Abstract
Background
Clinicians commonly prescribe antibiotics to prevent major adverse outcomes in children presenting in primary care with cough and respiratory symptoms, despite limited meaningful evidence of impact on these outcomes.
Aim
To estimate the effect of children’s antibiotic prescribing on adverse outcomes within 30 days of initial consultation.
Design and setting
Secondary analysis of 8320 children in a multicentre prospective cohort study, aged 3 months to <16 years, presenting in primary care across England with acute cough and other respiratory symptoms.
Method
Baseline clinical characteristics and antibiotic prescribing data were collected, and generalised linear models were used to estimate the effect of antibiotic prescribing on adverse outcomes within 30 days (subsequent hospitalisations and reconsultation for deterioration), controlling for clustering and clinicians’ propensity to prescribe antibiotics.
Results
Sixty-five (0.8%) children were hospitalised and 350 (4%) reconsulted for deterioration. Clinicians prescribed immediate and delayed antibiotics to 2313 (28%) and 771 (9%), respectively. Compared with no antibiotics, there was no clear evidence that antibiotics reduced hospitalisations (immediate antibiotic risk ratio [RR] 0.83, 95% confidence interval [CI] = 0.47 to 1.45; delayed RR 0.70, 95% CI = 0.26 to 1.90, overall P = 0.44). There was evidence that delayed (rather than immediate) antibiotics reduced reconsultations for deterioration (immediate RR 0.82, 95% CI = 0.65 to 1.07; delayed RR 0.55, 95% CI = 0.34 to 0.88, overall P = 0.024).
Conclusion
Most children presenting with acute cough and respiratory symptoms in primary care are not at risk of hospitalisation, and antibiotics may not reduce the risk. If an antibiotic is considered, a delayed antibiotic prescription may be preferable as it is likely to reduce reconsultation for deterioration.
Keywords: adverse outcomes, antibiotics, children, cohort studies, primary care, respiratory tract infections
INTRODUCTION
Children presenting with cough and other symptoms of respiratory tract infection (RTI) are the most frequent attenders to general practice internationally, are almost all managed in primary care, and the majority still receive antibiotics.1–3 A very small percentage of children are hospitalised for serious bacterial illnesses or complications.4,5 However, GPs are risk averse and report prescribing antibiotics at the point of presentation to this patient group ‘just in case’6,7 and in fear of a poor outcome.6–9
This uncertainty is fuelled by the very limited experimental or observational evidence available regarding the impact of different antibiotic prescribing strategies on major adverse outcomes among children. Available systematic reviews suggest that antibiotics have limited efficacy in treating a large proportion of upper RTIs10–13 but the reviews are underpowered to assess complications and there is little evidence for bronchitis, in particular. Although there is some evidence for adults,14–19 there is almost no meaningful evidence in children regarding complications if antibiotics are withheld for respiratory infections. The major problem with continuing to prescribe for respiratory infections in children is that primary care antibiotic use is a major driver of antibiotic resistance internationally.20
Two large prospective cohort studies of adults with RTI symptoms demonstrated that either immediate or delayed antibiotic prescriptions can modify health outcomes.18,19 The authors were aware of no comparable data in children. This paper used data from a large cohort study to establish whether an immediate or delayed antibiotic prescription given to children with acute cough and RTI in primary care modifies risk of subsequent hospitalisation or reconsultation with deterioration.
METHOD
A large, four-centre (England, UK) prospective cohort study was conducted that recruited children aged 3 months to <16 years presenting to primary care with acute cough and RTI between July 2011 and May 2013. The results from the primary aim of the study have been published.5 Here are presented findings from a secondary analysis.
How this fits in
Antibiotic prescribing to children in primary care is one of the key areas of inappropriate prescribing. This is mainly due to the lack of evidence for, and uncertainty regarding, which children are at risk of poor outcome. This study investigated whether antibiotic prescribing had an impact on two adverse health outcomes for children: hospitalisation for respiratory tract infections and reconsultation for deteriorating symptoms. The study shows that there is little evidence to justify the use of antibiotics for reducing hospitalisation, which occurred very rarely, and supports previous research in adults that a delayed antibiotic prescribing strategy is likely to reduce reconsultation for deterioration.
The protocol has been described elsewhere.21 In summary, eligible children presenting to primary care were recruited by prescribing ‘clinicians’ (GPs and prescribing practice nurses) across four centres if they presented with acute cough as the most prominent symptom, combined with other symptoms or signs suggestive of RTI. Clinicians who self-reported prescribing antibiotics in ≤30% to children with RTIs were invited to participate. Following informed consent, clinicians completed a structured case report form (Appendix 1) that included sociodemographics, parent-reported symptoms, clinician-assessed signs, diagnosis, and whether an immediate or delayed antibiotic was prescribed (including number of days delayed) at the time of the consultation.
The main outcomes, hospitalisation for any RTI in the 30 days following recruitment and reconsultation for deterioration (a proxy marker for reconsultation for the same episode of RTI illness with evidence of worsening illness, shown to be reliably assessed),22 were collected via a detailed review of the child’s medical record. History of chronic conditions was also recorded. Medical record reviews were generally conducted 3 months post-recruitment for each child, to allow for adequate feedback to occur. On some occasions this was slightly longer than 3 months, and in all cases the period of time was sufficient to allow both reconsultations and complications to occur. Double, independent medical record review was undertaken in a random set of 1% of participants to estimate inter-reviewer error.
Data preparation
Children referred for acute hospitalisation at the consultation were excluded from the analysis, as clinicians’ prescribing behaviour was expected to differ for children whom they had decided to refer to hospital on the same day as the consultation, compared with those they did not.
Common clinical cut-offs were used for continuous data where possible (high temperature >37.8°C)23 and were age-related if appropriate (age-specific heart and respiratory rates and blood pressure).24 UK guidelines for low oxygen saturation level (≤95%) were used.25 Given the large number of variables, continuous variables were dichotomised using 25th or 75th percentile cut-offs as appropriate. For carer-reported symptom severity (mild, moderate, or severe) in the 24 hours prior to consultation, dichotomy for each variable was split, depending on the overall prevalence, to either ‘severe’ if more than 5% of the whole cohort fell into this category or ‘moderate and severe’ if the proportion was smaller. This pragmatic cut-off was chosen prior to analysis to avoid variables with very low prevalence. Capillary refill time (CRT) was coded as normal (≤2 seconds) or long (≥3 seconds).26,27 Multiple deprivation score was based on the family postcode using the UK Indices of Multiple Deprivation 2007.28
Covariates
Variables measured at the baseline consultation (symptoms, signs, demographics) were identified as possible confounders/covariates. These variables were considered during the analysis of secondary outcomes (Appendix 2).
