Abstract
Ageing is the primary risk factor for osteoarthritis (OA). A decline in the ageing-associated process of autophagy is suggested as a potential contributor to OA development. Polyamines such as spermidine decrease during ageing, contributing to impaired autophagy and reduced cellular function. However, the role of polyamines and their effect on the regulatory mechanism governing autophagy in aged and arthritic cartilage tissue has not been established. Elucidating if polyamine regulation of autophagy is impaired during ageing and OA in chondrocytes may lead to improved treatment approaches to protect against cartilage degradation. Our results indicate that polyamine synthesis was decreased in aged and OA cartilage, along with reduced autophagy activity, evidenced by decreased autophagy-related gene and protein expression and autophagosome formation. Importantly, spermidine treatment increased the expression of the acetyltransferase EP300, which binds to crucial autophagy proteins, Beclin1 and LC3, and elevates chondrocyte autophagy. Our data indicate spermidine prevents the ageing- and OA-related decrease in autophagy and may protect against OA development.
Aging: toward a natural treatment for osteoarthritis
The naturally occurring small molecule spermidine might prevent and repair the cartilage damage in osteoarthritis, commonly associated with aging. Spermidine was first found in semen but is widely distributed in animals and plants and is known to promote longevity in mammals. During normal aging, a process of “autophagy” in which cells degrade and recycle used materials is known to decline in chrondrocytes, cells which produce and maintain cartilage. Researchers in the UK led by James Edwards at the University of Oxford have uncovered details of the biochemical mechanisms linking spermidine and autophagy. Treating cultured human and mouse chrondrocytes with spermidine can reverse the decline in autophagy. The researchers identified specific genes and proteins involved in spermidine’s effects. Spermidine treatment might restore normal autophagy, combatting osteoarthritis.
Introduction
Osteoarthritis (OA) is the most common form of arthritis characterised by cartilage degradation, synovitis and pain1. There are no effective therapeutic options for OA1. The primary risk factor for OA is increased age, where chondrocytes, the only resident cells in cartilage, fail to maintain extracellular matrix turnover and cartilage integrity diminishes over time2. The well-conserved cellular process of autophagy (self-eating), where unwanted or dysfunctional cytoplasmic constituents are isolated, degraded and recycled, decreases with age, leading to an accumulation of toxic and non-functional protein elements3. Autophagy decreases in OA4,5 and mice deficient in the key autophagy protein ATG5, have been shown to develop premature OA6. Spermidine an organic polyamine, found in peas and whole grains, has previously been shown to enhance autophagy and protect against age-related pathologies7. However, the mechanistic regulation of autophagy by spermidine is not fully understood and the role of polyamines in OA is not established. Previously, the regulation of the epigenetic modifier and acetyltransferase EP300, has been linked to changes in autophagic flux, and where inhibition of EP300 by spermidine treatment may influence normal cell biology through post-translational modifications of essential autophagy-related protein complexes8,9. Importantly, the role of EP300 in chondrocytes is also not established. This study investigates the expression and autophagy-related protein interactions in ageing- and OA-cartilage and whether spermidine-induced EP300 regulation might provide a mechanism through which chondrocyte autophagy in aged cartilage and OA might be reactivated.
Material and methods
Isolation of human chondrocytes
Healthy or OA knee/hip cartilage was obtained from surgical patients. Tissue samples were collected with informed donor consent in full compliance with national and institutional ethical requirements, the United Kingdom Human Tissue Act, and the Declaration of Helsinki. Dissected cartilage pieces were incubated overnight in DMEM with 1 mg/ml Collagenase A (Roche Pharmaceuticals) at 37 °C for 5–6 h to isolate cells. Cells used in experiments were at passage 1.
Murine studies
Wild-type young (2 months) or old (16 months) C57BL/6 mice (Jackson Laboratory) were used. All animal procedures were approved by the British Home Office, under the United Kingdom Animal (Scientific Procedures) Act 1986. Femoral heads of mice were avulsed and placed in culture media, Dulbecco’s modified Eagle’s medium (DMEM), for ex vivo experiments.
Cell culture
Isolated human chondrocytes and HTB-94 chondrosarcoma cells (American Type Culture Collection) were cultured in DMEM containing 4.5 g/l of glucose and L-glutamine (Lonza), 10% foetal calf serum (PAA Laboratories), 1% Penicillin and Streptomycin (Cambrex), Amphotericin B (Gibco) and HEPES solution (Cambrex) unless otherwise stated.
siRNA transfection
Healthy isolated human chondrocytes were wet reverse transfected with Dharmacon ON-TARGETplus SMARTpool (4 oligos) human EP300 siRNA (25 nM) or ON-TARGETplus EP300 siRNA (Dharmacon Technologies) for 24 h. A scramble oligo sequence was used as control (25 nM) (Dharmacon Technologies).
