Abstract
Type III secretion system (T3SS) is a protein translocator complex family including pathogenic injectisome or bacterial flagellum. The inejectisomal T3SS serves to deliver virulence proteins into host cell and the flagellar T3SS constructs the flagellar axial structure. Although earlier studies have provided many findings on the molecular mechanism of the Type III protein export, they were not sufficient to reveal energy transduction mechanism due to difficulties in controlling measurement conditions in vivo. Recently, we developed an in vitro flagellar Type III protein transport assay system using inverted membrane vesicles (IMVs), and analyzed protein export by using the in vitro method. We reproduced protein export of the flagellar T3SS, hook assembly and substrate specificity switch in IMV to a similar extent to what is seen in living cell. Furthermore, we demonstrated that ATP-hydrolysis energy can drive protein transport even in the absence of proton-motive force (PMF). In this mini-review, we will summarize our new in vitro Type III transport assay method and our findings on the molecular mechanism of Type III protein export.
Keywords: In vitro reconstitution, Inverted membrane vesicle, Type III secretion system, injectisome, bacterial flagellum
Significance.
Bacterial Type III secretion system (T3SS) is a protein translocator to deliver virulence factors or its own axial structure components. The molecular mechanism of protein export is still obscure due to difficulties in controlling measurement conditions in vivo. We developed an in vitro protein transport assay system using inverted membrane vesicles (IMVs). Our method reproduced protein export of the flagellar T3SS in IMV and the sub-sequent flagellar assembly. We demonstrated that T3SS can drive protein export by using either ATP-hydrolysis energy or proton motive force. Our method will give us novel insights into the functional mechanisms of T3SS.
Type III secretion system (T3SS) is one of the bacterial protein translocators and its family contains two types of supramolecular complexes, the injectisome (or the needle complex) and the bacterial flagellum [1,2]. The bacterial flagellum is a filamentous organelle with a rotary motor in the cell envelope and responsible for motility in aqueous environments or on wet surfaces. The flagellum is composed of a basal body ring structure consisting of the MS-ring and the C-ring, and a filamentous axial structure consisting of the rod, the hook, the junction and the filament (Fig. 1). The axial structure is assembled from more than 20,000 protein subunits of 9 different proteins with the help of 3 different scaffold proteins. The axial proteins and their scaffold proteins are translocated through the flagellar T3SS, called the export apparatus, into the central tubular space across the cell membrane. The exported proteins diffuse through the central tube toward the growing end of the flagellum [3,4]. The export apparatus consists of the transmembrane export gate complex formed by FlhA, FlhB, FliP, FliQ and FliR and the cytoplasmic ATPase complex formed by FliH, FliI and FliJ [1,5,6]. The export gate is located inside of the transmembrane MS ring of the basal body and exports the proteins by proton-motive force [7,8]. The ATPase complex