Abstract
NKG2D is an activating immune receptor expressed by NK and effector T cells. Induced expression of NKG2D ligand on tumor cell surface during oncogenic insults renders cancer cells susceptible to immune destruction. In advanced human cancers, tumor cells shed NKG2D ligand to produce an immune soluble form as a means of immune evasion. Soluble NKG2D ligands have been associated with poor clinical prognosis in cancer patients. Harnessing NKG2D pathway is considered a viable avenue in cancer immunotherapy over recent years. In this review, we will discuss the progress and perspectives.
Introduction
NKG2D is an activating immune receptor which regulates both innate and adoptive immune responses. In human, NKG2D is normally expressed on all NK cells, CD8 T cells, subsets of γδ T cells and some autoreactive CD4 T cells [1,2]. NKG2D serves as a major activating receptor for NK cells [3]. Activation of NKG2D provides co-stimulatory signal for CD8 T cells [4], NKT cells and in some cases for γδ T cells.
NKG2D receptor recognizes a broad and structurally diverse range of ligands with differential binding affinities [1,5,6]. Human NKG2D recognizes family of MHC I Chain-related molecules A and B (MICA and MICB, generally termed MIC) and family of six cytomegalovirus UL16-binding proteins (ULBP1–6) [7,8]. NKG2D ligands are generally absent on the surface of normal cells, but often are induced by tumorigenic insults and are further upregulated by chemotherapy or radiation. Expression of NKG2D ligands on tumor cell surface sensitizes tumor cells to immune cell-mediated destruction by engaging NKG2D to activate NK cells and costimulate effector T cells. However, tumor cells frequently escape the immune surveillance of NKG2D pathways by proteolytic-mediated shedding of NKG2D ligands from tumor cell surface or exosome-mediated secretion to release the soluble form of NKG2D ligands [9]. The shed form of NKG2D ligands, sNKG2D-L, has been demonstrated to be highly immune suppressive [2]. High levels of circulating sNKG2D-L were shown to be associated with poor disease prognosis in multiple tumor types, and poor response to immune checkpoint therapy shown in clinical and pre-clinical studies [10●,11●●,12–15]. Currently, there are many ongoing efforts to target the NKG2D pathway for cancer immunotherapy. This review will focus on our understanding of the immune suppressive role of soluble NKG2D ligands and ongoing translational perspectives of targeting NKG2D pathway for cancer immunotherapy. We will also discuss the controversies regarding the impact of NKG2D ligands in tumor immunity.
NKG2D signaling
NKG2D-mediated activation signals can override inhibitory signals by NK inhibitory receptors, hence NKG2D is defined as the master activating NK cell receptor [2,16]. NKG2D does not possess any signaling elements within its intracellular domain. NKG2D forms a homodimer and associates with adaptor proteins in its transmembrane domain to a hexameric complex structure and initiate signaling cascades [17,18]. In both humans and mice, stable NKG2D surface expression requires formation of a complex between NKG2D and DNAX-activating protein of 10 kDa (DAP10) [18,19]. In mice, NKG2D can also transiently associate with ITAM (immunoreceptor tyrosine-based activation motif)-containing DAP12, depending on the activation status of the cells [20,21]. Upon ligand engagement, Tyr-X-X-Meth (YXXM) motif within the cytoplasmic domain of DAP10 recruits PI3K and Grb2 to activate NK cell cytotoxicity pathways. DAP12 on the other hand, signals through an immunoreceptor tyrosine-based activation motif which after phosphorylation recruits ZAP70 and Syk to trigger NK cell effector functions. Crystal structure of the NKG2DNKG2DL complex revealed the rigid adaptation and structural plasticity of NKG2D receptor [22]. Upon binding to its ligands, NKG2D undergoes conformational changes, resulting in different configurations adopted by identical NKG2D residues in the receptor subunits. This plasticity was thought to explain the ability of NKG2D to recognize such structurally diverse ligands [22,23].
NKG2D ligands in human cancer
The expression of NKG2D ligands is tightly regulated to prevent autoimmune tissue damage. Normal tissues generally do not express NKG2D ligands. Although mRNA or intracellular expression of various NKG2D ligands was detected in some non-tumor tissues, such as gut epithelium and airway epithelium [24,25]. Surface expression of these ligands is rare or only at a threshold level unless under pathogenic insults [24,25]. Among the diversified NKG2D ligands, the MIC family molecules are the best characterized ligands and most prevalently expressed ligands by human tumors, among which MICA is more frequently and abundantly expressed on tumor cell surface than MICB (Table 1). The frequency of surface expression of MICB on tumor cells may depend on the status of HCMV-positivity in individuals, since HCMV protein UL16 was shown to bind and retain MICB in the cytoplasm. [26]. The surface expression of ULBP family members is rather sporadic and less well characterized in human tumors. Orthologs of the MIC family ligands are not identified in rodents whereas homologs of human ULBP family members are expressed by rodents [27–31].
