Abstract
Pre-clinical and clinical studies suggest black raspberries (BRBs) may inhibit the development of oral cancer. Lyophilized BRB powder is commonly used in these studies, but processed BRB products are more often consumed. The objective of this work was to understand how storage conditions influence the phytochemical profile and anti-proliferative activity of a BRB nectar beverage. Untargeted UHPLC-Q-TOF-MS based metabolomics analyses demonstrated that large chemical variation was introduced by storage above −20 °C over 60 days. However, minimal change in anti-proliferative activity was observed when stored nectar extracts were applied to SCC-83-01-82 premalignant oral epithelial cells. As proof of concept, cyanidin-3-O-rutinoside and its degradation product, protocatechuic acid, were administered in different ratios maintaining an equimolar dose, and anti-proliferative activity was maintained. This study shows the utility of metabolomics to profile global chemical changes in foods, while demonstrating that isolated phytochemicals do not explain the complete bioactivity of a complex food product.
Graphical Abstract
1. Introduction
Black raspberries (BRBs) are extensively studied for their cancer preventative properties 1,2. Their bioactivity has been attributed to their rich phytochemical profile inclusive of anthocyanins, ellagitannins, organic acids, and quercetin among other phenolic compounds 3. It has been hypothesized that these components elicit a complex series of biological responses that result in a net inhibition of cancer growth 4. Because of their high concentration of phenolic compounds, BRBs have been the subject of many studies on food-based chemoprevention strategies.
Much of the interest in the chemopreventative properties of BRBs has focused on oral cancer 5–9. The oral cavity presents unique opportunities for chemoprevention through dietary means due to direct exposure of tissues to food phytochemicals. Oral cancer is prevalent throughout the world, with higher incidence observed in men and people in less developed regions 10,11. The majority of oral cancers are squamous cell carcinomas (SCCs), and risk factors include tobacco use, alcohol consumption, human papillomavirus infection, and chronic periodontal disease 12. Pre-clinical models have consistently shown reduction of oral SCC incidence and multiplicity using whole freeze-dried BRBs, likely due to engagement of a number of biological mechanisms 13. A human clinical trial with a BRB-based mucoadhesive gel demonstrated the ability of BRBs to reduce the size and severity of precancerous oral lesions 8, while a relative reduction in the expression of molecular biomarkers indicative of SCC was observed after patients were treated with BRB-based troches for two weeks 6. These studies support a role for BRB-mediated efficacy in oral cancer prevention strategies.
Most research using BRBs has been conducted with minimally processed, lyophilized BRB powder. In practical terms, consumers mostly encounter BRBs after they have been incorporated into shelf-stable food products and stored for varying lengths of time, during which the phytochemical profile may be altered 14. Research on the stability of the phytochemical profile in BRB-based food products is limited to a short defined list of compounds 14–16, while effects on the global phytochemical profile and bioactivity of these products are unknown. Metabolomics is an emerging approach to chemical analysis in which hundreds to thousands of compounds within a food system are profiled, with the potential to provide new insight into the relationship between food phytochemicals and health outcomes 17. The objective of the current study is to use an untargeted metabolomics approach to understand the global differences in the phytochemical profile of a BRB nectar beverage over storage time and temperature variations, and how these changes relate to the growth inhibition activity in an in vitro oral premalignacy model.
2. Experimental
2.1 Chemicals
All solvents were of HPLC-MS grade from Fischer Scientific (Pittsburgh, PA) unless otherwise noted. Cyanidin-3-O-rutinoside (C3R) and protocatechuic acid (PA) standards were from Sigma Aldrich (St. Louis, MO). Cell culture-grade dimethyl sulfoxide and water were also from Sigma Aldrich.
2.2 Nectar Processing, Storage, and Sampling
Nectar was prepared in the pilot plant facilities located at The Ohio State University (Columbus, OH) using a formula similar to that described by Gu and colleagues, as shown in Table 1 16. The BRB powder used was produced from whole BRBs harvested at Stokes Berry Farm (Wilmington, OH). All components were combined in a high shear mixer for 20 min, and the nectar was subsequently pasteurized using a MicroThermics UHT/HTSTLab-25HV Hybrid unit (MicroThermics, Inc., Raleigh, NC, USA). The processing specifications mirrored industry practices for pasteurization of this product by which the nectar was held at 100 °C (± 1.1 °C) for 23 sec, immediately cooled, and aseptically filled into pre-sterilized 50 mL conical centrifuge tubes.
Table 1.
BRB nectar beverage formulation
| Ingredients | % Wet Basis |
|---|---|
| Water | 89.9 |
| Sucrose | 3.0 |
| Pectin | 0.5 |
| Corn Syrup | 1.0 |
| BRB Powder | 5.6 |
| TOTAL | 100.0 |
Nectar was stored at −20 °C, 4 °C, 10 °C, 25 °C, or 35 °C for 60 d with samples (n=4) removed from each condition at 5 d, 10 d, and subsequently in 10 d intervals. Two months has been described as an appropriate amount of time to study stability in foods intended for clinical trials, as time is often needed for subject recruitment and intervention 16. At each time point the nectar was centrifuged (1000 x g) for 5 min, partitioned into smaller aliquots, and stored at −80 °C prior to use. Samples of freshly produced nectar were also stored at −80 °C at the time of production as a t0 sample. Aerobic plate counts and yeast and mold counts were obtained for the 35 °C incubated samples at each time point with 3M Petrifilm (3M Company; Maplewood, MN), according to manufacturer instructions.
