 | 1.3 | 1.0 | .32 |
| Clinical presentation | ||||||
| Atypical chest pain/presentation | 5.2 | 7.1 | .001 | 2.4 | 1.9 | .36 |
| HR at admission, mean (SD), bpm | 80.1 (16.3) | 80.9 (17.3) | .03 | 79.8 (15.0) | 79.9 (15.1) | .87 |
| SBP at admission, mean (SD), mm Hg | 139.4 (23.3) | 139.1 (24.8) | .61 | 141.1 (22.4) | 140.8 (23.1) | .63 |
| Serum creatinine levels at admission >2.5 mg/dL | 1.5 | 1.7 | .33 | 0.9 | 1.1 | .38 |
| Killip Class ≥2 | 23.8 | 30.9 | <.001 | 17.4 | 22.6 | .001 |
| Time from symptoms onset to admission ≤2 h | 27.1 | 21.5 | <.001 | 32.0 | 26.1 | <.001 |
| Time from symptoms onset to admission ≤12 h | 80.9 | 76.4 | <.001 | NA | NA | NA |
| Patient treatment and medications within 24 h | ||||||
| Fibrinolysis | 12.8 | 10.9 | .01 | NA | NA | NA |
| Primary PCI | 65.8 | 57.6 | <.001 | NA | NA | NA |
| Aspirin | 97.8 | 96.0 | <.001 | 99.6 | 99.3 | .31 |
| Clopidogrel | 91.6 | 88.4 | <.001 | 97.7 | 98.6 | .04 |
| UFH | 57.7 | 52.0 | <.001 | 65.5 | 61.3 | <.001 |
| Enoxaparin | 30.5 | 30.5 | .98 | 27.1 | 26.7 | .77 |
| Outcomes | ||||||
| 30-d mortality | 6.0 | 11.6 | <.001 | 3.3 | 7.1 | <.001 |
Abbreviations: bpm, beats per minute; CABG, coronary artery bypass graft; HR, heart rate; MI, myocardial infarction; NA, not applicable; PAD, peripheral artery disease; PCI, percutaneous coronary intervention; SBP, systolic blood pressure; SD, standard deviation; UFH, unfractionated heparin.
SI conversion factor: To convert serum creatinine to μmol/L, multiply by 88.4.
Table 2. Multivariable Analysis of Factors Associated With 30-Day All-cause Mortality.
| Variable | Odds Ratio (95% CI) | P Value |
|---|---|---|
| Women | 1.32 (1.04-1.68) | .02 |
| Age, y | 1.04 (1.03-1.06) | <.001 |
| Diabetes mellitus | 1.31 (1.02-1.67) | .03 |
| Hypertension | 0.66 (0.52-0.85) | .001 |
| Current smoking | 0.73 (0.54-0.98) | .04 |
| Prior angina pectoris | 0.59 (0.42-0.83) | .003 |
| Prior MI | 1.19 (0.86-1.64) | .28 |
| Prior PCI | 1.40 (1.01-2.01) | .04 |
| Prior CABG | 1.05 (0.46-2.39) | .89 |
| Prior stroke | 1.86 (1.27-2.71) | .001 |
| Prior HF | 0.73 (0.47-1.15) | .18 |
| Killip Class ≥2 | 6.34 (4.96-8.10) | <.001 |
| HR at admission (SD increment)a | 1.11 (1.01-1.22) | .02 |
| SBP at admission (SD increment)a | 0.71 (0.65-0.78) | <.001 |
| Time to admission ≤2 h | 0.86 (0.64-1.16) | .34 |
Abbreviations: CABG, coronary artery bypass graft; HF, heart failure; HR, heart rate; MI, myocardial infarction; PCI, percutaneous coronary intervention; SBP, systolic blood pressure; SD, standard deviation.
Standard deviations for heart rate and systolic blood pressure in the overall population were 16.6 bpm and 23.8 mm Hg.
Results
From January 2010 through January 2016, a total of 8834 STEMI patients at 41 sites in 12 countries were enrolled in the ISACS-TC registry. Of these patients, 682 died at 30-day follow-up. eFigure 1 in the Supplement shows the flow diagram of patients entered into the study.
