Abstract
Background
Thyroid function tests (TFTs) are among the most requested tests internationally. However, testing practice is inconsistent, and potentially suboptimal and overly costly. The natural history of thyroid function remains poorly understood.
Aim
To establish the stability of thyroid function over time, and identify predictors of development of overt thyroid dysfunction.
Design and setting
Longitudinal follow-up in 19 general practices in the UK.
Method
A total of 2936 participants from the Birmingham Elderly Thyroid Study (BETS 1) with a baseline TFT result indicating euthyroid or subclinical state were re-tested after approximately 5 years. Change in thyroid-stimulating hormone (TSH), free thyroxine (FT4), and thyroid status between baseline and follow-up was determined. Predictors of progression to overt dysfunction were modelled.
Results
Participants contributed 12 919 person-years; 17 cases of overt thyroid dysfunction were identified, 13 having been classified at baseline as euthyroid and four as having subclinical thyroid dysfunction. Individuals with subclinical results at baseline were 10- and 16-fold more likely to develop overt hypothyroidism and hyperthyroidism, respectively, compared with euthyroid individuals. TSH and FT4 demonstrated significant stability over time, with 61% of participants having a repeat TSH concentration within 0.5 mIU/L of their original result. Predictors of overt hypothyroidism included new treatment with amiodarone (odds ratio [OR] 92.1), a new diagnosis of atrial fibrillation (OR 7.4), or renal disease (OR 4.8).
Conclusion
High stability of thyroid function demonstrated over the 5-year interval period should discourage repeat testing, especially when a euthyroid result is in the recent clinical record. Reduced repeat TFTs in older individuals is possible without conferring risk, and could result in significant cost savings.
Keywords: ageing, general practice, primary health care, subclinical thyroid dysfunction, symptoms, thyroid function test
INTRODUCTION
Disorders of the thyroid include both overt and subclinical hypothyroidism and hyperthyroidism. Subclinical thyroid dysfunction is common among older people, characterised by serum thyroid-stimulating hormone (TSH) outside the reference range, in association with serum thyroid hormone (free thyroxine [FT4] and triiodothyronine [FT3]) concentrations within the reference range.1–3 Both subclinical thyroid states are of limited clinical relevance. In overt thyroid disease states clinicians are generally more concerned about hypothyroidism, because onset is often non-specific and insidious, so the diagnosis is often missed. In contrast, hyperthyroidism will normally present with less common symptoms and be diagnosed promptly.
Within the UK, systematic screening is not recommended, partly because ‘The natural history of thyroid dysfunction remains unclear’.4 Because there is also uncertainty over management of subclinical disease, current practice is both variable and potentially suboptimal or excessively costly.5–7
Recommendations for how often thyroid function tests (TFTs) should be repeated after a previously normal or subclinical test result are lacking. The annual estimated cost of TFTs in the UK is £30 million, with the majority originating in primary care.5,8–9 Recent work undertaken by the authors suggests that, annually in UK general practice, TFTs are requested for around 30% of older patients without overt thyroid dysfunction (unpublished data). Available evidence suggests that primary care physicians (PCPs) repeatedly request TFTs in this patient group, in response to vague symptoms, previously mildly abnormal tests, or as part of other routine care monitoring.8 Few studies have explored the natural history of thyroid dysfunction, or the value of a single TFT within a primary care population. One small single-site primary care-based study followed 73 patients with subclinical hypothyroidism for 12 months, reporting that 17.8% developed overt disease and 5.5% reverted to a euthyroid state.1 Follow-up of the Whickham cohort identified increased odds of development of overt disease if an elevated TSH had previously been reported, but the 20-year interval used in this study and all-age cohort makes application of findings to an older population difficult.10
Secondary care studies are conflicting, with one study reporting an incidence of 9.9 overt cases per 100 patient–years, and a study with longer follow-up suggesting an annual incidence of 5.6, although the female-only population derived from secondary care limits generalisability.11,12 A further female-only cohort study of 252 individuals referred for elevated TSH reported a similar progression, with 19% requiring treatment for overt dysfunction or persistent elevated TSH (>10 mIU/L) over a 5-year interval.13 This Brazilian study was conducted in a region of iodine intake inadequacy, and relevance to the UK may be lacking. Although both populations and biochemical definitions of thyroid dysfunction differ, the much lower annual incidence reported in secondary care populations compared with the primary care study indicates more evidence is needed to identify predictors of overt disease outside of the secondary care setting.
How this fits in
Thyroid tests are commonly requested in the routine care of older adults. Subclinical thyroid dysfunction is a relatively common biochemical finding among older patients. Current practice for the management of a single test indicating mildly abnormal thyroid dysfunction in the older population is variable, and potentially suboptimal and overly costly. This large, population-based survey demonstrates significant stability in thyroid function over a period of up to 5 years in the older population, with 96% of individuals who were euthyroid at baseline remaining so, and <0.5% developing an overt hyperthyroid or hypothyroid status. Based on this evidence, routine repeat thyroid function testing among older individuals who have a recent (within 5 years) euthyroid result in their clinical record is not advised, unless clinically indicated.