interacts with the export gate and the cytoplasmic C ring of the basal body [9]. FliI is a Walker-type ATPase and forms a homo-hexameric ring [10,11]. FliH is a negative regulator of FliI ATPase and interacts with the N-terminal region of FliI [12–14]. FliH also interacts with FlhA and a C ring component, FliN, to anchor the ATPase complex to the basal body [15]. FliJ fits into a central hole of the hexameric FliI ring [16]. The ATPase complex is structurally similar to the F1- and V1-ATPase, suggesting that it hydrolyzes ATP in a similar manner as F1- and V1-ATPase [11,12,16]. While FliH, FliI and FliJ form the ATPase complex in the export apparatus, FliH and FliI form the FliH2/FliI hetero-trimeric complex in cytoplasm and interacts with the filament-type proteins in complex with their specific cognate chaperons, which allow the filament-type proteins to somehow come close to the export gate [17–19]. Two different energy sources, proton-motive force (PMF) and ATP-hydrolysis energy, are utilized to export the substrate proteins through the export gate [7,8,15,20]. Since addition of CCCP, which is a proton ionophore to disrupt PMF, inhibits the export of the substrate proteins, PMF is a primary driving energy for protein export. The export gate acts as a proton/protein antiporter to couple the proton translocation to the protein export [21,22]. The proton is thought to be translocated through an export gate component, FlhA. On the other hand, the role of ATP-hydrolysis energy remains controversial. The ATP hydrolysis by FliI ATPase is not an essential event for exporting the substrate proteins across the cell membrane because fliH-fliI double null mutant cells formed the flagella [7,8]. PMF is defined as the sum of ΔΨ and ΔpH. The wild-type cells can export the proteins by only ΔΨ, whereas the fliH-fliI double null mutant cells require both ΔΨ and ΔpH for protein export [7,8,20]. Infrequent ATP hydrolyzing mutant, FliI E211D still transported enough proteins to construct the axial structure, hence ATP hydrolysis energy may be used for activation of the export gate and switching of the export gate into a highly efficient ΔΨ-driven engine [23]. In the injectisome, however, the ATP-hydrolysis energy is suggested to be utilized for unfolding substrate proteins for export [24]. Although genetic and biochemical in vivo experiments have revealed many aspects of the molecular mechanism of the T3SS, they are not enough to reveal the energy transduction mechanism for protein export due to difficulties in controlling measurement conditions in vivo. Therefore, an in vitro transport assay system is required for further understanding of the Type III protein transport. Here we summarize the new in vitro method to analyze the Type III protein transport and the energy utilization mechanism found by using our method [25].
Figure 1.
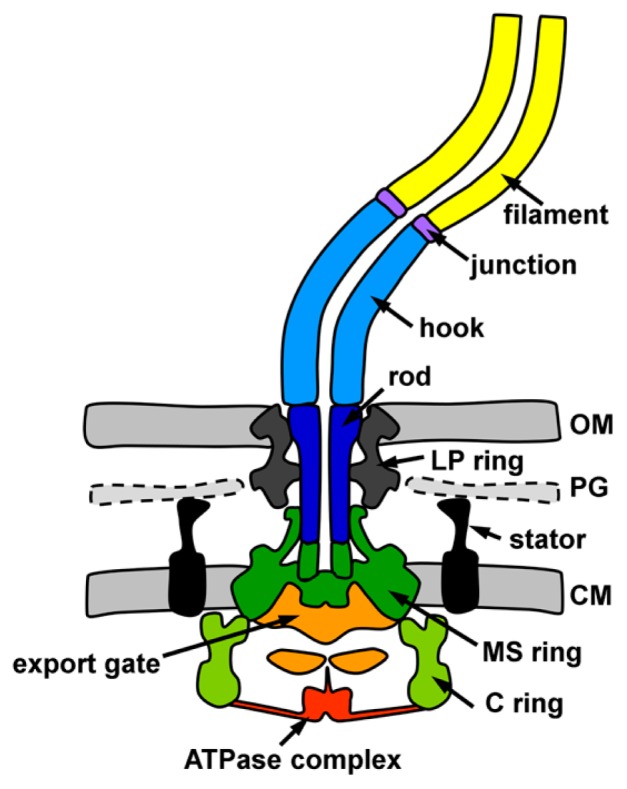
Cartoons diagram of the Salmonella flagellum. The functional parts of the flagellum are showed in following colors: MS ring, green; C ring, light green; transmembrane export gate, orange; cytoplasmic ATPase complex, red; rod, blue; hook, cyan; hook-filament junction, purple; filament, yellow; LP ring, gray; stator, black. CM, cytoplasmic membrane; PG, peptidoglycan layer; OM, outer membrane.