Table 1.
NKG2D ligands expression in human cancers
| Tumor type | Ligands identified | Known regulation |
|---|---|---|
| Carcinoma | ||
| • Ovarian | MICA/B, ULBP2 | Shedding |
| • Cervical cancer | MICA | Shedding |
| • Breast | MICA/B HER2/3, | Shedding |
| • Lung | MICA/B | Shedding |
| • Hepatocellular | MICA/B | Viral, retinoic acid |
| • Colon | MICA | Shedding |
| • Renel | MICA, MICB | ? |
| • Prostate | MICA (Hi), ULBP (lo) | Shedding |
| • Pancreatic | MICA/B | ? |
| • Head and neck cancer |
MICA/B | Shedding |
| Leukemia | MICA/B | Methylation |
| Lymphoma | MICA, ULBPS | Shedding |
| Multiple myeloma | MICA | Shedding |
| Melanoma | MICA, ULBP-2 | Shedding |
| Giloma | MICA/B,ULBP1–3 | TGF-b |
| OsteoSarcoma | MICA | ? |
| Neuroblastoma | MICA, ULBP-2 | Releasing soluble form |
Expression of human NKG2D ligands can be regulated at the transcriptional, post-transcriptional, or post-translational levels. Heat shock stress pathway has been implicated specifically in the regulation of MICA/B [32]. DNA damage has been implicated in the induction of NKG2D ligands via DNA damage response ATM and ATR pathway [33]. Post-transcriptional epigenetic modifications with histone deacetylase (HDAC) inhibitors along with DNA damaging low-dose chemotherapy or radiotherapy can upregulate MICA/B expression in an array of human tumor cells [34,35●●,36–39,40●,41●●]. Expression of MICA/B and ULBP3 can also be regulated by endogenous microRNAs [42–44]. At posttranscriptional level, NKG2D ligand expression in tumors is often shed from tumor cell surface to become a soluble form by proteolytic activity or exosomes [9].
High levels of circulating soluble MIC have been associated with poor clinical prognosis and metastasis in multiple cancer types, such as breast, colorectal, ovarian, prostate, lung and other cancers [13,45–47]. Shedding of other family of NKG2D ligands, such as ULBP2 has also been reported to correlate with poor prognosis in selected cancer of solid tumors and hematological malignancies [48–52]. Most recent clinical studies in cohorts of melanoma patients suggested that baseline serum soluble NKG2D ligands may negatively impact clinical outcome of immune checkpoint blockade therapy [10●]. Collectively, these data suggest that circulating soluble NKG2D ligands might be instrumental for cancer prognosis.
The controversy of NKG2D ligands in tumor immunity
The significance of ligand-induced NKG2D activation in controlling tumor initiation or progression was well demonstrated in experimental animal models more than a decade ago [53,54]. Ectopic expression of a NKG2D ligand alone on tumor cells was sufficient to induce tumor rejection, through activation of NK and in some cases CD8 T cells [53,54]. These studies signified the magnitude of ligand-induced activation of NKG2D in controlling tumor. There are ample subsequent studies to support the significance of NKG2D activation in controlling various stages of tumor development [55,56]. Clinically, tumor expression of membrane-bound NKG2D ligands generally correlated with better prognosis [57–59].
Controversy arose over NKG2D ligands in tumor immunity when different experimental model systems were used. In two studies, constitutive expression of membrane-bound NKG2D ligands systemically or in normal epithelium resulted in defects in NKG2D signaling, facilitating increased susceptibility to tumorigenesis [60,61]. On the basis of these observations, an alternative view was proposed indicating that membrane-bound ligands were responsible for the impairment of NKG2D function through chronic stimulation, raising the prospect of negative regulation of tumor immunity by NKG2D ligand expression. Considering the magnitude of NKG2D activation in controlling the survival of NKG2D ligandpositive cells, constitutive expression of NKG2D ligands in normal tissues would demand an inevitable selection process during embryogenesis to tune down NKG2D signaling to avoid NK-cell mediated suicidal signals. To resolve this controversy, Liu et al. took the advantage of the observation that mouse NKG2D recognizes human MICB and generated two lines of transgenic mouse models, TRAMP/MICB.A2 and TRAMP/MICB. The former expresses a mutated membrane-bound MIC form (MICB.A2) restricted in the prostate tumor in TRAMP mice, whereas MICB in TRAMP/MICB mice can be shed by tumors, resembling those in cancer patients. The TRAMP/MICB.A2 mice enjoyed tumor-free survival although the oncogene was expressed in the prostate epithelium, whereas a percentage of TRAMP/MICB mice developed aggressive prostate tumors [62]. Liu et al. further demonstrated that TRAMP/MICB mice with progressive and metastatic tumors all had elevated serum sMICB [62]. The study demonstrated the opposite impact of soluble MIC and membrane-bound MIC on tumor progression in clinically relevant experimental models (Figure 1).
