Abstract
Purpose
Beyond survival, achieving independence is a primary goal for adult survivors of pediatric CNS tumors. However, the prevalence of and risk factors for failure to achieve independence, assessed with multiple concurrent indicators, have not been examined.
Patients and Methods
Functional and social independence was assessed in 306 survivors (astrocytoma [n = 130], medulloblastoma [n = 77], ependymoma [n = 36], and other [n = 63]; median current age, 25.3 years [range, 18.9 to 53.1 years]; time since diagnosis, 16.8 years [range, 10.6 to 41.8 years]). Six observed indicators were used to identify latent classes of independence, which included employment, living independently, assistance with personal care, assistance with routine needs, obtaining a driver’s license, and marital status. Physical performance impairments were defined as scores < 10th percentile on measures of aerobic capacity, strength, flexibility, balance, mobility, and adaptive function. Multinomial logistic regression estimated odds ratios (ORs) and 95% CIs were calculated for associations of disease/treatment exposures and impairments in physical performance with nonindependence.
Results
Three classes of independence were identified as independent (40%), moderately independent (34%), and nonindependent (26%). In multivariable models, craniospinal irradiation (OR, 4.20; 95% CI, 1.69 to 10.44) and younger age at diagnosis (OR, 1.24; 95% CI, 1.14 to 1.35) were associated with risk of nonindependence versus independence. Beyond impaired IQ, limitations in aerobic capacity (OR, 5.47; 95% CI, 1.78 to 16.76), flexibility (OR, 3.66; 95% CI, 1.11 to 12.03), and adaptive physical function (OR, 11.54; 95% CI, 3.57 to 37.27) were associated with nonindependence versus independence. Nonindependent survivors reported reduced physical but not mental health-related quality of life compared with independent survivors.
Conclusion
Sixty percent of survivors of pediatric CNS tumors do not achieve complete independence as adults. Reduction in intensity of primary therapies and interventions that target physical performance and adaptive deficits may help survivors to achieve greater independence.
INTRODUCTION
CNS tumors account for approximately 20% of malignancies diagnosed in children < 19 years of age, with an incidence of 5.7 per 100,000 in the United States.1 With advances in treatment and supportive care, 5-year survival rates have increased from < 60% in 1980 to approximately 74% today,2 yet the consequences associated with long-term survival of a CNS tumor remain considerable. Deleterious effects of tumor location within the CNS and CNS-directed therapies include increased risk for late mortality, subsequent neoplasms, endocrinopathies, musculoskeletal abnormalities, sensory and neurologic deficits, neurocognitive impairment, and physical performance limitations, among others.3-7
Because large numbers of survivors of CNS tumors are now aging into adulthood, the inability of many to achieve independence is becoming increasingly apparent. Independence can be characterized by participation in functional activities (self-care, independent living), social engagement (partnership/marriage), and the establishment of economic autonomy from caregivers (education, employment). Results from the Childhood Cancer Survivor Study indicate that unemployment is 2.4 times greater among survivors of CNS tumors compared with siblings,8 78% never being married or living as married,9 and 87% less likely to live independently than survivors of Hodgkin Lymphoma.10 The International Classification of Functioning, Disability, and Health recognizes that personal physical factors (ie, physiologic functions of body systems) influence the execution of activities (eg, activities of daily living) and societal participation.11 Survivors of CNS tumors have well-documented personal deficits in strength, balance, and fitness, and these have been associated with discrete aspects of social participation/independence.7 Past studies have examined markers of independence in isolation without considering that individual indicators of independence often co-occur. Identification of specific patterns or degrees of functional independence (eg, independent, nonindependent) and associated factors may help to inform recommendations for clinical care and intervention-based approaches. In addition, although failure to attain independence in adulthood has been associated with poorer quality of life and psychological distress in noncancer populations,12 this has not been examined in survivors of pediatric CNS tumors.
The aims of the current study were to provide a comprehensive assessment of independence in survivors of CNS tumors by identifying profiles of functional and social independence using multiple concurrent indicators, examining the contribution of physical performance status to failure to achieve independence, and assessing the effect on survivors’ health-related quality of life.
PATIENTS AND METHODS
Participants
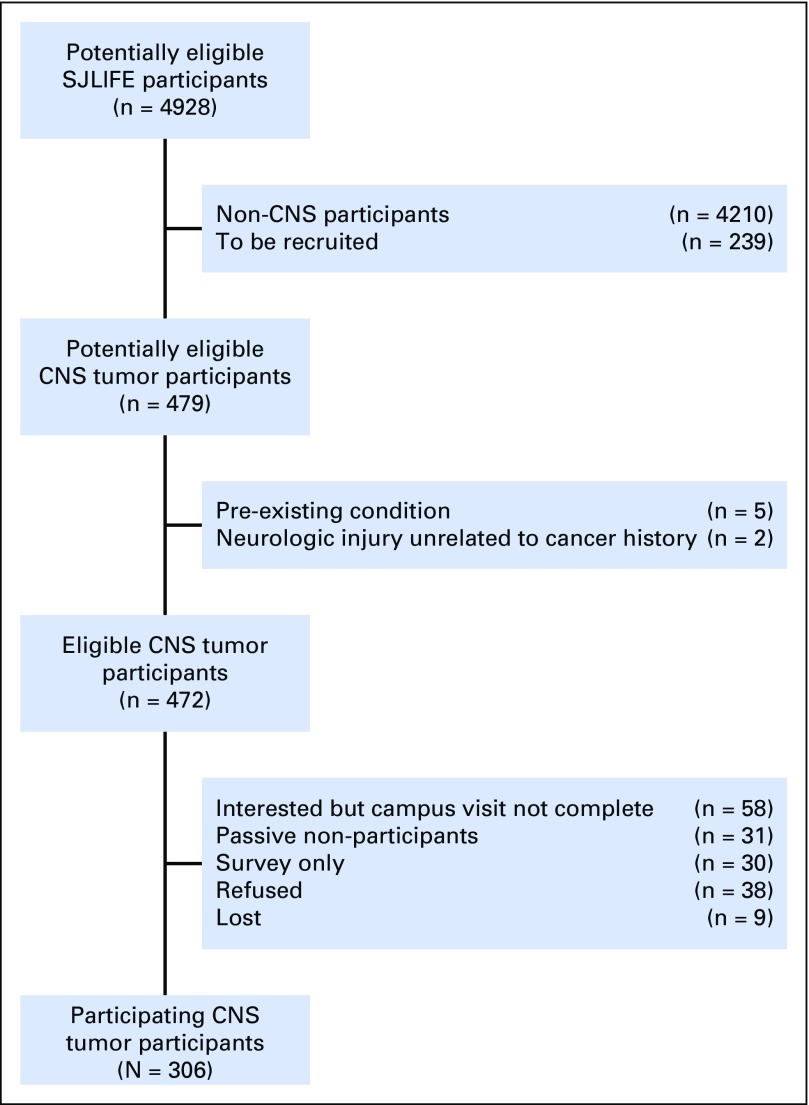
Participants included 306 adult survivors of childhood CNS tumors who completed baseline evaluations as part of the St Jude Lifetime Cohort Study (SJLIFE). SJLIFE—a dynamic cohort of childhood cancer survivors—is designed to facilitate a longitudinal evaluation of health outcomes. The study design has been previously described.13 For these analyses, participants were treated for a CNS tumor at St Jude Children’s Research Hospital (SJCRH), are currently ≥ 18 years of age, and are ≥ 10 years since their original diagnosis. The SJLIFE protocol was approved by the SJCRH institutional review board, and participants provided written informed consent. Survivors were excluded if they had a neurologic disorder or injury unrelated to their primary cancer diagnosis or its treatment and known to affect functional independence (Fig 1).