Construction of an in vitro protein transport assay system based on inverted membrane vesicles
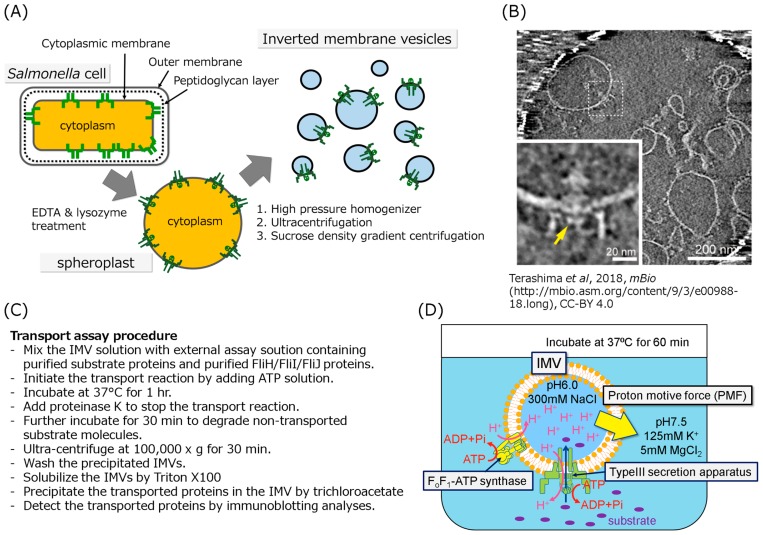
When people plan to construct a biochemical in vitro experiment, they often have to purify protein molecules or complexes of interest. The export apparatus, however, has never been purified as an active complex so far because the export apparatus with the base structure is a huge transmembrane protein complex with molecular mass of over 7 MDa. Therefore, an inverted membrane vesicle (IMV)-based protein transport assay system seems to be a possible in vitro method. Since the IMV is a membrane vesicle with inside-out orientation derived from the cytoplasmic membrane, we can precisely control measurement conditions for protein transport. To prepare the IMVs, we introduced the following mutations into Salmonella cells: fliT null mutation and exogenous FlhD/FlhC expression to increase the number of flagellum; flhB N269A mutation to lock the export apparatus in the rod-hook type secretion mode; flgD null mutation to use FlgD as an export substrate to evaluate the protein transport activity of IMV [26–28]. We removed the outer membrane and the peptidoglycan layer from the grown mutant cells with EDTA and lysozyme, respectively, to produce spheroplasts (Fig. 2A). The spheroplasts were disrupted by high-pressure cell homogenizer to invert the cytoplasmic membrane (Fig. 2A). To purify the IMVs, the IMVs were precipitated by ultra-centrifugation, then separated from the contaminated outer membrane, protein aggregations and DNA by sucrose density gradient centrifugation (Fig. 2A). To verify the orientation of the membrane vesicles, limited-proteolytic analysis with proteinase K was performed. FlhA and FliI, which are located at the cytoplasmic side, were degraded, indicating that they face outward on the vesicles. Furthermore, the basal body with the cytoplasmic C-ring exposed on the outside of the vesicle was observed by electron cryotomography (Fig. 2B). Therefore, we concluded that the orientation of the IMVs is inside out. Next, we designed a transport assay method using IMVs (Fig. 2C, 2D). Since protein export by T3SS is driven by primarily PMF, we applied the initial PMF by providing the pH and ionic strength differences between inside and outside of the IMVs, and maintained the PMF by proton pumping by reverse reaction of endogenous FoF1- ATP synthase. ATP-Mg2+ was added into the assay solution containing the IMVs, purified substrate proteins and FliJ to initiate the protein transport, and the solution was incubated at 37 degree Celsius for 60 min. To stop the protein transport and digest non-transported proteins, proteinase K was added in the assay solution. The substrate proteins transported into the IMVs were detected by immunoblotting.
Figure 2.
