Abstract
Purpose
To determine if women with overactive bladder (OAB) requiring 3rd line therapy demonstrate greater central sensitization, indexed by temporal summation to heat pain stimuli, than those with OAB.
Materials and Methods
We recruited 39 adult women with OAB from the Urology clinic who were planning to undergo interventional therapy for medication refractory OAB with either onabotulinumtoxinA bladder injection or sacral neuromodulation and 55 women with OAB, either newly seen in our Urology clinic or responding to advertisements for study participation. Participants underwent quantitative sensory testing using a thermal temporal summation protocol. The primary study outcome was the degree of temporal summation, as reflected in the magnitude of positive slope of the line fitted to the series of 10 stimuli at a 49°C target temperature. We compared the degree of temporal summation between study groups using linear regression.
Results
Women in the group undergoing 3rd line therapy demonstrated significantly higher standardized temporal summation slopes compared to those in the nontreatment group (beta = 1.57, 95% confidence interval = .18 - 2.96, t = 2.25, p = .027). On exploratory analyses, a history of incontinence surgery or hysterectomy were factors associated with significantly greater temporal summation.
Conclusions
In this study, the degree of temporal summation was elevated in women undergoing 3rd line OAB therapy compared to women with OAB not undergoing 3rd line therapy. These findings suggest there may be pathophysiologic differences, specifically in afferent nerve function and processing, in some women with OAB.
Mesh Terms/Key Words: Overactive Bladder, Urge Incontinence, Central Sensitization, Temporal Summation
Introduction
Overactive Bladder (OAB) is a common condition affecting U.S. women.1 However, the etiology of OAB remains unclear and likely involves both inherent elements of the bladder and functional aspects of motor and sensory nerve innervation.2 In line with the afferent nerve hypothesis,2 it is plausible that any condition which promotes increased responses to normal or abnormal stimuli may contribute to the development of or exacerbate existing OAB. We have postulated that central sensitization (CS), a phenomenon well-described in the chronic pain literature in which afferent C-fiber circuits display hyper-responsiveness,3 may play such a role in certain individuals with OAB.1
We have previously reported that women with OAB requiring 3rd line therapy with either onabotulinumtoxinA bladder injection or sacral neuromodulation exhibited greater thermal cutaneous temporal summation (TS; increasing perceived pain in response to rapid repetition of the same stimulus intensity) than women without OAB.4 Because elevated TS is believed to be a marker for CS,5,6 this finding suggested elevated CS in OAB patients when compared to non-OAB controls. Our present hypothesis is that the presence of CS may contribute to why some women with OAB are more likely to be refractory to first- and second-line OAB therapy than others. As the next step in this line of investigation, the aim of the current study was to determine whether women with OAB undergoing 3rd line therapy would exhibit greater TS than women with OAB not undergoing these treatments.
Materials and Methods
After obtaining Institutional Review Board approval, we recruited 39 adult (18 or older) women with OAB from the Urology clinic who were planning to undergo either onabotulinumtoxinA bladder injection or sacral neuromodulation and 55 women with OAB (confirmed with a score of ≥4 on the OAB-V3 awareness tool7) either newly seen in our Urology clinic or responding to community advertisements, who were not undergoing 3rd line therapy. We excluded women if they had diagnoses of neurologic conditions that might contribute to their urinary symptoms (e.g. spinal cord injury, multiple sclerosis, stroke, autonomic dysfunction), had a history of bladder cancer, pelvic irradiation, or bowel diversions, or were unable or unwilling to complete all study protocols. We also excluded women with interstitial cystitis/bladder pain syndrome, based on medical record review and/or whether they met the Rand Interstitial Cystitis Epidemiology case definition.8
Participants completed a standardized questionnaire assessing demographics and medical history, including: age, race/ethnicity, highest level of education, general health, prior history of incontinence surgery, hysterectomy, or prolapse surgery, and whether they were taking OAB medications. We assessed pelvic surgical history with yes or no questions: “Have you ever had…?: a hysterectomy, an operation to remove your uterus or womb; surgery for incontinence (urine leakage); surgery for repair of pelvic organ prolapse (pelvic floor disorder, cystocele, rectocele).”