Evidence for the progression of subclinical hyperthyroidism is similarly lacking. Sawin et al 14 reported that none of the 33 subclinically hyperthyroid patients they followed up for 4 years developed overt disease, and similar findings are reported by Woeber,15 with only one of 16 patients followed developing overt disease. Although these findings demonstrate consistency, numbers are small and a recent expert panel review concluded that there was insufficient evidence to comment on the natural history of subclinical hyperthyroidism.5
This study aimed to address some of the important gaps in the evidence base, namely to:
determine incidence of subclinical thyroid dysfunction in an older primary care population previously shown to be euthyroid;
establish the proportion of patients with subclinical thyroid dysfunction who revert to a euthyroid state, experience persisting subclinical dysfunction, or develop overt disease;
evaluate within-person variation in TSH and FT4 concentrations over a 5-year interval; and
identify predictors of progression to overt hyper/hypothyroidism.
METHOD
Background to the Birmingham Elderly Thyroid Study (BETS 1)
The present study comprises follow-up of the BETS 1 cohort, a screening study of 5881 patients aged >65 years from 20 practices representative of the UK, conducted between 2002 and 2004.16–19 Thyroid function tests measured TSH and FT4. Measurement of FT3 was undertaken as dictated by routine laboratory protocol.
Practice recruitment
A total of 19 of the original 20 practices in the BETS 1 study agreed to participate.
Inclusion criteria
Patients identified in BETS 1 as having thyroid function results within normal or subclinical ranges were included in this study.
A euthryroid status was defined as both TSH and FT4 being within ranges indicated in Table 1. Subclinical hypothyroidism and hyperthyroidism were defined as FT4 in range, and TSH being above or below reference range, respectively.
Table 1.
Reference ranges for thyroid function assays with their associated intra-assay coefficient of variation
| Test | Reference range | Intra-assay coefficient of variation, associated range (95% CI) |
|---|---|---|
| Thyroid-stimulating hormone (TSH) | 0.3–4.5 mlU/L | 1.5% (0.5 to 33.0 mlU/L) |
| Free thyroxine (FT4) | 10–22 pmol/L | 2.0–2.5% (9.0 to 66.0 pmol/L) |
| Free triiodothyronine (FT3) | 3.1–6.8 pmol/L | 2.0–3.5% (4.0 to 21.0 pmol/L) |
Exclusion criteria
BETS 1 participants were excluded if:
classification of thyroid status was not possible, or if they had overt thyroid dysfunction at baseline;
they were recruited to the active treatment arm of the randomised controlled trial (RCT) embedded within BETS 1;17
the responsible clinician deemed contact inappropriate; or
they were unable or unwilling to give informed consent.
Study procedure
All eligible patients were sent an invitation letter, patient information sheet, and response return slip, after receipt of which screening appointments were organised at their usual surgery. TFTs for BETS 2 occurred over a 9-month period, approximately 5 years after the initial screening.
Case note evaluation
Data on diagnoses and treatment, including known confounders such as amiodarone (which comprises 37% iodine), were collected from primary care records. All significant medical diagnoses were categorised in accordance with recognised major disease groups, as reported in BETS 1.16
The results of the TFTs immediately prior to commencement of treatment were extracted for participants having thyroid surgery, radioiodine therapy, or starting antithyroid drugs and thyroxine replacement therapy in the interval period. If one or both measurements had not been conducted immediately prior to initiation of treatment, medical records were reviewed to ascertain reason for commencement of therapy, and to enable classification of thyroid status.
Measurement and categorisation of thyroid function
TFTs were measured by electrochemiluminescent immunoassays (Roche E170, Roche Diagnostics, UK). The TSH assay was calibrated against the second International Reference Preparation 80/558 (lower limit of reporting 0.02 mIU/L, manufacturer’s quoted mean functional sensitivity 0.014 mIU/L). Laboratory reference ranges are reported in Table 1. Changes in the assays used to measure TSH, FT4, and FT3 occurred between the two studies.16 In parallel with this, a change to the standard reference ranges for TSH, FT4, and FT3 occurred. To enable comparison across the two timepoints, a correction factor was applied to the baseline TFT results, and subsequent reclassification of thyroid status was undertaken before data were compared across the time interval.
Thyroid status was classified as euthyroid, subclinically hypothyroid, overtly hypothyroid, subclinically hyperthyroid, or overtly hyperthyroid based on the reference ranges reported in Table 1.
Follow-up contribution in terms of patient years at risk was calculated for all subjects as the time interval between initial and follow-up screen. Those receiving thyroid function treatment were classified based on the results of the TFT immediately prior to commencement of treatment, and their contribution censored at this point.
Primary analysis
Incidence was calculated as number of cases divided by number of person-years at risk. Risks of developing disease were calculated, and risks compared for groups who were categorised as euthyroid and subclinical at BETS 1. Sensitivity analysis was performed reclassifying all patients who commenced therapy during the interval period as having overt thyroid dysfunction (for example, overt hyper- or hypothyroidism based on treatment given) and re-running analyses.