Drug treatments
Cells were seeded at 300,000 cells per well in six-well plates in serum starved media for 1 h then washed. Thereafter, cells were seeded in complete DMEM media treated with either spermidine (100 nM; Sigma-Aldrich) or the control of DMSO (Sigma-Aldrich) for 2 h. For fluorescence-activated cell sorting (FACS) experiments, Bafliomycin A1 (BAF; 10 nM; Sigma-Aldrich) was used alongside other treatments as a positive control. For green fluorescent protein (GFP) studies, groups of cells were seeded in serum starved media alongside drug treatments for positive controls. To evaluate the effect of spermidine on EP300, human chondrocytes were transfected with EP300 siRNA for 24 h then treated with spermidine (100 nM) for 2 h.
Fluorescence-activated cell sorting
Autophagy was also measured by quantifying Microtubule-associated proteins 1 A/1B light chain 3B (LC3)-II mean fluorescence intensity using the FlowCellect Autophagy LC3 Antibody-based Assay Kit (Merck-Millipore) according to the manufacturer’s instructions after treating the cells with either spermidine (100 nM), the vehicle control of DMSO or/and BAF (10 nM). Untreated cells were also used as a negative control. Use of this kit includes a step where cytosolic LC3-I is washed from the cell, leaving only membrane bound LC3-II prior to staining.
LC3-GFP studies
LC3-GFP murine tissues were generously gifted by Prof Katja Simon (University of Oxford). These commercially available transgenic mice were previously described by Mizushima10. Hips from LC3-GFP mice were avulsed and placed in DMEM media containing either spermidine (100 nM) or DMSO. For positive controls, serum free DMEM containing either spermidine (100 nM) or DMSO was used. After an incubation of 2 h, hips were embedded in Optimal Cutting Temperature (CellPath) and snap-frozen. Thereafter, samples were cryosectioned at 10 µm. Fluorescence images were taken using an excitation wavelength of 473 nm and a band-path of 490−540 nm. Bright field images were obtained using scattering wavelength of 635 nm. The cells were segmented in bright field, and GFP intensity was measured in fluorescent channel. Intensity of background was subtracted from intensities of individual cells and divided by intensity of background in order to normalise and score cells. The number of LC3 puncta per cell was counted throughout each z stacks by two blinded operators.
Protein analysis
Cells were homogenized in lysis buffer (RIPA buffer) (Sigma-Aldrich), Ethylenediaminetetraacetic acid free protease inhibitor (Roche Pharmaceuticals), Phosphatase inhibitor cocktail 2 and 3 (Sigma-Aldrich) and protein levels quantified by bicinchoninic acid assay (Thermo Fisher Scientific). Lysed protein samples were probed overnight for western blotting with primary antibody: E1A-associated protein p300 (EP300) (Novus Biologicals), Beta (β)-actin (Sigma-Aldrich), Serine/threonine-protein kinase (ULK1) (Novus Biologicals), Beclin1 (Novus Biologicals), LC3 (Novus Biologicals), Acetylated-Lysine (Cell Signalling Technology), Type II collagen (COL2A1) (Sigma-Aldrich) or Sex determining region Y-box9 (SOX9) (Abcam); Nucleoporin p62 (p62) (Novus Biologicals). Murine hips were avulsed and placed in ice cold protein lysis buffer (prepared as above). Samples were shaken for 2 h at 4 °C and the supernatants collected for western blot analysis as outlined above.
RNA isolation and quantitative PCR
Total ribonucleic acid (RNA) was isolated from cell cultures using RNeasy Mini Kit (Qiagen) as per manufacturer’s instructions. Microdissected cartilage pooled from six mouse knee joints was collected, placed in TRIzol® (Life technologies) and homogenised using PowerGen™ Model 125 Homogenizer (Fisher Scientific). Thereafter the RNeasy Mini Kit protocol was followed. Thereafter, a High Capacity reverse transcription complementary deoxyribonucleic acid (cDNA) kit (Applied Biosystems) according to the recommendations of the manufacturer was used. Real-time-quantitative polymerase chain reaction (RT-qPCR) was carried out on a ViiA™ 7 System (Applied Biosystems). Relative gene expression of messenger RNA (mRNA) was analysed by the ∆∆Ct method using 18s as an endogenous control gene (Table 1). A total of 100 μl reaction mixture containing 50 μl cDNA template (500 ng starting RNA) was used as previously described11.
Table 1.