Patient Characteristics
Baseline characteristics of the patients sorted by sex and use of primary PCI are shown in Table 1. When stratifying according to age, sex differences in adjunctive medical therapies were greater in the older than in the younger cohort (eFigure 2 in the Supplement). eTable 1 in the Supplement lists the angiographic and procedural characteristics of patients undergoing PCI. There was no sex difference in frequency of multivessel disease, acute vessel closure, acute thrombosis, and bifurcation lesions. When stratifying according to age, sex differences in multivessel disease were significantly greater in young women compared with their older counterparts (eFigure 3 in the Supplement).
Time From Symptoms Onset to Admission
Fewer women than men presented within 2 hours from symptom onset (P < .001) (Table 1) (Figure 1). Importantly, the proportion of women presenting within 2 hours after symptom onset was greater in the younger than in older cohorts (28.0% for those aged <60 years vs 21.0% and 15.2% for those aged 60 to 74 years, and ≥75 years, respectively).
Figure 1. Distributions of Time From Symptoms Onset to Admission of ≤2 Hours.
P value for comparison between women and men in the same age category.
Outcomes
Women had a significantly higher unadjusted 30-day mortality rate compared with men both in the overall (11.6% vs 6.0%, P < .001) and primary PCI (7.1% vs 3.3%, P < .001) STEMI population. Major bleeding occurred more frequently in women than in men (1.9% vs 1.1%). There were similar stroke rates between men and women (eFigure 4 in the Supplement). After multivariable adjustment for clinical characteristics (Table 2), and angiographic disease severity (eTable 2 in the Supplement), female sex was associated with 30-day all-cause mortality. The adjusted OR for mortality associated with female sex did not change when controlling for primary PCI and medications used at admission (eFigure 5 in the Supplement). There was no significant association between centers of countries with low vs high PHE value and sex differences in 30-day mortality rates (eFigure 6 in the Supplement).
Sex-Age Interactions
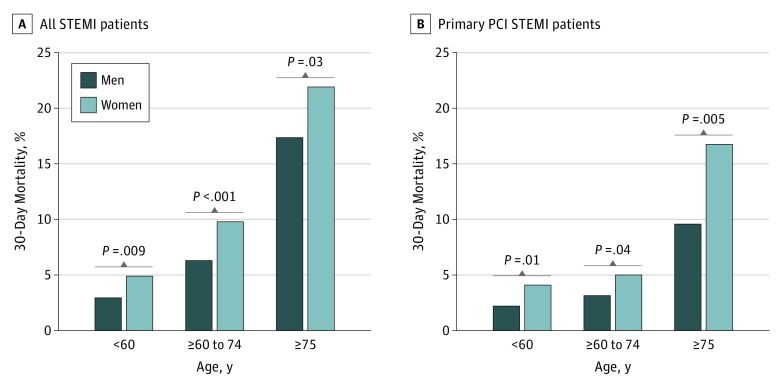
The role of a sex-age interaction was investigated by stratifying patients into 3 groups: age younger than 60 years, age between 60 to 74 years, and age 75 years or older. All-cause mortality increased with age (Figure 2). After multivariable adjustment, there were 5 factors positively associated with 30-day all-cause mortality in patients younger than 60 years: female sex, history of diabetes, prior PCI, heart rate, and Killip class of 2 or higher (Table 3). Women younger than 60 years had higher early mortality risk than men of the same group (OR, 1.88; 95% CI, 1.08-3.26; P = .02). Among older patients, there were fewer differences in comorbid conditions associated with outcomes. Differences in 30-day mortality between women vs men were not significant for those aged 60 to 74 years (OR, 1.28; 95% CI, 0.88-1.88; P = .19) and those aged 75 years or older (OR, 1.17; 95% CI, 0.80-1.73; P = .40). There were no significant interactions between age categories and sex on risk of outcomes (eTables 3, 4, and 5 in the Supplement). We also analyzed data using KNN algorithms. Women younger than 60 years had a remarkably higher early mortality risk than men of the same age category (OR, 1.52). The risk was lower (OR, 1.41) in the subgroup aged 60 to 74 years, and much lower in the subgroup aged 75 years or older (OR, 1.18) (eFigure 7 in the Supplement).
