Abstract
Hyaline cartilages, fibrocartilages and elastic cartilages play multiple roles in the human body including bearing loads in articular joints and intervertebral discs, providing joint lubrication, forming the external ears and nose, supporting the trachea, and forming the long bones during development and growth. The structure and organization of cartilage’s extracellular matrix (ECM) are the primary determinants of normal function. Most diseases involving cartilage lead to dramatic changes in the ECM which can govern disease progression (e.g., in osteoarthritis), cause the main symptoms of the disease (e.g., dwarfism caused by genetically inherited mutations) or occur as collateral damage in pathological processes occurring in other nearby tissues (e.g., osteochondritis dissecans and inflammatory arthropathies). Challenges associated with cartilage diseases include poor understanding of the etiology and pathogenesis, delayed diagnoses due to the aneural nature of the tissue and drug delivery challenges due to the avascular nature of adult cartilages. This narrative review provides an overview of the clinical and pathological features as well as current treatment options available for various cartilage diseases. Late breaking advances are also described in the quest for development and delivery of effective disease modifying drugs for cartilage diseases including osteoarthritis, the most common form of arthritis that affects hundreds of millions of people worldwide.
Keywords: Extracellular matrix, Cartilage, Diseases of cartilage, Growth plate fractures, Genetic disorders involving cartilage, Osteochondritis dissecans, Relapsing polychondritis, Chondrocalcinosis, Pseudogout Cartilaginous tumors, Inflammatory arthropathies, Osteoarthritis, Post-traumatic Osteoarthritis, Cartilage ECM-targeted drug carriers
1. Introduction: Cartilage Structure and Function
Cartilage is an avascular, aneural, alymphatic connective tissue found in the synovial joints, spine, ribs, external ears, nose, and airways, and in the growth plates of children and adolescents. There are three major types of cartilage found in humans: hyaline, fibrous and elastic [1] (see 1). All three types have a low density of cells (chondrocytes) [2] that synthesize and secrete the major components of the extracellular matrix (ECM) [3]. In order to perform the biomechanical functions of providing structural support and resistance to deformation, cartilage ECM contains a unique family of proteoglycans enmeshed within a highly hydrated collagen fibrillar network. Chondrocyte-mediated synthesis and assembly of this matrix is aided, in turn, by synthesis of dozens of additional non-collagenous proteins, proteoglycans and glycoproteins. The abundance, distribution and types of collagens and proteoglycans are different in each of the three types of cartilage, which gives rise to differences in appearance and biomechanical properties.
Hyaline cartilage has a glassy appearance and is the most common form of cartilage in the human body. It is found in the articulating surfaces of bones in synovial joints, and in the ribs, nose, trachea, bronchi, larynx, and growth plates. Articular hyaline cartilage enables joint movements by providing a lubricating surface with an extremely low coefficient of friction on the order of 0.001–0.01 [4] [5] [6] [7]. Water accounts for 60 to 85% of the wet weight of the tissue [8] [9]. Many different types of collagen molecules are expressed in articular cartilage, but the backbone type II collagen fibrillar network is described by Eyre et al. as a heteropolymeric structure with collagen IX molecules covalently linked to the surface of collagen II and collagen XI forming the inner filamentous template of the fibril as a whole [10]. This network accounts for 60 to 70% of the dry weight [9], with a hydrated spacing of ~100 nm between fibrils [11] [12], thereby providing the tissue with tensile and shear strength. The basic NC4 domain of collagen IX is thought to enable interactions between other collagen fibrils as well as other matrix macromolecules [13]. Type VI collagen is found in the pericellular matrix and enables chondrocytes to sense changes in the surrounding matrix and respond to them [14] [15]. Other collagens found in articular cartilage include type III, type X, type XI, type XII and type XIV [10].
The dominant proteoglycan in hyaline cartilage is aggrecan, comprised of a core protein substituted with over 100 chondroitin sulfate glycosaminoglycan (GAG) chains and a lower number of keratan sulfate chains, forming a bottle-brush structure [16] [17] [18]. The G1 globular domain at the N-terminus of the core protein binds non-covalently to hyaluronan (stabilized by adjacent binding of link protein) to form large aggrecan aggregates containing over 100 aggrecan monomers [16] [19]. These aggregates can achieve molecular weights as high as 350 MDa. The GAG chains of each aggrecan monomer are located between the G2 and G3 globular domains [18], giving each aggrecan a net negative charge of approximately −10,000 [20]. Visualized by atomic force microscopy, the protein core of aggrecan in immature and adult cartilage can range from 400–500 nm long, while the GAG chains range from 30–50 nm in length [21] [22]. Importantly, with respect to biomechanical and biophysical function of aggrecan, the spacing between GAG chains on the same aggrecan core protein molecule is ~3 nm [21], which is on the order of the electrical Debye length under physiological conditions. This close spacing, leads to extremely high electrostatic repulsive interactions between adjacent GAG chains along the same core protein and between adjacent core proteins. Taken together, these repulsive interactions at the molecular level provide compressive strength to cartilage at the tissue level, enabling it to act as a load bearing tissue in the joints.
Other proteoglycans found in cartilage include versican, perlecan [23](which is found in the hypertrophic chondrocyte regions in articular cartilage and growth plate cartilage), and small leucine-rich proteoglycans (SLRPs [24]) such as decorin, biglycan [25], epiphycan, lumican and fibromodulin [26] [15] [27]. Decorin, biglycan and epiphycan are dermatan-sulfate proteoglycans [26], while lumican and fibromodulin are keratan-sulfate proteoglycans, which are present in the glycoprotein form devoid of keratan sulfate in adults [26]. SLRPs can interact with collagen, regulating fibril assembly and interactions with additional ECM molecules. SLRPs can also regulate cell-signaling pathways by binding growth factors, cytokines and other ECM components [15]. Additional major non-collagenous proteins in cartilage ECM include cartilage oligomeric matrix protein (COMP), cartilage matrix protein (CMP) or matrilin-1, cartilage intermediate-layer protein (CILP), proline- and arginine-rich end leucine-rich repeat protein (PRELP), fibronectin [28] [29] [30] [31] [26] and cell surface heparan sulfate proteoglycans such as syndecan-4 (SDC4) [32]. A most important glycoprotein in the superficial zone of articular cartilage is lubricin (PRG4), which helps in reducing the friction between articulating surfaces [33]. Finally, ECM macromolecules are also extremely important mediators of cartilage mechanobiology [34], i.e., the ability of physiologic loading to induce cell-signaling responses that may be anabolic or catabolic.
Fibrous cartilage, found primarily in the intervertebral discs and in other locations such as the menisci, bone-tendon interfaces and ligament-tendon interfaces [35] [36], has a high density of type I collagen which gives it high tensile strength. This cartilage has type VI collagen in the pericellular matrix and relatively low amounts of type II collagen and proteoglycans throughout the ECM [1]. The extracellular matrix of elastic cartilage is predominantly made up of type II collagen, proteoglycans and elastin fibers [1]. The elastin fibers are responsible for the yellowish appearance of the tissue and also provide it with high elasticity. Elastic cartilage is found in the external ears, larynx and epiglottis.
Diseases affecting cartilage range from extremely common conditions such as osteoarthritis, which is estimated to affect as many as 37% of all adults in the United States [37] to rare genetic disorders such as Spondyloepimetaphyseal dysplasia (SEMD) aggrecan type, which has been reported in three patients worldwide [38]. The etiology and pathogenesis of many cartilage diseases is not fully understood, due to which treatment methods are not optimal in many cases. Damage to cartilage ECM is an important feature of most diseases affecting the tissue. However, since cartilage is aneural, symptoms usually appear only after significant structural destruction of the matrix, which also makes treatment challenging. The dense packing of ECM components also hinders the transport of drug molecules in the tissue which, along with the lack of vascularity, poses an additional challenge to the treatment of cartilage diseases. This review provides an overview of current knowledge regarding selected cartilage diseases and new developments in cartilage-targeted drug delivery strategies.
2. The role of cartilage in developmental disorders
Hyaline cartilage plays a crucial role in skeletal growth and development, first during endochondral bone formation in embryonic and fetal life and then in the form of growth plates which lead to the elongation of bones during childhood and adolescence [39] [15]. Fractures to the growth plates have the potential to severely disrupt normal growth. Growth plate cartilage is weaker than ligaments and tendons [40] [41], and thus injury patterns which would lead to ligament tears in adults end up causing growth plate fractures in children and adolescents. These fractures are reported to account for approximately 15% of all childhood fractures and between 1% and 30% of sports related injuries [40] [41]. The most widely accepted scheme for classifying these fractures based on the pattern of injury was first proposed in 1963 [40]. This scheme includes five types of fractures, and has proven to be important in determining the prognosis for continuation of normal growth. The classification scheme was expanded in 1982 and now includes 9 types of fractures [42].
In addition to external injuries, genetic mutations can also affect growth plate cartilage and thereby, disturb normal skeletal growth. These mutations can occur in genes encoding regulatory proteins/molecules or extracellular matrix molecules. Such genetic conditions as a group are called chondrodysplasias and, while each is a rare disorder, together they occur in approximately 1 in 4,000 births [43].
FGF (Fibroblast Growth factor) signaling is an important part of the regulatory pathway that controls chondrocyte proliferation and differentiation in the growth plate [39]. The most common form of dwarfism, achondroplasia, is caused by autosomal dominant point mutations in the transmembrane domain of FGFR3 (FGF receptor 3) [44] [45]. FGFR3 is expressed by proliferating chondrocytes in the growth plates and FGF signaling through this receptor inhibits proliferation [46] [39]. The mutations in achondroplasia lead to the formation of a receptor that remains activated even in the absence of a ligand, leading to reduced growth of endochondral bones without affecting cartilage in other parts of the body.
Most of these mutations arise sporadically in the germline cells of older men [45]. A single copy of the mutated gene leads to dwarfism, while having two copies leads to death early in life [47]. The frequency of achondroplasia is estimated to be between 1 in 15,000 and 1 in 28,000 live births [48] [49] [45]. Other autosomal dominant mutations in FGFR3 result in hypochondroplasia, which resembles a mild form of achondroplasia, and thanatotropic dysplasia which results in death in early life [50] [51]. In addition to FGF signaling, parathyroid hormone-related peptide (PTHrP) plays an important role in regulating chondrocyte proliferation in the growth plate [39]. Genetic mutations in the receptor for PTH/PTHrP lead to chondrodysplasias such as Jansen’s metaphyseal chondrodysplasia and Blomstrand’s lethal chondrodysplasia [52]. Mutations in SOX9, a transcription factor that is involved both in male sexual development and in the differentiation of chondrocytes [53] [54], leads to campomelic dysplasia [55]. This disease usually leads to death early in life due to respiratory failure caused by improperly formed cartilage in the respiratory tract [56].
In addition to regulatory proteins, mutations that affect extracellular matrix components also result in abnormal growth and, in some cases, early-onset osteoarthritis. Mutations in the Col2A1 gene for type II collagen predominantly affect cartilage and this spectrum of disorders is called type II collagenopathies. In addition to affecting cartilage, they are also typically accompanied by problems with skeletal development, vision and hearing. The most severe of these diseases are achondrogenesis type 2 (also called Langer-Saldino achondrogenesis), hypochondrogenesis and platyspondylic lethal skeletal dysplasia, Torrance type [57] [58] [59] [60]. Together, achondrogenesis type 2 and hypochondrogenesis occur in 1 in 40,000 to 1 in 60,000 newborns [61]. Platyspondylic lethal skeletal dysplasia, Torrance type is far rarer, with reports of only a few individuals in the world [62]. The mutations in these diseases prevent the secretion of type II collagen from chondrocytes, leading to its absence from the ECM. Instead, type I and type III collagen replace the missing type II collagen in the ECM in achondrogenesis type 2 and hypochondrogenesis [63] [64] [65]. All three diseases lead to severe skeletal under-development and result in death early in life [57] [66].
Less severe type II collagenopathies include Spondyloepiphyseal dysplasia congenita (SED congenita), Kniest dysplasia, SED with metatarsal shortening (also Czech dysplasia), Spondyloperipheral dysplasia, Spondyloepimetaphyseal dysplasia (SEMD) Strudwick type, Stickler syndrome type 1, and Mild SED with premature onset arthrosis [67]. In SED congenita and Kniest dysplasia, the collagen II content of cartilage is less than normal since the secretion from chondrocytes is reduced. These two conditions lead to dwarfism in the affected patients. Stickler syndrome type I is the mildest and most common form of type II collagenopathies that is seen in 1 in 10,000 live births [68]. Patients usually grow to a normal height and the disease is associated with early onset osteoarthritis, vitreous humor abnormalities and congenital myopia. Other variable features of type 1 Stickler syndrome include a flat midface, increased risk for retinal detachment, deafness, slender extremities and joints that are hypermobile [69]. The specific mutations in the Col2A1 gene that lead to type II collagenopathies are discussed in detail in [68].
Type × collagen is expressed by hypertrophic chondrocytes in the growth plates as well as in the deep and calcified zones of articular cartilage [39] [1]. Mutations in the Col10A1 gene leads to Schmid-type metaphyseal dysplasia, an autosomal dominant disorder characterized by short stature, reduction in the angle between the femoral head and the shaft and bow legs [70]. Due to the similarity in symptoms, it may be misdiagnosed as rickets (caused by Vitamin D deficiency) [71].
Disorders associated with mutations in aggrecan are extremely rare. It was thought that aggrecan mutations did not cause diseases in humans until it was first identified in 2005 that a mutation in the AGC1 gene caused Spondyloepiphyseal dysplasia (SED) Kimberly type [72]. Patients with SED Kimberly type are typically short in stature and have early onset osteoarthritis (OA) [73]. Since 2005, two other disorders - familial osteochondritis dissecans (OCD) [74] and Spondyloepimetaphyseal dysplasia (SEMD), aggrecan type [38] - have been identified as being caused by mutations in AGC1. Similar to SED Kimberly type, familial OCD is also associated with dwarfism and early onset OA [75]. SEMD aggrecan type leads to a decrease in height both in homozygous patients and heterozygous carriers, but does not lead to early OA. A thorough review on the current state of knowledge regarding these aggrecanopathies can be found in [76].