Binary logistic regressions were performed to identify predictors of development of overt hyper/hypothyroidism. The forward stepwise logistic regression (LR) method was used to identify variables with a significance level of 5% for inclusion and 10% for removal. Two additional variables were created, one to indicate subclinical thyroid status at BETS 1 and the other to indicate whether BETS 1 thyroid function status had been reclassified due to the application of the laboratory-defined correction factors. In total, 31 variables were available for construction of r models, including medical conditions (classed as absent/pre-existing/new [occurring in the interval between studies]), amiodarone use, alcohol intake, smoking status, family history of thyroid dysfunction, age, and deprivation measure (Index of Multiple Deprivation [IMD] 2004 score).20 Analyses were undertaken using SPSS (version 15.0).
Exploratory analysis
To explore stability of TSH and FT4 over time, the change in both measures between BETS 1 and BETS 2 was calculated for each participant, and BETS 1 values plotted against BETS 2 values to explore within-person change.
RESULTS
Eligibility for follow-up
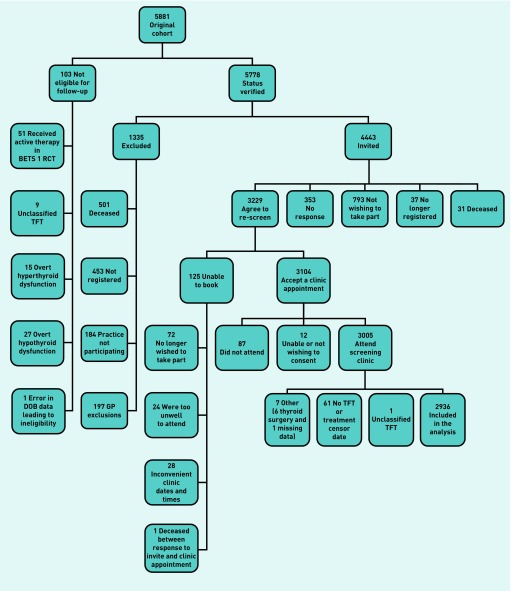
Overall, 103 BETS 1 participants were ineligible for follow-up (Figure 1). A further 1335 were deceased or excluded prior to invitation. Just under 50% of the BETS 1 cohort (n = 2936) fulfilled the inclusion criteria and attended for follow-up. Those available for follow-up demonstrated a statistically significant difference in deprivation scores and age compared with those not available for follow-up.
Figure 1.
Consort diagram. BETS 1 = Birmingham Elderly Thyroid Study 1. DOB = date of birth. RCT = randomised controlled trial. TFT = thyroid function tests.
The screened cohort had baseline IMD scores indicative of marginal greater affluence (mean IMD 21.65 versus 25.65) and were, on average, 1.82 years younger. This difference is likely to be attributable to greater mortality in older and less affluent participants, but given the large sample and 5-year follow-up period of an older adult cohort these difference are unlikely to impact findings.
Population characteristics
Age ranged from 68.7–96.4 years, with a mean of 76.9 years (standard deviation [SD] 5.03), and 49% were female. Socioeconomic status ranged from 3.16 (most affluent) to 74.4 (least affluent), mean IMD score 21.79 (SD 15.14).
Overall, 92.3% (2709/2936) were classified as euthyroid, 1% (n = 29) subclinically hyperthyroid, 6.2% (n = 181) subclinically hypothyroid, 0.3% (n = 8) as overtly hyperthyroid and 0.3% (n = 9) overtly hypothyroid. In addition, 1.8% (53/2936) had a thyroid diagnosis or treatment in the interim period, and were classified based on the TFT immediately prior to treatment commencement.
Status change over time
Overall, 95.5% (2644/2768, 95% confidence interval [CI] = 94.7 to 96.3%) of the individuals classified as euthyroid at baseline retained euthyroid status at follow-up. Six (0.2%, 95% CI = 0.1 to 0.5%) classified as euthyroid at baseline had developed overt hypothyroidism, and seven (0.3%, 95% CI = 0.1 to 0.5%) had developed overt hyperthyroidism. Only 3.5% (98/2768, 95% CI = 2.9 to 4.3%) had follow-up results indicative of a change from euthyroid to subclinical hypothyroidism, and 0.5% (13/2768, 95% CI = 0.3 to 0.8%) to subclinical hyperthyroidism (Table 2).
Table 2.
Thyroid status at baseline and follow-upa
| n= 2936 | Follow-up (BETS 2) status (1 unclassified) | |||||
|---|---|---|---|---|---|---|
| Overt hypothyroid, % n= 9 (0.3%) | Subclinical hypothyroid, % n= 181 (6.2%) | Euthyroid, % n= 2709 (92.3%) | Subclinical hyperthyroid, % n= 29 (1.0%) | Overt hyperthyroid, % n= 8 (0.3%) | ||
| Baseline (BETS 1) status | Subclinical hypothyroid (n= 143) | 3 (2.0) | 83 (58.0) | 57 (40) | 0 | 0 |
| Euthyroid (n= 2768) | 6 (0.2) | 98 (3.5) | 2644 (95.5) | 13 (0.5) | 7 (0.3) | |
| Subclinical hyperthyroid (n= 25) | 0 | 0 | 8 (32.0) | 16 (64.0) | 1 (4.0) | |
Shaded cells indicate no status change over the screening interval.
