Abstract
Although a fundamental goal of developmental science is to identify general processes of change, developmental scientists rarely generalize beyond their specific content domains. As a first step toward a more unified approach to development, we offer 15 suggestions gleaned from a century of research on infant walking. These suggestions collectively address the multi-leveled nature of change processes, cascades of real-time and developmental events, the diversity of developmental trajectories, inter- and intraindividual variability, starting and ending points of development, the natural input for learning, and the roles of body, environment, and sociocultural context. We argue that these 15 suggestions are not limited to motor development, and we encourage researchers to consider them within their own areas of research.
Infant Walking as a Model System for Development
Development is tricky. A primary goal of developmental research is to identify general processes of change. However, generalities are elusive because researchers must focus on particular behaviors in particular contexts. Researchers must study the development of something – moral reasoning, face perception, language, or whatnot. The difficulty arises in generalizing beyond the specific details of the particular behavior in question to concepts, methods, and theories that hold across content domains.
Since the 1920s researchers have used infant motor skill acquisition as a window into general developmental processes [1–7]. Infants’ motor behaviors are an especially promising model system because movements are directly observable and occur over multiple, nested time-scales [8]. In contrast to the covert nature of most psychological functions, motor actions occur out in the open. Whereas infants’ thoughts, percepts, emotions, and linguistic representations must be inferred, the form and timing of their movements are directly accessible. Moreover, researchers can observe change in infants’ movements in real time and over development – the millisecond changes in joint angles and foot trajectory over a single step, the step-to-step changes across a walking path, and the changes in walking skill over months of practice.
15 Suggestions for Developmental Research
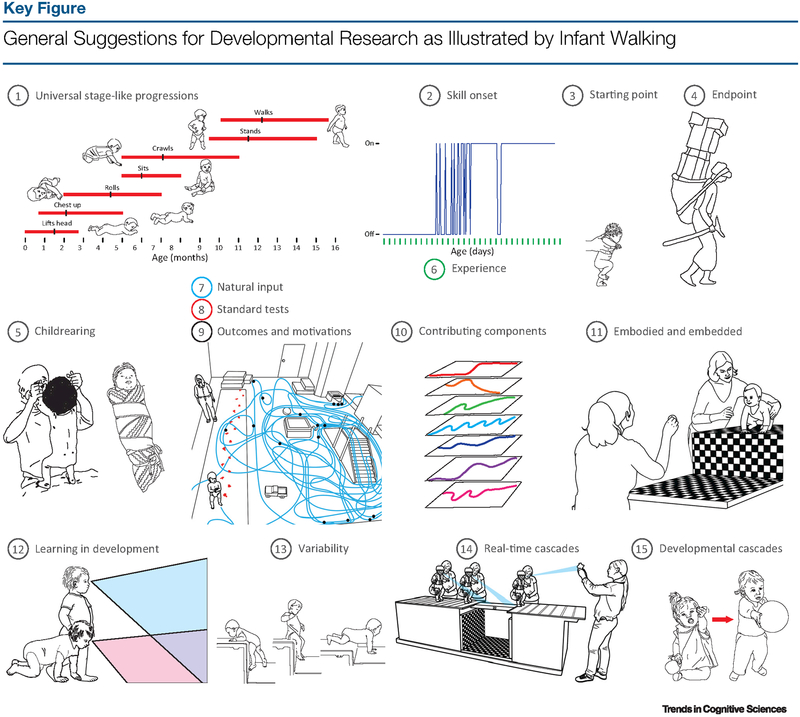
We offer 15 suggestions for the study of development gleaned from a century of research on infant walking (Figure 1, Key Figure). We do not presume that this list is exhaustive or that every suggestion holds true for every developmental phenomenon. However, we do propose that these suggestions have implications for phenomena far outside the realm of infant walking and even outside the domain of motor development (Box 1 provides a demonstration of how each suggestion applies to the development of language). Some of our suggestions build on those of previous authors; others differ radically.
Figure 1.
Numbers correspond to suggestions. (1) Universal stage-like progressions do not represent individual development: motor milestone chart. (2) Skill onset is not an on–off switch: variable developmental trajectory derived from daily observation. (3) Starting points are arbitrary: newborn stepping. (4) Endpoints are arbitrary: woman carrying a heavy load without incurring increased energetic cost. (5) Childrearing alters the onset age and form of skill acquisition: infant exercise and restraint. (6) Experience is not the mere passage of time: the x axis depicts the number of days since skill onset. (7) Natural input shapes learning: the blue line depicts the path of a typical infant during free play. (8) Standard tests are not natural activity: compare red infant footsteps over the gait carpet to blue line in spontaneous walking. (9) Developmental outcomes are not real-time motivations: infants often stop walking without reaching a destination or goal (see location of dots on blue line). (10) Many developing components contribute to skill acquisition: abstraction of multiple, interacting components, each with its own developmental trajectory. (11) Behavior happens in a body in an environment: infant deciding whether and how to descend at the edge of an adjustable drop-off. Possibilities for walking depend on infants’ leg length, balance, and strength relative to drop-off height. (12) Learning occurs in the context of development: overlay of infants’ field of view while crawling and walking.(13) Variability is inherent to development: infants use different strategies to descend a high drop-off (e.g., backing, scooting, crawling). (14) Behavior is a cascade of real-time events: exploratory activity as an infant approaches and navigates an obstacle. (15) New skills instigate a cascade of developmental events: a crawler’s stationary bid for a caregiver’s attention, and a walker’s moving bid for a caregiver’s attention.
Box 1. Walking and Talking.
Our 1 suggestions based on the development of walking also hold true for the development of talking.
-
(1)
Universal Stage-Like Progressions. As in motor development, researchers have identified developmental stages or ‘milestones’ in language production (e.g., cooing, babbling, one-word utterances, simple multi-word utterances, and finally grammatically complete sentences). Children can skip language milestones, revert to earlier forms, display overlap among forms, and display behaviors that are not on the standard milestone chart (e.g., combined word–gesture ‘utterances’) [121,122].
-
(2)
Skill Onset Is Not an On–Off Switch. Infant language development is not continuous. Infants may produce a word one day, but not the next [122].
-
(3/4)
Starting and Ending Points Are Arbitrary. Are infants’ first words the starting point for expressive language? Or is babbling the starting point? Or the instantiation of the vocal cords? Is the endpoint being able to hold a conversation? Or to engage in elaborate narratives? What about children who are learning two languages? Do different starting and ending points exist for each language?
-
(5)
Childrearing Alters the Onset Age and Form of Skill Acquisition. The amount and quality of language input – tokens, types, contingency, embedded clauses – shape the timing and rate of growth in the vocabulary and grammar of children [123,124].
-
(6/7)
Experience Is Not the Mere Passage of Time; Natural Input Shapes Everyday Learning. A large literature and shared corpora document the natural input for language learning [125]. Current work emphasizes language input in the context of everyday activities in the home [126].