In many of the chondrodysplasias with mutations in ECM components, disease progression is driven not only by abnormalities in the ECM structure, but also by the accumulation of the mutant molecules within chondrocytes [77] [43]. Specifically, abnormally folded proteins collect in the endoplasmic reticulum (ER) which triggers ER stress. This in turn induces the unfolded protein response (UPR). The UPR is a set of signaling pathways that attempt to eliminate mutant proteins by upregulating degradation of the accumulating proteins and increasing the synthesis of chaperones that assist with protein folding. At the same time, UPR leads to a downregulation of overall protein synthesis in order to reduce the expression of mutant proteins [78]. This downregulation could affect not just the mutant ECM components, but also other ECM molecules which are unaffected by the mutation [77]. Consequently, the ECM may lack sufficient levels of multiple proteins which would compromise its structure and function, thereby contributing to the pathological changes that are observed in these disorders. Additionally, if the mechanisms described above are not successful in reducing ER stress, UPR will eventually trigger apoptosis which can also contribute to the phenotypic changes that are seen in patients [77]. Developing drugs that can alleviate ER stress and target UPR could potentially lead to a significant clinical improvement in chondrodysplasias without requiring gene therapy [43].
3. Osteochondritis dissecans
Osteochondritis dissecans (OCD) was first described by König in 1888 as a condition that leads to the formation of loose bodies in the joints without any trauma [79] [80]. Most recently, OCD has been defined as “A focal, idiopathic alteration of subchondral bone with risk for instability and disruption of adjacent articular cartilage that may result in premature osteoarthritis" [81]. There are both juvenile and adult forms of the disease, which are defined based on whether or not the growth plates are open in the patient [82] [83]. The joint that is most commonly affected by OCD is the knee, followed by the ankle and the elbow. Within the knee, OCD lesions classically present on the lateral aspect of the medial femoral condyle [84] [85]. In one report of disease incidence in femoral condyles, the highest incidence was found in the 10–20 year old age group with 20 in 100,000 women and 30 in 100,000 men affected [86]. A more recent pediatric knee OCD epidemiological study was conducted involving over 1 million people aged between 2 and 19 years [87]. In this study, there was no incidence of knee OCD in the 2–5 year old children, while the incidence in the 6–19 year old age group was 9.5 in 100,000. Within the 6–19 age group, the incidence was much higher for males compared to females (15.4 vs 3.3 in 100,000).
The etiology of OCD has not yet been established conclusively. One proposed mechanism is that repetitive trauma leads to progression of the disease. This is supported by the findings that 55 to 60% of patients are involved regularly in sporting or athletic activities [88] [89] [90]. Additionally, repetitive contact with the tibial spine has been proposed as the etiology for OCD at the classic location on the medial condyle [84]. Other etiologies that have been proposed include repetitive trauma, inflammation, ischemia, hereditary factors, osteonecrosis and gradual development following an injury to the endochondral epiphyseal growth plate [75] [91] [90]. There is currently speculation that a single etiology may not explain all cases of OCD, and that the disease may be multifactorial [91].
OCD is diagnosed based on patient history, a physical exam and a radiograph. An MRI or an arthroscopy may also be used to supplement the findings on the radiograph. Once the disease is diagnosed, the treatment options considered depend on the stability of the lesion and the age of the patient. Non-surgical options are usually the first line of treatment for juvenile patients with stable lesions [92] [91]. If the stable lesion does not improve with non-surgical management, then surgery is considered. Surgical management is considered as the first line of treatment for cases where the lesion is unstable or when a stable lesion is found in an adult [92]. The various non-surgical and surgical treatment methods are covered in detail in other papers [85] [93] [94] [95], and we refer the reader to them.
4. Relapsing polychondritis
Relapsing polychondritis (RP) is a rare inflammatory disease that can affect all forms of cartilage and also organs containing proteoglycans, such as the eye and the heart. Its prevalence is estimated to be 2/million person-years in Hungary [96] and 3.5/million person-years in the United States [97]. The early symptoms of RP include one or more of the following: external ear pain that does not affect the non-cartilaginous lobule, temporary pain in one or more joints, nasal pain, throat pain, hoarse voice, eye involvement in the form of scleritis or episcleritis, vasculitis, skin symptoms, hearing impairment, dizziness and systemic symptoms such as fever and weight loss [98] [99]. These symptom(s) can appear as intermittent acute flares, and the disease follows a progressive course that can lead to complete destruction of the affected tissue [98]. For instance, destruction of nasal cartilage leads to the commonly observed “saddle nose" in these patients. The most common cause of death in RP patients is respiratory failure, followed by causes related to cardiovascular involvement. In rare cases, the disease also affects the kidneys and the prognosis is very poor in these patients [100].
The symptoms of RP can overlap with many other diseases and there is currently no specific test that can be used to conclusively diagnose this condition. Biopsy findings cannot be used to make a conclusive diagnosis, and the injury caused by a biopsy can further aggravate the disease. The diagnosis for RP is currently made solely on the basis of clinical presentation and by excluding other diseases based on symptoms or by running specific tests. However, this process leads to frequent misdiagnosis of RP in the initial stages, especially in cases where there is a single presenting symptom. The mean delay between the time a patient first presents with symptoms and the time a diagnosis for RP is made is 2.9 years [98]. The first clinical algorithm for confirming a diagnosis was published in 1976, in which 3 of the following 6 criteria were considered to be sufficient: (1) bilateral chondritis in the ears, (2) polyarthritis that is inflammatory and non-erosive, (3) nasal chondritis, (4) inflammation in the eyes, (5) chondritis involving cartilage in the larynx and/or the trachea and (6) damage to the cochlea or the vestibules in the ears [101]. Other authors have modified these criteria to include biopsy results, response to dapsone and steroid drugs and seronegative inflammatory arthritis [102] [103]. RP frequently occurs in conjunction with other autoimmune diseases, and these should be considered while making a diagnosis.
The etiology of relapsing polychondritis is not fully understood. But there is evidence that autoimmunity against cartilage specific components may play an important role and that there may be a genetic component that may increase susceptibility for the disease [104] [105]. Disease progression is measured using the Relapsing Polychondritis Disease Activity Index, which was developed in 2012 and uses 27 variables to generate a score [106]. Clinical progression and RPDAI are used to determine the management strategy for RP. In early stages, NSAIDs, dapsone and steroids are used to manage the symptoms. If these drugs do not induce a response, immunosuppressive drugs such as azathioprene and methotrexate are then administered. In recent years, biologic drugs such as TNF-α antagonists have been used in patients with severe RP that does not respond to other treatments [99]. Additionally, surgical interventions are used to rescue patients who are in respiratory collapse and to improve hearing and/or stability in those with inner ear involvement.
5. Chondrocalcinosis
Chondrocalcinosis is the deposition of calcium pyrophosphate dihydrate (CPPD) crystals in joints. This condition can stay asymptomatic, give rise to an acute inflammatory reaction that resembles gout (called pseudogout or acute CPP crystal arthritis) [107] [108], or chronic arthritis that may resemble osteoarthritis (OA) or rheumatoid arthritis [109]. In symptomatic patients, finding characteristic CPPD crystals in the synovial fluid confirms the diagnosis. Imaging techniques such as radiographs, ultrasonographs, CT scans and MRI scans are also used to diagnose the disease. The prevalence of chondrocalcinosis increases with age, and estimates range from 7% to 10% after the age of 60 years [110] [111] [112]. There is also a higher incidence of CPPD crystal deposition in joints with OA, but this has not been found to increase the rate of OA progression [113] [114] [115].
CPPD crystals are predominantly formed in the pericellular spaces surrounding chondrocytes in cartilaginous tissues [116] [109]. Chondrocytes release small amounts of inorganic pyrophosphate as well as ATP into the pericellular space [117] [118]. Extracellular ATP is broken down to form inorganic pyrophosphate by enzymes in the pericellular space [118] [119]. Inorganic pyrophosphate reacts with calcium to form CPPD crystals, which can change mechanical properties of cartilage. A transmembrane protein, ANKH, regulates the release of ATP and inorganic pyrophosphate from chondrocytes [120] and mutations in this have been found in genetically inherited cases of chondrocalcinosis [109].
When CPPD crystals are released from cartilage into the joint space, they can induce an acute inflammatory reaction that leads to pseudogout symptoms. The crystals can induce chondrocytes and synoviocytes to upregulate MMP and prostaglandin production and also activate innate immune responses by activating the NLRP3 inflammasome which leads to the release of proinflammatory cytokines [121] [122] [123] [124].
There are currently no treatments that can reduce the quantity of CPPD crystals being formed in the joints. The acute form of the disease affects approximately 25% of patients with chondrocalcinosis [125] and leads to joint pain, swelling and warmth in one or more joints. The knee is the most commonly affected joint in these patients. Current treatment options include intra-articular glucocorticoid injections, oral colchicine (which acts upstream of inflammasome activation) and NSAIDs [126]. The chronic presentation of the disease is more difficult to manage, and in addition to the drugs used for the acute disease, hydroxychloroquine and methotrexate have also been used [127] [128] [129]. Emerging therapeutics include anti-inflammatory drugs such as IL-1 inhibitors and drugs such as probenecid that can target the ANKH protein and reduce the production of inorganic pyrophosphate in the joint [130] [109].
6. Cartilaginous tumors
Tumors made of cartilaginous tissue constitute a major class of bone tumors. Benign cartilaginous tumors include osteochondroma, enchondroma, periosteal chondroma, multiple chondromatosis or enchondromatosis, chondroblastoma, and chondromyxoid fibroma. Enchondroma and osteochondroma are by far the most frequently occurring benign forms, accounting for 34% and 30% of all benign cartilaginous tumors, respectively. Malignant cartilaginous tumors (chondrosarcomas (CHS)) include central CHS (both primary and secondary), peripheral CHS, dedifferentiated CHS, mesenchymal CHS and clear cell CHS. Overall, bone sarcomas account for only about 0.2% of all malignancies in the United States [131] and primary CHS is the third most common primary bone malignancy, accounting for 20 to 27% of all cases. The epidemiology of benign cartilaginous tumors has not been well established, in part because many cases remain undiagnosed due to the absence of symptoms. When they are symptomatic, benign tumors can present with pain, swelling, lesions or a pathological fracture.
In osteochondroma, there is a marrow-containing bony projection covered with hyaline cartilage that gets thinner with increasing age. It usually occurs in the metaphyseal region of endochondral bones. In chondromas, hyaline cartilage can be found either inside the bone (called an enchondroma if it is a single lesion or enchondromatosis in the case of multiple lesions) or on the surface of bones (periosteal or juxta-cortical chondroma). Enchondromatosis and periosteal chondroma are far less common than enchondroma. There is emerging evidence that disturbances in the signaling pathways involved in the formation of new bone from growth plate cartilage contribute to the formation of osteochondroma and enchondroma [132]. Chondroblastoma is a rare benign tumor (less than 1% of all bone tumors) of unknown etiology that is usually found in the bone epiphysis and is made up of chondroblasts (immature chondrocytes). Chondromyxoid fibroma is an equally rare benign tumors comprising of lobules of chondroid and myxoid tissue with both spindle shaped cells and giant osteoclast-like cells. Benign cartilaginous tumors rarely progress to malignant forms and are usually treated through excision or curettage along with a bone graft if necessary. The radiological and histological features of all these tumors are covered in detail in [133], and these can be used to confirm the diagnosis. One exception is enchondroma, since it is still very challenging to differentiate enchondroma from low grade chondrosarcoma based on histology, imaging and clinical features [134] [135]. This is an important problem that needs to be solved since chondrosarcoma is a malignant tumor and a misdiagnosis can lead to terminal progression of the disease. Chondrosarcomas may be primary (arising de novo; these account for 85% of all cases) or secondary (arising from a pre-existing tumor) lesions made up of hyaline cartilage [136]. There are 3 grades of CHS and the morphology of the tissue deviates significantly from normal cartilage in the higher grades [136]. Histological analysis can be used to identify different grades as well as the different types of CHS. Chemotherapy and radiation are not very effective for treating CHS, and surgery is the main form of therapy [137] [138] [139].
7. Inflammatory arthropathies
There are several inflammatory rheumatic diseases that lead to arthritis and can severely damage cartilage tissue. These include rheumatoid arthritis, juvenile idiopathic arthritis, gout, systemic lupus erythematosus, and seronegative spondyloarthropathies.
Rheumatoid arthritis (RA), an autoimmune disease that affects 0.5 to 1% of people worldwide [140] [141], is characterized by chronic systemic inflammation and arthritis in multiple synovial joints. Left untreated, the disease leads to progressive destruction of cartilage and synovial joints. Serum markers for RA include rheumatoid factor (RF) and anticitrullinated peptide antibody (ACPA) [141]. Both adaptive and innate immune systems play a role in the progression of the disease and the major cytokines involved are TNF-α, IL-6, IL-1β and IL-17A [141]. Synthetic disease modifying anti-rheumatic drugs (DMARDs) include methotrexate (which is usually the first line of treatment for RA [142]), leflunomide, hydroxychloroquine and sulfasalazine [140]. Biologic drugs against TNF-α have constituted a major breakthrough for RA treatment, and constitute the first widespread and major success of the biologic DMARD era; they are effective in halting the progression of the disease in almost 65% of RA patients. Additional therapeutics, when TNF blockers are not found to be effective, include monoclonal antibodies against CD20 in B cells, IL-1 receptor antagonist and monoclonal antibodies against the IL-6 receptor [140].
Gout is the most common form of inflammatory arthritis [143], affecting approximately 8.3 million people, or 4% of the population in the United States [144]. It is caused by the deposition of uric acid crystals in the joint and is managed using non-steroidal anti-inflammatory drugs (NSAIDs), colchicine and lifestyle changes to reduce uric acid levels [145].
