Abstract
Background:
Ferric citrate, an iron-based phosphate binder, has been shown to improve both hyperphosphatemia and iron deficiency in adult chronic kidney disease patients, but its use in the pediatric dialysis population has not been described.
Methods:
This is a retrospective analysis of 11 unselected pediatric dialysis patients who received ferric citrate as a phosphate binder between 2015 and 2017. Time-averaged laboratory values were compared pre- and post-ferric citrate initiation using the Wilcoxon signed-rank test.
Results:
The median age of this cohort was 13 years old (range 4 – 17 years old). Five patients were on hemodialysis, and six patients were on peritoneal dialysis. The median duration of ferric citrate therapy was 214 days (range 39 – 654 days), with a median time-averaged ferric citrate dose of 3.5 tablets per day (range 1.5 – 8.4 tablets per day). Compared to the pre-ferric citrate period, ferric citrate treatment was associated with decreased serum phosphate (6.5 mg/dl to 5.2 mg/dl, p=0.014), decreased serum phosphate age-related standard deviation score (SDS) (2.3 to 0.9, p=0.019), increased transferrin saturation (26% to 34%, p=0.049), increased ferritin (107 ng/ml to 230 ng/ml, p=0.074), and maintenance of hematocrit.
Conclusions:
In pediatric dialysis patients, ferric citrate may be able to concurrently lower phosphate levels and treat iron deficiency. However, larger studies are needed to further evaluate safety and efficacy in the pediatric chronic kidney disease population.
Keywords: pediatric, dialysis, CKD-MBD, phosphate, iron deficiency, ferric citrate
Introduction:
Chronic kidney disease (CKD) is characterized by the development of mineral and bone disorder [1] and progressive anemia [2]. Hyperphosphatemia is a common manifestation of chronic kidney disease-mineral and bone disorder (CKD-MBD), and higher phosphate levels are associated with vascular calcification [3] and increased mortality rates in CKD populations [4, 5]. As such, the use of phosphate binders has been recommended. Iron deficiency is also common in CKD [6], contributes to anemia, and has been associated with increased mortality, independent of anemia [7].
Recently, iron-based phosphate binders have been developed [8], which may concurrently address the common CKD co-morbidities of hyperphosphatemia and iron deficiency. Indeed, one iron-based phosphate binder, ferric citrate (Auryxia®, Keryx Biopharmaceuticals), has been shown to bind enteral phosphate effectively and provide clinically significant iron supplementation. In randomized controlled clinical trials involving adult nondialysis-dependent CKD patients [9, 10] and adult dialysis-dependent patients [11, 12], ferric citrate controlled serum phosphate levels and improved iron parameters. In 2014, ferric citrate was approved by the U.S. Food and Drug Administration (FDA) for use as a phosphate binder in adult CKD patients on dialysis. In pediatric dialysis patients, the safety and efficacy of ferric citrate have not been established.
In the present study, we performed a retrospective, real-world data analysis from the off-label use of ferric citrate in a cohort of pediatric dialysis patients. We characterized the cohort, and evaluated changes in serum phosphate and hematologic parameters before and after ferric citrate initiation.
Methods:
Retrospective data was obtained from the medical records of 11 unselected pediatric (<18 years old) dialysis patients, from a single dialysis center, who were started on ferric citrate as a phosphate binder between 2015 and 2017. Institutional review board approval was obtained to review the medical records. Prior to ferric citrate initiation, all patients were on various phosphate binders. Ferric citrate was added to the original phosphate binder(s), or patients were switched from the original phosphate binder(s) to ferric citrate.
From the medical records, demographic, anthropomorphic, medication, and laboratory data were obtained. Medication data included doses of intravenous iron sucrose and doses of erythropoietin. Laboratory data included serum phosphate, transferrin saturation, ferritin, hematocrit, calcium, parathyroid hormone, and 25OH vitamin D.
Serum phosphate concentrations vary physiologically with age in the pediatric population, decreasing as children and adolescents age [13]. To adjust for the effects of age, we calculated age-related phosphate standard deviation scores (SDS) [13]. The SDS, also known as a z-score, is defined as the number of standard deviations an observation is above the mean.