Fig 1.
Study flow diagram. SJLIFE, St Jude Lifetime Cohort Study.
Primary Outcome
Functional and social independence were assessed by six observed indicators, selected on the basis of individual item face validity, use of similar constructs in past studies, and validated measures.10,14-17 These indicators were defined as independent living (yes: lives with spouse/partner or alone; no: lives with parents or other relatives [not including minor children]); employment (full time: working full time, caring for home or family, student; part time: working part time; unemployed: not currently working, unemployed and looking for work, unable to work because of disability or illness, retired); marital status (never married: single, never married; history of marriage: married, living as married, widowed, divorced, separated or no longer married or living as married); assistance with personal care needs, such as eating, bathing, dressing, or getting around one’s home (yes, no); assistance with routine needs, such as everyday chores, necessary business, shopping, and other purposes (yes, no); and current driver’s license (yes, no). Being unmarried or not living with a partner does not suggest the absence of social relationships but may limit the extent of an individual’s social network. Likewise, not having a driver’s license does not equate to social isolation but suggests greater dependence on others for social engagement.16 Because indicators of independence can be reliably observed by significant others, we accepted responses from surveys that were completed by direct as well as proxy report.
Secondary Outcomes
Quality of life.
Health-related quality of life (HRQOL) was assessed using the Medical Outcomes Survey 36-Item Short Form,18 which provides two composite scores (physical and mental) and subscale scores for eight domains (general health, role physical, physical function, bodily pain, vitality, mental health, social function, and role emotional). Age- and sex-specific normative data were used to calculate T scores (mean, 50; standard deviation [SD], 10). Scores ≤ 40 were classified as poor HRQOL.
Psychological distress.
Psychological distress was measured with the Brief Symptom Inventory 18,19 an 18-item questionnaire that uses a 5-point Likert response format to assess the presence of distress symptoms over the past 7 days. The measure provides an overall index of global psychological distress as well as subscales for anxiety, depression, and somatization. Sex-specific T scores (mean, 50; SD, 10) were calculated using normative data from a sample of adults in the northeastern United States. Scores ≥ 90th percentile (T score ≥ 63) were classified as representing acute distress.
Exposures
Disease and treatment exposures.
Consistent with previously published SJLIFE procedures,13 medical record abstraction was performed to codify disease and treatment exposures, including primary tumor location (infratentorial, supratentorial), surgical resection (none, biopsy, partial, gross total), hydrocephalus with shunt placement, and agent-specific chemotherapeutic exposure (yes, no). Mean radiation dose to four segments of the brain (posterior fossa, temporal, parieto-occipital, frontal) was estimated using established methods by the radiation physicists at MD Anderson Cancer Center.5
Physical performance.
Physical performance measures were completed during a 2-hour dedicated assessment by master’s level–certified clinical exercise physiologists (American College of Sports Medicine) and one physical therapist. This group performs inter- and intrarater reliability yearly for all measures. Participants were given standardized instructions and sufficient rest between tests to account for fatigue. Impairment on each outcome was defined as performance < 10th percentile (> 1.3 SD below the mean) using age- and sex-specific data from community comparisons.20
Aerobic capacity.
Aerobic endurance was assessed with the 6-minute walk test according to American Thoracic Society guidelines.21 Heart rate, oxygen saturation, and the Borg Rating of Perceived Exertion were recorded at baseline; at 2, 4, and 6 minutes, and after a 2-minute recovery. The outcome was distance (in meters) walked in 6 minutes.22
Strength.
Isokinetic knee extension and ankle dorsiflexion strength were measured while sitting (System 4 dynamometer; Biodex Medical Systems, Shirley, NY). Strength was recorded as peak torque (Newton meters per kilogram) of five repetitions at 60° per second. Isometric handgrip strength was measured using a hand-held dynamometer (Jamar; Patterson Medical, Warrenville, IL).23 The maximum value (in kilograms) from two trials was used for analysis.
Flexibility.
Low-back and hamstring flexibility was assessed using a Flex-Tester sit and reach box (Novel Products, Rockton, IL). The better of two trials (in centimeters) was used for analysis.24
Balance.
Balance was evaluated using the Sensory Organization Test (NeuroCom SMART EquiTest, Natus Medical, Pleasanton, CA). Percentage of time spent inside a 12.5° sway envelope was recorded.25,26
Mobility.
The Timed Up and Go test was used to assess mobility. Participants began seated in a standard chair and were instructed to rise, walk 3 m, turn, return to the chair, and sit. Time to complete the test was recorded.27
Adaptive physical function.
The Physical Performance Test,28 seven-item version, was used to evaluate activities of daily living. Participants were timed while writing a sentence, simulating eating, transferring an object to a shelf, dressing, retrieving an object from the floor, standing and turning, and walking. The score out of a maximum of 28 was recorded.
Covariates
Physical health status.
Chronic health conditions were classified using a modified version of the Common Terminology Criteria for Adverse Events (version 4.03) specific to childhood cancer survivors.29 We included the following organ systems (specific variables): cardiac (cardiomyopathy), pulmonary (corrected diffusing capacity of lung for carbon monoxide), endocrine (growth hormone deficiency, luteinizing hormone/follicle-stimulating hormone deficiencies, thyroid-stimulating hormone deficiency, adrenocorticotropic hormone deficiency), and neurologic (stroke, hemiplegia, paraplegia).
Intelligence.
General intelligence was assessed using the matrix reasoning and vocabulary subsets from the Wechsler Abbreviated Scale of Intelligence,30 which provides an abbreviated intelligence score (mean, 100; SD, 15) on the basis of age-specific national normative data. Scores < 10th percentile were considered impaired (standard score ≤ 81). Attention, memory, and executive functions also were assessed; domain-specific impairments were classified using previously published methods in our cohort.