Juvenile idiopathic arthritis (JIA) refers to any form of arthritis of unknown cause that occurs in a person younger than 16 years of age [146]. JIA is the most common form of arthritis in children and its prevalence is estimated to range from 16 to 150 per 100,000 in the developed world [146]. In the United States, juvenile arthritis and other rheumatological conditions affect approximately 1 in 250 children [147] [148]. There are six subtypes of JIA: systemic, oligoarticular, polyarticular (which may be positive or negative for rheumatoid factor), juvenile psoriatic, enthesis-related, and undifferentiated arthritis [146].
Systemic lupus erythematosus is an auto-immune disease characterized by the presence of circulating anti-nuclear antibodies (ANA) [149]. Approximately 20% of cases occur in childhood and the childhood onset form of the disease has a worse prognosis compared to the adult onset form [150]. SLE can affect multiple organs in the body including the kidneys, skin, lungs, heart, joints and brain [149]. Clinically, there may be a broad range of signs and symptoms; the criteria for making a diagnosis were proposed in 1971 and then revised and updated in 1982 and 1997 [151] [152]. A majority of patients experience arthropathy [153], and 3 to 6% of patients have Jaccoud's arthropathy which leads to severe deformation of the fingers [154] [155].
Seronegative spondyloarthropathies, which comprise of ankylosing spondylitis, psoriatic arthritis, reactive arthritis, arthritis associated with inflammatory bowel disease, and a subtype of juvenile idiopathic arthritis [156]. These conditions are characterized by an absence of rheumatoid factor, a strong correlation with carrying the HLA-B27 gene and clinical features that include inflammatory back pain, inflammation of insertion sites of tendons and ligaments, peripheral arthritis and eye inflammation [157] [156]. The overall prevalence of spondyloarthritides in North America is approximately 1% [158] and the most common form of spondyloarthritis is ankylosing spondylitis [156] [159]. The prevalence of psoriatic arthritis in the United States is 0.1% [158] and it is seen in 10 to 40% of people with psoriasis [160]. In a majority of patients, the arthritis develops after psoriasis develops [160]. In addition to arthritis and skin lesions, these patients may also experience eye inflammation, tendinitis and pitting of the fingernails [161]. Importantly, anti-TNF biologics have been found to be very effective in treatment of ankylosing spondylitis as well as psoriatic arthritis [162].
8. Osteoarthritis
Osteoarthritis (OA) is the most common form of arthritis, affecting approximately 90 million adults (36.8% of the adult population) in the United States alone [37] and hundreds of millions of people worldwide. The disease primarily affects articular hyaline cartilage in load bearing joints such as the knee, hip and shoulder, and is also commonly found in the hands and feet. Other tissues such as the joint capsule synovium and subchondral bone are also affected and contribute to disease progression. As the disease advances, there is severe degeneration of cartilage, narrowing of the joint space, subchondral bone thickening, formation of osteophytes [163] or bone spurs, and inflammation in the joint accompanied by swelling and pain [164]. While debates continue as to whether disease initiation primarily involves cartilage versus subchondral bone, we limit this review to processes and mechanisms involving cartilage degeneration, as a separate review in this series will focus entirely on OA.
The risk factors for OA are diverse and include obesity, female gender, age, congenital structural abnormalities in the joint and acute joint trauma [164]. In 2011, the total direct healthcare expenditure associated with OA in the United States was $ 41.7 billion [165]. Despite the tremendous disease burden worldwide, there are currently no disease modifying drugs (DMOADS) to alter or halt disease progression, which usually takes place over multiple years [166]. Early OA diagnosis also remains challenging because cartilage changes involved in the earliest stages do not cause pain, due to the aneural nature of the tissue. Thus, patients may remain symptom-free until more advanced stages of the disease occur, with significant and irreparable joint damage [167]. Progress is being made in improving imaging modalities and discovering molecular biomarkers to enable earlier disease diagnosis [168] [169] [170]. One exception where early intervention is currently possible is post-traumatic osteoarthritis (PTOA), the sub-type of OA that develops in approximately 50% of all patients who experience a traumatic joint injury such as an ACL tear [171]. PTOA accounts for ~12% of all OA cases [172] and typically affects a population that is much younger and otherwise healthier than other forms of OA [171]. Since PTOA usually takes 10–15 years to progress from the time of the joint injury to fully developed OA, effective clinical intervention immediately after the injury could potentially prevent PTOA from developing in these patients. Even so, developing DMOADs for PTOA remains challenging as there are no approved methods to delivery drugs directly inside of cartilage, either to chondrocytes or ECM targets [173].
The etiopathogenesis of OA is not completely understood. It is known that OA is a multifactorial disease involving the entire joint and not just cartilage [164] [174] [175]. Mechanical, biological and structural factors are all known to play a role in disease initiation and progression. For example, in the case of PTOA, the initial joint injury results in damaging mechanical forces on multiple joint tissues. While surgical intervention may enable mechanical stabilization of the joint and repair of the initial injury, it is known that surgery does not alter progression to OA. The initial injury can result in permanent structural changes to the joint which can lead to abnormal loads on cartilage and thereby contribute further to PTOA progression [176]. The acute mechanical trauma can also lead to cell death, loss of aggrecan from cartilage and bruising of the subchondral bone [177] [178]. In addition to mechanical damage, there is a sudden increase in the levels of pro-inflammatory cytokines in the joint in the days following the initial injury [179], such as TNF-α, IL-6, and IL-1β. These cytokines can diffuse into cartilage and cause upregulation of protease activity, leading to degradation of multiple macromolecules of cartilage ECM by the matrix metalloproteinases [180] and aggrecanases [178] [179].
Inflammation also plays an important role in other types of OA [181] [182], and there are reports of both innate immune responses (e.g., complement cascade [183], recruitment of macrophages [184], and the activation of pattern recognition receptors (such as toll-like receptors) [185] [186] [187]) and adaptive immune responses (B cell responses [188] and T cell responses [189]). Inflammation can cause oxidative stress through the action of reactive oxygen species that can damage cartilage tissue and cells [190], also leading to catabolic degradation of ECM and loss of aggrecan and collagen [178] as well as other matrix degradation products [191]. Ultimately, this breakdown compromises the mechanical strength of the tissue and makes it susceptible to further damage and degradation. Since tissue fragments and macromolecules released by mechanical damage can trigger innate immune responses, these factors can amplify each other’s effect and form a vicious cycle leading to rapid disease progression [182]. The effect of inflammation on the joint capsule is also a frequent source of pain for OA patients. Finally, changes in the subchondral bone at early stages contribute to disease progression [192] [193].
Based on recent advances in understanding OA pathogenesis, several DMOAD candidates are currently under investigation. Based on evidence that subchondral bone may play a crucial role in OA progression, drugs such as bisphosphonates, calcitonin and strontium ranelate that can reduce bone resorption and increase bone formation are under consideration [166] [194] [195]. Inflammation and mechanical injury in OA leads to excessive activation of Wnt signaling, which increases transformation of mesenchymal stem cells (MSCs) to bone and induces MMP production, leading to cartilage degeneration. Thus, molecule inhibitors of Wnt signaling, are also being tested [196], and were found to promote in vitro differentiation of MSCs into chondrocytes and to inhibit production of MMPs by chondrocytes treated with cytokines [196] [197]. Another potential therapeutic, Kartogenin, induces MSCs to selectively differentiate into chondrocytes [198], and reduces nitric oxide production and GAG loss from bovine cartilage explants in the presence of cytokines [198]. In a rat PTOA model [199], Kartogenin decreased cartilage degeneration and matrix turnover. To promote cartilage regeneration, growth factors such as IGF-1 and FGF-18 are being tested in-vitro and in-vivo [200] [201]. Phase I clinical trials have been completed for FGF18 in advanced OA patients where it was found to be safe for use and also promote cartilage growth while preventing its loss [202]. In a separate phase I trial [203], there were no differences found in the cartilage thickness in the medial femorotibial compartment of the treatment versus placebo groups. However, there was a dose dependent increase in the cartilage thickness and reduction in the joint space narrowing in the lateral femorotibial compartment [203]. A location-independent analysis showed that FGF18 reduced cartilage loss and improved cartilage thickness in this trial [204].
To manage OA pain, acetaminophen has been found to be ineffective [205] and NSAIDs (non-steroidal anti-inflammatory drugs) are currently the preferred treatment. β-nerve growth factor (NGF) is a neurotrophin that causes pain and a monoclonal antibody inhibitor of NGF (tanezumab) has been studied in clinical trials [206]. Phase III clinical trial results showed that tanezumab treatment significantly reduced hip and knee OA pain but caused rapid OA progression in some patients [206] [207]. Similar results were found in a phase II–III trial with fasinumab, another anti-NGF monoclonal antibody [206] [208]. Further studies are warranted, as increased OA progression could have been due to higher activity and loading of joints in the absence of pain, neuropathic arthropathy or upregulation of TLR (toll-like receptor) inflammatory response [206].
While pain medications may alleviate OA symptoms, they do not resolve the underlying pathological processes. To target the inflammatory and catabolic aspects of OA, MMP inhibitors and aggrecanase inhibitors were developed, but early trials of systemically administered MMP inhibitors were not successful and were discontinued [166]. Monoclonal antibody (mAb) inhibitors targeting the aggrecanase ADAMTS-5 are still undergoing preclinical testing [166] [209]. Other mAbs targeting the pro-inflammatory cytokines IL-1, TNF-α and IL-6 have yet to be found dramatically effective [166]. But a possible reason for this is that mAbs are too large in size to penetrate cartilage tissue rapidly enough before being cleared from the joint capsule. Emerging targets for anti-inflammatory therapy include reactive oxygen species, senescent chondrocytes and signaling receptors and pathways involved in pro-inflammatory cytokine production (examples: TLR2 and TLR4 receptors, p38 MAPK signaling pathway) [175] [210] [185] [187].
Corticosteroids are currently injected into joints to manage pain, but due to their broad anti-inflammatory effects they are also being studied as potential DMOADs. In an in-vitro bovine cartilage cytokine injury model, dexamethasone was found to rescue cartilage viability and have anti-catabolic effects [200]. In the same study, a combination therapy of Dexamethasone and IGF-1 was found to have both anti-catabolic and pro-anabolic effects, and the combination was superior to either drug alone using human cartilage. A clinical trial to study the efficacy of Dexamethasone in preventing or reducing the severity of PTOA following a wrist fracture was initiated based in part on such data [211]. Another corticosteroid, triamcinolone, was injected into the joints of patients with knee OA in a phase IV clinical trial [212]. Using standard intra-articular injection (with no direct delivery system into cartilage), patients receiving triamcinolone did not have any difference in pain scores compared to controls receiving saline. Furthermore, the triamcinolone group revealed loss of cartilage volume (by MRI) at the end of two years compared to the controls. In contrast, in a different Phase IIb clinical trial in which triamcinolone was injected using an extended release micro-particle formulation, there was a significant reduction in the pain during the first 11 weeks [213].
The difference in the results of the above trials highlights the challenge of drug delivery to targets inside avascular cartilage. Drugs administered systemically will not reach the tissue in an effective manner due to the lack of blood vessels. A method to overcome this challenge is intra-articular (IA) injection, where drugs are injected directly into the synovial fluid in joints [214]. This has the added advantage of being a localized delivery method that will prevent any systemic side effects associated with the drug. However, drugs injected into the joints are cleared out rapidly by the lymphatics and vessels in the joint capsule, leading to poor cartilage penetration [214]. The dense extracellular matrix of cartilage also provides steric hindrance to the transport of drug molecules, which further reduces drug penetration into the tissue. To achieve therapeutic levels of the drug inside cartilage, repeated injections with high doses would be required. But these are not recommended clinically as they would cause systemic side effects and increase the risk of infection.
To improve the retention time of drugs in the joint, multiple strategies including entrapment of hydrophobic drugs inside liposomes [215], encapsulation inside polymer based micro- or nano-particles [216], and encapsulation in PEGylated single walled carbon nanotubes (PEG SWCNTs) [217] have been studied. A recent development has been the discovery that electrostatic interactions between positively charged nano-carriers and negatively charged aggrecan proteoglycans can dramatically increase both penetration and retention of drugs in cartilage [218] [219]. Avidin, a 66 kDa positively charged protein, has been demonstrated to be an effective carrier for dexamethasone, both in-vitro (in cytokine injury models) and in a rabbit PTOA model [220] [221]. Increased interfacial partitioning of the cationic nano-carrier, and thus increased diffusive transport into cartilage occurs with increasing positive charge on the nano-carrier. At the same time, highly charged cationic carriers may bind strongly to the negatively charged matrix, which slows transport into cartilage, though increasing retention time [173]. The carrier charge can be fine-tuned to balance these two effects and provide optimal uptake, penetration and retention in the tissue and this is currently a very active area of research. Successful delivery of drugs may potentially transform several of the DMOAD candidates discussed above into effective DMOADs and thereby aid in the development of the first treatment method to slow down or even halt the progression of OA.
9. Conclusions
Cartilage is predominantly made of extracellular matrix with a low density of cells, and matrix changes play an important role in the progression of diseases affecting the tissue. Taken together, cartilage diseases represent one of the most common clinical conditions affecting quality of life in the developed world, with osteoarthritis alone affecting over 90 million people in the United States. In this review, we have provided an overview of the structure of cartilage, changes in cartilage ECM under different disease conditions, and the etiology, pathology, clinical features and currently available treatments for the these diseases. Anti-inflammatory biologic drugs have emerged as a highly effective and groundbreaking treatment to slow down the progression of inflammatory arthropathies such as psoriatic and rheumatoid arthritis. However, there are still no effective disease modifying drugs for most diseases affecting cartilage, and the factors contributing to this include a lack of comprehensive understanding of etiology and pathogenesis, delays in diagnosis owing to the aneural nature of the tissue and challenges associated with delivering potential therapeutics due to the avascular nature of cartilage. There is an urgent need for further investigation into the mechanisms leading to the initiation and progression of cartilage diseases, especially in the case of relatively rare conditions such as relapsing polychondritis and osteochondritis dissecans. In the case of osteoarthritis, mechanistic studies are yielding insights into drug targets and are leading to the development of potential therapeutic candidates. However, there remains a lack of effective methods to deliver appropriate drugs. Cationic carriers for cartilage-targeted drug delivery are now emerging as a promising approach to aid in the identification and improved efficacy of potential DMOADs. Such delivery methods have the added advantage of enabling localized, sustained treatment that is effective at low doses, which minimizes systemic side effects. Since the mechanism of enhanced delivery for these charged carriers depends on their interaction with matrix proteoglycans, the same principles could be applied to deliver drugs for other diseases affecting cartilage. Potential applications include targeted delivery of toxic chemotherapeutic drugs to cartilage forming tumors in the bone without affecting normal tissues, and delivery of gene therapies and/or drugs targeting ER stress to chondrocytes in patients with genetic disorders affecting cartilage.
