Key Points
Question
What is the relative cardiovascular safety of smoking cessation medications comparing varenicline, bupropion, nicotine replacement therapy, and placebo?
Findings
In this randomized clinical trial including 8058 individuals who smoked, the incidence of major cardiovascular events during treatment and follow-up was low and did not differ significantly by treatment.
Meaning
These findings provide evidence that, in a general population of smokers, smoking cessation medications do not increase the risk of serious cardiovascular events.
Abstract
Importance
Quitting smoking is enhanced by the use of pharmacotherapies, but concerns have been raised regarding the cardiovascular safety of such medications.
Objective
To compare the relative cardiovascular safety risk of smoking cessation treatments.
Design, Setting, and Participants
A double-blind, randomized, triple-dummy, placebo- and active-controlled trial (Evaluating Adverse Events in a Global Smoking Cessation Study [EAGLES]) and its nontreatment extension trial was conducted at 140 multinational centers. Smokers, with or without established psychiatric diagnoses, who received at least 1 dose of study medication (n = 8058), as well as a subset of those who completed 12 weeks of treatment plus 12 weeks of follow up and agreed to be followed up for an additional 28 weeks (n = 4595), were included.
Interventions
Varenicline, 1 mg twice daily; bupropion hydrochloride, 150 mg twice daily; and nicotine replacement therapy, 21-mg/d patch with tapering.
Main Outcomes and Measures
The primary end point was the time to development of a major adverse cardiovascular event (MACE: cardiovascular death, nonfatal myocardial infarction, or nonfatal stroke) during treatment; secondary end points were the occurrence of MACE and other pertinent cardiovascular events (MACE+: MACE or new-onset or worsening peripheral vascular disease requiring intervention, coronary revascularization, or hospitalization for unstable angina).
Results
Of the 8058 participants, 3553 (44.1%) were male (mean [SD] age, 46.5 [12.3] years). The incidence of cardiovascular events during treatment and follow-up was low (<0.5% for MACE; <0.8% for MACE+) and did not differ significantly by treatment. No significant treatment differences were observed in time to cardiovascular events, blood pressure, or heart rate. There was no significant difference in time to onset of MACE for either varenicline or bupropion treatment vs placebo (varenicline: hazard ratio, 0.29; 95% CI, 0.05-1.68 and bupropion: hazard ratio, 0.50; 95% CI, 0.10-2.50).
Conclusions and Relevance
No evidence that the use of smoking cessation pharmacotherapies increased the risk of serious cardiovascular adverse events during or after treatment was observed. The findings of EAGLES and its extension trial provide further evidence that smoking cessation medications do not increase the risk of serious cardiovascular events in the general population of smokers.
Trial Registration
clinicaltrials.gov Identifier: NCT01574703
This randomized clinical trial evaluates the cardiovascular risk associated with use of varenicline, bupropion, and nicotine replacement therapy in individuals who smoke.
Introduction
Cigarette smoking is associated with an increased risk of myocardial infarction (MI), stroke, peripheral vascular disease, atrial fibrillation, sudden death, worsening heart failure, and increased rates of thrombosis following coronary revascularization.1,2,3,4 Quitting is the single most important step a cigarette smoker can take to protect and enhance cardiovascular (CV) health. The US Public Health Service Clinical Practice Guideline for Smoking Cessation, as well as guidelines from other countries, recommend smoking cessation pharmacotherapy for all smokers making a quit attempt.5,6,7,8
Despite the proven efficacy of smoking cessation medications, many clinicians have been hesitant to prescribe them because of concerns regarding adverse events (AEs), including CV safety. Initial concerns regarding the risk of MI if a person smoked while wearing a nicotine patch9 were dispelled, and nicotine replacement therapy (NRT) is now recognized as a safe treatment for smokers with CV disease (CVD).9,10,11 Bupropion hydrochloride can increase blood pressure,12 and the package label includes precautions about hypertension. However, clinical trials of bupropion in smokers with CVD have not identified an increased incidence of CV AEs.13 Early clinical trials of varenicline, including a study of smokers with CVD, found rates of CV events (including MIs and strokes) to be low and not significantly higher than for placebo.14 However, in 2011, the US Food and Drug Administration (FDA) mandated strengthened product warnings addressing the possibility of an increased CV event risk in smokers with established CVD.15 Subsequently, a number of publications have reached mixed conclusions regarding the safety of varenicline, including several meta-analyses,11,16,17,18 a retrospective cohort study,19 a clinical trial among smokers with acute coronary syndrome,20 and most recently, an observational study using pharmacy and health record administrative databases.21
The FDA and the European Medicines Agency requested the manufacturers of varenicline and bupropion to conduct a randomized clinical trial to assess neuropsychiatric AEs to these medications vs an active control (NRT)—the results of which have been published22—and that the Evaluating Adverse Events in a Global Smoking Cessation Study (EAGLES) randomized clinical trial (NCT01456936) be extended to allow for CV event monitoring during and after treatment. We report the CV safety findings from EAGLES and its extension trial.
Methods
The EAGLES extension trial is a nontreatment extension of EAGLES. It provides CV safety data for all participants enrolled in EAGLES, beginning with the first dose of medication and, among those who completed the full 24 weeks of EAGLES, continuing an additional 28 weeks of observation (eFigure in Supplement 1).
Conducted at 140 multinational centers, EAGLES was a 24-week randomized, double-blind, triple-dummy, placebo- and active-controlled trial in cohorts of smokers with psychiatric (PC) and without psychiatric (NPC) disease assessing the safety and efficacy of varenicline, 1 mg twice daily, and bupropion, 150 mg twice daily, for smoking cessation. A tapering regimen with NRT, 21-mg/d patch, was the active control. Full details of the EAGLES methodology have been published.22 At the request of the FDA and the European Medicines Agency, the EAGLES protocol was amended to permit the collection of additional CV safety data and independent adjudication of CV events. To allow for earlier analysis of the neuropsychiatric safety data from EAGLES and provide for CV safety data collection for up to 52 weeks, the EAGLES extension trial was established as a separate protocol. The study adhered to the Declaration of Helsinki,23 the final protocol (Supplement 2) were approved by the institutional review boards and/or independent ethics committees at each participating investigational center (eAppendix in Supplement 1), and all participants provided written informed consent. Participants may have received reimbursement for personal expenses, such as travel costs, if approved by the institutional review board or ethics committee at each site.