Participants also completed the Overactive Bladder Questionnaire9 and the International Consultation on Incontinence Modular Questionnaire-Female Lower Urinary Tract Symptoms (ICIQ-FLUTS).10 To assess psychosocial and somatic characteristics, participants completed the NIH Patient-Reported Outcomes Measurement Information System (PROMIS) short form v1.0 instruments for depression (8a), anxiety (8a) and pain intensity (3a), as well as the Somatic Symptom Scale-8.11 We also assessed the presence of co-morbid functional somatic conditions by self-report (i.e. low back pain)or validated patient-reported diagnostic measures, including migraine headache;12 fibromyalgia;13 and irritable bowel syndrome.14
Quantitative Sensory Testing
All quantitative sensory testing protocols used a Medoc TSAII NeuroSensory Analyzer (Medoc U.S., Minneapolis, MN) with a 9-cm2 peltier thermode applied to the volar forearm. To determine heat pain threshold and tolerance levels, a series of four pain trials was conducted, during which the probe temperature increased from a baseline temperature of 32°C at a rate of 0.5°C per second. For the heat pain threshold test, the participant indicated the temperature at which the heat was first perceived as “painful”, while for the heat pain tolerance trials, the participant indicated when the pain became “intolerable.” Means for the four threshold and tolerance trials were then separately derived.
Our quantitative sensory techniques to measure TS are similar to those that are standard in the pain literature6,15 and have been previously detailed in the context of OAB.4 Briefly, we administered a sequence of 10 successive 0.5 second heat pulses to the forearm, during which the temperature rapidly increased and decreased from a temperature of 40°C to 49°C at a frequency of 0.4 Hz, a frequency known to elicit C-fiber mediated temporal summation in the dorsal horn of the spinal cord. Immediately after the peak of every heat pulse, subjects provided a verbal numeric pain intensity rating using a 0 – 100 visual analog scale (VAS) anchored with 0 = “No Pain or Warmth” and 100 = “Worst Possible Pain.” The standardized slope of change in pain ratings over the series of 10 stimuli was derived for each patient as an index of TS. A positive slope (i.e., slope > 0) demonstrates the presence of TS, while a negative slope (i.e., slope < 0) denotes habituation.
Statistical analyses
Statistical analyses were performed using Stata/SE 14.1 (StataCorp, College Station, TX). We used Student's t-tests to compare continuous variables and Chi-Squared tests for categorical variables. Our independent variable in all primary analyses was OAB group (OAB vs. OAB undergoing 3rd line therapy). We created linear regression models with the pain response index as the dependent variable (i.e. pain threshold, tolerance, or TS), OAB group as the independent variable, and age and OABq score as control variables. For the analysis of TS, we also included the initial VAS pain rating for the first TS stimulus to control for confounding due to baseline effects on observed slopes (i.e., ceiling effects). Finally, we explored possible factors that may be associated with observed degree of TS using linear regression models, in which the slope of TS was the dependent variable. Analyses used a two-tailed P < 0.05 criterion for statistical significance.
Results
Participant characteristics
Study participants who were undergoing 3rd line OAB therapy were older, slightly less educated and reported less general good health, while they did not differ by race/ethnicity (See Table 1). More of the women in the 3rd line therapy group had undergone prior pelvic surgery, although specific details on these procedures were not available, and significantly more were taking OAB medications. Urinary symptoms were more severe in the 3rd line therapy group, as reflected in a higher mean OABq symptom scores, total ICIQ FLUTS scores and ICIQ urge incontinence scores, with this group also reporting lower mean OABq quality of life scores (Table 2).
Table 1.