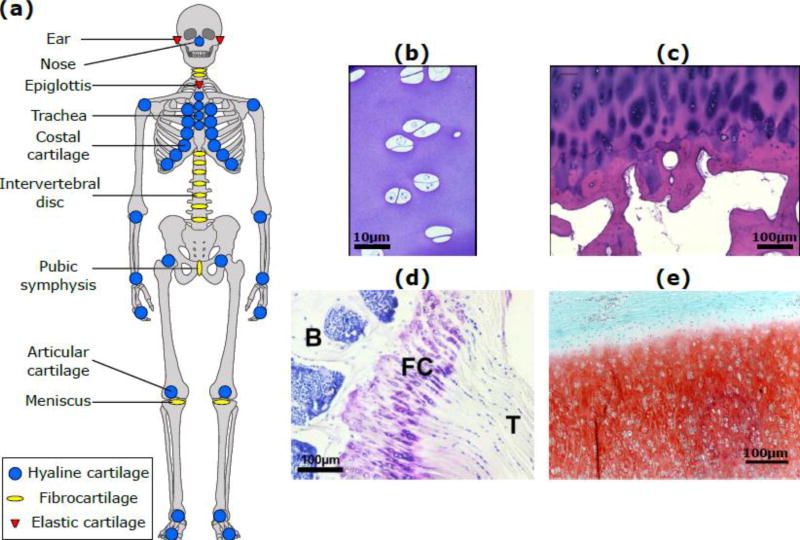
Figure 1. Anatomical locations and microscopic structure of cartilage.
(a) Locations of hyaline cartilage (blue), fibrocartilage (yellow) and elastic cartilage (red) in the human body; (b) Immature bovine articular cartilage; (c) Human adult articular cartilage (83 year old male donor distal femur) showing chondrocyte clustering near the tidemark, typical of osteoarthritis; (d) Murine fibrous cartilage (FC) at the interface of the supraspinatus tendon (T) and the humeral head of the humerus bone (B); (e) Elastic cartilage of neonatal bovine ear; elastin fibers appear as unstained lines between the safranin-O staining for GAGs
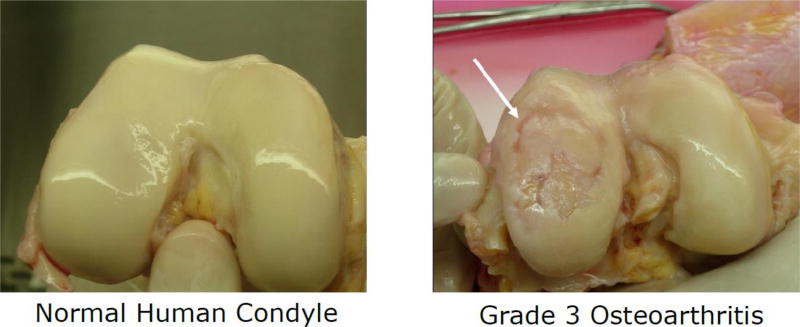
Figure 2.
Macroscopic cartilage degradation in human femoral cartilage caused by osteoarthritis
Highlights.
Cartilage diseases range from extremely rare conditions with three cases reported worldwide to the most common, Osteoarthritis (OA), which affects hundreds of millions of people
Dramatic extracellular matrix changes in these diseases impair normal tissue function and lead to disease progression
There are no disease modifying drugs available for osteoarthritis
Potential disease modifying OA drugs (DMOADs) are being developed based on new advances in understanding OA pathogenesis
Cartilage-targeted drug delivery systems can potentially transform unsuccessful DMOAD candidates into effective therapeutics
Acknowledgments
The authors would like to thank Dr. Brianne Connizzo and Dr. Lawrence Bonassar for providing original images of fibrocartilage and elastic cartilage. This work was supported by the NIH National Institute of Biomedical Imaging and Bioengineering grant EB017755, the National Science Foundation Materials Research Science and Engineering Centers (MRSEC) grant DMR-1419807, the NIH National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS) grant AR060331, the Department of Defense (DoD) Congressionally Directed Medical Research Programs (CDMRP) grant W81XWH-14-1-0544 and the NIH/NCATS grant UG3 TR002186.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributions
Both authors performed a literature search, selected and summarized articles, prepared the figures and wrote the manuscript.
Declarations of interest
None
References
- 1.Wachsmuth L, Söder S, Fan Z, Finger F, Aigner T. Immunolocalization of matrix proteins in different human cartilage subtypes. Histol Histopathol. 2006;21(4–6):477–485. doi: 10.14670/HH-21.477. [DOI] [PubMed] [Google Scholar]
- 2.Stockwell R. The cell density of human articular and costal cartilage. Journal of anatomy. 1967;101(Pt 4):753–763. doi: 10.1111/(ISSN)1469-7580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Naba A, Clauser KR, Ding H, Whittaker CA, Carr SA, Hynes RO. The extracellular matrix: tools and insights for the “ömics” era. Matrix Biol. 2016;49:10–24. doi: 10.1016/j.matbio.2015.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Linn FC. Lubrication of animal joints. I. The arthrotripsometer. The Journal of bone and joint surgery. American. 1967;49(6):1079–98. doi: 10.1080/10643389.2012.728825. [DOI] [PubMed] [Google Scholar]
- 5.Charnley J. The Lubrication of Animal Joints in Relation to Surgical Reconstruction by Arthroplasty. Annals of the Rheumatic Diseases. 1960;19(1):10–19. doi: 10.1136/ard.19.1.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Unsworth A, Dowson D, Wright V. Some new evidence on human joint lubrication. Annals of the Rheumatic Diseases. 1975;34(4):277–285. doi: 10.1136/ard.34.4.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Unsworth A, Dowson D, Wright V. The Frictional Behavior of Human Synovial joints–part I: Natural Joints. Journal of Lubrication Technology. 1975;97(3):369–376. doi: 10.1115/1.3452605. [DOI] [Google Scholar]
- 8.Mow VC, Ratcliffe A, Robin Poole A. Cartilage and diarthrodial joints as paradigms for hierarchical materials and structures. Biomaterials. 1992;13(2):67–97. doi: 10.1016/0142-9612(92)90001-5. [DOI] [PubMed] [Google Scholar]
- 9.Mansour JM. Biomechanics of Cartilage. Kinesiology: the mechanics and pathomechanics of human movement. 2009:66–79. doi: 10.1002/art.23548. [DOI] [Google Scholar]
- 10.Eyre DR, Weis MA, Wu J-J. Articular cartilage collagen: an irreplaceable framework? European cells & materials. 2006;12:57–63. doi: 10.22203/ecm.v012a07. [DOI] [PubMed] [Google Scholar]
- 11.Maroudas A. Transport of solutes through cartilage: permeability to large molecules. Journal of anatomy. 1976;122(Pt 2):335–347. [PMC free article] [PubMed] [Google Scholar]
- 12.Han L, Frank EH, Greene JJ, Lee HY, Hung HHK, Grodzinsky AJ, Ortiz C. Time-dependent nanomechanics of cartilage. Biophysical Journal. 2011;100(7):1846–1854. doi: 10.1016/j.bpj.2011.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Olsen BR. Collagen IX. The international journal of biochemistry & cell biology. 1997;29(4):555–8. doi: 10.1016/s1357-2725(96)00100-8. [DOI] [PubMed] [Google Scholar]
- 14.Eyre D. Collagen of articular cartilage. Arthritis research. 2002;4(1):30–5. doi: 10.1186/ar380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Melrose J, Shu C, Whitelock JM, Lord MS. The cartilage extracellular matrix as a transient developmental scaffold for growth plate maturation. Matrix Biology. 2016;52–54:363–383. doi: 10.1016/j.matbio.2016.01.008. [DOI] [PubMed] [Google Scholar]
- 16.Hardingham TE, Fosang AJ. Proteoglycans: many forms and many functions. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 1992;6(3):861–70. [PubMed] [Google Scholar]
- 17.Dudhia J. Aggrecan, aging and assembly in articular cartilage. Cellular and Molecular Life Sciences. 2005;62(19–20):2241–2256. doi: 10.1007/s00018-005-5217-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Roughley PJ. The structure and function of cartilage. European cells & materials. 2006;12:92–101. doi: 10.22203/ecm.v012a11. doi:vol012a11 [pii] [DOI] [PubMed] [Google Scholar]
- 19.Huang Y, Askew EB, Knudson CB, Knudson W. CRISPR/Cas9 knockout of HAS2 in rat chondrosarcoma chondrocytes demonstrates the requirement of hyaluronan for aggrecan retention. Matrix Biology. 2016;56:74–94. doi: 10.1016/j.matbio.2016.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Muir H. Proteoglycans as organizers of the intercellular matrix. Biochemical Society transactions. 1983;11(6):613–622. doi: 10.1042/bst0110613. [DOI] [PubMed] [Google Scholar]
- 21.Ng L, Grodzinsky AJ, Patwari P, Sandy J, Plaas A, Ortiz C. Individual cartilage aggrecan macromolecules and their constituent glycosaminoglycans visualized via atomic force microscopy. Journal of Structural Biology. 2003;143(3):242–257. doi: 10.1016/j.jsb.2003.08.006. [DOI] [PubMed] [Google Scholar]
- 22.Lee HY, Han L, Roughley PJ, Grodzinsky AJ, Ortiz C. Age-related nanostructural and nanomechanical changes of individual human cartilage aggrecan monomers and their glycosaminoglycan side chains. Journal of Structural Biology. 2013;181(3):264–273. doi: 10.1016/j.jsb.2012.12.008. arXiv:NIHMS150003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gubbiotti MA, Neill T, Iozzo RV. A current view of perlecan in physiology and pathology: A mosaic of functions. Matrix Biology. 2017;57–58:285–298. doi: 10.1016/j.matbio.2016.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Paracuellos P, Kalamajski S, Bonna A, Bihan D, Farndale RW, Hohenester E. Structural and functional analysis of two small leucine-rich repeat proteoglycans, fibromodulin and chondroadherin. Matrix Biology. 2017;63:106–116. doi: 10.1016/j.matbio.2017.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Robinson KA, Sun M, Barnum CE, Weiss SN, Huegel J, Shetye SS, Lin L, Saez D, Adams SM, Iozzo RV, Soslowsky LJ, Birk DE. Decorin and biglycan are necessary for maintaining collagen fibril structure, fiber realignment, and mechanical properties of mature tendons. Matrix Biology. 2017;64(class I):81–93. doi: 10.1016/j.matbio.2017.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Roughley PJ. Articular cartilage and changes in arthritis noncollagenous proteins and proteoglycans in the extracellular matrix of cartilage. Arthritis Research. 2001;3(6):342–347. doi: 10.1186/ar326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wilusz RE, Sanchez-Adams J, Guilak F. The structure and function of the pericellular matrix of articular cartilage. Matrix Biology. 2014;39:25–32. doi: 10.1016/j.matbio.2014.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hedbom E, Antonsson P, Hjerpe A, Aeschlimann D, Paulsson M, Rosa-Pimentel E, Sommarin Y, Wendel M, Oldberg Å, Heinegård D. Cartilage matrix proteins: An acidic oligomeric protein (COMP) detected only in cartilage. Journal of Biological Chemistry. 1992;267(9):6132–6136. [PubMed] [Google Scholar]
- 29.Paulsson M, Heinegård D. Matrix proteins bound to associatively prepared proteoglycans from bovine cartilage. The Biochemical journal. 1979;183(3):539–545. doi: 10.1042/bj1830539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lorenzo P, Bayliss MT, Heinegårdt D. A novel cartilage protein (CILP) present in the mid-zone of human articular cartilage increases with age. Journal of Biological Chemistry. 1998;273(36):23463–23468. doi: 10.1074/jbc.273.36.23463. [DOI] [PubMed] [Google Scholar]
- 31.Heinegard D, Larsson T, Sommarin Y, Franzén A, Paulsson M, Hedbom E. Two novel matrix proteins isolated from articular cartilage show wide distributions among connective tissues. Journal of Biological Chemistry. 1986;261(29):13866–13872. [PubMed] [Google Scholar]
- 32.Binch AL, Shapiro IM, Risbud MV. Syndecan-4 in intervertebral disc and cartilage: Saint or synner? Matrix Biology. 2016;52–54:355–362. doi: 10.1016/j.matbio.2016.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jay GD, Waller KA. The biology of Lubricin: Near frictionless joint motion. Matrix Biology. 2014;39:17–24. doi: 10.1016/j.matbio.2014.08.008. [DOI] [PubMed] [Google Scholar]
- 34.Ringer P, Colo G, Fässler R, Grashoff C. Sensing the mechano-chemical properties of the extracellular matrix. Matrix Biology. 2017;64:6–16. doi: 10.1016/j.matbio.2017.03.004. [DOI] [PubMed] [Google Scholar]
- 35.Benjamin M, Ralphs JR. Fibrocartilage in tendons and ligaments—an adaptation to compressive load. J. Anat. 1998;193:481–494. doi: 10.1046/j.1469-7580.1998.19340481.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Apostolakos J, Durant TJS, Dwyer CR, Russell RP, Weinreb JH, Alaee F, Beitzel K, McCarthy MB, Cote MP, Mazzocca AD. The enthesis: a review of the tendon-to-bone insertion. Muscles, Ligaments and Tendons Journal. 2014;4(3):333–342. [PMC free article] [PubMed] [Google Scholar]
- 37.Jafarzadeh SR, Felson DT. Updated Estimates Suggest a Much Higher Prevalence of Arthritis in United States Adults Than Previous Ones. Arthritis & Rheumatology. 2018;70(2):185–192. doi: 10.1002/art.40355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tompson SW, Merriman B, Funari VA, Fresquet M, Lachman RS, Rimoin DL, Nelson SF, Briggs MD, Cohn DH, Krakow D. A Recessive Skeletal Dysplasia, SEMD Aggrecan Type, Results from a Missense Mutation Affecting the C-Type Lectin Domain of Aggrecan. American Journal of Human Genetics. 2009;84(1):72–79. doi: 10.1016/j.ajhg.2008.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kronenberg HM. Developmental regulation of the growth plate. Nature. 2003;423(May):332–336. doi: 10.1038/nature01657. [DOI] [PubMed] [Google Scholar]
- 40.Salter RB, Harris WR. Injuries Involving the Epiphyseal Plate. JBJS. 1963;45-A(3):586–622. [Google Scholar]
- 41.Caine D, DiFiori J, Maffulli N. Physeal injuries in children’s and youth sports: Reasons for concern? British Journal of Sports Medicine. 2006;40(9):749–760. doi: 10.1136/bjsm.2005.017822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ogden JA. Skeletal growth mechanism injury patterns. Journal of pediatric orthopedics. 1982;2(4):371–7. doi: 10.1097/01241398-198210000-00004. [DOI] [PubMed] [Google Scholar]