-
(8)
Standard Tests Are Not Natural Activity. Laboratory observations of language input suggest that infants experience high, consistent, and responsive language, with few bouts of silence. However, during everyday home routines, the same infants experience fluctuating input, including long bouts of silence [126].
-
(9)
Developmental Outcomes Are Not Real-Time Motivations. Infants do not set out to build their vocabularies or to learn grammar. These long-term developmental outcomes are the result of many individual speech acts. Moreover, although language ultimately supports communication, conversing with others is not always the immediate goal – infants narrate events aloud, speak to objects, and talk or babble even when no one else is around [127].
-
(10)
Many Developing Components Contribute to Skill Acquisition. The onset of speech and communication is supported by myriad developing systems: the ability to perceive sights and sounds, the development of the articulatory apparatus, improvements in memory, capacity for categorization, motivation to communicate, acquisition of language tools (vocabulary), and more [128].
-
(11)
Behavior Happens in a Body in an Environment. Language is contextual. Speakers quickly and easily change the way they speak to suit the current environment (e.g., home versus school) or their speaking partner (e.g., adult versus child, Spanish versus English speaker) [128].
-
(12)
Learning Occurs in the Context of Development. In the moments around speaking, newly talking infants decrease their emotional expressions to concentrate their efforts on forming words. Later, more-experienced talkers decrease both their speech and emotional expressions to focus on object construction during play [129].
-
(13)
Variability Is Inherent to Development. The onset of speech and relative rates of vocabulary growth are highly variable between children. Within individuals, children may produce some words one day, but not the next [122– 124,128].
-
(14)
Behavior Is a Cascade of Real-Time Events. When infants vocalize, parents respond, and in turn infants react and change their behavior [130]. When parents’ social responses are delayed or decoupled, infants’ vocalizations are less mature [131].
-
(15)
New Skills Instigate a Cascade of Developmental Events. After children learn to talk, they gain access to new and more nuanced aspects of the social world. With this new skill, children can express their emotions, make requests, and ask questions [128,132].
Suggestion 1: Universal Stage-Like Progressions Do Not Represent Individual Development
From newborn to toddler, infants progress from lying with their face in the carpet to running across the floor. Early pioneers Gesell and McGraw recognized the pervasive variability, overlapping strategies, regressions, and individual differences in the form and timing of skill acquisition [1,2,4,5]. However, adherence to a theory of neuromuscular maturation led them to view development through a ‘filter’ that obscured the messy details. Instead of representing the development of individuals, they characterized the route to walking as a series of universal, age-related stages that are still widely depicted in modern ‘milestone’ charts and assessment scales (Figure 1.1) [9–11]. The World Health Organization (WHO) took these generalizations a step farther by assigning skill onset ages as standards (imperatives that all infants should meet) rather than norms (describing a given population) [12].
Of course, some things must develop before others, and milestones may be clinically useful for identifying motor delays (Box 2). But the orderly succession of skills on a milestone chart can be deceiving. Infants often skip milestones or perform them out of sequence [13–15]. Gesell anointed skills such as crawling as essential steps toward walking, but infants in some cultures skip crawling or crawl after walking [16–18]. Skills such as logrolling, hitching, and other forms of locomotion appear and disappear from the historical motor repertoire [19,20]. In fact, the items on milestone charts reflects the proclivities of their creators, more than fundamental facts of development. Stages and milestones are not a proxy or a mandate for a universal process of development.
Box 2. Clinical Implications.
Many of the suggestions and findings outlined here have implications for clinicians working with infants and young children. For example, onset ages and milestones (Figure 1.1) are useful for identifying motor delays in children with developmental disabilities. However, overly strict adherence to ‘standards’ and obligatory stages may cause undue stress for parents and serve as a red herring for clinical interventions. For example, cultures where infants never crawl refute the notion (central to the traditional Bobath method and other therapies) that infants must be taught to crawl before they learn to walk [112].
Perhaps most relevant for clinicians is the description of the natural ‘training regimen’ experienced by children with typical development – immense amounts of highly variable practice moving through complex, real-world environments, with frequent opportunities to experience the results of errors via falls and near falls. Although new interventions seek to replicate such complex and varied experience [113,114], the most prevalent interventions (e.g., neurodevelopmental treatment) focus on repetition of isolated, simplified movements (such as stepping on a treadmill) as a foundation for complex, goal-directed actions [115]. But typically developing children do not master simplified, isolated movements before advancing to more complex ones; they practice it all simultaneously. Indeed, the most effective interventions for children with disabilities incorporate child-initiated movement and environmental modification [114,116,117]
A related and equally crucial point concerns the sequelae of motor development. Children who lack independent mobility also lack the opportunity to explore the larger environment and to engage in social interactions. Infant go-carts and other infant-friendly, mobility-powered devices may offer children with disabilities the opportunity to access people and places at earlier ages than previously possible with electrically powered wheelchairs [118].
Suggestion 2: Skill Onset Is Not an On–Off Switch
Milestone charts depict skill acquisition as a step function – first infants cannot walk; then they can (Figure 1.1). The assumption is that motor skills have a punctate onset date. However, daily sampling shows that skill acquisition is not like turning on a switch [21,22]. Instead, skills stutter into infants’ repertoires, with variable trajectories that oscillate between skill expression and non-expression over several days, weeks, or months (Figure 1.2). Variable trajectories hold regardless of the criterion for onset (first step, five consecutive steps, walking across the room, etc.). Although convenient and useful, selecting the first day of expression (or any other day) to designate skill onset is arbitrary, and measures that rely on onset ages are crude approximations at best.
Wider sampling intervals (e.g., even weekly or monthly observations that are considered heroic in longitudinal studies) result in developmental trajectories that appear step-like [21,22]. The unfortunate consequence is that theories of development may rely on a mischaracterization of the shape and rate of developmental change.
Suggestion 3: Starting Points Are Arbitrary
To study the development of a skill (or anything else), researchers must pick a place to start. However, the starting point is always arbitrary because every behavior has a developmental history [23]. Before infants take their first independent walking steps, they typically exhibit other upright skills; before that, they display other forms of locomotion; and before that, they move their legs in alternation. Some researchers consider infants’ first struggle to conquer gravity to be the starting point for walking [2], as pictured at the bottom of Figure 1.1. Others focus on newborns’ alternating leg movements when held upright [5,24], as in Figure 1.3
Why focus on leg alternation in newborns? One reason is that their upright leg movements bear a striking resemblance to adult walking. Indeed, some researchers consider this similarity to be evidence for ‘core knowledge’ of walking [25,26]. However, before birth, fetuses move their legs. Before that, spinal circuitry exhibits patterns of alternation (at least in rats and chicks); and even earlier, the spinal circuitry exhibits other patterns [27]. Furthermore, the circuitry itself develops. And so on. Of course, researchers must start somewhere, but it is important to remember that the starting point is arbitrary. It could be newborn stepping, or it could be an earlier event such as the instantiation of spinal circuitry. Or it could be a later event such as rolling, crawling, or supported walking. Choosing a starting point is essential, but reifying that point is not.
