Abstract
Aims:
To establish an animal model of bladder underactivity induced by prolonged and intense stimulation of somatic afferent axons in the tibial nerve.
Methods:
In seven cats under α-chloralose anesthesia, tibial nerve stimulation (TNS) of 30-min duration was repeatedly (3–8 times) applied at 4–6 times threshold (T) intensity for inducing a toe twitch to produce bladder underactivity determined by cystometry. Naloxone (1 mg/kg, i.v.) was administered to examine the role of opioid receptors in TNS-induced bladder underactivity.
Results:
After prolonged (1.5–4 h) and intense (4–6T) TNS, a complete suppression of the micturition reflex occurred in six cats and an increase in bladder capacity to about 150% of control and a decrease in the micturition contraction amplitude to 50% of control occurred in one cat. The bladder underactivity was maintained for at least 1–1.5 h. Naloxone reversed the bladder underactivity, but an additional 30-min TNS removed the naloxone effect.
Conclusions:
The results indicate that prolonged and intense activation of somatic afferent axons in the tibial nerve can suppress the central reflex mechanisms controlling micturition. This animal model may be useful for examining the pathophysiology of neurogenic bladder underactivity and for development of new treatments for underactive bladder symptoms.
Keywords: bladder, cat, naloxone, tibial, underactivity
1 |. INTRODUCTION
Underactive bladder (UAB) is a symptom complex characterized by prolonged urination time with or without a sensation of incomplete bladder emptying, usually with hesitancy, reduced sensation on filling, and a slow stream.1,2 About 45–48% of the elderly population exhibit detrusor underactivity3,4 and 25–40% of the general population have UAB.5,6 Currently there are no medications to treat UAB, which significantly impacts the quality of life.7,8 Intermittent self-catheterization or indwelling catheters are used when urinary retention is present.1,9 The pathophysiology of UAB is not fully understood and is probably multifactorial including both neurogenic and myogenic factors. Therefore, development of animal models for different types of bladder underactivity would be useful for basic science research to better understand possible mechanisms of UAB and discover potential treatments.
Because neuromodulation therapies, which are used clinically to treat overactive bladder (OAB), are also effective in producing a persistent suppression of normal bladder reflexes in animal models, we have considered the possibility that studies of the inhibitory mechanisms of neuromodulation might provide insights into the pathophysiology of UAB. For example, tibial neuromdulation, an FDA-approved therapy for OAB that is applied for 30-min once a week for 12 weeks can elicit a prolonged suppression of OAB symptoms lasting for several weeks.10 Tibial nerve stimulation (TNS) also elicits a post-stimulation inhibition of the normal micturition reflex in anesthetized cats and significantly increases bladder capacity for at least 2 h.11,12 These results indicate that firing of somatic afferents in the tibial nerve can activate an inhibitory mechanism in the central nervous system that initiates persistent inhibition of bladder function that mimics some of the UAB symptoms. Therefore, it seems likely that further studies of TNS-induced persistent inhibition might provide insights into the possible contribution of somatic afferent inhibition to certain types of UAB.
In this study using anesthetized cats, we addressed the following questions: (i) can TNS completely suppress the micturition reflex if the stimulation is strong and prolonged (>30 min); (ii) what are the minimal stimulation parameters to produce complete inhibition and how long can the inhibition last; and (iii) does opioid receptor activity, which contributes to TNS suppression of bladder overactivity in cats,13 also play a role in the TNS-induced bladder underactivity?
2 |. METHOD AND MATERIALS
The experimental protocol and animal use in this study were approved by the Animal Care and Use Committee at the University of Pittsburgh.
2.1 |. Surgical procedures
A total of seven cats (four males and three females, 2.9–4 kg; Liberty Research, Waverly, NY) were used in this study. The animals were anesthetized with isoflurane (2–5% in oxygen) during surgery and then switched to α-chloralose anesthesia (initial 65 mg/kg i.v. and supplemented as needed) during data collection.14 The left cephalic vein was catheterized for administration of anesthetics and fluid. A tracheotomy was performed and a tube was inserted to keep the airway patent. A catheter was inserted into right carotid artery to monitor systemic blood pressure. Heart rate and blood oxygen were monitored by a pulse oximeter (9847V; NONIN Medical, Plymouth, MN) attached to the tongue. Through an abdominal incision, the ureters were isolated, tied, and cut for external drainage. A double lumen catheter was inserted into the bladder via a small cut in the proximal urethra and secured by a ligature around the urethra. One lumen was connected to a pump to infuse saline (1–2 mL/min) for bladder distention. The other lumen was attached to a pressure transducer to measure bladder pressure. The tibial nerve on the right side was dissected via a skin incision at the ankle. A tripolar cuff electrode (NC223pt, MicroProbe, Gaithersburg, MD) was implanted on the nerve and then the electrode was connected to an electrical stimulator (S88; Grass Medical Instruments, Quincy, MA) via a constant voltage stimulus isolator (SIU5; Grass Medical Instruments). After the surgery, the skin and muscle layers were closed by sutures.
