Abstract
Objective:
To evaluate the use of monosyllabic word recognition versus sentence recognition to determine candidacy and long-term benefit for cochlear implantation.
Study Design:
Prospective multi-center single-subject design.
Methods:
A total of 21 adults aged 18 years and older with bilateral moderate to profound sensorineural hearing loss and low monosyllabic word scores received unilateral cochlear implantation. The consonant-nucleus-consonant (CNC) word test was the central measure of pre- and postoperative performance. Additional speech understanding tests included the Hearing in Noise Test sentences in quiet and AzBio sentences +5 dB signal-to-noise ratio (SNR). Quality of life (QoL) was measured using the Abbreviated Profile of Hearing Aid Benefit and Health Utilities Index.
Results:
Performance on sentence recognition reached the ceiling of the test after only 3 months of implant use. In contrast, none of the participants in this study reached a score of 80% on CNC word recognition, even at the 12-month postoperative test interval. Measures of QoL related to hearing were also significantly improved following implantation.
Conclusion:
Results of this study demonstrate that monosyllabic words are appropriate for determining preoperative candidate and measuring long-term postoperative speech recognition performance.
Level of Evidence:
2c.
Keywords: Cochlear implant, speech recognition, quality of life, revised indications, word recognition
INTRODUCTION
Since 1985, indications for adult cochlear implantation have evolved from bilateral profound sensorineural hearing loss with 0% open-set sentence recognition to bilateral moderate to profound sensorineural hearing loss with limited functional benefit from amplification. Limited benefit is currently defined by scores of 50% correct or less in the ear to be implanted (60% or less in the best-aided condition) on recorded tests of sentence recognition.1 These criteria have not been updated since the most recent postmarket approval study (PMA) in 2005 (PMA# P970051/S028).
Technological advancements have allowed cochlear implant (CI) patients to access more acoustic cues and to achieve high levels of speech understanding.2 Current outcomes challenge whether implant candidacy criterion should be broadened once again. In fact, recent work has suggested that sentence recognition may be insufficient for determining CI candidacy3,4 and suggests that word recognition may be a better tool for determining candidacy and measuring long-term benefit from a CI. Even more difficult sentences, such as the AzBio sentence test,5 have sufficient semantic content to allow higher levels of performance compared to monosyllabic words. Another study examining outcomes of 40 implanted postlingually deafened adults6 found that 62% of participants scored above 80% on the AzBio sentences test, whereas only 25% of the same individuals scored above 80% on the consonant-nucleus-consonant (CNC) word test. Speech is a highly complex signal comprised of frequency, intensity, and temporal information. Speech understanding, that is, the degree to which spoken language can be resolved, varies greatly in everyday communication. Factors such as noise, reverberation, distance, speaker style (e.g., rate, dialect, accent), and language proficiency all may contribute to variability in overall speech understanding. Sentence recognition relies heavily on top-down processing, during which listeners uses their available cognitive resources—including their knowledge of the language, topic of conversation, and previous experiences—to fill in missing pieces. The ability to use top-down processing may vary based on things such as linguistic knowledge.7 Sentence recognition scores, therefore, may not accurately reflect how well a person can detect and process the individual spectral and temporal components of speech—an example of bottom-up processing—but rather the ability to fill in missing pieces. Many studies investigating monosyllabic word recognition and sentence recognition in a within-subjects design have demonstrated significantly higher performance levels for sentences as compared to monosyllables.1,4,8–14 Higher sentence scores, as compared to word recognition scores, are even expected for complex sentences containing multiple talkers with lower context such as AzBio in quiet,10–13,15 as well as AzBio sentences at +10 dB signal-to-noise ratio (SNR).16 A primary theory explaining this phenomenon is related to the ease of language understanding17–19 by which incoming speech stimuli are grouped into known phonological representations and cross-checked against the listener’s semantic lexicon. The greater the context for the incoming speech stimulus, the simpler the retrieval process. On the other hand, it is not uncommon for much more difficult sentence measures, such as the TIMIT corpus, to yield equivalent or even lower scores than monosyllables (e.g., King et al.).20 However, the TIMIT sentences are not recognized as the industry standard for assessing pre- and postimplant performance for adult CI users, and thus this relationship does not hold immediate clinical relevance. Given the well-known relationship between word recognition and sentence recognition, even for the AzBio corpus, word recognition therefore may be a better metric for determining how well one can resolve the incoming speech stimulus in the absence of contextual cues.