Statistical analysis
All data were analysed using STATA (version 13.1). The κ statistic to assess inter-rater reliability of the two main outcomes was calculated. Generalised linear modelling with a log link to produce risk ratios (RR) was used, accounting for clustering by clinician and controlling for potential covariates associated with the prescription strategy and the two outcomes. Two models were generated: in the first, variables were selected using backward stepwise selection with variables retained if the P-value <0.05. In the second model, analyses were conducted post-hoc, where a stratified propensity score was created, which allowed for more rigorous control of potential confounding by indication.29,30
RESULTS
Ascertainment and baseline characteristics
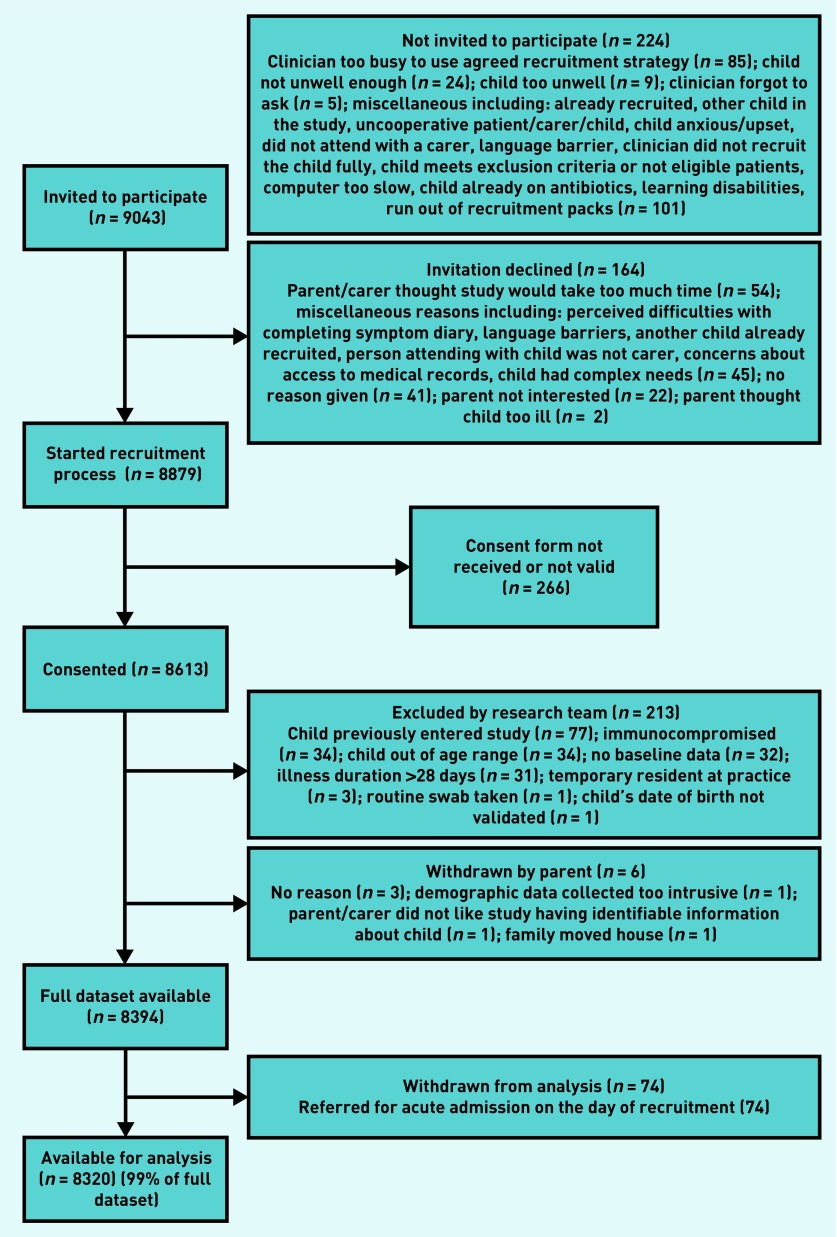
Between July 2011 and May 2013, 518 clinicians recruited children from 247 primary care practices across England. A total of 8613 children were recruited, and, of these, 219 (3%) children were excluded: 181 did not meet eligibility, 32 children did not have baseline data, and six children were withdrawn. Seventy-four children referred for acute hospital admission on the day of recruitment were excluded from the analysis, leaving a total of 8320 children. Antibiotic prescription data from the baseline consultation were available for 100% of these children and all analyses used this final sample of 8320. Figure 1 details the flow of participants through the study. The outcome of hospitalisation was obtained for 8320 (100%) children, and reconsultation for deterioration was obtained for 98% (n = 8136/8320).
Figure 1.
Flow of participants through the study.
Inter-reviewer agreement analysis for medical record data collection was assessed. For hospitalisation this was 90% (κ 0.80) and 84% for reconsultation within r the same episode of illness (κ 0.67). Missing data for candidate predictors were infrequent (<2%) with the exception of oxygen saturation (50% missing values) due to lack of available paediatric monitors.
Clinicians prescribed antibiotics for 3084/8320 children (37%), with 2313 (28%) children prescribed immediate and 771 (9%) delayed antibiotics. The range of days the prescription was delayed for was between 0–10, median 2 (interquartile range [IQR] 2–3).
Of the 8320 children included in the analysis, 65 (0.8%) were hospitalised for an RTI in the 30 days following recruitment. Median time to hospitalisation was 4 days (IQR 1–15) with 5% hospitalised on the day of recruitment (day 0), 52% on days 1–7, 17% on days 8–14, and 26% on days 15–30. Of the 65 children hospitalised, 25 (38.5%) had been prescribed an antibiotic.