Statistical Analysis
χ2, Fisher’s exact, and t tests were used to compare demographic and treatment characteristics between participants and nonparticipants. Latent class analysis (LCA) using the six indicators of independence was used to identify classes of independence without prespecification of a set number of classes.31,32 Models were fit with one to four classes, and multiple fit indices were evaluated to select the optimal class number, including the Bayesian information criterion (BIC), Vuong-Lo-Mendell-Rubin likelihood ratio test, entropy, and minimum posterior probability.33,34 Optimal fit emphasis was placed on adjusted Bayesian information criterion, adjusted Vuong-Lo-Mendell-Rubin P value, and substantive meaning. We required that the smallest class size be ≥ 10% to permit sufficient power for subsequent multivariable analyses. To confirm the robustness of class selection, we applied a similar LCA approach to a second population of acute lymphoblastic leukemia survivors who also received CNS-directed therapies. The proportion of survivors in each class with observed indicators of independence were examined and compared using χ2 tests. Multivariable log-binomial models were used to examine associations between treatment exposures and physical performance variables with classes of functional independence; odds ratios (ORs) and 95% CIs were calculated. For models that examined associations between classes of independence and quality of life and psychological distress outcomes, adjusted means and standard errors were calculated, and generalized linear regression models were used. A Bonferroni correction was applied to analyses of HRQOL and psychological distress, and P = .0035 was considered significant. Analyses were completed using SAS 9.3 statistical software (SAS Institute, Cary, NC).
RESULTS
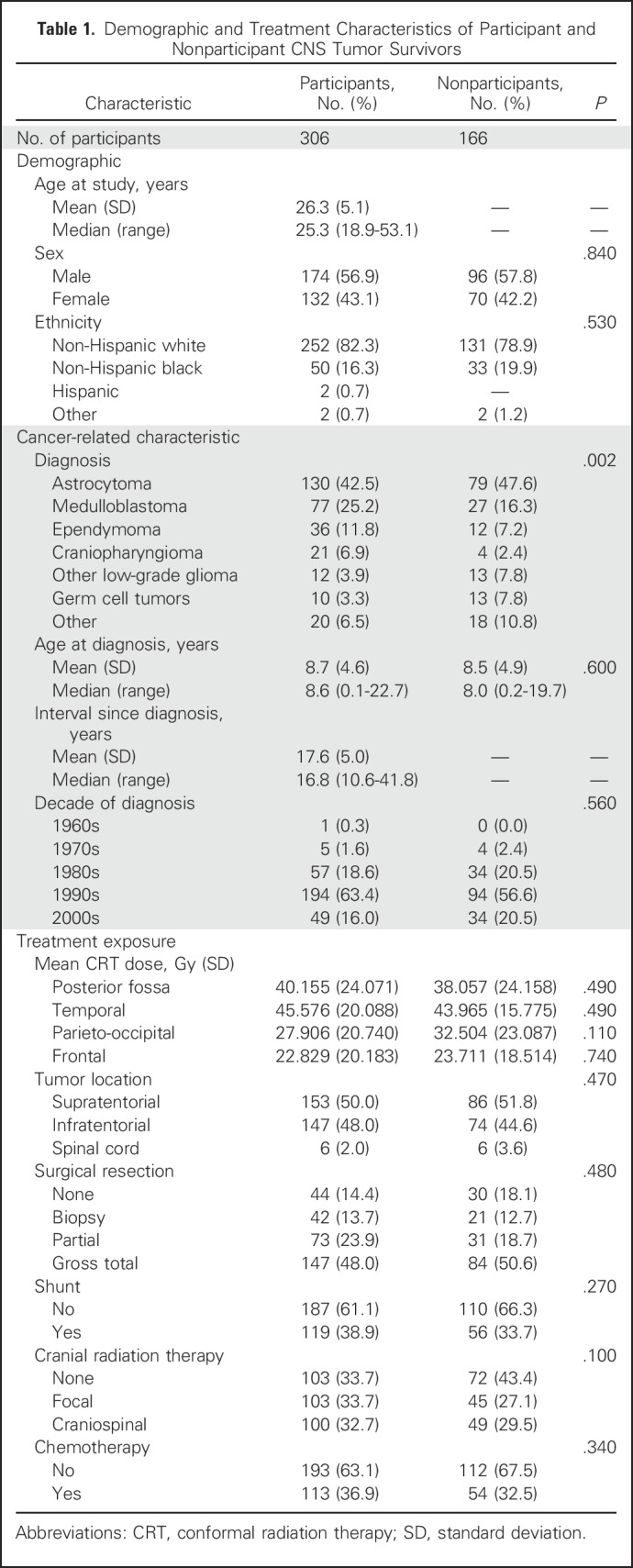
Of 472 potentially eligible CNS tumor survivors, 306 (65%) returned to SJCRH for a risk-based medical evaluation (Fig 1). Participants were, on average, 26.3 years of age and 17.6 years since their original diagnosis and were more likely than nonparticipants to be diagnosed with medulloblastoma (25% v 16%; Table 1). Approximately one third of participants received focal irradiation and one third craniospinal irradiation (CSI). Eighty-one percent of survivors completed study questionnaires independently; 19% were completed by significant other proxy report.
Table 1.
Demographic and Treatment Characteristics of Participant and Nonparticipant CNS Tumor Survivors
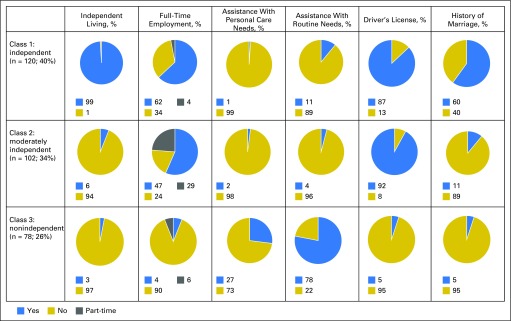
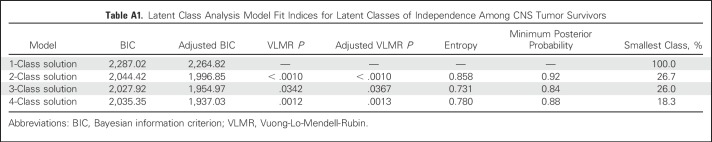
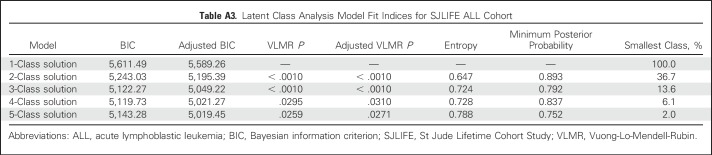
Three meaningful classes of functional independence were identified through LCA and reflected varying degrees of independence: independent (40%), moderately independent (34%), and nonindependent (26%). Appendix Table A1 (online only) lists the model fit indices for one through four classes. A similar three-class structure was observed for survivors of acute lymphoblastic leukemia treated with CNS-directed therapies (Appendix Tables A2 to A4, online only). Figure 2 shows the distribution of the six primary indicators by class of independence. A larger proportion of nonindependent survivors required assistance with personal care (independent, 1%; moderately independent, 2%; nonindependent, 27%; P < .001) or routine needs (independent, 11%; moderately independent, 4%; nonindependent, 78%; P < .001) and did not have a driver’s license (independent, 13%; moderately independent, 8%; nonindependent, 95%; P < .001). However, moderately independent survivors had comparable rates of employment, driving, and independence with routine and personal needs yet differed from those in the independent class in their inability to live alone (P < .001) and history of marriage (P < .001).
Fig 2.
Indicators of functional and social independence by latent classes.