Suggestion 4: Endpoints Are Arbitrary
Just as there is no de definitive starting point, development has no conclusive endpoint. As in other areas of psychology [28], researchers typically consider the mature endpoint of walking to be the behaviors of young adults recruited from introductory psychology courses. Children reach this ‘mature’ endpoint by 5–7 years [29,30].
However, walking in modern, Western college students does not represent mature walking across recorded history or around the world [31–33]. Chinese footbinding (eradicated in the 1920s) caused 1000 years of women to walk on narrow, foreshortened feet (as little as 3 to 4 inches in length) [34,35]. Children and adults in some cultures have tremendous endurance. The Tarahumara run the distance of several marathons back-to-back for sport [36–38]. Persistence hunters chase game until the animals drop from exhaustion [37,39]. African women and Nepalese porters carry prodigious loads for great distances (Figure 1.4), freeloading up to 30% of their bodyweight without increased energy expenditure [40,41]. These marathoners and load carriers, whose development eclipses that of Western college students, are the rank and file – not the Olympians – of their communities. Like footbinding, long-distance running and load carriage from an early age change the endpoint of mature walking.
Suggestion 5: Childrearing Alters the Onset Age and Form of Skill Acquisition
Even skills that share similar ‘endpoints’ across cultures are affected by everyday childrearing practices. Most Western caregivers hold newborns like a fragile carton of eggs. However, caregivers in some African and Caribbean cultures believe that rough handling and exercise are essential for healthy motor development (Figure 1.5) [31–33]. They lift infants by the arms, ankles, or head; they toss infants into the air, and swing them around. They formally train walking by exercising upright stepping [16–18]. In these ‘natural experiments’, infants walk at younger ages than expected based on Western norms or the WHO standards. In true experiments with random assignment, a few minutes of daily practice with upright stepping or gentle postural training results in earlier onset of independent walking [42,43]. Similarly, training Icelandic infants results in independent standing by 4 months of age [44].
Most Western caregivers assume that freedom to move is crucial for motor development. However, caregivers in some cultures believe that restricting infants’ movements is not harmful and can even be beneficial [31–33]. Caregivers in rural China bury supine infants up to their chests in sandbags [45,46]; in central Asia, caregivers bind infants neck to toe in a gahvora cradle [47]; the nomadic Ache in Paraguay carry infants nearly all their waking time [48]. Restricted movement – even without social deprivation – delays postural and locomotor skills relative to Western norms and the WHO standards.
One need not travel to exotic places to see effects of childrearing on motor development. In Western cultures, putting infants to sleep on their backs instead of their stomachs delays the emergence of crawling [49,50]. Merely wearing a diaper impedes walking compared with going naked [51]. Moreover, there is something ironic about attributing ‘acceleration’ or ‘delay’ to infants from cultures that are not represented in the norms. The WHO standards were based on data from infants in Ghana, India, Norway, Oman, and the USA – cultures that do not employ exercise or restriction [12]. Indeed, reliance on data from a limited sample of cultures is so pervasive that this criticism can be made for most research studies in the psychological literature [28,31–33], including those reported in this article.
Suggestion 6: Experience Is Not the Mere Passage of Time
Despite the arbitrary nature of onset dates, researchers commonly de ne ‘walking experience’ as the number of days since skill onset (Figure 1.6). This is tantamount to considering test age (number of days since birth) as ‘life experience.’ Although children’s test age is a powerful predictor of development, researchers have long recognized that age is not an explanatory variable [52]. Similarly, walking experience in days – that is, ‘walking age’ – is a powerful predictor of changes in walking skill (Box 3). But experience measured in days is essentially a black box – a placeholder for something else which is unknown or dif cult to measure. More conceptually useful measures would quantify the content of experience (e.g., number of steps taken, distance traveled, surfaces traversed) that play a causal role in driving development [8,32,33,53].
Box 3. Infants Learn to Walk.
Everyone says that ‘infants learn to walk.’ But do they? The early pioneers viewed the development of walking as a window into neuromuscular maturation – not learning at all [5,119]. However, a century of research shows that days of walking predict fluency and flexibility [32,33,58]. Fluency makes skills more automatic, smooth, and consistent during stable conditions. For example, with accumulated days of walking, infants’ steps become more mature and more consistent while walking over flat ground (compare short, wide, and variable steps of a novice infant in the first row of Figure I to the long, narrow, and consistent steps of the experienced infant in the second row). Flexibility makes skills more functional by allowing behavior to be tailored to changing body–environment conditions. With accumulated days of walking, infants learn to use perceptual information to modify their steps to navigate novel obstacles. For example, as shown in the bottom two rows of footprints of Figure I, infants brake forward momentum by shortening their step length and decreasing their step velocity while approaching and descending steep slopes, but not shallow ones [120]. Accumulated days of walking is a better predictor of infants’ fluency and flexibility than their test age or body dimensions, suggesting that infants truly ‘learn to walk.’ What is needed is a description of the content of accumulated days of walking – the aspects of walking experience that lead to increased fluency and flexibility.
Figure I. Learning to Walk.
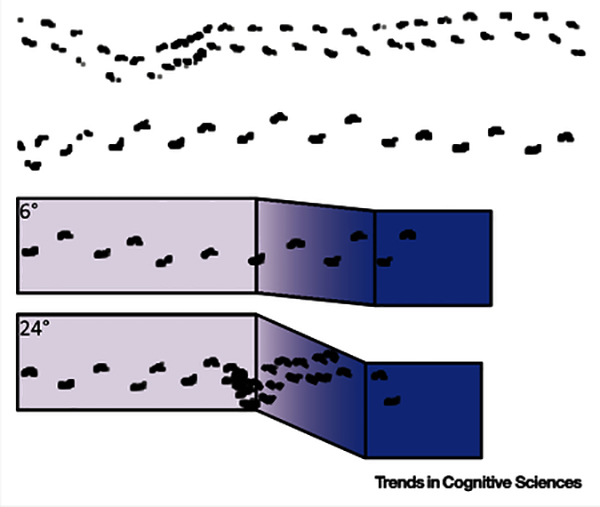
(First Row) Steps of a novice infant walking over at ground. (Second Row) Steps of an experienced infant walking over flat ground. (Third Row) Walking steps of an experienced infant approaching and navigating a shallow 6°slope. (Fourth Row) Walking steps of the same experienced infant approaching a steeper 24° slope.
Suggestion 7: Natural Input Shapes Everyday Learning
Placeholders, proxies, and assumptions about experience obscure the nature of development. For example, when clinicians and roboticists teach someone or something to walk, they typically assume that learning should begin with the simplest case – continuous, evenly paced, alternating, forward steps on a treadmill or along a straight, uniform path [54,55] (Box 2). However, research that documents the development of walking shows that, when infants learn to walk, they solve a completely different problem. The natural input for walking is massive and highly variable (Figure 1.7). The average toddler takes 2400 steps, travels the distance of 8 American football fields, and falls 17 times per hour during free play with a caregiver [53]. They step on most available surfaces (carpet, linoleum, etc.) and elevations (stairs, slopes, etc.).