2.3 Sample Preparation and Analysis for Untargeted Metabolomics
Aliquots (1 mL) of nectar were thawed in a room temperature water bath for 10 min. Once thawed, 750 μL were deposited into a glass vial followed by 2.25 mL of 0.1% formic acid in methanol. The mixture was homogenized with a probe sonicator (Branson Ultrasonics; Danbury, CT) for 10 sec and centrifuged for 5 min at 1000 x g (4 °C). The supernatant was decanted into a glass vial, and the pellet was extracted twice more with 3 mL of 75% methanol in water with 0.1% formic acid. Aliquots (200 μL) were deposited into 4 mL glass vials, dried under a stream of nitrogen, and stored at −20 °C until analysis.
Dried aliquots were solubilized in 100 μL 25% methanol in water with 0.1% formic acid and vortexed for 15 sec. Samples were then centrifuged at 21,130 x g for 4 min (4 °C) and placed in the autosampler of a 1290 Infinity II series UHPLC (Agilent Technologies, Santa Clara, CA) maintained at 4 °C until analysis. Samples were injected (5 μL) onto a 2.1 x 100 mm, 1.8 μm Acquity HSS T3 column (Waters, Milford, MA) maintained at 40 °C. The mobile phase consisted of A: 0.1% formic acid in water and B: 0.1% formic acid in acetonitrile with a flow rate of 0.5 mL/min. The linear gradient program was as follows: 0% B held for 1 min, increased to 60% B over 5 min, increased to 100% B over 2 min and hold for 1.5 min, immediately switched to 0% B and held for 2 min for a total run time of 11.5 min.
Eluent was directed to an Agilent iFunnel 6550 QTOF-MS interfaced with an electrospray ionization (ESI) source operated in negative ion mode. The first minute of flow from the UHPLC was directed to waste. Relevant MS settings were as follows: gas temp 150 °C, drying gas 18 L/min, nebulizer 30 psig, sheath gas temp 350 °C, sheath gas flow 12 L/min, VCap 4000 V, nozzle voltage 2000 V, acquisition mode was 2 GHz extended dynamic range with a mass range of 50–1700 m/z. Reference mass solution (Agilent Technologies) was concurrently infused into the source via a dedicated sprayer for continual mass correction. Sample run order was randomized. Quality control samples, composed of equal portions of each nectar sample, were run every 10 samples to monitor instrument performance over the run time (data not shown).
2.4 Data Pre-processing and Analysis for Untargeted Metabolomics
Raw spectral data was processed using the batch recursive feature extraction algorithm in Profinder (B.08.00, Agilent Technologies). Mass spectral features were picked and binned according to expected isotope patterns, adducts, and charge states. These molecular features were then aligned across all samples, and those appearing in less than three samples per time/temperature group were removed from further analysis. The raw data was then searched against this assembled list in a targeted manner to improve the quality of the data used for multivariate analysis. Further data pre-processing was performed in Mass Profiler Professional (version 14.5, Agilent Technologies), including removal of features present in sample blanks. To remove low quality peaks from the data, an additional abundance filter was applied which required a minimum peak height of 5.0 x 104 in 75% of the samples in at least one time/temperature sample group.
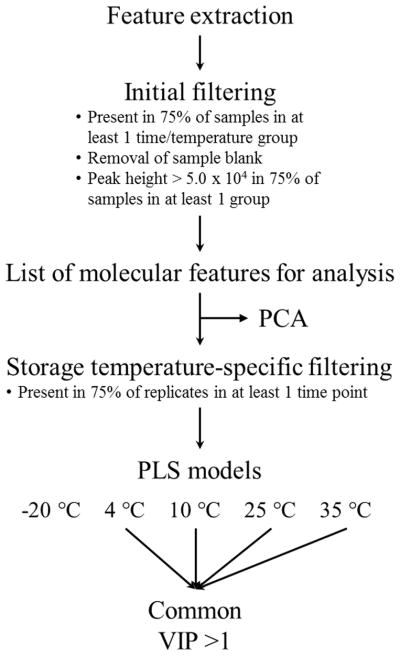
Multivariate analyses, including principal component analysis and partial least squares regression (PLS), were executed in R (version 3.2.3) with the ropls package using the autofit option to determine the optimal number of components18. Data were log10 transformed and Pareto scaled prior to analysis. PCA is a dimensional reduction technique that allows for analysis of multidimensional data in an easily-visualized space. PLS is a common multivariate modeling technique that builds off the dimensional reduction properties of PCA but in the framework of a linear regression 19. Separate PLS models were constructed for each storage condition. The X matrices were composed of features present in 75% of replicates from at least one time point in each storage condition, and the Y matrix was storage time. Performance of the PLS models was assessed using 8-segment cross validation, and statistical significance of each model was determined using permutation tests (n = 100). Features with a variable importance on projection value (VIP) ≥1 across all successful models were manually reviewed before further analysis. Similarly, features with a VIP ≥ 1 in only the 35 °C samples were also manually reviewed for further analysis. VIP scores are estimates of the relative importance of a chemical feature to a given PLS model, and features with a score ≥ 1 are typically considered to be important in the model. A data pre-treatment and analysis summary is shown in Figure 1.
Figure 1.
Summary of untargeted metabolomics data pre-treatment and analysis.
2.5 Targeted Compound Analysis
Cyanidin-3-O-rutinoside (C3R) and protocatechuic acid (PA) were quantified in the nectar samples from t0 and 60d at 35 °C. Extracts of BRB nectar were obtained as described for the untargeted metabolomics workflow, reconstituted in 5 mL of 5% aqueous formic acid, and filtered through a 0.22 μm nylon filter. Samples were then injected (0.5 μL) into an Agilent 1290 Infinity II UHPLC coupled to an Agilent 6495 triple quadrupole MS equipped with an ESI source operated in positive and negative ion modes. The mobile phase consisted of A: 5% aqueous formic acid and B: 5% formic acid in acetonitrile. The column and gradient program were identical to that was described here for untargeted analyses. MS parameters included gas temp: 150 °C, gas flow: 18 L/min, nebulizer: 45 psi, sheath gas heater: 375 °C, sheath gas flow: 12 L/min, capillary: 3000 V, fragmentor: 350. Quantitation was performed using standard curves constructed from serial dilutions of authentic standards. The transitions used for each compound were as follows: C3R: 595 [M+] →287 (CE = 17 V), PCA: 153 [M-H]− →109 (CE = 10).
