Abstract
Positive allosteric modulators (PAMs) of the M1 subtype of muscarinic acetylcholine receptor have attracted intense interest as an exciting new approach for improving the cognitive deficits in schizophrenia and Alzheimer’s disease. Recent evidence suggests that the presence of intrinsic agonist activity of some M1 PAMs may reduce efficacy and contribute to adverse effect liability. However, the M1 PAM PF-06827443 was reported to have only weak agonist activity at human M1 receptors but produced M1-dependent adverse effects. We now report that PF-06827443 is an allosteric agonist in cell lines expressing rat, dog, and human M1 and use of inducible cell lines shows that agonist activity of PF-06827443 is dependent on receptor reserve. Furthermore, PF-06827443 is an agonist in native tissue preparations and induces behavioral convulsions in mice similar to other ago-PAMs. These findings suggest that PF-06827443 is a robust ago-PAM, independent of species, in cell lines and native systems.
Keywords: Positive allosteric modulator, muscarinic acetylcholine receptor, M1 prefrontal cortex, long-term depression, ago-PAM, schizophrenia, Alzheimer’s disease
Graphical abstract
The cognitive disturbances that dramatically disrupt quality of life in patients suffering from Alzheimer’s disease (AD) and schizophrenia remain untreated by currently approved medications. Interestingly, clinical studies with the M1/M4 muscarinic acetylcholine (mACh) receptor partial agonist xanomeline suggested that selective activation of muscarinic receptors may provide a novel approach for improving cognition in these disorders. While the potential of xanomeline to improve the cognitive disturbances in Alzheimer’s disease1 and schizophrenia2 appeared promising, xanomeline exhibited severe dose-limiting adverse effects thought to be mediated by nonselective agonist activity, specifically via activation of peripheral M2 and M3 receptors.3 While efforts to develop highly selective M1/M4 orthosteric agonists failed due to the highly conserved acetylcholine binding site, several groups have developed subtype-selective positive allosteric modulators (PAMs), which act at structurally distinct sites from the orthosteric binding site (see reviews4,5).
Since the M1 receptor is the most abundant of the five mACh receptors in brain regions critically involved in cognition6,7 such as the prefrontal cortex (PFC) and hippocampus, several groups have focused on developing M1 PAMs. Further rationale for targeting M1 is evidenced by the cognitive disturbances induced by blockade8,9 or genetic deletion10 of M1 in mice. Additionally, multiple studies provide strong evidence that M1 PAMs can reverse cognitive deficits in animal models relevant for AD11,12 and schizophrenia.13–15 While these preclinical studies are exciting, recent evidence suggests that intrinsic agonist activity (i.e., agonist activity in the absence of the orthosteric ligand) is detrimental to the cognition-enhancing efficacy of M1 PAMs and may be responsible for the adverse effects ascribed to some M1 ago-PAMs such as PF-06764427 and MK-7622.16
Interestingly, PF-06827443 was recently disclosed as a highly selective and potent M1 PAM with weak agonist activity at the human M1 receptor.7 While this work has shown that high doses of PF-06827443 induce minimal adverse effects when administered to rats, PF-06827443 induces severe seizures when administered to dogs.7 Based on the relatively weak agonist activity of PF-06827443, the authors suggested that severe adverse effect liability is not dependent on agonist activity of M1 PAMs. However, this study relied on a single cell line expressing human M1, and PF-06827443 was not extensively characterized to establish the level of agonist activity of this M1 PAM in other preclinical systems. Allosteric agonist activity is highly dependent on receptor expression levels,17,18 and there are documented species differences in the pharmacological profiles for other muscarinic allosteric modulators.19,20 Since animal models, such as rodent, dog and monkey often drive preclinical drug discovery, it is therefore critical to fully assess agonist activity across different levels of receptor expression, in different species, and in native systems to fully evaluate intrinsic agonist activity of M1 PAMs. We now report that PF-06827443 displays robust agonist activity across cell lines expressing rat, dog and human M1. Furthermore, we used an inducible cell line to control M1 receptor expression and found that PF-06827443 displays agonist activity in systems with moderate to high receptor reserve. In contrast, VU0550164, an M1 PAM optimized to avoid ago-PAM activity16 exhibits no agonist activity at any expression level tested. Finally, unlike recently reported M1 PAMs optimized to eliminate agonist activity,16 PF-06827443 displays robust agonist activity in mouse brain slices and induces behavioral convulsions in mice that is similar to other previously described ago-PAMs (e.g., MK-7622 and PF-06764427). Taken together, these findings reveal that PF-06827443 is a robust M1 ago-PAM and add further support to the hypothesis that intrinsic agonist activity may be a detrimental quality for M1 PAM clinical candidates.