- 43.Patterson SE, Dealy CN. Mechanisms and models of endoplasmic reticulum stress in chondrodysplasia. Developmental Dynamics. 2014;243(7):875–893. doi: 10.1002/dvdy.24131. [DOI] [PubMed] [Google Scholar]
- 44.Shiang R, Thompson LM, Zhu Y-Z, Church DM, Fielder TJ, Bocian M, Winokur ST, Wasmuth JJ. Mutations in the transmembrane domain of FGFR3 cause the most common genetic form of dwarfism, achondroplasia. Cell. 1994;78(2):335–342. doi: 10.1016/0092-8674(94)90302-6. [DOI] [PubMed] [Google Scholar]
- 45.Rousseau F, Bonaventure J, Legeai-Mallet L, Pelet A, Rozet JM, Maroteaux P, Le Merrer M, Munnich A. Mutations in the gene encoding fibroblast growth factor receptor-3 in achondroplasia. Nature. 1994;371(6494):252–4. doi: 10.1038/371252a0. [DOI] [PubMed] [Google Scholar]
- 46.Deng C, Wynshaw-Boris A, Zhou F, Kuo A, Leder P. Fibroblast growth factor receptor 3 is a negative regulator of bone growth. Cell. 1996;84(6):911–921. doi: 10.1016/S0092-8674(00)81069-7. [DOI] [PubMed] [Google Scholar]
- 47.Stanescu R, Stanescu V, Maroteaux P. Homozygous achondroplasia: morphologic and biochemical study of cartilage. American Journal of Medical Genetics. 1990;37(3):412–21. doi: 10.1002/ajmg.1320370323. [DOI] [PubMed] [Google Scholar]
- 48.Oberklaid F, Danks DM, Jensen F, Stace L, Rosshandler S. Achondroplasia and hypochondroplasia. Comments on frequency, mutation rate, and radiological features in skull and spine. Journal of Medical Genetics. 1979;16(2):140–6. doi: 10.1136/jmg.16.2.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Waller DK, Correa A, Vo TM, Wang Y, Hobbs C, Langlois PH, Pearson K, Romitti PA, Shaw GM, Hecht JT. The population-based prevalence of achondroplasia and thanatophoric dysplasia in selected regions of the US. American Journal of Medical Genetics, Part A. 2008;146A(18):2385–2389. doi: 10.1002/ajmg.a.32485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ornitz DM, Marie PJ. FGF signaling pathways in endochondral and intramembranous bone development and human genetic disease. Genes & Development. 2002;16:1446–1465. doi: 10.1101/gad.990702.ized. [DOI] [PubMed] [Google Scholar]
- 51.Mundlos S, Olsen BR. Heritable diseases of the skeleton. Part I: Molecular insights into skeletal development-transcription factors and signaling pathways. The FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 1997;11(2):125–132. doi: 10.1096/FASEBJ.11.2.9039954. [DOI] [PubMed] [Google Scholar]
- 52.Schipani E, Provot S. PTHrP, PTH, and the PTH/PTHrP receptor in endochondral bone development. Birth defects research. Part C, Embryo today : reviews. 2003;69(4):352–62. doi: 10.1002/bdrc.10028. [DOI] [PubMed] [Google Scholar]
- 53.Lefrebvre V, de Crombrugghe B. Toward understanding S0X9 function in chondrocyte differentiation. Matrix Biology. 1998;16(9):529–540. doi: 10.1016/S0945-053X(98)90065-8. [DOI] [PubMed] [Google Scholar]
- 54.Akiyama H, Chaboissier M-C, Martin JF, Schedl A, de Crombrugghe B. The transcription factor Sox9 has essential roles in successive steps of the chondrocyte differentiation pathway and is required for expression of Sox5 and Sox6. Genes & development. 2002;16(21):2813–28. doi: 10.1101/gad.1017802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Giordano J, Prior HM, Bamforth JS, Walter MA. Genetic study of SOX9 in a case of campomelic dysplasia. American Journal of Medical Genetics. 2001;98(2):176–181. doi: 10.1002/1096-8628(20010115)98:2<176::AID-AJMG1027>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 56.Provot S, Schipani E. Molecular mechanisms of endochondral bone development. Biochemical and Biophysical Research Communications. 2005;328(3):658–665. doi: 10.1016/j.bbrc.2004.11.068. [DOI] [PubMed] [Google Scholar]
- 57.Spranger J, Winterpacht A, Zabel B. The type II collagenopathies: A spectrum of chondrodysplasias. European Journal of Pediatrics. 1994;153(2):56–65. doi: 10.1007/BF01959208. [DOI] [PubMed] [Google Scholar]
- 58.Neumann L, Kunze J, Uhl M, Stöver B, Zabel B, Spranger J, Neumann L. Survival to adulthood and dominant inheritance of platyspondylic skeletal dysplasia, Torrance-Luton type. Pediatric Radiology. 2003;33(11):786–790. doi: 10.1007/s00247-003-1055-x. [DOI] [PubMed] [Google Scholar]
- 59.Nishimura G, Nakashima E, Mabuchi a, Shimamoto K, Shimamoto T, Shimao Y, Nagai T, Yamaguchi T, Kosaki R, Ohashi H, Makita Y, Ikegawa S. Identification of COL2A1 mutations in platyspondylic skeletal dysplasia, Torrance type. Journal of medical genetics. 2004;41(1):75–79. doi: 10.1136/jmg.2003.013722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zankl A, Neumann L, Ignatius J, Nikkels P, Schrander-Stumpel C, Mortier G, Omran H, Wright M, Hilbert K, Bonafé L, Spranger J, Zabel B, Superti-Furga A. Dominant negative mutations in the C-propeptide of COL2A1 cause platyspondylic lethal skeletal dysplasia, torrance type, and define a novel subfamily within the type 2 collagenopathies. American Journal of Medical Genetics. 2005;133 A(1):61–67. doi: 10.1002/ajmg.a.30531. [DOI] [PubMed] [Google Scholar]
- 61.Achond rogenesis. 2018 URL https://ghr.nlm.nih.gov/condition/achondrogenesis#statistics.
- 62.Platyspondylic lethal skeletal dysplasia, Torrance type. 2018 URL https://ghr.nlm.nih.gov/condition/platyspondylic-lethal-skeletal-dysplasia-torrance-type#statistics.
- 63.Eyre DR, Upton MP, Shapiro FD, Wilkinson RH, Vawter GF. Nonexpression of cartilage type II collagen in a case of Langer-Saldino achondrogenesis. Am J Hum Genet. 1986;39(1):52–67. [PMC free article] [PubMed] [Google Scholar]
- 64.Chan D, Cole WG, Chow CW, Mundlos S, Bateman JF. A COL2A1 mutation in achondrogenesis type II results in the replacement of type II collagen by type I and III collagens in cartilage. 1995 doi: 10.1074/jbc.270.4.1747. [DOI] [PubMed] [Google Scholar]
- 65.Mundlos S, Chan D, McGill J, Bateman JF. An α1(II) Gly913 to Cys substitution prevents the matrix incorporation of type II collagen which is replaced with type I and III collagens in cartilage from a patient with hypochondrogenesis. American Journal of Medical Genetics. 1996;63(1):129–136. doi: 10.1002/(SICI)1096-8628(19960503)63:1<129::AID-AJMG23>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 66.Mundlos S, Olsen BR. Heritable diseases of the skeleton. Part II: Molecular insights into skeletal development-matrix components and their homeostasis. The FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 1997;11(4):227–233. [PubMed] [Google Scholar]
- 67.Bonafe L, Cormier-Daire V, Hall C, Lachman R, Mortier G, Mundlos S, Nishimura G, Sangiorgi L, Savarirayan R, Sillence D, Spranger J, Superti-Furga A, Warman M, Unger S. Nosology and classification of genetic skeletal disorders: 2015 revision. American Journal of Medical Genetics, Part A. 2015;167A:2869–2892. doi: 10.1002/ajmg.a.37365. [DOI] [PubMed] [Google Scholar]
- 68.Barat-Houari M, Sarrabay G, Gatinois V, Fabre A, Dumont B, Genevieve D, Touitou I. Mutation Update for COL2A1 Gene Variants Associated with Type II Collagenopathies. Human Mutation. 2016;37(1):7–15. doi: 10.1002/humu.22915. [DOI] [PubMed] [Google Scholar]
- 69.Snead MP, Yates JRW. Clinical and molecular genetics of Stickler syndrome. J Med Genet. 1999;36(1):353–359. doi: 10.1136/JMG.36.5.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Warman ML, Abbott M, Apte SS, Hefferon T, McIntosh I, Cohn DH, Hecht JT, Olsen BR, Francomano CA. A type × collagen mutation causes schmid metaphyseal chondrodysplasia. Nature Genetics. 1993;5(1):79–82. doi: 10.1038/ng0993-79. [DOI] [PubMed] [Google Scholar]
- 71.Alves C, Sobral MM, Ney-Oliveira F. Metaphyseal condrodysplasia, Schmid-type, a differential diagnosis with rickets. Journal of pediatric endocrinology & metabolism : JPEM. 2010;23(4):331–2. doi: 10.1080/10643389.2012.728825. [DOI] [PubMed] [Google Scholar]
- 72.Gleghorn L, Ramesar R, Beighton P, Wallis G. A Mutation in the Variable Repeat Region of the Aggrecan Gene (AGC1) Causes a Form of Spondyloepiphyseal Dysplasia Associated with Severe, Premature Osteoarthritis. The American Journal of Human Genetics. 2005;77(3):484–490. doi: 10.1086/444401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Anderson IJ, Tsipouras P, Scher C, Ramesar RS, Martell RW, Beighton P. Spondyloepiphyseal dysplasia, mild autosomal dominant type is not due to primary defects of type II collagen. American Journal of Medical Genetics. 1990;37(2):272–276. doi: 10.1002/ajmg.1320370223. [DOI] [PubMed] [Google Scholar]
- 74.Stattin EL, Wiklund F, Lindblom K, Önnerfjord P, Jonsson BA, Tegner Y, Sasaki T, Struglics A, Lohmander S, Dahl N, Heinegård D, Aspberg A. A Missense Mutation in the Aggrecan C-type Lectin Domain Disrupts Extracellular Matrix Interactions and Causes Dominant Familial Osteochondritis Dissecans. American Journal of Human Genetics. 2010;86(2):126–137. doi: 10.1016/j.ajhg.2009.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Stattin EL, Tegner Y, Domellöf M, Dahl N. Familial osteochondritis dissecans associated with early osteoarthritis and disproportionate short stature. Osteoarthritis and Cartilage. 2008;16(8):890–896. doi: 10.1016/j.joca.2007.11.009. [DOI] [PubMed] [Google Scholar]
- 76.Gibson BG, Briggs MD. The aggrecanopathies; An evolving phenotypic spectrum of human genetic skeletal diseases. Orphanet Journal of Rare Diseases. 11(1) doi: 10.1186/s13023-016-0459-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Boot-Handford RP, Briggs MD. The unfolded protein response and its relevance to connective tissue diseases. Cell and Tissue Research. 2010;339(1):197–211. doi: 10.1007/s00441-009-0877-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hughes A, Oxford AE, Tawara K, Jorcyk CL, Oxford JT. Endoplasmic reticulum stress and unfolded protein response in cartilage pathophysiology; contributing factors to apoptosis and osteoarthritis. International Journal of Molecular Sciences. 18(3) doi: 10.3390/ijms18030665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.König Ueber freie Körper in den Gelenken. Deutsche Zeitschrift für Chirurgie. 1888;27(1–2):90–109. doi: 10.1007/BF02792135. [DOI] [Google Scholar]
- 80.König F, Brand RA. The classic: On loose bodies in the joint. Clinical Orthopaedics and Related Research. 2013;471(4):1107–1115. doi: 10.1007/s11999-013-2824-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Edmonds EW, Shea KG. Osteochondritis dissecans: Editorial comment. Clinical Orthopaedics and Related Research. 2013;471(4):1105–1106. doi: 10.1007/s11999-013-2837-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Schenck RC, Goodnight JM. Osteochondritis dissecans. The Journal of bone and joint surgery. American volume. 1996;78(3):439–56. [PubMed] [Google Scholar]
- 83.Uppstrom TJ, Gausden EB, Green DW. Classification and assessment of juvenile osteochondritis dissecans knee lesions. Current opinion in pediatrics. 2016;28(1):60–7. doi: 10.1097/MOP.0000000000000308. [DOI] [PubMed] [Google Scholar]
- 84.Fairbank HAT. Osteo-chondritis dissecans. British Journal of Surgery. 1933;21(81):67–82. doi: 10.1002/bjs.1800218108. [DOI] [Google Scholar]
- 85.Carey JL, Grimm NL. Treatment algorithm for osteochondritis dissecans of the knee. Clinics in sports medicine. 2014;33(2):375–82. doi: 10.1016/j.csm.2014.01.002. [DOI] [PubMed] [Google Scholar]
- 86.Lindén B. the Incidence of Osteochondritis Dissecans in the Condyles of the Femur. Acta Orthopaedica. 1976;47(6):664–667. doi: 10.3109/17453677608988756. [DOI] [PubMed] [Google Scholar]
- 87.Kessler JI, Nikizad H, Shea KG, Jacobs JC, Bebchuk JD, Weiss JM. The demographics and epidemiology of osteochondritis dissecans of the knee in children and adolescents. American Journal of Sports Medicine. 2014;42(2):320–326. doi: 10.1177/0363546513510390. [DOI] [PubMed] [Google Scholar]