Demographic and clinical information comparing women with OAB and those with OAB undergoing 3rd line OAB therapy. Data presented a mean (95% confidence interval) or number (%).
| OAB | 3rd Line OAB | P Value | |
|---|---|---|---|
| Number of women | 55 | 39 | |
| Age | 47.0 (43.2 - 50.8) | 56.3 (51.4 - 61.3) | 0.0029 |
| Race/ethnicity | |||
| Non-Hispanic White | 44 (80.0) | 29 (74.4) | 0.615 |
| Non-Hispanic Black | 9 (16.4) | 10 (25.6) | |
| Asian | 1 (1.8) | 0 | |
| Hispanic | 1 (1.8) | 0 | |
| Education | |||
| 9th to 11th grade | 0 | 2 (5.1) | 0.002 |
| High school | 2 (3.6) | 11 (28.2) | |
| Some college | 17 (30.9) | 12 (30.8) | |
| College Graduate | 25 (45.5) | 9 (23.1) | |
| Graduate or professional | 11 (20.) | 5 (12.8) | |
| General Health | |||
| Excellent | 4 (7.3) | 4 (10.3) | 0.018 |
| Very good | 30 (54.6) | 11 (28.2) | |
| Good | 17 (30.9) | 13 (33.3) | |
| Fair | 3 (5.5) | 10 (25.6) | |
| Poor | 1 (1.8) | 1 (2.6) | |
| Prior Pelvic Surgery | |||
| Incontinence Surgery | 2 (3.6) | 13 (33.3) | <.001 |
| Hysterectomy | 15 (27.3) | 21 (53.9) | 0.011 |
| Prolapse Surgery | 3 (5.5) | 6 (15.4) | 0.156 |
| Current OAB medication | 5 (9) | 20 (51) | <.001 |
| Anticholinergic medication | 4 (4) | 17 (44) | <.001 |
Table 2.
Urinary symptoms and co-morbidity compared between groups. Data presented as mean (95% confidence interval) or number (%)
| OAB | 3rd Line OAB | P Value | |
|---|---|---|---|
| OABq Symptom Score | 43.5 (38.1 - 48.8) | 61.7 (54.8 - 68.7) | <.001 |
| OABq Quality of Life | 71.5 (65.5 - 77.4) | 45.9 (37.6 - 54.2) | <.001 |
| ICIQ-FLUTS Total Score | 14.4 (12.6 - 16.1) | 19.4 (17.1 - 21.7) | <.001 |
| Urge Incontinence* | 1.7 (1.4 – 1.9) | 2.5 (2.1 – 2.8) | <.001 |
| Stress incontinence* | 1.8 (1.5 - 2.2) | 1.9 (1.4 - 2.3) | 0.8 |
| Somatic Symptom Scale | 7.8 (6.2 - 9.3) | 10.2 (8.2 - 12.3) | 0.052 |
| PROMIS Anxiety | 51.2 (49.1 - 53.3) | 50.8 (47.8 - 53.8) | 0.81 |
| PROMIS Depression | 46.7 (44.7 - 48.7) | 47.4 (45.0 - 49.8) | 0.65 |
| PROMIS Pain intensity | 42.1 (39.5 - 44.6) | 44.1 (40.6 - 47.6) | 0.33 |
| Fibromyalgia | 2 (4) | 5 (14) | 0.11 |
| Irritable Bowel Disease | 6 (11) | 4 (10) | 1 |
| Migraine Headaches | 13 (24) | 11 (28) | 0.6 |
| Chronic Low Back Pain | 23 (41) | 21 (54) | 0.3 |
Abbr: OABq – Overactive Bladder Questionnaire; ICIQ-FLUTS – International Consultation on Incontinence Questionnaire Female Lower Urinary Tract Symptoms.
Measured by individual items from the ICIQ-FLUTS questionnaire
Heat Pain Threshold and Tolerance
The mean (95% confidence interval) thermal pain threshold and tolerance temperatures were similar between the women undergoing versus not undergoing 3rd line OAB therapy: 44.0 (43.2 - 44.8) vs. 44.3 (43.2 - 45.4) and 47.4 (46.9 - 47.8) vs.47.3 (46.8 - 47.8), respectively. After adjustment for age and OABq symptom scores, neither pain threshold nor pain tolerance levels differed between the two groups (both p's>.10; data not shown).