Schematic drawing of the typical in vitro protein transport assay. (A) Schematic drawing of the IMV preparation. The cultivated cells were converted to spheroplasts by treatment with EDTA and lysozyme to remove the outer membrane and the peptidoglycan layer, respectively, then disrupted by a high pressure homogenizer to form the inside-out vesicles. The vesicles were purified by ultracentrifugation and sucrose density gradient centrifugation. (B) Electron cryotomographic image of the typical IMV in which the flagellar basal structure is embedded. The lower left panel is an enlarged image of the basal body in the IMV. The disk like density formed by FlhA cytoplasmic domain is indicated by yellow arrow. This image was cited from Terashima et al. In Vitro Reconstitution of Functional Type III Protein Export and Insights into Flagellar Assembly. MBio. 9, e00988–18. (2018) [25]. Please read the full license for further details at—https://creativecommons.org/licenses/by/4.0/. (C) (D) The in vitro Type III protein transport assay method. (C) The procedures for the in vitro transport assay was described as a list. (D) To apply the initial PMF to the IMV, the IMVs filled with 300 mM Na+ solution at pH 6.0 were suspended in solution at pH 7.5 containing 125 mM K+ and 5 mM MgCl2. The inverted membrane vesicle is mixed with the export substrates, ATP-Mg2+, the FliH2/FliI complex and/or FliJ depending on experiment purpose. Addition of ATP induces proton pumping into the IMVs by reverse reaction of the endogenous FoF1-ATP synthase, resulting in generating PMF across the membrane. The PMF and ATP hydrolysis drive the protein transport into the IMV. The transported proteins are detected by immunoblotting.
The IMV-based assay system preserved protein transport ability as well as that seen in cells
First of all, we confirmed the protein transport ability of the IMV using purified FlgD, FlgE and FliK. FlgE is a hook protein and FlgD is a scaffolding protein for hook formation [28]. FlgE is incorporated into the growing hook beneath the FlgD cap complex that is attached on the tip of the hook [29]. FliK is a molecular ruler protein which regulates the hook length at c.a. 55 (±6) nm [30]. FlgD was transported into the IMVs in the presence of ATP-Mg2+ but not in the absence of ATP-Mg2+. Addition of CCCP, a proton ionophore, hindered the protein transport, indicating that protein transport in IMV was driven by PMF as well as that in cell. Next, we investigated whether the FliH2/FliI complex and FliJ in the assay solution affect on the protein transport because FliI shows turn-over between the ATPase complex attached on the export gate and the cytoplasmic pool [31]. Transport activity was significantly enhanced when both the FliH2/FliI complex and FliJ were added in the assay solution, but transport activity was little changed by adding the FliH2/FliI complex or FliJ alone in the assay solution. The IMVs pre-incubated with the FliH2/FliI complex and FliJ showed only slight enhancement of transport activity. These results indicate that the presence of FliH, FliI and FliJ in the cytoplasmic pool is required for efficient protein export. Although the IMVs show protein export activity, it is still obscure whether the protein export proceeds as in the cell. If the protein export process in IMV fully retains that in cell, the transported proteins would be assembled into the axial structure. Therefore, we added FlgE with/without FlgD to the assay solution, and then observed the IMVs by electron cryotomography. The transported FlgE molecules assembled into the hook on the rod in IMV. Next, we tried to regulate the hook length by adding FliK in the assay solution. IMVs were prepared from a Salmonella ΔflhB ΔflgD ΔfliT mutant strain expressing wild-type FlhB, instead of FlhB N269A, to switch the substrate specificity upon completion of the hook. In the absence of FliK, the polyhook, the abnormally long hook structure, was formed in IMV, but in the presence of 4 μM FliK, the average length of the hook was controlled at c.a. 55 (±22) nm. These results indicate that only FliK is required to terminate the transport of the rodhook type proteins. These in vitro experiments clearly show that our in vitro assay system fully reproduces the T3SS protein export and following events, such as the hook assembly process and substrate specificity switching as in the cell.