2.6 Extract Preparation for Cell Study
Extraction of the nectar was scaled up from the procedure used in the untargeted metabolomics workflow to ensure sufficient extract mass. Briefly, nectar replicates were pooled and 1 mL aliquots were deposited into glass vials followed by 3 mL of 0.1% formic acid in methanol. The mixture was homogenized with a probe sonicator and centrifuged at 3220 × g (4 °C) for 7 min. The supernatant was decanted into a glass vial, and the pellet was extracted once with 75% aqueous methanol with 0.1% formic acid. The pooled supernatants were dried using a Genevac EZ2 vacuum evaporation system (SP Scientific; Ipswich, United Kingdom) set at 30 °C. Remaining water was removed by lyophilization on a Labconco FreeZone 12 Plus system (Kansas City, MO). Nectar extracts were reconstituted in 1:1 DMSO/water, sonicated for 15 sec, and diluted to a concentration of 2 mg extract/mL in cell culture media.
2.7 Cell Culture and Growth Inhibition Assay
Premalignant human oral epithelial cells (SCC-83-01-82) were maintained in modified minimal essential medium (MEM) with 10% fetal bovine serum and 5% antibiotic/antimycotic solution including penicillin (10,000 U/mL), streptomycin (10,000 U/mL), and amphotericin B (25 μg/mL) as previously described 20,21. The characteristics of this cell line have been previously described 22. Cell cultures were incubated at 37 °C in a 90% humidified environment with 5% CO2 atmosphere.
Cells were seeded at a density of 1000 per well in 96-well plates. After 24 hr, the media was replaced to deliver 200 μg extract/well or standards of C3G or PA at concentrations ranging from 3–100 μg/mL using previous work with crude berry product extracts and their isolated components as a guide 23. Control samples were composed of an equivalent amount of 1:1 DMSO/water diluted in MEM. All samples were incubated for 72 hr.
Growth inhibition was determined using a WST-1 assay (Roche; Pleasanton, CA) according to manufacturer instructions. Growth inhibition was calculated as 1 - ((Atrt-Atrt blank)/(Acontrol-Acontrol blank)). Treatment blanks were made by incubating sterile media with the corresponding dose of nectar extracts or phytochemicals in identical conditions as the treated cells. Technical replicates were performed in quadruplicate, while biological replicates were performed in triplicate. Cytotoxic activity was evaluated using the Clontech LDH Cytotoxicity Detection Kit (Mountain View, CA) according to manufacturer instructions. Data were analyzed using the generalized linear model procedure in SAS version 9.4. The data were fitted with an ANOVA model with terms corresponding to nectar incubation time, temperature, and their interaction with significance reported at P<0.05. Differences between treatments were assessed using Tukey’s post hoc test with α = 0.05.
3 Results and Discussion
We report on the phytochemical stability of a BRB nectar over storage using targeted and untargeted metabolomics, and we relate these chemical changes to their bioactive properties on premalignant oral epithelial cell proliferation. The product was a viscous liquid with pH of 3.4 and soluble solids reading of 9 °Brix. Microbial growth observed during storage was below the limit of quantitation (data not shown), indicating that any chemical changes incurred over storage were not due to microbial metabolism. Untargeted metabolomics has been used by others to understand the chemistry of foods in several applications including food authentication, effects of different production practices, the dynamics of fermentation processes, and recently, changes in flavor attributes during storage 24–26. Here we use the technique to understand how the chemistry of BRB nectar, as impacted by storage, may relate to the biological activity of the product.
3.1 Untargeted Metabolomics Revealed Large Chemical Variation with Elevated Storage Temperatures
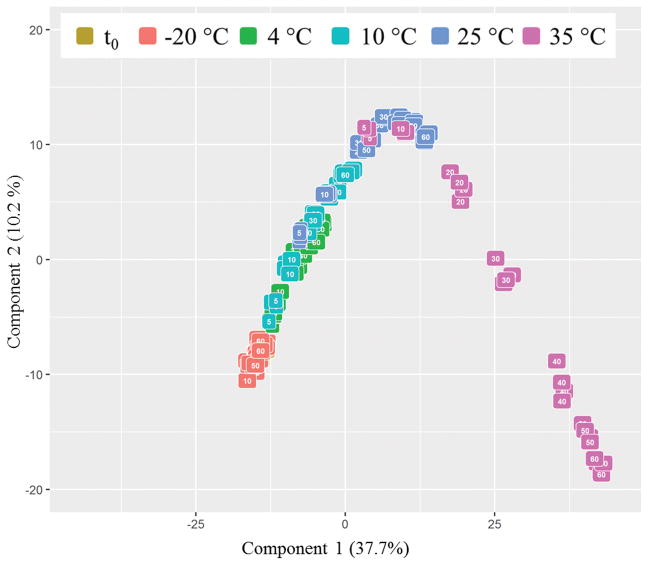
Full scan UHPLC-MS-QTOF data was acquired for all nectar samples. Following the extraction, alignment, binning, and filtering of peaks in the data, a total of 1,712 molecular features were considered for further analyses. Overall trends across the dataset were visualized using principal component analysis (PCA) autofitted to three components. Only the first two comonents are displayed in Figure 2 to simplify data interpretation, as the third component only explained 3.8% of the variation. The scores plot in Figure 2 indicates that the samples stored at −20 °C were relatively stable over 60 days of storage as demonstrated by their close clustering and proximity to the samples from t0. Samples stored at higher temperatures for longer amounts of time were further separated from the t0 samples along the first component, which explained 37.7% of the variation, suggesting that considerable chemical variation was introduced with elevated temperature and time.