2.2 |. Stimulation protocol
At the beginning of each experiment, uniphasic rectangular pulses (5 Hz frequency, 0.2 ms pulse width) were used to determine the intensity threshold (T) for TNS to induce observable toe twitches. Based on our previous studies11,12 showing that 30-min TNS of 2–4T intensity can significantly increase bladder capacity for at least 2 hr following the stimulation, stronger TNS (4–6T intensity) was used in this study with the goal of completely suppressing the micturition contraction.
Initially, multiple cystometrograms (CMGs) were performed by slowly infusing the bladder with saline to determine the bladder capacity that was defined as the volume threshold to induce a micturition reflex contraction of large amplitude (>30 cmH2O) and long duration (>20 s). Once the control bladder capacity was determined, TNS (5 Hz, 0.2 ms, 4T) of 30-min duration was applied three times at 15–20 min intervals while the bladder was distended with a volume equal to the bladder capacity. After each 30-min TNS, the bladder was emptied and a control CMG was performed to determine if the micturition reflex was completely suppressed during the post-TNS period. A complete suppression is defined as the absence of a large amplitude (>30 cmH2O) and long duration (>20 s) bladder contraction even when the baseline bladder pressure between small non-micturition contractions reached 40 cmH2O at which time the CMG was terminated and the bladder capacity was recorded as the infused volume. This end point was selected because clinical studies revealed that sustained bladder storage pressures greater than 40 cmH2O are associated with increased risk of upper tract damage.15 In two animals in which the micturition reflex was blocked after three periods of TNS, five control CMGs were performed over a 1–1.5 h period to determine if the inhibition was reversible. In five other animals, which exhibited inhibition after the first three periods of TNS, the repeated TNS protocol was continued for 1–5 additional periods at 6T intensity to examine reproducibility of the inhibitory responses, and then was followed by five control CMGs to examine possible recovery from the inhibition. After the fifth control CMG in each animal, naloxone (an opioid receptor antagonist) was administered (1 mg/kg, i.v.). Five minutes after administering naloxone, a control CMG was performed to determine the naloxone effect. Because naloxone reversed the persistent TNS inhibition, a 30-min TNS (5 Hz, 0.2 ms, 6T) was applied one more time and a control CMG was performed following the 30-min TNS to determine if the naloxone effect could be reversed. The bladder was emptied after each CMG and a waiting period of 2–3 min was inserted after each emptying to allow the bladder muscle to recover from distention.
2.3 |. Data analysis
Repeated measurements (2–3 CMGs) of control bladder capacity before any treatment were averaged in the same animal. Then, the bladder capacity was measured during every CMG and normalized to the averaged control capacity in each cat. The amplitude of bladder contractions was also measured in each CMG and normalized to the averaged values obtained during control CMGs in each cat. After repeated 30-min TNS, some animals (6 of 7 cats) exhibited a complete suppression of micturition reflex contraction and the bladder infusion was stopped when the baseline bladder pressure reached 40 cmH2O. When the micturition reflex was completely suppressed, the infused volume was used as bladder capacity and the amplitude of the bladder contraction was measured as zero. The data from different animals are presented as mean ± standard error. Statistical significance (P < 0.05) was determined by t-test or repeated-measures one-way ANOVA followed by Dunnett's multiple comparison.