Inclusion and Exclusion Criteria
Participants were aged 18 to 75 years, smoked 10 or more cigarettes per day, were interested in quitting smoking, and had been randomized to treatment in—and had completed the week 24 visit of—EAGLES. By definition, these participants met the inclusion or exclusion criteria for EAGLES.22 Participants were eligible for inclusion if they stopped study medication prematurely during EAGLES, so long as they had completed all EAGLES study visits. Exclusion criteria for EAGLES entry included unstable psychiatric illness, active substance abuse, clinically significant CVD in the 2 months prior to study entry (eg, MI or coronary artery bypass graft), clinically significant cerebrovascular disease in the 2 months prior to study entry (eg, stroke or documented transient ischemic attack), or inadequate control of hypertension as judged by investigators at screening and baseline.
Objectives and End Points
The primary objective was to characterize the CV safety profiles of varenicline and bupropion vs placebo. Secondary objectives were to compare the CV safety profiles of NRT vs placebo, varenicline vs bupropion, varenicline vs NRT, and bupropion vs NRT.
The primary end point was time to a major adverse CV event (MACE)—defined as a CV death, a nonfatal MI, or a nonfatal stroke—during treatment (ie, starting with the first dose and up to the date of the last dose of study drug). It was anticipated that the number of such events would be small and that the time-to-an-event approach would permit a more sensitive means of identifying medication-related CV events. Time to MACE was also evaluated (1) up to the date of the last dose of study drug plus 30 days (treatment-emergent) and (2) until the end of the study (up to 52 weeks for those who enrolled in the extension and up to 24 weeks for those who did not).
Secondary end points included the occurrence of MACEs (assessed over the same 3 time intervals) and evaluation of MACE+ (any MACE, a new onset of peripheral vascular disease [PVD], or a worsening of PVD requiring intervention, a need for coronary revascularization, or hospitalization for unstable angina). In addition, CV deaths, nonfatal MI, and nonfatal stroke (the components of MACE) were evaluated individually, as were hospitalizations for congestive heart failure and serious arrhythmias. The definitions of MACE and MACE+ had been previously developed in consultation with the FDA and were used in an earlier CV meta-analysis of varenicline studies.18
Procedures
During the EAGLES screening visit, detailed information on pre-existing CV risk factors was collected, and Framingham CV risk scores (high risk, >20% 10-year risk; medium risk, 10%-20% 10-year risk; and low risk, <10% 10-year risk of having a nonfatal MI and coronary heart disease death) were calculated.24 The week 24 visit of EAGLES served as the initiation visit for the extension trial; subsequent clinic visits occurred every 4 weeks up to week 52, maintaining the original week numbering (eg, week 28, 32). The focus of these visits was the identification of any AEs as well as measurement of blood pressure, heart rate, self-reported nicotine use, and exhaled carbon monoxide. If a participant reported a potential CV event at any time from the baseline EAGLES visit up to the week 52 visit, site investigators were to collect all medical records and other relevant information to permit event adjudication. In addition, study participants received physical examinations, clinical laboratory tests, and electrocardiograms.
Cardiovascular AEs were reviewed and adjudicated by an independent adjudication committee comprising 2 cardiologists and a neurologist. The committee members were blinded to study treatment allocation; they confirmed diagnoses of CV events of interest based on a review of the documentation provided by study investigators. All deaths were reviewed by the adjudication committee to determine whether they were likely of CV or non-CV origin. Event adjudication occurred throughout EAGLES and the EAGLES extension trial, but no adjudicated events were analyzed until after database lock at the completion of the EAGLES extension trial.
Statistical Analysis
The primary safety end point was analyzed using a stratified log-rank test with PC and NPC as strata. The overall estimated log-rank statistics were used to derive hazard ratios (HRs) and associated 95% CIs for the key pairwise comparisons of varenicline vs placebo and bupropion vs placebo. Time started at the date of the first dose of study medication; censoring occurred at the date of the last dose of study medication for participants not experiencing a MACE during treatment. Analysis was performed using the safety analysis set (ie, all participants who received ≥1 partial dose of study medication). Secondary analyses of time to MACE included a treatment-emergent censoring (to last dose plus 30 days) and end-of-study censoring, also using the safety analysis set. Relative to the comparator, an HR lower than 1 means a longer time to CV event.
Additional secondary analyses included the occurrence of MACE, MACE+, CV deaths, nonfatal MI, nonfatal stroke, hospitalization for congestive heart failure, and serious cardiac arrhythmia; each was assessed using logistic regression based on the safety analysis set and considering 3 time intervals: during treatment, treatment emergent, and end of study. Model terms included treatment, cohort, region, baseline CV risk (Framingham category), and the treatment-by-cohort interaction. Analyses were completed by cohort and overall for both the EAGLES safety study population and the subset of safety participants who transitioned to the extension trial. Level of significance was 2-sided and at 5%, with no matched pairs. EAGLES extension data were analyzed using SAS, version 9 (SAS Institute Inc).