- 88.Aichroth P. Osteochondritis dissecans of the knee. A clinical survey. The Journal of bone and joint surgery. British. 1971;53(3):440–7. [PubMed] [Google Scholar]
- 89.Hefti F, Beguiristain J, Krauspe R, Möller-Madsen B, Riccio V, Tschauner C, Wetzel R, Zeller R. Osteochondritis dissecans: A multicenter study of the European pediatric orthopedic society. 1999 doi: 10.1097/01202412-199910000-00001. [DOI] [PubMed] [Google Scholar]
- 90.Grimm NL, Weiss JM, Kessler JI, Aoki SK. Osteochondritis dissecans of the knee: pathoanatomy, epidemiology, and diagnosis. Clinics in sports medicine. 2014;33(2):181–8. doi: 10.1016/j.csm.2013.11.006. [DOI] [PubMed] [Google Scholar]
- 91.Edmonds EW, Polousky J. A review of knowledge in osteochondritis dissecans: 123 years of minimal evolution from König to the ROCK study group. Clinical Orthopaedics and Related Research. 2013;471(4):1118–1126. doi: 10.1007/s11999-012-2290-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Heyworth BE, Kocher MS. Osteochondritis Dissecans of the Knee. JBJS reviews. 2015;3(7):1–12. doi: 10.2106/JBJS.RVW.N.00095. [DOI] [PubMed] [Google Scholar]
- 93.Heyworth BE, Edmonds EW, Murnaghan ML, Kocher MS. Drilling techniques for osteochondritis dissecans. Clinics in sports medicine. 2014;33(2):305–12. doi: 10.1016/j.csm.2013.11.007. [DOI] [PubMed] [Google Scholar]
- 94.Polousky JD, Albright J. Salvage techniques in osteochondritis dissecans. Clinics in sports medicine. 2014;33(2):321–33. doi: 10.1016/j.csm.2014.01.004. [DOI] [PubMed] [Google Scholar]
- 95.Bauer KL, Polousky JD. Management of Osteochondritis Dissecans Lesions of the Knee, Elbow and Ankle. Clinics in sports medicine. 2017;36(3):469–487. doi: 10.1016/j.csm.2017.02.005. [DOI] [PubMed] [Google Scholar]
- 96.Horvath A, Pall N, Molnar K, Kovats T, Surjan G, Vicsek T, Pollner P. A nationwide study of the epidemiology of relapsing polychondritis. Clinical Epidemiology Volume. 2016;8:211–230. doi: 10.2147/CLEP.S91439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kent PD, Michet CJ, Luthra HS. Relapsing polychondritis. Current opinion in rheumatology. 2004;16(1):56–61. doi: 10.1080/10643389.2012.728825. [DOI] [PubMed] [Google Scholar]
- 98.Trentham DE, Le CH. Relapsing polychondritis. Annals of internal medicine. 1998;129(2):114–22. doi: 10.1016/B978-1-4160-4048-4.50055-6. [DOI] [PubMed] [Google Scholar]
- 99.Cuestas D, Peñaranda E, Mora S, Cortes C, Galvis I, Patiño M, Velasquez O. Relapsing polychondritis, an underestimated dermatological urgency: case report and literature review. International Journal of Dermatology. 2017;56(12):1379–1386. doi: 10.1111/ijd.13755. [DOI] [PubMed] [Google Scholar]
- 100.Sharma A, Gnanapandithan K, Sharma K, Sharma S. Relapsing polychondritis: A review. Clinical Rheumatology. 2013;32(11):1575–1583. doi: 10.1007/s10067-013-2328-x. [DOI] [PubMed] [Google Scholar]
- 101.McAdam LP, O’Hanlan MA, Bluestone R, Pearson CM. Relapsing polychondritis: prospective study of 23 patients and a review of the literature. Medicine. 1976;55(3):193–215. [PubMed] [Google Scholar]
- 102.Damiani JM, Levine HL. Relapsing polychondritis–report of ten cases. The Laryngoscope. 1979;89(6 Pt 1):929–46. doi: 10.1177/0192513X12437708. [DOI] [PubMed] [Google Scholar]
- 103.Michet CJ, McKenna CH, Luthra HS, O’Fallon WM. Relapsing polychondritis. Survival and predictive role of early disease manifestations. Annals of internal medicine. 1986;104(1):74–8. doi: 10.7326/0003-4819-104-1-74. [DOI] [PubMed] [Google Scholar]
- 104.Foidart JM, Abe S, Martin GR, Zizic TM, Barnett EV, Lawley TJ, Katz SI. Antibodies to type II collagen in relapsing polychondritis. The New England journal of medicine. 1978;299(22):1203–7. doi: 10.1056/NEJM197811302992202. [DOI] [PubMed] [Google Scholar]
- 105.Lang B, Rothenfusser A, Lanchbury JS, Rauh G, Breedveld FC, Urlacher A, Albert ED, Peter H, Melchers I. Susceptibility to relapsing polychondritis is associated with HLA–DR4. Arthritis & Rheumatism. 1993;36(5):660–664. doi: 10.1002/art.1780360513. [DOI] [PubMed] [Google Scholar]
- 106.Arnaud L, Devilliers H, Peng SL, Mathian A, Costedoat-Chalumeau N, Buckner J, Dagna L, Michet C, Sharma A, Cervera R, Haroche J, Papo T, D’Cruz D, Arlet P, Zwerina J, Belot A, Suzuki N, Harle J-R, Moots R, Jayne D, Hachulla E, Marie I, Tanaka T, Lebovics R, Scott D, Kucharz EJ, Birchall M, Kong KO, Gorochov G, Amoura Z R. study group. The Relapsing Polychondritis Disease Activity Index: development of a disease activity score for relapsing polychondritis. Autoimmunity reviews. 2012;12(2):204–9. doi: 10.1016/j.autrev.2012.06.005. [DOI] [PubMed] [Google Scholar]
- 107.McCarty DJ, Kohn NN, Faires JS. The Significance of Calcium Phosphate Crystals in the Synovial Fluid of Arthritic Patients: The "Pseudogout Syndrome": I. Clinical Aspects. Annals of Internal Medicine. 1962;56(5_Part_1):711–737. doi: 10.7326/0003-4819-56-5-711. [DOI] [PubMed] [Google Scholar]
- 108.Kohn NN, Hughes RE, McCarty DJ, Faires JS. The significance of calcium phosphate crystals in the synovial fluid of arthritic patients: the "pseudogout syndrome". II. Identification of crystals. Annals of internal medicine. 1962;56:738–45. doi: 10.7326/0003-4819-56-5-738. [DOI] [PubMed] [Google Scholar]
- 109.Rosenthal AK, Ryan LM. Calcium Pyrophosphate Deposition Disease. New England Journal of Medicine. 2016;374(26):2575–2584. doi: 10.1056/NEJMra1511117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Felson DT, Anderson JJ, Naimark A, Kannel W, Meenan RF. The prevalence of chondrocalcinosis in the elderly and its association with knee osteoarthritis: the Framingham Study. The Journal of rheumatology. 1989;16(9):1241–5. [Google Scholar]
- 111.Sanmarti R, Panella D, Brancos MA, Canela J, Collado A, Brugues J. Prevalence of articular chondrocalcinosis in elderly subjects in a rural area of Catalonia. Annals of the Rheumatic Diseases. 1993;52(6):418–422. doi: 10.1136/ard.52.6.418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Neame RL, Carr AJ, Muir K, Doherty M. UK community prevalence of knee chondrocalcinosis: Evidence that correlation with osteoarthritis is through a shared association with osteophyte. Annals of the Rheumatic Diseases. 2003;62(6):513–518. doi: 10.1136/ard.62.6.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Derfus BA, Kurian JB, Butler JJ, Daft LJ, Carrera GF, Ryan LM, Rosenthal AK. The high prevalence of pathologic calcium crystals in pre-operative knees. The Journal of rheumatology. 2002;29(3):570–4. [PubMed] [Google Scholar]
- 114.Nalbant S, Martinez JA, Kitumnuaypong T, Clayburne G, Sieck M, Schumacher HR. Synovial fluid features and their relations to osteoarthritis severity: New findings from sequential studies. Osteoarthritis and Cartilage. 2003;11(1):50–54. doi: 10.1053/joca.2002.0861. [DOI] [PubMed] [Google Scholar]
- 115.Viriyavejkul P, Wilairatana V, Tanavalee A, Jaovisidha K. Comparison of characteristics of patients with and without calcium pyrophosphate dihydrate crystal deposition disease who underwent total knee replacement surgery for osteoarthritis. Osteoarthritis and Cartilage. 2007;15(2):232–235. doi: 10.1016/j.joca.2006.08.012. [DOI] [PubMed] [Google Scholar]
- 116.Derfus BA, Rachow JW, Mandel NS, Boskey AL, Buday M, Kushnaryov VM, Ryan LM. Articular Cartilage Vesicles Generate Calcium Pyrophosphate Dihydrate― Like Crystals in Vitro. Arthritis & Rheumatism. 1992;35(2):231–240. doi: 10.1002/art.1780350218. [DOI] [PubMed] [Google Scholar]
- 117.Caswell A, Guilland-Cumming DF, Hearn PR, McGuire MK, Russell RG. Pathogenesis of chondrocalcinosis and pseudogout. Metabolism of inorganic pyrophosphate and production of calcium pyrophosphate dihydrate crystals. Annals of the rheumatic diseases. 1983;42(Suppl 1):27–37. doi: 10.1136/ard.42.suppl_1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Ryan LM, Rosenthal AK. Metabolism of extracellular pyrophosphate. Current Opinion in Rheumatology. 2003;15(3):311–314. doi: 10.1097/00002281-200305000-00020. [DOI] [PubMed] [Google Scholar]
- 119.Costello JC, Rosenthal AK, Kurup IV, Masuda I, Medhora M, Ryan LM. Parallel regulation of extracellular ATP and inorganic pyrophosphate: Roles of growth factors, transduction modulators, and ANK. Connective Tissue Research. 2011;52(2):139–146. doi: 10.3109/03008207.2010.491928. [DOI] [PubMed] [Google Scholar]
- 120.Rosenthal AK, Gohr CM, Mitton-Fitzgerald E, Lutz MK, Dubyak GR, Ryan LM. The progressive ankylosis gene product ANK regulates extracellular ATP levels in primary articular chondrocytes. Arthritis Research and Therapy. 15(5) doi: 10.1186/ar4337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Reuben PM, Wenger L, Cruz M, Cheung HS. Induction of matrix metalloproteinase-8 in human fibroblasts by basic calcium phosphate and calcium pyrophosphate dihydrate crystals: effect of phosphocitrate. Connective tissue research. 2001;42(1):1–12. doi: 10.3109/03008200109014244. [DOI] [PubMed] [Google Scholar]
- 122.Liu-Bryan R, Pritzker K, Firestein GS, Terkeltaub R. TLR2 Signaling in Chondrocytes Drives Calcium Pyrophosphate Dihydrate and Monosodium Urate Crystal-Induced Nitric Oxide Generation. The Journal of Immunology. 2005;174(8):5016–5023. doi: 10.4049/jimmunol.174.8.5016. [DOI] [PubMed] [Google Scholar]
- 123.Macmullan P, Mccarthy G. Treatment and management of pseudogout: Insights for the clinician. Therapeutic Advances in Musculoskeletal Disease. 2012;4(2):121–131. doi: 10.1177/1759720X11432559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Pang L, Hayes CP, Buac K, Yoo D-g, Rada B. Pseudogout-Associated Inflammatory Calcium Pyrophosphate Dihydrate Microcrystals Induce Formation of Neutrophil Extracellular Traps. The Journal of Immunology. 2013;190(12):6488–6500. doi: 10.4049/jimmunol.1203215. [DOI] [PubMed] [Google Scholar]
- 125.McCarty D. Diagnostic mimicry in arthritis: patterns of joint involvement associated with calcium pyrophosphate dihydrate crystal deposits. Bulletin on the Rheumatic Diseases. 1974;25(5):804–809. [Google Scholar]
- 126.Choy G. An update on the treatment options for gout and calcium pyrophosphate deposition. Expert opinion on pharmacotherapy. 2005;6(14):2443–53. doi: 10.1517/14656566.6.14.2443. [DOI] [PubMed] [Google Scholar]
- 127.Rothschild B, Yakubov LE. Prospective 6-month, double-blind trial of hydroxychloroquine treatment of CPDD. Comprehensive therapy. 1997;23(5):327–31. [PubMed] [Google Scholar]
- 128.Chollet-Janin A, Finckh A, Dudler J, Guerne P-A. Methotrexate as an alternative therapy for chronic calcium pyrophosphate deposition disease: an exploratory analysis. Arthritis and rheumatism. 2007;56(2):688–92. doi: 10.1002/art.22389. [DOI] [PubMed] [Google Scholar]
- 129.Finckh A, Mc Carthy GM, Madigan A, Van Linthoudt D, Weber M, Neto D, Rappoport G, Blumhardt S, Kyburz D, Guerne PA. Methotrexate in chronic-recurrent calcium pyrophosphate deposition disease: no significant effect in a randomized crossover trial. Arthritis research & therapy. 2014;16(5):458. doi: 10.1186/s13075-014-0458-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Announ N, Guerne PA. Treating difficult crystal pyrophosphate dihydrate deposition disease. Current Rheumatology Reports. 2008;10(3):228–234. doi: 10.1007/s11926-008-0037-2. [DOI] [PubMed] [Google Scholar]
- 131.Franchi A. Epidemiology and classification of bone tumors. Clinical Cases in Mineral and Bone Metabolism. 2012;9(2):92–95. [PMC free article] [PubMed] [Google Scholar]
- 132.Bovée JVMG, Hogendoorn PCW, Wunder JS, Alman BA. Cartilage tumours and bone development: molecular pathology and possible therapeutic targets. Nature Reviews Cancer. 2010;10(7):481–488. doi: 10.1038/nrc2869. [DOI] [PubMed] [Google Scholar]
- 133.Fletcher CDM, Unni KK, Mertens F. World Health Organization Classification of Tumours. Pathology and Genetics of Tumours of Soft Tissue and Bone. 2002 [Google Scholar]