3 |. RESULTS
3.1 |. Bladder underactivity produced by repeated application of 30-min TNS
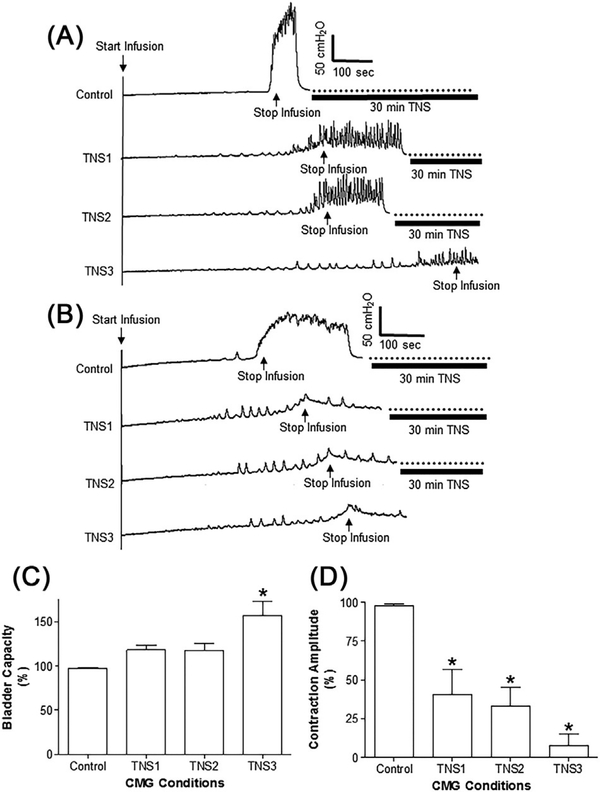
Repeated (three times) application of 30-min periods of TNS at 4T intensity produced in six cats bladder underactivity evident as a complete suppression of the micturition reflex (Figure 1) and produced in one cat a 50% decrease in the amplitude of the micturition contraction without changing the bladder capacity. However, among the six cats the effects of TNS varied in different animals. In three cats bladder capacity increased after the first and second 30-min periods of TNS; and complete suppression of the micturition reflex occurred after three applications of 30-min TNS (Figure 1A). In three other cats a complete suppression occurred after the first TNS application (Figure 1B). On average (N = 7 cats) during the three periods of TNS the amplitude of bladder contractions gradually declined to less than 10% of control (Figure 1D) and the bladder capacity significantly (P < 0.01) increased to 157 ± 10% of control (Figure 1C).
FIGURE 1.
Effect of repeated application of 30-min tibial nerve stimulation (TNS) on the micturition reflex. A, Repeated cystometrogram (CMG) traces showing that the amplitude of micturition contraction was partially reduced after the first and second 30-min TNS, but was completely suppressed after the third 30-min TNS. Infusion rate = 1 mL/min. TNS: 5 Hz, 0.2 ms, 4T = 2.4 V. B, Repeated CMGs showing that the amplitude of micturition contraction was completely suppressed after the first 30-min TNS. Infusion rate = 2 mL/min. TNS: 5 Hz, 0.2 ms, 4T = 4.4 V. C and D, summarized results showing the effects on bladder capacity (C) or contraction amplitude (D) after each application of 30-min tibial nerve stimulation (TNS). TNS: 5 Hz, 0.2 ms, 4T = 2.0–4.8 V. T—threshold intensity for inducing toe twitch. *Indicates significantly (P < 0.05) different from control (one-way ANOVA). N = 7 cats
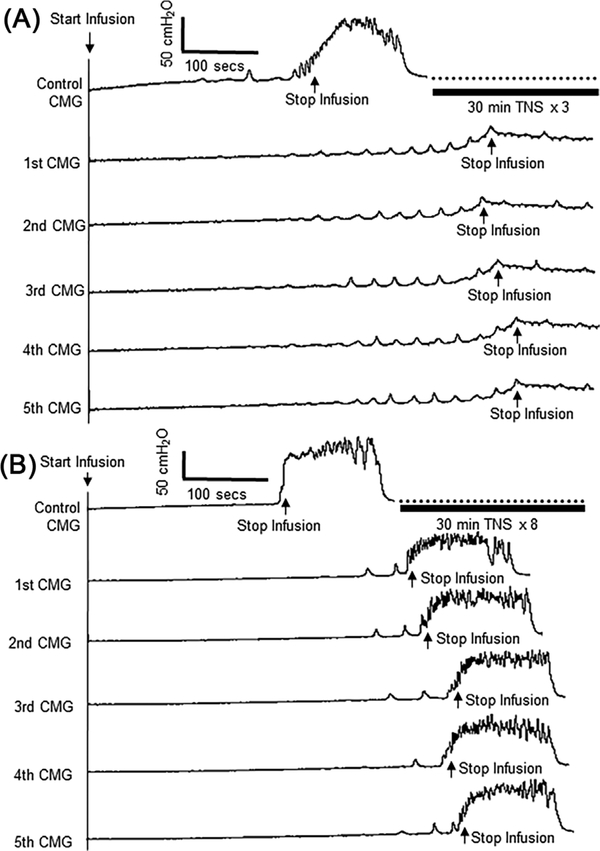
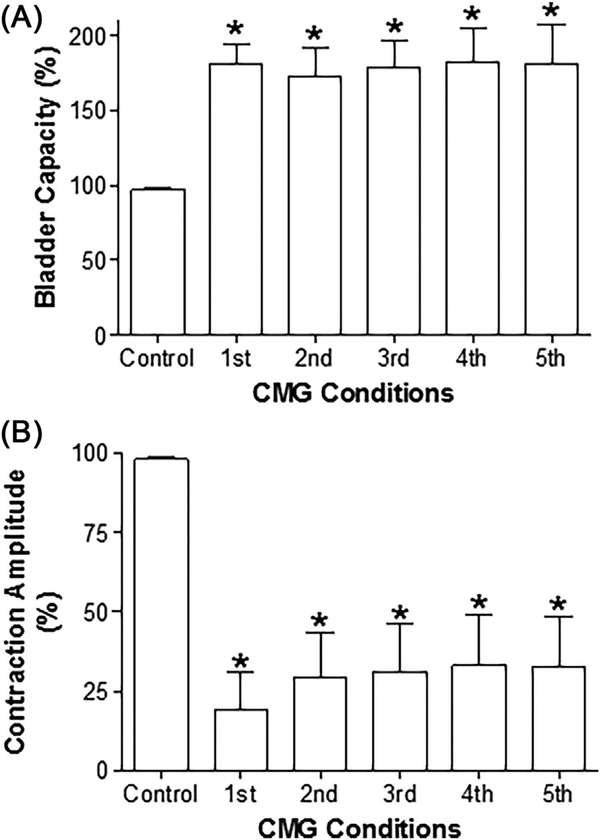