Of the 25 individuals classified as subclinically hyperthyroid at baseline, 16 (64.0%, 95% CI = 42.5 to 82.0%) retained this classification, eight (32.0%, 95% CI = 15.0 to 53.5%) reverted to euthyroid status, and one individual (4.0%, 95% CI = 0.1 to 20.4%) developed overt hyperthyroidism. A similar proportion, 58.0% (83/143, 95% CI = 49.5 to 66.2%), classified as being subclinically hypothyroid at baseline remained so, 40.0% (n = 57, 95% CI = 31.8 to 48.4%) reverted to euthyroid status, whereas 2.0% (n = 3, 95% CI = 0.4 to 6.0%) developed overt hypothyroidism (Table 2).
Sensitivity analysis
Because therapy may have disrupted natural history and dysfunction progression, all treated cases were re-categorised as having overt thyroid dysfunction. The total number of cases of assumed overt hypothyroidism therefore increases to 51 (26 being euthyroid and 25 subclinical at baseline), suggesting that a maximum of 1.7% may have developed overt hypothyroidism compared with the 0.3% estimate based on TFT results alone. The total number of cases of overt hyperthyroidism remains the same, because in all cases therapy was commenced based on TFT results in the overt range.
Cases of overt dysfunction
Overall, 2936 participants contributed 12 919 person-years for analysis. The risk of developing overt hypothyroidism in the subclinical hypothyroid group was 51.5 per 10 000 person-years at risk (95% CI = 38.8 to 67.1), compared with 4.9 (95% CI = 1.6 to 10.2) per 10 000 person-years at risk in the euthyroid group, making the subclinical group 10 times more likely to develop overt hypothyroid dysfunction.
Sensitivity analyses increase risk for an individual with a subclinical hypothyroid status to 20 times that for a euthyroid individual 429.5 (95% CI = 390.3 to 471.9) versus 21.3 (95% CI = 13.8 to 32.1) per 10 000 person-years at risk, respectively. It is noted that true risk is likely to be magnified in sensitivity analyses due to inclusion of 20 individuals who were euthyroid at baseline but treated with thyroxine during the interval period based on a subclinical result.
The risk of an individual with a subclinical hyperthyroid status developing overt hyperthyroidism was approximately 16 times greater than that of an individual with a euthyroid status — 95.4 (95% CI = 7.8 to 116.1) versus 5.7 (95% CI = 2.2 to 11.7) per 10 000 person-years at risk, respectively.
Predictors of development of overt thyroid dysfunction
Given the low number of events (overt thyroid disease), it was not possible to produce a robust model to predict development of overt dysfunction. However, given that, to achieve sufficient events for modelling, >14 000 individuals would require follow-up (from a 28 000 baseline population, based on the authors’ follow-up), the authors have provided a cautious model based on available data to identify factors that appear to increase risk of development of hypothyroidism (Table 3). Individuals with higher TSH or lower FT4 values at baseline are at greatest risk of development of overt hypothyroid status. Later diagnoses of atrial fibrillation (AF) or renal disease, or commencement of amiodarone, also increase the likelihood of progression.
Table 3.
Logistic regression: factors associated with development of hypothyroidism and associated likelihooda
| Variable | Coefficient | P-value | OR | 95% CI |
|---|---|---|---|---|
| Baseline TSH | 0.38 | 0.001 | 1.46 | 1.17 to 1.81 |
| Baseline FT4 | −0.86 | <0.001 | 0.42 | 0.27 to 0.66 |
| Newb amiodarone prescription | 4.61 | 0.001 | 92.1 | 5.64 to 1501.39 |
| Newb AF diagnosis | 2.00 | 0.012 | 7.41 | 1.56 to 35.14 |
| Newb renal disease diagnosis | 1.57 | 0.044 | 4.81 | 1.04 to 22.22 |
In all, 31 variables were available for construction of logistic regression models, comprising 22 disease categories, amiodarone use, alcohol use, smoking status, family history of thyroid dysfunction, age, sex, IMD score, and baseline TSH and FT4. Seven disease groups and family history were removed to maximise the dataset available for analysis where data were missing for ≥1% of the population. The forward stepwise method was used with a significance level of 5% for variable inclusion, and 10% for variable removal. Eight variables were entered to the final model, though existing (at baseline) diagnoses of AF, renal disease, or amiodarone use did not make a significant contribution to the final model.
Prescription or diagnosis occurring for the first time after the initial baseline screening episode. AF = atrial fibrillation. FT4 = free thyroxine. IMD = Index of Multiple Deprivation. OR = odds ratio. TSH = thyroid stimulating hormone.
The model constructed to predict development of hyperthyroidism failed to demonstrate any predictors, which is not surprising given the very low event rate.