The field of cochlear implants also sees a need to move beyond tests of speech recognition and evaluate the whole patient. Specifically, the impact on ease of communication and QoL should be addressed. Previous studies have unanimously demonstrated significantly higher health-related quality of life (HRQoL) scores among implanted adults at postoperative compared to preoperative intervals.21–24 To this end, measures of HRQoL are now considered essential in outcome-based research.
The aim of the current study was to determine if word recognition, versus sentence recognition, was a more appropriate tool for measuring candidacy and long-term benefit for cochlear implantation. Specifically, this study aimed to compare word recognition using the CNC monosyllabic word test25 and sentence recognition using both the Hearing in Noise Test (HINT)26 and AzBio sentences5 in both the pre- and postimplant period. The primary hypothesis was that participants would approach ceiling level (≥ 80%) on sentence recognition shortly after activation, whereas performance on CNC word recognition would not reach ceiling level for the majority of participants.
MATERIALS AND METHODS
This was a U.S. Food and Drug Administration (FDA)-approved multi-center, single-arm, repeated measures clinical trial. Recruitment for participants began in 2012. Each participating center obtained institutional review board approval prior to enrolling participants. The 10 participating sites implanted between one and five patients each.
The protocol was initially approved for use of the Cochlear Nucleus Freedom device with Contour Advance electrode [CI24RE(CA); Cochlear Limited, Lake Cove, NSW, Australia]. Shortly after the study began, however, the Cochlear Nucleus CI422 electrode was released, which impacted subject accrual. Thus, this first phase of the revised indications study with CI24RE(CA) was ultimately closed and re-opened using the CI422. The new study protocol using the CI422 did not match the current study; therefore, the data from the two cannot be combined.
Participants
All participants had bilateral moderate to profound sensorineural hearing loss in the low frequencies (up to 1,000 Hz) and profound sensorineural hearing loss at 3,000 Hz and above. Speech recognition criteria required preoperative aided CNC word recognition (mean of two, 50-item lists) between 10% and 40% in the ear to be implanted, and no greater than 50% in the contralateral ear
Informed consent was given by 43 participants, although 22 did not meet the inclusion criteria for the study. There were 11 who had hearing thresholds that fell below (i.e., too good) the inclusion criterion; eight who had conductive components exceeding 15 dB at two or more frequencies; and three who had risk of cognitive decline, as determined by a medical doctor prior to implantation per the clinic’s own protocol and which included open-ended, probing questions regarding temporal and spatial orientation. The final group of 21 participants included 17 males and four females, ranging in age from 32 to 88 years (mean = 77 years). Duration of severe-to-profound high frequency sensorineural hearing loss was determined by patient report or medical records and ranged between 4.1 and 65.2 years, with an average of 25.4 years. These details can be found for each participant in Table I. All participants were implanted with a CI24RE(CA) and used a CP810 sound processor.
TABLE I.
Participant Demographics.
| Subject No | Gender | Ear Implanted | Age at Implant | Duration Deafness | Etiology |
|---|---|---|---|---|---|
| 1 | Male | Left | 79.3 | 19.3 | Noise exposure |
| 2 | Male | Right | 64.4 | 30.4 | Unknown |
| 3 | Male | Right | 87.7 | 32.7 | Noise exposure |
| 4 | Female | Right | 88.0 | 33.0 | Noise exposure |
| 5 | Male | Right | 59.5 | 19.5 | Noise exposure |
| 6 | Male | Right | 83.2 | 18.2 | Unknown |
| 7 | Female | Left | 32.7 | 13.7 | Wolfram’s Syndrome |
| 8 | Female | Left | 72.0 | 13.0 | Unknown |
| 9 | Male | Right | 68.3 | 21.3 | Unknown |
| 10 | Male | Right | 83.0 | 7.39 | Noise exposure |
| 11 | Male | Left | 63.3 | 5.09 | Unknown |
| 12 | Male | Left | 50.5 | 10.5 | Familial |
| 13 | Male | Left | 73.3 | 10.3 | Unknown |
| 14 | Male | Right | 82.0 | 4.0 | Noise exposure |
| 15 | Female | Left | 71.7 | 31.7 | Noise exposure |
| 16 | Male | Right | 79.0 | 29.0 | Noise exposure |
| 17 | Male | Right | 75.9 | 55.9 | Noise exposure |
| 18 | Male | Right | 67.8 | 5.8 | Unknown |
| 19 | Male | Right | 69.1 | 61.1 | Measles |
| 20 | Male | Right | 61.4 | 61.4 | Unknown |
| 21 | Male | Right | 69.0 | 23.0 | Noise exposure |
Materials
Speech understanding in quiet was assessed using CNC words and HINT sentences. The CNC Monosyllabic Word Test is an open-set measure of word recognition consisting of 10 lists of 50 words. The HINT is comprised of 25 lists of 10 sentences. HINT sentences were chosen because they were the industry-standard sentence test for candidacy testing at the time of this protocol approval by the FDA in March, 2011, prior to the release of the adult minimum speech test battery (MSTB, 2011) in July 2011, which specified AzBio sentences for pre- and post-implant assessment. The ability to understand speech in noise (SIN) was assessed using AzBio sentences.5 The AzBio Sentence Test is comprised of 33 lists of 20 sentences, each produced by two male and two female talkers and scored for each word repeated correctly.