Figure 2. Unadjusted 30-Day Mortality Rates in Age Categories Sorted by Sex.
Abbreviations: PCI, percutaneous coronary interventions; STEMI, ST-segment elevation myocardial infarction.
Table 3. Multivariable Analysis of Factors Associated With 30-Day All-Cause Mortality in the Selected Age Categories.
| Variable | Age <60 Years (n = 3806) | Age ≥60 to 74 Years (n = 3556) | Age ≥75 Years (n = 1472) | |||
|---|---|---|---|---|---|---|
| OR (95% CI) | P Value | OR (95% CI) | P Value | OR (95% CI) | P Value | |
| Women | 1.88 (1.08-3.26) | .02 | 1.28 (0.88-1.88) | .19 | 1.17 (0.80-1.73) | .40 |
| Age, y | 1.04 (0.99-1.08) | .05 | 1.08 (1.04-1.13) | <.001 | 1.06 (1.02-1.11) | .002 |
| Diabetes mellitus | 2.04 (1.18-3.54) | .01 | 1.18 (0.80-1.75) | .39 | 1.24 (0.83-1.87) | .28 |
| Hypertension | 0.66 (0.39-1.13) | .13 | 0.60 (0.40-0.90) | .02 | 0.76 (0.50-1.14) | .20 |
| Current smoking | 0.52 (0.31-0.89) | .02 | 1.01 (0.66-1.55) | .93 | 0.66 (0.33-1.32) | .25 |
| Prior angina pectoris | 0.49 (0.21-1.13) | .10 | 0.62 (0.35-1.08) | .10 | 0.65 (0.39-1.08) | .10 |
| Prior MI | 0.81 (0.38-1.72) | .59 | 1.90 (1.19-3.03) | .007 | 0.76 (0.41-1.38) | .37 |
| Prior PCI | 2.97 (1.51-5.83) | .002 | 1.08 (0.62-1.86) | .77 | 1.26 (0.67-2.40) | .46 |
| Prior CABG | 0.74 (0.07-7.47) | .80 | 1.28 (0.44-3.71) | .64 | 1.02 (0.20-5.23) | .98 |
| Prior stroke | 1.04 (0.27-3.87) | .95 | 1.72 (0.96-3.08) | .07 | 2.26 (1.28-3.98) | .004 |
| Prior HF | 1.10 (0.41-2.95) | .84 | 0.45 (0.19-1.06) | .07 | 0.77 (0.40-1.48) | .45 |
| Killip class ≥2 | 11.25 (6.54-19.35) | <.001 | 7.72 (5.21-11.44) | <.001 | 3.46 (2.33-5.15) | <.001 |
| HR at admission (SD increment)a | 1.43 (1.17-1.75) | .001 | 1.08 (0.93-1.25) | .26 | 1.01 (0.87-1.18) | .82 |
| SBP at admission (SD increment)a | 0.76 (0.61-0.94) | .01 | 0.70 (0.61-0.80) | <.001 | 0.71 (0.60-0.83) | <.001 |
| Time to admission ≤2 h | 1.15 (0.66-2.00) | .61 | 0.94 (0.59-1.50) | .82 | 0.57 (0.31-1.02) | .06 |
Abbreviations: CABG, coronary artery bypass graft; HF, heart failure; HR, heart rate; MI, myocardial infarction; PCI, percutaneous coronary intervention; SBP, systolic blood pressure; SD, standard deviation.
Standard deviations for heart rate and systolic blood pressure in the overall population were 16.6 bpm and 23.8 mm Hg.
Inverse Probability of Treatment Weighting
Baseline characteristics were well matched between women and men. In the sample, the largest absolute standardized difference was “prior PCI” (10.9% vs 11.4%; P = .64) (eTables 6, 7, and 8 in the Supplement). The estimated odds ratios for death of women vs men were 1.56 (95% CI, 1.05-2.32) for patients younger than 60 years, 1.49 (95% CI, 1.15-1.92) for those aged 60 to 74 years, and 1.21. (95% CI, 0.93-1.57) for those aged 75 years or older.