RESULTS AND DISCUSSION
PF-06827443 was previously demonstrated to display minimal agonist activity in cell lines expressing human M1 but induced severe adverse effects in some preclinical animal models;7 thus, we evaluated the ability of PF-06827443 to directly activate M1 as assessed by mobilization of intracellular calcium (Ca2+) in Chinese hamster ovary (CHO) cells stably expressing the rat M1 receptor. PF-06827443 induces a robust increase in intracellular Ca2+ in the absence of an orthosteric mACh receptor agonist (rat ago EC50 1900 nM; 81 ± 5% ACh Max, Figure 1) and is a potent PAM in the presence of an EC20 concentration of acetylcholine (rat PAM EC50 36.1 ± 4.9 nM; 97 ± 1% ACh Max, Figure 1B). Additionally, PF-06827443 displays similar agonist and PAM activities at the dog and human M1 receptors (Supplemental). Furthermore, unlike other M1 PAMs devoid of agonist activity, PF-06827443 inhibits [3H]-NMS binding, which likely reflects negative cooperativity with antagonist binding at the orthosteric binding site (Supporting Information).
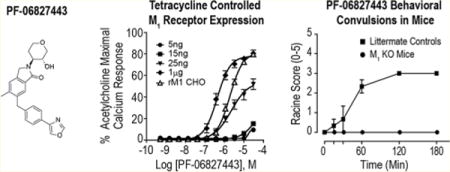
Figure 1.
PF-06827443 displays intrinsic agonist activity in rM1-CHO cells with high receptor expression. (A) Representative raw calcium traces following the addition of 30 μM PF-06827443 (solid line) and the subsequent additions of EC20 and EC80 concentrations of acetylcholine (ACh) (dotted line). (B) Concentration–response curves of rM1-CHO calcium mobilization assay for PF-06827443 in the absence of ACh (agonist mode; black) and the presence of an EC20 of ACh (PAM mode; red). Concentration–response curves for (C) PF-06827443 and (D) VU0550164, in the absence of ACh, in cell lines treated with 5 ng, 15 ng, 25 ng, and 1 μg of tetracycline as well as rM1 CHO cells. Data represent mean ± SEM from three independent experiments performed in triplicate.
While PF-06827443 was previously demonstrated to have minimal agonist activity at human M1,7 this discrepancy could be due to the use of a cell line with lower M1 expression as agonist activity of GPCR PAMs is dependent on receptor expression levels.17,18,21–23 To determine whether the robust agonist activity is dependent on levels of receptor expression, we used a TREx-CHO cell line in which the rM1 receptor is under control of the tetracycline (TET) repressor protein, enabling us to systematically increase M1 expression by adding increasing amounts of TET. This permits the measurement of M1 activation in a single cellular background with different levels of receptor reserve. As shown in the Supporting Information, increases in M1 receptor expression was observed in a TET-concentration dependent manner and cells treated with 25 ng/mL TET show comparable M1 expression levels as CHO cells stably expressing rM1 and hM1. Similarly, increasing concentrations of TET led to a progressive increase in M1 receptor expression, and a leftward shift in the ACh potency (Supporting Information). PF-06827443 shows comparable agonist activity in the cells treated with 25 ng TET (ago EC50 = 2300 nM; 54 ± 5% ACh Max, Figure 1C) to one in rat M1-CHO. However, in cells with 1 μg TET, PF-06827443 exhibits robust agonist activity at high receptor expression levels (ago EC50 = 400 nM ; 78 ± 2% ACh Max), which is not as evident at lower expression levels, e.g. 5 ng and 15 ng TET (Figure 1C). In contrast, a previously characterized M1 PAM optimized to lack agonist activity, VU0550164, does not exhibit agonism at any receptor expression level (Figure 1D).