Analysis of energy transduction mechanism by using the in vitro protein transport assay system
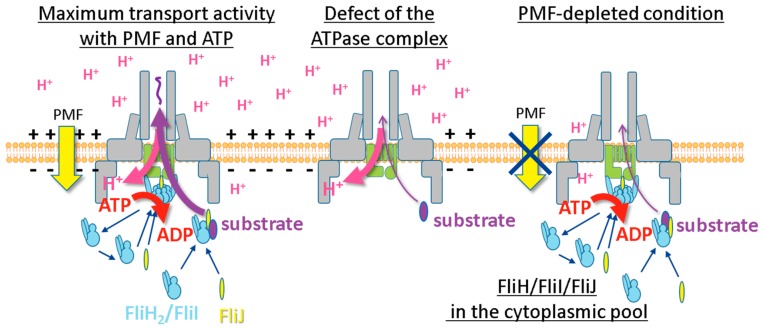
Protein export by T3SS is thought to be primarily driven by PMF and facilitated by ATP hydrolysis energy [7,8,15,20], but the roles of these energies are still not clear. To clarify the role of ATP hydrolysis energy produced by the T3SS ATPase complex, we tried to examine the protein transport without PMF. Since our IMV includes endogenous FoF1-ATP synthase, we genetically disrupted both fliH-fliI genes and FoF1-ATP synthase locus (subunit α, β, γ, δ, ɛ, A, B, C and I) from the Salmonella Δ flhB Δ flgD Δ fliT strain expressing FlhB N269A and FlhD/FlhC, then prepared the IMVs without the pH and ion concentration gradient. In the PMF-depleted condition, surprisingly, FlgD transport activity was still retained when the FliH2/FliI complex, FliJ and ATP-Mg2+ were present in the external solution. In order to further confirm the protein export without PMF, we added CCCP in the assay solution with the FliH2/FliI complex, FliJ and ATP-Mg2+. FlgD transport was not affected by CCCP addition. Moreover, the polyhook formation was observed in the PMF-depleted condition when FlgD and FlgE were added in the assay solution, indicating that the flagellar T3SS can transport proteins without PMF if the FliH2/FliI complex, FliJ and ATP-Mg2+ are present in solution. To confirm whether ATP hydrolysis by FliI ATPase contributes to protein export, FliI E211Q mutant protein, which binds ATP but does not hydrolyze it, was added to the assay solution. Unlike wild-type FliI, the protein transport didn’t occur. These results indicate that protein export by T3SS can be driven by only ATP hydrolysis energy produced by FliI ATPase even in the absence of PMF (Fig. 3). Our study clearly revealed that T3SS can drive protein export by using either PMF or ATP hydrolysis energy, although they are both needed for the maximum export activity. It is still unclear how the ATP hydrolysis energy by FliI ATPase is utilized to drive protein export. Thus, further investigations are needed for understanding the role of ATP hydrolysis energy.
Figure 3.
Three possilble energy transduction modes of T3SS. The left panel, protein export by cooperative use of PMF and the ATP hydrolysis energy in wild-type cells; the middle panel, protein export by PMF without the ATP hydrolysis energy in the fliH-fliI null mutant cell; the right panel, protein export by the ATP hydrolysis energy without PMF.
Conclusion
Our in vitro protein transport assay system based on IMVs reproduced protein export and flagellar assembly as well as those seen in cell. Although the previous in vivo studies have shown that T3SS is primarily driven by PMF and facilitated by ATP hydrolysis energy, the transport analyses using IMVs revealed that the export apparatus can export the substrates using only ATP hydrolysis energy. This suggests that T3SS is a “hybrid-engined” protein translocator that is capable of using either PMF or ATP hydrolysis energy as the energy source. The energetic robustness may contribute to maintain the protein export activity of T3SS against environmental perturbations.
Acknowledgements
We gratefully thank Tohru Minamino, Akihiro Kawamoto, Keiichi Namba and Michio Homma for continuous support and encouragement. Our research is supported in part by JSPS KAKENHI Grant Numbers JP15H02386 (to K. I.) and MEXT KAKENHI Grant Numbers 23115008 (to K. I.).
Footnotes
Conflict of Interest
The authors declare no conflict of interests.