Measures used to assess self-perceived benefit included the Abbreviated Profile of Hearing Aid Benefit (APHAB27) and the Health Utility Index Mark 3 (HUI328). The APHAB is a 24-item, self-assessment scored in four subscales: ease of communication, reverberation, background noise, and aversiveness to sounds. The HUI3 is a 15-item, population-based health utility instrument that postulates the domains of health as hearing, vision, speech, emotion, pain, ambulation, dexterity, cognition, and self-care.
Procedures
Speech understanding in quiet was measured using recorded stimuli at a calibrated presentation level of 60 dB sound pressure level (SPL, A-weighted), whereas SIN was measured in a +5 dB SNR with speech at 65 dB SPL(A). The CNC, HINT, and AzBio tests were administered in the unilateral CI-only condition at the baseline appointment and again at 3-, 6-, and 12-months postactivation. The same measures were administered in the bimodal condition, although study protocol excluded bimodal testing of CNC word recognition from the 3-month test interval. The APHAB was administered at both baseline and 6-months postactivation; and the HUI3 was conducted at baseline, 6-months, and 12-months postactivation.
Candidacy testing was completed using hearing aids (personal or loaners) set to National Acoustic Laboratories29 prescriptive targets within 5 dB.
RESULTS
Statistical analyses were completed using IBM SPSS Statistical Package, version 21.0.0 (IBM Corp., Armonk, NY). An alpha level of 0.05 was used to determine statistical significance, though the alpha level was adjusted in cases where Bonferroni corrections were used. The study protocol was completed by all 21 participants at the preoperative and 3-month postactivation interval, 18 participants at the 6-month interval, and 14 participants at the 12-month interval.
Speech Understanding
Speech perception scores were calculated as percent correct and then subjected to an arcsine transformation30 prior to statistical analysis. Data are presented either as percent correct or as rationalized arcsine units (RAU).
CNC Word Test in Quiet.
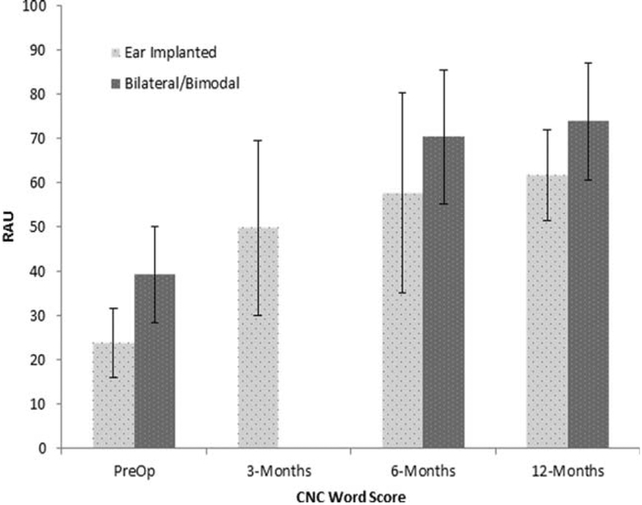
Mean CNC word scores, in percent correct, were 23.6% standard deviation (SD) (11.1%) at the preoperative interval, 49.6% (SD 14.6%) at the 3-month postactivation interval, 57.1% (SD 21.2%) at 6-month postactivation interval, and 65.1% (SD 12%) at the 12-month postactivation interval. Following arcsine transformation, the data were analyzed using a repeated measures analysis of variance (RM-ANOVA) using CNC word score in RAU, as the dependent variable, and test interval (preoperative, 3-, 6-, and 12-months postactivation) as a within-subjects variable. The group averages can be found in Figure 1. Results showed a main effect of test interval, F (3, 36) = 29.0, P < 0.001, r = .76. Te main effect of time interval was followed up with post-hoc pairwise comparisons between each test interval using Bonferroni corrections. Results showed significant improvement in CNC word recognition in the unilateral condition between the preoperative and 3-months postactivation intervals, P < 0.001; between the 3-month and 6-month postactivation intervals, P = 0.002; and between the 3-month and 12-month intervals, P = .001; but not between the 6-month and 12-month postactivation intervals, P = 0.74.