Primers used in RT-qPCR experiments
| Gene | Human | Murine |
|---|---|---|
| 18S | Hs03003631_g1 | Mm02601776_g1 |
| ACAN | Hs00153936_m1 | Mm00545794_m1 |
| ATG5 | Hs00169468_m1 | Mm00504340_m1 |
| ATG7 | Hs00893766_m1 | Mm00512209_m1 |
| BECN1 | Hs00177504_m1 | Mm01265461_m1 |
| COL2A1 | Hs00264051_m1 | Mm01309565_m1 |
| LC3 | Hs01076567_g1 | Mm00458724_m1 |
| SSAT | Hs00971739_g1 | Mm00485911_g1 |
| SPDS | Hs00171253_m1 | Mm 00445061_m1 |
| ODC1 | Hs00159739_m1 | Mm02019269_g1 |
| POA | Hs00382210_m1 | Mm00464096_m1 |
| EP300 | Hs00914223_m1 | Mm00625535_m1 |
| SOX-9 | Hs01001343_g1 | Mm00448840_m1 |
| ULK1 | Hs00177504_m1 | Mm00437238_m1 |
ACAN aggrecan, ATG5 autophagy related 5, ATG7 autophagy related 7, BECN1 Beclin1, SSAT spermidine/spermine N-1 acetyl transferase, SPDS spermidine synthase, ODC1 ornithine decarboxylase 1, POA polyamine oxidase
Electron microscopy
Microdissected cartilage from mice was fixed using 2.5% glutaraldehyde + 4% paraformaldehyde (stocks solutions both from Agar Scientific) in 0.1 M 1,4 Piperazine bis (2-ethanosulfonic acid) (Sigma-Aldrich) buffer at pH 7.2 at room temperature for 1–2 h, then at 4 °C until further processing. Samples were prepared as described previously [10] and imaged on an FEI company Tecnai 12 TEM with a Gatan US1000 camera. Thereafter, autophagosomes from images were counted from cells.
Immunohistochemistry
Heat mediated antigen retrieval was performed on paraffin embedded sections using citric acid buffer (Sigma-Aldrich) warmed in a water bath at 100 °C for 20 mins. LC3 (Novus Biologics) antibody and rabbit Immunoglobulin G (IgG) control (Santa Cruz Biotechnology) were used at 1:200 dilution. Sections were counter stained with Mayers Haematoxylin (Sigma-Aldrich). Slides were imaged using a light microscope (Olympus) at the magnification of x20. Thereafter, quantification of LC3 positive cells/total cells were counted.
Immunoprecipitation
EP300 or autophagy-related proteins were immunoprecipitated after 24 h treatment of HTB-94 cells treated spermidine (100 nM) or DMSO (control) using a commercially available immunoprecipitation kit (Pierce). Total acetyl lysine or autophagy targets for the respective experiments were detected by western blotting. β-Actin from input whole lysate was used as loading control.
Statistical analysis
All data are expressed as mean ± standard error of mean (S.E.M) of n observations. Experiments were statistically analysed utilising the Students unpaired t-test for parametric data with independent groups compared with their specific controls or time-matched controls. One-way analysis of variance (ANOVA) with the Bonferroni post hoc test or ANOVA with Tukeys comparison test was used to compare three or more groups. A significant difference was accepted when p < 0.05, p < 0.01, p < 0.001 or p < 0.0001 represented in all tables and Figures as *, **, *** or **** respectively. NS, non–significant. Data analysis was performed using GraphPad Prism® 5.0 (GraphPad Software, California, U.S.A).
Results
Decreased autophagy and polyamine synthesis in aged and OA human and murine cartilage
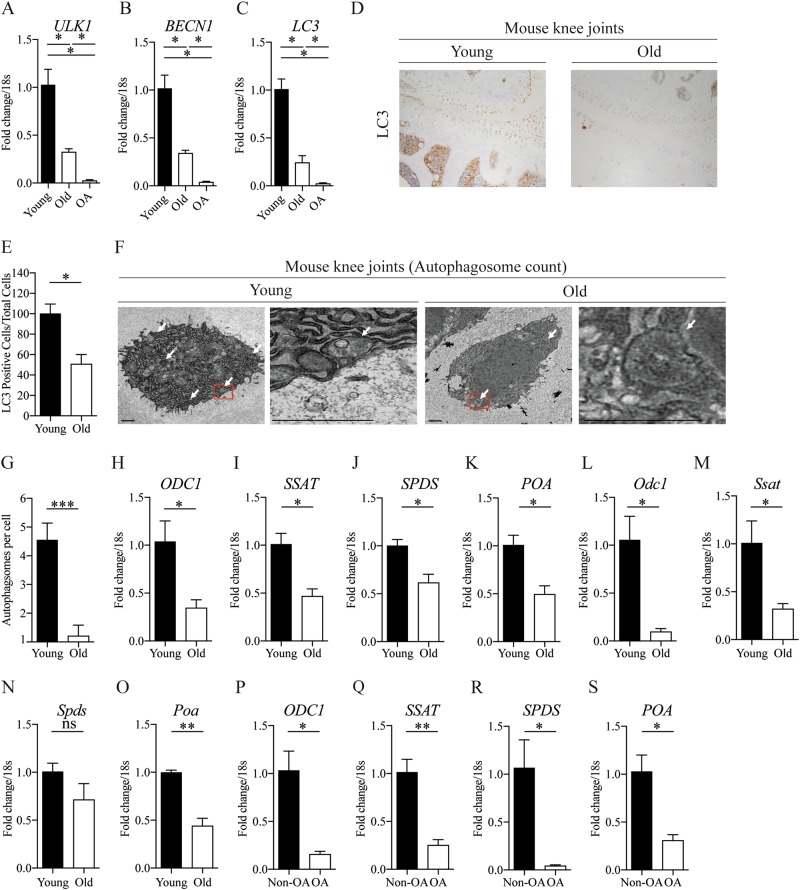
Normal human cartilage samples from aged individuals showed decreased expression of autophagy-related genes Ulk1, beclin1 and LC3 compared with samples of young cartilage. Autophagy-related protein expression was further reduced in OA samples compared to normal controls (Fig. 1a–c and Supplementary Figure 1a). Immunohistochemistry confirmed a similar decrease in LC3 protein expression within articular chondrocytes along with decreased autophagosome formation by scanning electron microscope in cartilage from aged mice compared to young control animals (Fig. 1d–g and Supplementary Figure 1b). Importantly, the mRNA expression of key rate limiting enzymes responsible for polyamine synthesis was also reduced, correlating with the decrease in autophagy levels seen in the same samples (Fig. 1h–s).