Change in TSH and T4
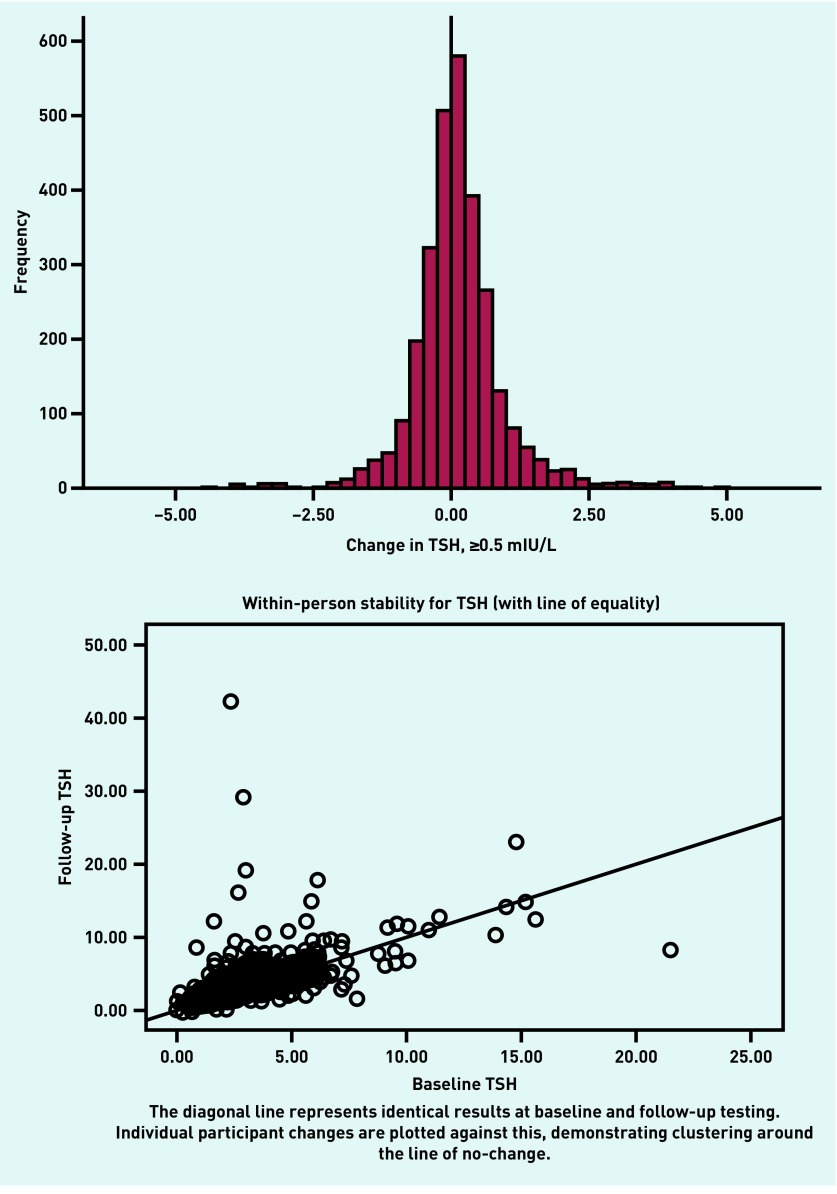
Change in TSH between BETS 1 and BETS 2 was calculated and explored using an arbitrary definition of a shift of ≥0.5 units being classified as ‘change’. Using this definition, 61% of participants showed no change between BETS 1 and BETS 2. Removal of outliers further demonstrates equal numbers of individuals experiencing an increase and decrease in TSH between timepoints, and, for the majority, TSH remains relatively stable (Figure 2).
Figure 2.
Frequency graphs of change in TSH during the period of follow-up (approximately 5 years). TSH = thyroid-stimulating hormone.
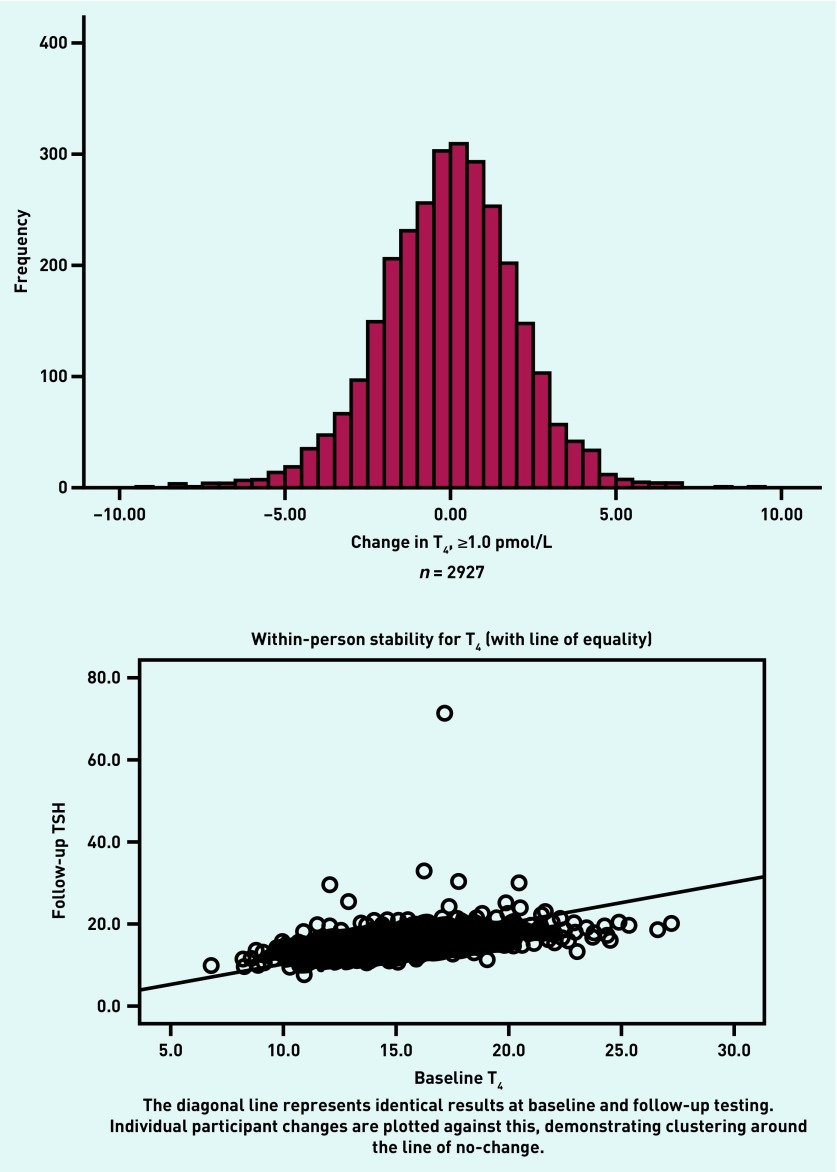
Change in FT4 was calculated based on a definition of change as ≥1.0 unit — 39.6% (n = 1162) of participants showed no change, with very few individuals demonstrating large shifts in FT4 (Figure 3).
Figure 3.
Frequency graphs of change in free T4 (FT4) during the period of follow-up (approximately 5 years). FT4 = free thyroxine. T4 = thyroxine
DISCUSSION
Summary
This large population-based study provides the most comprehensive data yet on the long-term dynamics of thyroid function among older patients in primary care. The prevalence of subclinical hypothyroidism in this study population was 6.2%, similar to that reported in smaller studies.21 Subclinical hyperthyroidism was less common, with just 1% of individuals being affected. BETS 2 confirms the incident findings of BETS 1, that subclinical thyroid dysfunction is a relatively common biochemical finding among older patients, and therefore understanding the natural course of the disease is important for both clinicians and patients.16
This study also demonstrates significant stability of thyroid function in this ageing population over a long (mean 5-year) time interval, with 96% of individuals who were euthyroid at baseline remaining so, and <0.5% developing an overt hyper- or hypothyroid status.
Strengths and limitations
This is the largest study to date following the natural history of thyroid functioning in an unselected primary care cohort of more older adults, with >2900 individuals followed over a 5-year period. One limitation of this study was that thyroid status on each occasion was based on a single sample. There are several factors that can influence the reproducibility of TFTs, including seasonality22 and intercurrent illness.23 However, any subtle or transient change that may have occurred is unlikely to have influenced the authors’ findings in any significant fashion due to the large sample size and the likelihood of transient fluctuations impacting at both timepoints. The predictive