Fig. 1.
Mean and standard deviations for the CNC word test for the pre-operative and 3-, 6-, and 12-months postactivation test intervals. Scores are expressed in RAU.
CNC = consonant-nucleus-consonant. RAU = rationalized arcsine units.
Mean CNC word recognition in the bilateral configuration was 38.7% (SD 11.1) at the preoperative interval, 70.1% (SD 14.6) at 6-months postactivation, and 73.6% (SD 12) at 12-months postactivation. A RM-ANOVA was used to analyze performance over time using the word score in RAU as the dependent variable, and test interval (preoperative, 6-months postactivation, 12-months postactivation) as the within-subjects variable. Results demonstrated a significant main effect of test interval, F(2,22) = 31.9, P < 0.001. Pairwise comparisons using Bonferroni corrections showed significant differences in the bilateral listening condition between the preoperative and 6-month postactivation interval, P = 0.001, and between the 6-month and 12-month postactivation intervals, P = 0.02.
Matched pairs t tests were used to test for CNC word recognition performance differences, in RAU, between unilateral and bilateral configurations. Bonferonni adjustments were used to correct for possible type I error from multiple t tests. Results showed significantly higher performance for the bilateral compared to unilateral conditions at the preoperative interval (t (20) = 5.7, P < 0.001) and 6-month postactivation interval (t (16) = 2.2, P = 0.04), but not the 12-month interval (t (9) = 2.1, P = 0.06).
Hearing in Noise Test in Quiet.
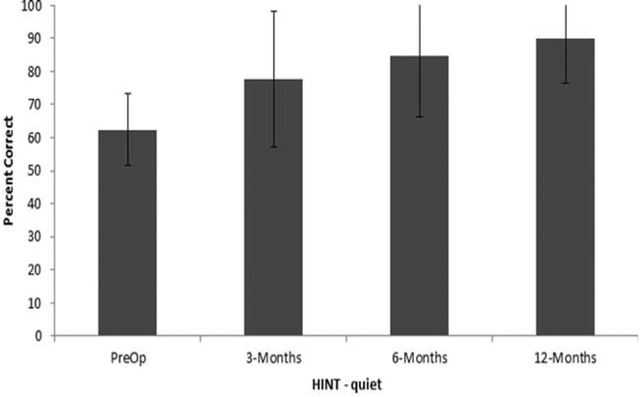
HINT sentence recognition in quiet, in percent correct, is shown in Figure 2. The preoperative group mean was 62.3% (SD = 10.8). The mean scores at the 3-, 6-, and 12-months postactivation intervals were 77.8% (SD = 20.5), 84.6% (SD = 18), and 89.7% (SD = 13.4), respectively. The data, in RAU, were subjected to RM-ANOVA, with time as the within-subjects factor revealing a significant main effect of time, F(3, 36) = 12.0, P < 0.001, r = .5. Pairwise comparisons with Bonferonni corrections showed significant increase in performance at the 3-month test interval compared to the preoperative test interval, P = .01, but no change in performance between the 3- and 6-month interval, P = 0.10, or between the 6-month and 12-month interval, P = 0.27.
Fig. 2.
Mean percent correct and standard deviations for the HINT sentence test in quiet at the preoperative and 3-, 6-, and 12-months postactivation test intervals.
HINT = Hearing in Noise Test.
AzBio Sentence Recognition in Noise.