Figure 1: Opposite impact of membrane-bound and soluble NKG2D ligands on tumor immunity.
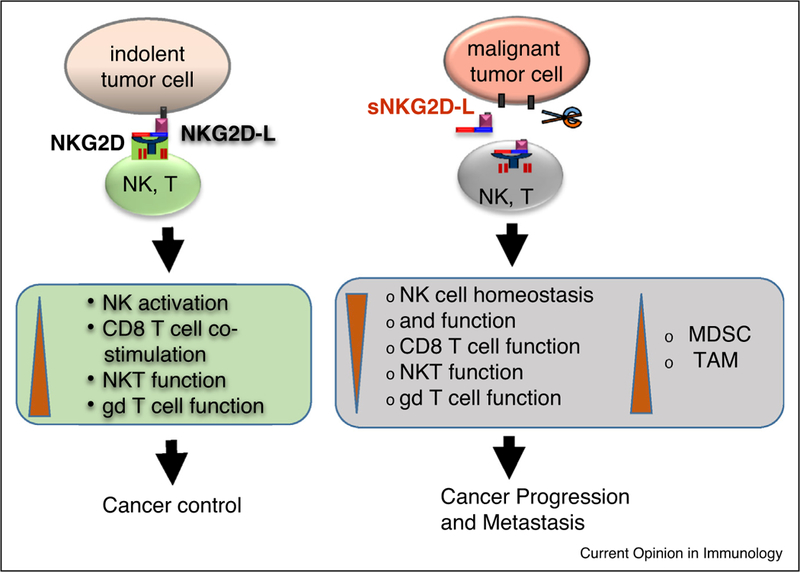
Membrane-bound NKG2D ligands (NKG2D-L) stimulate tumor immunity by activating NK cells, co-stimulating CD8 T cell, NKT cell, and subsets of γδ T cells. Soluble NKG2D ligands (sNKG2D-L) incapacitate tumor immunity through perturbing NK cell hemostasis and function, impairing effector function of CD8 T, NKT, and γδ T cells, and expanding MDSC and TAMS.
Soluble NKG2D as a cancer immunotherapeutic target
Since MIC is the most expressed NKG2D ligand in human tumors, the impact of sMIC on tumor immunity has therefore been studied the most. sMIC was shown to suppress anti-tumor immune responses through multiple mechanisms, such as down-modulating surface NKG2D expression on effector NK and T cells [47,63], destabilizing the critical CD3/TCR signaling component CD3ζ on CD8 T cells [64], perturbing NK cell homeostasis [62], facilitating the expansion of myeloid suppressor cells, and skewing macrophages to alternative tumor-promoting phenotype in the tumor microenvironment [65●]. These findings clearly suggested that targeting pathways related to sMIC release or immune suppressive function might be a viable approach for cancer immunotherapy.
Various approaches have been proposed to target sMIC pathway for cancer immunotherapy. These include blocking tumor shedding of MIC through targeting sequences in the α3 domain of MIC that regulates shedding or the thiol isomerase ERp5 that regulates proteinase access to MIC [66,67], removing plasma sMIC through absorption apheresis to precondition patients for adoptive cell therapy [68●●], and clearing sMIC with monoclonal antibodies [69●●]. The challenge for pre-clinical validation of these approaches is the requirement for genetically engineered mouse model (GEM) which expresses MIC in tumors, since no MIC homolog is found in rodents.
Antibody clearance of sMIC has been tested to be an effective, safe, and feasible therapeutic approach for metastatic cancers. Using the engineered clinically relevant TRAMP/MICB model, Lu et al. demonstrated that clearing sMIC with a monoclonal antibody B10G5 significantly reduced primary tumor burden and eliminated metastasis [69●●]. Mechanistically, B10G5 clearance of sMIC revived and re-invigorated host anti-tumor response by a complex mechanism (Figure 2). It was shown that clearing sMIC stabilized NKG2D surface expression on effector cells, restored NK cell homeostasis and function, overcame tumor-induced tolerance of antigen-specific CD8 T cells, and remodeled tumor microenvironment through elimination of MDSC and arginase+ macrophages [69●●]. Clearing sMIC with B10G5 demonstrated synergistic therapeutic effect with IL-15 agonist ATL-803 and significantly enhanced tumor response to anti-CTLA4 checkpoint blockade therapy in clinically relevant pre-clinical models [11●●,70].