The most common RTI discharge diagnoses (Table 1) were bronchiolitis (20%), lower RTI (14%), and upper RTI (12%); other diagnoses included viral wheeze, exacerbation of asthma, tonsillitis, croup, unspecified viral illness, chest infection, bronchiolitis and bronchitis, viral pneumonitis, pyrexia, and febrile convulsions.
Table 1.
Hospital discharge diagnoses in the 30 days post-recruitment for children who were and were not prescribed an antibiotic at the baseline general practice consultation
| Hospital diagnosis | Number of children | |||
|---|---|---|---|---|
| Immediate | Delayed | Not prescribed | Total | |
| Bronchiolitis | 1 | 2 | 10 | 13 |
| LRTI | 6 | 0 | 3 | 9 |
| URTI | 0 | 3 | 5 | 8 |
| Exacerbation of asthma | 2 | 0 | 4 | 6 |
| Tonsillitis | 3 | 0 | 3 | 6 |
| Viral wheeze | 2 | 0 | 4 | 6 |
| Croup | 1 | 1 | 3 | 5 |
| Unspecified viral illness | 1 | 0 | 2 | 3 |
| Chest infection | 1 | 0 | 1 | 2 |
| Bronchiolitis and bronchitis | 0 | 0 | 1 | 1 |
| LRTI/viral pneumonitis | 1 | 0 | 0 | 1 |
| Pyrexia | 1 | 0 | 0 | 1 |
| URTI and febrile convulsions | 0 | 0 | 1 | 1 |
| No record | 0 | 0 | 3 | 3 |
| Total | 19 | 6 | 40 | 65 |
LRTI = lower respiratory tract infection. URTI = upper respiratory tract infection.
Just over one-fifth (22.5%; 1830/8136) of children reconsulted for any RTI symptoms in the 30 days after consultation, 14% (1163/8136) reconsulted for the same episode of RTI illness, and 4% (350/8136) reconsulted for the same RTI with evidence in their medical records of deteriorating symptoms.
Appendix 3 shows the clinical history, sociodemographics, parent/carer-reported symptoms, clinical signs observed by the clinician, and adverse health outcomes (in the 30 days post-baseline) for the children with different antibiotic strategies at the baseline consultation. There is wide variation in the number of children prescribed an immediate, delayed, or no antibiotic with regard to parent-reported symptoms and clinical signs.
Relationships between baseline characteristics and health outcomes
Hospitalisation
Table 1 shows the discharge diagnoses for the hospitalised children and whether they received an antibiotic or not. There was no evidence of a difference between hospital diagnoses in children prescribed an antibiotic compared with those who were not (χ2 test: P = 0.46).
Table 2 details the univariable and multivariable relationships between antibiotic prescribing at the baseline consultation and subsequent hospitalisation. There was no clear evidence at the univariable level or multivariable level that prescribing immediate or delayed antibiotics reduced the risk of a child being hospitalised in the 30 days post-baseline consultation (immediate RR 0.83, 95% confidence interval [CI] = 0.47 to 1.45; delayed RR 0.70, 95% CI = 0.26 to 1.90, overall P = 0.44).
Table 2.
Association between children’s antibiotic prescription strategies and hospitalisation in the 30 days following the baseline consultation
| Not hospitalised | Hospitalised | Univariable analysis clustering by clinician | Multivariable analysis accounting for covariates where P<0.05 and clustering by clinician | Analysis stratified by propensity score and accounting for clustering by clinician | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | % | n | % | RR | 95% CI | P-valuea | RR | 95% CI | P-valuea | RR | 95% CI | P-valuea | |
| No antibiotic | 5196/8255 | 62.9 | 40/65 | 61.5 | Ref | Ref | 0.53 (2 df) | Ref | Ref | 0.31 (2 df)b | Ref | Ref | 0.44 (2 df) |
| Immediate | 2292/8255 | 27.8 | 21/65 | 32.3 | 1.19 | 0. 70 to 1.88 | 0.81 | 0.40 to 1.32 | 0.83 | 0.47 to 1.45 | |||
| Delayed | 767/8255 | 9.3 | 4/65 | 6.2 | 0.68 | 0.24 to 1.88 | 0.62 | 0.22 to 1.66 | 0.70 | 0.26 to 1.90 | |||
Overall P-value.
Covariates included (<0.05): age<(2 years), current asthma, short<(3 days) illness duration prior to baseline, moderate/severe vomiting in the 24 hours before baseline, clinician-reported wheeze, high temperature (age-related cut-offs). df = degrees of freedom. Ref = reference. RR = risk ratio.
Reconsultation within 30 days for deterioration
Table 3 describes the univariable and multivariable relationships between prescription at the baseline consultation and reconsultation for deterioration. Both univariable and multivariable analysis, accounting for clinician clustering, indicate there is evidence to suggest a difference in those reconsulting with deteriorating symptoms in the subsequent 30 days, for those prescribed an antibiotic compared with those who were not (immediate risk ratio [RR] 0.82, CI = 0.65 to 1.07; delayed RR 0.55, CI = 0.34 to 0.88, overall P = 0.02). Delayed antibiotics reduced reconsultation with deterioration by almost half and, although the point estimate for those prescribed immediate antibiotics suggests a reduction, the 95% CI means the absence of an effect cannot be ruled out.
Table 3.
Association between children’s antibiotic prescription strategies and reconsulting for the same RTI illness with evidence of deterioration in the 30 days following the baseline consultation
| No reconsultation | Reconsulted for deterioration | Univariable analysis clustering by clinician | Multivariable analysis accounting for where P<0.05 and clustering by clinician | Analysis stratified by propensity score and accounting for clustering by clinician | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | % | n | % | RR | 95% CI | P-valuea | RR | 95% CI | P-valuea | RR | 95% CI | P-valuea | |
| No antibiotic | 4864/7786 | 62.5 | 240/350 | 68.6 | Ref | Ref | 0.008 (2 df) | Ref | Ref | 0.007 (2 df)b | Ref | Ref | 0.024 (2 d.f) |
| Immediate | 2175/7786 | 27.9 | 91/350 | 26.0 | 0.85 | 0.67 to 1.09 | 0.78 | 0.61 to 0.99 | 0.82 | 0.65 to 1.07 | |||
| Delayed | 747/7786 | 9.6 | 19/350 | 5.4 | 0.52 | 0.32 to 0.87 | 0.56 | 0.34 to 0.91 | 0.55 | 0.34 to 0.88 | |||
Overall P-value.