Discussion
The main finding of the current study is that younger age (<60 years) in women with STEMI was independently associated with higher 30-day mortality compared with men after adjustment for demographics, comorbidities, medications, and severity of coronary disease. The association persisted even in patients treated with primary PCI. Sex difference in mortality declined after age 60 years and could not be observed in oldest patients.
Prior Work
Specific data on STEMI were reported by the Get With the Guidelines–Coronary Artery Disease from 25 353 patients in 420 US hospitals from 2001 to 2006.30 In the STEMI cohort, there was a significant difference in mortality, 10.2% in women vs 5.5% in men. Higher rates of early deaths among women compared with men were seen only on the first 24 hours of hospitalization. Reasonably, the authors were unable to effectively control for some hidden variables that may be responsible for the relationship between sex and outcome. Indeed, women are less likely to receive aspirin, β-blockers, and reperfusion therapies at admission. Thus, the extent to which mortality rate differences between men and women are related to disparities in treatment or dissimilarities in pathophysiology remain a matter of debate. Previous studies in STEMI also have found that when the angiographic severity of coronary artery disease was included in the 30-day mortality models containing baseline characteristics, there were no significant associations between sex and mortality nor significant interaction between sex and age on mortality risk.9 Further, a recent meta-analysis on sex differences in all-cause mortality among patients treated with primary PCI concluded that there was an increased mortality in women, but this difference was likely to be confounded by baseline cardiovascular risk factors and differences in clinical profile.31 In summary, most studies have supported the claim that women with STEMI are at greater risk for mortality than men. There is controversy regarding whether this difference persists after adjusting for baseline characteristics and treatment, and about whether sex differences in outcomes are age dependent. Subgrouping in young and old women is not so straightforward and may be subjective. Results of prior work should, therefore, stimulate further research.
Unexplained Higher Mortality Rates in Women
Besides differences in baseline cardiovascular risk and differences in clinical profile, other contributing factors may explain the observed sex differences in outcome after STEMI. These factors may include, but are not limited to, race, delay to hospital presentation, and delayed diagnosis among women compared with men. Few studies have investigated the associations between the overall treatment delay and mortality.32,33 Even fewer studies have evaluated the effect of treatment delays on sex differences in guideline-recommended time of PCI and early cardiac mortality.11,12 Further, care may influence results. When rates of coronary revascularization and evidence-based medications differ between men and women, the value of sex as a prognostic factor may be underestimated or overestimated by analyses that include patients with such markedly different treatment. Finally, the results of subgroup analyses by age can result in either consistent or insignificant findings depending on the definition of age used in the study.34 No subgroup analysis will attain practical usefulness when undertaken without any explanatory background, no matter how significant the results may be.35
Study Design and Patient Selection
In this study, the 3 subgroups based on age were predefined on a rational indication. The WHO’s Minimum Data Set Project on Aging and Health agreed on a cut-off of 60 years to refer to the older population.36 In developed countries, most people in their 60s and early 70s are still active and able to care for themselves. However, after 75 years, they will become increasingly frail.19 Therefore, rather than lumping together all people who have been defined as old, we acknowledged the diversity of old age by distinguishing the young old (≥60 to 74 years) from the old patients aged 75 years or older. More age boundaries and consequently more subgroup analyses would have generated small sample sizes and therefore rendered analyses underpowered, making the results unreliable. Further, to minimize the effect of inception time bias, we explored the association between duration from onset of symptoms to hospital presentation and clinical outcomes after STEMI in 2 patient categories according to the American College of Cardiology (ACC)/American Heart Association (AHA) Guidelines37: those with mild to moderate delay (2 hours or less after symptom onset) and those with severe delay (more than 2 hours). Lastly, we checked for comparability of prognostic factors in subgroup analyses and used IPTW for comparing outcomes across subgroups of women and men with balanced prognostic factors.