Categorical data are presented as numbers and percentages. Continuous data are presented as medians and ranges or interquartile ranges. Time-averaged data obtained pre-ferric citrate was compared to time-averaged data obtained post-ferric citrate, using the Wilcoxon signed-rank test.
Results:
Cohort characteristics
This cohort included 11 pediatric dialysis patients, with a median age of 13 years old (range 4 – 17 years old) at the time of ferric citrate initiation (Table 1). Patients were treated with ferric citrate for a median of 214 days (range 39 – 654 days), with a median time-averaged ferric citrate dose of 3.5 tablets per day (range 1.5 – 8.4 tablets per day). Each ferric citrate tablet contains 1 gram ferric citrate (210 mg elemental iron). Patients were e–xposed to a median time-averaged elemental iron dose of 27 mg/kg/day (range 10 – 38 mg/kg/day), with a median maximum exposure at any time point of 28 mg/kg/day (range 10 – 95 mg/kg/day). While on ferric citrate, the median maximum transferrin saturation (TSAT) recorded was 53% (range 29 – 100%), and the median maximum ferritin concentration recorded was 394 ng/ml (range 161 – 1550 ng/ml).
Table 1:
Cohort characteristics
| Number | 11 |
| Age at FC initiation (years) | 13 (4 – 17) |
| Sex | 4 male (36%), 7 female (64%) |
| Primary kidney disease | 3 dysplasia, 2 FSGS, 1 obstructive uropathy, 1 reflux nephropathy, 1 C3 glomerulopathy, 1 primary hyperoxaluria, 1 medullary cystic kidney disease, 1 unknown |
| Dialysis modality | 5 HD (45%), 6 PD (55%) |
| Pre-FC dialysis duration (months) | 18.3 (1.8 – 105.1) |
| Residual renal function | 6 anuric (55%), 5 non-anuric (45%) |
| Pre-FC phosphate binder | 4 calcium carbonate (36%), 2 sevelamer (18%), 5 both (45%) |
| Weight (kg) | 47.6 (11.4 – 67.0) |
| Weight standard deviation score | −1.1 (−4.0 – 1.2) |
| Height (cm) | 149.5 (84.2 – 176.0) |
| Height standard deviation score | −1.3 (−4.7 – 0.0) |
| Days on FC | 214 (39 – 654) |
| Starting FC dose (tabs/day) | 3.0 (1.5 – 6.0) |
| Time-averaged FC dose (tabs/day) | 3.5 (1.5 – 8.4) |
| Maximum FC dose (tabs/day) | 6.0 (1.5 – 9.0) |
| Time-averaged elemental iron dose (mg/kg/day) | 27 (10 – 38) |
| Maximum elemental iron dose (mg/kg/day) | 28 (10 – 95) |
| Time-averaged transferrin saturation on FC (%) | 35 (20 – 55) |
| Maximum transferrin saturation on FC (%) | 53 (29 – 100) |
| Time-averaged ferritin on FC (ng/ml) | 260 (94 – 1162) |
| Maximum ferritin on FC (ng/ml) | 394 (161 – 1550) |
| Data presented as percentages or medians (range). FC ferric citrate. | |
While on ferric citrate, four patients received intravenous (IV) iron sucrose administration. The first patient, weighing 61.5 kg, received 62 doses of 50 mg iron sucrose (0.8 mg/kg/dose) over all 637 days of ferric citrate administration (average of one dose approximately every 10 days). This patient had a maximum TSAT of 32% and maximum ferritin of 1366 ng/ml. The other three patients had more limited exposure to IV iron. The second patient, weighing 20.0 kg, received 7 doses of 12.5 mg iron sucrose (0.6 mg/kg/dose) over 49 days (19.5%) of the 251 total days of ferric citrate administration. During this time, TSAT and ferritin remained within normal ranges. The third patient, weighing 11.4 kg, received only two doses of 12.5 mg iron sucrose (1.1 mg/kg/dose) over all 46 days of ferric citrate administration. The fourth patient, weighing 64.0 kg, received a single dose of 100 mg iron sucrose (1.6 mg/kg/dose) during all 214 days of ferric citrate administration.