Native systems often have a high receptor reserve for M1;21,23 thus, it is important to evaluate the potential agonist activity of PF-06827443 in a native system relevant to rodent cognition. Previously, we and others found that cholinergic agonists, as well as M1 ago-PAMs, can induce an M1-dependent long-term depression (LTD) of layer V field excitatory post synaptic potentials (fEPSPs) electrically evoked by stimulation of layer II/III in the mouse PFC (Figure 2A).13,14,16,24 Therefore, we tested the hypothesis that PF-06827443 would induce a LTD of fEPSPs at this cortical synapse similar to previously described ago-PAMs.16 Consistent with previous studies, 1 μM (77.8 ± 4.27%, Figure 2B) and 10 μM PF-06827443 (51.8 ± 3.78%, Figure 2C) induce a sustained LTD of fEPSPs in the PFC. This effect of PF-06827443 was completely blocked by the highly selective M1 antagonist VU0255035,25 confirming that this PF-06827443 induced-LTD is M1-dependent (101.6 ± 9.30%, Figure 2D). Quantification of LTD measured at 46–50 min after drug washout (shaded area) indicates a significant difference in the magnitude of LTD observed with application of 1 μM PF-06827443 + 10 μM VU0255035 compared to 1 μM PF-06827443 alone (Figure 2E, one-way ANOVA, p < 0.05). Therefore, similar to other M1 ago-PAMs, PF-06827443 displays robust agonist activity in the mouse PFC, a brain region heavily implicated in cognition. In addition, we performed studies to test the hypothesis that bath application of PF-06827443 would increase the frequency of spontaneous excitatory postsynaptic currents (sEPSCs) measured in layer V pyramidal cells of the PFC (Figure 3A) similar to previously characterized M1 ago-PAMs.16 In agreement with previous studies, 10 μM PF-06827443 decreases the interevent-interval (IEI) (Figure 3B) and, consequently, significantly increases sEPSC frequency in layer V pyramidal cells (Figure 3C, paired t test, p < 0.05). Together, these results show that PF-06827443 displays robust agonist activity in two native tissue electrophysiological assays.
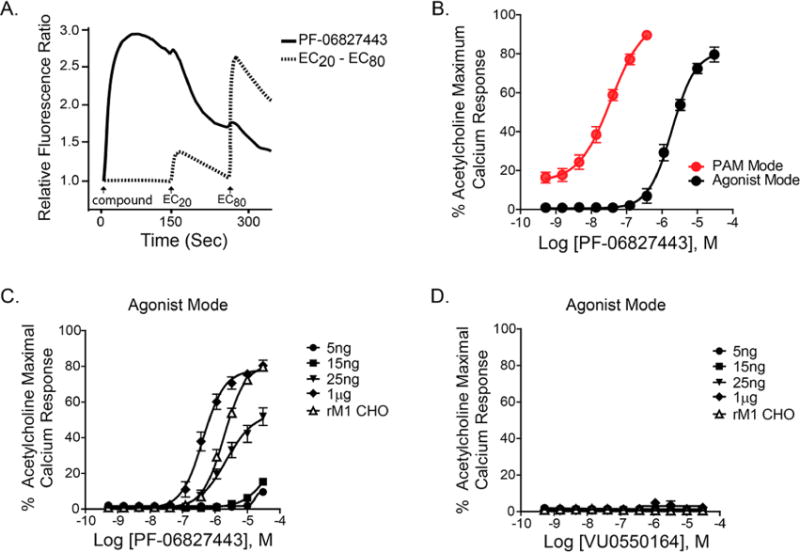
Figure 2.
PF-06827443 robustly depresses fEPSP slopes recorded in layer V of the prelimbic mPFC evoked by electrical stimulation in layer II/III. (A) Schematic the fEPSPs recorded from layer V of the mouse PFC in response to electrical stimulation in the superficial layers II–III. Time-course graph showing that bath application of (B) 1 μM PF-06827443 and (C) 10 μM PF-06827443 for 20 min leads to a robust LTD of fEPSP slope. (D) Time course graph showing that bath application of 1 μM PF-06827443 fails to induce LTD in the presence of 10 μM VU0255035, a highly selective M1 antagonist. Insert contains fEPSP sample traces during baseline (red) and 46–50 min following drug washout (black). Scale bars denote 0.2 mV and 5 s. n = 6–8 brain slice experiments per group. (E) Quantification of LTD (normalized fEPSP slopes) 46–50 min after drug washout (shaded area) of the different experimental groups. There was a significant difference in the magnitude of LTD observed with application of 1 μM PF-06827443 + 10 μM VU0255035 compared to 1 μM PF-06827443 alone. One-way ANOVA was carried out with Dunnett’s posthoc test, 1 μM PF-06827443 (black bar) as the control group. *p < 0.05.