Fig. 1. Decreased autophagy and polyamine synthesis in aged and OA human and murine cartilage.
a–c Autophagy gene expression in isolated chondrocytes from young (21–37 years), old (62–68 ) and OA (49–86 years) knee joints (n = 3). d Representative images (x20) of LC3 protein expression in knee joints of WT mice at 2 months (young) or 16 months (old) of age. e Quantification of LC3 positive cells/total from murine knee joints (n = 4). f Representative EM images of chondrocytes from microdissected cartilage obtained from knee joints of WT young (2 months) or old (16 months) mice. White arrows highlight autophagosomes. Red boxes highlight the area of magnification. Scale bars: 1 μm. g Quantification of autophagosomes in chondrocytes (microdissected cartilage) from murine knee joints (n = 18 cells per mouse. six mice used). h–k RT-qPCR analysis gene expression of key enzymes involved in polyamine synthesis from isolated chondrocytes from young healthy (21–37 years) or old (62–68 years) (n = 3). l–o RT-qPCR analysis gene expression of key enzymes involved in spermidine synthesis in microdissected cartilage obtained from knee joints of WT young (2 months) or old mice (16 months) (n = 3). p–s RT-qPCR analysis of gene expression of key enzymes involved in spermidine synthesis in non-OA and OA human chondrocytes (n = 3). All RT-qPCR gene expressions were normalised to the endogenous level of 18s. All data are expressed as mean ± S.E.M of n observations. Students unpaired t-test was used for statistical analysis. p < 0.05, p < 0.01 or p < 0.001 represented in all tables and Figures as *, **, or ***, respectively. NS non–significant
Spermidine activates chondrocyte autophagy
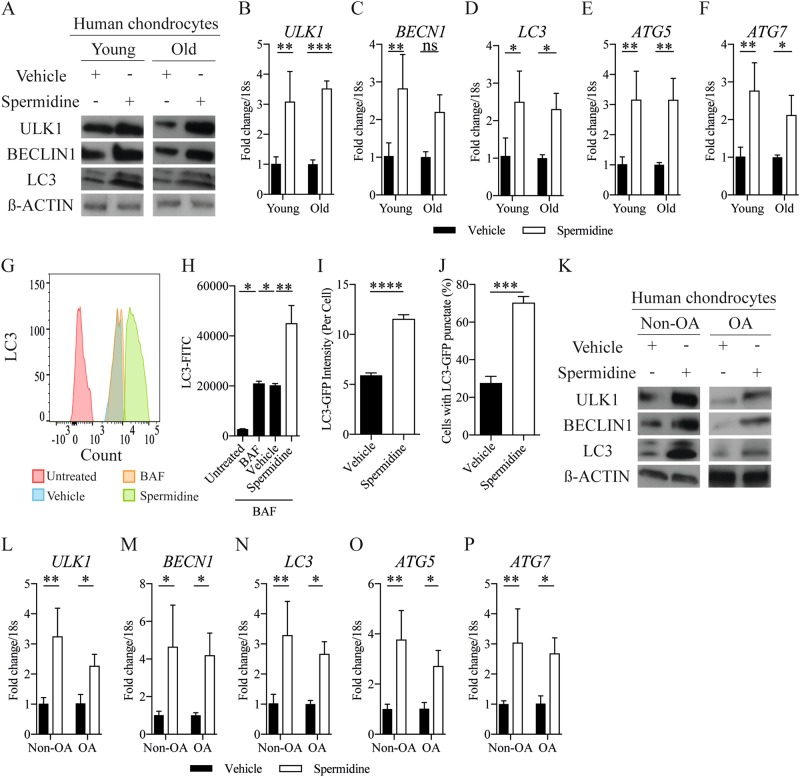
To determine whether stimulation with spermidine might activate autophagy in this system, protein and gene expression of critical autophagy-related molecules (ULK1, BECLIN1, LC3, ATG5 and ATG7) were examined following spermidine treatment. In both human and murine isolated chondrocytes, autophagy markers were significantly elevated compared to vehicle-treated control cells, this occurred in chondrocytes from both aged and young cartilage (Fig. 2a–f and Supplementary Figure 2a–f). Whereas, P62 (indicates impaired autophagy) protein expression decreased in young and old human isolated chondrocytes after spermidine stimulation (supplementary Figure 2g). The conversion from LC3-I to LC3-II is indicative of increased autophagy12. LC3 II mean fluorescent intensity increased in chondrocytes treated with spermidine (Fig. 2g and h). This finding was supported using LC3-GFP reporter mice, where isolated primary chondrocytes showed increased LC3-GFP + puncta (95.37%) and staining intensity per cell (154.17%) following spermidine stimulation (Fig. 2i–j and Supplementary Figure 2h-i). We also observed spermidine also increased protein and gene expression of key autophagy molecules in human isolated chondrocytes from OA patients compared to untreated control cells (Fig. 2k–p). Collectively, this suggests that the decline in chondrocyte autophagy and cellular function with ageing and OA might be reversible depending on the correct stimuli.
