Abstract
Background:
It is well accepted that mold exposure is a major contributor to the development of asthma, and beta-glucans are often used as a surrogate for mold exposure in the environment. Beta-glucans are an important component of mold spores and are recognized by the immune system by their receptor, Dectin-1. Cladosporium cladosporioides spores have a high beta-glucan content, but the beta-glucans are not available on the surface of live spores.
Objective:
We sought to determine whether altering the exposure of beta-glucans in C cladosporioides through heat killing could alter the immune response through binding to Dectin-1.
Methods:
In a murine model of mold-induced asthma, mice were repeatedly exposed to either live or heat-killed C cladosporioides and the phenotype was determined by the measurement of airway hyperresponsiveness, airway inflammation, and cytokine production. Pro-inflammatory cytokines from dendritic cells were measured by using quantitative PCR and ELISA.
Results:
Live C cladosporioides induced robust airway hyperresponsiveness, eosinophilia, and a predominately TH2 response, while heat-killed C cladosporioides induced a strong TH17 response and neutrophilic inflammation, but very mild airway hyperresponsiveness. Heat killing of C cladosporioides spores effectively exposed beta-glucans on the surface of the spores and increased binding to Dectin-1. In the absence of Dectin-1, heat-killed spores induced a predominantly TH2 response analogous to live spores. Furthermore, the production of TH17-skewing IL-6, IL-23, and TNF-α by dendritic cells in response to heat-killed C cladosporioides was dependent on Dectin-1.
Conclusions:
The host immune response to C cladosporioides is dependent on the surface availability of beta-glucans rather than the total beta-glucan content. (J Allergy Clin Immunol 2013;132:159-69.)
Keywords: Mold, Cladosporium cladosporioides, beta-glucans, asthma, Dectin-1
Fungal spores are ubiquitously distributed in both the indoor and outdoor environment and are often associated with respiratory disease.1 Numerous studies have implicated mold exposure in the development and prevalence of asthma. Chronic mold exposure in a high-risk birth cohort was associated with persistent wheeze,2 and persistent childhood asthma was associated with sensitivity to mold in another pediatric cohort.3 In adult asthma, allergic sensitization to molds was associated with more hospital and intensive care unit admissions due to asthma.4,5 These studies underscore the importance of mold exposure as a public health concern and the relevance of mold exposure to asthma.
A major component of fungal cell walls is (1-3)-beta-D-glucans, and these are commonly used as a marker of mold exposure in the environment.6 Beta-glucans account for up to 60% of the weight of the cell wall,7 and they are important for the recognition of molds by the immune system. Dectin-1, the receptor for beta-glucans,8 is expressed on macrophages, monocytes, neutrophils, and dendritic cells.9,10 Signaling through Dectin-1 promotes fungal immunity, specifically by inducing dendritic cells to polarize T cells toward TH17 cells.11-14 Accordingly, deficiency in IL-17A or Dectin-1 has been associated with an increased risk for fungal infections in humans.15-20 Results from animal studies also demonstrate that the absence of Dectin-1 leads to increased susceptibility to fungal infections21-23 and decreased production of IL-17A.23,24
Several studies indicate that the beta-glucans in mold spores are important for the development of an immune response and type of immune response. Removal of rodA, either genetically or chemically, from Aspergillus fumigatus spores increases both the activation of dendritic cells and the binding of Dectin-1.25 Heat-killed Candida albicans has greater binding to Dectin-1 than do live spores, and the heat-killed spores are better at activating antigen-presenting cells.26,27 Another study demonstrated that distinct immune responses develop in response to live and heat-killed mold spores,28 suggesting that exposure of beta-glucans on the surface of spores may alter the immune response. Furthermore, recognition of different stages of mold growth is dependent on the accessibility of beta-glucan and binding to Dectin-1.29 We recently reported that Dectin-1 and IL-17A prevent the development of asthma in mice after exposure to A versicolor, but not C cladosporioides, due to differences in the surface availability of beta-glucans between the 2 spores and, therefore, differences in binding to Dectin-1.30 Collectively, these studies indicate that the surface exposure of beta-glucans alters the immune response to molds.
In this study, we hypothesized that exposure of beta-glucans on C cladosporioides spore surface by heat killing will prevent the development of asthma through a Dectin-1-dependent mechanism. Our data demonstrate that heat-killed C cladosporioides spores display more surface beta-glucans and Dectin-1 binding than do live spores. Furthermore, heat-killed C cladosporioides spores induced an attenuated asthma phenotype compared with live spores. The inflammatory phenotype induced by heat-killed C cladosporioides spores was predominately a TH17 response marked by neutrophilic inflammation in the airways, and this response was dependent on Dectin-1.
METHODS
Mice
Dectin-1−/− mice have been previously described and were generously provided by Dr Gordon Brown.21 Age- and sex-matched wild-type BALB/c and C57Bl/6 mice were purchased from Harlan Laboratories (Indianapolis, Ind). All mice were housed in a specific pathogen-free environment in the animal facility at Cincinnati Children’s Hospital Medical Center. All procedures were performed in accordance with the ethical guidelines in the Guide for the Care and Use of Laboratory Animals of the Institutional Animal Care and Use Committee approved by the Veterinary Services Department of the Cincinnati Children’s Hospital Medical Center.
Preparation of mold spores and spore challenge model
C cladosporioides isolate 6721 (American Type Culture Collection, Manassas, Va) was grown on malt extract agar for 4 to 6 weeks at 25°C. The spores were collected by agitating the surface of fungal cultures with glass beads and rinsing the beads with saline supplemented with 0.05% Tween 80. The spores were stored at −80°C until use, and upon thawing were counted and resuspended in saline at a concentration of 2 × 107 spores/mL. Heat killing of spores was achieved by autoclaving the spores. Mice were lightly anesthetized with isoflurane and exposed to saline, 106 live C cladosporioides or 106 heat-killed C cladosporioides spores in 50 μL intratracheally 3 times a week for 3 weeks, and then were assessed 2 days after the last exposure. The mold spore dose used is the same as previously published.30
Assessment of airway hyperresponsiveness
Airway hyperresponsiveness (AHR) to methacholine (acetyl-b-methyl-cholinechloride; Sigma, St Louis, Mo) was assessed in mice by using flexiVent (SCIREQ, Montreal, Quebec, Canada) as previously described.30
Bone marrow-derived dendritic cells
Bone marrow–derived dendritic cells (BMDCs) were generated as previously described.31 BMDCs were stimulated with either live or heat-killed C cladosporioides spores at a 1:2 cell-to-spore ratio. After 24 hours, supernatants and RNA were collected.
Bronchoalveolar lavage fluid collection and analysis
Bronchoalveolar lavage fluid (BALF) was collected and analyzed as previously described.32 For detection of total serum IgE, plasma was diluted at 1:50 and the ELISA was performed as previously reported.32 Detection of IL-4 and IL-17A was performed according to manufacturer instructions (BioLegend, San Diego, Calif) in undiluted BALF. IL-6 and TNF-α were also detected according to manufacturer instructions (BioLegend) in supernatants from BMDCs diluted 1:5.