Covariates included (P<0.05): moderate/severe vomiting in the 24 hours before baseline, white ethnicity, age<(2 years), short< (3 days) illness duration prior to baseline, clinician-reported wheeze, parent-reported disturbed sleep in the previous 24 hours, moderate or severe vomiting and severe blocked nose in the previous 24 hours. df = degrees of freedom. Ref = reference. RR = risk ratio.
DISCUSSION
Summary
This is the first cohort evidence available to date to indicate that prescribing immediate or delayed antibiotics in children does not prevent RTI-related hospitalisation in the 30 days post primary care consultation. Hospital admissions in the 30 days after the baseline consultation were rare and almost none of the reasons for admission were related to the withholding of antibiotics. This has demonstrated that delayed antibiotics reduced the risk of the child reconsulting for the same illness with deterioration. For those given immediate antibiotics, the trend was in the same direction, although no clear evidence was found; it is not clear if this is due to a lack of power or a true finding. This supports previous research in adults that also suggests delayed prescribing should be considered if an antibiotic is being prescribed.
Strengths and limitations
The study’s large observational dataset reflects a realistic primary care setting and the findings are likely to be generalisable to general practice in other high-income countries. Follow-up and case ascertainment were high. The study has several potential limitations. First, prescribing rates were relatively low in this cohort, particularly delayed prescribing, which may impact on the generalisability. The low prescribing rates are likely to be because clinicians who self-classified themselves as ‘low prescribers’ were eligible to recruit to the study. Second, establishing whether prescribed antibiotics were dispensed and consumed was not possible, although previous studies suggest that immediate prescriptions commonly are consumed.31 Third, both health outcomes were rare and event rates low (as expected), particularly hospitalisation, which unavoidably limits analytic power. Fourth, as with any secondary analysis of observational data there may be residual confounding, although only a few variables predicted hospitalisation, which lessens any effect of confounding by indication. For reconsulting for deterioration, very little change in risk ratios were recorded when a wide range of potential covariates were included in the model, which suggests that confounding, for those variables that were recorded, was not a major issue.
Comparison with existing literature
The authors did not find evidence to support the use of an immediate antibiotic prescription as a means of clearly reducing hospitalisations for RTIs. Even if the lower confidence intervals for the estimate are taken, more than 200 children would need to be given an immediate antibiotic for one hospitalisation to be prevented. These findings are in agreement with evidence from systematic reviews11–13,32 where little or no evidence was found to support their use in children or adults. The authors found similar estimates for reconsultations for deterioration with that of one large cohort study investigating new or non-resolving symptoms in adult sore throat.18,19 Similarly, this evidence supports the idea that a delayed antibiotic script is not necessarily equivalent to a ‘no prescription’ strategy and can be a useful means to reduce reconsultations18,19,33,34 as well as the use of antibiotics.31,33–36 Evidence from this cohort demonstrated which symptoms and signs predict complications in children presenting to general practice with acute cough and RTI.5 This may reduce uncertainty around distinguishing which children might benefit from antibiotics, from those who are at a much lower risk of poor health outcomes where the clinician can safely make a ‘no prescription’ decision.21 However, a multifaceted approach and more complex behavioural interventions may be required to support clinicians to reduce their prescribing to children.37–39
Qualitative evidence suggests that the relationship between parents and clinicians, in relation to antibiotic prescribing for their child’s RTI, is complex. Studies show that clinicians are prescribing ‘just in case’,6 feel uncertain about prognostic outcomes,7 and perceive pressure from parents to prescribe when parents want symptomatic relief and safety-netting advice.40,41 The authors’ evidence indicates a delayed prescription reduces the likelihood of a parent reconsulting with their child with deterioration. The reasons for this are not entirely clear, but may represent the timely access to antibiotics if illness is not settling, or prompt treatment of a secondary bacterial infection following an initial viral infection.
Implications for practice
These findings suggest that there is little evidence that antibiotics substantially reduce the risk of hospitalisation in children presenting to primary care; and that these risks are extremely low for the majority of children presenting with acute cough and RTI. The rates of prescribing in this cohort, even for self-classified ‘low prescribers’, indicate continued need for interventions and strategies to better target antibiotics. These results provide reassurance that, when faced with a child and uncertain prognosis, delayed prescribing can be a safe and effective method to reduce the child’s probability of reconsulting with deterioration and can act as part of safety-netting strategies for parents.
The implications for clinical practice are that the majority of children presenting with acute cough and respiratory symptoms in primary care are not at risk of hospitalisation, and antibiotics may not reduce the risk. If clinicians are considering an antibiotic, a delayed prescription may be preferable as it is likely to reduce reconsultation for deterioration.
Acknowledgments
Index of Multiple Deprivation data: ©Crown Copyright 2006. Source: National Statistics/Ordnance Survey. Extracts are Crown Copyright and may only be reproduced by permission (https://www.ukdataservice.ac.uk/use-data/citing-data). The authors are extremely grateful to the children, parents/carers, and families who have participated in the study, all general practices including recruiting clinicians, administrative and research contacts, and all other staff whose participation made this study possible. We thank all our colleagues from the TARGET Programme, the TARGET Programme Management Group, and the TARGET Programme Steering Committee for their time, expertise, and support. We are grateful to the many individuals who have supported in any way the cohort study across the study centres. The TARGET study team acknowledges the support of the NIHR Clinical Research Network, and the Guy’s and St Thomas’ Biomedical Research Centre in supporting the web-based data collection.
Appendix 1. Case report form used to record baseline data for the prospective TARGET cohort study (a similar online version was also used).