Author Contribution
H. T. and K. I. wrote the paper.
References
- 1.Minamino T, Imada K, Namba K. Mechanisms of type III protein export for bacterial flagellar assembly. Mol Biosyst. 2008;4:1105–1115. doi: 10.1039/b808065h. [DOI] [PubMed] [Google Scholar]
- 2.Cornelis GR. The type III secretion injectisome. Nat Rev Microbiol. 2006;4:811–825. doi: 10.1038/nrmicro1526. [DOI] [PubMed] [Google Scholar]
- 3.Iino T. Assembly of Salmonella flagellin in vitro and in vivo. J Supramol Struct. 1974;2:372–384. doi: 10.1002/jss.400020226. [DOI] [PubMed] [Google Scholar]
- 4.Zhao Z, Zhao Y, Zhuang XY, Lo WC, Baker MAB, Lo CJ, et al. Frequent pauses in Escherichia coli flagella elongation revealed by single cell real-time fluorescence imaging Nat Commun 918852018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Macnab RM. Type III flagellar protein export and flagellar assembly. Biochem Biophys Acta. 2004;1694:207–217. doi: 10.1016/j.bbamcr.2004.04.005. [DOI] [PubMed] [Google Scholar]
- 6.Terashima H, Kojima S, Homma M. Flagellar motility in bacteria: structure and function of flagellar motor. Int Rev Cell Mol Biol. 2008;270:39–85. doi: 10.1016/S1937-6448(08)01402-0. [DOI] [PubMed] [Google Scholar]
- 7.Minamino T, Namba K. Distinct roles of the FliI ATPase and proton motive force in bacterial flagellar protein export. Nature. 2008;451:485–488. doi: 10.1038/nature06449. [DOI] [PubMed] [Google Scholar]
- 8.Paul K, Erhardt M, Hirano T, Blair DF, Hughes KT. Energy source of flagellar type III secretion. Nature. 2008;451:489–492. doi: 10.1038/nature06497. [DOI] [PubMed] [Google Scholar]
- 9.Kawamoto A, Morimoto YV, Miyata T, Minamino T, Hughes KT, Kato T, et al. Common and distinct structural features of Salmonella injectisome and flagellar basal body. Sci Rep. 2013;3:3369. doi: 10.1038/srep03369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fan F, Macnab RM. Enzymatic characterization of FliI: an ATPase involved in flagellar assembly in Salmonella typhimurium. J Biol Chem. 1996;271:31981–31988. doi: 10.1074/jbc.271.50.31981. [DOI] [PubMed] [Google Scholar]
- 11.Imada K, Minamino T, Tahara A, Namba K. Structural similarity between the flagellar type III ATPase FliI and F1-ATPase subunits. Proc Natl Acad Sci USA. 2007;104:485–490. doi: 10.1073/pnas.0608090104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Imada K, Minamino T, Uchida Y, Kinoshita M, Namba K. Insight into the flagella type III export revealed by the complex structure of the type III ATPase and its regulator. Proc Natl Acad Sci USA. 2016;113:3633–3638. doi: 10.1073/pnas.1524025113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Minamino T, Macnab RM. FliH, a soluble component of the type III flagellar export apparatus of Salmonella, forms a complex with FliI and inhibits its ATPase activity. Mol Microbiol. 2000;37:1494–1503. doi: 10.1046/j.1365-2958.2000.02106.x. [DOI] [PubMed] [Google Scholar]
- 14.González-Pedrajo B, Fraser GM, Minamino T, Macnab RM. Molecular dissection of Salmonella FliH, a regulator of the ATPase FliI and the type III flagellar protein export pathway. Mol Microbiol. 2002;45:967–982. doi: 10.1046/j.1365-2958.2002.03047.x. [DOI] [PubMed] [Google Scholar]
- 15.Minamino T, Kinoshita M, Inoue Y, Morimoto YV, Ihara K, Koya S, et al. FliH and FliI ensure efficient energy coupling of flagellar type III protein export in Salmonella. Microbiologyopen. 2016;5:424–435. doi: 10.1002/mbo3.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ibuki T, Imada K, Minamino T, Kato T, Miyata T, Namba K. Common architecture of the flagellar type III protein export apparatus and F- and V-type ATPases. Nat Struct Mol Biol. 2011;18:277–282. doi: 10.1038/nsmb.1977. [DOI] [PubMed] [Google Scholar]