Isolation of lung cells and flow cytometry
Lungs were removed, and the upper right lobe was minced and incubated at 37°C for 25 to 30 minutes in 2 mL of RPMI1640 containing Liberase DL (0.5 mg/mL; Roche Diagnostics, Indianapolis, Ind) and DNAse I (0.5 mg/mL; Sigma). Lung cells were passed through a 70-μm cell strainer. Cells were centrifuged and resuspended in 2 mL of RPMI. Cell viability was confirmed by trypan blue exclusion.
Approximately 106 lung cells were transferred into a V-bottom 96-well plate on ice, centrifuged, and resuspended in PBS containing FcBlock (2.4G2 mAb, Biolegend) after stimulation with phorbol 12-myristate 13-acetate (50 ng/mL; Sigma) and ionomycin (500 ng/mL; Sigma) in the presence of Brefeldin A (eBioscience, San Diego, Calif) and monensin (eBioscience) diluted 1:1000. Cells were labeled with LIVE/DEAD Fixable Aqua Dead Cell Stain Kit according to manufacturer’s instructions (Invitrogen by Life Technologies, Carlsbad, Calif). T cells were stained with CD4-Pacific Blue (BioLegend). Intracellular staining for IL13-PE (eBioscience) and IL17A-AF647 (BioLegend) was done by using reagents from eBioscience. All flow cytometric data were acquired by using the LSR Fortessa (Becton Dickinson, Mountain View, Calif), maintained by the Research Flow Cytometry Core in the Division of Rheumatology at Cincinnati Children’s Hospital Medical Center. Data were analyzed by using FlowJo software (Tree Star, Ashland, Ore).
RT-PCR
RNA was extracted from the lungs or from BMDCs by using TRIzol reagent (Life Technologies, Grand Island, NY). Reverse transcription was performed by using iScript Reverse Transcription Supermix (BioRad, Hercules, Calif). Real-time PCR was done by using the SYBR Green Master Kit and a LightCycler 480 instrument (Roche Diagnostics). Murine hypoxanthine phosphoribosyltransferase was used to normalize expression and was specifically amplified with forward primer 5′-TGC CGA GGA TTT GGA AAA AG-3′ and reverse primer 5′-CCC CCC TTG AGC ACA CAG-3′. cDNA for murine IL-4 was specifically amplified by using forward primer 5′-CTG TAG GGC TTC CAA GGT GCT TCG-3′ and reverse primer 5′-CCA TTT GCA TGA TGC TCT TTA GGC-3′. cDNA for murine IL-17A was specifically amplified by using forward primer 5′-ACT ACC TCA ACC GTT CCA CG-3′ and reverse primer 5′-AGA ATT CAT GTG GTG GTC CA-3′. cDNA for murine IL-6 was specifically amplified by using forward primer 5′-TGA TGC ACT TGC AGA AAA CA-3′ and reverse primer 5′-ACC AGA GGA AAT TTT CAA TAG GC-3′. cDNA for murine TNF-α was specifically amplified by using forward primer 5′-TGT GCT CAG AGC TTT CAA CAA-3′ and reverse primer 5′-CTT GAT GGT GGT GCA TGA GA-3′. cDNA for murine IL-23p19 was specifically amplified by using forward primer 5′-ACT CAG CCA ACT CCT CCA GCC AG-3′ and reverse primer 5′-CTG CTC CGT GGG CAA AGA CCC-3′.
Beta-glucan staining and Dectin-1 pulldown
Binding to recombinant murine Dectin-1 (R&D Systems, Minneapolis, Minn) was determined as previously described.30 Briefly, mold spores were labeled with Alexa Fluor 488 dye (Life Technologies), incubated with recombinant murine Dectin-1 (R&D Systems), and pulled down with magnetic nickel beads (Life Technologies). The amount of spores pulled down with Dectin-1 was determined by reading the fluorescence on a Synergy H1 Hybrid Reader (BioTek, Winooski, Vt) and normalizing to a standard curve.
To determine the amount and localization of exposed beta-glucans on the spores, 106 spores were incubated with murine anti–beta-glucan antibody, primary antibody (Biosupplies, Bundoora, Australia), at a concentration of 1 μg/mL in 1% goat serum in 0.01% PBS Tween-20, and the secondary antibody was goat antimouse DyLight 594 (BioLegend). Images were acquired at 1000× by using a Nikon 90i Fully Automated Upright Microscope System with a Nikon DSQiMc camera, with Z-sequence taken every 0.5 μm. Nikon Elements Quantitative Analysis software was used to acquire the images. For the measurement of mean fluorescence intensity, the fluorescence of each spore in10 fields of view was measured by using Nikon Elements Quantitative Analysis software. The mean was calculated by adding the fluorescence of each individual spore and dividing by the total number of spores.
Statistical analysis
All statistical analyses were done by using PRISM software (GraphPad Software, Inc, La Jolla, Calif). Statistical significance was assessed by using 1-way ANOVA followed by a Neuman-Kohls posttest or a 2-way ANOVA followed by a Bonferroni posttest for dose-response data.
RESULTS
Heat-killed C cladosporioides spores induce neutrophilic inflammation in the lungs of mice
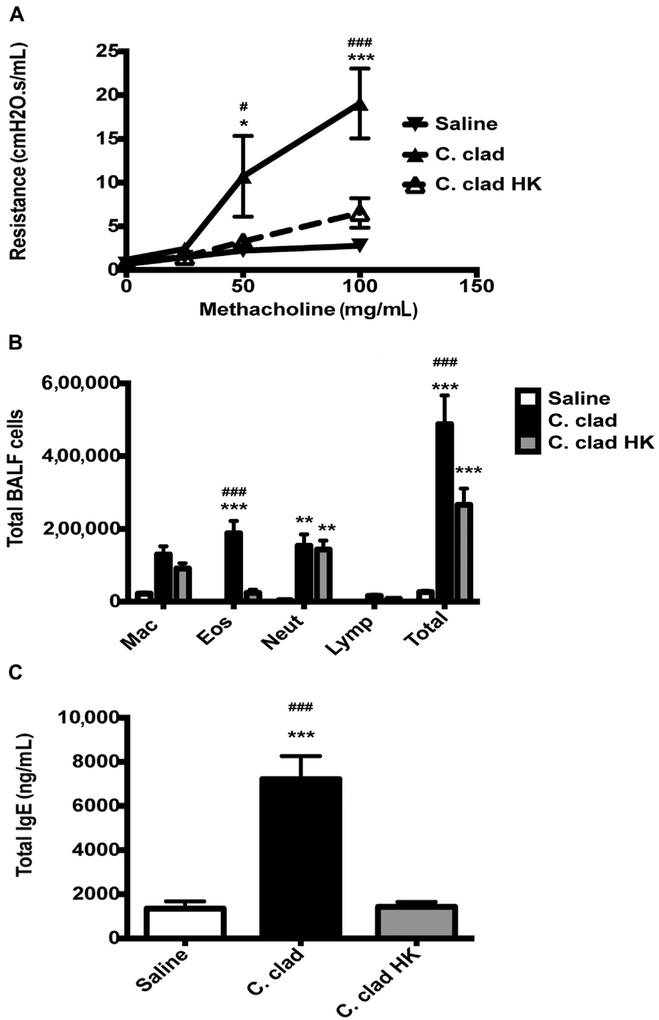
Our previous data indicate that Dectin-1 prevents the development of murine asthma in response to the environmental mold A versicolor but not C cladosporioides. This was due to differences in the surface exposure of beta-glucans on the 2 molds and thus the differing capabilities of the molds to bind to Dectin-1.30 Previous studies reported that heat killing of Candia albicans results in exposure of beta-glucans.26,27 Therefore, we wanted to determine whether heat-killed C cladosporioides spores would induce an inflammatory phenotype in the lungs of mice similar to A versicolor, which express higher levels of surface beta-glucans.30 Exposure of mice to live C cladosporioides spores induced robust AHR, a strong eosinophilic response, and an increase in total IgE (Fig 1), as we previously reported.30 In contrast, exposure of mice to heat-killed C cladosporioides spores resulted in the development of minimal AHR, a predominately neutrophilic response, and no increase in serum IgE (Fig 1). Thus, heat-killed C cladosporioides spores induce a predominantly neutrophilic response in the lungs of mice similar to our published data with A versicolor.30
FIG 1.