Two patients received oral ferrous sulfate concurrently with ferric citrate. The first patient was on ferrous sulfate for 33.5% of the time she was receiving ferric citrate, which provided 1.1 mg/kg/day elemental iron. The second patient was on ferrous sulfate for only 5.4% of the time she was treated with ferric citrate, which provided 1.3 mg/kg/day elemental iron.
Changes pre- and post-ferric citrate initiation
With ferric citrate treatment, the median time-averaged serum phosphate concentration significantly decreased from 6.5 (interquartile range 5.5, 7.0) mg/dl to 5.2 (5.1, 6.3) mg/dl (p=0.014) (Table 2 and Figure 1). The median time-averaged, age-adjusted phosphate standard deviation score (SDS) significantly decreased from 2.3 (1.5, 3.6) to 0.9 (0.0, 2.4) (p=0.019). Concurrently, transferrin saturation (TSAT) significantly increased from 26 (17, 34) % to 34 (28, 46) % (p=0.049), and ferritin tended to increase from 107 (86, 675) ng/ml to 230 (113, 716) ng/ml (p=0.074). No significant changes were observed in hematocrit (p=0.83), serum calcium (p=0.32), parathyroid hormone (p=0.13), or 25OH vitamin D (p=1.00). After excluding the five patients who received any other iron preparations (IV iron sucrose and/or oral ferrous sulfate) for any duration while on ferric citrate, we still observed that ferric citrate treatment tended to increase TSAT (26 (22, 34) % to 39 (33, 47) %, p=0.063) and ferritin (91 (86, 496) ng/ml to 122 (113, 726) ng/ml, p=0.063).
Table 2:
Cohort data pre- and post-ferric citrate initiation
| Pre-Ferric Citrate Initiation | Post-Ferric Citrate Initiation | p value | |
|---|---|---|---|
| Days (median (range)) | 95 (2 – 273) | 214 (39 – 654) | n/a |
| Number of values averaged over time (median (range)): | n/a | ||
| Phosphate (n=11) | 5 (1 – 13) | 7 (1 – 46) | n/a |
| Transferrin saturation (n=10) | 3 (1 – 9) | 7 (1 – 21) | n/a |
| Ferritin (n=9) | 3 (1 – 9) | 6 (1 – 21) | n/a |
| Hematocrit (n=11) | 5 (1 – 9) | 6 (2 – 45) | n/a |
| Calcium (n=11) | 5 (1 −13) | 7 (3 – 46) | n/a |
| Parathyroid hormone (n=10) | 3 (1 – 9) | 7 (1 – 23) | n/a |
| 250H vitamin D (n=10) | 3 (1 – 9) | 6 (1 – 23) | n/a |
| Time-averaged parameters pre - and post-ferric citrate (median (interquartile range)): | |||
| Phosphate (mg/dl) | 6.5 (5.5, 7.0) | 5.2 (5.1, 6.3) | 0.014 |
| Phosphate standard deviation score | 2.3 (1.5, 3.6) | 0.9 (0.0, 2.4) | 0.019 |
| Transferrin saturation (%) | 26 (17, 34) | 34 (28, 46) | 0.049 |
| Ferritin (ng/ml) | 107 (86, 675) | 230 (113, 716) | 0.074 |
| Hematocrit (%) | 36.7 (32.9, 40.9) | 36.5 (34.6, 37.8) | 0.83 |
| Calcium (mg/dl) | 9.4 (8.8, 10.0) | 9.4 (8.9, 9.6) | 0.32 |
| Parathyroid hormone (pg/ml) | 408 (251, 610) | 716 (306, 1200) | 0.13 |
| 250H vitamin D (ng/ml) | 26 (22, 35) | 27 (21, 34) | 1.00 |
| Erythropoietin (EPO) use (n=6): | |||
| Number of weeks averaged (median (range)) | 19 (2 – 44) | 48 (4 – 92) | n/a |
| EPO dose (units/kg/week) (median (interquartile range)) | 286 (152, 521) | 381 (174, 630) | 0.49 |
Figure 1: Changes in serum parameters pre-ferric citrate initiation and post-ferric citrate initiation.