Appendix 2. Potential covariates associated with hospitalisation and reconsultation in the 30 days following baseline
| Characteristic | Data source | |
|---|---|---|
| Sociodemographic variables | ||
| Age | <2 years versus ≥2 years | Parent |
| Sex | Male versus female | Parent |
| Age of mother at child’s birth | ≤26 years versus >26 years | Parent |
| Breastfed for ≥3 months | Yes versus no | Parent |
| Mother smokes | Yes versus no | Parent |
| Children in the home | ≥2 versus <2 | Parent |
| IMD score | High, top quintile versus quintiles 1 to 4 | Parent |
| Ethnicity | White versus mixed, Asian or Asian British, black or black British, Chinese, or other ethnic groups | Parent |
| Past medical history | ||
| Consultations for RTI in the 12 months prior to baseline | ≥2 versus <2 | General practice medical notes |
| Asthma (current diagnosis) | Yes versus No | General practice medical notes |
| Chronic conditions (any) | Yes versus No | General practice medical notes |
| Asthma (previous diagnosis) | Yes versus No | General practice medical notes |
| Parent-reported symptoms (present during the illness) | ||
| Illness duration prior to baseline (days) | <3 versus 3 | Parent |
| Breathing faster than normal | Present versus absent | Parent |
| High parent illness severity score | ≥7 versus<7 | Parent |
| Low energy/fatigue/lethargy | Present versus absent | Parent |
| Fever | Present versus absent | Parent |
| Eating less | Present versus absent | Parent |
| Illness much worse recently | Yes versus no | Parent |
| Disturbed sleep | Present versus absent | Parent |
| Wheezing or whistling in the chest | Present versus absent | Parent |
| Chills/shivering | Present versus absent | Parent |
| Taken fewer fluids/milk feeds | Present versus absent | Parent |
| Productive wet cough | Present versus absent | Parent |
| Vomiting (including after a cough) | Present versus absent | Parent |
| Passing urine less often/drier nappies | Present versus absent | Parent |
| Change in cry | Present versus absent | Parent |
| Dry cough | Present versus absent | Parent |
| Diarrhoea | Present versus absent | Parent |
| Barking/croupy cough | Present versus absent | Parent |
| Blocked/runny nose | Present versus absent | Parent |
| Parent-reported symptoms (last 24 hours) | ||
| Change in cry (moderate/severe) | Present versus absent | Parent |
| Vomiting (moderate/severe) | Present versus absent | Parent |
| Disturbed sleep (severe) | Present versus absent | Parent |
| Taking fewer fluids/milk feeds (moderate/severe) | Present versus absent | Parent |
| Passing urine less often/drier nappies (moderate/severe) | Present versus absent | Parent |
| Productive wet cough (severe) | Present versus absent | Parent |
| Chills/shivering (moderate/severe) | Present versus absent | Parent |
| Eating less (severe) | Present versus absent | Parent |
| Low energy/fatigue/lethargy (moderate/severe) | Present versus absent | Parent |
| Wheeze (moderate/severe) | Present versus absent | Parent |
| Fever (severe) | Present versus absent | Parent |
| Breathing faster than normal (moderate/severe) | Present versus absent | Parent |
| Blocked/runny nose (severe) | Present versus absent | Parent |
| Dry cough (severe) | Present versus absent | Parent |
| Barking/croupy cough (moderate/severe) | Present versus absent | Parent |
| Diarrhoea (moderate/severe) | Present versus absent | Parent |
| Clinical signs | ||
| Inter/subcostal recession | Present versus absent | Clinician |
| Bronchial breathing (unilateral/bilateral) | Present versus absent | Clinician |
| Nasal flaring | Present versus absent | Clinician |
| Pallor | Present versus absent | Clinician |
| Wheeze (unilateral/bilateral) | Present versus absent | Clinician |
| Abnormal consciousness | Yes versus no | Clinician |
| High temperature | ≥37.8°C versus <37.8°C | Clinician |
| High respiratory rate (age-related cut-offs) | Present versus absent | Clinician |
| High pulse (age-related cut-offs) | Present versus absent | Clinician |
| Inflamed pharynx | Present versus absent | Clinician |
| Grunting | Present versus absent | Clinician |
| Crackles/crepitations (unilateral/bilateral) | Present versus absent | Clinician |
| Slow capillary refill time | ≥3 seconds versus ≤2 seconds | Clinician |
| Stridor | Present versus absent | Clinician |
| High clinician illness severity score | ≥4 versus <4 | Clinician |
| Clinician gut feeling that ‘something is wrong’ | Yes versus no | Clinician |
IMD = Index of Multiple Deprivation. RTI = respiratory tract infection.
Appendix 3. Characteristics of the children and antibiotic prescribing strategies at the baseline general practice consultation
| No antibiotic | Immediate antibiotics | Delayed antibiotics | ||||
|---|---|---|---|---|---|---|
| n/N | % | n/N | % | n/N | % | |
| Clinical history | ||||||
| RTI consultations in the 12 months prior to baseline (≥2 consultations) | 1739/5106 | 34 | 839/2269 | 37 | 262/766 | 34 |
| Any chronic conditiona | 916/5235 | 18 | 492/2311 | 21 | 157/771 | 20 |