Temporal Summation
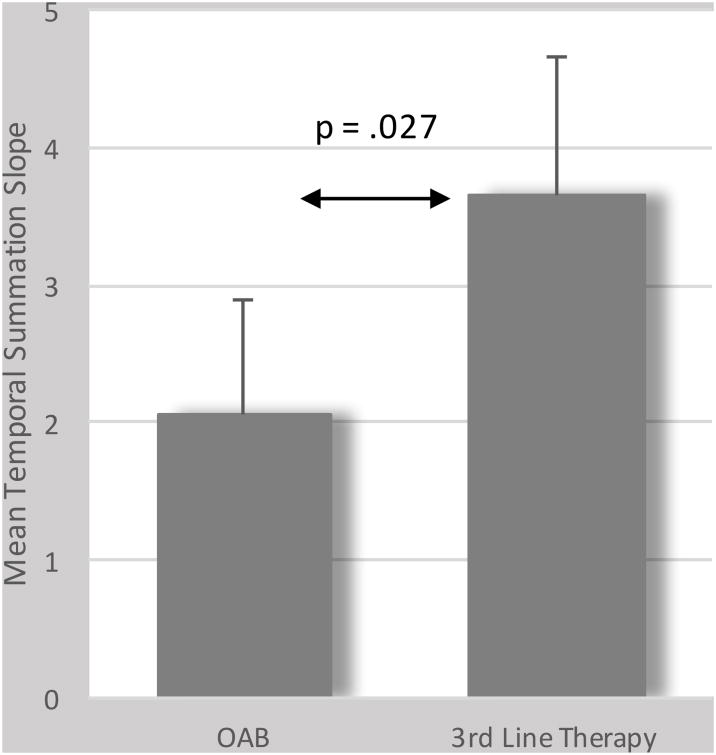
During the TS protocol, the mean (95% CI) VAS pain intensity ratings in response to application of the first heat pain stimulus were similar for the OAB group and the 3rd line therapy group [35.8 (27.9 - 43.6) vs. 39.3 (31.0 - 47.5), respectively]. In the OAB group, four subjects (7%) displayed negative slopes, four (7%) exhibited no change in pain ratings, and 47 (86%) displayed positive slopes (range 0.1–9.4). In the 3rd line therapy group, four subjects (10%) displayed negative slopes, one (3%) exhibited no change, and 34 (87%) displayed positive slopes (range 0.1–13.0). The unadjusted mean ± standard deviation of the standardized TS slopes was significantly greater for the 3rd line treatment group compared to the OAB only group (3.5 ± 3.8 vs. 2.2 ± 2.4, respectively, t = -2.11, df = 92, p = .037). Even after we controlled for the group differences in age, OABq scores, and initial VAS pain intensity rating in our linear regression model, this difference in TS slopes between groups was preserved (beta = 1.57, 95% confidence interval = .18 – 2.96, t = 2.25, p = .027) (see Figure 1).
Figure 1.
Comparison of means of temporal summation slopes between women with OAB undergoing and not undergoing 3rd line therapy. Bar represents the mean, adjusted for age, OAB symptom severity, and initial VAS pain scores during the temporal summation protocol while the stem represents the standard deviation.
Exploratory analyses
We examined several factors that may be associated with elevated TS across the two treatment groups (see Table 3). For these analyses, we excluded participants with a negative slope (n=8), as these individuals, by definition, did not exhibit TS. In addition, because of the recognized potential for ceiling effects on TS slopes due to higher initial VAS pain ratings in some individuals and the potential confounding due to age, we adjusted for these variables in the analyses. Symptom severity, either as an aggregate score (e.g. OABq symptom score) or as individual symptoms (e.g. urgency, urge incontinence, etc.), was, in general, not associated with degree of TS, with the exception of a positive association between TS and bladder pain (beta = .78, 95% CI: .02, 1.54). PROMIS anxiety scores (beta = .09, 95% CI: .02, .16) were also associated with increased TS, while somatic symptom burden, co-morbid conditions, and PROMIS depression and overall bodily pain intensity scores were not. A prior self-reported history of incontinence surgery (beta = 1.96; 95% CI: .14, 3.79) and hysterectomy (beta = 2.07; 95% CI: .38, 3.75) were both associated with increased TS.
Table 3.