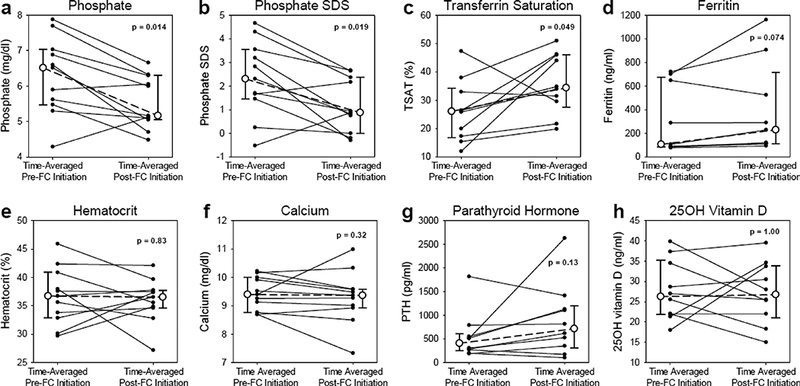
Serum parameters shown are (a) phosphate, (b) age-adjusted phosphate standard deviation score (SDS), (c) transferrin saturation (TSAT), (d) ferritin, (e) hematocrit, (f) calcium, (g) parathyroid hormone (PTH), and (h) 25OH vitamin D. With ferric citrate (FC) treatment, phosphate decreased, phosphate SDS decreased, TSAT increased, ferritin tended to increase, and hematocrit, calcium, PTH, and 25OH vitamin D did not significantly change. For each patient, the time-averaged parameter values before and after FC are indicated by the small, closed circles. For each parameter, the median time-averaged values before and after FC are indicated by large, open circles; the error bars represent the interquartile range (IQR), with the lower error bar indi cating the 25th percentile, and the upper error bar indicating the 75th percentile. The Wilcoxon signed-rank test was used to compare the median values before and after FC initiation. For phosphate, phosphate SDS, hematocrit, and calcium, n=11 patients; for TSAT, PTH, and 25OH vitamin D, n=10 patients; and for ferritin, n=9 patients
We also assessed erythropoietin (EPO) use in our cohort. Two patients stopped EPO prior to ferric citrate administration, two patients started EPO after ferric citrate initiation, and seven patients remained on EPO pre- and post-ferric citrate administration. Of the patients on EPO pre- and post-ferric citrate administration, one patient was on darbepoetin alfa, and the dose decreased from 60 mcg weekly to 45 mcg weekly with ferric citrate administration. The remaining six patients were on epoetin alfa, and the median epoetin alfa dose did not differ pre- and post-ferric administration (p=0.49).
Discussion:
In this study, we present retrospective real-world data from the off-label use of ferric citrate in a cohort of pediatric dialysis-dependent patients. As in the studies of adult dialysis patients [11, 12], with ferric citrate treatment, we observed decreased serum phosphate, improved iron parameters, and maintenance of hematocrit. Notably, with ferric citrate treatment, the median age-adjusted phosphate SDS decreased from 2.3 standard deviations above the mean for age (99th percentile) to 0.9 standard deviation above the mean for age (81st percentile). Although not all patients achieved time-averaged serum phosphate concentrations <6.0 mg/dl or time-averaged, age-adjusted phosphate SDS <2.0 with ferric citrate, our observations suggest that ferric citrate can lower phosphate levels, as well as improve iron status, in pediatric dialysis patients.
The main adverse effects associated with ferric citrate treatment are gastrointestinal symptoms (diarrhea, nausea, emesis, constipation) [9]. However, in our pediatric cohort, ferric citrate seemed to be well-tolerated, as no patient developed adverse gastrointestinal effects severe enough to warrant dose reduction or discontinuation of therapy. In the two randomized, placebo-controlled trials of ferric citrate conducted in adult CKD patients not on dialysis, gastrointestinal adverse events were reported in 49.5% of patients treated with ferric citrate vs. 27.7% of patients treated with placebo [14]. Compared to the placebo group, patients on ferric citrate reported more discolored feces (21.6% vs. 0.0%), diarrhea (20.5% vs. 12.2%), constipation (18.4% vs. 10.1%), and nausea (9.5% vs. 4.3%) [14]. In a randomized, active-controlled trial of ferric citrate conducted in adult dialysis patients, serious gastrointestinal adverse events recorded any time after drug initiation occurred in 8.3% of the ferric citrate group vs. 12.8% of the active control group, and non-serious gastrointestinal adverse events occurred in 48.8% of the ferric citrate group vs. 36.9% of the active control group [11].