Albeit massive, infants’ practice regimen is not a continuous marathon. It looks nothing like a straight, uniform path. Walking is broken by frequent starts and stops, including many (30–50%) one- to three-step bouts [53,56,57]. Infants rarely walk in a straight line. Most paths (73%) are curved, meaning that the two sides of the body do different things [57]. Most bouts contain backward and sideways – not only forward – steps (82%), meaning that infants initiate disequilibrium in every direction [57]. Short bouts, curved paths, and omnidirectional steps are equally present in novice and experienced walkers, indicating that these phenomena are not a byproduct of poor walking skill. Thus, theories based on continuous, forward, alternating leg movements [25,58] miss essential characteristics of walking. Moreover, immense varied input is conducive for learning. Simulated robots trained on infant-like paths or in varied environments perform better in new, untrained settings [59–61].
Suggestion 8: Standard Tests Are Not Natural Activity
Although every motor development researcher knows that short bouts, curved paths, and omnidirectional steps are prevalent during natural activity, they typically measure walking skill by coaxing infants to walk along a straight path [62–67] (Figure 1.8). Similarly, every clinician aims to improve walking during functional, everyday activity in cluttered real-life environments. However, in clinical gait laboratories, children and adults with disabilities walk along straight, unobstructed paths to assess effects of interventions [54,68–70] (Box 2). This standard ‘straight-path’ test serves practical functions. It allows researchers to compare walking skill across infants and timepoints. A century of research using the straight-path test shows that infant gait becomes more mature (faster, longer, narrower steps, etc.) with experience [32,33,65,71] (Box 3). Furthermore, until recently, available technology only allowed researchers to collect data within a limited recording area. Today, the availability of a large instrumented floor allows researchers to measure the same gait parameters during natural activity, and to study the development of walking in all its richness and complexity – presumably the phenomenon researchers wish to explain and clinicians wish to train [57]. More generally, all researchers could benefit from an increased focus on ecological validity [72,73].
Suggestion 9: Developmental Outcomes Are Not Real-Time Motivations
Massive amounts of varied walking experience serve long-term, developmental functions. Over months of walking, infants learn about their developing bodies, skills, and the larger physical and social environment. However, infants do not set out to accumulate locomotor experience or the long-term benefits it provides. Instead, locomotor experience is the byproduct of many independently motivated, real-time bouts of locomotion (Figure 1.9). Moreover, real-time motivations may not match researchers’ assumptions.
Historically, researchers assumed that infants locomote to reach distant people, places, or things [74–77]. Infants can and do go to destinations, but destination-directed walking does not characterize natural activity – only Ȉ40% of their bouts [56]. Instead, most bouts consist of steps in place or end in the middle of the room out of arms’ reach of any discernable destination. Like human infants, infant animals engage in locomotor play in the absence of a short-term goal [78,79]. Although effortful and costly, infant animals spend about 20% of their time and 10% of their energy on gross motor play [80]. This propensity for locomotor play helps infants to accumulate the vast amount of varied experience needed to hone their locomotor skills. Thus, infants’ short-term motivations (e.g., to play) do not necessarily reflect developmental outcomes (e.g., flexible and proficient skill acquisition).
Suggestion 10: Many Developing Components Contribute to Skill Acquisition
Because developmental outcomes are the byproducts of many real-time motivations and events, every developmental achievement reflects the contributions of multiple components. Each component has its own developmental trajectory, and thus development across the set is typically asynchronous [6] (Figure 1.10). Infants’ first independent walking steps await suffi cient strength and balance to support the body on one leg [24,29]. Other factors are sufficient long before – the ability to move the legs, to do so in alternation, sensitivity to optic flow, motivation to go somewhere, myelination of the corticospinal tract, and so on. Some components can be hastened (e.g., accelerating leg strength by exercising upright stepping [42]; increasing discrimination of lamellar optic flow via experience with a baby go-cart [81]), whereas others (e.g., pendular energy exchange [66,82]) emerge in due time.
Suggestion 11: Behavior Happens in a Body in an Environment
Two crucial components in the development of walking are the body and the environment. Skill acquisition is both embodied and embedded. The current status of the body and the environment affects the biomechanical constraints on walking [32,33]. Changes in body size and in the location of the center of mass require modifications of posture and foot placement [83]. Merely lifting an arm or tilting the head can throw a new walker off balance. Carrying a toy or wearing clunky shoes, heavy clothes, or even a diaper can do likewise [51,84–87]. Changes in environmental layout (e.g., drop-offs, stairs) and ground surfaces (sloping, slippery, narrow, deformable, etc.) require infants to modify their gait or to select an alternative method of locomotion [81,88–94] (Figure 1.11 and Box 3).
Because infants’ bodies and environments are continually changing, relying on simple alternating leg movements is not viable. Instead, walking is a creative act. Each step and each bout must be tailored to suit the current body–environment relations. To do so, infants must generate perceptual information to plan and guide their movements. They must create a variety of means to navigate each new challenge. Eventually, infants also learn to integrate social information from caregivers with self-generated perceptual information when deciding whether and how to tackle an obstacle [89,95]. Thus, behavior is shaped from moment to moment by the immediate context—changes in infants’ bodies and in their physical and social environments.
Suggestion 12: Learning Occurs in the Context of Development
Infants acquire new skills in a developing body in a developing environment [33]. Infants can wake up today to find themselves nearly 2 cm taller than yesterday [96]. Features of the environment that were previously inaccessible can come into view and into reach as infants acquire postural and locomotor skills [97]. While crawling, for example, infants see the floor in front of their hands, but while walking the whole room swoops into view [98] (Figure 1.12). What is impossible earlier in development can be possible later, and vice versa. Thus, learning fixed facts about the body or environment – and what actions are possible or not – would be maladaptive. Instead, infants must learn to detect possibilities for locomotion in the moment, a process of ‘learning to learn’ [33].
Suggestion 13: Variability Is Inherent to Development
Given that infants’ bodies and environments are continually changing, it should come as no surprise that variable behavior is the rule, not the exception, in development. Interindividual variability is apparent in the wide age ranges for skill acquisition (Figure 1.1), different manifestations of mature walking (Figure 1.4), altered onset ages and days of ‘experience’ due to childrearing practices (Figure 1.5/6), and the uneven development of components (Figure 1.10). Indeed, infants exhibit different strategies to first solve the problem of walking. ‘Steppers’ propel themselves forward in short, tiny steps, ‘fallers’ lean forward on tiptoe and fall into each step, and ‘twisters’ twist their torso to swing their legs around from each side [99,100].
Intraindividual variability is similarly apparent in the deviations from perfect alternation in newborn stepping (Figure 1.3), variable expression and non-expression of new skills (Figure 1.2), twisting, turning walking paths in natural activity (Figure 1.7), step-to-step differences on flat ground and while approaching obstacles (Figure 1.8 and Box 3), seemingly random initiation and termination of walking bouts (Figure 1.9), variable bodies and environments across development (Figure 1.11/12), and the variety of strategies infants use from trial to trial to navigate obstacles. For example, the same infant in the same session frequently descends a drop-off by walking, crawling, scooting, and backing (Figure 1.13). In short, all aspects of walking development are variable. Both inter- and intraindividual variability may decrease or increase over development [101], but they are always present.