model needs to be interpreted with caution given low event rates. An automatic selection process to create a set of variables with the strongest association with the outcome was used, given this low event rate. However, such a process is data driven and may create a biased selection. But confidence is derived from the fact that all variables associated with development of hypothyroidism have strong biological plausibility.
Comparison with existing literature
Of those individuals classified as subclinically hypothyroid at baseline, only 2% progressed to overt hypothyroidism, which is lower than has been previously reported.24 This study demonstrated 40% of such patients revert to euthryoid status, which is in line with the 15–65% estimate by Somwaru et al,25 and the majority of those with subclinical hypothyroidism at baseline retain a subclinical state despite the long screening interval. Similarly, just 4% of those who were subclinically hyperthyroid at baseline developed overt hyperthyroidism.
To further support stability over time, the authors demonstrate here that most individuals (61%) had almost no change in TSH (≤0.5 mIU/L), with equal numbers of individuals experiencing either increasing or decreasing TSH. This contrasts with findings from previous work that suggests TSH increases with age.26 In a similar manner, the authors also demonstrate that FT4 is remarkably stable over time in older subjects.
In this study, development of overt hypothyroid status was significantly more likely among individuals with a new diagnosis of either AF or renal disease, or recent commencement of amiodarone. This supports a previous finding that prevalence of subclinical hypothyroidism is higher among patients with chronic renal disease.27 These factors, along with previously high–normal TSH or low–normal FT4, increase the likelihood of hypothyroid dysfunction and may represent triggers for repeat testing, although further work is needed to describe any benefit accrued from such a targeted approach. The large odds ratio, with wide confidence intervals, observed for commencement of amiodarone during the follow-up period (92.1, 95% CI = 5.64 to 1501.39) should, however, be interpreted with caution, as it is likely an artefact of the very low prevalence of amiodarone use in the study cohort.
Predictors of development of hyperthyroidism could not be determined from this study but, considering the low incidence of hyperthyroidism, there is nothing to support repeat testing of individuals with a normal test result within the previous 5 years to identify hyperthyroidism in the absence of substantive clinical signs and symptoms.