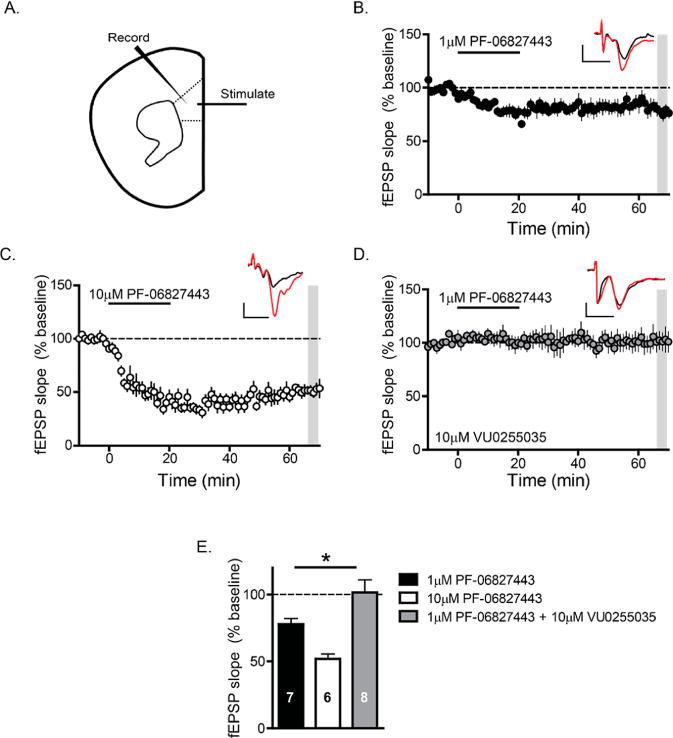
Figure 3.
PF-06827443 increases sEPSC frequency in layer V prelimbic mPFC neurons. (A) Whole-cell recordings from pyramidal neurons (regular spiking firing) clamped at −70 mV were performed in layer V of the prelimbic prefrontal mouse cortex. A sample trace of baseline (upper-trace) and during drug add (bottom-trace) for a typical cell are shown. Scale bars denote 50 pA and 2 s. (B) Histogram summarizing the change in the interevent-interval of baseline (black) to the drug peak effect (gray). (C) 10 μM PF-06827443 produced a statistically significant increase in sEPSC frequency. n = 8 slices. Student’s t test; *p < 0.05.
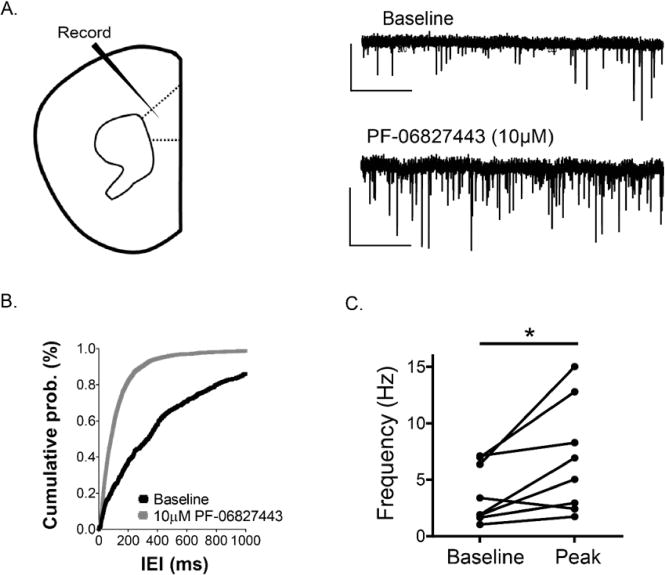
As seen with other ago-PAMs,16,26 we hypothesized that this overactivation of M1 by PF-06827443 in native brain tissue preparations is responsible for M1-induced behavioral convulsions. We next tested the hypothesis that the agonist activity of PF-06827443 seen in in vitro and native tissue assays would correlate to behavioral convulsions when administered in mice. Therefore, we performed a single high dose (100 mg/kg) PF-06827443 study in M1 knockout (KO) mice and littermate controls to assess seizure liability. Consistent with our previous ago-PAM studies, 100 mg/kg PF-06827443 induced behavioral convulsions that reached stage 3 on the modified Racine scale26,27 in wild type littermate mice that were absent in M1 KO mice (Figure 4). Collectively, these findings demonstrate that high doses of PF-06827443 induce behavioral convulsions in mice similar to the adverse effects previously published in dogs.7
Figure 4.