Results
Participant Characteristics and Smoking Abstinence Rates
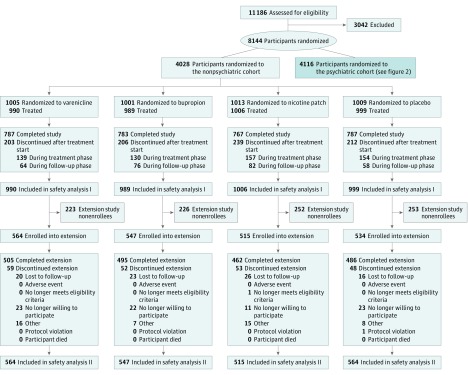
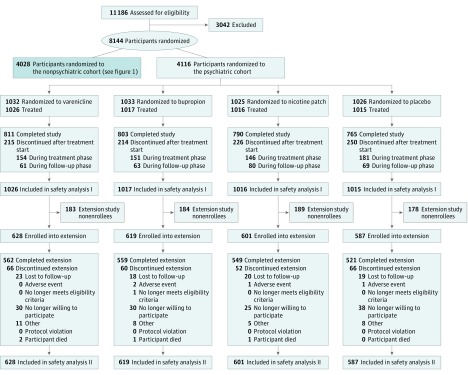
Detailed characteristics of the 11 186 smokers screened between November 30, 2011, and January 13, 2015, have been published.22 Briefly, of the 3984 NPC participants and the 4074 PC participants who received treatment, 2016 received varenicline; 2006, bupropion; 2022, NRT; and 2014, placebo (Table 1; Figure 1 and Figure 2). Of these participants, 3553 (44.1%) were men; mean (SD) age was 46.5 (12.3) years. Study completion rates for the 24-week EAGLES trial were similar across all treatment arms, with a high of 79.3% (varenicline) vs a low of 77.0% (NRT). Of those who completed EAGLES, 964 NPC participants and 734 PC participants declined enrollment in the 28-week extension trial. Thus, 4595 participants (73.0% of EAGLES completers or 56.4% of those randomized to EAGLES) enrolled in the extension trial; similar numbers of participants enrolled in each of the 4 treatment arms (Figure 1 and Figure 2). Extension trial completion rates were high (4139 of 4595 [90.1%]) and similar across the 4 treatment groups. All baseline characteristics of participants randomized in EAGLES and those who extended participation in the extension trial were similar (Table 1).
Table 1. Baseline Characteristics of Participants.
| Characteristic | EAGLES (n = 8058) | EAGLES Extension Trial (n = 4595) | ||||||
|---|---|---|---|---|---|---|---|---|
| Varenicline (n = 2016) | Bupropion (n = 2006) | NRT (n = 2022) | Placebo (n = 2014) | Varenicline (n = 1192) | Bupropion (n = 1166) | NRT (n = 1116) | Placebo (n = 1121) | |
| Demographics | ||||||||
| Age, mean (SD), y | 46.5 (12.4) | 46.3 (12.6) | 46.9 (12.2) | 46.4 (12.1) | 48.1 (12.2) | 47.7 (12.5) | 48.3 (11.9) | 47.5 (12.2) |
| Male, No. (%) | 902 (44.7) | 892 (44.5) | 883 (43.7) | 876 (43.5) | 533 (44.7) | 518 (44.4) | 493 (44.2) | 500 (44.6) |
| White race, No. (%) | 1668 (82.7) | 1636 (81.6) | 1641 (81.2) | 1639 (81.4) | 978 (82.0) | 946 (81.1) | 904 (81.0) | 893 (79.7) |
| BMI, mean (SD) | 28.1 (6.4) | 28.1 (6.4) | 28.0 (6.3) | 28.3 (6.4) | 28.6 (6.4) | 28.5 (6.6) | 28.6 (6.6) | 28.6 (6.6) |
| NPC, No. (%) | 990 (49.1) | 989 (49.3) | 1006 (49.8) | 999 (49.6) | 564 (47.3) | 547 (46.9) | 515 (46.1) | 534 (47.6) |
| PC, No. (%) | 1026 (50.9) | 1017 (50.7) | 1016 (50.2) | 1015 (50.4) | 628 (52.7) | 619 (53.1) | 601 (53.9) | 587 (52.4) |
| CV Risk Factors, No. (%) | ||||||||
| Diabetes | 122 (6.1) | 133 (6.6) | 118 (5.8) | 127 (6.3) | 71 (6.0) | 79 (6.8) | 68 (6.1) | 79 (7.0) |
| Type 1 | 3 (0.1) | 3 (0.1) | 1 (<0.1) | 0 | 0 | 1 (0.1) | 1 (0.1) | 0 |
| Type 2 | 119 (5.9) | 130 (6.5) | 117 (5.8) | 127 (6.3) | 71 (6.0) | 78 (6.7) | 67 (6.0) | 79 (7.0) |
| CHDa | 94 (4.7) | 96 (4.8) | 88 (4.4) | 87 (4.3) | 58 (4.9) | 58 (5.0) | 52 (4.7) | 51 (4.5) |
| Carotid artery diseaseb | 17 (0.8) | 9 (0.4) | 15 (0.7) | 12 (0.6) | 12 (1.0) | 6 (0.5) | 6 (0.5) | 10 (0.9) |
| Family history of premature CHDc | 304 (15.1) | 277 (13.8) | 280 (13.8) | 300 (14.9) | 181 (15.2) | 153 (13.1) | 163 (14.6) | 162 (14.5) |
| Baseline CV risk score, mean (SD)d | 8.3 (7.6) | 8.4 (8.2) | 8.4 (7.8) | 8.2 (7.6) | 8.8 (7.9) | 8.6 (7.9) | 9.0 (8.1) | 8.6 (8.0) |
| Baseline CV risk category, No. (SD)d | ||||||||
| Low risk (<10%) | 1403 (69.6) | 1410 (70.3) | 1408 (69.6) | 1444 (71.7) | 798 (66.9) | 809 (69.4) | 747 (66.9) | 787 (70.2) |
| Medium risk (10%-20%) | 460 (22.8) | 426 (21.2) | 451 (22.3) | 412 (20.5) | 298 (25.0) | 259 (22.2) | 270 (24.2) | 235 (21.0) |
| High risk (>20%) | 153 (7.6) | 170 (8.5) | 163 (8.1) | 158 (7.8) | 96 (8.1) | 98 (8.4) | 99 (8.9) | 99 (8.8) |
| CV Medical History, No. (%) | ||||||||
| Participants with ≥1 disease/syndrome | 676 (33.5) | 671 (33.4) | 681 (33.7) | 663 (32.9) | 422 (35.4) | 405 (34.7) | 398 (35.7) | 400 (35.7) |
| Atrial fibrillation | 3 (0.1) | 3 (0.1) | 3 (0.1) | 1 (<0.1) | 2 (0.2) | 1 (0.1) | 2 (0.2) | 1 (0.1) |
| Congestive cardiac failure | 2 (0.1) | 2 (0.1) | 3 (0.1) | 3 (0.1) | 0 | 1 (0.1) | 3 (0.3) | 1 (0.1) |