Heat killing of C cladosporioides prevents the development of murine asthma. A, BALB/c mice were exposed i.t. to 106 live or heat-killed C cladosporioides (C clad or C clad HK) spores 3 times a week for 3 weeks and 48 hours after the last challenge, AHR was measured by flexiVent. B, BALF was analyzed for total and differential counts. C, Total serum IgE. Data are representative of 3 independent experiments and are expressed as mean and SEM. n = 4 to 6 mice per group. Eos, Eosinophils; HK, heat killed; i.t., intratracheally; Lymp, lymphocytes; Mac, macrophages; Neut, neutrophils. *P < .05, **P < .01, and ***P < .001 as compared with saline. #P < .05 and ###P < .001 as compared with C clad HK.
Lack of a TH2 cytokine response in the lungs of mice exposed to heat-killed C cladosporioides
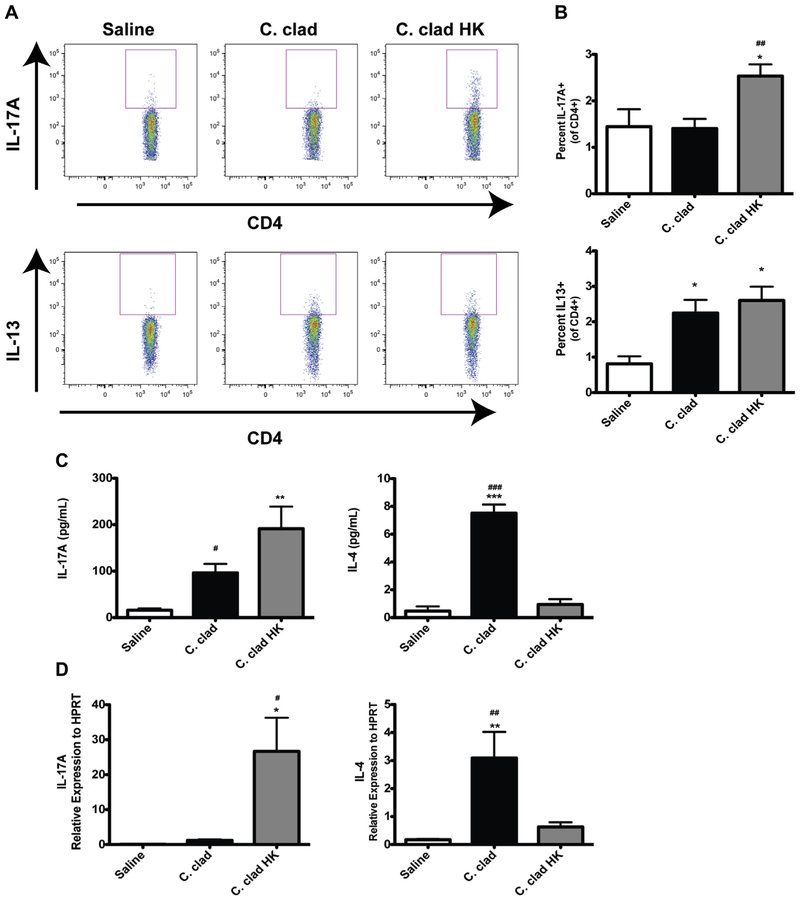
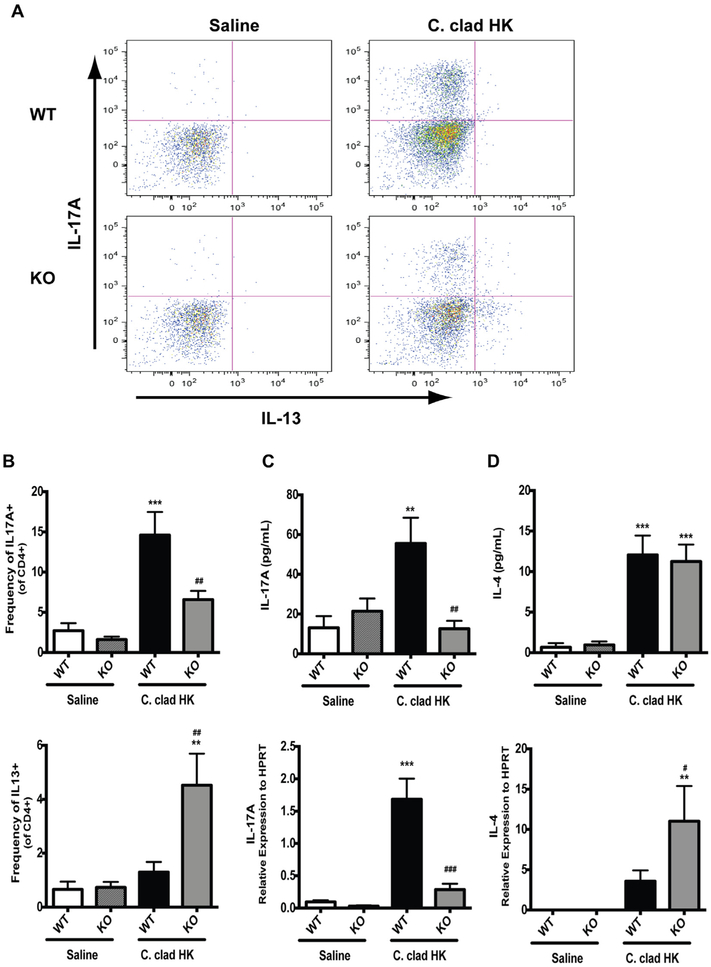
We next investigated the nature of the immune responses that developed in the lungs of mice after exposure to live versus heat-killed C cladosporioides spores. While both live and heat-killed spores were able to induce TH2 cells (Fig 2, B), only the heat-killed spores induced TH17 cells (Fig 2, A). The gating strategy is demonstrated in Fig E1 in this article’s Online Repository at www.jacionline.org. However, we found by analyzing IL-17A and IL-4 in the BALF by ELISA (Fig 2, C) or in the lungs by quantitative PCR (Fig 2, D) that heat-killed spores induced significant amounts of IL-17A and very little IL-4 than did live spores (Fig 2, C and D). Thus, heat-killed spores induce a strong TH17 response and a relatively weak TH2 response than do live spores.
FIG 2.