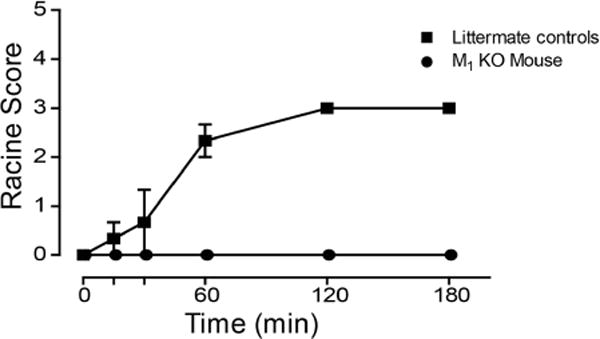
PF-06827443 induces behavioral convulsions in mice. C57Bl6/J mice were administered a single 100 mg/kg dose of PF-06827443 and behavioral convulsions were measured for 3 h using the modified Racine scale (0–5). M1-KO mice treated with PF-06827443 exhibit no behavioral convulsions, suggesting that PF-06827443 induces behavioral convulsions in an M1-dependent fashion. Compounds were formulated in 10% Tween 80 and delivered intraperitoneally. Data represent mean ± SEM (n = 3 mice per dose).
In conclusion, PF-06827443 exerts considerable agonist activity in vitro as assessed by calcium mobilization in mammalian M1-expressing cell lines and this agonist activity is most robust in high expressing cell lines. Additionally, PF-06827443 displays agonist effects in two native tissue electrophysiological assays. Lastly, PF-06827443 induces M1-dependent behavioral convulsions in adult mice. Therefore, we conclude that the intrinsic agonist activity of some M1 PAMs appears to contribute to adverse effects and the limited in vivo utility of ago-PAMs. Furthermore, as receptor expression levels may vary between cell lines and between research groups, it is critical to evaluate M1 preclinical candidates in systems with varying degrees of receptor reserve (i.e., both in vitro and in native tissue) to fully characterize potential ago-PAM activity.
METHODS
Cell Line and Calcium Mobilization Assay
Stable CHO cell lines constitutively expressing rat, dog, and human M1 were used in binding and Ca2+assays. Generation of a TET-inducible (TRex) human M1 mACh receptor stable cell line was previously described.21 Detailed methods are described in the Supplemental Methods.
Radioligand Binding Assay
Competition binding assays were performed using [3H]-N-methylscopolamine ([3H]-NMS, PerkinElmer. Boston, MA) as previously described.22,26 Compounds were serial diluted 1:3 in DMSO for an 11-point CRC and then further diluted for a final top concentration of 30 μM in binding buffer (20 mM HEPES, 10 mM MgCl2, and 100 mM NaCl, pH 7.4). Membranes from rat M1- CHO cells (10 μg) were incubated with the serially diluted compounds in the presence of a Kd concentration of [3H]-NMS, 0.088 nM, at room temperature for 3 h with constant shaking. Nonspecific binding was determined in the presence of 10 μM atropine. Similarly, saturation binding was performed using varying concentration of [3H]-NMS, 3 nM to 0.003 nM. Detailed methods are described in the Supplemental Methods.
Animals
All animal studies were approved by the Vanderbilt University Medical Center Institutional Animal Care and Use Committee and were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. For the electrophysiology studies, 6–10 week old adult male C57BL6/J mice (Jackson laboratories) were used. For the behavioral studies, M1 receptor knockout (KO) mice and littermate controls were used (with permission from J. Wess, National Institutes of Health–National Institute of Diabetes and Digestive and Kidney Diseases, Bethesda, MD). Mice were group housed 4–5 per cage, maintained on a 12 h light/dark cycle, and food and water were provided ad libitum.