STEMI-related Death Within 30 Days: Association With Age and Sex
The study showed that women with STEMI died at a higher rate than men, with 309 (11.6%) of 2657 women in the study dying over 30-day follow-up compared with 373 (6%) of 6177 men. Mortality dramatically decreased in both sexes if restricting the analysis to women and men undergoing primary PCI (7.1% vs 3.3%). Yet there was still a sex difference with a 30% higher mortality in women even after controlling for clinical characteristics, primary PCI, and medications used at admission (OR, 1.29; 95% CI, 1.01-1.65). Although death rates for women were greater than those for men at all ages, this estimate is an average of the individual outcomes. The different twist to this study is that we evaluated quantitatively the age subgroup effects of STEMI and found that women aged under 60 years have an unacceptable higher (OR, 1.88; 95% CI, 1.04-3.26) early mortality risk than men of the same age category. Older women experienced less sex gap in mortality than the reported average.
Likelihood of Death Among Young Women vs Young Men
There could be several reasons for why younger women were more likely to die than young men. Some previous work speculated that men may be more likely than women to die before arriving to hospital, so outcomes among men could be attributed to only the less severe cases reaching the health care system.6 There are no data available in our or other studies for supporting or refuting this hypothesis. Another likely reason for the higher 30-day mortality in younger women with STEMI could be the lower use of revascularization and adjunctive medical therapies.30,38,39 However, in our study, the association between younger age and excess mortality in women persisted in patients treated with primary PCI. In addition, there was a significant inverse relationship between treatment and sex in the defined age categories. Sex differences in adjunctive medical therapies were greater in the older than in the younger cohort, which does not support the hypothesis that differences in early treatment may account for difference in risk of mortality. Other possible explanations for the poor prognosis among younger women include a lack of awareness of symptoms and, consequently, suboptimal total ischemic time to PCI.11,12 We found that women with STEMI were less often admitted to hospital within 2 hours from symptom onset than their male counterparts. However, longer delays to hospital presentation were mainly observed in the older women, which, in turn, is in keeping with previous observations on delay times and age.40 Thus, there is not any one of the above-mentioned factors able to explain the increase in mortality in the young women, but a number of issues are not covered by our covariates, including those describing the pathophysiology of ischemic heart disease in women.
Pathophysiological Issues
Other aspects that may account for differences in outcomes between women and men with STEMI are related to vascular biological factors such as a smaller vessel size, less collateral flow, and more vascular stiffness in women.41,42 In addition, younger women have less extensive coronary artery disease and, therefore, may have lesser myocardial ischemic preconditioning, resulting in a greater susceptibility to ischemia.12 Moreover, women and men with STEMI have different distributions of cardiovascular risk factors and, more importantly, risk factors may have a different impact on risk of mortality depending on age. We found that diabetes was a factor associated with increased risk of 30-day all-cause mortality in the young, but not the old population. Women in this age group are also more likely to have depression.43 The coexistence of both conditions, diabetes and depression, might identify high-risk in women,44 and may help explain why young women die at a disproportionately higher rate than men after STEMI.
Age-Sex Subgrouping
For continuous variables such as age there are many ways of creating groups. Exploratory examination of many such subgroups may generate some spurious significant interactions because imbalances regarding prognostic factors may be present after stratification owing to chance.45 Our approach avoided post hoc definitions or interpretations that retroactively fit the data. We explained why this age division was made. We checked the subgroups for comparability of prognostic factors after age stratification, especially for those factors that were found to correlate with 30-day mortality. Because diabetes was a predictor of adverse outcome only in the younger population, the subgroup analyses were adjusted with regression techniques. Further, we examined the association of sex with age subgroups by assessing estimates of the same populations derived from IPTW analyses in which the distribution of measured baseline covariates were similar between women and men. Yet, mortality difference by sex was still large (OR, 1.56) in younger patients (<60 years). Sex difference in mortality became lower (OR, 1.49) in the young old (≥60 to 74 years) patients, and even lower (OR, 1.21; P = .14) in the old (≥75 years) patients. There is, therefore, good evidence to support different outcomes in younger vs older women. A disproportionate burden of coronary risk factors and comorbidities is a clear feature of younger women with STEMI, but does not account for age-dependent difference in mortality.