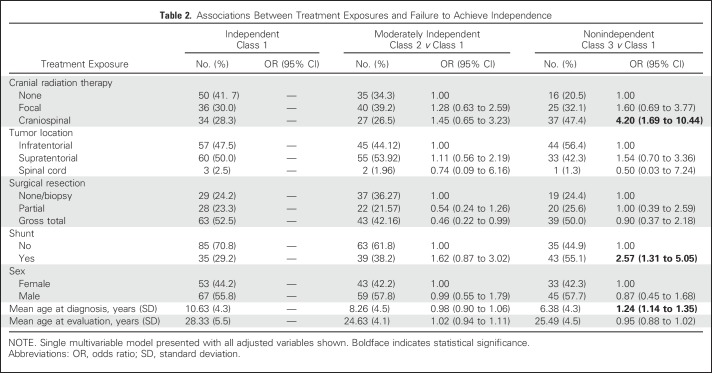
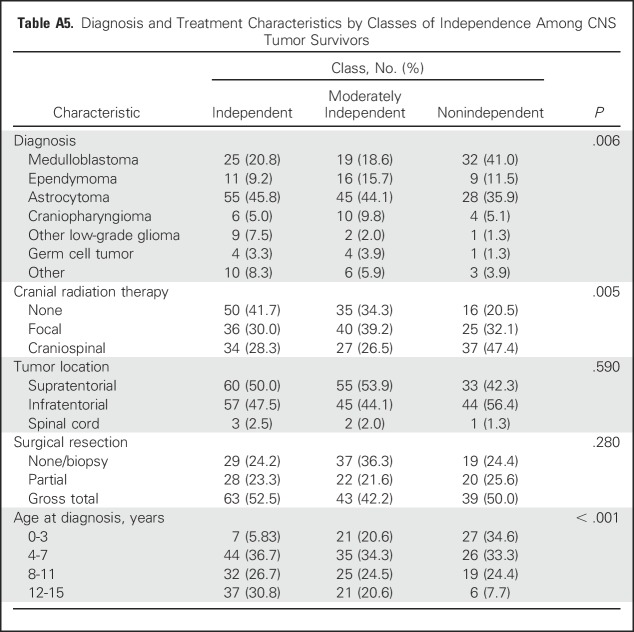
In multivariable models, treatment with CSI was associated with a 4.2-fold increased likelihood of nonindependence (OR, 4.20; 95% CI, 1.69 to 10.44). Hydrocephalus with shunting (OR, 2.57; 95% CI, 1.31 to 5.05) and younger age at diagnosis (OR, 1.24; 95% CI, 1.14 to 1.35) also were associated with an increased likelihood of survivors being nonindependent compared with independent (Table 2). Appendix Table A5 (online only) lists diagnostic and treatment characteristics by latent class. Appendix Table A6 (online only) lists a comparison of class 2 (moderately independent) and class 3 (nonindependent) with respect to treatment exposures.
Table 2.
Associations Between Treatment Exposures and Failure to Achieve Independence
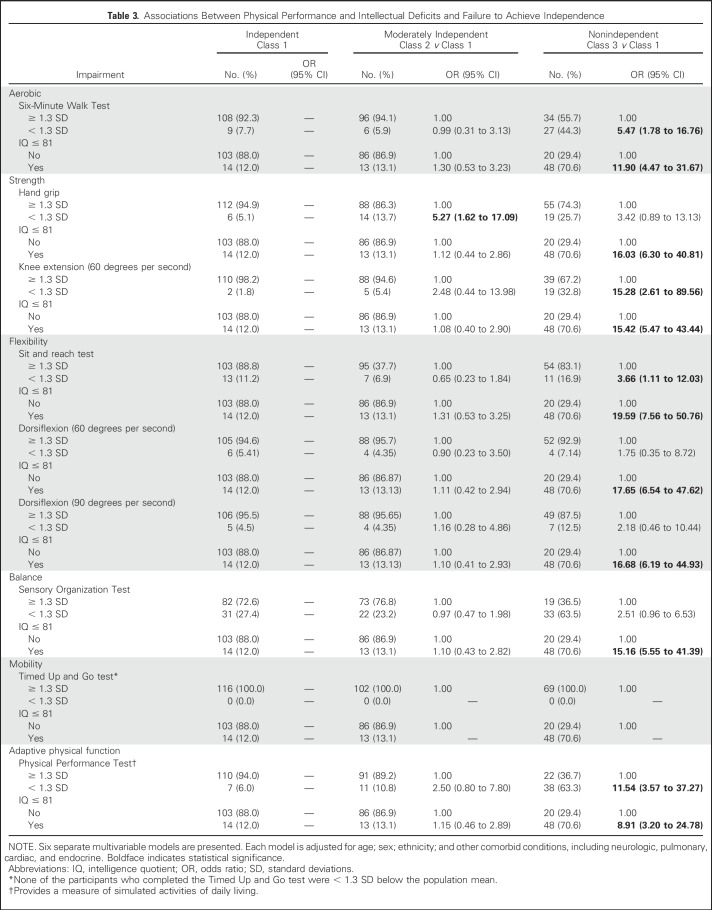
In multivariable models that considered associations between physical performance and failure to achieve independence (Table 3), measures of aerobic capacity (OR, 5.47; 95% CI, 1.78 to 16.76), leg strength (OR, 15.28; 95% CI, 2.61 to 89.56), flexibility (OR, 3.66; 95% CI, 1.11 to 12.03), and adaptive physical function (OR, 11.54; 95% CI, 3.57 to 37.27) were associated with an increased likelihood of nonindependence in survivors. These estimates were observed after adjustment for concurrent chronic health conditions and intellectual impairment. Of note, nonindependent survivors were more likely to have severe impairment in the domains of attention, memory, and executive function (Appendix Table A7, online only). Appendix Table A8 (online only) lists a comparison of class 2 (moderately independent) and class 3 (nonindependent) with respect to physical performance measures.
Table 3.
Associations Between Physical Performance and Intellectual Deficits and Failure to Achieve Independence
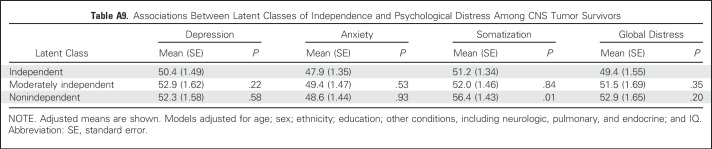
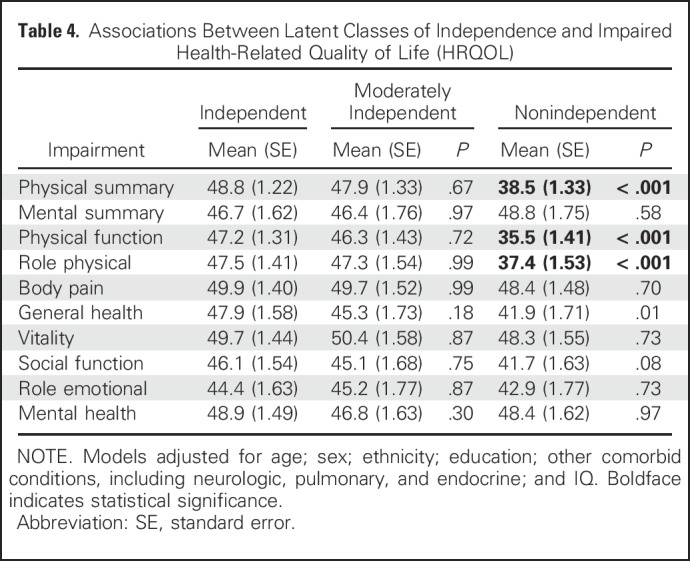
Degree of functional independence was associated with HRQOL but not with emotional distress symptoms (Table 4; Appendix Table A9, online only). Survivors who were nonindependent reported reduced physical HRQOL in some but not all domains (physical function, P < .001; role limitations as a result of general health, P < .001) compared with independent survivors.