After the three periods of TNS, stimulation was stopped in two cats and five control CMGs were performed over a 1–1.5 h period to determine if the inhibition was reversible. In these two cats the inhibition persisted (Figure 2A). In the remaining cats the intensity of TNS was increased to 6T and 1–5 additional 30-min periods of TNS were applied during a period ranging from 0.5–2.5 h in different cats. In the one cat in which the initial three periods of TNS only produced a 50% decrease in the amplitude of the micturition contraction without changing the bladder capacity, five additional periods of TNS increased bladder capacity 50% and the increase persisted throughout the post-stimulation period (Figure 2B). In the other four cats the micturition reflex was completely suppressed by additional periods of TNS as evident in the following five control CMGs (similar to Figure 2A), except for the occurrence of a micturition reflex after the fourth application of TNS at an increased bladder capacity in two cats. On average (N = 7 cats) the bladder capacity following the additional higher intensity of stimulation significantly (P < 0.05) increased to 181 ± 13% of control and the amplitude of the micturition contraction was reduced to about 25% of the control. The inhibitory effects persisted in the five control CMGs performed during the post-stimulation recovery period (Figure 3).
FIGURE 2.
Repeated application of 30-min tibial nerve stimulation (TNS) induced prolonged post-stimulation inhibition. A, TNS eliminated the micturition reflex contraction even when the saline infusion (2 mL/min) distended the bladder to a baseline pressure of 40 cmH2O. Repeated TNS: 5 Hz, 0.2 ms, 30 min at 4T applied for three times, T = 0.5 V. T—threshold intensity for inducing toe twitch. B, TNS significantly increased bladder capacity during saline infusion (1 mL/min). Repeated TNS: 5 Hz, 0.2 ms, 30 min at 4T applied three times followed by 6T five times, T = 0.5 V
FIGURE 3.