- 134.Marco RA, Gitelis S, Brebach GT, Healey JH. Cartilage tumors: evaluation and treatment. The Journal of the American Academy of Orthopaedic Surgeons. 1991;8(5):292–304. doi: 10.1080/10643389.2012.728825. [DOI] [PubMed] [Google Scholar]
- 135.Ferrer-Santacreu EM, Ortiz-Cruz EJ, Díaz-Almirón M, Pozo Kreilinger JJ. Enchondroma versus Chondrosarcoma in Long Bones of Appendicular Skeleton: Clinical and Radiological Criteria - A Follow-Up. Journal of Oncology. 2016 doi: 10.1155/2016/8262079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Riedel RF, Larrier N, Dodd L, Kirsch D, Martinez S, Brigman BE. The clinical management of chondrosarcoma. Current Treatment Options in Oncology. 2009;10(1–2):94–106. doi: 10.1007/s11864-009-0088-2. [DOI] [PubMed] [Google Scholar]
- 137.Bovée JVMG, Cleton-Jansen AM, Taminiau AHM, Hogendoorn PCW. Emerging pathways in the development of chondrosarcoma of bone and implications for targeted treatment. Lancet Oncology. 2005;6(8):599–607. doi: 10.1016/S1470-2045(05)70282-5. [DOI] [PubMed] [Google Scholar]
- 138.Gelderblom H, Hogendoorn PC, Dijkstra SD, van Rijswijk CS, Krol AD, Taminiau AH, Bovee JV. The Clinical Approach Towards Chondrosarcoma. The Oncologist. 2008;13(3):320–329. doi: 10.1634/theoncologist.2007-0237. [DOI] [PubMed] [Google Scholar]
- 139.Brown HK, Schiavone K, Gouin F, Heymann MF, Heymann D. Biology of Bone Sarcomas and New Therapeutic Developments. Calcified Tissue International. 2017;102(2):1–22. doi: 10.1007/s00223-017-0372-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Kahlenberg JM, Fox DA. Advances in the Medical Treatment of Rheumatoid Arthritis. Hand Clinics. 2011;27(1):11–20. doi: 10.1016/j.hcl.2010.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Gibofsky A. Overview of epidemiology, pathophysiology, and diagnosis of rheumatoid arthritis. The American journal of managed care. 2012;18(13 Suppl):S295–302. [PubMed] [Google Scholar]
- 142.Upchurch KS, Kay J. Evolution of treatment for rheumatoid arthritis. Rheumatology. 2012;51(suppl 6):vi28–vi36. doi: 10.1093/rheumatology/kes278. [DOI] [PubMed] [Google Scholar]
- 143.Roddy E, Doherty M. Epidemiology of gout. Arthritis research & therapy. 2010;12(6):223. doi: 10.1186/ar3199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Zhu Y, Pandya BJ, Choi HK. Prevalence of gout and hyperuricemia in the US general population: The National Health and Nutrition Examination Survey 2007–2008. Arthritis and Rheumatism. 2011;63(10):3136–3141. doi: 10.1002/art.30520. [DOI] [PubMed] [Google Scholar]
- 145.El-Zawawy H, Mandell BF. Update on Crystal-Induced Arthritides. Clinics in geriatric medicine. 2017;33(1):135–144. doi: 10.1016/j.cger.2016.08.010. [DOI] [PubMed] [Google Scholar]
- 146.Ravelli A, Martini A. Juvenile idiopathic arthritis. The Lancet. 2007;369(9563):767–778. doi: 10.1016/S0140-6736(07)60363-8. arXiv:NIHMS150003. [DOI] [PubMed] [Google Scholar]
- 147.Sacks JJ, Helmick CG, Luo YH, Ilowite NT, Bowyer S. Prevalence of and annual ambulatory health care visits for pediatric arthritis and other rheumatologic conditions in the United States in 2001–2004. Arthritis Care and Research. 2007;57(8):1439–1445. doi: 10.1002/art.23087. [DOI] [PubMed] [Google Scholar]
- 148.Moorthy LN, Peterson MG, Hassett AL, Lehman TJ. Burden of childhood-onset arthritis. Pediatric rheumatology online journal. 2010;8:20. doi: 10.1186/1546-0096-8-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Buyon JP. Primer on the Rheumatic Diseases. Springer New York; New York, NY: 2008. Systemic Lupus Erythematosus; pp. 303–338. [DOI] [Google Scholar]
- 150.Thakral A, Klein-Gitelman MS. An Update on Treatment and Management of Pediatric Systemic Lupus Erythematosus. Rheumatology and Therapy. 2016;3(2):209–219. doi: 10.1007/s40744-016-0044-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Tan EM, Cohen AS, Fries JF, Masi AT, Mcshane DJ, Rothfield NF, Schaller JG, Talal N, Winchester RJ. The 1982 revised criteria for the classification of systemic lupus erythematosus. Arthritis & Rheumatism. 1982;25(11):1271–1277. doi: 10.1002/art.1780251101. [DOI] [PubMed] [Google Scholar]
- 152.Hochberg MC. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis and rheumatism. 1997;40(9):1725. doi: 10.1002/1529-0131(199709)40:9. lt;1725::AID-ART29 gt;3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 153.Ostendorf B, Scherer A, Specker C, Mödder U, Schneider M. Jaccoud’s arthropathy in systemic lupus erythematosus: Differentiation of deforming and erosive patterns by magnetic resonance imaging. Arthritis and Rheumatism. 2003;48(1):157–165. doi: 10.1002/art.10753. [DOI] [PubMed] [Google Scholar]
- 154.Santiago MB, Galvão V. Jaccoud arthropathy in systemic lupus erythematosus: analysis of clinical characteristics and review of the literature. Medicine. 2008;87(1):37–44. doi: 10.1097/MD.0b013e3181632d18. [DOI] [PubMed] [Google Scholar]
- 155.Skare TL, Godoi Ade L, Ferreira VO. Jaccoud arthropathy in systemic lupus erythematosus: clinical and serological findings. Revista Da Associacao Medica Brasileira. 2012;58:489–492. doi: 10.1590/S0104-42302012000400022. [DOI] [PubMed] [Google Scholar]
- 156.Khan MA. Update on spondyloarthropathies. Annals of internal medicine. 2002;136(12):896–907. doi: 10.7326/0003-4819-136-12-200206180-00011. [DOI] [PubMed] [Google Scholar]
- 157.Dougados M, Linden SVD, Juhlin R, Huitfeldt B, Amor B, Calin A, Cats A, Dijkmans B, Olivieri I, Pasero G, Veys E, Zeidler H. The European Spondylarthropathy Study Group Preliminary Criteria for the Classification of Spondylarthropathy. Arthritis & Rheumatism. 1991;34(10):1218–1227. doi: 10.1002/art.1780341003. [DOI] [PubMed] [Google Scholar]
- 158.Reveille JD. Epidemiology of spondyloarthritis in North America. American Journal of the Medical Sciences. 2011;341(4):284–286. doi: 10.1097/MAJ.0b013e31820f8c99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Akgul O, Ozgocmen S. Classification criteria for spondyloarthropathies. World Journal of Orthopedics. 2011;2(12):107. doi: 10.5312/wjo.v2.i12.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Veale DJ. Psoriatic arthritis: Recent progress in pathophysiology and drug development. Arthritis Research and Therapy. 15(6) doi: 10.1186/ar4414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Gladman DD, Antoni C, Mease P, Clegg DO, Nash O. Psoriatic arthritis: Epidemiology, clinical features, course, and outcome. Annals of the Rheumatic Diseases. 2005;64(SUPPL. 2):14–18. doi: 10.1136/ard.2004.032482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Raychaudhuri SP, Wilken R, Sukhov AC, Raychaudhuri SK, Maverakis E. Management of psoriatic arthritis: Early diagnosis, monitoring of disease severity and cutting edge therapies. Journal of autoimmunity. 2017;76:21–37. doi: 10.1016/j.jaut.2016.10.009. [DOI] [PubMed] [Google Scholar]
- 163.Junker S, Frommer KW, Krumbholz G, Tsiklauri L, Gerstberger R, Rehart S, Steinmeyer J, Rickert M, Wenisch S, Schett G, Müller-Ladner U, Neumann E. Expression of adipokines in osteoarthritis osteophytes and their effect on osteoblasts. Matrix Biology. 2017;62(2017):75–91. doi: 10.1016/j.matbio.2016.11.005. [DOI] [PubMed] [Google Scholar]
- 164.Wieland HA, Michaelis M, Kirschbaum BJ, Rudolphi KA. OSTEOARTHRITIS — AN UNTREATABLE DISEASE ? Nature Reviews Drug Discovery. 2005;4:331–344. doi: 10.1038/nrd1693. [DOI] [PubMed] [Google Scholar]
- 165.Menon J, Mishra P. Health care resource use, health care expenditures and absenteeism costs associated with osteoarthritis in US healthcare system. Osteoarthritis and cartilage. doi: 10.1016/j.joca.2017.12.007. [DOI] [PubMed] [Google Scholar]
- 166.Karsdal MA, Michaelis M, Ladel C, Siebuhr AS, Bihlet AR, Andersen JR, Guehring H, Christiansen C, Bay-Jensen AC, Kraus VB. Disease-modifying treatments for osteoarthritis (DMOADs) of the knee and hip: lessons learned from failures and opportunities for the future. Osteoarthritis and Cartilage. 2016;24(12):2013–2021. doi: 10.1016/j.joca.2016.07.017. [DOI] [PubMed] [Google Scholar]
- 167.Bijlsma JW, Berenbaum F, Lafeber FP. Osteoarthritis: an update with relevance for clinical practice. The Lancet. 2011;377(9783):2115–2126. doi: 10.1016/S0140-6736(11)60243-2. [DOI] [PubMed] [Google Scholar]
- 168.Roemer FW, Crema MD, Trattnig S, Guermazi A. Advances in Imaging of Osteoarthritis and Cartilage. Radiology. 2011;260(2):332–354. doi: 10.1148/radiol.11101359. [DOI] [PubMed] [Google Scholar]
- 169.Braun HJ, Gold GE. Diagnosis of osteoarthritis: Imaging. Bone. 2012;51(2):278–288. doi: 10.1016/j.bone.2011.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Hunter DJ, Nevitt M, Losina E, Kraus V. Biomarkers for osteoarthritis: Current position and steps towards further validation. Best Practice & Research Clinical Rheumatology. 2014;28(1):61–71. doi: 10.1016/j.berh.2014.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Anderson DD, Chubinskaya S, Guilak F, Martin JA, Oegema TR, Olson SA, Buckwalter JA. Post-traumatic osteoarthritis: Improved understanding and opportunities for early intervention. Journal of Orthopaedic Research. 2011;29(June):802–809. doi: 10.1002/jor.21359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Brown T, Johnston J, Saltzman C, Marsh J, Buckwalter J. Post-traumatic osteoarthritis: a first estimate of incidence, prevalence, and burden of disease. Journal of Orthopaedic Trauma. 2006;20(10):739–744. doi: 10.1097/01.bot.0000246468.80635.ef. [DOI] [PubMed] [Google Scholar]
- 173.Bajpayee AG, Grodzinsky AJ. Cartilage-targeting drug delivery: can electrostatic interactions help? Nature reviews. Rheumatology. 2017;13:183–193. doi: 10.1038/nrrheum.2016.210. [DOI] [PubMed] [Google Scholar]
- 174.Poole RA. Osteoarthritis as a Whole Joint Disease. HSS Journal. 2012;8(1):4–6. doi: 10.1007/s11420-011-9248-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Poulet B, Beier F. Targeting oxidative stress to reduce osteoarthritis. Arthritis Research and Therapy. 2016;18(1):15–16. doi: 10.1186/s13075-015-0908-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Stiebel M, Miller L, Block J. Post-traumatic knee osteoarthritis in the young patient: therapeutic dilemmas and emerging technologies. Open Access Journal of Sports Medicine. 2014;5:73–79. doi: 10.2147/OAJSM.S61865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Johnson DL, Urban WP, Caborn DN, Vanarthos WJ, Carlson CS. Articular cartilage changes seen with magnetic resonance imaging-detected bone bruises associated with acute anterior cruciate ligament rupture. The American journal of sports medicine. 1991;26(3):409–14. doi: 10.1177/03635465980260031101. [DOI] [PubMed] [Google Scholar]
- 178.Lotz MK, Kraus VB. New developments in osteoarthritis. Posttraumatic osteoarthritis: pathogenesis and pharmacological treatment options. Arthritis research & therapy. 2010;12:211. doi: 10.1186/ar3046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Swärd P, Frobell R, Englund M, Roos H, Struglics A. Cartilage and bone markers and inflammatory cytokines are increased in synovial fluid in the acute phase of knee injury (hemarthrosis) - a cross-sectional analysis. Osteoarthritis and Cartilage. 2012;20(11):1302–1308. doi: 10.1016/j.joca.2012.07.021. [DOI] [PubMed] [Google Scholar]