Table 4.
Associations Between Latent Classes of Independence and Impaired Health-Related Quality of Life (HRQOL)
DISCUSSION
To our knowledge, this study is the first to comprehensively examine the ability of survivors of childhood CNS tumors to achieve independence as adults. By using multiple concurrent indicators, we identified three phenotypes that reflect varying degrees of functional and social independence. Only 40% of long-term survivors in our population achieved complete independence in adulthood. In this sample, nonindependent survivors were more likely to be living dependently, unemployed, require assistance with personal care and routine needs, unable to drive, and unmarried. However, we identified a substantial proportion (34%) as moderately independent. The identification of interventions and support to help this latter population to achieve autonomy is important as their caregivers, most commonly their parents, age.
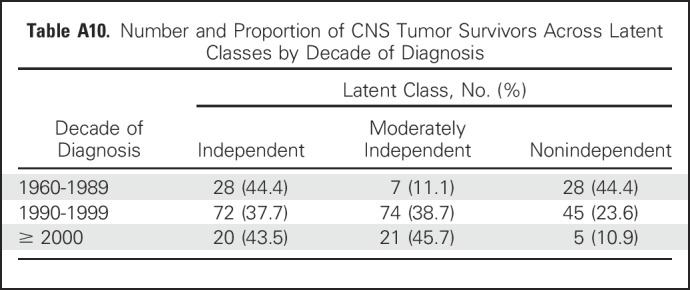
Treatment with CSI conferred a four-fold increased likelihood of nonindependence. This finding is not particularly surprising given the well-established cognitive morbidities associated with CSI, particularly among those exposed at younger ages.6,35 Tumors such as medulloblastomas are now classified by prognostic molecular characteristics. Such improved stratification may allow for the identification of favorable populations, such as the wingless (WNT) subgroup of medulloblastoma, in whom reductions in CSI exposure may be achieved without compromising tumor control. In fact, the current data suggest that a smaller proportion of survivors treated in more recent decades are nonindependent (Appendix Table A10, online only), which potentially corresponds to changes in cranial radiation dose and delivery parameters (ie, three-dimensional conformal radiation therapy).
This analysis identified both new targets (physical performance) and established targets (cognition) for intervention. Moderately independent survivors comprise a group potentially amenable to physical performance interventions designed to promote greater independence because intellectual impairment was largely unrelated to independence in this group, but large effect estimates (ie, ORs ≥ 2.5) were observed for associations between strength and adaptive function. Exercise programs have been shown to have positive effects on independence and adaptive living skills in adults with dementia,36-38 and strength training has been shown to preserve independence in older adults.39 We previously reported that survivors of pediatric brain tumors with physical performance limitations had restricted access to their physical environment.40 Efforts to improve physical performance deficits and reduce individual-level barriers associated with access to the environment may promote greater independence. Vocational rehabilitation efforts may be appropriate for survivors who are unable to acquire employment because childhood cancer survivors who receive job search assistance and on-the-job support are four times more likely to be employed after receipt of such services.41
Physical performance deficits, including aerobic capacity, strength, flexibility, and mobility, were significantly associated with classification in the nonindependent class in which intellectual deficits also had a substantial effect on this poor outcome. More than 60% of survivors in this class showed severe impairment on measures of attention, memory, and executive function, which is consistent with an earlier report from our group; in that report we considered measures of independence separately and found that survivors of CNS tumors with impaired neurocognitive function were less likely to graduate from college and live independently.6 The current results suggest that for this identified group of nonindependent survivors of CNS tumors, multimodal interventions that target both cognitive and physical performance deficits are warranted. In fact, interdisciplinary rehabilitation programs for the treatment of postacute traumatic brain injury in adults are generally effective at improving outcomes.42 However, for this group of survivors, some aspects of independence may be unattainable (eg, driver’s license in the presence of seizures), and interventions may need to focus on long-term supportive care both within and outside the home, including psychosocial support for primary caregivers of these survivors.
The results should be considered in the context of several limitations. A larger proportion of survivors of medulloblastoma were participants than nonparticipants. Because this diagnostic group is most likely to receive CSI, the results may overestimate the prevalence of nonindependence. Conversely, nonparticipants may have been unable to return to SJCRH because of reduced independence, so the results may underestimate the prevalence of nonindependence. Taken together, the effect of nonparticipation on the direction of our effect estimates is unclear. Future research is needed to understand the longitudinal trajectory of independence in survivors as well as the effect of changes in front-line therapies on degree of independence. Because survivors in this sample were a mean age of 26 years, they may acquire greater independence from their primary caregivers as they continue to transition through adulthood; however, with the heighten risk of developing chronic health conditions43 and frailty,44 survivors’ independence also may be compromised as they age. Finally, future research should incorporate the use of validated measures of independence and disability because normative population data may provide additional information about the magnitude of risk of nonindependence among survivors.
In conclusion, the findings show that for children with CNS tumors, 5- or even 10-year survival is not enough. The ultimate goal should be to deliver therapies that maximize both survival and opportunities for functional and social independence throughout the lifespan.
Appendix
Table A1.
Latent Class Analysis Model Fit Indices for Latent Classes of Independence Among CNS Tumor Survivors
Table A2.