Extracellular Field Electrophysiology
Extracellular field potential recordings were performed with 6–10-week-old male C57BL6/J mice (Jackson Laboratories) using 400 μm coronal slices containing the prelimbic prefrontal cortex recorded from layer V and evoked electrically by a concentric bipolar stimulating electrode (200 μs duration, 0.05 Hz; interpulse interval of 50 ms) in the superficial layers II–III. Input–output curves were generated to determine the stimulus intensity that produced approximately 70% of the maximum field excitatory postsynaptic potential (fEPSP) slope before each experiment, which was then used as the baseline stimulation. PF-06827443 was prepared in DMSO (<0.1% final) and subsequently diluted into ACSF to the appropriate concentration and applied to the bath for 20 min. Detailed methods are described in the Supporting Information.
Whole Cell Electrophysiology
Whole-cell patch-clamp recordings were performed using coronal PFC slices (300 μm) prepared from 6–10 week old male C57BL6/J mice (Jackson Laboratories) according to the methods above using pipettes filled with an intracellular solution consisting of the following (in mM): 125 K-gluconate, 4 NaCl, 10 HEPES, 4 MgATP, 0.3 NaGTP, 10 Tris-phosphocreatine and had resistances ranging from 3–5 MΩ. Pyramidal neurons were visualized based on morphology and were further identified by their regular spiking pattern following depolarizing current injections. Spontaneous EPSCs were recorded at a holding potential of −70 mV (the reversal potential Cl− through GABAA channels). After a stable baseline was recorded for 5 min, test compounds were applied to the bath for 5 min using a peristaltic pump perfusion system. Peak effect was calculated by averaging 3 min of peak effect change in interevent-interval (IEI) during drug application. Cumulative probability plots were constructed using 2 min episodes of baseline and peak affect during drug add IEI values.
Behavioral Manifestations of Seizure Activity
To evaluate induction of behavioral manifestation of seizure activity, C57Bl/6J mice received administration of vehicle or 100 mg/kg PF-06827443. Compounds were formulated in 10% Tween 80 (pH 7.0) at a concentration of 10 mg/mL and injected intraperitoneally (i.p.) (n = 3 per genotype). Animals were monitored continuously and scored for behavioral manifestations of seizure activity at 5, 10, 15, and 30 min and at 1 and 3 h postinjection. Behavioral manifestations of seizures were scored using a modified Racine scoring system.26,27
Supplementary Material
Acknowledgments
Funding
This work was supported by NIH F31 MH114368 (Moran), NIH T32 MH64913 (Moran), U01 MH087965 (Conn), R01 MH062646 (Conn), R01 MH073676 (Conn) and R01 MH082867 (Lindsley), CIHR DFS146189 (Maksymetz). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Mr. Moran also receives support from the Clinical Neuroscience Scholars program through the generous support of the Dan Marino Foundation.
ABBREVIATIONS
- AD
Alzheimer’s disease
- ACh
acetylcholine
- ANOVA
analysis of variance
- CHO
Chinese hamster ovary
- CNS
central nervous system
- CRC
concentration–response curve
- fEPSP
field excitatory post synaptic potentials
- GTP
guanosine triphosphate
- HEPES
(4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid)
- IEI
interevent interval
- i.p
intraperitoneally
- KO
knockout
- LTD
long-term depression
- M1
muscarinic receptor subtype 1
- mACh
muscarinic acetylcholine
- mg/kg
milligrams per kilograms
- mV
millivolts
- NMDG
N-methyl-D-glucamine
- NMS
N-methylscopolamine
- PAM
positive allosteric modulator
- PFC
prefrontal cortex
- sEPSC
spontaneous excitatory post synaptic current
- TET
tetracycline
Footnotes
Supporting Information
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acschemneuro.8b00106.
PF-06827443 intrinsic agonist data in CHO cells expressing dog and human M1; PF-06827443 competition binding assay at the rat M1 receptor; receptor densities determined from saturation binding assays; acetylcholine concentration required to elicit EC20 response in each TET condition (PDF)
ORCID
Sean P. Moran: 0000-0001-7672-242X
Craig W. Lindsley: 0000-0003-0168-1445
Author Contributions
S.P.M. and H.P.C. contributed equally. SPM, JMR, CWL, CMN and PJC designed experiments; SPM, HPC, DHR, JM, and JWD performed experiments; SPM, HPC, and PJC wrote the manuscript with input from all the authors.