Exploratory analyses of factors potentially influencing temporal summation independent of OAB treatment group, adjusted for age and initial pain rating. Significant results in bold.
| Beta coefficient (95% CI) | |
|---|---|
| Age | .02 (-.01, .06) |
| First Pain | -.03 (-.05, -.01) |
| OABq Symptom Score | .02 (-.01, .04) |
| OABq Quality of Life Score | -.01 (-.04, .01) |
| ICIQFLUTS total score | .08 (0, .17) |
| ICIQFLUTS Incontinence subscale | .05 (-.07, .17) |
| Nocturia* | .55 (-.05, 1.15) |
| Urgency* | .01 (-.64, .66) |
| Bladder pain* | .78 (.02, 1.54) |
| Frequency* | .30 (-.12, .72) |
| Urge incontinence* | -.04 (-.62, .54) |
| Stress incontinence* | .02 (-.44, .49) |
| Somatic Symptom Scale | .09 (-.01, .19) |
| PROMIS Anxiety | .09 (.02, .16) |
| PROMIS Depression | .07 (-.01, .15) |
| PROMIS Pain intensity | .02 (-.04, .08) |
| Incontinence surgery | 2.16 (.53, 3.79) |
| Prolapse surgery | -.34 (-2.37, 1.69) |
| Hysterectomy | 1.85 (.47, 3.23) |
| OAB medication use | 1.13 (-.19, 2.44) |
| Fibromyalgia | 2.25 (-.09, 4.58) |
| Irritable Bowel Disease | -.28 (-2.35, 1.80) |
| Migraine Headaches | .07 (-1.38, 1.53) |
| Chronic Low Back Pain | .25 (-1.01, 1.50) |
Abbreviations: OABq – Overactive Bladder Questionnaire; ICIQ-FLUTS – International Consultation on Incontinence Questionnaire Female Lower Urinary Tract Symptoms.
Measured by individual items from the ICIQ-FLUTS questionnaire
Discussion
In this study of women with OAB, we documented increased levels of TS to heat stimuli in a group of women who have failed first and second line therapies and are electing 3rd line OAB therapy. Because elevated TS is an indicator of increased CS, this finding suggests that some women undergoing advanced therapy for OAB may have a component of CS that contributes to the pathophysiology/nature of their bladder dysfunction. On an individual level, further investigation is needed to identify particular characteristics that may be associated with this finding of elevated TS.
We have previously postulated that CS may play a role in the pathophysiology of overactive bladder in some women, even though OAB is generally not associated with pain.1 CS is an induced state of spinal hypersensitivity and a well-recognized mechanism of centrally amplified pain perception, facilitating afferent signaling. Repetitive activation of afferent C fibers results in conditioning of second-order neurons in the dorsal horn of the spinal cord that then amplify afferent signals from low-threshold Aβ and Aδ mechanoreceptors and nociceptive C-fibers.3 Hypersensitive spinal neurons demonstrate reduced firing thresholds, increased receptive field sizes, persistent stimulus-independent activity, and greater evoked responses.16 In this context of CS, stimuli that generally do not provoke pain can produce pain (i.e. allodynia) and stimuli that normally provoke pain can produce pain at higher intensity (i.e. hyperalgesia). Sensory sensitivity can also be increased even without pain (i.e. hyperesthesia).
Changes related to CS like those described above may parallel to some degree the mechanisms proposed for OAB pathophysiology.2,17,18 OAB pathophysiology is thought to involve afferent nervous system dysfunction, reflected in either abnormally increased afferent signals from the bladder and/or decreased capacity to handle afferent signals in the central nervous system.2 Hypersensitive spinal neurons in OAB patients, like those seen in CS, could amplify bladder afferent signals, even from low- or sub-threshold mechanoreceptors like those involved in normal bladder homeostasis, resulting in increased bladder sensitivity.18 While the perception of pain, which is typically used to describe the effects of CS in chronic pain disorders (i.e. allodynia and hyperalgesia) may not be specifically relevant to the bladder in OAB, the afferent sensitivity inherent in CS may be very relevant for non-painful bladder sensations (i.e. hyperesthesia) that are the hallmark of OAB.