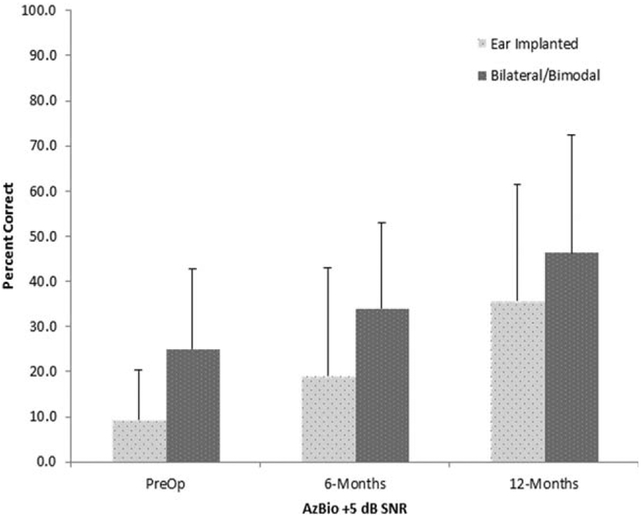
Mean AzBio sentence recognition scores at 15 dB SNR, in percent correct, are shown in Figure 3. Data were analyzed using RM-ANOVA with test interval and listening condition as within-subject factors. The results demonstrated significant main effects of test interval F(2,22) = 9.1, P < 0.001, r = .7, and listening condition, F(1,11) = 19.2, P < 0.001, r = .3; the interaction was not significant, F(2,22) = 0.51, P = 0.60. The main effect of test interval was followed up with pairwise comparisons with Bonferonni corrections, which showed significant improvement for the unilateral condition between preoperative and 6-month postactivation, P = 0.05, and between 6-month and 12-month postactivation, P = 0.03. Results obtained in the bilateral condition were not statistically different between the preoperative (M = 25.1%, SD = 17.1) and 6-month postactivation interval (M = 34.1, SD = 18.9), P = 0.12. Performance in the bilateral condition at the 12-month interval (M = 46.4, SD = 26) was significantly higher than the 6-month interval, P = 0.04.
Fig. 3.
Mean percent correct and standard deviations for the AzBio sentence test in 5 dB SNR for the preoperative, and 6-, and 12-month postactivation test intervals.
SNR = signal-to-noise ratio.
Self-Perceived Benefit
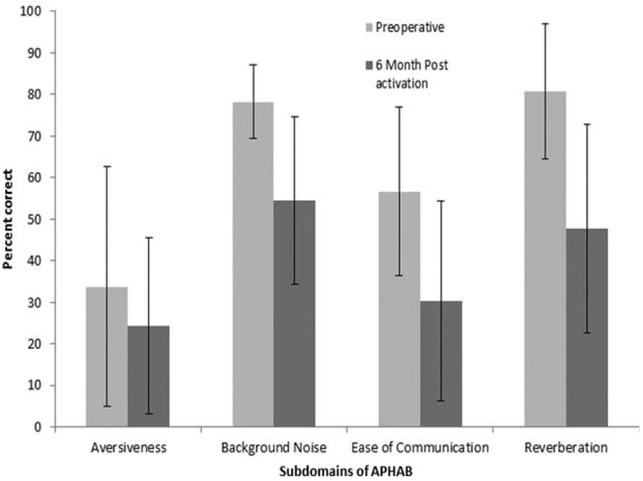
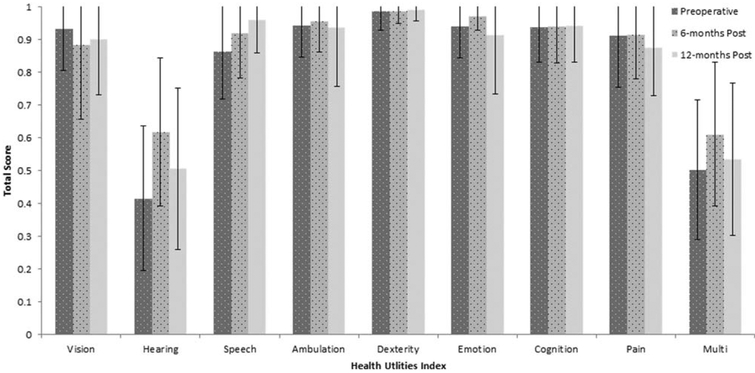
Mean total scores for each domain of the APHAB and HUI are shown in Figures 4 and 5, respectively. The scores from each domain were subjected to paired t test to determine statistical significance. Bonferroni adjustments were used to correct for possible type I error from multiple t tests. Analysis of the APHAB showed that the 6-month postactivation interval was significantly better than the preoperative interval for the domains of aversiveness, t(18) = 2.1, P = 0.05; background noise, t(18) = 5.1, P < 0.001; ease of listening, t(18) = 5.2, P < 0.001; and reverberation, t(18) = 5.8, P < 0.001. Results of the HUI revealed a significant improvement between pre- and 6-months postactivation, but only for the hearing domain, t(18) = −3.2, P < 0.001, and the multifactorial scores t(18) = 5.3, P = 0.03. Neither the hearing t(12) = 1.7, P = 0.27, or multifactorial scores t(12) = 2.4, P = 0.15, changed between the 6-month and 12-month test intervals.