Limitations
Our study does have some limitations. As an observational study, we cannot completely exclude residual confounding or selection bias, although we were able to adjust for a wide range of patient clinical and angiographic characteristics. Because catheterization data was mandated as part of the registry’ protocol only in centers with PCI facilities, patient selection may have introduced potential referral bias, and therefore should be interpreted with caution. Lastly, even though we found a complex sex-age association in a large database, results may not be definitive without replication.
Conclusions
The current study is the first investigation which demonstrates that differences between younger men and younger women in STEMI mortality rates are unrelated to disparities in treatment. This holds true even in patients undergoing primary PCI. Sex-related pathophysiological differences may contribute to the higher mortality in younger women compared with men of the same age category. These results reflect outcomes from 41 participating registry centers in 12 different countries and may be generalizable to other settings.
eMethods.
eResults.
eReferences.
References
- 1.Vaccarino V, Parsons L, Peterson ED, Rogers WJ, Kiefe CI, Canto J. Sex differences in mortality after acute myocardial infarction: changes from 1994 to 2006. Arch Intern Med. 2009;169(19):1767-1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vaccarino V, Parsons L, Every NR, Barron HV, Krumholz HM. Sex-based differences in early mortality after myocardial infarction. National Registry of Myocardial Infarction 2 Participants. N Engl J Med. 1999;341(4):217-225. [DOI] [PubMed] [Google Scholar]
- 3.Berger JS, Elliott L, Gallup D, et al. . Sex differences in mortality following acute coronary syndromes. JAMA. 2009;302(8):874-882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lichtman JH, Wang Y, Jones SB, et al. . Age and sex differences in inhospital complication rates and mortality after percutaneous coronary intervention procedures: evidence from the NCDR(®). Am Heart J. 2014;167(3):376-383. [DOI] [PubMed] [Google Scholar]
- 5.Smilowitz NR, Mahajan AM, Roe MT, et al. . Mortality of myocardial infarction by sex, age, and obstructive coronary artery disease status in the ACTION Registry-GWTG (Acute Coronary Treatment and Intervention Outcomes Network Registry-Get With the Guidelines). Circ Cardiovasc Qual Outcomes. 2017;10(12):e003443. [DOI] [PubMed] [Google Scholar]
- 6.Khera S, Kolte D, Gupta T, et al. . Temporal trends and sex differences in revascularization and outcomes of st-segment elevation myocardial infarction in younger adults in the United States. J Am Coll Cardiol. 2015;66(18):1961-1972. [DOI] [PubMed] [Google Scholar]
- 7.Lawesson SS, Alfredsson J, Fredrikson M, Swahn E. A gender perspective on short- and long term mortality in ST-elevation myocardial infarction—a report from the SWEDEHEART register. Int J Cardiol. 2013;168(2):1041-1047. [DOI] [PubMed] [Google Scholar]
- 8.Heer T, Hochadel M, Schmidt K, et al. . Sex differences in percutaneous coronary intervention-insights from the Coronary Angiography and PCI Registry of the German Society of Cardiology. J Am Heart Assoc. 2017;6(3):e004972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Berger JS, Brown DL. Gender-age interaction in early mortality following primary angioplasty for acute myocardial infarction. Am J Cardiol. 2006;98(9):1140-1143. [DOI] [PubMed] [Google Scholar]