The exploratory findings of possible links between pelvic surgery and elevated levels of TS is mechanistically intriguing, although preliminary. As noted, CS is believed to develop in response to sustained C-fiber input, typically associated with tissue injury and pain.3 We speculate that pelvic surgery, and the local tissue trauma and pain associated with such surgery, in some patients might be the trigger eliciting initial development of CS. Once developed, this CS could enhance responsiveness to non-painful bladder stimuli, and thereby contribute to OAB symptomatology that is more resistant to first and second-line treatments. Determining whether this hypothesis is correct will require replication and additional investigation of these issues. Unfortunately, lack of detailed information on the specific characteristics of prior pelvic surgeries makes it difficult to fully interpret these findings in the current work.
An important finding in this study is that while we demonstrated differences in TS between those with OAB and those planning to undergo 3rd line OAB therapy, we did not find any differences in thermal pain threshold or tolerance. The TS protocol used in this study is intended to be relatively specific to CS, whereas this is not the case for the protocols used to assess pain threshold or tolerance. Thus, our findings suggest a specific difference in CS rather than a difference in global pain responsiveness. A question can be raised regarding whether evaluating TS, pain threshold, and pain tolerance in the forearm, as in the current work, would be expected to be sensitive to afferent changes affecting the bladder. Because CS represents an alteration in centrally-mediated afferent processing, it is by definition centralized and should influence ascending afferent input from multiple dermatomes. Its effect should be observable in multiple body areas and not just at the level of clinical symptoms, in this case, the bladder. Applying the stimuli at a dermatome (i.e., the forearm) spatially removed from that of the bladder (i.e. the suprapubic area) follows a widely used and validated approach that has successfully demonstrated elevated TS in various chronic pain conditions relative to controls when tested well outside of the location of the clinical pain.19 However, further study is needed to examine the impact of segmental changes in afferent processing in women with OAB.
Considering limitations to our study, while the sample size is adequate to detect group differences, larger numbers of patients are needed to better phenotype women with OAB and clarify determinants of CS. Second, our study is cross-sectional, so we are unable to determine the causal relationship between OAB symptoms and elevated TS, such as whether CS that appears related to OAB reflects a pre-existing condition possibly related to some prior bladder or pelvic insult. Lastly, our study focused on TS and to a lesser extent, hyperalgesia, using thermal pain stimulation. It is unknown whether other stimulus modalities, such as mechanical pain stimuli, or psychophysical laboratory tests capturing other aspects of pain modulatory processes, such as conditioned pain modulation, would demonstrate findings consistent with ours.
Conclusions
In this study, the degree of TS was elevated in women undergoing 3rd line OAB therapy compared to women with OAB not undergoing those treatments. As there are no established objective markers that identify individuals with OAB or pathophysiologic mechanisms that may underlie OAB symptoms, our findings that some women with OAB do demonstrate CS may represent an important, preliminary step towards advancing the care of women with OAB. Future studies will need to address whether TS can identify patients early in the OAB disease process, specifically those who may not respond adequately to non-invasive treatment options and may need 3rd line therapy.
Acknowledgments
Research reported in this publication was supported by National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health; Grant number: K23DK103910; Grant sponsor: Society of Urodynamics, Female Pelvic Medicine and Urogenital Reconstruction Foundation; Grant sponsor: National Center for Advancing Translational Sciences; Grant number: UL1TR000445
References