Summarized results showing the effects of repeated tibial nerve stimulation (TNS) on bladder capacity (A) and contraction amplitude (B). Repeated TNS: 5Hz, 0.2 ms, 30 min at 4–6T applied 3–8 times, T = 0.5–1.2 V. T—threshold intensity for inducing toe twitch. *Indicates significantly (P < 0.05) different from control (one-way ANOVA). N = 7 cats
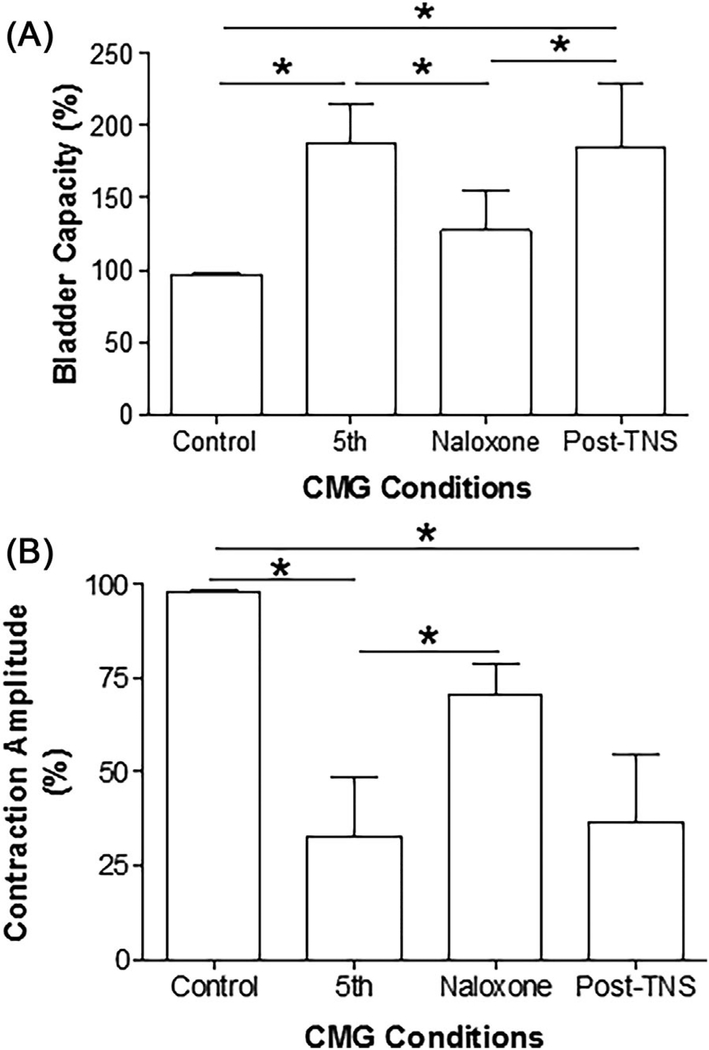
3.2 |. Effect of naloxone on TNS-induced bladder underactivity
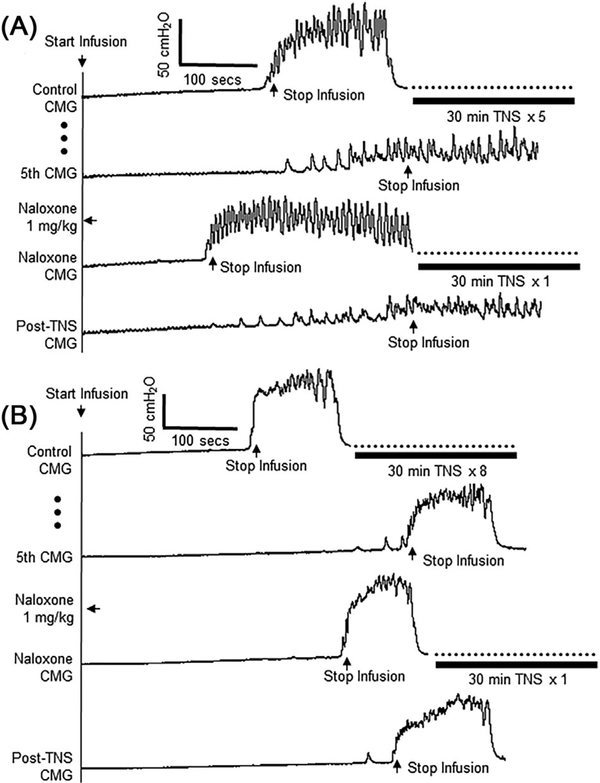
In six cats after the repeated application of 30-min TNS completely suppressed the micturition reflex, naloxone (1 mg/kg, i.v.) rapidly (within 15–20 min) reversed the inhibition and restored the reflex (see the naloxone CMG trace in Figure 4A). Fifteen to twenty minutes after naloxone administration, application of a 30-min TNS at 6T intensity reversed the effect of naloxone by re-establishing the complete inhibition in four cats (see the Post-TNS CMG trace in Figure 4A) and increasing bladder capacity in the other two cats.
FIGURE 4.
Typical effects of naloxone on prolonged TNS inhibition of micturition reflex. Naloxone (1 mg/kg, i.v.) either restored the inhibited micturition reflex (A) or reduced the bladder capacity (B). However, additional 30-min TNS removed the naloxone effect by either completely suppressing the micturition reflex (A) or increasing the bladder capacity (B). Repeated TNS before naloxone in A: 5 Hz, 0.2 ms, 30 min at 4T applied three times followed by 6T two times, T = 1.2 V. Repeated TNS before naloxone in B: 5 Hz, 0.2 ms, 30 min at 4T applied three times followed by 6T five times, T = 0.5 V. TNS after naloxone in both A and B: 5 Hz, 0.2 ms, 30 min at 6T applied one time. T—intensity threshold for inducing toe twitch
In the one cat where the repeated applications of 30-min TNS did not completely suppress the micturition reflex but only decreased the amplitude and increased bladder capacity, naloxone treatment reduced bladder capacity (Figure 4B). In this cat, an additional 30-min TNS reversed the naloxone effect by increasing the bladder capacity (Figure 4B).