Fig. 4.
Mean percent correct and standard deviations for the four domains of the APHAB at the pre-operative and 6-, and 12-months postactivation test intervals.
APHAB = Abbreviated Profile of Hearing Aid Benefit.
Fig. 5.
Total score and standard deviations for each domain of the Health Utilities Index at the preoperative and 6- and 12-month postactivation test intervals.
DISCUSSION
Overall, this study demonstrated that cochlear implantation yielded significant improvements in speech recognition in quiet and in noise, and self-perceived HRQoL for this group of adults who were evaluated for candidacy using CNC word recognition. The performance outcomes showed a trend for improved performance compared to results published by Balkany et al.,1 with the same internal device but with more restrictive candidacy criteria. In that study, they reported 6-month postoperative CNC and HINT scores of 57.4% and 78.2%, respectively, whereas the current study outcomes were 65.1% and 89.7%, respectively. This suggests that using a criterion of up to 40% CNC word recognition yields at least equivocal, if not higher outcomes, than patients implanted via existing FDA guidelines or up to 30% CNC, as outlined by the original CI24RE(CA) clinical trial (Balkany et al., 2007). Not only does this study suggest that the current indications are too strict, the data provide several examples supporting the need to revise current adult candidacy indications for cochlear implantation from a sentence test to a word test. For example, comparing CNC word and HINT sentence recognition demonstrates that simple sentence recognition tasks (sentences spoken slowly and clearly by a single talker) are not useful for tracking performance over time. Mean HINT sentence recognition improved significantly between the preoperative and 3-month postactivation test interval; however, no further improvements were noted beyond that point. One can easily see that sentence recognition scores quickly hit the ceiling. In fact, 11 of 20 participants (60%) of this study achieved HINT scores above 80% by the 3-month point. In contrast, CNC word recognition continued to improve over the entire first year following activation. Unlike performance on HINT test, not one patient scored above 80% on the CNC word test, even at 12-months postactivation in the unilateral CI condition—although an alternative explanation is that the relatively small sample size may have restricted our outcomes, as other studies have demonstrated unilateral CI recipients achieving ceiling-level performance for CNC word recognition. In these cases, however, we would expect that HINT sentence scores would have also reached ceiling (e.g., Gifford et al.13), and likely at an earlier postoperative time point. Thus, the clinical utility of CNC words for longitudinal postoperative assessment is greater than that of the HINT sentences.
Speech recognition in noise also improved significantly after implantation. In the unilateral condition, AzBio sentence recognition at 15 dB SNR improved from 9.4% preoperatively to 34.8% at 12-months postactivation. Previous studies have examined similar groups of postlingually deafened adults and reported mean AzBio sentence recognition at 15 dB SNR ranging from 49.2% to 58.6%, although most had several years of implant experience.6,31,32
Results from the APHAB were significantly improved at 6-months postactivation compared to the preoperative test interval. This finding is similar to previous reports, such as Skarzynkski et al.,33 who found a significant decrease in self-perceived hearing handicap scores on the APHAB among a group of 10 adult recipients. Regarding the HUI, only scores in the hearing domain improved significantly between the preoperative and 6-month postactivation test interval. Both outcomes are highly valuable. The APHAB provides critical outcome data on a disease-specific scale. The HUI, although more general, indicates that implantation among adults with better preoperative hearing positively influences hearing and does not negatively impact other areas of life, such as vision, dexterity, or cognition.
There are several limitations to the current study that are worth mentioning. First, this study had a low sample size of 23 participants. The effect size of cochlear implantation on CNC word performance proved to be quite high (r = .71), and significant results were obtained despite recruiting a relatively small number of participants. The study was, therefore, adequately powered, although a larger cohort study is needed in order to generalize the findings to the broader population. Another limitation is the use of sentence recognition in quiet was measured using the HINT sentences instead of the industry standard AzBio sentences. As mentioned previously, the HINT sentences were chosen because they were industry standard at the time the study protocol was approved by the FDA. The AzBio sentences are more difficult because they are spoken at a conversational rate and the lists are comprised of multiple talkers. It is possible that performance on AzBio sentences in quiet would not hit the ceiling as quickly as performance on the HINT sentences. However, there is an overall trend for candidacy to be based on single word recognition, as is the current FDA-labeled indications for Hybrid-L24. The trend to use monosyllabic word performance for determining implant candidacy has also been observed in European countries such as France, Germany, and Spain. Therefore, using word, as opposed to sentence recognition to determine candidacy for standard cochlear implantation, aligns well with other labeled indications for cochlear implantation in the United States, as well as being the standard criterion from a more global perspective.34–37
CONCLUSION
Overall, results of the current study show significant benefit of cochlear implantation among a group of postlingually deafened adults who had better preoperative hearing and speech understanding abilities compared to the current FDA candidacy guidelines. Moreover, these results show that the benefits of cochlear implantation reach beyond speech understanding in quiet into speech understanding in noise and QoL. In addition, the current study suggests that CNC word scores, rather than sentence scores, should be used to determine candidacy and measure long-term outcomes for adults with postlingual hearing loss.