Induction of a TH17 response by heat-killed C cladosporioides (C clad HK). Contour plots (A) and quantification of intracellular cytokine staining for IL-13 or IL-17A in CD4+ lung cells (B). C, Levels of IL-17A and IL-4 in BALF. D, Relative levels of mRNA for IL-17A and IL-4 in whole lung. Data are representative of 3 independent experiments and are expressed as mean and SEM. n = 4 to 6 mice per group. HPRT, Hypoxanthine phosphoribosyltransferase. *P < .05, **P < .01, and ***P < .001 as compared with saline. #P < .05, ##P < .01, and ###P < .001 as compared with C clad HK.
Heat killing of C cladosporioides exposes beta-glucans
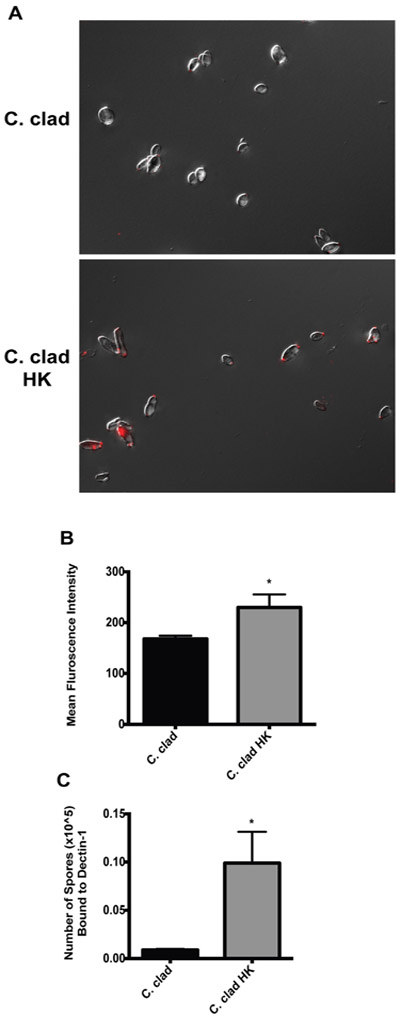
To establish the impact of heat killing on the level of beta-glucans exposed on the surface of C cladosporioides spores, we performed immunofluorescence on the spores by using an anti–beta-glucan antibody. In both live and heat-killed spores, we observed staining predominately at the ends of spores at the bud scars (Fig 3, A). Importantly, the heat-killed spores were intact and the process of heat killing did not disrupt the spores (Fig 3, A). Furthermore, we did not observe any autofluorescence in the AF488 channel because only spores stained with an AF488 dye demonstrated any fluorescence (see Fig E2 in this article’s Online Repository at www.jacionline.org). The staining intensity was stronger in the heat-killed spores, supporting that there are more beta-glucans exposed following heat killing (quantified in Fig 3, B). To confirm this, we directly examined the ability of the heat-killed spores to bind recombinant Dectin-1. As shown in Fig 3, C, heat-killed spores demonstrated increased binding to recombinant Dectin-1 than did live spores (Fig 3, C), consistent with the greater exposure of beta-glucans on heat-killed spores.
FIG 3.
Heat-killed C cladosporioides have increased surface exposure of beta-glucans and binding to Dectin-1. A, Immunofluorescence images of beta-glucan staining taken at ×1000 for live and heat-killed C cladosporioides spores. Scale bar is 10 μm. B, Quantification of beta-glucan fluorescence intensity per spore. C, Number of spores bound to recombinant Dectin-1. Data are representative of 2 independent experiments and are expressed as mean and SEM. n = 3 samples per group. C clad HK, Heat-killed C cladosporioides. *P < .05.
Dectin-1 prevents the induction of murine asthma in response to heat-killed spores
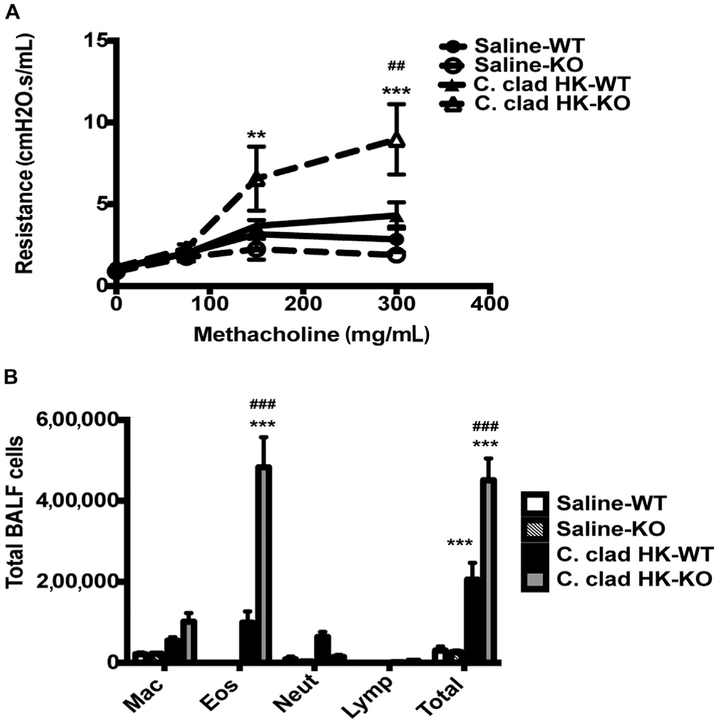
We investigated whether the phenotype induced by heat-killed C cladosporioides was dependent on Dectin-1. Wild-type mice exposed to heat-killed C cladosporioides spores developed a mixed neutrophilic/eosinophilic response but no AHR (Fig 4). Dectin-1−/− mice exposed to heat-killed C cladosporioides spores developed robust AHR and eosinophilia. Furthermore, they did not develop any neutrophilic inflammation (Fig 4). In contrast, Dectin-1 had minimal impact on the development of AHR and eosinophilia in response to live spores (data not shown and previously published30). Similar to our previously published data,30 C57B1/6 mice developed more robust eosinophilia than did BALB/c mice. Thus, these data indicate that the inflammatory phenotype induced by heat-killed C cladosporioides spores is dependent on Dectin-1.
FIG 4.
Heat-killed C cladosporioides prevents murine asthma in a Dectin-1–dependent manner. A, Wild-type C57BI/6 or Dectin-1−/− mice were exposed to heat-killed C cladosporioides spores, and AHR was assessed 48 hours later. B, Total and differential counts of BALF. Data are representative from 2 independent experiments and are expressed as mean and SEM. n = 4 to 6 mice per group. C clad HK, Heat-killed C cladosporioides; Eos, eosinophils; KO, knockout; Lymp, lymphocytes; Mac, macrophages; Neut, neutrophils; WT, wild type. **P < .01 and ***P < .001 as compared with saline. ##P < .01 and ###P < .001 as compared with wild-type mice exposed to C cladosporioides.