Figure 2.
Principal component analysis of all samples colored by storage temperature and labeled according to length of storage.
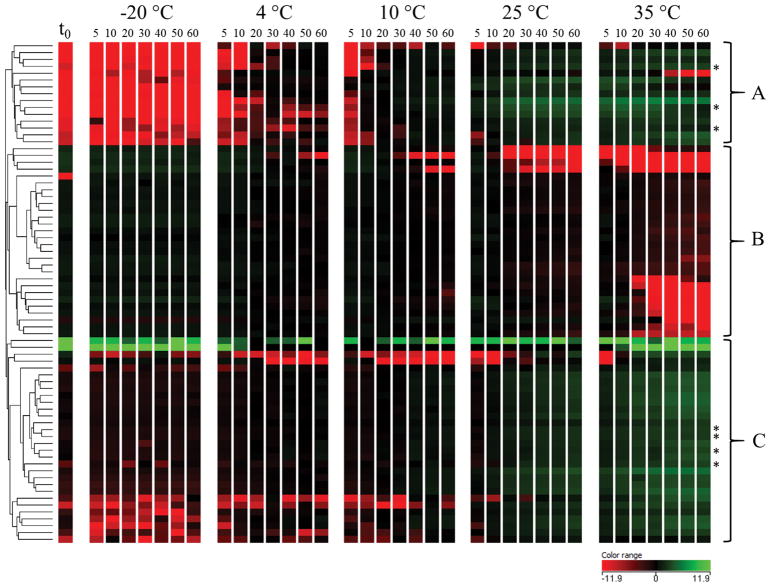
Partial least squares regression (PLS) was used to further understand how chemical profiles of BRB nectars stored at different temperatures changed over time. A separate model for each storage temperature was generated in which relative feature abundances were regressed against storage time, including t0 (Table 2). The model for samples stored at −20 °C was of poor quality (Q2 = 0.165; P = 0.03), indicating that storage time was not a strong predictor of chemical variation in these samples. This further demonstrated the stability of BRB nectar stored at −20 °C for 60 d. The models for samples stored at 4 °C–35 °C all had a Q2 > 0.9 (P = 0.01), which indicated good performance of these models. We focused our analysis on features that were influenced by storage time, regardless of storage temperature, by collating features with VIP ≥ 1 across all four PLS models. Following a manual data quality review, 73 features were found to contribute significantly to all four models. Figure 3 displays the mean relative abundance of these features at each time point across storage conditions. Features were clustered using Euclidian distance metric and Ward’s linkage rule. The heat map demonstrates that these significant features increased and decreased simultaneously at storage temperatures above −20 °C. These relative changes in abundance appeared to be more severe at 25 °C and 35 °C storage, as anticipated. The features in cluster A reflect a pattern of formation by which features were not present at t0 and were created over time, more so at higher temperatures. At 35 °C, some of these features decreased in abundance before day 60, indicating further degradation of these generated compounds. Cluster B contains features that degraded over time, some which degraded after 20 days at 35 °C. The features in cluster C increased in abundance continuously over time with elevated storage temperatures. Many of these features were present at low levels in the t0 samples. These data demonstrate that above −20 °C, the BRB nectar is a system in dynamic chemical flux over 60 days of storage.
Table 2.
PLS model cross validation results
| Storage Temperature | Q2 | P Q2 | RMSEE |
|---|---|---|---|
| −20 °C | 0.165 | 0.03 | 6.98 |
| 4 °C | 0.948 | 0.01 | 1.04 |
| 10 °C | 0.980 | 0.01 | 1.58 |
| 25 °C | 0.919 | 0.01 | 0.997 |
| 35 °C | 0.985 | 0.01 | 2.05 |
Figure 3.
Heat map of molecular features with VIP>1 in PLS models for storage at 4–35 °C. Features were clustered using Euclidian distance metrics and Ward’s linkage rule. * Denotes potential Maillard-related sugar fragmentation-phenolic degradation products determined after derivatization with o-phenylenediamine.
Tentative identifications were generated for some features based on plausible database matches from FooDB (www.foodb.ca), a component of the human metabolome database 27. Identities were confirmed by authentic standards or by collecting additional MS/MS fragmentation data and comparing to published values when authentic standards were unavailable. These techniques correspond to identification levels 1 and 2, respectively, as proposed by the Metabolomics Standard Initiative 28. Table 3 displays features that were identified using these methods, all of which have been previously reported in BRBs 29. Catechin and epicatechin are isomeric flavan-3-ols, and their levels have been shown to decline over storage in other products such as apple juice 30, and a variety of blueberry products 31. B-type procyanidins are oligomers of catechin and/or epicatechin linked by C-C bonds, and have also been reported to be unstable over storage 31. The MS/MS fragmentation patterns of the B-type procyanidins from the current work closely resembled those reported previously 32. PA is a B-ring cleavage product of cyanidin-based anthocyanins that can form as a result of heating or storage, but is also present in fresh BRBs 29,33.
Table 3.
Level 1 and 2 identified compounds from list of features with VIP>1 across all four PLS models
| Compound Name | Molecular Formula | Retention time (min) | [M-H]− | Δppm | Heat map cluster |
|---|---|---|---|---|---|
| Epicatechin1 | C15H14O6 | 3.6 | 289.0718 | 0 | B |
| Catechin1 | C15H14O6 | 3.3 | 289.0718 | 0 | B |
| B-type procyanidin dimer A2 | C30H26O12 | 3.6 | 577.1344 | 1 | B |
| B-type procyanidin dimer B2 | C30H26O12 | 3.4 | 577.1398 | 8 | B |
| Protocatechuic acid1 | C7H6O4 | 2.9 | 153.0196 | 2 | C |
Level 1 identified features,
Level 2 identified features
The lack of plausible database matches for many of the features in clusters A and C in Figure 3 led us to hypothesize that these entities may be uncharacterized degradation products of BRB components. The Maillard reaction is a prevalent reaction between reducing sugars and amino acids that occurs over processing and storage of foods. Intermediates in this reaction include reactive carbonyl species that can form adducts with phenolic compounds, such as epicatechin, in food products 34. Kokkinidou and Peterson demonstrated that phenolic-reactive carbonyl species adducts can be decomposed by derivatization with o-phenylenediamine 35. When o-phenylenediamine was added to BRB nectar extracts, the abundances of 7 features from clusters A and C in Figure 3 were significantly or completely reduced, suggesting that these may be Maillard-related sugar fragmentation-phenolic degradation products.