| Dyslipidemia | 374 (18.6) | 374 (18.6) | 383 (18.9) | 356 (17.7) | 241 (20.2) | 236 (20.2) | 221 (19.8) | 217 (19.4) |
| Hypertension | 451 (22.4) | 450 (22.4) | 474 (23.4) | 448 (22.2) | 273 (22.9) | 265 (22.7) | 284 (25.4) | 267 (23.8) |
| PVD | 20 (1.0) | 16 (0.8) | 16 (0.8) | 22 (1.1) | 15 (1.3) | 8 (0.7) | 10 (0.9) | 9 (0.8) |
| Coronary artery bypass | 2 (0.1) | 0 | 2 (0.1) | 1 (<0.1) | 0 | 0 | 1 (0.1) | 1 (0.1) |
| Percutaneous coronary intervention | 1 (<0.1) | 3 (0.1) | 1 (<0.1) | 3 (0.1) | 0) | 3 (0.3) | 0 | 1 (0.1) |
| Decreased ankle brachial index | 0 | 0 | 0 | 3 (0.1) | 0 | 0 | 0 | 1 (0.1) |
| Familial risk factor | 49 (2.4) | 43 (2.1) | 45 (2.2) | 42 (2.1) | 27 (2.3) | 29 (2.5) | 26 (2.3) | 22 (2.0) |
Abbreviations: BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); CHD, coronary heart disease; CV, cardiovascular; EAGLES, Evaluating Adverse Events in a Global Smoking Cessation Study; MI, myocardial infarction; NPC, nonpsychiatric cohort; NRT, nicotine replacement therapy (transdermal nicotine patch); PC, psychiatric cohort; PVD, peripheral vascular disease.
Includes MI, unstable angina, stable angina pectoris, coronary artery procedure (coronary angioplasty, stenting, or coronary artery surgery), silent MI, silent myocardial ischemia, other CHD, peripheral arterial disease, or abdominal aortic aneurysm.
Includes symptomatic carotid artery disease (transient ischemic attack or stroke of carotid origin) and greater than 50% stenosis.
Includes CHD in male first-degree relative younger than 55 years, female first-degree relative younger than 65 years (first-degree relatives include parents, offspring, and siblings), or participants with a parent who had an MI before age 60 years.
Ten-year Framingham risk score for total CHD. The generally accepted risk categories are high risk, greater than 20%; medium risk, 10% to 20%; and low risk, less than 10% of having a nonfatal MI and CHD death over 10 years.
Figure 1. CONSORT Diagram of Nonpsychiatric Disease Cohort.
Figure 2. CONSORT Diagram of Psychiatric Disease Cohort.
Among participants who received 1 or more partial dose of the assigned medication (n = 8058), the mean (SD) number of days of medication exposure (assessed by pill or patch count) was similar in all treatment groups: varenicline, 74.4 (23.1) days; bupropion, 73.7 (23.8) days; NRT, 73.7 (23.6) days; and placebo, 73.6 (23.6) days. Among the participants in the extension trial, the number and percentage of those with exposure to study drug for 78 or more days were varenicline, 1084 of 1192 (90.9%); bupropion, 1058 of 1166 (90.7%); NRT, 1005 of 1116 (90.1%); and placebo, 1025 of 1121 (91.4%).
As described in the EAGLES publication,22 the primary smoking cessation end points—continuous abstinence rates from weeks 9 to 12—were varenicline, 33.5%; bupropion, 22.6%; NRT, 23.4%; and placebo, 12.5%.
Safety Outcomes
There were no significant differences (log-rank test P > .05) in time to MACE or MACE+ overall or in either NPC or PC comparing active treatment with placebo across all observation periods (during treatment, end of treatment plus 30 days, and end of study (eTable 1 in Supplement 1). Consequently, the results presented herein are for overall analyses only. During the initial 12-week treatment phase, there was no significant difference in the primary safety end point—time to onset of MACE—for either varenicline or bupropion treatment vs placebo (varenicline: HR, 0.29; 95% CI, 0.05-1.68) and bupropion: HR, 0.50; 95% CI, 0.10-2.50) (eTable 1 in Supplement 1). Similar nonsignificant results were obtained for other observation periods (end of treatment plus 30 days and end of study). In addition, no statistically significant differences were observed for time to MACE+ for either varenicline or bupropion treatment vs placebo for all observation periods (during treatment, end of treatment plus 30 days, and end of study). Secondary comparisons between each active treatment group and comparing NRT with placebo for time to MACE and MACE+ likewise revealed no significant differences across all 3 observation periods.