Notes
The authors declare the following competing financial interest(s): The authors are developing M1 PAMs for the treatment of schizophrenia and AD, and have an open-IND/Phase I trial for the same as well as a patent portfolio of M1 PAMs.
References
- 1.Bodick NC, Offen WW, Levey AI, Cutler NR, Gauthier SG, Satlin A, Shannon HE, Tollefson GD, Rasmussen K, Bymaster FP, Hurley DJ, Potter WZ, Paul SM. Effects of xanomeline, a selective muscarinic receptor agonist, on cognitive function and behavioral symptoms in Alzheimer disease. Arch Neurol. 1997;54:465–473. doi: 10.1001/archneur.1997.00550160091022. [DOI] [PubMed] [Google Scholar]
- 2.Shekhar A, Potter WZ, Lightfoot J, Lienemann J, Dubé S, Mallinckrodt C, Bymaster FP, McKinzie DL, Felder CC. Selective muscarinic receptor agonist xanomeline as a novel treatment approach for schizophrenia. Am J Psychiatry. 2008;165:1033–1039. doi: 10.1176/appi.ajp.2008.06091591. [DOI] [PubMed] [Google Scholar]
- 3.Bymaster FP, McKinzie DL, Felder CC, Wess J. Use of M1-M5 muscarinic receptor knockout mice as novel tools to delineate the physiological roles of the muscarinic cholinergic system. Neurochem Res. 2003;28:437–442. doi: 10.1023/a:1022844517200. [DOI] [PubMed] [Google Scholar]
- 4.Conn PJ, Jones CK, Lindsley CW. Subtype-selective allosteric modulators of muscarinic receptors for the treatment of CNS disorders. Trends Pharmacol Sci. 2009;30:148–155. doi: 10.1016/j.tips.2008.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yohn SE, Conn PJ. Positive allosteric modulation of M1 and M4 muscarinic receptors as potential therapeutic treatments for schizophrenia. Neuropharmacology. 2017 doi: 10.1016/j.neuropharm.2017.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wei J, Walton EA, Milici A, Buccafusco JJ. m1-m5 muscarinic receptor distribution in rat CNS by RT-PCR and HPLC. J Neurochem. 1994;63:815–821. doi: 10.1046/j.1471-4159.1994.63030815.x. [DOI] [PubMed] [Google Scholar]
- 7.Davoren JE, Garnsey M, Pettersen B, Brodney MA, Edgerton JR, Fortin JP, Grimwood S, Harris AR, Jenkinson S, Kenakin T, Lazzaro JT, Lee CW, Lotarski SM, Nottebaum L, O’Neil SV, Popiolek M, Ramsey S, Steyn SJ, Thorn CA, Zhang L, Webb D. Design and Synthesis of γ- and δ-Lactam M1 Positive Allosteric Modulators (PAMs): Convulsion and Cholinergic Toxicity of an M1-Selective PAM with Weak Agonist Activity. J Med Chem. 2017;60:6649–6663. doi: 10.1021/acs.jmedchem.7b00597. [DOI] [PubMed] [Google Scholar]
- 8.Gould RW, Dencker D, Grannan M, Bubser M, Zhan X, Wess J, Xiang Z, Locuson C, Lindsley CW, Conn PJ, Jones CK. Role for the M1Muscarinic Acetylcholine Receptor in Top-Down Cognitive Processing Using a Touchscreen Visual Discrimination Task in Mice. ACS Chem Neurosci. 2015;6:1683–1695. doi: 10.1021/acschemneuro.5b00123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Miyauchi M, Neugebauer NM, Sato T, Ardehali H, Meltzer HY. Muscarinic receptor signaling contributes to atypical antipsychotic drug reversal of the phencyclidine-induced deficit in novel object recognition in rats. J Psychopharmacol (London, U K) 2017;31:1588–1604. doi: 10.1177/0269881117731278. [DOI] [PubMed] [Google Scholar]
- 10.Anagnostaras SG, Murphy GG, Hamilton SE, Mitchell SL, Rahnama NP, Nathanson NM, Silva AJ. Selective cognitive dysfunction in acetylcholine M1 muscarinic receptor mutant mice. Nat Neurosci. 2003;6:51–58. doi: 10.1038/nn992. [DOI] [PubMed] [Google Scholar]