- 180.Yamamoto K, Okano H, Miyagawa W, Visse R, Shitomi Y, Santamaria S, Dudhia J, Troeberg L, Strickland DK, Hirohata S, Nagase H. MMP-13 is constitutively produced in human chondrocytes and co-endocytosed with ADAMTS-5 and TIMP-3 by the endocytic receptor LRP1. Matrix Biology. 2016;56:57–73. doi: 10.1016/j.matbio.2016.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Sokolove J, Lepus CM. Role of inflammation in the pathogenesis of osteoarthritis: latest findings and interpretations. Therapeutic advances in musculoskeletal disease. 2013;5(2):77–94. doi: 10.1177/1759720X12467868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Orlowsky EW, Kraus VB. The role of innate immunity in osteoarthritis: When our first line of defense goes on the offensive. Journal of Rheumatology. 2015;42(3):363–371. doi: 10.3899/jrheum.140382. arXiv:15334406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Silawal S, Triebel J, Bertsch T, Schulze-Tanzil G. Osteoarthritis and the Complement Cascade. Clinical Medicine Insights: Arthritis and Musculoskeletal Disorders. 11 doi: 10.1177/1179544117751430. doi: 10.1177/1179544117751430. 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Bondeson J, Blom AB, Wainwright S, Hughes C, Caterson B, Van Den Berg WB. The role of synovial macrophages and macrophage-produced mediators in driving inflammatory and destructive responses in osteoarthritis. Arthritis and Rheumatism. 2010;62(3):647–657. doi: 10.1002/art.27290. [DOI] [PubMed] [Google Scholar]
- 185.Gómez R, Villalvilla A, Largo R, Gualillo O, Herrero-Beaumont G. TLR4 signalling in osteoarthritis-finding targets for candidate DMOADs. Nature reviews. Rheumatology. 2014;11(3):1–12. doi: 10.1038/nrrheum.2014.209. [DOI] [PubMed] [Google Scholar]
- 186.Liu-Bryan R, Terkeltaub R. Emerging regulators of the inflammatory process in osteoarthritis. Nature Reviews Rheumatology. 2015;11(1):35–44. doi: 10.1038/nrrheum.2014.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Philp AM, Davis ET, Jones SW. Developing anti-inflammatory therapeutics for patients with osteoarthritis. Rheumatology (Oxford, England) 2017;56(6):869–881. doi: 10.1093/rheumatology/kew278. [DOI] [PubMed] [Google Scholar]
- 188.Shiokawa S, Matsumoto N, Nishimura J. Clonal analysis of B cells in the osteoarthritis synovium. Ann Rheum Dis. 2001;60:802–805. doi: 10.1136/ard.60.8.802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Sakkas LI, Platsoucas CD. The role of T cells in the pathogenesis of osteoarthritis. Arthritis and Rheumatism. 2007;56(2):409–424. doi: 10.1002/art.22369. [DOI] [PubMed] [Google Scholar]
- 190.Lepetsos P, Papavassiliou AG. ROS/oxidative stress signaling in osteoarthritis. Biochimica et Biophysica Acta - Molecular Basis of Disease. 2016;1862(4):576–591. doi: 10.1016/j.bbadis.2016.01.003. [DOI] [PubMed] [Google Scholar]
- 191.Wang Y, Li Y, Khabut A, Chubinskaya S, Grodzinsky AJ, Önnerfjord P. Quantitative proteomics analysis of cartilage response to mechanical injury and cytokine treatment. Matrix Biology. 2017;63:11–22. doi: 10.1016/j.matbio.2016.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Li G, Yin J, Gao J, Cheng TS, Pavlos NJ, Zhang C, Zheng MH. Subchondral bone in osteoarthritis: Insight into risk factors and microstructural changes. Arthritis Research and Therapy. 15(6) doi: 10.1186/ar4405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Mahjoub M, Berenbaum F, Houard X. Why subchondral bone in osteoarthritis? the importance of the cartilage bone interface in osteoarthritis. Osteoporosis International. 23(8 SUPPL) doi: 10.1007/s00198-012-2161-0. [DOI] [PubMed] [Google Scholar]
- 194.Han W, Fan S, Bai X, Ding C. Strontium ranelate, a promising disease modifying osteoarthritis drug. Expert Opinion on Investigational Drugs. 2017;26(3):375–380. doi: 10.1080/13543784.2017.1283403. [DOI] [PubMed] [Google Scholar]
- 195.Haj-Mirzaian A, Guermazi A, Roemer FW, Bowes MA, Conaghan PG, Demehri S. Bisphosphonates intake and its association with changes of periarticular bone area and three-dimensional shape: data from the Osteoarthritis Initiative (OAI) Osteoarthritis and cartilage. 2018;44(6):638–704. doi: 10.1016/j.joca.2018.01.005. [DOI] [PubMed] [Google Scholar]
- 196.Deshmukh V, Hu H, Barroga C, Bossard C, KC S, Dellamary L, Stewart J, Chiu K, Ibanez M, Pedraza M, Seo T, Do L, Cho S, Cahiwat J, Tam B, Tambiah JR, Hood J, Lane NE, Yazici Y. A small-molecule inhibitor of the Wnt pathway (SM04690) as a potential disease modifying agent for the treatment of osteoarthritis of the knee. Osteoarthritis and Cartilage. 2018;26(1):18–27. doi: 10.1016/j.joca.2017.08.015. [DOI] [PubMed] [Google Scholar]
- 197.Yazici Y, McAlindon TE, Fleischmann R, Gibofsky A, Lane NE, Kivitz AJ, Skrepnik N, Armas E, Swearingen CJ, DiFrancesco A, Tambiah JRS, Hood J, Hochberg MC. A novel Wnt pathway inhibitor, SM04690, for the treatment of moderate to severe osteoarthritis of the knee: results of a 24-week, randomized, controlled, phase 1 study. Osteoarthritis and cartilage. 2017;25(10):1598–1606. doi: 10.1016/j.joca.2017.07.006. [DOI] [PubMed] [Google Scholar]
- 198.Johnson K, Zhu S, Tremblay MS, Payette JN, Wang J, Bouchez LC, Meeusen S, Althage A, Cho CY, Wu X, Schultz PG. A Stem Cell-Based Approach to Cartilage Repair. Science. 2012;336(6082):717–721. doi: 10.1126/science.1215157. [DOI] [PubMed] [Google Scholar]
- 199.Mohan G, Magnitsky S, Melkus G, Subburaj K, Kazakia G, Burghardt AJ, Dang A, Lane NE, Majumdar S. Kartogenin treatment prevented joint degeneration in a rodent model of osteoarthritis: A pilot study. Journal of Orthopaedic Research. 2016;34(10):1780–1789. doi: 10.1002/jor.23197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Li Y, Wang Y, Chubinskaya S, Schoeberl B, Florine E, Kopesky P, Grodzinsky AJ. Effects of insulin-like growth factor-1 and dexamethasone on cytokine-challenged cartilage: Relevance to post-traumatic osteoarthritis. Osteoarthritis and Cartilage. 2015;23(2):266–274. doi: 10.1016/j.joca.2014.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Reker D, Kjelgaard-Petersen CF, Siebuhr AS, Michaelis M, Gigout A, Karsdal MA, Ladel C, Bay-Jensen AC. Sprifermin (rhFGF18) modulates extracellular matrix turnover in cartilage explants ex vivo. Journal of Translational Medicine. 2017;15(1):1–12. doi: 10.1186/s12967-017-1356-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Dahlberg LE, Aydemir A, Muurahainen N, Gühring H, Fredberg Edebo H, Krarup-Jensen N, Ladel CH, Jurvelin JS. A first-in-human, double-blind, randomised, placebo-controlled, dose ascending study of intra-articular rhFGF18 (sprifermin) in patients with advanced knee osteoarthritis. Clinical and experimental rheumatology. 2016;34(3):445–50. [PubMed] [Google Scholar]
- 203.Lohmander LS, Hellot S, Dreher D, Krantz EFW, Kruger DS, Guermazi A, Eckstein F. Intraarticular Sprifermin (Recombinant Human Fibroblast Growth Factor 18) in Knee Osteoarthritis: A Randomized, Double-Blind, Placebo-Controlled Trial. Arthritis & Rheumatology. 2014;66(7):1820–1831. doi: 10.1002/art.38614. [DOI] [PubMed] [Google Scholar]
- 204.Eckstein F, Wirth W, Guermazi A, Maschek S, Aydemir A. Brief report: Intraarticular sprifermin not only increases cartilage thickness, but also reduces cartilage loss: Location-independent post hoc analysis using magnetic resonance imaging. Arthritis and Rheumatology. 2015;67(11):2916–2922. doi: 10.1002/art.39265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Moore N, Salvo F, Duong M, Gulmez SE. Does paracetamol still have a future in osteoarthritis? The Lancet. 2016;387(10033):2065–2066. doi: 10.1016/S0140-6736(15)01170-8. [DOI] [PubMed] [Google Scholar]
- 206.Lane NE, Corr M. Osteoarthritis in 2016: Anti-NGF treatments for pain — two steps forward, one step back? Nature Reviews Rheumatology. 2017;13(2):76–78. doi: 10.1038/nrrheum.2016.224. [DOI] [PubMed] [Google Scholar]
- 207.Hochberg MC, Tive LA, Abramson SB, Vignon E, Verburg KM, West CR, Smith MD, Hungerford DS. When Is Osteonecrosis Not Osteonecrosis?: Adjudication of Reported Serious Adverse Joint Events in the Tanezumab Clinical Development Program. Arthritis and Rheumatology. 2016;68(2):382–391. doi: 10.1002/art.39492. [DOI] [PubMed] [Google Scholar]
- 208.Maloney J, Kivitz A, Schnitzer T, Dakin P, Stehman-Breen C, Geba G. Efficacy and Safety of Fasinumab for Osteoarthritic Pain in Patients with Moderate to Severe Osteoarthritis of the Knees or Hips [abstract] Arthritis Rheumatol. 2016;68(suppl 10) [Google Scholar]
- 209.Zheng S, Hunter DJ, Xu J, Ding C. Monoclonal antibodies for the treatment of osteoarthritis. Expert Opinion on Biological Therapy. 2016;16(12):1529–1540. doi: 10.1080/14712598.2016.1229774. [DOI] [PubMed] [Google Scholar]
- 210.Jeon OH, Kim C, Laberge RM, Demaria M, Rathod S, Vasserot AP, Chung JW, Kim DH, Poon Y, David N, Baker DJ, Van Deursen JM, Campisi J, Elisseeff JH. Local clearance of senescent cells attenuates the development of post-traumatic osteoarthritis and creates a pro-regenerative environment. Nature Medicine. 2017;23(6):775–781. doi: 10.1038/nm.4324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Grodzinsky AJ, Wang Y, Kakar S, Vrahas MS, Evans CH. Intra-articular dexamethasone to inhibit the development of post-traumatic osteoarthritis. Journal of Orthopaedic Research. 2017;35(3):406–411. doi: 10.1002/jor.23295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.McAlindon TE, LaValley MP, Harvey WF, Price LL, Driban JB, Zhang M, Ward RJ. Effect of intra-articular triamcinolone vs saline on knee cartilage volume and pain in patients with knee osteoarthritis a randomized clinical trial. JAMA - Journal of the American Medical Association. 2017;317(19):1967–1975. doi: 10.1001/jama.2017.5283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Conaghan PG, Cohen SB, Berenbaum F, Lufkin J, Johnson JR, Bodick N. A Phase IIb Trial of a Novel Extended-Release Microsphere Formulation of Triamcinolone Acetonide for Intraarticular Injection in Knee Osteoarthritis. Arthritis and Rheumatology. 2017;70(2):204–211. doi: 10.1002/art.40364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Evans CH, Kraus VB, Setton LA. Progress in intra-articular therapy. Nature reviews. Rheumatology. 2014;10(1):11–22. doi: 10.1038/nrrheum.2013.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Gerwin N, Hops C, Lucke A. Intraarticular drug delivery in osteoarthritis. Advanced Drug Delivery Reviews. 2006;58(2):226–242. doi: 10.1016/j.addr.2006.01.018. [DOI] [PubMed] [Google Scholar]
- 216.Lam J, Lu S, Kasper FK, Mikos AG. Strategies for controlled delivery of biologics for cartilage repair. Advanced Drug Delivery Reviews. 2015;84:123–134. doi: 10.1016/j.addr.2014.06.006. arXiv:15334406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Sacchetti C, Liu-Bryan R, Magrini A, Rosato N, Bottini N, Bottini M. Polyethylene-glycol-modified single-walled carbon nanotubes for intra-articular delivery to chondrocytes. ACS Nano. 2014;8(12):12280–12291. doi: 10.1021/nn504537b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Bajpayee AG, Wong CR, Bawendi MG, Frank EH, Grodzinsky AJ. Avidin as a model for charge driven transport into cartilage and drug delivery for treating early stage post-traumatic osteoarthritis. Biomaterials. 2014;35(1):538–49. doi: 10.1016/j.biomaterials.2013.09.091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Bajpayee AG, Scheu M, Grodzinsky AJ, Porter RM. Electrostatic Interactions Enable Rapid Penetration, Enhanced Uptake and Retention of Intra-Articular Injected Avidin in Rat Knee Joints. Journal of Orthopaedic Research. 2014;32(8):1044–1051. doi: 10.1002/jor.22630. [DOI] [PubMed] [Google Scholar]
- 220.Bajpayee AG, Quadir MA, Hammond PT, Grodzinsky AJ. Charge based intra-cartilage delivery of single dose dexamethasone using Avidin nano-carriers suppresses cytokine-induced catabolism long term. Osteoarthritis and Cartilage. 2016;24(1):71–81. doi: 10.1016/j.joca.2015.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Bajpayee A, De la Vega R, Scheu M, Varady N, Yannatos I, Brown L, Krishnan Y, Fitzsimons T, Bhattacharya P, Frank E, Grodzinsky A, Porter R. Sustained intra-cartilage delivery of low dose dexamethasone using a cationic carrier for treatment of post traumatic osteoarthritis. European Cells and Materials. 2017;34:341–364. doi: 10.22203/eCM.v034a21. [DOI] [PMC free article] [PubMed] [Google Scholar]