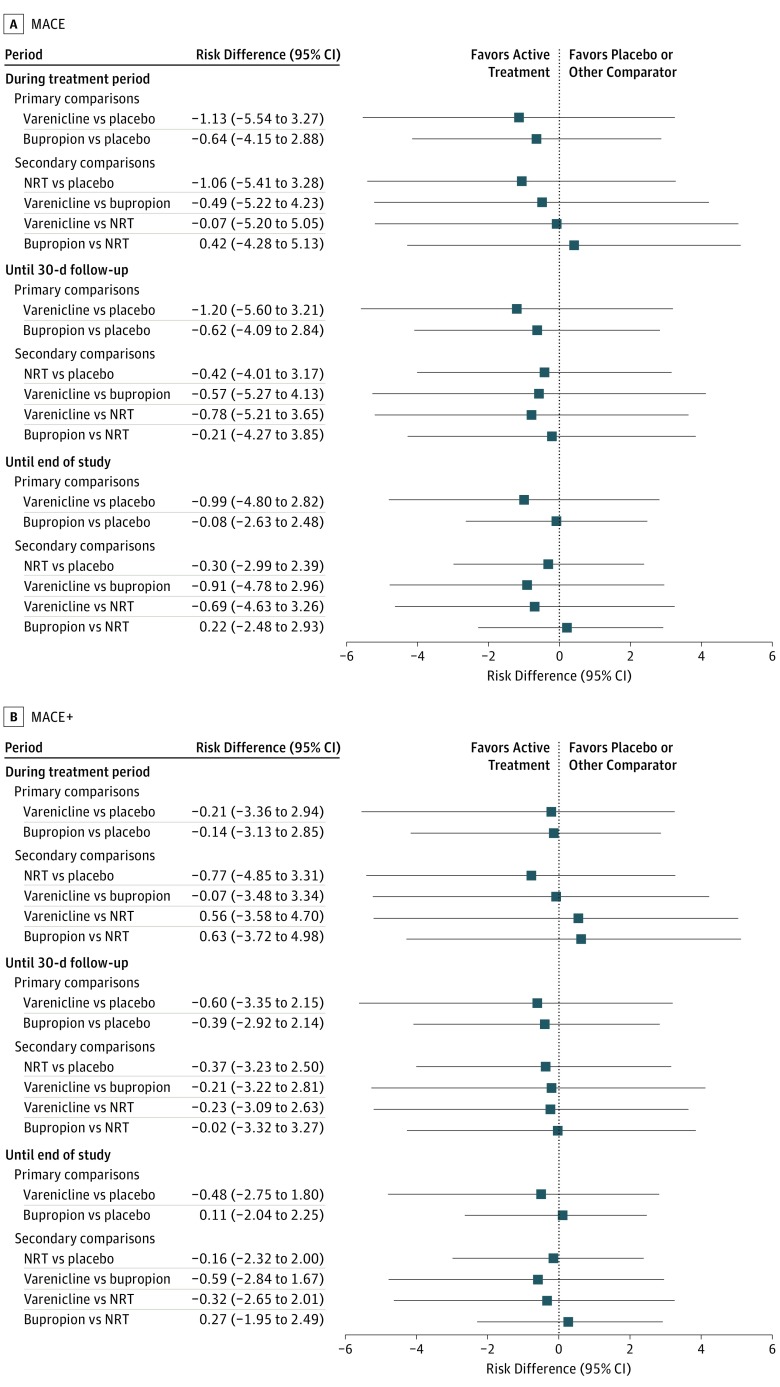
The observed incidence of MACE, MACE+, and all component CV safety end points was low across all treatment groups and observation periods (<0.5% for MACE; <0.8% for MACE+) (Table 2). The total number of adjudicated composite and component CV events through to the end of the study were MACE, 26; MACE+, 47; nonfatal MI, 14; nonfatal stroke, 8; new or worsening peripheral vascular disease, 11; coronary revascularization, 18; hospitalization for unstable angina, 3; serious cardiac arrhythmia, 18; and hospitalization for congestive heart failure, 7. There was no observable difference in the incidence of any of these events across treatment groups (Table 2). While the number of events was greatest in participants in the highest Framingham risk category, there were no significant differences in the incidence of CV events by treatment group when evaluated according to low, medium, or high Framingham CV risk scores. The risk differences for MACE and MACE+ across all observation periods were not significantly different for varenicline, bupropion, or NRT vs placebo, and similar results were obtained comparing varenicline with bupropion and NRT (Figure 3).
Table 2. Overall Occurrence of CV End Points During Treatment and 52 Weeks of Follow-up.
| End Point | No. (%) | |||
|---|---|---|---|---|
| Varenicline (n = 2016) | Bupropion (n = 2006) | NRT (n = 2022) | Placebo (n = 2014) | |
| MACE | ||||
| During treatment period | 1 (<0.1) | 2 (0.1) | 1 (<0.1) | 4 (0.2) |
| Until 30-d follow-up | 1 (<0.1) | 2 (0.1) | 2 (0.1) | 4 (0.2) |
| End of studya | 3 (0.1) | 9 (0.4) | 6 (0.3) | 8 (0.4) |
| MACE+ | ||||
| During treatment period | 5 (0.2) | 4 (0.2) | 2 (0.1) | 5 (0.2) |
| Until 30-d follow-up | 5 (0.2) | 4 (0.2) | 3 (0.1) | 7 (0.3) |
| End of studya | 10 (0.5) | 15 (0.7) | 10 (0.5) | 12 (0.6) |
| CV Death | ||||
| During treatment period | 0 | 1 (<0.1) | 0 | 1 (<0.1) |
| Until 30-d follow-up | 0 | 1 (<0.1) | 0 | 1 (<0.1) |
| End of studya | 1 (<0.1) | 2 (0.1) | 0 | 2 (0.1) |
| Nonfatal MI | ||||
| During treatment period | 1 (<0.1) | 1 (<0.1) | 1 (<0.1) | 3 (0.1) |
| Until 30-d follow-up | 1 (<0.1) | 1 (<0.1) | 1 (<0.1) | 3 (0.1) |
| End of studya | 2 (0.1) | 4 (0.2) | 3 (0.1) | 5 (0.2) |
| Nonfatal Stroke | ||||
| During treatment period | 0 | 0 | 0 | 0 |
| Until 30-d follow-up | 0 | 0 | 1 (<0.1) | 0 |
| End of studya | 0 | 4 (0.2) | 3 (0.1) | 1 (<0.1) |
| New-Onset or Worsening PVD Requiring Intervention | ||||
| During treatment period | 0 | 1 (<0.1) | 1 (<0.1) | 0 |
| Until 30-d follow-up | 0 | 1 (<0.1) | 1 (<0.1) | 2 (0.1) |
| End of studya | 3 (0.1) | 3 (0.1) | 3 (0.1) | 2 (0.1) |
| Hospitalization for Unstable Angina | ||||
| During treatment period | 1 (<0.1) | 0 | 0 | 0 |
| Until 30-d follow-up | 1 (<0.1) | 0 | 0 | 0 |
| End of studya | 1 (<0.1) | 2 (0.1) | 0 | 0 |
| Coronary Revascularization | ||||
| During treatment period | 4 (0.2) | 2 (0.1) | 1 (<0.1) | 4 (0.2) |
| Until 30-d follow-up | 4 (0.2) | 2 (0.1) | 1 (<0.1) | 4 (0.2) |
| End of studya | 4 (0.2) | 5 (0.2) | 2 (0.1) | 7 (0.3) |
| Serious Cardiac Arrhythmia | ||||
| During treatment period | 2 (0.1) | 1 (<0.1) | 3 (0.1) | 0 |
| Until 30-d follow-up | 3 (0.1) | 2 (0.1) | 4 (0.2) | 0 |
| End of studya | 5 (0.2) | 2 (0.1) | 8 (0.4) | 3 (0.1) |
| Hospitalization for CHF | ||||
| During treatment period | 0 | 0 | 0 | 3 (0.1) |
| Until 30-d follow-up | 0 | 0 | 0 | 4 (0.2) |
| End of studya | 0 | 1 (<0.1) | 1 (<0.1) | 5 (0.2) |
Abbreviations: CHF, congestive heart failure; CV, cardiovascular; MACE, major adverse CV event; MACE+, any MACE or a new-onset or worsening peripheral vascular disease (PVD) requiring intervention, a need for coronary revascularization, or hospitalization for unstable angina; MI, myocardial infarction; NRT, nicotine replacement therapy (transdermal nicotine patch).
Last visit in the EAGLES extension trial or in EAGLES for individuals not enrolled in the EAGLES extension trial.
Figure 3. Estimated Risk Difference for the Overall Incidence of a Major Adverse Cardiovascular Event (MACE) and Any MACE or a New-Onset or Worsening Peripheral Vascular Disease Requiring Intervention, a Need for Coronary Revascularization, or Hospitalization for Unstable Angina (MACE+).
Risk differences in MACE (A) and MACE+ (B) treatment groups. NRT indicates nicotine replacement therapy (transdermal nicotine patch).
A total of 13 participants died during the 52-week study period: varenicline group, 2; bupropion group, 4; NRT group, 3; and placebo group, 4. Five of these deaths were judged to be CV-associated deaths: varenicline group, 1; bupropion group, 2; and placebo group, 2 (Table 2 and eTable 2 in Supplement 1).