Figure 2: Proposed mechanism of action of clearing sMIC to re-invigorate the anti-tumor responses.
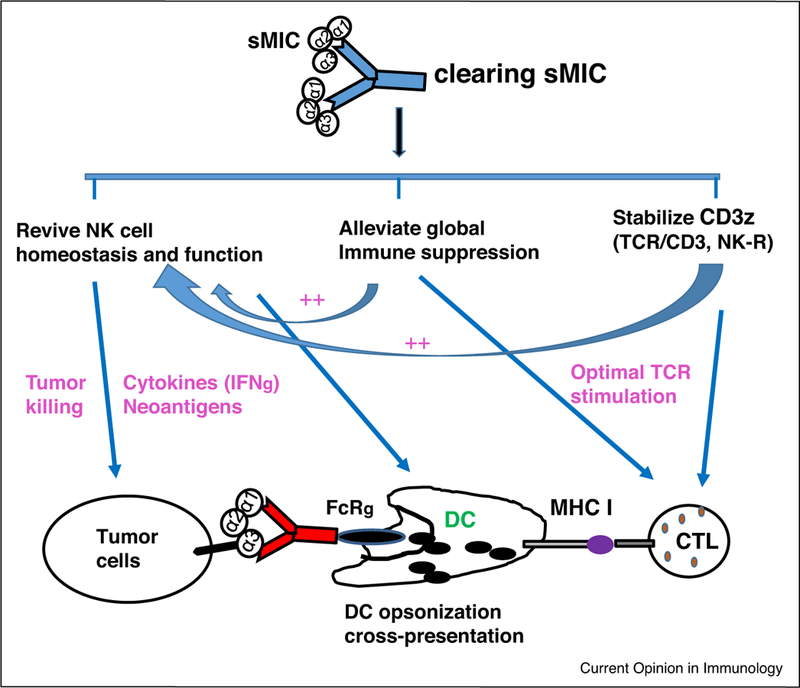
Clearance of sMIC was shown to alleviate global immune suppression, restore NK cell anti-tumor immunity, promote DC maturation, and augment antigen-specific CD8 T cell function.
NKG2D-CAR T cell and NK cell for cancer immunotherapy
NKG2D-CAR-redirected T cells and NK cells are currently being explored and under evaluation for safety and efficacy for targeting NKG2D ligand-positive tumors. Variations of NKG2D-CARs have been generated based on the signaling platforms, whether with DAP10 adaptor molecule or with CD28 or 4–1BB signaling platform [71]. All the NKG2D-CARs contain CD3ζ as the intracellular signaling component. NKG2D-CARs were shown by different research groups to effectively control the growth of a number of tumor types in pre-clinical mouse models, including multiple myeloma [72], ovarian carcinoma [73], and osteosarcoma [74●]. All the NKG2D-CARs presented effective cytotoxicity against tumor cells, but produced different cytokine profiles with DAP10 signaling from CD28 or 4–1BB signaling platform [71]. NKG2D-CARdirected NK cells were shown to greatly enhance NK cell activity in general, compared to NK cells with endogenous NKG2D receptor [75]. Toxicity associated with NKG2D-CAR has been observed in preclinical studies [76], but mostly is related to cytokine release syndrome [77]. NKG2D-CAR T cell therapy with DAP10 signaling platform is currently under clinical evaluation for treating metastatic tumors [78].
Concluding remarks
NKG2D-NKG2DL pathway involves multiple effector cell types in controlling tumor progression and can be enhanced by HDAC inhibitors, low dose chemotherapy or irradiation. In patients with metastatic diseases, NKG2D ligands also exist as highly immune suppressive soluble forms in the circulation. Antibody clearance of soluble NKG2D ligands has recently been demonstrated to be an effective and safe method, alone or in combination with immune checkpoint blockade therapy, in preclinical models to treat metastatic tumors. NKG2D-CARredirected T cell and NK cell therapy for metastatic tumors is currently in clinical evaluation for safety and efficacy. It is expected that targeting soluble NKG2D ligands will be in clinical evaluation in the near future as a single cancer therapy agent or in combination with immune checkpoint blockade therapy. Therapeutic advantage of targeting NKG2D-NKG2DL pathway in combination with HDAC inhibitors or low dose chemotherapy or radiation therapy awaits future investigation.
Acknowledgements
Supported by National Institute of Health (grant number R01CA 208246 and R01CA204021) and DOD USARMC (Award W81XWH150406) to JDW.