Characteristics of SJLIFE ALL Cohort
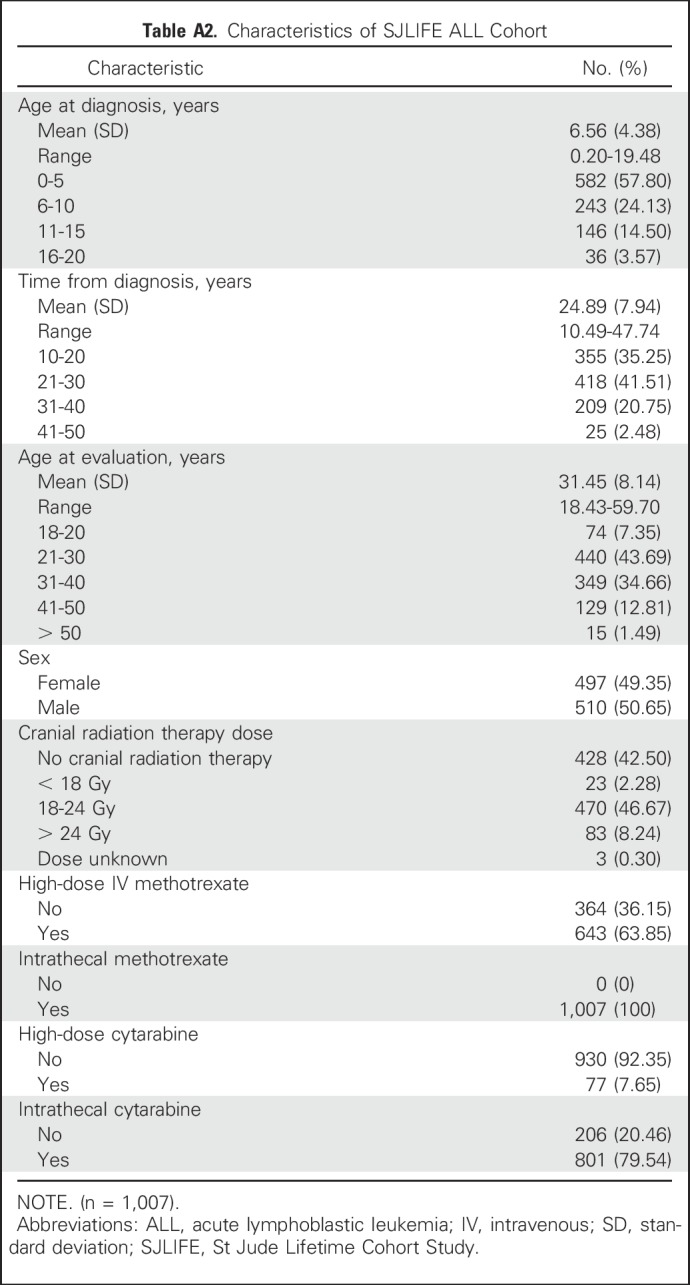
Table A3.
Latent Class Analysis Model Fit Indices for SJLIFE ALL Cohort
Table A4.
Classes of Functional Independence in SJLIFE ALL Cohort
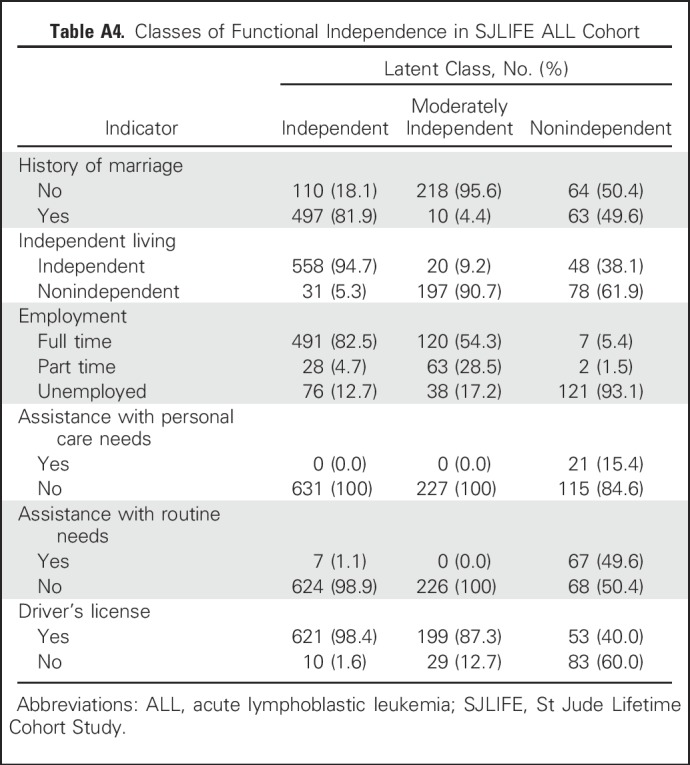
Table A5.
Diagnosis and Treatment Characteristics by Classes of Independence Among CNS Tumor Survivors
Table A6.
Associations Between Treatment Exposures and Failure to Achieve Independence (Class 2 v Class 3) Among CNS Tumor Survivors
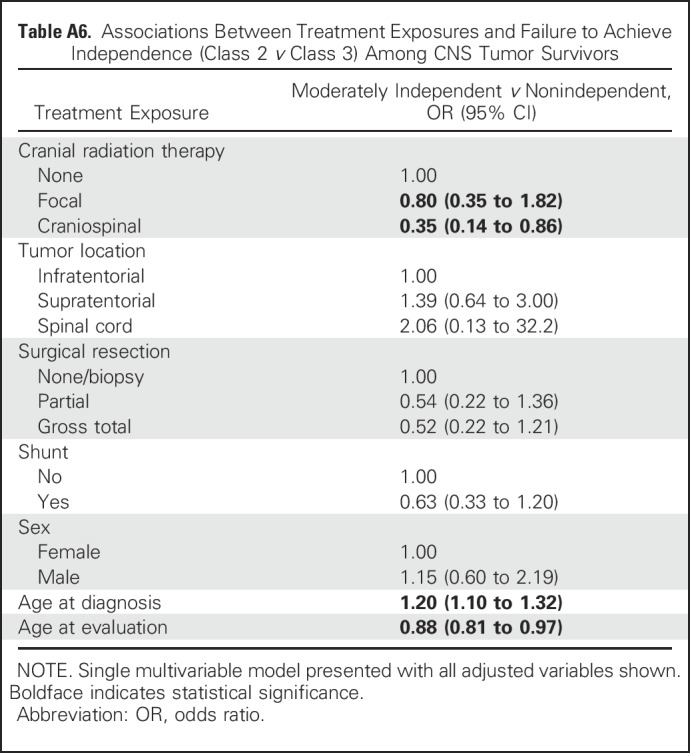
Table A7.
Number and Proportion of CNS Tumor Survivors With Severe Impairment in Attention, Memory, and Executive Function Across Latent Classes
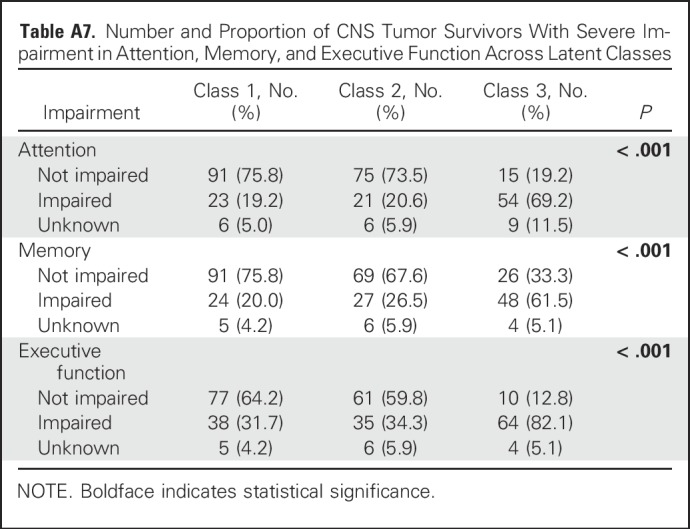
Table A8.