| Current asthma diagnosisb | 415/5236 | 8 | 247/2313 | 11 | 77/771 | 10 |
| Previous asthma diagnosis | 184/5235 | 4 | 124/2313 | 5 | 41/771 | 5 |
| Sociodemographics | ||||||
| Sex (male) | 2693/5236 | 51 | 1230/2313 | 53 | 365/771 | 47 |
| Age (<2 years) | 1875/5236 | 36 | 715/2313 | 31 | 212/771 | 28 |
| Children in the home (>1) | 3292/5213 | 63 | 1644/2303 | 71 | 526/765 | 69 |
| Breastfeeding (at 3 months) | 2132/4887 | 44 | 934/2117 | 44 | 350/718 | 49 |
| Ethnicity (white) | 4015/5212 | 77 | 1889/2298 | 82 | 585/766 | 76 |
| Mother smokes | 914/5178 | 18 | 447/2277 | 20 | 115/759 | 15 |
| Young mother | 1566/5222 | 30 | 652/2304 | 28 | 197/768 | 26 |
| IMD quintile (most deprived) | 1066/5236 | 20 | 436/2313 | 19 | 117/771 | 15 |
| Parent-reported symptoms present at any time during the illness | ||||||
| High severity score (parent: ≥7/10) | 993/5218 | 19 | 914/2305 | 40 | 209/771 | 27 |
| Short duration of illness (≤3 days) | 1598/5233 | 31 | 533/2312 | 23 | 234/771 | 30 |
| Illness worsened recently | 3114/5230 | 60 | 1835/2310 | 79 | 527/770 | 68 |
| Dry cough | 3326/5234 | 64 | 1205/2309 | 52 | 447/771 | 58 |
| Productive wet cough | 2556/5230 | 49 | 1455/2310 | 63 | 440/770 | 57 |
| Barking/croupy cough | 1357/5232 | 26 | 605/2307 | 26 | 161/771 | 21 |
| Blocked/runny nose | 4202/5234 | 80 | 1833/2311 | 79 | 620/770 | 81 |
| Change in cry | 850/5221 | 16 | 385/2302 | 17 | 131/766 | 17 |
| Breathing quicklyc | 1602/5235 | 31 | 1057/2311 | 46 | 279/771 | 36 |
| Wheezing/whistling in chest | 1885/5232 | 36 | 1058/2311 | 46 | 303/771 | 39 |
| Chills | 948/5233 | 18 | 679/2310 | 29 | 212/770 | 28 |
| Fever | 2865/5234 | 55 | 1733/2311 | 75 | 533/771 | 69 |
| Diarrhoea | 783/5233 | 15 | 340/2311 | 15 | 101/771 | 13 |
| Vomitingd | 1349/5234 | 26 | 765/2311 | 33 | 201/771 | 26 |
| Eating less than normal | 2855/5232 | 55 | 1627/2310 | 70 | 498/771 | 65 |
| Fewer fluids | 1529/5232 | 29 | 834/2309 | 36 | 253/771 | 33 |
| Low energy | 2512/5234 | 48 | 1475/2310 | 64 | 483/771 | 63 |
| Disturbed sleep | 3880/5234 | 74 | 1926/2311 | 83 | 592/770 | 77 |
| Less urine than normal | 652/5223 | 13 | 348/2307 | 15 | 131/770 | 17 |
| Parent-reported symptoms present in the last 24 hours (severe) | ||||||
| Dry cough | 337/5215 | 6 | 174/2306 | 8 | 40/768 | 5 |
| Productive wet cough | 329/5215 | 6 | 270/2304 | 12 | 68/770 | 9 |
| Blocked/runny nose | 406/5202 | 8 | 201/2304 | 9 | 52/765 | 7 |
| Fever | 228/5217 | 4 | 236/2302 | 10 | 70/768 | 9 |
| Eating less | 208/5213 | 4 | 175/2299 | 8 | 38/769 | 5 |
| Disturbed sleep | 784/5208 | 15 | 430/2305 | 19 | 116/765 | 15 |
| Parent-reported symptoms present in the last 24 hours (moderate or severe) | ||||||
| Barking cough | 957/5226 | 18 | 446/2303 | 19 | 111/771 | 14 |
| Change in cry | 480/5212 | 9 | 224/2301 | 10 | 73/765 | 10 |
| Chills/shivering | 382/5229 | 7 | 362/2305 | 16 | 84/769 | 11 |
| Breathing quicklyc | 836/5224 | 16 | 619/2308 | 27 | 146/771 | 19 |
| Wheeze | 878/5225 | 17 | 585/2305 | 25 | 128/771 | 17 |
| Diarrhoea | 216/5229 | 4 | 103/2309 | 5 | 22/771 | 3 |
| Vomitingd | 460/5227 | 9 | 290/2310 | 13 | 74/770 | 10 |
| Taking fewer fluids/milk feeds | 641/5224 | 12 | 388/2302 | 17 | 107/769 | 14 |
| Low energy/fatigue/lethargy | 1192/5213 | 23 | 824/2301 | 36 | 229/768 | 30 |
| Passing urine less often | 256/5213 | 5 | 158/2306 | 7 | 42/769 | 6 |
| Physical examination signs | ||||||
| Pallor | 284/5227 | 5 | 439/2311 | 19 | 84/771 | 11 |
| Nasal flaring | 39/5228 | 1 | 51/2311 | 2 | 6/771 | 1 |
| Grunting | 25/5227 | 0 | 40/2310 | 2 | 6/771 | 1 |
| Inter/subcostal recession | 131/5227 | 3 | 226/2310 | 10 | 21/771 | 3 |
| Wheeze | 498/5228 | 10 | 624/2308 | 27 | 87/771 | 11 |
| Crackles/crepitations | 128/5227 | 2 | 1300/2310 | 56 | 130/770 | 17 |
| Bronchial breathing | 43/5225 | 1 | 210/2307 | 9 | 21/769 | 3 |
| Inflamed pharynx | 1250/5212 | 24 | 828/2308 | 36 | 299/771 | 39 |
| Stridor | 25/5226 | 0 | 11/2310 | 0 | 5/771 | 1 |
| Abnormal consciousness | 42/5229 | 1 | 73/2308 | 3 | 7/768 | 1 |
| High respiratory rate | 619/5212 | 12 | 492/2300 | 21 | 107/763 | 14 |
| High temperature ≥37.8°Ce | 346/5223 | 7 | 567/2307 | 25 | 116/770 | 15 |
| High pulse | 170/5203 | 3 | 178/2297 | 8 | 33/766 | 4 |
| Capillary refill rate (≥3 seconds) | 41/5216 | 1 | 18/2304 | 1 | 6/763 | 1 |
| High severity score (clinician: (≥4/10) | 1038/5233 | 20 | 1502/2296 | 65 | 341/768 | 44 |
| Gut feeling something is wrong | 273/5230 | 5 | 1265/2307 | 55 | 110/766 | 14 |
| Adverse health outcomes in the 30 days post-baseline | ||||||
| Hospitalised | 40/5236 | 1 | 21/2313 | 1 | 4/771 | 1 |
| Reconsulted general practice for the same RTI illness with evidence of symptom deterioration | 240/5104 | 5 | 91/2266 | 4 | 19/766 | 2 |
Includes both current and previous asthma diagnosis.
Defined as present if asthma in medical notes problem list and asthma medication issued in the previous 12 months.
Faster than normal.
Including after a cough.
High temperature (age-related cut-offs). IMD = Index of Multiple Deprivation. RTI = respiratory tract infection.