Footnotes
Conflict of interest
The authors declare that they have no competing interest.
Recommended Reading
-
•of special interest
-
••of outstanding interest
References
- 1.Raulet DH: Roles of the NKG2D immunoreceptor and its ligands. Nat Rev Immunol 2003, 3:781–790. [DOI] [PubMed] [Google Scholar]
- 2.Zhang J, Basher F, Wu JD: NKG2D ligands in tumor immunity: two sides of a coin. Front Immunol 2015, 6:p97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bauer S et al. : Activation of NK cells and T cells by NKG2D, a receptor for stress-inducible MICA. Science 1999, 285:727–729. [PubMed] [Google Scholar]
- 4.Groh V et al. : Costimulation of CD8alphabeta T cells by NKG2D via engagement by MIC induced on virus-infected cells. Nat Immunol 2001, 2:255–260. [DOI] [PubMed] [Google Scholar]
- 5.Champsaur M, Lanier LL: Effect of NKG2D ligand expression on host immune responses. Immunol Rev 2010, 235:267–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Le Bert N, Gasser S: Advances in NKG2D ligand recognition and responses by NK cells. Immunol Cell Biol 2014, 92:230–236. [DOI] [PubMed] [Google Scholar]
- 7.Eagle RA, Trowsdale J: Promiscuity and the single receptor: NKG2D. Nat Rev Immunol 2007, 7:737–744. [DOI] [PubMed] [Google Scholar]
- 8.El-Gazzar A, Groh V, Spies T: Immunobiology and conflicting roles of the human NKG2D lymphocyte receptor and its ligands in cancer. J Immunol 2013, 191:1509–1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baragano Raneros A, Suarez-Alvarez B, Lopez-Larrea C: Secretory pathways generating immunosuppressive NKG2D ligands: new targets for therapeutic intervention. Oncoimmunology 2014, 3:e28497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.●.Maccalli C et al. : Soluble NKG2D ligands are biomarkers associated with the clinical outcome to immune checkpoint blockade therapy of metastatic melanoma patients. Oncoimmunology 2017, 6:e1323618 This study describes that serum soluble NKG2D-L in melanoma patients before therapy correlated with reduced survival in response to immune checkpoint blockade therapy, suggesting that serum sNKG2D-L can be biomarker to select melanoma patients for immune checkpoint therapy. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.●●.Zhang J et al. : Antibody-mediated neutralization of soluble MIC significantly enhances CTLA4 blockade therapy. Sci Adv 2017, 3:e1602133 Two major findings in this pre-clinical study using clinically relevant animal model: high levels of serum sMIC at base level induce colitis in response to anti-CTLA therapy and confer poor tumor response; and antibody clearing sMIC significantly enhanced tumor response to antiCTLA4 therapy and alleviated colitis. The findings suggest that cancer patients should be pre-screened for serum sMIC to select better responders. The study also endorsed a new combination immunotherapy. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kumar V et al. : Soluble MICA and a MICA variation as possible prognostic biomarkers for HBV-induced hepatocellular carcinoma. PLoS ONE 2012, 7:e44743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tamaki S et al. : Soluble MICB serum levels correlate with disease stage and survival rate in patients with oral squamous cell carcinoma. Anticancer Res 2010, 30:4097–4101. [PubMed] [Google Scholar]
- 14.Vyas M et al. : Soluble NKG2D ligands in the ovarian cancer microenvironment are associated with an adverse clinical outcome and decreased memory effector T cells independent of NKG2D downregulation. Oncoimmunology 2017, 6:e1339854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tamaki S et al. : Association between soluble MICA levels and disease stage IV oral squamous cell carcinoma in Japanese patients. Hum Immunol 2008, 69:88–93. [DOI] [PubMed] [Google Scholar]
- 16.Nausch N, Cerwenka A: NKG2D ligands in tumor immunity. Oncogene 2008, 27:5944–5958. [DOI] [PubMed] [Google Scholar]
- 17.Garrity D et al. : The activating NKG2D receptor assembles in the membrane with two signaling dimers into a hexameric structure. Proc Natl Acad Sci U S A 2005, 102:7641–7646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wu J et al. : An activating immunoreceptor complex formed by NKG2D and DAP10. Science 1999, 285:730–732. [DOI] [PubMed] [Google Scholar]
- 19.Ogasawara K, Lanier LL: NKG2D in NK and T cell-mediated immunity. J Clin Immunol 2005, 25:534–540. [DOI] [PubMed] [Google Scholar]
- 20.Lanier LL et al. : Immunoreceptor DAP12 bearing a tyrosinebased activation motif is involved in activating NK cells. Nature 1998, 391:703–707. [DOI] [PubMed] [Google Scholar]