- 17.Thomas J, Stafford GP, Hughes C. Docking of cytosolic chaperone-substrate complexes at the membrane ATPase during flagellar type III protein export. Proc Natl Acad Sci USA. 2004;101:3945–3950. doi: 10.1073/pnas.0307223101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Imada K, Minamino T, Kinoshita M, Furukawa Y, Namba K. Structural insight into the regulatory mechanisms of interactions of the flagellar type III chaperone FliT with its binding partners. Proc Natl Acad Sci USA. 2010;107:8812–8817. doi: 10.1073/pnas.1001866107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Minamino T, Kinoshita M, Imada K, Namba K. Interaction between FliI ATPase and a flagellar chaperone FliT during bacterial flagellar protein export. Mol Microbiol. 2012;83:168–178. doi: 10.1111/j.1365-2958.2011.07924.x. [DOI] [PubMed] [Google Scholar]
- 20.Minamino T, Morimoto YV, Hara N, Namba K. An energy transduction mechanism used in bacterial flagellar type III protein export. Nat Commun. 2011;2:475. doi: 10.1038/ncomms1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Morimoto YV, Kami-Ike N, Miyata T, Kawamoto A, Kato T, Namba K, et al. High-resolution pH imaging of living bacterial cells to detect local pH differences MBio 7e01911–e019162016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Minamino T, Morimoto YV, Hara N, Aldridge PD, Namba K. The bacterial flagellar type III export gate complex is a dual fuel engine that can use both H+ and Na+ for flagellar protein export. PLoS Pathog. 2016;12:e1005495. doi: 10.1371/journal.ppat.1005495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Minamino T, Morimoto YV, Kinoshita M, Aldridge PD, Namba K. The bacterial flagellar protein export apparatus processively transports flagellar proteins even with extremely infrequent ATP hydrolysis. Sci Rep. 2014;4:7579. doi: 10.1038/srep07579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Akeda Y, Galán JE. Chaperone release and unfolding of substrates in type III secretion. Nature. 2005;437:911–915. doi: 10.1038/nature03992. [DOI] [PubMed] [Google Scholar]
- 25.Terashima H, Kawamoto A, Tatsumi C, Namba K, Minamino T, Imada K. In vitro reconstitution of functional type III protein export and insights into flagellar assembly. MBio. 2018;9:e00988–18. doi: 10.1128/mBio.00988-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Aldridge C, Poonchareon K, Saini S, Ewen T, Soloyva A, Rao CV, et al. The interaction dynamics of a negative feedback loop regulates flagellar number in Salmonella enterica serovar Typhimurium. Mol Microbiol. 2010;78:1416–1430. doi: 10.1111/j.1365-2958.2010.07415.x. [DOI] [PubMed] [Google Scholar]
- 27.Fraser GM, Hirano T, Ferris HU, Devgan LL, Kihara M, Macnab RM. Substrate specificity of type III flagellar protein export in Salmonella is controlled by subdomain interactions in FlhB. Mol Microbiol. 2003;48:1043–1057. doi: 10.1046/j.1365-2958.2003.03487.x. [DOI] [PubMed] [Google Scholar]
- 28.Ohnishi K, Ohto Y, Aizawa S, Macnab RM, Iino T. FlgD is a scaffolding protein needed for flagellar hook assembly in Salmonella typhimurium. J Bacteriol. 1994;176:2272–2281. doi: 10.1128/jb.176.8.2272-2281.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Homma M, Iino T. Locations of hook-associated proteins in flagellar structures of Salmonella typhimurium. J Bacteriol. 1985;162:183–189. doi: 10.1128/jb.162.1.183-189.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moriya N, Minamino T, Hughes KT, Macnab RM, Namba K. The type III flagellar export specificity switch is dependent on FliK ruler and a molecular clock. J Mol Biol. 2006;359:466–477. doi: 10.1016/j.jmb.2006.03.025. [DOI] [PubMed] [Google Scholar]
- 31.Bai F, Morimoto YV, Yoshimura SD, Hara N, Kami-Ike N, Namba K, et al. Assembly dynamics and the roles of FliI ATPase of the bacterial flagellar export apparatus. Sci Rep. 2014;4:6528. doi: 10.1038/srep06528. [DOI] [PMC free article] [PubMed] [Google Scholar]