On average (N = 7 cats), the naloxone treatment significantly (P < 0.05) decreased bladder capacity (Figure 5A) and increased contraction amplitude (Figure 5B). The additional 30-min TNS after naloxone treatment removed the naloxone effect by increasing bladder capacity (Figure 5A) and decreasing contraction amplitude (Figure 5B).
FIGURE 5.
Summarized results showing the effects of naloxone on bladder capacity (A) and contraction amplitude (B), and the reversal effects of additional 30-min tibial nerve stimulation (TNS). TNS after naloxone: 5 Hz, 0.2 ms, 30 min at 6T applied one time, T = 0.5–1.2. T—intensity threshold for inducing toe twitch. *Indicates a significant difference (P < 0.05, t-test). N = 7 cats
4 |. DISCUSSION
This study in cats shows that prolonged (1.5–4 h) and intense (4–6T) activation of myelinated somatic afferent axons in the tibial nerve can induce a long-lasting (>1–1.5 h) poststimulation inhibition evident as: (i) a significant increase in the bladder volume threshold for triggering the micturition reflex; (ii) a decrease in the amplitude of the micturition reflex; or (iii) a complete suppression of the micturition reflex. These effects of TNS can be reversed by blocking opioid receptors with naloxone; and the effect of naloxone can in turn be reversed by an additional period of TNS.
One type of UAB in young women (ie, Fowler's syndrome) is suggested to be due in part to an upregulation of opioid inhibitory mechanisms in the central nervous system.16 Considerable animal data also support a physiological role for opioid mechanisms in the regulation of bladder function. Previous experiments in anesthetized and decerebrate cats17–20 revealed that intracerebroventricular or intrathecal administration of opioid receptor agonists suppresses reflex bladder activity and that administration of naloxone (an opioid receptor antagonist) at the same sites enhances bladder activity, indicating that the bladder reflex pathway is suppressed by a tonically active opioid inhibitory mechanism presumably mediated by the release of enkephalins. Opioid receptor mediated inhibition has also been identified in bladder parasympathetic ganglia21 but not in the bladder smooth muscle.22 Thus, it is reasonable to conclude that the naloxonesensitive post-stimulation inhibition elicited in the present experiments by prolonged TNS is mediated by an action on the neural pathways controlling the bladder and most likely occurs at a site in the central nervous system possibly in the pontine micturition center (PMC) which regulates bladder capacity by acting as a switching center for the micturition reflex.18–20 The prolonged effect of TNS could be viewed as a type of short term memory or neuroplasticity which would be consistent with a TNS site of action in the brain where these phenomena are more prominent than in the spinal cord.
However, alternate interpretations of the data are possible. For example, because naloxone has an excitatory effect on reflex bladder activity, it could reverse a TNS inhibitory response that is mediated by non-opioid inhibitory neurotransmitters (eg, GABA, glycine, or serotonin) by increasing the excitability of the micturition reflex pathway. This mechanism could also explain the observation that an additional 30-min TNS reversed the effect of naloxone at the end of our experiments after naloxone was administered in a large dose (1 mg/kg, i.v.) that should have completely blocked opioid receptors and therefore negated any further effect of TNS due to release of enkephalins. Thus TNS: (i) may release other inhibitory transmitters that compensate for the suppression of opioid inhibition by naloxone; or (ii) may release additional enkephalins in the CNS that overcome the competitive blockade of opioid receptors produced by naloxone. Although more experiments are needed to establish the neurotransmitter mechanisms contributing to TNS inhibition, this study clearly indicates that the opioid receptors are directly or indirectly involved in the bladder underactivity induced by somatic afferent activity in the tibial nerve.
The types of somatic afferents that trigger the prolonged TNS inhibitory response are also of interest. This was evaluated by first determining the electrical stimulus threshold (T) for TNS activation of the large diameter muscle afferents and efferents that elicit motor responses and then using multiples of those thresholds to evoke TNS inhibition. The tibial nerve contains different types and sizes of axons (groups I-IV) innervating both muscle and skin. Group I (Aα) nerve fibers are largest muscle afferent and motor axons with the lowest stimulation threshold (T) which was determined by a toe twitch in this study. The Group II nerve fibers consist of large non-nociceptive mechanosensitive afferent axons (Aβ); whereas groups III (Aδ) and IV (C) nerve fibers consist in part of small nociceptive afferent axons that would elicit painful sensation if excited.23 A previous study in cats showed that 2T intensity is required to excite the group II large afferent axons and >10T intensity is required to excite the groups III and IV small afferent axons.24 Therefore, in this study the TNS at 4–6T intensity must have excited the group I muscle afferents and the non-nociceptive mechanosensitive group II afferent axons but not the group III and IV nociceptive small afferent axons. This result implies that bladder underactivity can be caused by prolonged non-nociceptive afferent firing in the tibial nerve.