- 21.Diefenbach A et al. : Selective associations with signaling proteins determine stimulatory versus costimulatory activity of NKG2D. Nat Immunol 2002, 3:1142–1149. [DOI] [PubMed] [Google Scholar]
- 22.McFarland BJ et al. : Symmetry recognizing asymmetry: analysis of the interactions between the C-type lectin-like immunoreceptor NKG2D and MHC class I-like ligands. Structure 2003, 11:411–422. [DOI] [PubMed] [Google Scholar]
- 23.Radaev S et al. : Making sense of the diverse ligand recognition by NKG2D. J Immunol 2002, 169:6279–6285. [DOI] [PubMed] [Google Scholar]
- 24.Kraetzel K et al. : NKG2D-dependent effector function of bronchial epithelium-activated alloreactive T-cells. Eur Respir J 2008, 32:563–570. [DOI] [PubMed] [Google Scholar]
- 25.Hue S et al. : A direct role for NKG2D/MICA interaction in villous atrophy during celiac disease. Immunity 2004, 21:367–377. [DOI] [PubMed] [Google Scholar]
- 26.Wu J et al. : Intracellular retention of the MHC class I-related chain B ligand of NKG2D by the human cytomegalovirus UL16 glycoprotein. J Immunol 2003, 170:4196–4200. [DOI] [PubMed] [Google Scholar]
- 27.Cerwenka A et al. : Retinoic acid early inducible genes define a ligand family for the activating NKG2D receptor in mice. Immunity 2000, 12:721–727. [DOI] [PubMed] [Google Scholar]
- 28.Diefenbach A et al. : Ligands for the murine NKG2D receptor: expression by tumor cells and activation of NK cells and macrophages. Nat Immunol 2000, 1:119–126. [DOI] [PubMed] [Google Scholar]
- 29.Carayannopoulos LN et al. : Cutting edge: murine UL16-binding protein-like transcript 1: a newly described transcript encoding a high-affinity ligand for murine NKG2D. J Immunol 2002, 169:4079–4083. [DOI] [PubMed] [Google Scholar]
- 30.Takada A et al. : Two novel NKG2D ligands of the mouse H60 family with differential expression patterns and binding affinities to NKG2D. J Immunol 2008, 180:1678–1685. [DOI] [PubMed] [Google Scholar]
- 31.Lanier LL: NKG2D receptor and its ligands in host defense. Cancer Immunol Res 2015, 3:575–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Venkataraman GM et al. : Promoter region architecture and transcriptional regulation of the genes for the MHC class Irelated chain A and B ligands of NKG2D. J Immunol 2007, 178:961–969. [DOI] [PubMed] [Google Scholar]
- 33.Gasser S, Raulet DH: Activation and self-tolerance of natural killer cells. Immunol Rev 2006, 214:130–142. [DOI] [PubMed] [Google Scholar]
- 34.Armeanu S et al. : Natural killer cell-mediated lysis of hepatoma cells via specific induction of NKG2D ligands by the histone deacetylase inhibitor sodium valproate. Cancer Res 2005, 65:6321–6329. [DOI] [PubMed] [Google Scholar]
- 35.●●.Miyashita T et al. : Low-dose valproic acid with low-dose gemcitabine augments MHC class I-related chain A/B expression without inducing the release of soluble MHC class I-related chain A/B. Oncol Lett 2017, 14:5918–5926. Major finding: low dose HDAC inhibitor (valproic acid) and low-dose nucleoside analogue chemoreagent synergistically upregulate MIC/A expression in MICA/B-positive pancreatic tumor cells without inducing MIC shedding to sensitize pancreatic tumor to NKG2D-mediated cell cytotoxicity. The findings suggest epigenetic modification of NKG2D pathway may be combined with immunotherapy for treating pancreatic cancer. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shi P et al. : Valproic acid sensitizes pancreatic cancer cells to natural killer cell-mediated lysis by upregulating MICA and MICB via the PI3K/Akt signaling pathway. BMC Cancer 2014, 14:370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wu X et al. : Valproic acid upregulates NKG2D ligand expression through an ERK-dependent mechanism and potentially enhances NK cell-mediated lysis of myeloma. Neoplasia 2012, 14:1178–1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yamanegi K et al. : Sodium valproate, a histone deacetylase inhibitor, augments the expression of cell-surface NKG2D ligands, MICA/B, without increasing their soluble forms to enhance susceptibility of human osteosarcoma cells to NK cell-mediated cytotoxicity. Oncol Rep 2010, 24:1621–1627. [DOI] [PubMed] [Google Scholar]