3.2 All Extracts of Stored BRB Nectar Inhibited SCC-83-01-82 Cell Growth Similarly
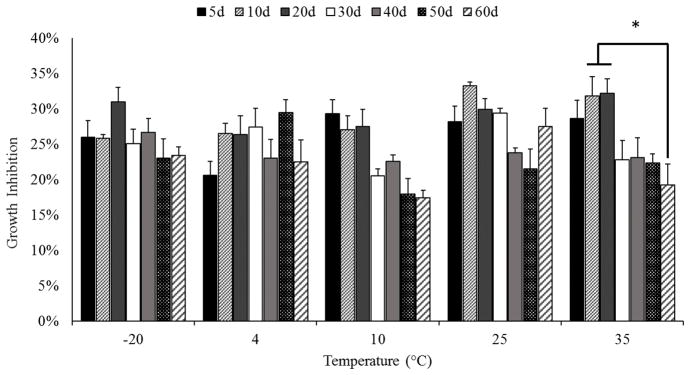
BRB nectar extracts were applied to SCC-83-01-82 premalignant oral epithelial cells to assess the effects of storage time and temperature on their cell growth activities. Extracts of the t0 nectar samples inhibited cell growth 27.8 ± 2.8% with inhibition of the stored samples shown in Figure 4. Data were evaluated using a two-way ANOVA model including terms for nectar storage time, temperature, and their interaction. The terms for time (P < 0.01), temperature (P < 0.01), and their interaction (P<0.0001) were significant. Few significant differences, however, were seen across time within any one storage condition, except for a 13% difference between samples stored for 10, 20, and 60 days at 35 °C (Figure 4). Non-significant trends emerged in the dataset, but were inconsistent among storage conditions. For example, a non-significant decrease in cell growth activity was observed for nectars stored at 10 °C, but this same trend was not maintained with storage at 25 °C. Thus, the capacity of BRB nectar extracts to inhibit SCC-83-01-82 cell growth after 72 hr of incubation was relatively unaffected by nectar storage conditions, despite the large variation seen in the nectar chemical profiles.
Figure 4.
Growth inhibition of SCC-83-01-82 cells by extracts of BRB nectar stored at increasing temperatures. ANOVA terms for storage time, temperature, and their interaction were significant (P<0.01). Only significant differences within each storage temperature are denoted (*).
Few studies have investigated the relationship between storage conditions of berry products, their corresponding chemical profiles, and bioactivity. A decrease in total anthocyanin content was observed over 60 days in blueberry juice produced from two different cultivars and stored at 6 °C and 23 °C. When the anthocyanin fraction of the juice was isolated and applied to HT-29 colorectal adenocarcinoma cells, the authors observed significant decreases in anti-proliferative activity after 30–90 days of storage. Only a slight decrease in anti-proliferative activity was noted in samples stored at 23 °C for 60 days, but it was concluded that storage conditions influenced the anthocyanin profiles and biological activities of the juices 36. The untargeted metabolomics approach employed in the present work aims to elucidate the relationship between the chemical profile and biological activity of a berry product in a more comprehensive way. BRBs contain a complex mixture of phytochemicals, thus it is unlikely that any single chemical component can account for the complete bioactivity of the fruit. For example, feeding whole BRB powder, anthocyanin-rich BRB extract, or anthocyanin-deplete extract all suppressed the growth of tumors to an identical amount in a rat model of esophageal cancer 37. Paudel and colleagues used NMR-based metabolomics to understand the effects of BRB cultivar and degree of ripeness on bioactivity in HT-29 colon cancer cells. They observed a myriad of biologically active BRB components including anthocyanins, other flavonoids, organic acids, and ellagic acid derivatives 38. Our data support these findings in that we observed nominal changes in bioactivity of BRB nectar products with considerably different phytochemical profiles, further demonstrating that a number of phytochemicals are responsible for this bioactivity.
3.3 C3R and its degradation product PA Equally Contribute to the Bioactivity of BRB Nectar
Since the anti-proliferative activity of stored nectars was relatively unchanged despite large changes in chemical profiles, we hypothesized that parent phytochemicals, as well as their degradation products, both contribute to the bioactivity of the product due to similarity in structural motifs. Anthocyanins constitute a large portion of the total polyphenols of BRBs, with C3R as a predominant species 39–41. Given that PA is a reported degradation product of C3R and was identified as an important feature in our PLS models, we focused on these two compounds in a model system as proof-of-concept that parent compounds and their associated degradation products can be complementarily bioactive.