Although patients on ferric citrate are exposed to high amounts of elemental iron, much of the ferric iron binds enteral phosphate, precipitating as ferric phosphate, an insoluble compound that is excreted. Yet, some iron is absorbed, necessitating close monitoring of serum iron parameters, such as TSAT and ferritin. In pediatric dialysis patients, optimal TSAT and ferritin ranges remain undefined. However, in a large study of over 58,000 adult hemodialysis patients, after time-dependent and multivariable adjustment, TSAT levels between 30–50% and ferritin levels between 200 and 1200 ng/ml were associated with the lowest mortality rates [15]. In our cohort, only two patients had a time-averaged TSAT >50% while on ferric citrate (51% and 55%), and one of these patients was receiving concurrent ferrous sulfate. TSAT is calculated by dividing serum iron by the total iron binding capacity (TIBC), and TIBC is a negative acute-phase reactant [16]; therefore, inflammation, which is common in CKD, can result in falsely elevated TSAT calculations. Furthermore, TSAT may be acutely increased if measured within four hours of oral iron administration [17]; in the present study, timing of TSAT measurements in relation to ferric citrate administration was not recorded. In our cohort, no patient had time- averaged ferritin >1200 ng/ml while on ferric citrate. As ferritin is an acute -phase reactant, inflammation can increase ferritin concentrations, confounding the use of ferritin as a marker of iron status [16].
Both hyperphosphatemia [18–21] and iron deficiency [22–25] can increase production of fibroblast growth factor 23 (FGF23), a bone-derived phosphaturic hormone. In both adult and pediatric CKD, FGF23 levels increase early [20, 26–28], with higher levels associated with faster CKD progression [29–31] and increased cardiovascular morbidity [32–35]. In adult CKD populations [29, 36], and in the general population [37], higher FGF23 levels are also independently associated with increased mortality rates. Ferric citrate can lower phosphate levels and increase iron parameters, therefore providing two possible mechanisms by which pathologically elevated FGF23 levels may be decreased. Indeed, in adult non-dialysis dependent CKD patients, ferric citrate treatment decreased FGF23 levels by 30–35% from baseline [9, 38]. Also, a small, prospective study of 27 iron-deficient, adult hemodialysis patients demonstrated that ferric citrate treatment controlled serum phosphate, improved iron parameters, and decreased FGF23 levels [39]. Therefore, via phosphate control and/or iron supplementation, ferric citrate may be able to affect FGF23 levels in CKD. In the pediatric CKD population, longitudinal studies are required to determine whether or not ferric citrate use can lower FGF23 levels or prevent increases in FGF23 over time.
In this small, retrospective study, we characterize our real world experience with ferric citrate in a group of pediatric dialysis patients. In our cohort, ferric citrate was well-tolerated and was associated with decreased phosphate levels and improved iron parameters. Limitations of this observational study include small sample size, variable subject characteristics (e.g. on different dialysis modalities for varying durations), and incomplete subject characterization (e.g. lack of inflammatory markers). Larger, controlled clinical trials are needed to further assess the safety and long-term efficacy of ferric citrate use in pediatric CKD patients.