Associations Between Physical Performance and Intellectual Deficits and Failure to Achieve Independence (Class 2 v Class 3) Among CNS Tumor Survivors
Table A9.
Associations Between Latent Classes of Independence and Psychological Distress Among CNS Tumor Survivors
Table A10.
Number and Proportion of CNS Tumor Survivors Across Latent Classes by Decade of Diagnosis
Footnotes
Supported by National Cancer Institute Grant No. CA195547 (L.L.R. and M.M.H., principal investigators). Support to St Jude Children’s Research Hospital also provided by Cancer Center Support (CORE) Grant No. CA21765 (C. Roberts, principal investigator) and the American Lebanese Syrian Associated Charities.
AUTHOR CONTRIBUTIONS
Conception and design: Tara M. Brinkman, Kirsten K. Ness, Leslie L. Robison, Melissa M. Hudson, Gregory T. Armstrong
Financial support: Leslie L. Robison, Melissa M. Hudson
Provision of study materials or patients: Melissa M. Hudson
Collection and assembly of data: Kirsten K. Ness, Kevin R. Krull, Robyn E. Partin, Melissa M. Hudson
Data analysis and interpretation: Tara M. Brinkman, Kirsten K. Ness, Zhenghong Li, I-Chan Huang, Kevin R. Krull, Amar Gajjar, Thomas E. Merchant, James L. Klosky, Ingrid Tonning Olsson, Frederick Boop, Paul Klimo Jr, Wassim Chemaitilly, Raja B. Khan, Deokumar Srivastava, Leslie L. Robison, Melissa M. Hudson, Gregory T. Armstrong
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Attainment of Functional and Social Independence in Adult Survivors of Pediatric CNS Tumors: A Report From the St Jude Lifetime Cohort Study
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/site/ifc.
Tara M. Brinkman
No relationship to disclose
Kirsten K. Ness
No relationship to disclose
Zhenghong Li
No relationship to disclose
I-Chan Huang
No relationship to disclose
Kevin R. Krull
Patents, Royalties, Other Intellectual Property: Royalties from Wolters Kluwer
Amar Gajjar
Research Funding: Genentech (Inst)
Thomas E. Merchant
No relationship to disclose
James L. Klosky
No relationship to disclose
Robyn E. Partin
No relationship to disclose
Ingrid Tonning Olsson
No relationship to disclose
Frederick Boop
Consulting or Advisory Role: Medtronics
Paul Klimo Jr
No relationship to disclose
Wassim Chemaitilly
Honoraria: Pfizer
Consulting or Advisory Role: Pfizer
Raja B. Khan
No relationship to disclose
Deokumar Srivastava
No relationship to disclose
Leslie L. Robison
No relationship to disclose
Melissa M. Hudson
Consulting or Advisory Role: Coleman Supportive Oncology Initiative for Children With Cancer, Oncology Research Information Exchange Network, Princess Máxima Center
Gregory T. Armstrong
No relationship to disclose
REFERENCES
- 1.Ostrom QT, Gittleman H, Xu J, et al. CBTRUS statistical report: Primary brain and other central nervous system tumors diagnosed in the United States in 2009-2013. Neuro Oncol. 2016;18(suppl_5):v1–v75. doi: 10.1093/neuonc/now207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Howlader N, Noone AM, Krapcho M, et al: SEER Cancer Statistics Review, 1975-2014. Bethesda, MD, National Cancer Institute, 2017. [Google Scholar]
- 3.Armstrong GT, Liu Q, Yasui Y, et al. Long-term outcomes among adult survivors of childhood central nervous system malignancies in the Childhood Cancer Survivor Study. J Natl Cancer Inst. 2009;101:946–958. doi: 10.1093/jnci/djp148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chemaitilly W, Li Z, Huang S, et al. Anterior hypopituitarism in adult survivors of childhood cancers treated with cranial radiotherapy: A report from the St Jude Lifetime Cohort study. J Clin Oncol. 2015;33:492–500. doi: 10.1200/JCO.2014.56.7933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Packer RJ, Gurney JG, Punyko JA, et al. Long-term neurologic and neurosensory sequelae in adult survivors of a childhood brain tumor: Childhood Cancer Survivor Study. J Clin Oncol. 2003;21:3255–3261. doi: 10.1200/JCO.2003.01.202. [DOI] [PubMed] [Google Scholar]
- 6.Brinkman TM, Krasin MJ, Liu W, et al. Long-term neurocognitive functioning and social attainment in adult survivors of pediatric CNS tumors: Results from the St. Jude Lifetime Cohort Study . J Clin Oncol. 2016;34:1358–1367. doi: 10.1200/JCO.2015.62.2589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ness KK, Morris EB, Nolan VG, et al. Physical performance limitations among adult survivors of childhood brain tumors. Cancer. 2010;116:3034–3044. doi: 10.1002/cncr.25051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kirchhoff AC, Leisenring W, Krull KR, et al. Unemployment among adult survivors of childhood cancer: A report from the Childhood Cancer Survivor Study. Med Care. 2010;48:1015–1025. doi: 10.1097/MLR.0b013e3181eaf880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Janson C, Leisenring W, Cox C, et al. Predictors of marriage and divorce in adult survivors of childhood cancers: A report from the Childhood Cancer Survivor Study. Cancer Epidemiol Biomarkers Prev. 2009;18:2626–2635. doi: 10.1158/1055-9965.EPI-08-0959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kunin-Batson A, Kadan-Lottick N, Zhu L, et al. Predictors of independent living status in adult survivors of childhood cancer: A report from the Childhood Cancer Survivor Study. Pediatr Blood Cancer. 2011;57:1197–1203. doi: 10.1002/pbc.22982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. World Health Organization: International Classification of Functioning, Disability, and Health (ICF). Geneva, Switzerland, World Health Organization, 2001. [Google Scholar]
- 12.Williamson ML, Elliott TR, Berry JW, et al. Predictors of health-related quality-of-life following traumatic brain injury. Brain Inj. 2013;27:992–999. doi: 10.3109/02699052.2013.801512. [DOI] [PubMed] [Google Scholar]
- 13.Hudson MM, Ness KK, Nolan VG, et al. Prospective medical assessment of adults surviving childhood cancer: Study design, cohort characteristics, and feasibility of the St. Jude Lifetime Cohort study. Pediatr Blood Cancer. 2011;56:825–836. doi: 10.1002/pbc.22875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Koch SV, Kejs AM, Engholm G, et al. Leaving home after cancer in childhood: A measure of social independence in early adulthood. Pediatr Blood Cancer. 2006;47:61–70. doi: 10.1002/pbc.20827. [DOI] [PubMed] [Google Scholar]
- 15.Khan F, Amatya B. Factors associated with long-term functional outcomes, psychological sequelae and quality of life in persons after primary brain tumour. J Neurooncol. 2013;111:355–366. doi: 10.1007/s11060-012-1024-z. [DOI] [PubMed] [Google Scholar]