Suggestion 14: Behavior Is a Cascade of Real-Time Events
Behaviors that develop over days, months, and years are the products of many real-time perception–action cycles. For instance, when navigating challenging terrain (e.g., bridges of varying widths) infants must generate information to decide whether and how to cross. To gather this information, infants modify their gait and engage in various forms of exploratory activity – looking, touching, and testing alternatives (Figure 1.14). Exploratory activity sequentially ‘ramps up’, from less to more costly forms, as new perceptual information is generated[92]. At the start of the trial, infants brie y glance at the bridge from a distance. These quick, low-cost looks provide an initial assessment that prompts more costly forms of exploratory activity for narrow bridges. When the path looks risky, infants collect additional information – they slow down, shorten their steps, and stop to feel the bridge beneath their feet. If haptic exploration suggests that walking is impossible, they test alternative routes and methods of locomotion. Thus, actions that occur earlier in the perception–action cycle have consequences for those downstream.
Suggestion 15: New Skills Instigate a Cascade of Developmental Events
The onset of independent locomotion instigates and facilitates cascades of change across multiple developmental systems [74,102]. Mobile infants are less dependent on their caregivers to gain access to the larger physical and social world. Walking, in particular, allows infants to go more, see more, do more, and play more [102]. Compared with crawlers, walking infants spend more time in motion, travel farther distances, visit more places in the environment [53], spend more time away from caregivers [103], and see more of the environment [98]. Walkers more frequently retrieve distal objects [104], and carry them to new locations [105,106] (Figure 1.15). The onset of walking is also related to increases in infant-initiated joint engagement and attention to caregivers’ joint engagement cues (e.g., following gaze and points) [107]. Caregivers, in turn, are more likely to respond to moving bids for attention with action directives about what infants can do with an object in hand [108]. Caregivers of mobile infants are also more likely to express anger, make demands, or admonish infants [109]. Finally, days of walking predict parental reports of infant receptive and productive language independently of infant age [110]. Thus, independent mobility can instigate a cascade of developmental events in domains far afield from motor development. Indeed, disabilities that hamper locomotion impede opportunities for learning about things in the environment and limit social engagements with caregivers and peers [111] (Box 2).
Concluding Remarks
Using infant walking as a model system, we presented 15 general suggestions for developmental researchers. Motor skills are particularly well suited for this task because their form and timing are directly observable. Thus, infants’ movements can provide a unique window into development writ large. At its heart, all behavior is motor behavior! Our suggestions raise new questions both about the development of walking and other areas of developmental research (see Outstanding Questions). Not every suggestion will hold true for every domain, but most are likely applicable. The development of walking, for example, shares many commonalties with the development of talking (Box 1). Furthermore, we do not propose that the research that served as the foundations for our suggestions is necessarily wrong. Instead, we propose that the developmental story is incomplete. We hope our suggestions will inspire developmental researchers outside the field of motor development to search for general principles of change and to think about their own research in new ways.
Highlights.
A fundamental goal of developmental science is to identify general principles of change, but developmental scientists rarely generalize beyond their specific content domains.
Because infant motor skill acquisition is directly observable over multiple, nested timescales, it can be used as a model system for development.
We offer 15 suggestions for developmental research based on motor skill acquisition that collectively address the multilevel nature of change processes, cascades of real-time and developmental events, the diversity of developmental trajectories, inter- and intraindividual variability, starting and ending points of development, the natural input for learning, and the roles of body, environment, and sociocultural context.
We argue that these 15 suggestions generalize across developmental domains and have important implications for clinical work. We encourage researchers to consider them within their own areas of research.
Outstanding Questions.
When is development most sensitive to insults and responsive to interventions? In motor development, early training facilitates later skill acquisition (e.g., exercise of newborn stepping accelerates later walking [42]), but effects of early constraint (e.g., swaddling) on later skill acquisition are unclear [133].
What does development look like in the rest of the world? Researchers’ focus on young, middle-class adults in Western cultures (and their children) has created a literature that is missing 95% of the world population [28]. Researchers do not yet know the extent of human potential or developmental routes for achieving it.
What is the natural input for learning? With the exception of language acquisition, researchers know little about the natural input for learning. Research on infant walking offers only a rough sketch [53,56,57]. To capture the full picture, we need detailed daily observations of infants in their natural home environments.
What types of experiences promote fluency and flexibility in the acquisition of basic cognitive, social, linguistic, and motor skills? For walking, the natural input is both massive and variable, but what are the relative contributions of the amount and variety of practice? Which types of variability promote learning and which contribute to noise?
How do unique developmental trajectories converge on common solutions? Despite striking inter- and intraindividual variability, most children acquire basic skills such as learning to walk.
What are the relations among developmental domains? Locomotion is rarely studied in the context of other motor actions (e.g., reaching, carrying) or psychological processes (e.g., perceptual guidance, social interactions, spatial cognition).
What are the mechanisms of development? Developmental research boasts a long history of detailed description. We know a lot about the ‘what’ and ‘when’, but far less about the ‘how.’ Future research should focus on identifying developmental mechanisms.
Acknowledgments
This work was supported by grants to K. E. A. from the National Institute of Child Health (NICHD); R37-HD033486 and R01-HD086034), the National Science Foundation (NSF; BCS-1528831 and BCS-1627993), and the LEGO Foundation. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NICHD, NSF, or LEGO Foundation. We thank Catherine Tamis-LeMonda for her insights about parallels between walking and talking, Orit Herzberg for her wonderful figure illustrations and design, Marcella Ruthe for her drawings of descent methods, and Mark Blumberg for his comments.