- 1.Reynolds WS, Dmochowski R, Wein A, Bruehl S. Does central sensitization help explain idiopathic overactive bladder? Nature reviews Urology. 2016;13:481–91. doi: 10.1038/nrurol.2016.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chapple C. Chapter 2: Pathophysiology of neurogenic detrusor overactivity and the symptom complex of “overactive bladder”. Neurourology and urodynamics. 2014;33(3):S6–13. doi: 10.1002/nau.22635. [DOI] [PubMed] [Google Scholar]
- 3.Woolf CJ. Central sensitization: implications for the diagnosis and treatment of pain. Pain. 2011;152:S2–15. doi: 10.1016/j.pain.2010.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Reynolds WS, Brown ET, Danford J, et al. Temporal summation to thermal stimuli is elevated in women with overactive bladder syndrome. Neurourology and urodynamics. 2017;36:1108–12. doi: 10.1002/nau.23059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cruz-Almeida Y, Fillingim RB. Can quantitative sensory testing move us closer to mechanism-based pain management? Pain medicine. 2014;15:61–72. doi: 10.1111/pme.12230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Anderson RJ, Craggs JG, Bialosky JE, et al. Temporal summation of second pain: variability in responses to a fixed protocol. Eur J Pain. 2013;17:67–74. doi: 10.1002/j.1532-2149.2012.00190.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Coyne KS, Margolis MK, Bavendam T, Roberts R, Elinoff V. Validation of a 3-item OAB awareness tool. International journal of clinical practice. 2011;65:219–24. doi: 10.1111/j.1742-1241.2010.02561.x. [DOI] [PubMed] [Google Scholar]
- 8.Berry SH, Elliott MN, Suttorp M, et al. Prevalence of symptoms of bladder pain syndrome/interstitial cystitis among adult females in the United States. The Journal of urology. 2011;186:540–4. doi: 10.1016/j.juro.2011.03.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Coyne K, Revicki D, Hunt T, et al. Psychometric validation of an overactive bladder symptom and health-related quality of life questionnaire: the OAB-q. Quality of life research : an international journal of quality of life aspects of treatment, care and rehabilitation. 2002;11:563–74. doi: 10.1023/a:1016370925601. [DOI] [PubMed] [Google Scholar]
- 10.Jackson S, Donovan J, Brookes S, Eckford S, Swithinbank L, Abrams P. The Bristol Female Lower Urinary Tract Symptoms questionnaire: development and psychometric testing. British journal of urology. 1996;77:805–12. doi: 10.1046/j.1464-410x.1996.00186.x. [DOI] [PubMed] [Google Scholar]
- 11.Gierk B, Kohlmann S, Kroenke K, et al. The somatic symptom scale-8 (SSS-8): a brief measure of somatic symptom burden. JAMA Intern Med. 2014;174:399–407. doi: 10.1001/jamainternmed.2013.12179. [DOI] [PubMed] [Google Scholar]
- 12.Lipton RB, Dodick D, Sadovsky R, et al. A self-administered screener for migraine in primary care: The ID Migraine validation study. Neurology. 2003;61:375–82. doi: 10.1212/01.wnl.0000078940.53438.83. [DOI] [PubMed] [Google Scholar]
- 13.Wolfe F, Clauw DJ, Fitzcharles MA, et al. The American College of Rheumatology preliminary diagnostic criteria for fibromyalgia and measurement of symptom severity. Arthritis care & research. 2010;62:600–10. doi: 10.1002/acr.20140. [DOI] [PubMed] [Google Scholar]
- 14.Longstreth GF, Thompson WG, Chey WD, Houghton LA, Mearin F, Spiller RC. Functional bowel disorders. Gastroenterology. 2006;130:1480–91. doi: 10.1053/j.gastro.2005.11.061. [DOI] [PubMed] [Google Scholar]
- 15.Dengler-Crish CM, Bruehl S, Walker LS. Increased wind-up to heat pain in women with a childhood history of functional abdominal pain. Pain. 2011;152:802–8. doi: 10.1016/j.pain.2010.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Baron R, Hans G, Dickenson AH. Peripheral input and its importance for central sensitization. Annals of neurology. 2013;74:630–6. doi: 10.1002/ana.24017. [DOI] [PubMed] [Google Scholar]
- 17.Birder L, de Groat W, Mills I, Morrison J, Thor K, Drake M. Neural control of the lower urinary tract: peripheral and spinal mechanisms. Neurourology and urodynamics. 2010;29:128–39. doi: 10.1002/nau.20837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kanai A. Afferent Mechanism in the Urinary Tract. In: Andersson KE, Michel MC, editors. Handbook of experimental pharmacology. Heidelberg: Springer; 2011. pp. 171–206. [DOI] [PubMed] [Google Scholar]
- 19.Cardoso JS, Riley JL, 3rd, Glover T, et al. Experimental pain phenotyping in community-dwelling individuals with knee osteoarthritis. Pain. 2016;157:2104–14. doi: 10.1097/j.pain.0000000000000625. [DOI] [PMC free article] [PubMed] [Google Scholar]