Fig. 2. Spermidine activates chondrocyte autophagy.
(a) Autophagy protein expression and (b–f) autophagy gene expression (RT-qPCR) in isolated chondrocytes from young (21–37 years) and old (62–68 years) human knee joints treated with DMSO control or spermidine (100 nM) (n = 3). g Histogram and (h) quantification of MFI of LC3-II in HTB-94 cells either untreated or treated with BAF (10 nM), alongside DMSO (vehicle control) or spermidine (100 nM), for 2 h (n = 3). i Total LC3-GFP intensity and (j) quantification of percentage of chondrocytes with LC3 positive punctate per chondrocyte obtained from avulsed femoral heads of LC3-GFP mice. Femoral heads were treated with either DMSO control or spermidine (100 nM) for 2 h before fixation (n = 15–37 cells from three mice per treatment group). k Protein expression and (l–p) gene expression (RT-qPCR) of key autophagy proteins in isolated chondrocytes from non-OA control or OA human knee joints. Cells were either treated with DMSO control or spermidine (100 nM) for 2 h (n = 3). All RT-qPCR gene expressions were normalised to the endogenous level of 18s. All data are expressed as mean ± S.E.M of n observations. Students unpaired t-test or ANOVA with Tukeys comparison were used for statistical analysis. p < 0.05, p < 0.01, p < 0.001 or p < 0.0001 represented in all tables and Figures as *, **, *** or ****, respectively. NS non–significant
Spermidine increases the expression of key chondrogenesis markers
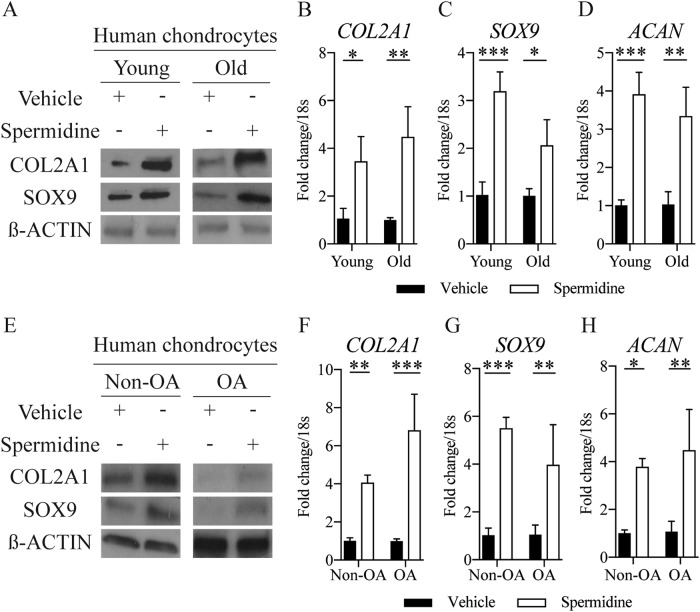
We also sought to understand if important chondrogenesis markers (COL2A1, AGGRECAN, SOX9) could be increased by spermidine alongside the expression of autophagy markers. To our surprise, in young and old human and murine chondrocytes and in human isolated OA chondrocytes, we observed significantly elevated markers of chondrogenesis in spermidine-treated groups compared to vehicle-treated controls (Fig. 3a–h and Supplementary Figure 2j-m). These indicate that spermidine may induce a potential repair response in cartilage by activating autophagy and key chondrogenesis markers.
Fig. 3. Spermidine increases the expression of key chondrogenesis markers.