References
- 1.Gesell A (1939) Reciprocal interweaving in neuromotor development. J. Comp. Neurol 70, 161–180 [Google Scholar]
- 2.Gesell A (1946) The ontogenesis of infant behavior In Manual of Child Psychology (Carmichael L, ed.), pp. 295–331, John Wiley [Google Scholar]
- 3.Gibson EJ (1997) An ecological psychologist’s prolegomena for perceptual development: a functional approach In Evolving Explanations of Development: Ecological Approaches to Organism–Environment Systems (Dent-Read C and Zukow-Goldring P, eds), pp. 23–45, American Psychological Association [Google Scholar]
- 4.McGraw MB (1935) Growth: A Study of Johnny and Jimmy, Appleton-Century Crofts
- 5.McGraw MB (1945) The Neuromuscular Maturation of the Human Infant, Columbia University Press [Google Scholar]
- 6.Thelen E and Ulrich BD (1991) Hidden skills: a dynamic systems analysis of treadmill stepping during the first year. Monogr. Soc. Res. Child Dev 56, i–103 [PubMed] [Google Scholar]
- 7.Thelen E and Smith LB (1994) A Dynamic Systems Approach to the Development of Cognition and Action, MIT Press; [DOI] [PubMed] [Google Scholar]
- 8.Adolph KE and Berger SE (2006) Motor development In Handbook of Child Psychology Vol. 2: Cognition, Perception, and Language (6th edn) (Kuhn D and Siegler RS, eds), pp. 161–213, Wiley [Google Scholar]
- 9.Bayley N (2006) Bayley Scales of Infant and Toddler Development (Bayley-III), Pearson [Google Scholar]
- 10.Frankenburg WK et al. (1992) Denver II Sceening Manual, Denver Developmental Materials
- 11.Piper MC and Darrah J (1994) Motor Assessment of the Developing Infant, WB Saunders [Google Scholar]
- 12.Martorell R et al. (2006) WHO motor development study: windows of achievement for six gross motor development milestones. Acta Paediatr. 95 (S450), 86–95 [DOI] [PubMed] [Google Scholar]
- 13.Adolph KE et al. (2011) Developmental continuity? Crawling, cruising, and walking. Dev. Sci 14, 306–318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Atun-Einy O et al. (2012) Pulling to stand: common trajectories and individual differences. Dev. Psychobiol 54, 187–198 [DOI] [PubMed] [Google Scholar]
- 15.Berger SE et al. (2007) How and when infants learn to climb stairs. Infant Behav. Dev 30, 36–49 [DOI] [PubMed] [Google Scholar]
- 16.Hopkins B and Westra T (1990) Motor development, maternal expectations, and the role of handling. Infant Behav. Dev 13, 117–122 [Google Scholar]
- 17.Super CM (1976) Environmental effects on motor development: the case of ‘African infant precocity’. Dev. Med. Child Neurol 18, 561–567 [DOI] [PubMed] [Google Scholar]
- 18.Hopkins B and Westra T (1988) Maternal handling and motor development: an intracultural study Genetic. Genet. Soc. Gen. Psychol. Monogr 114, 379–408 [PubMed] [Google Scholar]
- 19.Fox AT et al. (2002) Do ‘shuf ebottoms’ bottom shuf e? Arch. Dis. Child 87, 552–554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Trettien AW (1900) Creeping and walking. Am. J. Psychol 12, 1–57 [Google Scholar]
- 21.Adolph KE et al. (2008) What is the shape of developmental change? Psychol. Rev 115, 527–543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Adolph KE and Robinson SR (2011) Sampling development J. Cogn. Dev 12, 411–423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Adolph KE and Robinson SR (2008) In defense of change processes. Child Dev 79, 1648–1653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thelen E (1983) Learning to walk is still an ‘old’ problem: a reply to Zelazo (1983). J. Mot. Behav 15, 139–161 [DOI] [PubMed] [Google Scholar]
- 25.Dominici N et al. (2011) Locomotor primitives in newborn babies and their development. Science 334, 997–999 [DOI] [PubMed] [Google Scholar]
- 26.Spelke ES and Newport EL (1998) Nativism, empiricism, and the development of knowledge In Handbook of Child Psychology Vol. 1: Theoretical Models of Human Development (5th edn) (Damon W, ed.), pp. 275–340, John Wiley & Sons [Google Scholar]
- 27.Bekoff A (1992) Neuroethological approaches to the study of motor development in chicks: achievements and challenges. J. Neurobiol 23, 1486–1505 [DOI] [PubMed] [Google Scholar]
- 28.Heinrich J et al. (2010) The weirdest people in the world? Behav. Brain Sci 33, 61–83 [DOI] [PubMed] [Google Scholar]
- 29.Breniere Y and Bril B (1998) Development of postural control of gravity forces in children during the first 5 years of walking. Exp. Brain Res 121, 255–262 [DOI] [PubMed] [Google Scholar]
- 30.Sutherland DH et al. (1988) The Development of Mature Walking, Mac Keith Press [Google Scholar]
- 31.Adolph KE et al. (2010) Motor skills In Handbook of Cultural Development Science Vol. 1: Domains of Development across Cultures (Bornstein MH, ed.), pp. 61–88, Taylor and Francis [Google Scholar]
- 32.Adolph KE and Robinson SR (2013) The road to walking: what learning to walk tells us about development In Oxford Handbook of Developmental Psychology (Zelazo P, ed.), pp. 403–443, Oxford University Press [Google Scholar]
- 33.Adolph KE and Robinson SR (2015) Motor development In Handbook of Child Psychology and Developmental Science (7th edn) (Liben L and Muller U, eds), pp. 114–157, Wiley [Google Scholar]
- 34.Fang HSY and Yu FYK (1960) Foot binding in Chinese women. Can. J. Surg 293, 195–202 [PubMed] [Google Scholar]
- 35.Reznikov N et al. (2017) Functional adaptation of the calcaneus in historical foot binding. J. Bone Miner. Res 32, 1915–1925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bennett WC and Zingg RM (1935) The Tarahumara, an Indian Tribe of Northern Mexico, University of Chicago Press [Google Scholar]
- 37.Devine J (1985) The versatility of human locomotion. Am. Anthropol 87, 550–570 [Google Scholar]
- 38.McDougall C (2009) Born to Run: A Hidden Tribe, Superathletes, and the Greatest Race the World Has Never Seen, Alfred A. Knopf [Google Scholar]
- 39.Liebenberg L (2006) Persistence hunting by modern hunter-gatherers. Curr. Anthropol 47, 1017–1025 [Google Scholar]
- 40.Bastien GJ et al. (2005) Energetics of load carrying in Nepalese porters. Science 308, 1755. [DOI] [PubMed] [Google Scholar]
- 41.Heglund NC et al. (1995) Energy-saving gait mechanics with head-supported loads. Nature 375, 52–54 [DOI] [PubMed] [Google Scholar]
- 42.Zelazo PR et al. (1972) ‘Walking’ in the newborn. Science 176, 314–315 [DOI] [PubMed] [Google Scholar]
- 43.Lobo MA and Galloway JC (2012) Enhanced handling and positioning in early infancy advances development throughout the first year. Child Dev. 83, 1290–1302 [DOI] [PubMed] [Google Scholar]
- 44.Sigmundsson H et al. (2017) Exploring task-specific independent standing in 3- to 5-month-old infants. Front. Psychol 8, 657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Xie Q and Young ME (1999) Integrated Child Development in Rural China, The World Bank [Google Scholar]
- 46.Mei J (1994) The Northern Chinese custom of rearing babies in sandbags: implications for motor and intellectual development In Motor Development: Aspects of Normal and Delayed Development (van Rossum JHA and Laszlo JI, eds), pp. 41–48, VU Uitgeverij [Google Scholar]