Footnotes
Compliance with ethical standards
Institutional review board approval was obtained to review the medical records
Conflict of interest
IBS serves as a consultant for Akebia, Keryx, and Ultragenyx, and has received research funding from Abbvie and Amgen
References
- 1.KDIGO clinical practice guideline for the diagnosis, evaluation, prevention, and treatment of Chronic Kidney Disease-Mineral and Bone Disorder (CKD-MBD) (2009). Kidney Int Suppl:S1–130. [DOI] [PubMed] [Google Scholar]
- 2.Hsu CY, McCulloch CE, Curhan GC (2002) Epidemiology of anemia associated with chronic renal insufficiency among adults in the United States: results from the Third National Health and Nutrition Examination Survey. J Am Soc Nephrol 13:504–510. [DOI] [PubMed] [Google Scholar]
- 3.Yamada S, Giachelli CM (2017) Vascular calcification in CKD-MBD: Rolesfor phosphate, FGF23, and Klotho. Bone 100:87–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kestenbaum B, Sampson JN, Rudser KD, Patterson DJ, Seliger SL, Young B, Sherrard DJ, Andress DL (2005) Serum phosphate levels and mortality risk among people with chronic kidney disease. J Am Soc Nephrol 16:520–528. [DOI] [PubMed] [Google Scholar]
- 5.Block GA, Klassen PS, Lazarus JM, Ofsthun N, Lowrie EG, Chertow GM (2004) Mineral metabolism, mortality, and morbidity in maintenance hemodialysis. J Am Soc Nephrol 15:2208–2218. [DOI] [PubMed] [Google Scholar]
- 6.Fishbane S, Pollack S, Feldman HI, Joffe MM (2009) Iron indices in chronic kidney disease in the National Health and Nutritional Examination Survey 1988–2004. Clin J Am Soc Nephrol 4:57–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Klip IT, Jankowska EA, Enjuanes C, Voors AA, Banasiak W, Bruguera J, Rozentryt P, Polonski L, van Veldhuisen DJ, Ponikowski P, Comin-Colet J, van der Meer P (2014) The additive burden of iron deficiency in the cardiorenal-anaemia axis: scope of a problem and its consequences. Eur Journal Heart Fail 16:655–662. [DOI] [PubMed] [Google Scholar]
- 8.Shah HH, Hazzan AD, Fishbane S (2015) Novel iron-based phosphate binders in patients with chronic kidney disease. Curr Opin Nephrol Hypertens 24:330–335. [DOI] [PubMed] [Google Scholar]
- 9.Block GA, Fishbane S, Rodriguez M, Smits G, Shemesh S, Pergola PE, Wolf M, Chertow GM (2015) A 12-week, double-blind, placebo-controlled trial of ferric citrate for the treatment of iron deficiency anemia and reduction of serum phosphate in patients with CKD Stages 3–5. Am J Kidney Dis 65:728–736. [DOI] [PubMed] [Google Scholar]
- 10.Yokoyama K, Akiba T, Fukagawa M, Nakayama M, Sawada K, Kumagai Y, Chertow GM, Hirakata H (2014) Long-term safety and efficacy of a novel iron-containing phosphate binder, JTT-751, in patients receiving hemodialysis. J Ren Nutr 24:261–267. [DOI] [PubMed] [Google Scholar]
- 11.Lewis JB, Sika M, Koury MJ, Chuang P, Schulman G, Smith MT, Whittier FC, Linfert DR, Galphin CM, Athreya BP, Nossuli AK, Chang IJ, Blumenthal SS, Manley J, Zeig S, Kant KS, Olivero JJ, Greene T, Dwyer JP (2015) Ferric citrate controls phosphorus and delivers iron in patients on dialysis. J Am Soc Nephrol 26:493–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yokoyama K, Akiba T, Fukagawa M, Nakayama M, Sawada K, Kumagai Y, Chertow GM, Hirakata H (2014) A randomized trial of JTT-751 versus sevelamer hydrochloride in patients on hemodialysis. Nephrol Dial Transplant 29:1053–1060. [DOI] [PubMed] [Google Scholar]
- 13.Ardeshirpour L, Cole DE, Carpenter TO (2007) Evaluation of bone and mineral disorders. Pediatr Endocrinol Rev Suppl 1:584–598. [PubMed] [Google Scholar]
- 14.Chertow GM, Block GA, Neylan JF, Pergola PE, Uhlig K, Fishbane S (2017) Safety and efficacy of ferric citrate in patients with nondialysis-dependent chronic kidney disease. PloS One 12:e0188712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kalantar-Zadeh K, Regidor DL, McAllister CJ, Michael B, Warnock DG (2005) Time-dependent associations between iron and mortality in hemodialysis patients. J Am Soc Nephrol 16:3070–3080. [DOI] [PubMed] [Google Scholar]