- 10.Bugiardini R, Yan AT, Yan RT, et al. ; Canadian Acute Coronary Syndrome Registry I and II Investigators . Factors influencing underutilization of evidence-based therapies in women. Eur Heart J. 2011;32(11):1337-1344. [DOI] [PubMed] [Google Scholar]
- 11.D’Onofrio G, Safdar B, Lichtman JH, et al. . Sex differences in reperfusion in young patients with ST-segment-elevation myocardial infarction: results from the VIRGO study. Circulation. 2015;131(15):1324-1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bugiardini R, Ricci B, Cenko E, et al. . Delayed care and mortality among women and men with myocardial infarction. J Am Heart Assoc. 2017;6(8):e005968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bugiardini R, Badimon L; ISACS-TC Investigators and Coordinators . The international survey of acute coronary syndromes in transitional countries (ISACS-TC): 2010-2015. Int J Cardiol. 2016;217(suppl):S1-S6. [DOI] [PubMed] [Google Scholar]
- 14.Cenko E, Ricci B, Kedev S, et al. . The no-reflow phenomenon in the young and in the elderly. Int J Cardiol. 2016;222:1122-1128. [DOI] [PubMed] [Google Scholar]
- 15.Bugiardini R, Cenko E, Ricci B, et al. . Comparison of early versus delayed oral β blockers in acute coronary syndromes and effect on outcomes. Am J Cardiol. 2016;117(5):760-767. [DOI] [PubMed] [Google Scholar]
- 16.Bugiardini R, Dorobantu M, Vasiljevic Z, et al. ; ISACS-TC Investigators . Unfractionated heparin-clopidogrel combination in ST-elevation myocardial infarction not receiving reperfusion therapy. Atherosclerosis. 2015;241(1):151-156. [DOI] [PubMed] [Google Scholar]
- 17.Cenko E, Ricci B, Kedev S, et al. . Reperfusion therapy for ST-elevation acute myocardial infarction in Eastern Europe: the ISACS-TC registry. Eur Heart J Qual Care Clin Outcomes. 2016;2(1):45-51. [DOI] [PubMed] [Google Scholar]
- 18.Gliklich RE, Leavy MB, Levy D, Karl J, Campion DM, Taylor T. Registry of Patient Registries (RoPR) Policies and Procedures. Effective Health Care Program Research Report No. 41. (Prepared by Outcome DEcIDE Center under Contract No. HHSA 290-2005-0035-1.) AHRQ Publication No. 12-EHC066-EF. Rockville, MD: Agency for Healthcare Research and Quality; 2012https://effectivehealthcare.ahrq.gov/topics/registry-of-patient-registries/research-2012-2. Accessed January 11, 2018. [Google Scholar]
- 19.Clegg A, Young J, Iliffe S, Rikkert MO, Rockwood K. Frailty in elderly people. Lancet. 2013;381(9868):752-762. doi: 10.1016/S0140-6736(12)62167-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wiviott SD, Braunwald E, McCabe CH, et al. ; TRITON-TIMI 38 Investigators . Prasugrel versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2007;357(20):2001-2015. doi: 10.1056/NEJMoa0706482 [DOI] [PubMed] [Google Scholar]
- 21.Savedoff W. How Much Should Countries Spend on Health? World Health Organization, Department “health system financing, expenditure and resource allocation” (FER), cluster “evidence and information for policy” (EIP). 2003. http://www.who.int/health_financing/en/how_much_should_dp_03_2.pdf. Accessed January 11, 2018.
- 22.Raghunathan TE, Solenberger PW, Van Hoeyk J IVEware: Imputation and Variance Estimation Software. Michigan: Survey Research Center, Institute for Social Research University of Michigan. 2002. ftp://ftp.isr.umich.edu/pub/src/smp/ive/ive_user.pdf. Accessed August 04, 2017.