- 47.Save the Children (2011) Harmful Traditional Practices in Tajikistan, Save the Children [Google Scholar]
- 48.Kaplan H and Dove H (1987) Infant development among the Ache of Eastern Paraguay. Dev. Psychol 23, 190–198 [Google Scholar]
- 49.Davis BE et al. (1998) Effects of sleep position on infant motor development. Pediatrics 102, 1135–1140 [DOI] [PubMed] [Google Scholar]
- 50.Majnemer A and Barr R (2006) Association between sleep position and early motor development. J. Pediatr 149, 623–629 [DOI] [PubMed] [Google Scholar]
- 51.Cole WG et al. (2012) Go naked: diapers affect infant walking. Dev. Sci 15, 783–790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wohlwill JP (1970) The age variable in psychological research. Psychol. Rev 77, 49–64 [Google Scholar]
- 53.Adolph KE et al. (2012) How do you learn to walk? Thousands of steps and dozens of falls per day. Psychol. Sci 23, 1387–1394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Willoughby KL et al. (2010) Ef cacy of partial body weight-supported treadmill training compared with overground walking practice for children with cerebral palsy: a randomized controlled trial. Arch. Phys. Med. Rehabil 91, 333–339 [DOI] [PubMed] [Google Scholar]
- 55.Hirose M and Ogawa K (2007) Honda humanoid robots development. Philos. Trans. R. Soc. Lond. A Math. Phys. Eng. Sci 365, 11–19 [DOI] [PubMed] [Google Scholar]
- 56.Cole WG et al. (2016) Bouts of steps: the organization of infant exploration. Dev. Psychobiol 58, 341–354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lee DK, Cole WG, Golenia L and Adolph KE (2018) The cost of simplifying complex developmental phenomena: a new perspective on learning to walk. Dev. Sci 21, e12615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lacquaniti F et al. (2012) Development of human locomotion. Curr. Opin. Neurobiol 22, 822–828 [DOI] [PubMed] [Google Scholar]
- 59.Ossmy O et al. (2018) Variety wins: soccer-playing robots and infant walking. Front. Neurorobot 12, 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Heess N et al. (2017) Emergence of locomotion behaviours in rich environments. arXiv
- 61.MacAlpine P et al. (2012) Design and optimization of an omnidirectional humanoid walk: a winning approach at the RoboCup 2011 3D simulation competition In Proceedings of the 26th AAAI Conference, pp. 1047–1053, Association for the Advancement of Arti cial Intelligence [Google Scholar]
- 62.Adolph KE et al. (2003) What changes in infant walking and why. Child Dev. 74, 474–497 [DOI] [PubMed] [Google Scholar]
- 63.Bisi MC and Stagni R (2015) Evaluation of toddler different strategies during the first six-months of independent walking: a longitudinal study. Gait Posture 41, 574–579 [DOI] [PubMed] [Google Scholar]
- 64.Bril B et al. (2015) Learning to tune the antero-posterior propulsive forces during walking: a necessary skill for mastering upright locomotion in toddlers. Exp. Brain Res 233, 2903–2912 [DOI] [PubMed] [Google Scholar]
- 65.Hallemans A et al. (2006) Changes in 3D joint dynamics during the first 5 months after the onset of independent walking: a longitudinal follow-up study. Gait Posture 24, 270–279 [DOI] [PubMed] [Google Scholar]
- 66.Ivanenko YP et al. (2004) Development of pendulum mechanism and kinematic coordination from the rst unsupported steps in toddlers. J. Exp. Biol 207, 3797–3810 [DOI] [PubMed] [Google Scholar]
- 67.Chang CL et al. (2006) Early changes in muscle activation patterns of toddlers during walking. Infant Behav. Dev 29, 175–188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Cherng RJ et al. (2007) Effect of treadmill training with body weight support on gait and gross motor function in children with spastic cerebral palsy. Am. J. Phys. Med. Rehabil 86, 548–555 [DOI] [PubMed] [Google Scholar]
- 69.Ulrich DA et al. (2008) Effects of intensity of treadmill training on developmental outcomes and stepping in infants with Down syndrome: a randomized trial. Phys. Ther 88, 114–122 [DOI] [PubMed] [Google Scholar]
- 70.Reisman DS et al. (2009) Split-belt treadmill adaptation transfers to overground walking in persons poststroke. Neurorehabil. Neural Repair 23, 735–744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ivanenko YP et al. (2007) Development of independent walking in toddlers. Exerc. Sport Sci. Rev 35, 67–73 [DOI] [PubMed] [Google Scholar]
- 72.Bronfenbrenner U (2009) The Ecology of Human Development, Harvard University Press [Google Scholar]
- 73.Gibson JJ (1979) The Ecological Approach to Visual Perception, Houghton Mifflin [Google Scholar]
- 74.Campos JJ et al. (2000) Travel broadens the mind. Infancy 1, 149–219 [DOI] [PubMed] [Google Scholar]
- 75.Gibson EJ (1978) C’est moi. Contemp. Psychol 23, 609–614 [Google Scholar]
- 76.Gibson EJ and Schmuckler MA (1989) Going somewhere: an ecological and experimental approach to development of mobility. Ecol. Psychol 1, 3–25 [Google Scholar]
- 77.Piaget J (1954) The Construction of Reality in the Child, Basic Books [Google Scholar]
- 78.Burghardt GM (2005) The Genesis of Animal Play: Testing the Limits, MIT Press [Google Scholar]
- 79.Pellegrini AD (2013) Play In The Oxford Handbook of Developmental Psychology (Vol. 2) (Zelazo PD, ed.), pp. 276–299, Oxford University Press [Google Scholar]
- 80.Fagen R (1981) Animal Play Behavior, Oxford University Press [Google Scholar]
- 81.Dahl A et al. (2013) The epigenesis of wariness of heights. Psychol. Sci 24, 1361–1367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hallemans A et al. (2004) Mechanical energy in toddler gait: a trade-off between economy and stability? J. Exp. Biol 207, 2417–2431 [DOI] [PubMed] [Google Scholar]
- 83.Shumway-Cook A and Woollacott MH (2017) Motor Control: Translating Research into Clinical Practice, Wolters Kluwer [Google Scholar]
- 84.Hsu WH et al. (2016) Toddlers actively reorganize their whole body coordination to maintain walking stability while carrying an object. Gait Posture 50, 75–81 [DOI] [PubMed] [Google Scholar]
- 85.Mangalindan DM et al. (2014) The impact of object carriage on independent locomotion. Infancy 37, 76–85 [DOI] [PubMed] [Google Scholar]
- 86.Cole WG et al. (2014) Coping with asymmetry: how infants and adults walk with one elongated leg. Infant Behav. Dev 37, 305–314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Theveniau N et al. (2014) The effects of clothes on independent walking in toddlers. Gait Posture 39, 659–661 [DOI] [PubMed] [Google Scholar]
- 88.Burnay C and Cordovil R (2016) Crawling experience predicts avoidance of real cliffs and water cliffs: insight from a new paradigm. Infancy 21, 677–684 [Google Scholar]
- 89.Karasik LB et al. (2016) Decisions at the brink: locomotor experience affects infants’ use of social information on an adjustable drop-off. Front. Psychol 7, 797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kretch KS and Adolph KE (2013) Cliff or step? Posture-specific learning at the edge of a drop-off. Child Dev. 84, 226–240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kretch KS and Adolph KE (2013) No bridge too high: infants decide whether to cross based on the probability of falling not the severity of the potential fall. Dev. Sci 16, 336–351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kretch KS and Adolph KE (2017) The organization of exploratory behaviors in infant locomotor planning. Dev. Sci 20, e12421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Dominici N et al. (2010) Kinematic strategies in newly walking toddlers stepping over different support surfaces. J. Neurophysiol 103, 1673–1684 [DOI] [PubMed] [Google Scholar]