a Protein expression and (b–d) gene expression (RT-qPCR) of key chondrogenesis markers in isolated chondrocytes from young (21–37 years) and old (62–68 years) human knee joints treated with DMSO control or spermidine (100 nM) (n = 3). e Protein expression and (f–h) gene expression (RT-qPCR) of key chondrogenesis markers in isolated chondrocytes from non-OA control or OA human knee joints. Cells were either treated with DMSO control or spermidine (100 nM) for 2 h (n = 3). All RT-qPCR gene expressions were normalised to the endogenous level of 18s. All data are expressed as mean ± S.E.M of n observations. Students unpaired t-test or ANOVA with Tukeys comparison were used for statistical analysis. p < 0.05, p < 0.01 or p < 0.001 represented in all tables and Figures as *, ** or ***, respectively. NS non–significant
Spermidine activates chondrocyte autophagy via EP300
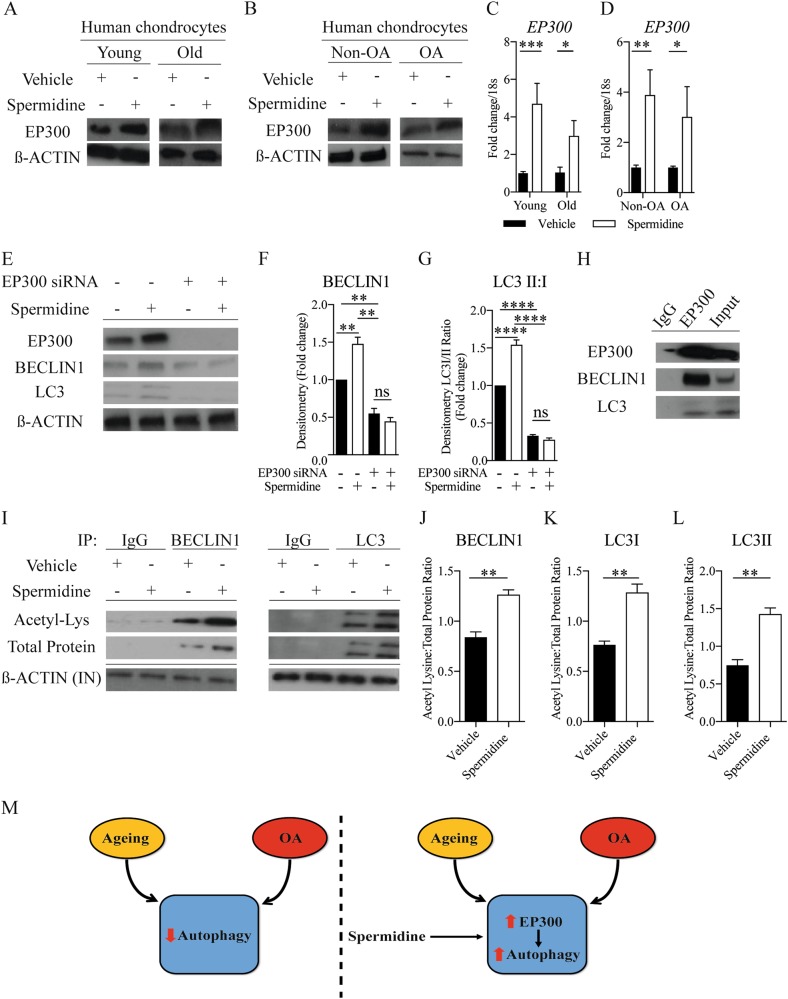
Previous studies have demonstrated that spermidine can activate autophagy via the acetyltransferase EP3008,13. Hence, we sought to determine whether spermidine activates (and re-activates) autophagy in chondrocytes by interacting with EP300 in this system. Following spermidine treatment, gene and protein expression of EP300 increased in human and murine young and old isolated chondrocytes and in human OA chondrocytes, compared to respective untreated control cells (Fig. 4a–d and Supplementary Figure 3a and b). To validate if spermidine activates chondrocyte autophagy via EP300, we silenced EP300 in chondrocytes prior to spermidine treatment. This led to the drug failing to increase the expression of autophagy proteins and, interestingly, knockdown of EP300 decreased expression of autophagy proteins beclin 1 and LC3 compared to control cells (Fig. 4e–g). Immunoprecipitation analysis determined that EP300 directly interacts with autophagy-related proteins, beclin 1 and LC3 (Fig. 4h). Moreover, both BECLIN1 and LC3 proteins were functionally modified by spermidine treatment, showing increased acetylation of lysine residues associated with enhanced protein activity and total protein expression overall (Fig. 4i–m).
Fig. 4. Spermidine activates chondrocyte autophagy via EP300.