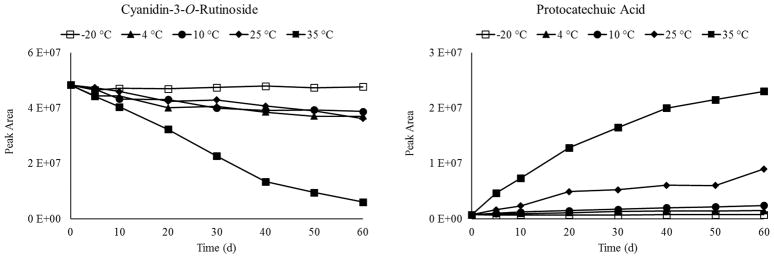
To understand how these two related compounds changed in the nectar over time, we extracted information about their abundances from the untargeted UHPLC-MS-QTOF dataset (Figure 5). The relative change in abundance over time was greater at higher storage temperatures for both compounds, consistent with prior findings on anthocyanin degradation 42. C3R and PA were subsequently quantitated at t0 and 60 d of storage at 35 °C using UHPLC-MS/MS and authentic standard curves (Table 4). Interestingly, C3R decreased by 13.1 nmol/mg, while PA increased by 14.9 nmol/mg during storage, demonstrating that these two bioactive compounds exchanged in near-equimolar amounts in the BRB nectar. It must be noted that anthocyanins can degrade via a multitude of mechanisms to form several different products apart from PA, while PA can also be a degradation product from other phenolics 42
Figure 5.
Averaged relative abundances of C3R and PA over time in each storage condition.
Table 4.
Quantitative analysis of C3R and PA in nectar from t0 and 60 days at 35 °C
| Time (d) | C3R (μg/mg extract) | PCA (μg/mg extract) |
|---|---|---|
| 0 | 8.02 ± 0.20 | 3.27 × 10−2 ± 8.1 × 10−3 |
| 60 | 0.20 ± 5.7 × 10−4 | 2.33 ± 0.13 |
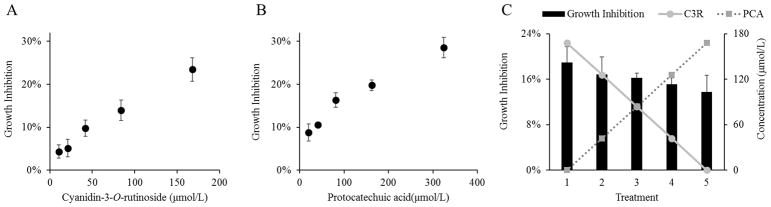
Independently, C3R and PA each inhibited the growth of SCC-83-01-82 cells in a dose-dependent manner (Figure 6A, B), with increasing concentrations corresponding with increased growth inhibition. The growth inhibition by C3R is similar to levels previously reported on cyanidin-3-O-glucoside isolated from strawberries, which inhibited the growth of CAL-27 malignant oral cancer cells by approximately 50% at a level of 100 μg/mL (222 μmol/L) 43. The current work further validates the bioactivity of cyanidin-based anthocyanins to inhibit cell growth in human oral cell lines. The anticancer activity of PA against oral cancer has previously been demonstrated in animal models 44. While the concentrations we tested in vitro are higher than those found in the BRB nectar, our results show that SCC-83-01-82 cells respond to individual treatments of C3R or PA in a dose-dependent manner.
Figure 6.
Dose-response relationship between increasing levels of C3R (A) and PA (B) and growth inhibition of SCC-83-01-82 cells. (C) When the ratio of C3R and PA were varied in equimolar solutions (molarity on right y-axis), growth inhibition (left y-axis) was maintained (P = 0.092 for differences among treatments).
To demonstrate that BRB phytochemicals and their degradation products can each contribute to the biological activity of the nectar, we delivered doses of equal molarity but differing molar ratios of C3R:PA. The conditions used mirror the equimolar exchange of these two compounds observed in the nectar. As shown in Figure 6C, after a starting dose of 170 μmol/L, C3R was reduced by 25% in successive treatments, while in parallel the concentrations of PA were increased in 25% increments to a final treatment dose of 170 μM. A consistent level of growth inhibition was maintained across treatments (P = 0.092 for differences among treatments) despite differing molar ratios of C3R:PCA. Consequently, our data demonstrates that the loss in bioactivity of a parent phytochemical (C3R) may be recovered by increased levels of their degradation products (PA) (Figure 6C). Previous studies with other cancer models have found the ortho-dihydroxyphenyl structural element of some anthocyanidins, such as cyanidin, to be critical for anti-cancer properties of these compounds 45. Our data suggest that this structural moiety, the main molecular structure maintained between C3R and PA, may also play a role in suppressing the growth of SCC-83-01-82 cells. Partial degradation of cyanidin-3-glucoside in cell culture media has been previously reported, with PA as the primary degradation product 46. While this represents an inherent limitation of studying anthocyanins in vitro, it further validates the idea that phytochemical degradation products can maintain active chemical moieties, and thus bioactivity.. We speculate that this phenomenon of degrading phytochemicals while maintaining active chemical moieties occurs on a larger scale with other components of the nectar. Additionally, it is plausible that phytochemicals that remain unchanged throughout storage contribute significantly to bioactivity. And while not addressed in the current study, it is also conceivable that the biochemical signaling and activation mechanisms underlying the growth inhibition shifted with changing nectar chemical profiles. In addition, our bioassay was an in vitro model with oral cells that can be directly exposed to BRB phytochemicals in vivo. Not addressed in this study is the impact that storage-induced changes in BRB phytochemicals affects their bioaccessibility and bioavailability in the remainder of the GI tract, which could have implications for their actions elsewhere in the body.
4. Conclusions
We investigated the impact of storage on the phytochemical stability and bioactivity of a BRB nectar product. Our data demonstrate that nectar stored at −20 °C is chemically stable over 60 days, but storage above this temperature introduces large amounts of chemical variation through a variety of mechanisms including cleavage of phenolic compounds and potential adduct formation with reactive carbonyl species. Despite the large chemical variation observed using untargeted metabolomics, storage conditions had minimal impact on the ability of the nectar to differentially inhibit growth in premalignant oral epithelial cells. Exploration of this phenomenon in vitro supports our hypothesis that degradation products of bioactive phytochemicals also demonstrate bioactivity, allowing maintenance of growth inhibition capacity, through independent, cooperative, or redundant mechanisms. This work demonstrates that BRBs are a complex mixture of compounds with potential anticancer activities. Assigning functional activity to a single black raspberry compound or metabolite fails to explain and appreciate this fluidity, as different compounds increase and decrease with the dynamics of storage. It remains important to dissect these pleiotropic phytochemical bioactives to fully understand the health benefits and consequences of consuming BRBs and their components.