In our previous experiments,11,12 TNS at 2–4T intensities at the same frequency (5 Hz) used in the present experiments but for shorter periods of time (30 min) increased bladder capacity but did not completely block or even decrease the amplitude of the micturition reflex. Thus, the more prominent inhibition evoked in the present experiments may be attributable to: (i) increased numbers of axons activated because some animals (N = 3 cats) exhibited a complete block with only one 30-min period of stimulation (Figure 1B); or (ii) increased duration of stimulation because complete block in other animals required 90 min of stimulation (Figure 1A). Since the 30-min TNS of 4T intensity was applied three times followed by additional 1–5 times application at a stronger intensity (6T), it is difficult to know which factor (duration or intensity) is playing an important role in maintaining the complete suppression for at least 1–1.5 h. Further tests will be needed to determine the effects of prolonged TNS at different fixed intensities (2T, 4T, or 6T). Furthermore, due to a closed urethral outlet in this study voiding efficiency could not be evaluated during the bladder underactivity. However, it can be assumed that during the complete suppression of the micturition reflex complete urinary retention would occur with a possible overflow incontinence when the bladder baseline pressure reached 40 cmH2O (see Figures 1B and 2A).
The clinical observations in patients with Fowler's syndrome provide further support for a possible link between abnormal or prolonged somatic afferent input and pathophysiology of UAB. These patients have a severe form of UAB—non-obstructive urinary retention (NOUR), which is believed to be caused by abnormal tonic muscle afferent activity passing through the pudendal nerve from the external urethral sphincter.25 Sacral neuromodulation is a very effective treatment to relieve the NOUR condition in Fowler’s syndrome patients.9 Our recent study in cats14 confirmed that sacral neuromodulation can reverse the bladder inhibition produced by electrical stimulation of the pudendal nerve. The current study raises the possibility that other populations of somatic afferents such as tibial afferents may also contribute to NOUR. However, the abnormal pudendal afferent activity in Fowler's syndrome is tonic, while the current study in cats suggests that tibial afferent activity might not have to be tonic to cause bladder underactivity because prolonged TNS triggered a longlasting post-stimulation inhibitory mechanism. More investigation is certainly warranted to further understand the neural mechanism underlying the long-lasting inhibitory effect produced by prolonged and intense somatic afferent activity.
In summary, this study revealed that abnormal somatic afferent activity in the tibial nerve could be a contributing factor in the generation of “idiopathic” UAB. In addition, this study also established an animal model of bladder underactivity that does not require damage to the bladder smooth muscle or neural pathways controlling the lower urinary tract. This animal model could be very useful for understanding the possible role of somatic afferent inhibition in certain types of UAB and for development of new treatments for UAB.
ACKNOWLEDGMENT
This study is supported by the National Institutes of Diabetes, Digestive, and Kidney Diseases under grants DK-102427 and DK-111382.
Funding information
National Institute of Diabetes and Digestive and Kidney Diseases, Grant numbers: DK-102427, DK-111382
Footnotes
CONFLICTS OF INTEREST
Nothing to disclose.
Limin Liao led the peer-review process as the Associate Editor responsible for the paper.
REFERENCES
- 1.Chapple CR, Osman NI, Birder L, et al. The underactive bladder: anew clinical concept? Eur Urol. 2015;68:351–353. [DOI] [PubMed] [Google Scholar]
- 2.Smith PP, Birder LA, Abrams P, Wein AJ, Chapple CR. Detrusor underactivity and the underactive bladder: symptoms, function, cause-what do we mean? ICI-RS think tank 2014. Neurourol Urodyn. 2016;35:312–317. [DOI] [PubMed] [Google Scholar]
- 3.Abarbanel J, Marcus EL. Impaired detrusor contractility in community-dwelling elderly presenting with lower urinary tract symptoms. Urology. 2007;69:436–440. [DOI] [PubMed] [Google Scholar]