Acknowledgments
This research was funded by Cochlear Corporation.
Footnotes
The authors have no other funding, financial relationships, or conflicts of interest to disclose.
BIBLIOGRAPHY
- 1.Balkany T, Hodges A, Menapace C, et al. Nucleus Freedom North American clinical trial. Otolaryngol Head Neck Surg 2007;136:757–762. [DOI] [PubMed] [Google Scholar]
- 2.Holden LK, Finley CC, Firszt JB, et al. Factors affecting open-set word recognition in adults with cochlear implants. Ear Hear 2013;34:342–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gifford RH, Dorman M, Shallop J, Sydlowski S. Evidence for the expansion of adult cochlear implant candidacy. Ear Hear 2010;31:186–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Firszt JB, Holden LK, Skinner MW, et al. Recognition of speech presented at soft to loud levels by adult cochlear implant recipients of three cochlear implant systems. Ear Hear 2004;25:375–87. [DOI] [PubMed] [Google Scholar]
- 5.Spahr AJ, Dorman MF, et al. Development and validation of the Azbio sentence lists. Ear Hear 2012;33:112–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sladen DP, Zappler A. Older and younger adult cochlear implant users: speech recognition in quiet and noise, quality of life, and music perception. Am J Audiol 2014;24:31–39. [DOI] [PubMed] [Google Scholar]
- 7.Gonsalves C, Pichora-Fuller MK. The effect of hearing loss and hearing aids on the use of information and communication technologies by community-living older adults. Can J Aging 2008;27:145–157. [DOI] [PubMed] [Google Scholar]
- 8.Skinner MW, Holden LK, Whitford LA, Plant KL, Psarras C, Holden TA. Speech recognition with the nucleus 24 SPEAK, ACE, and CIS speech coding strategies in newly implanted adults. Ear Hear 2002;23:207–223. [DOI] [PubMed] [Google Scholar]
- 9.Cullen RD, Higgins C, Buss E, Clark M, Pillsbury HC 3rd, Buchman CA. Cochlear implantation in patients with substantial residual hearing. Laryngoscope 2004;114:2218–2223. [DOI] [PubMed] [Google Scholar]
- 10.Carlson ML, Breen JT, Gifford RH, et al. Cochlear implantation in the octogenarian and nonagenarian. Otol Neurotol 2010;31:1343–1349. [DOI] [PubMed] [Google Scholar]
- 11.Gifford RH, Dorman MF, McKarns SA, Spahr AJ. Combined electric and contralateral acoustic hearing: word and sentence recognition with bimodal hearing. J Speech Lang Hear Res 2007;50:835–843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gifford RH, Dorman MF, Spahr AJ, Bacon SP. Auditory function and speech understanding in listeners who qualify for EAS surgery. Ear Hear 2007;28:114S–118S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gifford RH, Shallop JK, Peterson AM. Speech recognition materials and ceiling effects: considerations for cochlear implant programs. Audiol Neurootol 2008;13:193–205. [DOI] [PubMed] [Google Scholar]
- 14.Sheffield SW, Jahn K, Gifford RH. Preserved acoustic hearing in cochlear implantation improves speech perception. J Am Acad Audiol 2015;26: 145–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gifford RH, Driscoll CL, Davis TJ, Fiebig P, Micco A, Dorman MF. A within-subject comparison of bimodal hearing, bilateral cochlear implantation, and bilateral cochlear implantation with bilateral hearing preservation: high-performing patients. Otol Neurotol 2015;36:1331–1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sheffield SW, Gifford RH. The benefits of bimodal hearing: effect of frequency region and acoustic bandwidth. Audiol Neurootol 2014;19: 151–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ronnberg J Cognition in the hearing impaired and deaf as bridge between signal and dialogue: a framework an a model. Int J Audiol 2003;42: S68–S76. [DOI] [PubMed] [Google Scholar]