Acknowledgments
The authors would like to thank David M. Phinney, M.S. for his assistance in processing the nectar utilized in this study. This work was supported by USDA-NIFA National Needs Fellowship (2014-38420-21844), the Lisa and Dan Wampler Endowed Fellowship for Food and Health Research and Foods for Health, a focus area of the Discovery Themes Initiative at The Ohio State University.
Abbreviations used
- BRB
black raspberry
- SCC
squamous cell carcinoma
- C3R
cyanidin-3-O-rutinoside
- PA
protocatechuic acid
- PLS
partial least squares
- PCA
principal components analysis
- VIP
variable importance on projection
Footnotes
Conflicts of Interest
The authors have no conflicts of interest to declare.
References
- 1.Kula M, Krauze-Baranowska M. Nutr Cancer. 2016;68:18–28. doi: 10.1080/01635581.2016.1115095. [DOI] [PubMed] [Google Scholar]
- 2.Kresty LA, Mallery SR, Stoner GD. J Berry Res. 2016;6:251–261. doi: 10.3233/JBR-160125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stoner GD. Cancer Prev Res. 2009;2:187–194. doi: 10.1158/1940-6207.CAPR-08-0226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liu RH. Am J Clin Nutr. 2003;78:517S–520S. doi: 10.1093/ajcn/78.3.517S. [DOI] [PubMed] [Google Scholar]
- 5.Warner BM, Casto BC, Knobloch TJ, Accurso BT, Weghorst CM. Oral Surg Oral Med Oral Pathol Oral Radiol. 2014;118:674–683. doi: 10.1016/j.oooo.2014.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Knobloch TJ, Uhrig LK, Pearl DK, Casto BC, Warner BM, Clinton SK, Sardo-Molmenti CL, Ferguson JM, Daly BT, Riedl K, Schwartz SJ, Vodovotz Y, Buchta AJ, Schuller DE, Ozer E, Agrawal A, Weghorst CM. Cancer Prev Res. 2016;9:159–171. doi: 10.1158/1940-6207.CAPR-15-0187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Oghumu S, Casto BC, Ahn-Jarvis J, Weghorst LC, Maloney J, Geuy P, Horvath KZ, Bollinger CE, Warner BM, Summersgill KF, Weghorst CM, Knobloch TJ. Front Immunol. 2017;8 doi: 10.3389/fimmu.2017.01325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mallery SR, Tong M, Shumway BS, Curran AE, Larsen PE, Ness GM, Kennedy KS, Blakey GH, Kushner GM, Vickers AM, Han B, Pei P, Stoner GD. Clin Cancer Res. 2014;20:1910–1924. doi: 10.1158/1078-0432.CCR-13-3159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.El-Bayoumy K, Chen KM, Zhang SM, Sun YW, Amin S, Stoner G, Guttenplan JB. Chem Res Toxicol. 2017;30:126–144. doi: 10.1021/acs.chemrestox.6b00306. [DOI] [PubMed] [Google Scholar]
- 10.Petersen PE, Bourgeois D, Ogawa H, Estupinan-Day S, Ndiaye C. Bull World Health Organ. 2005;83:661–669. [PMC free article] [PubMed] [Google Scholar]
- 11.Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. Int J Cancer. 2015;136:E359–E386. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 12.Bsoul S, Huber MA, Terezhalmy GT. J Contemp Dent Pract. 2005;6:1–16. [PubMed] [Google Scholar]
- 13.Casto BC, Knobloch TJ, Weghorst CM. In: Berries and Cancer Prevention. Stoner GD, Seeram N, editors. Springer Science+Business Media; New York, NY: 2011. pp. 189–207. [Google Scholar]
- 14.Howard LR, Prior RL, Liyanage R, Lay JO. J Agric Food Chem. 2012;60:6678–6693. doi: 10.1021/jf2046575. [DOI] [PubMed] [Google Scholar]
- 15.Hager A, Howard LR, Prior RL, Brownmiller C. J Food Sci. 2008;73:H134–H140. doi: 10.1111/j.1750-3841.2008.00855.x. [DOI] [PubMed] [Google Scholar]
- 16.Gu J, Ahn-Jarvis J, Riedl KM, Schwartz SJ, Clinton SK, Vodovotz Y. J Agric Food Chem. 2014;62:3997–4006. doi: 10.1021/jf404566p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Manach C, Hubert J, Llorach R, Scalbert A. Mol Nutr Food Res. 2009;53:1303–1315. doi: 10.1002/mnfr.200800516. [DOI] [PubMed] [Google Scholar]
- 18.Thévenot EA, Roux A, Xu Y, Ezan E, Junot C. J Proteome Res. 2015;14:3322–3335. doi: 10.1021/acs.jproteome.5b00354. [DOI] [PubMed] [Google Scholar]
- 19.Kemsley EK, Le Gall G, Dainty JR, Watson AD, Harvey LJ, Tapp HS, Colquhoun IJ. Br J Nutr. 2007;98:1–14. doi: 10.1017/S0007114507685365. [DOI] [PubMed] [Google Scholar]
- 20.Han C, Ding H, Casto B, Stoner GD, D’Ambrosio SM. Nutr Cancer. 2005;51:207–217. doi: 10.1207/s15327914nc5102_11. [DOI] [PubMed] [Google Scholar]