EP300 protein expression in (a) isolated chondrocytes from young (21–37 years) and old (62–68 years) human knee joints (n = 3) or (b) from non-OA control and OA human knee joints (n = 3) treated with DMSO control or spermidine (100 nM) for 2 h. EP300 gene expression (RT-qPCR) (c) in isolated chondrocytes from young (21–37 years) and old (62–68 years) human knee joints (n = 3) or (d) in non-OA control and OA human knee joints (n = 3) treated with DMSO control or spermidine (100 nM) for 2 h. e Representative western blot (f and g) densitometry quantification of autophagy protein expressions in chondrocytes following EP300 siRNA or control siRNA transfection +/− spermidine (100 nM) or DMSO treatment for 2 h (n = 3). h Representative western blot showing the binding of autophagy markers using immunoprecipitation assays against EP300 in HTB-94 cells. i Western blots showing representative acetylation status (total acetyl lysine) and total protein expression of immunoprecipitated (IP) BECLIN1 and LC3 in HTB-94 cells treated with DMSO (control) or spermidine (100 nM) for 2 h. β-Actin from input (IN) lysate is shown as loading control. j–l Ratio of acetyl lysine compared to total protein of autophagy markers in HTB-94 cells treated with DMSO (control) or spermidine (100 nM) for 2 h (n = 3). m Ageing and OA leads to impaired regulation of autophagy. Pharmacologically targeting this impairment with spermidine upregulates chondrocyte autophagy via EP300. All RT-qPCR gene expressions were normalised to the endogenous level of 18s. All data are expressed as mean ± S.E.M of n observations. Students unpaired t-test or ANOVA with Tukeys comparison were used for statistical analysis. p < 0.05, p < 0.01, p < 0.001 or p < 0.0001 represented in all tables and Figures as *, **, ***, or ****, respectively. NS non–significant
Discussion
Collectively, our data suggest the decreasing levels of autophagy and polyamine synthesis occur in ageing and OA chondrocytes, and can be replenished by spermidine. Polyamines are polycations that are found in cells in many organisms and have been shown to decrease during ageing7. Transgenic animals overexpressing ODC and SSAT, the enzymes responsible for polyamine synthesis, demonstrated increased ageing phenotypes14. These findings support our data where a decrease in polyamine synthesis was observed in aged chondrocytes compared to young cells and in OA samples compared to non-OA samples. The decrease in enzymes-related to polyamine synthesis was accompanied directly by a decrease in autophagy. Previous studies have shown how decreased chondrocyte autophagy leads to cartilage damage and exacerbated OA4,6,15,16. Hence, we explored whether replenishing the deficiency of polyamines in aged chondrocytes can activate autophagy.
Spermidine is a naturally occurring compound, found in high concentrations in peas, brown rice and beans and decreases in expression during ageing7, whilst supplementation with spermidine increases lifespan in lower organisms9. Our data indicate spermidine increases autophagy in both young and old chondrocytes, indicating that the lower levels of polyamine-related enzymes in aged chondrocytes might be reversible when such cells are supplied with the correct stimuli, to restore adequate cellular function. However, OA-derived chondrocytes are typically aberrant and unresponsive. Surprisingly, spermidine increased autophagy in cells from both non-OA and OA patients, and indicating the potential to use polyamines to activate autophagy, and thereby reduce the development of OA. Previously, Carames et al.15 demonstrated the autophagy activator rapamycin protected against cartilage damage in vivo. However, unlike spermidine, rapamycin has toxicity concerns and many off-target mechanisms, including immunosuppression in humans17–19. Spermidine also increased markers of chondrogenesis in both healthy (young and old) and OA chondrocytes. These data may suggest polyamines could be used to promote repair or regenerate cartilage tissue.
Spermidine has previously been shown to function (in part) via activation of the EP300 acetyltransferase8,9. We noticed an increase in EP300 expression after spermidine treatment of chondrocyte, and simultaneous to autophagy activation, whereas silencing EP300 reduced autophagy-related protein expression, suggesting that EP300 is regulating spermidine-induced autophagy in this system. As a component of the TIP60 complex, EP300 has been shown to modify acetylation of autophagy-related proteins in lower organisms20, supporting our finding that EP300 binds directly to autophagy proteins to increase their acetylation status and activity in mammalian chondrocytes. Interestingly, spermidine has previously been used in vivo to enhance autophagy in a dose-dependent manner and protect against myopathy21, neurodegeneration22, and age-related defects in T cell response23. Further pre-clinical studies are required to investigate whether spermidine could prevent OA development in experimental animal models of arthritic disease.
Together our data indicate that polyamine synthesis is reduced in chondrocytes during ageing and replenishing this defect by spermidine treatment activates autophagy and promotes chondrogenesis. We have identified new direct interactions between the epigenetic modifier EP300 and autophagy-related proteins, where functional post-translational modifications occur to increase chondrocyte autophagy. Targeting autophagy pharmacologically with spermidine or other polyamines may therefore represent a viable, cost-effective and well-tolerated approach to the management of OA.
Electronic supplementary material
Acknowledgements
We want to thank Dr Erin Johnson and Dr Anna Pielach (University of Oxford) for helping us with electron microscopy. We also want to thank Dr Volodymyr Nechyporuk-Zloy (University of Oxford) for his assistance with confocal microscopy. We also express our thanks to Prof Katja Simon (University of Oxford) for generously providing us with LC3-GFP murine tissues. This work was supported by the Arthritis Research U.K. career developmental fellowship (20631) and Orthopaedic Research U.K. grant (509). Dr Pradeep Kumar Sacitharan was supported by a European Institute of Innovation & Technology transitional fellowship and a U.K.-U.S. all disciplines Fulbright research scholar award.
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Supplementary Information accompanies this paper at 10.1038/s12276-018-0149-3.