Funding
The National Institute for Health Research (NIHR) funds the Programme Grant for Applied Research TARGET Programme grant at the University of Bristol and NHS Bristol Clinical Commissioning Group. This article summarises independent research funded by the NIHR under its Programme Grants for Applied Research Programme (Grant Reference Number RP-PG-0608-10018). Niamh M Redmond’s time is supported by the NIHR Collaboration for Leadership in Applied Health Research and Care West (CLAHRC West) at University Hospitals Bristol NHS Foundation Trust. Hannah Christensen is supported by the NIHR Health Protection Research Unit in Evaluation of Interventions at the University of Bristol, in partnership with Public Health England. The views expressed are those of the authors and not necessarily those of the NHS, the NIHR, the Department of Health and Social Care, or Public Health England.
Ethical approval
The study was approved by the South West Central Bristol Research Ethics Committee, UK (reference number: 10/H0102/54) and research governance approvals obtained across all areas prior to the start of recruitment in those areas. The TARGET cohort study was sponsored by Research Enterprise and Development Department, University of Bristol, UK. The cohort study is registered on UK NIHR Clinical Research Network Portfolio as ‘The TARGET study’ (reference number: 9334).
Provenance
Freely submitted; externally peer reviewed.
Competing interests
Hannah Christensen reports receiving honoraria from Sanofi Pasteur, and consultancy fees from IMS Health, AstraZeneca, and GSK all paid to her employer. Matthew Thompson has received consultancy fees and research funding from Roche Molecular Diagnostics and from Alere.
Discuss this article
Contribute and read comments about this article: bjgp.org/letters
REFERENCES
- 1.Okkes IM, Oskam SK, Lamberts H. The probability of specific diagnoses for patients presenting with common symptoms to Dutch family physicians. J Fam Pract. 2002;51(1):31–36. [PubMed] [Google Scholar]
- 2.Hay AD, Heron J, Ness A, ALSPAC Study Team. The prevalence of symptoms and consultations in pre-school children in the Avon Longitudinal Study of Parents and Children (ALSPAC): a prospective cohort study. Fam Pract. 2005;22(4):367–374. doi: 10.1093/fampra/cmi035. [DOI] [PubMed] [Google Scholar]
- 3.Hersh AL, Shapiro DJ, Pavia AT, Shah SS. Antibiotic prescribing in ambulatory pediatrics in the United States. Pediatrics. 2011;128(6):1053–1061. doi: 10.1542/peds.2011-1337. [DOI] [PubMed] [Google Scholar]
- 4.Pearson GA, editor. Why children die: a pilot study 2006; England (South West, North East and West Midlands), Wales and Northern Ireland. London: Confidential Enquiry into Maternal and Child Health; 2008. [Google Scholar]
- 5.Hay AD, Redmond NM, Turnbull S, et al. Development and internal validation of a clinical rule to improve antibiotic use in children presenting to primary care with acute respiratory tract infection and cough: a prognostic cohort study. Lancet Respir Med. 2016;4(11):902–910. doi: 10.1016/S2213-2600(16)30223-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lucas PJ, Cabral C, Hay AD, Horwood J. A systematic review of parent and clinician views and perceptions that influence prescribing decisions in relation to acute childhood infections in primary care. Scand J Prim Health Care. 2015;33(1):11–20. doi: 10.3109/02813432.2015.1001942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Horwood J, Cabral C, Hay AD, Ingram J. Primary care clinician antibiotic prescribing decisions in consultations for children with RTIs: a qualitative interview study. Br J Gen Pract. 2016. . [DOI] [PMC free article] [PubMed]
- 8.Kumar S, Little P, Britten N. Why do general practitioners prescribe antibiotics for sore throat? Grounded theory interview study. BMJ. 2003;326(7381):138. doi: 10.1136/bmj.326.7381.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Whaley LE, Businger AC, Dempsey PP, Linder JA. Visit complexity, diagnostic uncertainty, and antibiotic prescribing for acute cough in primary care: a retrospective study. BMC Fam Pract. 2013;14:120. doi: 10.1186/1471-2296-14-120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fahey T, Stocks N, Thomas T. Systematic review of the treatment of upper respiratory tract infection. Arch Dis Child. 1998;79(3):225–230. doi: 10.1136/adc.79.3.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alves Galvão MG, Rocha Crispino Santos MA, Alves da Cunha AJ. Antibiotics for preventing suppurative complications from undifferentiated acute respiratory infections in children under five years of age. Cochrane Database Syst Rev. 2014;(2):CD007880. doi: 10.1002/14651858.CD007880.pub2. [DOI] [PubMed] [Google Scholar]
- 12.Ng GJY, Tan S, Vu AN, et al. Antibiotics for preventing recurrent sore throat. Cochrane Database Syst Rev. 2015;(7):CD008911. doi: 10.1002/14651858.CD008911.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Venekamp RP, Sanders SL, Glasziou PP, et al. Antibiotics for acute otitis media in children. Cochrane Database Syst Rev. 2015;(6):CD000219. doi: 10.1002/14651858.CD000219.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hay AD, Wilson A, Fahey T, Peters TJ. The duration of acute cough in pre-school children presenting to primary care: a prospective cohort study. Fam Pract. 2003;20(6):696–705. doi: 10.1093/fampra/cmg613. [DOI] [PubMed] [Google Scholar]
- 15.Little P, Moore M, Warner G, et al. Longer term outcomes from a randomised trial of prescribing strategies in otitis media. Br J Gen Pract. 2006;56(524):176–182. [PMC free article] [PubMed] [Google Scholar]
- 16.National Institute for Health and Clinical Excellence . Respiratory tract infections (self-limiting): prescribing antibiotics CG69. London: NICE; 2008. https://www.nice.org.uk/guidance/cg69/resources/respiratory-tract-infections-selflimiting-prescribing-antibiotics-pdf-975576354757 (accessed 1 Aug 2018) [PubMed] [Google Scholar]
- 17.Keith T, Saxena S, Murray J, Sharland M. Risk-benefit analysis of restricting antimicrobial prescribing in children: what do we really know? Curr Opin Infect Dis. 2010;23(3):242–248. doi: 10.1097/QCO.0b013e328338c46d. [DOI] [PubMed] [Google Scholar]