TH17 response induced by heat-killed C cladosporioides spores is dependent on Dectin-1
We next determined whether the development of a TH17 response following exposure to heat-killed C cladosporioides spores (Fig 2) was dependent on Dectin-1. We characterized the T cells in the lungs of wild-type and Dectin-1−/− mice following repeated exposure to heat-killed C cladosporioides spores. As shown in Fig 5, there was a significant decrease in the frequency of TH17 cells in Dectin-1−/− mice than in wild-type mice (Fig 5, A and B). Similarly, IL-17A was decreased in the BALF and IL-17A expression was decreased in the lungs of Dectin-1−/− mice, but not wild-type mice, exposed to heat-killed C cladosporioides spores (Fig 5, C). Furthermore, there was an increase in TH2 cells in Dectin-1−/− mice than in wild-type mice exposed to heat-killed C cladosporioides spores (Fig 5, A and B). While there was no difference in IL-4 BALF levels between wild-type and Dectin-1−/− mice, IL-4 mRNA levels were increased in the lungs of Dectin-1−/− versus wild-type mice following exposure to heat-killed C cladosporioides spores (Fig 5, D). Induction of TH2 cytokines by live C cladosporioides spores is independent of Dectin-1 (data not shown and previously published30). Thus, the TH17 response induced by heat-killed spores is dependent on Dectin-1. In the absence of Dectin-1, the frequency of TH2 cells and IL-4 expression in the lungs were increased.
FIG 5.
In the absence of Dectin-1, heat-killed C cladosporioides induces a TH2 response. Contour plots (A) and quantification of intracellular cytokine staining for IL-13 or IL-17A in CD4+ lung cells (B). C, Levels of IL-17A in BALF and mRNA in whole lung. D, Levels of IL-4 in BALF and mRNA in whole lung. Data are representative from 2 independent experiments and are expressed as mean and SEM. n = 4 to 6 mice per group. C clad HK, Heat-killed C cladosporioides; KO, knockout; WT, wild type. **P < .01 and ***P < .001 as compared with saline. #P < .05, ##P < .01, and ###P < .001 as compared with wild-type mice exposed to Ccladosporioides.
TH17-skewing cytokines are decreased in the lungs of Dectin-1-deficient mice
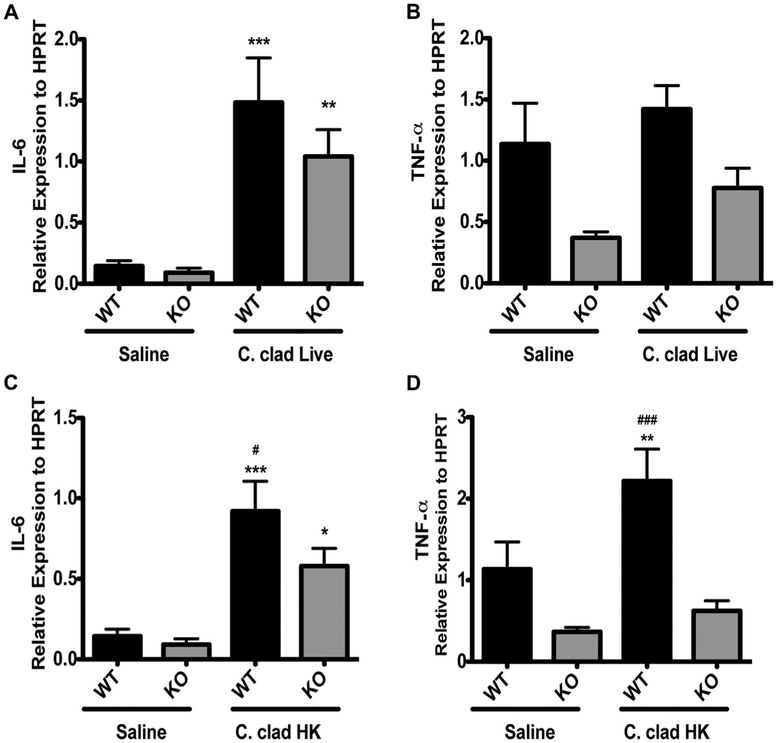
Because the TH17 response induced by heat-killed C cladosporioides spores is dependent on Dectin-1, we hypothesized that altered upstream expression of TH17-skewing cytokines by heat-killed versus live spores may be the mechanistic basis for our observations. IL-6 is important for priming TH17 cells in the mucosal tissues,33 and TNF-α production by dendritic cells is important for regulating IL-17 levels in the lungs.34 Therefore, we examined the expression of IL-6 and TNF-α in the lungs of mice exposed to live or heat-killed C cladosporioides spores in wild-type and Dectin-1−/− mice. Mice exposed to live C cladosporioides spores had a significant increase in the expression of IL-6 than did saline-exposed mice, and this increase was independent of Dectin-1. There was no significant difference between wild-type mice and Dectin-1−/− mice exposed to live C cladosporioides spores (Fig 6, A). There was only a slight, nonsignificant, induction of TNF-α by live C cladosporioides spores in both wild-type and Dectin-1−/− mice (Fig 6, B). However, heat-killed C cladosporioides spores induced expression of both IL-6 and TNF-α in a Dectin-1–dependent manner (Fig 6, C and D). In the absence of Dectin-1, expression of IL-6 and TNF-α was significantly reduced (Fig 6, C and D). Thus, Dectin-1 promotes the induction of TH17-skewing cytokines, but only in response to heat-killed spores that express surface beta-glucans.
FIG 6.
Expression of TH17-skewing cytokines in the lungs is dependent on Dectin-1. A and C, Expression of IL-6 in the lungs of mice exposed to live (Fig 6, A) or heat-killed (Fig 6, C) C cladosporioides. B and D, Expression of TNF-α in the lungs of mice exposed to live (Fig 6, B) or heat-killed (Fig 6, D) C cladosporioides. Data are representative from 2 independent experiments and are expressed as mean and SEM. n = 4 to 6 mice per group. C clad HK, Heat-killed C cladosporioides; HPRT, hypoxanthine phosphoribosyltransferase; KO, knockout; WT, wild type. *P < .05, **P < .01, and ***P < .001 as compared with saline. #P < .05 and ###P < .001 as compared with Dectin-1−/− mice exposed to C cladosporioides.