- 4.Jeong SJ, Kim HJ, Lee YJ, et al. Prevalence and clinical features ofdetrusor underactivity among elderly with lower urinary tract symptoms: a comparison between men and women. Korean J Urol. 2012;53:342–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Faraj K, Doo F, Boura J, Vereecke A, Chancellor MB. A cross-sectional study in the USA of the epidemiology and quality of life of underactive bladder symptoms. IntUrolNephrol. 2016;48:1797–1802. [DOI] [PubMed] [Google Scholar]
- 6.Valente S, DuBeau C, Chancellor D, et al. Epidemiology and demographics of the underactive bladder: a cross-sectional survey. Int Urol Nephrol. 2014;46:S7–S10. [DOI] [PubMed] [Google Scholar]
- 7.Juszczak K, Drewa T. Pharmacotherapy in detrusor underactivity: a new challenge for urologists and pharmacologists (from lab to clinic). Pharmacol Rep. 2016;68:703–706. [DOI] [PubMed] [Google Scholar]
- 8.Smith PP, Tyagi P, Kuchel GA, et al. Advanced therapeutic directions to treat the underactive bladder. Int Urol Nephrol. 2014;46:S35–S44. [DOI] [PubMed] [Google Scholar]
- 9.Kessler TM, Fowler CJ. Sacral neuromodulation for urinary retention. Nat Clin Pract Urol. 2008;5:657–666. [DOI] [PubMed] [Google Scholar]
- 10.Peters KM, MacDiarmid SA, Wooldridge LS, et al. Randomizedtrial of percutaneous tibial nerve stimulation versus extended-release tolterodine: results from the overactive bladder innovative therapy trial. J Urol. 2009;182:1055–1061. [DOI] [PubMed] [Google Scholar]
- 11.Tai C, Shen B, Chen M, Wang J, Roppolo JR, de Groat WC. Prolonged poststimulation inhibition of bladder activity induced by tibial nerve stimulation in cats. Am J Physiol Renal Physiol. 2011; 300:F385–F392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ferroni MC, Slater RC, Shen B, et al. Role of the brain stem in tibial inhibition of the micturition reflex in cats. Am J Physiol Renal Physiol. 2015;309:F242–F250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tai C, Larson JA, Ogagan PD, et al. Differential role of opioid receptors in tibial nerve inhibition of nociceptive and non-nociceptive bladder reflexes in cats. Am J Physiol Renal Physiol. 2012;302:F1090–F1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li X, Uy J, Yu M, et al. Sacral neuromodulation blocks pudendal inhibition of reflex bladder activity in cats: insight into the efficacy of sacral neuromodulation in Fowler's syndrome. Am J Physiol Regul Integr Comp Physiol. 2018;314:R34–R42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McGuire EJ, Woodside JR, Borden TA, Weiss RM. Prognostic value of urodynamic testing in myelodysplastic patients. J Urol. 1981;126:205–209. [DOI] [PubMed] [Google Scholar]
- 16.Panicker JN, Game X, Khan S, et al. The possible role of opiates in women with chronic urinary retention: observations from a prospective clinical study. J Urol. 2012;188:480–484. [DOI] [PubMed] [Google Scholar]
- 17.Booth AM, Hisamitsu T, Kawatani M, de Groat WC. Regulation of urinary bladder capacity by endogenous opioid peptides. J Urol. 1985;133:339–342. [DOI] [PubMed] [Google Scholar]
- 18.Hisamitsu T, de Groat WC. The inhibitory effect of opioid peptides and morphine applied intrathecally and intracerebroventricularly on the micturition reflex in the cat. Brain Res. 1984;298:51–65. [DOI] [PubMed] [Google Scholar]
- 19.Noto H, Roppolo JR, de Groat WC, Nishizawa O, Sugaya K, Tsuchida S. Opioid modulation of the micturition reflex at the level of the pontine micturition center. Urol Int. 1991;47:19–22. [DOI] [PubMed] [Google Scholar]
- 20.Roppolo JR, Booth AM, de Groat WC. The effects of naloxone on the neural control of the urinary bladder of the cat. Brain Res. 1983;264:355–358. [DOI] [PubMed] [Google Scholar]
- 21.de Groat WC, Kawatani M. Enkephalinergic inhibition in parasympathetic ganglia of the urinary bladder of the cat. J Physiol. 1989;413:13–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sillén U, Rubenson A. Central and peripheral motor effects of morphine on the rat urinary bladder. Acta Physiol Scand. 1986;126: 181–187. [DOI] [PubMed] [Google Scholar]
- 23.Whitwan JG. Classification of peripheral nerve fibres. Anaesth. 1976;31:495–503. [DOI] [PubMed] [Google Scholar]
- 24.Sato A, Sato Y, Schmidt RF. Reflex bladder activity induced by electrical stimulation of hind limb somatic afferents in the cat. J Auton Nerv Syst. 1980;1:229–241. [DOI] [PubMed] [Google Scholar]
- 25.Fowler CJ, Christmas TJ, Chapple CR, Parkhouse HF, Kirby RS,Jacobs HS. Abnormal electromyographic activity of the urethral sphincter, voiding dysfunction, and polycystic ovaries: a new syndrome? BMJ. 1988;297:1436–1438. [DOI] [PMC free article] [PubMed] [Google Scholar]