References
- 1.Glyn-Jones S, et al. Osteoarthritis. Lancet. 2015;386:376–387. doi: 10.1016/S0140-6736(14)60802-3. [DOI] [PubMed] [Google Scholar]
- 2.Wieland H, Michaelis M, Kirschbaum BJ, Rudolphi K. Osteoarthritis - an untreatable disease? Nat. Rev. Drug. Discov. 2005;4:331–344. doi: 10.1038/nrd1693. [DOI] [PubMed] [Google Scholar]
- 3.Rubinsztein DC, Mariño G, Kroemer G. Autophagy and aging. Cell. 2011;146:682–695. doi: 10.1016/j.cell.2011.07.030. [DOI] [PubMed] [Google Scholar]
- 4.Caramés B, Taniguchi N, Otsuki S, Blanco FJ, Lotz M. Autophagy is a protective mechanism in normal cartilage, and its aging-related loss is linked with cell death and osteoarthritis. Arthritis Rheum. 2010;62:791–801. doi: 10.1002/art.27305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Caramés B, Olmer M, Kiosses WB, Lotz MK. The relationship of autophagy defects to cartilage damage during joint aging in a mouse model. Arthritis Rheumatol. 2015;67:1568–1576. doi: 10.1002/art.39073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bouderlique T, et al. Targeted deletion of Atg5 in chondrocytes promotes age-related osteoarthritis. Ann. Rheum. Dis. 2015;3:627–631. doi: 10.1136/annrheumdis-2015-207742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Minois N, Carmona-Gutierrez D, Madeo F. Polyamines in aging and disease. Aging. 2011;3:716–732. doi: 10.18632/aging.100361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pietrocola F, et al. Spermidine induces autophagy by inhibiting the acetyltransferase EP300. Cell Death Differ. 2015;22:509–516. doi: 10.1038/cdd.2014.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eisenberg T, et al. Induction of autophagy by spermidine promotes longevity. Nat. Cell Biol. 2009;11:1305–1314. doi: 10.1038/ncb1975. [DOI] [PubMed] [Google Scholar]
- 10.Mizushima N. Chapter 2 methods for monitoring autophagy using GFP-LC3 transgenic mice. Methods Enzymol. 2009;451:13–23. doi: 10.1016/S0076-6879(08)03602-1. [DOI] [PubMed] [Google Scholar]
- 11.Burleigh A, et al. Joint immobilization prevents murine osteoarthritis and reveals the highly mechanosensitive nature of protease expression in vivo. Arthritis Rheum. 2012;64:2278–2288. doi: 10.1002/art.34420. [DOI] [PubMed] [Google Scholar]
- 12.Tanida I, Ueno T, Kominami E. LC3 and autophagy. Methods Mol. Biol. 2008;445:77–88. doi: 10.1007/978-1-59745-157-4_4. [DOI] [PubMed] [Google Scholar]
- 13.Yang Y, et al. Induction of autophagy by spermidine is neuroprotective via inhibition of caspase 3-mediated Beclin 1 cleavage. Cell Death Dis. 2017;8:e2738. doi: 10.1038/cddis.2017.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Suppola S, et al. Overexpression of spermidine/spermine N1-acetyltransferase under the control of mouse metallothionein I promoter in transgenic mice: evidence for a striking post-transcriptional regulation of transgene expression by a polyamine analogue. Biochem. J. 1999;338(Pt 2):311–316. doi: 10.1042/bj3380311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Carames B, et al. Autophagy activation by rapamycin reduces severity of experimental osteoarthritis. Ann. Rheum. Dis. 2012;71:575–581. doi: 10.1136/annrheumdis-2011-200557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang Y, et al. Cartilage-specific deletion of mTOR upregulates autophagy and protects mice from osteoarthritis. Ann. Rheum. Dis. 2014;7:1432–1440. doi: 10.1136/annrheumdis-2013-204599. [DOI] [PubMed] [Google Scholar]
- 17.Baroja-Mazo A, Revilla-Nuin B, Ramirez P, Pons JA. Immunosuppressive potency of mechanistic target of rapamycin inhibitors in solid-organ transplantation. World J. Transplant. 2016;6:183–192. doi: 10.5500/wjt.v6.i1.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li J, Kim SG, Blenis J. Rapamycin: one drug, many effects. Cell. Metab. 2014;19:373–379. doi: 10.1016/j.cmet.2014.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Casero RA, Marton LJ. Targeting polyamine metabolism and function in cancer and other hyperproliferative diseases. Nat. Rev. Drug. Discov. 2007;6:373–390. doi: 10.1038/nrd2243. [DOI] [PubMed] [Google Scholar]
- 20.Lin SY, et al. GSK3-TIP60-ULK1 signaling pathway links growth factor deprivation to autophagy. Science. 2012;336:477–481. doi: 10.1126/science.1217032. [DOI] [PubMed] [Google Scholar]
- 21.Chrisam M, et al. Reactivation of autophagy by spermidine ameliorates the myopathic defects of collagen VI-null mice. Autophagy. 2015;11:2142–2152. doi: 10.1080/15548627.2015.1108508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Büttner S, et al. Spermidine protects against α-synuclein neurotoxicity. Cell Cycle. 2014;13:3903–3908. doi: 10.4161/15384101.2014.973309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Puleston DJ, et al. Autophagy is a critical regulator of memory CD8(+) T cell formation. eLife. 2014;3:e03706. doi: 10.7554/eLife.03706. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.