- 39.Yang F et al. : Valproic acid upregulates NKG2D ligand expression and enhances susceptibility of human renal carcinoma cells to NK cell-mediated cytotoxicity. Arch Med Sci 2013, 9:323–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.●.Nakajima NI et al. : Inhibition of the HDAC/Suv39/G9a pathway restores the expression of DNA damage-dependent major histocompatibility complex class I-related chain A and B in cancer cells. Oncol Rep 2017, 38:693–702. Major finding: inhibitors to HDAC pathway can re-sensitive tumor cells that are non-response to IR-induction of MICA/B expression. The findings suggest that inhibition of HDAC pathway with low-dose IR can potentiate immunotherapy to control tumors. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.●●.Weiss T et al. : NKG2D-dependent anti-tumor effects of chemotherapy and radiotherapy against glioblastoma. Clin Cancer Res 2017. This study demonstrates standard care with temozolomide and radiotherapy induces NKG2D-L expression on primary and recurrent glioblastoma cells and confers survival benefit in pre-clinical models. The study provides a rationale to combine NKG2D-based immunotherapies with TMZ and IR for glioblastoma. [DOI] [PubMed]
- 42.Stern-Ginossar N et al. : Human microRNAs regulate stressinduced immune responses mediated by the receptor NKG2D. Nat Immunol 2008, 9:1065–1073. [DOI] [PubMed] [Google Scholar]
- 43.Breunig C et al. : MicroRNA-519a-3p mediates apoptosis resistance in breast cancer cells and their escape from recognition by natural killer cells. Cell Death Dis 2017, 8:e2973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kishikawa T et al. : Regulation of the expression of the liver cancer susceptibility gene MICA by microRNAs. Sci Rep 2013, 3:2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhao YK et al. : Expression and clinical value of the soluble major histocompatibility complex class I-related chain A molecule in the serum of patients with renal tumors. Genet Mol Res 2015, 14:7233–7240. [DOI] [PubMed] [Google Scholar]
- 46.Holdenrieder S et al. : Soluble MICA in malignant diseases. Int J Cancer 2006, 118:684–687. [DOI] [PubMed] [Google Scholar]
- 47.Wu JD et al. : Prevalent expression of the immunostimulatory MHC class I chain-related molecule is counteracted by shedding in prostate cancer. J Clin Invest 2004, 114:560–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Paschen A et al. : Differential clinical significance of individual NKG2D ligands in melanoma: soluble ULBP2 as an indicator of poor prognosis superior to S100B. Clin Cancer Res 2009, 15:5208–5215. [DOI] [PubMed] [Google Scholar]
- 49.Li K et al. : Clinical significance of the NKG2D ligands, MICA/B and ULBP2 in ovarian cancer: high expression of ULBP2 is an indicator of poor prognosis. Cancer Immunol Immunother 2009, 58:641–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nuckel H et al. : The prognostic significance of soluble NKG2D ligands in B-cell chronic lymphocytic leukemia. Leukemia 2010, 24:1152–1159. [DOI] [PubMed] [Google Scholar]
- 51.Wang R, Sun PD: Natural killer cell-mediated shedding of ULBP2. PLoS ONE 2014, 9:e91133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yamaguchi K et al. : Diagnostic and prognostic impact of serum-soluble UL16-binding protein 2 in lung cancer patients. Cancer Sci 2012, 103:1405–1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cerwenka A, Baron JL, Lanier LL: Ectopic expression of retinoic acid early inducible-1 gene (RAE-1) permits natural killer cellmediated rejection of a MHC class I-bearing tumor in vivo. Proc Natl Acad Sci U S A 2001, 98:11521–11526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Diefenbach A et al. : Rae1 and H60 ligands of the NKG2D receptor stimulate tumour immunity. Nature 2001, 413:165–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Smyth MJ et al. : NKG2D function protects the host from tumor initiation. J Exp Med 2005, 202:583–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Guerra N et al. : NKG2D-deficient mice are defective in tumor surveillance in models of spontaneous malignancy. Immunity 2008, 28:571–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.McGilvray RW et al. : NKG2D ligand expression in human colorectal cancer reveals associations with prognosis and evidence for immunoediting. Clin Cancer Res 2009, 15:69937002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Vetter CS et al. : Expression of stress-induced MHC class I related chain molecules on human melanoma. J Invest Dermatol 2002, 118:600–605. [DOI] [PubMed] [Google Scholar]
- 59.Vetter CS et al. : Loss of nonclassical MHC molecules MIC-A/B expression during progression of uveal melanoma. Br J Cancer 2004, 91:1495–1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Oppenheim DE et al. : Sustained localized expression of ligand for the activating NKG2D receptor impairs natural cytotoxicity in vivo and reduces tumor immunosurveillance. Nat Immunol 2005, 6:928–937. [DOI] [PubMed] [Google Scholar]