- 18.Little P, Stuart B, Hobbs FD, et al. Antibiotic prescription strategies for acute sore throat: a prospective observational cohort study. Lancet Infect Dis. 2014;14(3):213–219. doi: 10.1016/S1473-3099(13)70294-9. [DOI] [PubMed] [Google Scholar]
- 19.Little P, Stuart B, Smith S, et al. Antibiotic prescription strategies and adverse outcome for uncomplicated lower respiratory tract infections: prospective cough complication cohort (3C) study. BMJ. 2017;357:j2148. doi: 10.1136/bmj.j2148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goossens H, Ferech M, Vander Stichele R, et al. Outpatient antibiotic use in Europe and association with resistance: a cross-national database study. Lancet. 2005;365(9459):579–587. doi: 10.1016/S0140-6736(05)17907-0. [DOI] [PubMed] [Google Scholar]
- 21.Redmond NM, Davies R, Christensen H, et al. The TARGET cohort study protocol: a prospective primary care cohort study to derive and validate a clinical prediction rule to improve the targeting of antibiotics in children with respiratory tract illnesses. BMC Health Serv Res. 2013;13:322. doi: 10.1186/1472-6963-13-322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gillespie D, Hood K, Farewell D, et al. Adherence-adjusted estimates of benefits and harms from treatment with amoxicillin for LRTI: secondary analysis of a 12-country randomised placebo-controlled trial using randomisation-based efficacy estimators. BMJ Open. 2015;5(3):e006160. doi: 10.1136/bmjopen-2014-006160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Craig JV, Lancaster GA, Williamson PR, Smyth RL. Temperature measured at the axilla compared with rectum in children and young people: systematic review. BMJ. 2000;320(7243):1174–1178. doi: 10.1136/bmj.320.7243.1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ferguson J. Advanced paediatric life support. 3rd edn. London: BMJ Books; 2001. [Google Scholar]
- 25.National Institute for Health and Clinical Excellence . Feverish illness in children: assessment and initial management in children younger than 5 years. London: NICE; 2007. [Google Scholar]
- 26.Strozik KS, Pieper CH, Roller J. Capillary refilling time in newborn babies: normal values. Arch Dis Child Fetal Neonatal Ed. 1997;76(3):F193–F196. doi: 10.1136/fn.76.3.f193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tibby SM, Hatherill M, Murdoch IA. Capillary refill and core-peripheral temperature gap as indicators of haemodynamic status in paediatric intensive care patients. Arch Dis Child. 1999;80(2):163–166. doi: 10.1136/adc.80.2.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ministry of Housing, Communities and Local Government Index of Multiple Deprivation Score. 2007. https://data.gov.uk/dataset/index-of-multiple-deprivation-score-2007 (accessed 1 Aug 2018)
- 29.Rosenbaum PR, Rubin DB. Reducing bias in observational studies using subclassification on the propensity score. J Am Stat Assoc. 1984;79(387):516–524. [Google Scholar]
- 30.Stuart BL, Grebel LEN, Butler CC, et al. Comparison between treatment effects in a randomised controlled trial and an observational study using propensity scores in primary care. Br J Gen Pract. 2017. . [DOI] [PMC free article] [PubMed]
- 31.Francis NA, Gillespie D, Nuttall J, et al. Delayed antibiotic prescribing and associated antibiotic consumption in adults with acute cough. Br J Gen Pract. 2012. . [DOI] [PMC free article] [PubMed]
- 32.Spurling GK, Doust J, Del Mar CB, Eriksson L. Antibiotics for bronchiolitis in children. Cochrane Database Syst Rev. 2011;(6):CD005189. doi: 10.1002/14651858.CD005189.pub3. [DOI] [PubMed] [Google Scholar]
- 33.Little P, Rumsby K, Kelly J, et al. Information leaflet and antibiotic prescribing strategies for acute lower respiratory tract infection: a randomized controlled trial. JAMA. 2005;293(24):3029–3035. doi: 10.1001/jama.293.24.3029. [DOI] [PubMed] [Google Scholar]
- 34.Moore M, Little P, Rumsby K, et al. Effect of antibiotic prescribing strategies and an information leaflet on longer-term reconsultation for acute lower respiratory tract infection. Br J Gen Pract. 2009. . [DOI] [PMC free article] [PubMed]
- 35.Dowell J, Pitkethly M, Bain J, Martin S. A randomised controlled trial of delayed antibiotic prescribing as a strategy for managing uncomplicated respiratory tract infection in primary care. Br J Gen Pract. 2001;51(464):200–205. [PMC free article] [PubMed] [Google Scholar]
- 36.Little P, Moore M, Kelly J, et al. Delayed antibiotic prescribing strategies for respiratory tract infections in primary care: pragmatic, factorial, randomised controlled trial. BMJ. 2014;348:g1606. doi: 10.1136/bmj.g1606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Turnbull SL, Redmond NM, Lucas P, et al. The CHICO (Children’s Cough) Trial protocol: a feasibility randomised controlled trial investigating the clinical and cost-effectiveness of a complex intervention to improve the management of children presenting to primary care with acute respiratory tract infection. BMJ Open. 2015;5(9):e008615. doi: 10.1136/bmjopen-2015-008615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Coxeter P, Del Mar CB, McGregor L, et al. Interventions to facilitate shared decision making to address antibiotic use for acute respiratory infections in primary care. Cochrane Database Syst Rev. 2015;(11):CD010907. doi: 10.1002/14651858.CD010907.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lucas PJ, Ingram J, Redmond NM, et al. Development of an intervention to reduce antibiotic use for childhood coughs in UK primary care using critical synthesis of multi-method research. BMC Med Res Methodol. 2017;17(1):175. doi: 10.1186/s12874-017-0455-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ingram J, Cabral C, Hay AD, et al. Parents’ information needs, self-efficacy and influences on consulting for childhood respiratory tract infections: a qualitative study. BMC Fam Pract. 2013;14:106. doi: 10.1186/1471-2296-14-106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cabral C, Ingram J, Hay AD, Horwood J. ‘They just say everything’s a virus’ — parent’s judgment of the credibility of clinician communication in primary care consultations for respiratory tract infections in children: a qualitative study. Patient Educ Couns. 2014;95(2):248–253. doi: 10.1016/j.pec.2014.01.010. [DOI] [PubMed] [Google Scholar]