Minor changes from baseline were noted in body weight, blood pressure, and heart rate at weeks 12, 24, and 52. There was no significant difference in any of these measures across the treatment groups. Further data are presented in eTable 3 in Supplement 1. No significant differences in clinical laboratory test or electrocardiogram results across treatment groups were identified, and no new safety concerns were observed.
Discussion
The EAGLES with extension trial is, to our knowledge, the first study comparing the CV safety of varenicline, bupropion, and NRT head to head using a placebo comparator. The rate of CV events during 12 weeks of treatment, 30 days post treatment, and up to 52 weeks of follow-up was low and did not differ significantly by treatment group. Furthermore, we found no effect of any drug vs placebo on time to CV event, blood pressure, or heart rate.
Participants were in generally good health, but many had baseline CVD risk factors—hypertension (23%), dyslipidemia (18%), and diabetes (6%). Their CV risk profile determined by Framingham score was high risk in 8% and medium risk in 22%. This population is likely similar to smokers who are encountered in general medical practice25,26 and includes a significant percentage of individuals with mental health conditions who are prone to CV- and other smoking-related health risks.27
Our primary end point—time to MACE, which includes CV death, nonfatal MI, or nonfatal stroke—was not significantly different across treatment groups. The incidence of MACE+ during treatment and in the 30 days immediately post treatment was approximately 0.2% overall. At 1 year, the number of events was greater than during treatment, as anticipated, but was still only approximately 0.6%. As expected, MACE+ rates were greater in the high-risk Framingham CV risk group, with a 1.8% to 2.9% incidence of events at 1 year (eTable 4 in Supplement 1). There were only 5 CV deaths throughout the study.
The pharmacologic effects of NRT, bupropion, and varenicline provide some biological plausibility for concerns about CV AEs with smoking cessation medications that need to be considered. Nicotine acts on the α4β2 nicotinic acetylcholinergic receptor (nAChR), which mediates nicotine’s reinforcing effects and nicotine addiction, and on the α3β4 nAChR, which mediates sympathetic neural stimulation.28 Thus, nicotine increases heart rate, blood pressure, and myocardial work; may constrict coronary arteries to reduce myocardial blood supply; and releases epinephrine systemically.29 These effects could increase the risk of myocardial ischemia or MI and arrhythmogenesis. However, individuals who smoke develop a degree of tolerance to the CV effects of nicotine and generally receive less nicotine using NRT than with cigarette smoking.30
Bupropion is a sympathomimetic amphetamine analog. Bupropion may increase heart rate and blood pressure and could contribute to CV events.9,12 Varenicline binds relatively selectively to the α4β2 nAChR, with little expected CV effect via the α3β4 nAChR.31 However, varenicline binds to the α7 homomeric nAChR, and there is some evidence that actions on nonneuronal endothelial α7 nAChRs could produce adverse effects on endothelial function and/or angiogenesis, thereby contributing to CV AEs.32,33 We found no evidence of an effect of any smoking cessation medication on heart rate, blood pressure, incidence of CV events, or time to CV events, which is consistent with prior reports.9,34 Our results suggesting minimal risk of serious CV AEs support the findings of most other researchers who have analyzed the potential CV toxic effects of smoking cessation medications.10,11,13,14,16,17,18,19
Limitations and Strengths
A limitation of our study is that participants had no acute or unstable CVD. However, we included smokers with CV risk factors in addition to smoking or with a history of stable CVD. As noted previously, the prevalence of major CV risk factors, such as diabetes and hypertension, in the study population is similar to that found in participants in other smoking cessation trials and similar to the prevalence of these conditions in the general population. Smoking cessation trials in smokers with stable CVD found no evidence of risk from NRT,10 bupropion,13 or varenicline.14 A trial of varenicline in smokers with acute coronary syndrome found no evidence of increased risk of CV AEs.20 A recent observational trial using the Ontario Drug Benefit program database reported a 34% increased risk of CV hospitalizations and emergency department visits during varenicline use compared with a self-controlled posttreatment interval,21 although Kalhan et al35 provide a cautionary response regarding limitations of this design and findings.