Dendritic cell production of proinflammatory cytokines is dependent on Dectin-1
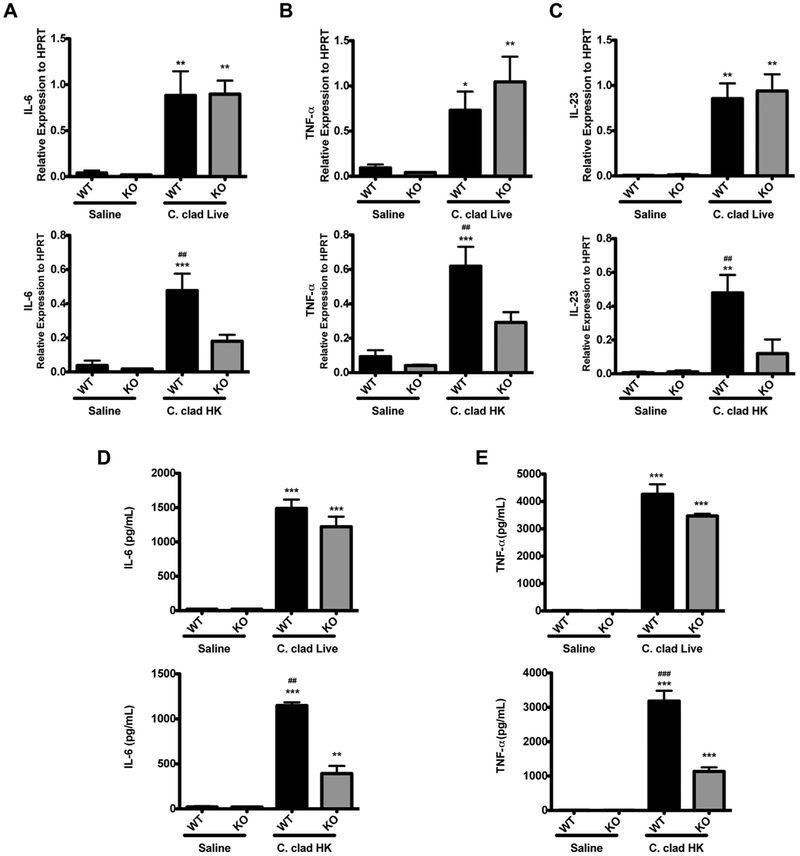
Because we observed altered expression of IL-6 and TNF-α in response to heat-killed spores in Dectin-1−/− mice, we wanted to determine whether the expression of Dectin-1 in dendritic cells could contribute to altered production of TH17-skewing cytokines. Signaling through Dectin-1 on dendritic cells has been shown to induce pro-TH17 factors including TNF-α, IL-6, and IL-23.13,21,35,36 Therefore, we investigated the role of Dectin-1 in the response of dendritic cells to heat-killed versus live C cladosporioides spores. When BMDCs were exposed to live spores, the presence or absence of Dectin-1 did not impact the induction of IL-6, TNF-α, or IL-23 (Fig 7). In contrast, on exposure to heat-killed spores, BMDCs from Dectin-1−/− mice showed significantly attenuated expression of IL-6, IL-23, and TNF-α compared with wild-type mice (Fig 7, A-C). Similarly, in the absence of Dectin-1, BMDCs secreted significantly less IL-6 and TNF-α (Fig 7, D and E). Thus, Dectin-1 promotes the induction of proinflammatory cytokines, but only in response to heat-killed spores that express surface beta-glucans, not in response to live spores.
FIG 7.
Proinflammatory cytokine production in BMDCs is dependent on Dectin-1. Expression of IL-6 (A), TNF-α (B), and IL-23 (C) in BMDCs. Level of IL-6 (D) and TNF-α (E) in culture supernatants of BMDCs. Data are representative of 3 independent experiments and are expressed as mean and SEM. n = 4 samples per group. C clad HK, Heat-killed C cladosporioides; HPRT, hypoxanthine phosphoribosyltransferase; KO, knockout; WT, wild type. *P < .05, **P < .01, and ***P < .001 as compared with saline. ##P < 0.01 and ###P < .001 as compared with Dectin-1−/− BMDCs exposed to C cladosporioides.
DISCUSSION
These data demonstrate that the surface availability of beta-glucans is a critical determinant of the type of inflammatory response induced by specific mold species. Our previous work demonstrated that the distinct pulmonary inflammatory phenotypes are induced by A versicolor and C cladosporioides, common environmental molds. Ccladosporioides induces strong eosinophilic inflammation and AHR through a Dectin-1-independent mechanism, while A versicolor induces neutrophilic inflammation and mild AHR and this is dependent on Dectin-1. In the absence of Dectin-1, A versicolor induces a response similar to that of C cladosporioides, indicating that Dectin-1 inhibits eosinophilic inflammation and AHR in response to A versicolor.30 In this study, we demonstrate that exposure of beta-glucans in C cladosporioides by heat killing induces a neutrophilic inflammatory phenotype characterized by TH17 cytokines similar to that induced by A versicolor. In the absence of Dectin-1, heat-killed C cladosporioides induce strong AHR, eosinophilia, and TH2 cytokines. Thus, the availability of beta-glucans on the surface of a mold, and not the viability of the mold spores, is an important determinant in the type of inflammatory response that ensues.
Furthermore, our data support a mechanistic role for dendritic cells in the observed differences in mold-induced inflammation. The presence or absence of Dectin-1 did not impact the induction of proinflammatory cytokines by live C cladosporioides spores, but induction of proinflammatory cytokines IL-6, IL-23, and TNF-α, all of which have been shown to be important in the development of TH17 inflammatory responses,33,34,37,38 was significantly attenuated in the absence of Dectin-1 following exposure to heat-killed C cladosporioides spores. Despite the production of IL-6, IL-23, and TNF-α dendritic cells after exposure to live C cladosporioides spores, we did not observe a significant increase in TH17 cells in mice exposed to live spores. It is likely that live C cladosporioides spores are inducing these cytokines through a non–Dectin-1−/− pathway that does not contribute to the development of TH17 responses.
Quantification of beta-glucans in the environment is often used as a surrogate for mold exposure.39-41 Several studies have examined the direct relationship between beta-glucans and health effects (reviewed in Douwes7) with inconsistent results. One study found that low levels of beta-glucans were positively correlated with a positive asthma predictive index but high levels of beta-glucans were negatively correlated with a positive asthma predictive index.42 A study in Germany similarly found a negative association between beta-glucan levels in mattress dust and sensitization to aeroallergens.41 Another study also found a negative correlation because there was a trend toward increased IFN-γ in patients exposed to beta-glucans rather than TH2 cytokines.40 Studies examining airway function and inflammation also contradict each other.7 The inconsistencies in the literature may be explained by the fact that it is not the amount of beta-glucans present but rather the surface availability of beta-glucans on the mold spores in the samples taken. Notably, C cladosporioides has a higher beta-glucan content than does A versicolor43; thus, one might predict that C cladosporioides would mediate its effects through Dectin-1 binding; however, C cladosporioides induces a strong asthma phenotype independent of Dectin-1.30 It is the surface availability of beta-glucans that is important for determining the immune response, not the beta-glucan content of spores. This study demonstrates that in the same mold species, increasing the availability of beta-glucans on the surface of the mold spore (without changing the total beta-glucan content) alters the immune response.
Several studies support the concept that exposure of beta-glucans on spore surface modulates the immune response. Heat killing of C albicans exposes the beta-glucans and alters the immune response,26,27 and exposure of beta-glucans in A fumigatus by genetic or chemical depletion alters the immunogenicity of mold spores.25 Our data now add to the current literature and demonstrate that differences in the exposure of surface beta-glucans in the same species of mold can alter the immune response in a Dectin-1-dependent manner. In addition, our data suggest that when quantifying mold exposure, it is important to consider to develop methods that assess the availability of beta-glucans on the molds, in addition to the total content of beta-glucans present. It is intriguing to speculate that one may be able to modify the immune response to molds by altering the beta-glucan availability.
Extended Data
FIG E1.
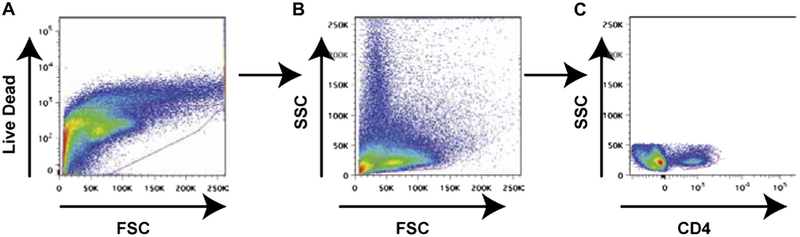
A, Live cells gating with live dead stain (Invitrogen). B, Gating of lymphocytes in the live population based on forward scatter (FSC) and side scatter (SSC). C, CD4 gating from the lymphocyte population.