- 94.Franchak JM and Adolph KE (2012) What infants know and what they do: perceiving possibilities for walking through openings. Dev. Psychol 48, 1254–1261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Adolph KE et al. (2008) Locomotor experience and use of social information are posture specific. Dev. Psychol 44, 1705–1714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lampl M (1993) Evidence of saltatory growth in infancy. Am. J. Hum. Biol 5, 641–652 [DOI] [PubMed] [Google Scholar]
- 97.Gibson EJ (1988) Exploratory behavior in the development of perceiving, acting, and the acquiring of knowledge. Annu. Rev. Psychol 39, 1–41 [Google Scholar]
- 98.Kretch KS et al. (2014) Crawling and walking infants see the world differently. Child Dev. 85, 1503–1518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.McCollum G et al. (1995) Forms of early walking. J. Theor. Biol 176, 373–390 [DOI] [PubMed] [Google Scholar]
- 100.Snapp-Childs W and Corbetta D (2009) Evidence of early strategies in learning to walk. Infancy 14, 101–116 [DOI] [PubMed] [Google Scholar]
- 101.Adolph KE et al. (2015) Intraindividual variability in the development of motor skills in childhood In Handbook of Intraindividual Variability Across the Life Span (Diehl M, ed.), pp. 59–83, Routledge/Taylor & Francis [Google Scholar]
- 102.Adolph KE and Tamis-LeMonda CS (2014) The costs and benefits of development: the transition from crawling to walking. Child Dev. Perspect 8, 187–192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Biringen Z et al. (2008) Development of autonomy: role of walking onset and its timing. Percept. Mot. Skills 106, 395–414 [DOI] [PubMed] [Google Scholar]
- 104.Dosso JA and Boudreau JP (2014) Crawling and walking infants encounter objects differently in a multi-target environment. Exp. Brain Res 232, 3047–3054 [DOI] [PubMed] [Google Scholar]
- 105.Karasik LB et al. (2012) Carry on: spontaneous object carrying in 13-month-old crawling and walking infants. Dev. Psychol 48, 389–397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Karasik LB et al. (2011) Transition from crawling to walking and infants’ actions with objects and people. Child Dev. 82, 1199–1209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Walle EA (2016) Infant social development across the transition from crawling to walking. Front. Psychol 7, 960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Karasik LB et al. (2014) Crawling and walking infants elicit different verbal responses from mothers. Dev. Sci 17, 388–395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Biringen Z et al. (1995) Affective reorganization in the infant, the mother, and the dyad: the role of upright locomotion and its timing. Child Dev. 66, 499–514 [PubMed] [Google Scholar]
- 110.Walle EA and Campos JJ (2014) Infant language development is related to the acquisition of walking. Dev. Psychol 50, 336–348 [DOI] [PubMed] [Google Scholar]
- 111.Logan S et al. (2016) Why we move: social mobility behaviors of non-disabled and disabled children across childcare contexts. Front. Public Health 4, 204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Bobath K (1980) A Neurophysiological Basis for the Treatment of Cerebral Palsy, Heinemann Medical [Google Scholar]
- 113.Bleyenheuft Y et al. (2017) Intensive upper- and lower-extremity training for children with bilateral cerebral palsy: a quasi-randomized trial. Dev. Med. Child Neurol 59, 625–633 [DOI] [PubMed] [Google Scholar]
- 114.Morgan C et al. (2016) Effectiveness of motor interventions in infants with cerebral palsy: a systematic review. Dev. Med. Child Neurol 58, 900–909 [DOI] [PubMed] [Google Scholar]
- 115.Zanon MA et al. (2015) Neurodevelopmental treatment approaches for children with cerebral palsy. Cochrane Database Syst. Rev 2015, 1–23 [Google Scholar]
- 116.Novak I et al. (2013) A systematic review of interventions for children with cerebral palsy: state of the evidence. Dev. Med. Child Neurol 55, 885–910 [DOI] [PubMed] [Google Scholar]
- 117.Morgan C et al. (2013) Enriched environments and motor outcomes in cerebral palsy: systematic review and meta-analysis. Pediatrics 132, e735–e746 [DOI] [PubMed] [Google Scholar]
- 118.Logan SW et al. (2018) Modifed ride-on car use by young children with disabilities. Pediatr. Phys. Ther 30, 50–56 [DOI] [PubMed] [Google Scholar]
- 119.Gesell A (1933) Maturation and the patterning of behavior In A Handbook of Child Psychology (2nd edn) (Murchison C, ed.), pp. 209–235, Clark University Press [Google Scholar]
- 120.Gill SV et al. (2009) Change in action: how infants learn to walk down slopes. Dev. Sci 12, 888–902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Nelson K (1973) Stucture and strategy in learning to talk. Monogr. Soc. Res. Child Dev 38, 1–135 [Google Scholar]
- 122.Brown R (1973) A First Language: The Early Stages, Harvard University Press [Google Scholar]
- 123.Fernald A et al. (2013) SES differences in language processing skill and vocabulary are evident at 18 months. Dev. Sci 16, 234–248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Huttenlocher J et al. (2010) Sources of variability in children’s language growth. Cognit. Psychol 61, 343–365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.MacWhinney B (2000) The CHILDES Project: Tools for Analyzing Talk, Erlbaum [Google Scholar]
- 126.Tamis-LeMonda CS et al. (2017) Power in methods: language to infants in structured and naturalistic contexts. Dev. Sci 20, e12456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Nelson K (1989) Narratives from the Crib, Harvard University Press [Google Scholar]
- 128.Hoff E (2014) Language Development, Wadsworth [Google Scholar]
- 129.Bloom L and Tinker E (2001) The intentionality model and language acquisition: engagement, effort, and the essential tension in development. Monogr. Soc. Res. Child Dev 66, i–101 [PubMed] [Google Scholar]
- 130.Bornstein MH et al. (2008) Maternal responsiveness to young children at three ages: longitudinal analysis of a multidimensional, modular, and speci c parenting construct. Dev. Psychol 44, 867–874 [DOI] [PubMed] [Google Scholar]
- 131.Goldstein MH et al. (2003) Social interaction shapes babbling: testing parallels between birdsong and speech. Proc. Natl. Acad. Sci. U. S. A 100, 8030–8035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Bloom L and Beckwith R (1989) Talking with feeling: integrating affective and linguistic expression in early language development. Cogn. Emot 3, 313–342 [Google Scholar]
- 133.Manaseki-Holland S et al. (2010) Effects of traditional swaddling on development: a randomized controlled trial. Pediatrics 126, 1485–1492 [DOI] [PubMed] [Google Scholar]