The strengths of our study include the large number of participants treated with the 3 major classes of recommended smoking cessation pharmacotherapies; follow-up for 1 year in a multicenter, multinational, placebo-controlled clinical trial, including a large cohort of smokers with psychiatric disorders; and the independent adjudication of all CV events.
Conclusions
In what we believe to be the largest smoking cessation clinical trial and the only trial comparing NRT, bupropion, and varenicline vs placebo, we found no signal that smoking cessation pharmacotherapy increases the risk of serious CVD or CV AEs in a general population of smokers. While the number of events was small, the incidence of serious CV events was low, suggesting that any absolute increase in risk that we might have missed would be low and not clinically meaningful. Our findings are consistent with and support previously published findings from meta-analyses and small clinical trials in smokers with known CVD. Because our study excluded smokers with acute or unstable CVD, no conclusions can be drawn regarding this population.
Quitting smoking is arguably the most important action a smoker can take to reduce the risk of CV and other smoking-induced diseases. National guidelines recommend that health care professionals offer smoking cessation behavioral support and pharmacotherapy to their patients who smoke; such treatment substantially increases the likelihood of long-term tobacco abstinence and can significantly lower CV risk. We conclude that, in the general population of individuals who smoke, the benefit of improved CV health from pharmacotherapy-assisted smoking cessation exceeds any risk of medication-induced CV harm.
Supplementary Appendix
eFigure. Study Overview
eAppendix. Independent Ethics Committee or Institutional Review Board
eTable 1. Hazard Ratios for Time to Occurrence of MACE and MACE+
eTable 2. Cardiovascular Deaths
eTable 3. Median Change From Baseline in Weight and Vital Signs by Treatment Group
eTable 4. MACE and MACE+ Until End of Study Stratified by Baseline Framingham Cardiovascular Risk
Clinical Trial Protocol
References
- 1.National Center for Chronic Disease Prevention and Health Promotion (US) Office on Smoking and Health The health consequences of smoking—50 years of progress: a report of the Surgeon General. http://www.ncbi.nlm.nih.gov/pubmed/24455788. Published 2014. Accessed January 12, 2017.
- 2.Centers for Disease Control and Prevention (US); National Center for Chronic Disease Prevention and Health Promotion (US) ; Office on Smoking and Health (US). How tobacco smoke causes disease: the biology and behavioral basis for smoking-attributable disease. 2010. https://www.ncbi.nlm.nih.gov/books/NBK53017/. Published 2010. Accessed January 12, 2017. [PubMed]
- 3.Jha P, Ramasundarahettige C, Landsman V, et al. 21st-century hazards of smoking and benefits of cessation in the United States. N Engl J Med. 2013;368(4):341-350. [DOI] [PubMed] [Google Scholar]
- 4.Morris PB, Ference BA, Jahangir E, et al. Cardiovascular effects of exposure to cigarette smoke and electronic cigarettes: clinical perspectives from the Prevention of Cardiovascular Disease Section Leadership Council and Early Career Councils of the American College of Cardiology. J Am Coll Cardiol. 2015;66(12):1378-1391. [DOI] [PubMed] [Google Scholar]
- 5.Clinical Practice Guideline Treating Tobacco Use and Dependence 2008 Update Panel, Liaisons, and Staff A clinical practice guideline for treating tobacco use and dependence: 2008 update: a US Public Health Service report. Am J Prev Med. 2008;35(2):158-176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.West R, McNeill A, Raw M; Health Education Authority . Smoking cessation guidelines for health professionals: an update. Thorax. 2000;55(12):987-999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.European Network for Smoking and Tobacco Prevention (ENSP) European Smoking Cessation Guidelines: the authoritative guide to a comprehensive understanding of the implications and implementation of treatments and strategies to treat tobacco dependence. http://ensp.org/wp-content/uploads/2016/12/ENSP-ESCG_FINAL.pdf. Accessed January 12, 2017.
- 8.Zwar N, Richmond R, Borland R, et al. Supporting smoking cessation: a guide for health professionals. http://whyquit.com/guidelines/2011_Australia_Guide.pdf. Updated 2012. Accessed January 12, 2017.
- 9.Sobieraj DM, White WB, Baker WL. Cardiovascular effects of pharmacologic therapies for smoking cessation. J Am Soc Hypertens. 2013;7(1):61-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Joseph AM, Norman SM, Ferry LH, et al. The safety of transdermal nicotine as an aid to smoking cessation in patients with cardiac disease. N Engl J Med. 1996;335(24):1792-1798. [DOI] [PubMed] [Google Scholar]
- 11.Mills EJ, Thorlund K, Eapen S, Wu P, Prochaska JJ. Cardiovascular events associated with smoking cessation pharmacotherapies: a network meta-analysis. Circulation. 2014;129(1):28-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Roose SP, Dalack GW, Glassman AH, Woodring S, Walsh BT, Giardina EG. Cardiovascular effects of bupropion in depressed patients with heart disease. Am J Psychiatry. 1991;148(4):512-516. [DOI] [PubMed] [Google Scholar]
- 13.Tonstad S, Farsang C, Klaene G, et al. Bupropion SR for smoking cessation in smokers with cardiovascular disease: a multicentre, randomised study. Eur Heart J. 2003;24(10):946-955. [DOI] [PubMed] [Google Scholar]
- 14.Rigotti NA, Pipe AL, Benowitz NL, Arteaga C, Garza D, Tonstad S. Efficacy and safety of varenicline for smoking cessation in patients with cardiovascular disease: a randomized trial. Circulation. 2010;121(2):221-229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.US Food and Drug Administration FDA Drug Safety Communication: Chantix (varenicline) may increase the risk of certain cardiovascular adverse events in patients with cardiovascular disease. http://www.fda.gov/Drugs/DrugSafety/ucm259161.htm. Published July 22, 2011. Accessed Jan 12, 2017.