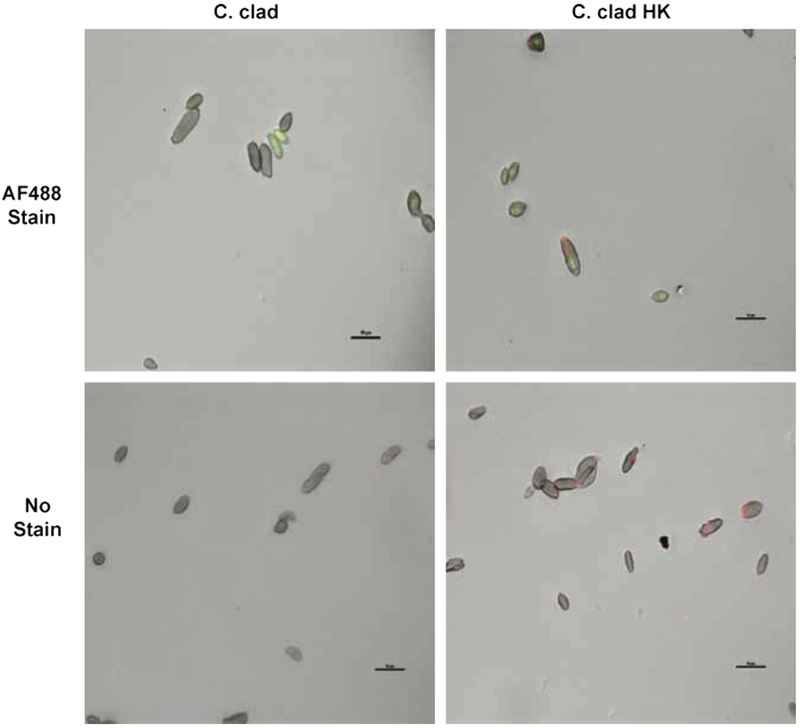
FIG E2.
C clad or C clad HK spores were stained with an AF488 dye (green) or not stained and then incubated with anti-beta glucan and a secondary antibody (red). C clad HK, Heat-killed C cladosporioides.
Key messages.
Heat-killed C cladosporioides spores effectively expose beta-glucans on the surface of spores.
Alteration of beta-glucan surface availability influences the host response to C cladosporioides through differential binding to Dectin-1.
Beta-glucan exposure and signaling through Dectin-1 attenuates the induction of allergic disease, likely through the promotion of TH17 responses.
Acknowledgments
We acknowledge the assistance of the Research Flow Cytometry Core in the Division of Rheumatology at Cincinnati Children’s Hospital Medical Center, supported in part by NIH AR-47363, NIH DK78392, and NIH DK90971, and Dr Gordon Brown for his generous gift of the Dectin-1−/− mice. In addition, we thank Drs Marsha Wills-Karp, Kimberly Risma, Claire Chougnet, and Umasundari Sivaprasad for insightful discussions and Cynthia Chappell for editorial assistance.
This work was supported by NIAID grant no. 2U19AI70235-06 (to G.K.K.H.), NHBLI grant no. F30 HL103087 (to R.A.M.C.), and NIEHS grant no. T32 E5010956 (to E.B.B.).
Abbreviations used
- AHR
Airway hyperresponsiveness
- BALF
Bronchoalveolar lavage fluid
- BMDCs
Bone marrow–derived dendritic cells
Footnotes
Disclosure of potential conflict of interest: R. A. Mintz-Cole has been supported by a Ruth L. Kirschstein NRSA Individual Fellowship. T. Reponen has received one or more grants from or has one or more grants pending with the US HUD (contract from the Centers for Disease Control and Prevention). G. K. Khurana Hershey has received one or more grants from or has one or more grants pending with the National Institutes of Health. The rest of the authors declare that they have no relevant conflicts of interest.
REFERENCES
- 1.Eduard W Fungal spores: a critical review of the toxicological and epidemiological evidence as a basis for occupational exposure limit setting. Crit Rev Toxicol 2009;39:799–864. [DOI] [PubMed] [Google Scholar]
- 2.Belanger K, Beckett W, Triche E, Bracken MB, Holford T, Ren P, et al. Symptoms of wheeze and persistent cough in the first year of life: associations with indoor allergens, air contaminants, and maternal history of asthma. Am J Epidemiol 2003;158:195–202. [DOI] [PubMed] [Google Scholar]
- 3.Halonen M, Stern DA, Wright AL, Taussig LM, Martinez FD. Alternaria as a major allergen for asthma in children raised in a desert environment. Am J Respir Crit Care Med 1997;155:1356–61. [DOI] [PubMed] [Google Scholar]
- 4.O’Driscoll BR, Hopkinson LC, Denning DW. Mold sensitization is common amongst patients with severe asthma requiring multiple hospital admissions. BMC Pulm Med 2005;5:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Black PN, Udy AA, Brodie SM. Sensitivity to fungal allergens is a risk factor for life-threatening asthma. Allergy 2000;55:501–4. [DOI] [PubMed] [Google Scholar]
- 6.Tischer C, Chen CM, Heinrich J. Association between domestic mould and mould components, and asthma and allergy in children: a systematic review. Eur Respir J 2011;38:812–24. [DOI] [PubMed] [Google Scholar]
- 7.Douwes J (1->3)-Beta-D-glucans and respiratory health: a review of the scientific evidence. Indoor Air 2005;15:160–9. [DOI] [PubMed] [Google Scholar]
- 8.Brown GD, Gordon S. Immune recognition: a new receptor for beta-glucans. Nature 2001;413:36–7. [DOI] [PubMed] [Google Scholar]
- 9.Taylor PR, Brown GD, Reid DM, Willment JA, Martinez-Pomares L, Gordon S, et al. The beta-glucan receptor, Dectin-1, is predominantly expressed on the surface of cells of the monocyte/macrophage and neutrophil lineages. J Immunol 2002; 169:3876–82. [DOI] [PubMed] [Google Scholar]
- 10.Ariizumi K, Shen GL, Shikano S, Xu S, Ritter R III, Kumamoto T, et al. Identification of a novel, dendritic cell-associated molecule, Dectin-1, by subtractive cDNA cloning. J Biol Chem 2000;275:20157–67. [DOI] [PubMed] [Google Scholar]
- 11.Tassi I, Cella M, Castro I, Gilfillan S, Khan WN, Colonna M. Requirement of phospholipase C-gamma2 (PLCgamma2) for Dectin-1-induced antigen presentation and induction of TH1/TH17 polarization. Eur J Immunol 2009;39:1369–78. [DOI] [PubMed] [Google Scholar]
- 12.Fuller GL, Williams JA, Tomlinson MG, Eble JA, Hanna SL, Pohlmann S, et al. The C-type lectin receptors CLEC-2 and Dectin-1, but not DC-SIGN, signal via a novel YXXL-dependent signaling cascade. J Biol Chem 2007;282:12397–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dennehy KM, Willment JA, Williams DL, Brown GD. Reciprocal regulation of IL-23 and IL-12 following co-activation of Dectin-1 and TLR signaling pathways. Eur J Immunol 2009;39:1379–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Osorio F, Leibund Gut-Landmann S, Lochner M, Lahl K, Sparwasser T, Eberl G, et al. DC activated via Dectin-1 convert Treg into IL-17 producers. Eur J Immunol 2008;38:3274–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kisand K, Boe Wolff AS, Podkrajsek KT, Tserel L, Link M, Kisand KV, et al. Chronic mucocutaneous candidiasis in APECED or thymoma patients correlates with autoimmunity to Th17-associated cytokines. J Exp Med 2010;207:299–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Puel A, Doffinger R, Natividad A, Chrabieh M, Barcenas-Morales G, Picard C, et al. Autoantibodies against IL-17A, IL-17F, and IL-22 in patients with chronic mucocutaneous candidiasis and autoimmune polyendocrine syndrome type I. J Exp Med 2010;207:291–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Grimbacher B, Holland SM, Gallin JI, Greenberg F, Hill SC, Malech HL, et al. Hyper-IgE syndrome with recurrent infections-an autosomal dominant multisystem disorder. N Engl J Med 1999;340:692–702. [DOI] [PubMed] [Google Scholar]