- 21.Ding H, Han C, Guo D, Chin YW, Ding Y, Kinghorn AD, D’Ambrosio SM. Nutr Cancer. 2009;61:348–356. doi: 10.1080/01635580802567158. [DOI] [PubMed] [Google Scholar]
- 22.Lee H, Li D, Prior T, Casto BC, Weghorst CM, Shuler CF, Milo GE. Med Biochem. 1997;13:419–434. doi: 10.1023/a:1007419810705. [DOI] [PubMed] [Google Scholar]
- 23.Bishayee A, Haskell Y, Do C, Siveen KS, Mohandas N, Sethi G, Stoner GD. Crit Rev Food Sci Nutr. 2016;56:1753–1775. doi: 10.1080/10408398.2014.982243. [DOI] [PubMed] [Google Scholar]
- 24.Cevallos-Cevallos JM, Reyes-De-Corcuera JI, Etxeberria E, Danyluk MD, Rodrick GE. Trends Food Sci Technol. 2009;20:557–566. [Google Scholar]
- 25.Ronningen I, Miller M, Xia Y, Peterson DG. J Agric Food Chem. doi: 10.1021/acs.jafc.7b00093. ePub ahead. [DOI] [PubMed] [Google Scholar]
- 26.Ronningen I, Peterson DG. J Agric Food Chem. 2018;66:682–688. doi: 10.1021/acs.jafc.7b04450. [DOI] [PubMed] [Google Scholar]
- 27.Wishart DS, Jewison T, Guo AC, Wilson M, Knox C, Liu Y, Djoumbou Y, Mandal R, Aziat F, Dong E, Bouatra S, Sinelnikov I, Arndt D, Xia J, Liu P, Yallou F, Bjorndahl T, Perez-Pineiro R, Eisner R, Allen F, Neveu V, Greiner R, Scalbert A. Nucleic Acids Res. 2013;41:801–807. doi: 10.1093/nar/gks992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sumner LW, Samuel T, Noble R, Gmbh SD, Barrett D, Beale MH, Hardy N, Harnly J, Higashi R, Kopka J, Lane AN, Lindon J, Marriott P, Nicholls AW, Reily MD, Thaden JJ, Viant MR. Metabolomics. 2007;3:211–221. [Google Scholar]
- 29.Kula M, Majdan M, Głód D, Krauze-Baranowska M. J Food Compos Anal. 2016;52:74–82. [Google Scholar]
- 30.Van Der Sluis AA, Dekker M, Van Boekel MAJS. J Agric Food Chem. 2005;53:1073–1080. doi: 10.1021/jf040270r. [DOI] [PubMed] [Google Scholar]
- 31.Brownmiller C, Howard LR, Prior RL. J Agric Food Chem. 2009;57:1896–1902. doi: 10.1021/jf803015s. [DOI] [PubMed] [Google Scholar]
- 32.Gu L, Kelm MA, Hammerstone JF, Beecher G, Holden J, Haytowitz D, Prior RL. J Agric Food Chem. 2003;51:7513–7521. doi: 10.1021/jf034815d. [DOI] [PubMed] [Google Scholar]
- 33.Stintzing FC, Carle R. Trends Food Sci Technol. 2004;15:19–38. [Google Scholar]
- 34.Totlani VM, Peterson DG. J Agric Food Chem. 2005;53:4130–4135. doi: 10.1021/jf050044x. [DOI] [PubMed] [Google Scholar]
- 35.Kokkinidou S, Peterson DG. Food Funct. 2013;4:1093. doi: 10.1039/c3fo60032g. [DOI] [PubMed] [Google Scholar]
- 36.Srivastava A, Akoh CC, Yi W, Fischer J, Krewer G. J Agric Food Chem. 2007;55:2705–2713. doi: 10.1021/jf062914w. [DOI] [PubMed] [Google Scholar]
- 37.Wang L, Hecht SS, Carmella SG, Yu N, Larue B, Henry C, Mcintyre C, Rocha C, Lechner JF, Stoner GD. Cancer Prev Res. 2009;2:84–93. doi: 10.1158/1940-6207.CAPR-08-0155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Paudel L, Wyzgoski FJ, Giusti MM, Johnson JL, Rinaldi PL, Scheerens JC, Chanon AM, Bomser JA, Miller AR, Hardy JK, Reese RN. J Agric Food Chem. 2014;62:1989–1998. doi: 10.1021/jf404998k. [DOI] [PubMed] [Google Scholar]
- 39.Rothwell JA, Perez-Jimenez J, Neveu V, Medina-Remón A, M’Hiri N, García-Lobato P, Manach C, Knox C, Eisner R, Wishart DS, Scalbert A. Database. 2013;2013:1–8. doi: 10.1093/database/bat070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tulio AZ, Reese RN, Wyzgoski FJ, Rtnaldi PL, Fu R, Scheerens JC, Miller AR. J Agric Food Chem. 2008;56:1880–1888. doi: 10.1021/jf072313k. [DOI] [PubMed] [Google Scholar]
- 41.Torre LC, Barritt BH. J Food Sci. 1977;42:488–490. [Google Scholar]
- 42.Patras A, Brunton NP, O’Donnell C, Tiwari BK. Trends Food Sci Technol. 2010;21:3–11. [Google Scholar]
- 43.Zhang YJ, Seeram NP, Lee R, Feng L, Heber D. J Agric Food Chem. 2008;56:670–675. doi: 10.1021/jf071989c. [DOI] [PubMed] [Google Scholar]
- 44.Tanaka T, Tanaka T, Tanaka M. J Exp Clin Med. 2011;3:27–33. [Google Scholar]
- 45.Hou DX, Kai K, Li JJ, Lin S, Terahara N, Wakamatsu M, Fujii M, Young MR, Colburn N. Carcinogenesis. 2003;25:29–36. doi: 10.1093/carcin/bgg184. [DOI] [PubMed] [Google Scholar]
- 46.Kay CD, Kroon PA, Cassidy A. Mol Nutr Food Res. 2009;53:92–101. doi: 10.1002/mnfr.200800461. [DOI] [PubMed] [Google Scholar]