Implications for research and practice
This study confirms that older patients with subclinical thyroid dysfunction are only at a small relative risk of progression to overt dysfunction, and absolute risk is very low. TFTs are remarkably stable over extended periods and repeat routine testing or screening should be avoided in this population, particularly where a previous euthyroid result is reported in the clinical record. Considering this evidence, clinicians should minimise repeat TFT, unless clinically indicated, among older individuals who have a recent (within 5 years) euthyroid result in their clinical record. This is an important finding, given that almost one-third of older patients without overt thyroid dysfunction have at least one TFT performed annually (unpublished data).
Further research is required into whether there are benefits to be had from a targeted approach to repeat testing based on triggers such as a new diagnosis of AF or renal disease, recent commencement of amiodarone, and previously high–normal TSH or low–normal FT4.
Acknowledgments
The authors would like to thank the patients and GPs who participated, the general practice staff who facilitated collection of data, and Mr Peter Lewis, Dr Penelope Clark, and the staff at the Regional Endocrine Laboratory who conducted the thyroid function testing. The co-investigators for BETS 1 and/or BETS 2 are Professor Sue Wilson, Professor Jayne Franklyn, Professor Michael Gammage, Mr Roger Holder, Mrs Andrea Roalfe, and Dr Helen Pattison.
Funding
The study was funded by the National Institute for Health Research (NIHR) School for Primary Care Research (SPCR). The funders of the study had no role in the study design, data collection, data analyses, data interpretation, or writing of the manuscript. FD Richard Hobbs acknowledges part-support from the NIHR SPCR, the NIHR Collaboration for Leadership in Applied Research in Health and Care (CLARHC) Oxford, and the NIHR Oxford Biomedical Research Centre (BRC).
Ethical approval
Ethical approval for the study was obtained from the North Staffordshire Local Research Ethics Committee (reference number: 07/H1204/136, approval date 19 Dec 2007). Research and development (R&D) approval was obtained from the Birmingham and Solihull Primary Care Trust (PCT) Consortium (153/1108/P) and Coventry Teaching PCT (COV100907).
Provenance
Freely submitted; externally peer reviewed.
Competing interests
The authors have declared no competing interests.
Discuss this article
Contribute and read comments about this article: bjgp.org/letters
REFERENCES
- 1.Parle JV, Franklyn JA, Cross KW, et al. Prevalence and follow-up of abnormal thyrotrophin (TSH) concentrations in the elderly in the United Kingdom. Clin Endocrinol. 1991;34(1):77–83. doi: 10.1111/j.1365-2265.1991.tb01739.x. [DOI] [PubMed] [Google Scholar]
- 2.Koutras DA. Subclinical hyperthyroidism. Thyroid. 1999;9(3):311–315. doi: 10.1089/thy.1999.9.311. [DOI] [PubMed] [Google Scholar]
- 3.Eggertsen R, Petersen K, Lundberg PA, et al. Screening for thyroid disease in a primary care unit with a thyroid stimulating hormone assay with a low detection limit. BMJ. 1988;297(6663):1586–1592. doi: 10.1136/bmj.297.6663.1586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.UK National Screening Committee The UK NSC recommendation on thyroid disease screening in adults. 2018 https://legacyscreening.phe.org.uk/thyroid (accessed 8 Aug 2018) [Google Scholar]
- 5.Surks MI, Ortiz E, Daniels GH, et al. Subclinical thyroid disease: scientific review and guidelines for diagnosis and management. JAMA. 2004;291(2):228–238. doi: 10.1001/jama.291.2.228. [DOI] [PubMed] [Google Scholar]
- 6.Vaidya B, Ukoumunne OC, Shuttleworth J, et al. Variability in thyroid function test requests across general practices in south-west England. Qual Prim Care. 2013;21(3):143–148. [PubMed] [Google Scholar]
- 7.Public Health England The NHS atlas of variation in diagnostic services Reducing unwarranted variation to increase value and improve quality. 2013 https://ukgtn.nhs.uk/fileadmin/uploads/ukgtn/Documents/Resources/Library/Reports_Guidelines/Right_Care_Diagnostics_Atlas_2013.pdf (accessed 8 Aug 2018) [Google Scholar]
- 8.Allport J, McCahon D, Hobbs FDR, Roberts LM. Why are GPs treating subclinical hypothyroidism? Case note review and GP survey. Primary Health Care Res Dev. 2013;14(2):175–184. doi: 10.1017/S1463423612000230. [DOI] [PubMed] [Google Scholar]