- 16.Kalantar-Zadeh K, Kalantar-Zadeh K, Lee GH (2006) The fascinating but deceptive ferritin: to measure it or not to measure it in chronic kidney disease? Clin J Am Soc Nephrol 1 Suppl 1:S9–18. [DOI] [PubMed] [Google Scholar]
- 17.Girelli D, Trombini P, Busti F, Campostrini N, Sandri M, Pelucchi S, Westerman M, Ganz T, Nemeth E, Piperno A, Camaschella C (2011) A time course of hepcidin response to iron challenge in patients with HFE and TFR2 hemochromatosis. Haematologica 96:500–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Saito H, Maeda A, Ohtomo S, Hirata M, Kusano K, Kato S, Ogata E, Segawa H, Miyamoto K, Fukushima N (2005) Circulating FGF-23 is regulated by 1alpha,25-dihydroxyvitamin D3 and phosphorus in vivo. J Biol Chem 280:2543–2549. [DOI] [PubMed] [Google Scholar]
- 19.Antoniucci DM, Yamashita T, Portale AA (2006) Dietary phosphorus regulates serum fibroblast growth factor-23 concentrations in healthy men. J Clin Endocrinol Metab 91:3144–3149. [DOI] [PubMed] [Google Scholar]
- 20.Gutierrez O, Isakova T, Rhee E, Shah A, Holmes J, Collerone G, Juppner H, Wolf M (2005) Fibroblast growth factor-23 mitigateshyperphosphatemia but accentuates calcitriol deficiency in chronic kidney disease. J Am Soc Nephrol 16:2205–2215. [DOI] [PubMed] [Google Scholar]
- 21.Gupta A, Winer K, Econs MJ, Marx SJ, Collins MT (2004) FGF-23 is elevated by chronic hyperphosphatemia. The J Clin Endocrinol Metab 89:4489–4492. [DOI] [PubMed] [Google Scholar]
- 22.Farrow EG, Yu X, Summers LJ, Davis SI, Fleet JC, Allen MR, Robling AG, Stayrook KR, Jideonwo V, Magers MJ, Garringer HJ, Vidal R, Chan RJ, Goodwin CB, Hui SL, Peacock M, White KE (2011) Iron deficiency drives an autosomal dominant hypophosphatemic rickets (ADHR) phenotype in fibroblast growthfactor-23 (Fgf23) knock in mice Proc Natl Acad Sci U S A 108:E1146–1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Clinkenbeard EL, Farrow EG, Summers LJ, Cass TA, Roberts JL, Bayt CA, Lahm T, Albrecht M, Allen MR, Peacock M, White KE (2014) Neonatal iron deficiency causes abnormal phosphate metabolism by elevating FGF23 in normal and ADHR mice. J Bone Miner Res 29:361–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.David V, Martin A, Isakova T, Spaulding C, Qi L, Ramirez V, Zumbrennen-Bullough KB, Sun CC, Lin HY, Babitt JL, Wolf M (2016) Inflammation and functional iron deficiency regulate fibroblast growthfactor 23 production. Kidney Int 89:135–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hanudel MR, Chua K, Rappaport M, Gabayan V, Valore E, Goltzman D, Ganz T, Nemeth E, Salusky IB (2016) Effects of dietary iron intake and chronic kidney disease on fibroblast growthfactor 23 metabolism in wild-type and hepcidin knockout mice. American journal of physiology Renal Physiol 311:F1369–f1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Isakova T, Wahl P, Vargas GS, Gutierrez OM, Scialla J, Xie H, Appleby D, Nessel L, Bellovich K, Chen J, Hamm L, Gadegbeku C, Horwitz E, Townsend RR, Anderson CA, Lash JP, Hsu CY, Leonard MB, Wolf M (2011) Fibroblast growth factor 23 is elevated before parathyroid hormone and phosphate in chronic kidney disease. Kidney Int 79:1370–1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Larsson T, Nisbeth U, Ljunggren O, Juppner H, Jonsson KB (2003) Circulating concentration of FGF-23 increases as renal function declines in patients with chronic kidney disease, but does not change in response to variation in phosphate intake in healthy volunteers. Kidney Int 64:2272–2279. [DOI] [PubMed] [Google Scholar]