- 16.Corrigan JD, Cuthbert JP, Harrison-Felix C, et al. US population estimates of health and social outcomes 5 years after rehabilitation for traumatic brain injury. J Head Trauma Rehabil. 2014;29:E1–E9. doi: 10.1097/HTR.0000000000000020. [DOI] [PubMed] [Google Scholar]
- 17. World Health Organization: Measuring Health and Disability. Manual for WHO Disability Assessment Schedule. Geneva, Switzerland, World Health Organization, 2010. [Google Scholar]
- 18.Ware JE, Jr, Sherbourne CD. The MOS 36-Item Short-Form Health Survey (SF-36): I. Conceptual framework and item selection. Med Care. 1992;30:473–483. [PubMed] [Google Scholar]
- 19.Derogatis L. Brief Symptom Inventory - 18 (BSI - 18) - Administration, Scoring, and Procedures Manual. Minneapolis, MN: NCS Pearson; 2000. [Google Scholar]
- 20.Ness KK, DeLany JP, Kaste SC, et al. Energy balance and fitness in adult survivors of childhood acute lymphoblastic leukemia. Blood. 2015;125:3411–3419. doi: 10.1182/blood-2015-01-621680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. American Thoracic Society: ATS statement: Guidelines for the six-minute walk test Am J Respir Crit Care Med 166:111–117.2002 [DOI] [PubMed] [Google Scholar]
- 22.Cole CR, Blackstone EH, Pashkow FJ, et al. Heart-rate recovery immediately after exercise as a predictor of mortality. N Engl J Med. 1999;341:1351–1357. doi: 10.1056/NEJM199910283411804. [DOI] [PubMed] [Google Scholar]
- 23.Mathiowetz V, Weber K, Volland G, et al. Reliability and validity of grip and pinch strength evaluations. J Hand Surg Am. 1984;9:222–226. doi: 10.1016/s0363-5023(84)80146-x. [DOI] [PubMed] [Google Scholar]
- 24.American College of Sports Medicine . ACSM’s Guidelines for Exercise Testing and Prescription. Baltimore, MD: Lippincott Williams & Wilkins; 2013. [DOI] [PubMed] [Google Scholar]
- 25.Ford-Smith CD, Wyman JF, Elswick RK, Jr, et al. Test-retest reliability of the sensory organization test in noninstitutionalized older adults. Arch Phys Med Rehabil. 1995;76:77–81. doi: 10.1016/s0003-9993(95)80047-6. [DOI] [PubMed] [Google Scholar]
- 26. Nashner LM: Computerized dynamic posturography, in Jacobson GP, Newman CW, Kartush JM (eds): Handbook of Balance Function Testing. Norwich, UK, Singular, 1993, p 280-307. [Google Scholar]
- 27.Kear BM, Guck TP, McGaha AL: Timed Up and Go (TUG) Test: Normative reference values for ages 20 to 59 years and relationships with physical and mental health risk factors. J Prim Care Community Health 10.1177/2150131916659282 [epub ahead of print on July 22, 2016] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Reuben DB, Siu AL. An objective measure of physical function of elderly outpatients. The Physical Performance Test. J Am Geriatr Soc. 1990;38:1105–1112. doi: 10.1111/j.1532-5415.1990.tb01373.x. [DOI] [PubMed] [Google Scholar]
- 29.Hudson MM, Ehrhardt MJ, Bhakta N, et al. Approach for classification and severity grading of long-term and late-onset health events among childhood cancer survivors in the St. Jude Lifetime Cohort. Cancer Epidemiol Biomarkers Prev. 2017;26:666–674. doi: 10.1158/1055-9965.EPI-16-0812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wechsler D. Wechsler Abbreviated Scale of Intelligence. San Antonio, TX: Psychological Corporation; 1999. [Google Scholar]
- 31.Jung T, Wickrama K. An introduction to latent class growth analysis and growth mixture modeling. Soc Personal Psychol Compass. 2008;2:302–317. [Google Scholar]
- 32. McCutcheon AL: Latent Class Analysis. Newbury Park, CA, Sage, 1987. [Google Scholar]
- 33.Tein JY, Coxe S, Cham H. Statistical power to detect the correct number of classes in latent profile analysis. Struct Equ Modeling. 2013;20:640–657. doi: 10.1080/10705511.2013.824781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. https://www.statmodel.com/examples/webnotes/webnote14.pdf Asparouhov T, Muthén B: Using Mplus TECH11 and TECH14 to test the number of latent classes, 2012.
- 35.Mulhern RK, Palmer SL, Merchant TE, et al. Neurocognitive consequences of risk-adapted therapy for childhood medulloblastoma. J Clin Oncol. 2005;23:5511–5519. doi: 10.1200/JCO.2005.00.703. [DOI] [PubMed] [Google Scholar]
- 36.Forbes D, Thiessen EJ, Blake CM, et al. Exercise programs for people with dementia. Cochrane Database Syst Rev. 2013;(12):CD006489. doi: 10.1002/14651858.CD006489.pub3. [DOI] [PubMed] [Google Scholar]
- 37.Barnes DE, Mehling W, Wu E, et al. Preventing Loss of Independence Through Exercise (PLIÉ): A pilot clinical trial in older adults with dementia. PLoS One. 2015;10:e0113367. doi: 10.1371/journal.pone.0113367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Toots A, Littbrand H, Lindelöf N, et al. Effects of a high-intensity functional exercise program on dependence in activities of daily living and balance in older adults with dementia. J Am Geriatr Soc. 2016;64:55–64. doi: 10.1111/jgs.13880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. doi: 10.1016/s0749-3797(03)00177-6. Seguin R, Nelson ME: The benefits of strength training for older adults. Am J Prev Med 25:141-149, 2003 (suppl 2) [DOI] [PubMed] [Google Scholar]
- 40.Brinkman TM, Li Z, Neglia JP, et al. Restricted access to the environment and quality of life in adult survivors of childhood brain tumors. J Neurooncol. 2013;111:195–203. doi: 10.1007/s11060-012-1001-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Strauser D, Feuerstein M, Chan F, et al. Vocational services associated with competitive employment in 18-25 year old cancer survivors. J Cancer Surviv. 2010;4:179–186. doi: 10.1007/s11764-010-0119-9. [DOI] [PubMed] [Google Scholar]
- 42.Lu J, Gary KW, Neimeier JP, et al. Randomized controlled trials in adult traumatic brain injury. Brain Inj. 2012;26:1523–1548. doi: 10.3109/02699052.2012.722257. [DOI] [PubMed] [Google Scholar]
- 43.Bhakta N, Liu Q, Ness KK, et al. The cumulative burden of surviving childhood cancer: An initial report from the St Jude Lifetime Cohort Study (SJLIFE) Lancet. 2017;390:2569–2582. doi: 10.1016/S0140-6736(17)31610-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ness KK, Krull KR, Jones KE, et al. Physiologic frailty as a sign of accelerated aging among adult survivors of childhood cancer: A report from the St. Jude Lifetime Cohort Study. J Clin Oncol. 2013;31:4496–503. doi: 10.1200/JCO.2013.52.2268. [DOI] [PMC free article] [PubMed] [Google Scholar]