- 61.Wiemann K et al. : Systemic NKG2D down-regulation impairs NK and CD8 T cell responses in vivo. J Immunol 2005, 175:720729. [DOI] [PubMed] [Google Scholar]
- 62.Liu G et al. : Perturbation of NK cell peripheral homeostasis accelerates prostate carcinoma metastasis. J Clin Invest 2013, 123:4410–4422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Groh V et al. : Tumour-derived soluble MIC ligands impair expression of NKG2D and T-cell activation. Nature 2002, 419:734–738. [DOI] [PubMed] [Google Scholar]
- 64.Hanaoka N et al. : NKG2D initiates caspase-mediated CD3zeta degradation and lymphocyte receptor impairments associated with human cancer and autoimmune disease. J Immunol 2010, 185:5732–5742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.●.Xiao G et al. : Soluble NKG2D ligand promotes MDSC expansion and skews macrophage to the alternatively activated phenotype. J Hematol Oncol 2015, 8:13 The study demonstrated that sNKG2D-L, sMIC, can skew myeloid cell differentiation to more immune suppressive phenotypes and uncovers a new mechanism whereby sMIC is immune suppressive. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wang X et al. : An six-amino acid motif in the alpha3 domain of MICA is the cancer therapeutic target to inhibit shedding. Biochem Biophys Res Commun 2009, 387:476–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kaiser BK et al. : Disulphide-isomerase-enabled shedding of tumour-associated NKG2D ligands. Nature 2007, 447:482–486. [DOI] [PubMed] [Google Scholar]
- 68.●●.Weil S et al. : Natural killer group 2D ligand depletion reconstitutes natural killer cell immunosurveillance of head and neck squamous cell carcinoma. Front Immunol 2017, 8:387 The study demonstrated that highly levels of sNKG2D-L prevent NK cell infiltration into HNSCC tumors and suggest the removal of plasma sNKG2D-L will pre-condition HNSCC patient for better outcome of cellular cancer immunotherapy. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.●●.Lu S et al. : Nonblocking monoclonal antibody targeting soluble MIC revamps endogenous innate and adaptive antitumor responses and eliminates primary and metastatic tumors. Clin Cancer Res 2015, 21:4819–4830. The study demonstrated that antibody clearance of sMIC can revive host innate and adoptive immune response and debulk established primary tumors and eliminate metastasis. The finding provides proof-of-concept that sMIC is a viable target for cancer immunotherapy. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Basher F et al. : Cooperative therapeutic anti-tumor effect of IL15 agonist ALT-803 and co-targeting soluble NKG2D ligand sMIC. Oncotarget 2016, 7:814–830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sentman CL, Meehan KR: NKG2D CARs as cell therapy for cancer. Cancer J 2014, 20:156–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Barber A et al. : Chimeric NKG2D receptor-expressing T cells as an immunotherapy for multiple myeloma. Exp Hematol 2008, 36:1318–1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Barber A et al. : Chimeric NKG2D receptor-bearing T cells as immunotherapy for ovarian cancer. Cancer Res 2007, 67:50035008. [DOI] [PubMed] [Google Scholar]
- 74.●.Fernandez L et al. : Memory T cells expressing an NKG2D-CAR efficiently target osteosarcoma cells. Clin Cancer Res 2017, 23:5824–5835. Using an orthotopic osteosarcoma preclinical model, this study demonstrated the NKG2D-CAR-directed human CD45RA (4–1BB-CD3 platform) memory T cells exert potent and safe antitumor activity to primary and rechallenged tumors. The findings provide the rationale to treatment patients with osteosarcoma with NKG2D-CAR redirected memory T cells. [DOI] [PubMed] [Google Scholar]
- 75.Chang YH et al. : A chimeric receptor with NKG2D specificity enhances natural killer cell activation and killing of tumor cells. Cancer Res 2013, 73:1777–1786. [DOI] [PubMed] [Google Scholar]
- 76.VanSeggelen H et al. : T cells engineered with chimeric antigen receptors targeting NKG2D ligands display lethal toxicity in mice. Mol Ther 2015, 23:1600–1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sentman ML et al. : Mechanisms of acute toxicity in NKG2D chimeric antigen receptor T cell-treated mice. J Immunol 2016, 197:4674–4685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lonez C et al. : Study protocol for THINK: a multinational openlabel phase I study to assess the safety and clinical activity of multiple administrations of NKR-2 in patients with different metastatic tumour types. BMJ Open 2017, 7:e017075. [DOI] [PMC free article] [PubMed] [Google Scholar]