- 16.Prochaska JJ, Hilton JF. Risk of cardiovascular serious adverse events associated with varenicline use for tobacco cessation: systematic review and meta-analysis. BMJ. 2012;344:e2856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sterling LH, Windle SB, Filion KB, Touma L, Eisenberg MJ. Varenicline and adverse cardiovascular events: a systematic review and meta-analysis of randomized controlled trials. J Am Heart Assoc. 2016;5(2):e002849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ware JH, Vetrovec GW, Miller AB, et al. Cardiovascular safety of varenicline: patient-level meta-analysis of randomized, blinded, placebo-controlled trials. Am J Ther. 2013;20(3):235-246. [DOI] [PubMed] [Google Scholar]
- 19.Kotz D, Viechtbauer W, Simpson C, van Schayck OC, West R, Sheikh A. Cardiovascular and neuropsychiatric risks of varenicline: a retrospective cohort study. Lancet Respir Med. 2015;3(10):761-768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Eisenberg MJ, Windle SB, Roy N, et al. ; EVITA Investigators . Varenicline for smoking cessation in hospitalized patients with acute coronary syndrome. Circulation. 2016;133(1):21-30. [DOI] [PubMed] [Google Scholar]
- 21.Gershon AS, Campitelli MA, Hawken S, et al. Cardiovascular and neuropsychiatric events following varenicline use for smoking cessation [published online December 20, 2017]. Am J Respir Crit Care Med. 2017. [DOI] [PubMed] [Google Scholar]
- 22.Anthenelli RM, Benowitz NL, West R, et al. Neuropsychiatric safety and efficacy of varenicline, bupropion, and nicotine patch in smokers with and without psychiatric disorders (EAGLES): a double-blind, randomised, placebo-controlled clinical trial. Lancet. 2016;387(10037):2507-2520. [DOI] [PubMed] [Google Scholar]
- 23.World Medical Association World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191-2194. [DOI] [PubMed] [Google Scholar]
- 24.D’Agostino RB Sr, Vasan RS, Pencina MJ, et al. General cardiovascular risk profile for use in primary care: the Framingham Heart Study. Circulation. 2008;117(6):743-753. [DOI] [PubMed] [Google Scholar]
- 25.Gillespie CD, Hurvitz KA; Centers for Disease Control and Prevention (CDC) . Prevalence of hypertension and controlled hypertension—United States, 2007-2010. MMWR Suppl. 2013;62(3):144-148. [PubMed] [Google Scholar]
- 26.Beckles GL, Chou CF. Disparities in the prevalence of diagnosed diabetes—United States, 1999-2002 and 2011-2014. MMWR Morb Mortal Wkly Rep. 2016;65(45):1265-1269. [DOI] [PubMed] [Google Scholar]
- 27.Colton CW, Manderscheid RW. Congruencies in increased mortality rates, years of potential life lost, and causes of death among public mental health clients in eight states. Prev Chronic Dis. 2006;3(2):A42. [PMC free article] [PubMed] [Google Scholar]
- 28.Benowitz NL. Clinical pharmacology of nicotine: implications for understanding, preventing, and treating tobacco addiction. Clin Pharmacol Ther. 2008;83(4):531-541. [DOI] [PubMed] [Google Scholar]
- 29.Benowitz NL, Burbank AD. Cardiovascular toxicity of nicotine: implications for electronic cigarette use. Trends Cardiovasc Med. 2016;26(6):515-523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Benowitz NL, Gourlay SG. Cardiovascular toxicity of nicotine: implications for nicotine replacement therapy. J Am Coll Cardiol. 1997;29(7):1422-1431. [DOI] [PubMed] [Google Scholar]
- 31.Rollema H, Chambers LK, Coe JW, et al. Pharmacological profile of the α4β2 nicotinic acetylcholine receptor partial agonist varenicline, an effective smoking cessation aid. Neuropharmacology. 2007;52(3):985-994. [DOI] [PubMed] [Google Scholar]
- 32.Mihalak KB, Carroll FI, Luetje CW. Varenicline is a partial agonist at α4β2 and a full agonist at α7 neuronal nicotinic receptors. Mol Pharmacol. 2006;70(3):801-805. [DOI] [PubMed] [Google Scholar]
- 33.Lee J, Cooke JP. Nicotine and pathological angiogenesis. Life Sci. 2012;91(21-22):1058-1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Silva AP, Scholz J, Abe TO, et al. Influence of smoking cessation drugs on blood pressure and heart rate in patients with cardiovascular disease or high risk score: real life setting. BMC Cardiovasc Disord. 2016;16:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kalhan R, Wilkins JT, Hitsman BL. Tobacco smoking is a medical problem: we ought to treat it like one [published online December 20, 2017]. Am J Respir Crit Care Med. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Appendix
eFigure. Study Overview
eAppendix. Independent Ethics Committee or Institutional Review Board
eTable 1. Hazard Ratios for Time to Occurrence of MACE and MACE+
eTable 2. Cardiovascular Deaths
eTable 3. Median Change From Baseline in Weight and Vital Signs by Treatment Group
eTable 4. MACE and MACE+ Until End of Study Stratified by Baseline Framingham Cardiovascular Risk
Clinical Trial Protocol