- 9.Beckett G, Toft AD. First line thyroid function tests — TSH alone is not enough. Clin Endocrinol. 2003;58(1):20–21. doi: 10.1046/j.1365-2265.2003.01690.x. [DOI] [PubMed] [Google Scholar]
- 10.Vanderpump MP, Tunbridge WM, French JM, et al. The incidence of thyroid disorders in the community: a twenty-year follow-up of the Whickham Survey. Clin Endocrinol. 1995;43(1):55–68. doi: 10.1111/j.1365-2265.1995.tb01894.x. [DOI] [PubMed] [Google Scholar]
- 11.Diez JJ, Iglesias P, Burman KD. Spontaneous normalization of thyrotropin concentrations in patients with subclinical hypothyroidism. J Clin Endocrinol Metab. 2005;90(7):4124–4127. doi: 10.1210/jc.2005-0375. [DOI] [PubMed] [Google Scholar]
- 12.Huber G, Staub JJ, Meier C, et al. Prospective study of the spontaneous course of subclinical hypothyroidism: prognostic value of thyrotropin, thyroid reserve, and thyroid antibodies. J Clin Endocrinol Metab. 2002;87(7):3221–3226. doi: 10.1210/jcem.87.7.8678. [DOI] [PubMed] [Google Scholar]
- 13.Rosario PW, Carvalho M, Calsolari MR. Natural history of subclinical hypothyroidism with TSH ≤10 mIU/l: a prospective study. Clin Endocrinol. 2016;84(6):878–881. doi: 10.1111/cen.12939. [DOI] [PubMed] [Google Scholar]
- 14.Sawin CT, Geller A, Kaplan MM, et al. Low serum thyrotropin (thyroid-stimulating hormone) in older persons without hyperthyroidism. Arch Intern Med. 1991;151(1):165–168. [PubMed] [Google Scholar]
- 15.Woeber KA. Observations concerning the natural history of subclinical hyperthyroidism. Thyroid. 2005;15(7):687–691. doi: 10.1089/thy.2005.15.687. [DOI] [PubMed] [Google Scholar]
- 16.Wilson S, Parle JV, Roberts LM, et al. Prevalence of subclinical thyroid dysfunction and its relation to socioeconomic deprivation in the elderly: a community-based cross-sectional survey. J Clin Endocrinol Metab. 2006;91(12):4809–4816. doi: 10.1210/jc.2006-1557. [DOI] [PubMed] [Google Scholar]
- 17.Parle J, Roberts L, Wilson S, et al. A randomized controlled trial of the effect of thyroxine replacement on cognitive function in community-living elderly subjects with subclinical hypothyroidism: the Birmingham Elderly Thyroid study. J Clin Endocrinol Metab. 2010;95(8):3623–3632. doi: 10.1210/jc.2009-2571. [DOI] [PubMed] [Google Scholar]
- 18.Gammage MD, Parle JV, Holder RL, et al. Association between serum free thyroxine concentration and atrial fibrillation. Arch Intern Med. 2007;167(9):928–934. doi: 10.1001/archinte.167.9.928. [DOI] [PubMed] [Google Scholar]
- 19.Roberts LM, Pattison H, Roalfe A, et al. Is subclinical thyroid dysfunction in the elderly associated with depression or cognitive dysfunction? Ann Intern Med. 2006;145(8):573–581. doi: 10.7326/0003-4819-145-8-200610170-00006. [DOI] [PubMed] [Google Scholar]
- 20.Office of the Deputy Prime Minister The English Indices of Deprivation 2004 (revised) 2003 http://www.simonpoulter.co.uk/iod/iodpdf/odpm_urbpol_029534.pdf (accessed 8 Aug 2018) [Google Scholar]
- 21.Biondi B, Cooper DS. The clinical significance of subclinical thyroid dysfunction. Endocr Rev. 2008;29(1):76–131. doi: 10.1210/er.2006-0043. [DOI] [PubMed] [Google Scholar]
- 22.Kim TH, Kim KW, Ahn HY, et al. Effect of seasonal changes on the transition between subclinical hypothyroid and euthyroid status. J Clin Endocrinol Metab. 2013;98(8):3420–3429. doi: 10.1210/jc.2013-1607. [DOI] [PubMed] [Google Scholar]
- 23.Wong ET, Bradley SG, Schultz AL. Elevations of thyroid-stimulating hormone during acute nonthyroidal illness. Arch Intern Med. 1981;141(7):873–875. [PubMed] [Google Scholar]
- 24.Diez JJ, Iglesias P. Spontaneous subclinical hypothyroidism in patients older than 55 years: an analysis of natural course and risk factors for the development of overt thyroid failure. J Clin Endocrinol Metab. 2004;89(10):4890–4897. doi: 10.1210/jc.2003-032061. [DOI] [PubMed] [Google Scholar]
- 25.Somwaru LL, Rariy CM, Arnold AM, Cappola AR. The natural history of subclinical hypothyroidism in the elderly: the cardiovascular health study. J Clin Endocrinol Metab. 2012;97(6):1962–1969. doi: 10.1210/jc.2011-3047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Surks MI, Boucai L. Age- and race-based serum thyrotropin reference limits. J Clin Endocrinol Metab. 2010;95(2):496–502. doi: 10.1210/jc.2009-1845. [DOI] [PubMed] [Google Scholar]
- 27.Chonchol M, Lippi G, Salvagno G, et al. Prevalence of subclinical hypothyroidism in patients with chronic kidney disease. Clin J Am Soc Nephrol. 2008;3(5):1296–1300. doi: 10.2215/CJN.00800208. [DOI] [PMC free article] [PubMed] [Google Scholar]