- 18.Ronnberg J, Lunner T, Zekveld A, et al. The ease of language understanding (ELU) model: theoretical, empirical, and clinical advances. Front Syst Neurosci 2013;13:7–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ronnberg J, Rudner M, Foo C, Lunner T. Cognition counts: a working memory system for ease of language understanding (ELU). Int J Audiol 2008;47:S99–S105. [DOI] [PubMed] [Google Scholar]
- 20.King SE, Firszt JB, Reeder RM, Holden LK, Strube M. Evaluation of TIMIT sentence list equivalency with adult cochlear implant recipients. J Am Acad Audiol 2012;23:313–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Noble W, Tyler RS, Dunn CC, et al. Younger and older-age adults with unilateral and bilateral cochlear implants: speech and spatial hearing self-ratings and performance. Otol Neurotol 2009;30:921–929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Orabi AA, Mawman D, AlZoubi F, et al. Cochlear implant outcomes and quality of life in the elderly: Manchester experience over 13 years. Clin Otolaryngol 2006;31:116–122. [DOI] [PubMed] [Google Scholar]
- 23.Poissant SF, Beaudoin F, Huang J, et al. Impact of cochlear implantation on speech understanding, depression and loneliness in the elderly. J Otolaryngol Head Neck Surg 2008;37:488–494. [PubMed] [Google Scholar]
- 24.Vermeire K, Brokx JP, Wuyts FL, et al. Quality of life benefit from cochlear implantation in the elderly. Otol Neurotol 2005;26:188–195. [DOI] [PubMed] [Google Scholar]
- 25.Peterson FE, Lehiste I. Revised CNC lists for auditory tests. J Speech Hear Disorders 1962;27:62–70. [DOI] [PubMed] [Google Scholar]
- 26.Nilsson M, Soli SD, Sullivan J. Development of the hearing in noise test for the measurement of speech reception thresholds in quiet and in noise. J Acoust Soc Am 1994;95:1085–1099. [DOI] [PubMed] [Google Scholar]
- 27.Cox R, Alexander G. The abbreviated profile of hearing aid benefit. Ear Hear 1995;16:176–186. [DOI] [PubMed] [Google Scholar]
- 28.Furlong W, Feeny D, Torrance GW, Barr RD. The Health Utilities Index (HUI) system for assessing health-related quality of life in clinical studies. Ann Med 2001;33:375–384. [DOI] [PubMed] [Google Scholar]
- 29.Byrne D, Parkinson A, Newall P. Hearing aid gain and frequency response requirements for the severely/profoundly hearing-impaired. Ear Hear 1990;11:40–49. [DOI] [PubMed] [Google Scholar]
- 30.Studebaker GA. A “rationalized” arcsine transform. J Speech Hear Res 1985;28:455–462. [DOI] [PubMed] [Google Scholar]
- 31.Gifford RH, Dorman MF, Sheffield SW, Teece K, Olund AP. Availability of binaural cues for bilateral implant recipients and bimodal listeners with and without preserved hearing in the implanted ear. Audiol Neurotol 2014;19:57–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dorman MF, Gifford RH, Spahr AJ, McKarns SH. The benefits of combin ing acoustic and electric stimulation for the recognition of speech, voice, and melodies. Audiol Neurotol 2008;13:105–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Skarynkski H, Lorens A, Piotrowska A, Anderson I. Partial deafness cochlear implantation provides benefit to a new population of individuals with hearing loss. Acta Otolaryngol 2006;1–7 [DOI] [PubMed] [Google Scholar]
- 34.Aschendorff A, Kromeier J, Klenzer T, et al. Quality control after insertion of the nucleus contour and contour advance electrode in adults. Ear Hear 2007;28:75S–79S. [DOI] [PubMed] [Google Scholar]
- 35.H aumann S, Hohmann V, Meis M, Herzke T, Lenarz T, Buchner A. Indication criteria for cochlear implants and hearing aids: impact of audiological and non-audiological findings. Audiol Res 2012;2:e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Teschner S, Polite C, Lenarz T, Lustig L. Cochlear implantation in different health-care systems: disparities between Germany and the United States. Otol Neurotol 2013;34:66–74. [DOI] [PubMed] [Google Scholar]
- 37.Fraysse B, Dillier N, Klenzner T, et al. Cochlear implant for adults obtaining marginal benefit from acoustic amplification; a European study. Am J Otol 1998;19:591–597. [PubMed] [Google Scholar]