- 28.Portale AA, Wolf M, Juppner H, Messinger S, Kumar J, Wesseling-Perry K, Schwartz GJ, Furth SL, Warady BA, Salusky IB (2014) Disordered FGF23 and mineral metabolism in children with CKD. Clin J Am Soc Nephrol 9:344–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Isakova T, Xie H, Yang W, Xie D, Anderson AH, Scialla J, Wahl P, Gutierrez OM, Steigerwalt S, He J, Schwartz S, Lo J, Ojo A, Sondheimer J, Hsu CY, Lash J, Leonard M, Kusek JW, Feldman HI, Wolf M (2011) Fibroblast growth factor 23 and risks of mortality and end-stage renal disease in patients with chronic kidney disease. JAMA 305:2432–2439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fliser D, Kollerits B, Neyer U, Ankerst DP, Lhotta K, Lingenhel A, Ritz E, Kronenberg F, Kuen E, Konig P, Kraatz G, Mann JF, Muller GA, Kohler H, Riegler P (2007) Fibroblast growth factor 23 (FGF23) predicts progression of chronic kidney disease: the Mild to Moderate Kidney Disease (M MKD) Study. J Am Soc Nephrol 18:2600–2608. [DOI] [PubMed] [Google Scholar]
- 31.Portale AA, Wolf MS, Messinger S, Perwad F, Juppner H, Warady BA, Furth SL, Salusky IB (2016) Fibroblast Growth Factor 23 and Risk of CKD Progression in Children. Clin J Am Soc Nephrol 11:1989–1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Faul C, Amaral AP, Oskouei B, Hu MC, Sloan A, Isakova T, Gutierrez OM, Aguillon-Prada R, Lincoln J, Hare JM, Mundel P, Morales A, Scialla J, Fischer M, Soliman EZ, Chen J, Go AS, Rosas SE, Nessel L, Townsend RR, Feldman HI, St John Sutton M, Ojo A, Gadegbeku C, Di Marco GS, Reuter S, Kentrup D, Tiemann K, Brand M, Hill JA, Moe OW, Kuro OM, Kusek JW, Keane MG, Wolf M (2011) FGF23 induces left ventricular hypertrophy. J Clin Invest 121:4393–4408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gutierrez OM, Januzzi JL, Isakova T, Laliberte K, Smith K, Collerone G, Sarwar A, Hoffmann U, Coglianese E, Christenson R, Wang TJ, deFilippi C, Wolf M (2009) Fibroblast growth factor 23 and left ventricular hypertrophy in chronic kidney disease. Circulation 119:2545–2552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mehta R, Cai X, Lee J, Scialla JJ, Bansal N, Sondheimer JH, Chen J, Hamm LL, Ricardo AC, Navaneethan SD, Deo R, Rahman M, Feldman HI, Go AS, Isakova T, Wolf M (2016) Association of Fibroblast Growth Factor 23 With Atrial Fibrillation in Chronic Kidney Disease, From the Chronic Renal Insufficiency Cohort Study. JAMA Cardiol 1:548–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mitsnefes MM, Betoko A, Schneider MF, Salusky IB, Wolf MS, Juppner H, Warady BA, Furth SL, Portale AA (2018) FGF23 and Left Ventricular Hypertrophy in Children with CKD. Clin J Am Soc Nephrol 13:45–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gutierrez OM, Mannstadt M, Isakova T, Rauh-Hain JA, Tamez H, Shah A, Smith K, Lee H, Thadhani R, Juppner H, Wolf M (2008) Fibroblast growthfactor 23 and mortalityamong patients undergoing hemodialysis. N Engl J Med 359:584–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Souma N, Isakova T, Lipiszko D, Sacco RL, Elkind MS, DeRosa JT, Silverberg SJ, Mendez AJ, Dong C, Wright CB, Wolf M (2016) Fibroblast Growth Factor 23 and Cause-Specific Mortality in the General Population: The Northern Manhattan Study. J Clin Endocrinol Metab 101:3779–3786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yokoyama K, Hirakata H, Akiba T, Fukagawa M, Nakayama M, Sawada K, Kumagai Y, Block GA (2014) Ferric citrate hydrate for the treatment of hyperphosphatemia in nondialysis-dependent CKD. Clin J Am Soc Nephrol 9:543–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Iguchi A, Kazama JJ, Yamamoto S, Yoshita K, Watanabe Y, Iino N, Narita I (2015) Administration of Ferric Citrate Hydrate Decreases Circulating FGF23 Levels Independently of Serum Phosphate Levels in Hemodialysis Patients with Iron Deficiency. Nephron 131:161–166. [DOI] [PubMed] [Google Scholar]