- 18.Ma CS, Chew GY, Simpson N, Priyadarshi A, Wong M, Grimbacher B, et al. Deficiency of Th17 cells in hyper IgE syndrome due to mutations in STAT3. J Exp Med 2008;205:1551–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ferwerda B, Ferwerda G, Plantinga TS, Willment JA, van Spriel AB, Venselaar H, et al. Human Dectin-1 deficiency and mucocutaneous fungal infections. N Engl J Med 2009;361:1760–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cunha C, Di Ianni M, Bozza S, Giovannini G, Zagarella S, Zelante T, et al. Dectin-1 Y238X polymorphism associates with susceptibility to invasive aspergillosis in hematopoietic transplantation through impairment of both recipient- and donor-dependent mechanisms of antifungal immunity. Blood 2010;116:5394–402. [DOI] [PubMed] [Google Scholar]
- 21.Taylor PR, Tsoni SV, Willment JA, Dennehy KM, Rosas M, Findon H, et al. Dectin-1 is required for beta-glucan recognition and control of fungal infection. Nat Immunol 2007;8:31–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Saijo S, Fujikado N, Furuta T, Chung SH, Kotaki H, Seki K, et al. Dectin-1 is required for host defense against Pneumocystis carinii but not against Candida albicans. Nat Immunol 2007;8:39–46. [DOI] [PubMed] [Google Scholar]
- 23.Werner JL, Metz AE, Horn D, Schoeb TR, Hewitt MM, Schwiebert LM, et al. Requisite role for the Dectin-1 beta-glucan receptor in pulmonary defense against Aspergillus fumigatus. J Immunol 2009;182:4938–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Werner JL, Gessner MA, Lilly LM, Nelson MP, Metz AE, Horn D, et al. Neutrophils produce interleukin 17A (IL-17A) in a Dectin-1 and IL-23-dependent manner during invasive fungal infection. Infect Immun 2011;79:3966–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Aimanianda V, Bayry J, Bozza S, Kniemeyer O, Perruccio K, Elluru SR, et al. Surface hydrophobin prevents immune recognition of airborne fungal spores. Nature 2009;460:1117–21. [DOI] [PubMed] [Google Scholar]
- 26.Gow NA, Netea MG, Munro CA, Ferwerda G, Bates S, Mora-Montes HM, et al. Immune recognition of Candida albicans beta-glucan by Dectin-1. J Infect Dis 2007;196:1565–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gantner BN, Simmons RM, Underhill DM. Dectin-1 mediates macrophage recognition of Candida albicans yeast but not filaments. EMBO J 2005;24:1277–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rivera A, Van Epps HL, Hohl TM, Rizzuto G, Pamer EG. Distinct CD4+-T-cell responses to live and heat-inactivated Aspergillus fumigatus conidia. Infect Immun 2005;73:7170–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hohl TM, Van Epps HL, Rivera A, Morgan LA, Chen PL, Feldmesser M, et al. Aspergillus fumigatus triggers inflammatory responses by stage-specific beta-glucan display. PLoS Pathog 2005;1:e30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mintz-Cole RA, Gibson AM, Bass SA, Budelsky AL, Reponen T, Hershey GK. Dectin-1 and IL-17A suppress murine asthma induced by Aspergillus versicolor but not Cladosporium cladosporioides due to differences in β-glucan surface exposure. J Immunol 2012;189:3609–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lewkowich IP, Lajoie S, Clark JR, Herman NS, Sproles AA, Wills-Karp M. Allergen uptake, activation, and IL-23 production by pulmonary myeloid DCs drives airway hyperresponsiveness in asthma-susceptible mice. PLoS ONE 2008;3:e3879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sivaprasad U, Askew DJ, Ericksen MB, Gibson AM, Stier MT, Brandt EB, et al. A nonredundant role for mouse Serpinb3a in the induction of mucus production in asthma. J Allergy Clin Immunol 2011;127:254–61, 261.e1-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hu W, Troutman TD, Edukulla R, Pasare C. Priming microenvironments dictate cytokine requirements for Thelper 17 cell lineage commitment.Immunity 2011;35:1010–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fei M, Bhatia S, Oriss TB, Yarlagadda M, Khare A, Akira S, et al. TNF-alpha from inflammatory dendritic cells (DCs) regulates lung IL-17A/IL-5 levels and neutrophilia versus eosinophilia during persistent fungal infection. Proc Natl Acad Sci U S A 2011;108:5360–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Steele C, Rapaka RR, Metz A, Pop SM, Williams DL, Gordon S, et al. The beta-glucan receptor Dectin-1 recognizes specific morphologies of Aspergillus fumigatus. PLoS Pathog 2005;1:e42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Goodridge HS, Shimada T, Wolf AJ, Hsu YM, Becker CA, Lin X, et al. Differential use of CARD9 by Dectin-1 in macrophages and dendritic cells. J Immunol 2009;182:1146–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M, et al. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature 2006;441:235–8. [DOI] [PubMed] [Google Scholar]
- 38.Manel N, Unutmaz D, Littman DR. The differentiation of human T(H)-17 cells requires transforming growth factor-beta and induction of the nuclear receptor ROR-gammat. Nat Immunol 2008;9:641–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Iossifova Y, Reponen T, Sucharew H, Succop P, Vesper S. Use of (1-3)-beta-d-glucan concentrations in dust as a surrogate method for estimating specific fungal exposures. Indoor Air 2008;18:225–32. [DOI] [PubMed] [Google Scholar]
- 40.Beijer L, Thorn J, Rylander R. Mould exposure at home relates to inflammatory markers in blood. Eur Respir J 2003;21:317–22. [DOI] [PubMed] [Google Scholar]
- 41.Gehring U, Douwes J, Doekes G, Koch A, Bischof W, Fahlbusch B, et al. Beta(1–>3)-glucan in house dust of German homes: housing characteristics, occupant behavior, and relations with endotoxins, allergens, and molds. Environ Health Perspect 2001;109:139–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Iossifova YY, Reponen T, Ryan PH, Levin L, Bernstein DI, Lockey JE, et al. Mold exposure during infancy as a predictor of potential asthma development. Ann Allergy Asthma Immunol 2009;102:131–7. [DOI] [PubMed] [Google Scholar]
- 43.Iossifova Y, Reponen T, Daines MO, Levin L, Khurana Hershey GK. Comparison of two analytical methods for detecting (1-3)-β-D-glucan in pure fungal cultures and in home dust samples. Open Allergy J 2008;1:26–34. [Google Scholar]