- 23.Liu Y, Gopalakrishnan V. An Overview and evaluation of recent machine learning imputation methods using cardiac imaging data. Data (Basel). 2017;2(1):8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Newgard CD, Lewis RJ. Missing data: how to best account for what is not known. JAMA. 2015;314(9):940-941. [DOI] [PubMed] [Google Scholar]
- 25.Troyanskaya O, Cantor M, Sherlock G, et al. . Missing value estimation methods for DNA microarrays. Bioinformatics. 2001;17(6):520-525. [DOI] [PubMed] [Google Scholar]
- 26.Beretta L, Santaniello A. Nearest neighbor imputation algorithms: a critical evaluation. BMC Med Inform Decis Mak. 2016;16(suppl 3):74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kononenko I, Simec E, Robnik-Sikonja M. Overcoming the myopia of inductive learning algorithms with RELIEFF. Appl Intell. 1997;7:39-55. [Google Scholar]
- 28.Kraemer HC, Frank E, Kupfer DJ. Moderators of treatment outcomes: clinical, research, and policy importance. JAMA. 2006;296(10):1286-1289. [DOI] [PubMed] [Google Scholar]
- 29.Altman DG, Bland JM. Interaction revisited: the difference between two estimates. BMJ. 2003;326(7382):219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jneid H, Fonarow GC, Cannon CP, et al. ; Get With the Guidelines Steering Committee and Investigators . Sex differences in medical care and early death after acute myocardial infarction. Circulation. 2008;118(25):2803-2810. [DOI] [PubMed] [Google Scholar]
- 31.Pancholy SB, Shantha GP, Patel T, Cheskin LJ. Sex differences in short-term and long-term all-cause mortality among patients with ST-segment elevation myocardial infarction treated by primary percutaneous intervention: a meta-analysis. JAMA Intern Med. 2014;174(11):1822-1830. [DOI] [PubMed] [Google Scholar]
- 32.McNamara RL, Herrin J, Wang Y, et al. . Impact of delay in door-to-needle time on mortality in patients with ST-segment elevation myocardial infarction. Am J Cardiol. 2007;100(8):1227-1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Terkelsen CJ, Sørensen JT, Maeng M, et al. . System delay and mortality among patients with STEMI treated with primary percutaneous coronary intervention. JAMA. 2010;304(7):763-771. [DOI] [PubMed] [Google Scholar]
- 34.Yusuf S, Wittes J, Probstfield J, Tyroler HA. Analysis and interpretation of treatment effects in subgroups of patients in randomized clinical trials. JAMA. 1991;266(1):93-98. [PubMed] [Google Scholar]
- 35.Oxman AD, Guyatt GH. A consumer’s guide to subgroup analyses. Ann Intern Med. 1992;116(1):78-84. [DOI] [PubMed] [Google Scholar]
- 36.World Health Organization (WHO), Minimum Data Set (MDS) http://www.who.int/healthinfo/survey/ageingdefnolder/en/. Accessed August 28, 2017.
- 37.O’Gara PT, Kushner FG, Ascheim DD, et al. ; American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines . 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2013;127(4):e362-e425. [DOI] [PubMed] [Google Scholar]
- 38.Vaccarino V, Badimon L, Corti R, et al. . Presentation, management, and outcomes of ischaemic heart disease in women. Nat Rev Cardiol. 2013;10(9):508-518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vaccarino V, Rathore SS, Wenger NK, et al. ; National Registry of Myocardial Infarction Investigators . Sex and racial differences in the management of acute myocardial infarction, 1994 through 2002. N Engl J Med. 2005;353(7):671-682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ting HH, Bradley EH, Wang Y, et al. . Factors associated with longer time from symptom onset to hospital presentation for patients with ST-elevation myocardial infarction. Arch Intern Med. 2008;168(9):959-968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shaw LJ, Bugiardini R, Merz CN. Women and ischemic heart disease: evolving knowledge. J Am Coll Cardiol. 2009;54(17):1561-1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mehta LS, Beckie TM, DeVon HA, et al. ; American Heart Association Cardiovascular Disease in Women and Special Populations Committee of the Council on Clinical Cardiology, Council on Epidemiology and Prevention, Council on Cardiovascular and Stroke Nursing, and Council on Quality of Care and Outcomes Research . Acute myocardial infarction in women: a scientific statement from the American Heart Association. Circulation. 2016;133(9):916-947. [DOI] [PubMed] [Google Scholar]
- 43.Shah AJ, Ghasemzadeh N, Zaragoza-Macias E, et al. . Sex and age differences in the association of depression with obstructive coronary artery disease and adverse cardiovascular events. J Am Heart Assoc. 2014;3(3):e000741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pan A, Lucas M, Sun Q, et al. . Increased mortality risk in women with depression and diabetes mellitus. Arch Gen Psychiatry. 2011;68(1):42-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cai T, Tian L, Wong PH, Wei LJ. Analysis of randomized comparative clinical trial data for personalized treatment selections. Biostatistics. 2011;12(2):270-282. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods.
eResults.
eReferences.