- 11.Ma L, Seager MA, Wittmann M, Jacobson M, Bickel D, Burno M, Jones K, Graufelds VK, Xu G, Pearson M, McCampbell A, Gaspar R, Shughrue P, Danziger A, Regan C, Flick R, Pascarella D, Garson S, Doran S, Kreatsoulas C, Veng L, Lindsley CW, Shipe W, Kuduk S, Sur C, Kinney G, Seabrook GR, Ray WJ. Selective activation of the M1 muscarinic acetylcholine receptor achieved by allosteric potentiation. Proc Natl Acad Sci U S A. 2009;106:15950–15955. doi: 10.1073/pnas.0900903106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shirey JK, Brady AE, Jones PJ, Davis AA, Bridges TM, Kennedy JP, Jadhav SB, Menon UN, Xiang Z, Watson ML, Christian EP, Doherty JJ, Quirk MC, Snyder DH, Lah JJ, Levey AI, Nicolle MM, Lindsley CW, Conn PJ. A selective allosteric potentiator of the M1 muscarinic acetylcholine receptor increases activity of medial prefrontal cortical neurons and restores impairments in reversal learning. J Neurosci. 2009;29:14271–14286. doi: 10.1523/JNEUROSCI.3930-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ghoshal A, Rook JM, Dickerson JW, Roop GN, Morrison RD, Jalan-Sakrikar N, Lamsal A, Noetzel MJ, Poslusney MS, Wood MR, Melancon BJ, Stauffer SR, Xiang Z, Daniels JS, Niswender CM, Jones CK, Lindsley CW, Conn PJ. Potentiation of M1 muscarinic receptor reverses plasticity deficits and negative and cognitive symptoms in a schizophrenia mouse model. Neuropsychopharmacology. 2016;41:598–610. doi: 10.1038/npp.2015.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grannan MD, Mielnik CA, Moran SP, Gould RW, Ball J, Lu Z, Bubser M, Ramsey AJ, Abe M, Cho HP, Nance KD, Blobaum AL, Niswender CM, Conn PJ, Lindsley CW, Jones CK. Prefrontal Cortex-Mediated Impairments in a Genetic Model of NMDA Receptor Hypofunction Are Reversed by the Novel M1 PAM VU6004256. ACS Chem Neurosci. 2016;7:1706–1716. doi: 10.1021/acschemneuro.6b00230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Choy KHC, Shackleford DM, Malone DT, Mistry SN, Patil RT, Scammells PJ, Langmead CJ, Pantelis C, Sexton PM, Lane JR, Christopoulos A. Positive allosteric modulation of the muscarinic M1 receptor improves efficacy of antipsychotics in mouse glutamatergic deficit models of behavior. J Pharmacol Exp Ther. 2016;359:354–365. doi: 10.1124/jpet.116.235788. [DOI] [PubMed] [Google Scholar]
- 16.Moran SP, Dickerson JW, Cho HP, Xiang Z, Maksymetz J, Remke DH, Lv X, Doyle CA, Rajan DH, Niswender CM, Engers DW, Lindsley CW, Rook JM, Conn PJ. M1-positive allosteric modulators lacking agonist activity provide the optimal profile for enhancing cognition. Neuropsychopharmacology. 2018 doi: 10.1038/s41386-018-0033-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Noetzel MJ, Rook JM, Vinson PN, Cho HP, Days E, Zhou Y, Rodriguez AL, Lavreysen H, Stauffer SR, Niswender CM, Xiang Z, Daniels JS, Jones CK, Lindsley CW, Weaver CD, Conn PJ. Functional impact of allosteric agonist activity of selective positive allosteric modulators of metabotropic glutamate receptor subtype 5 in regulating central nervous system function. Mol Pharmacol. 2012;81:120–133. doi: 10.1124/mol.111.075184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rook JM, Noetzel MJ, Pouliot WA, Bridges TM, Vinson PN, Cho HP, Zhou Y, Gogliotti RD, Manka JT, Gregory KJ, Stauffer SR, Dudek FE, Xiang Z, Niswender CM, Daniels JS, Jones CK, Lindsley CW, Conn PJ. Unique signaling profiles of positive allosteric modulators of metabotropic glutamate receptor subtype 5 determine differences in in vivo activity. Biol Psychiatry. 2013;73:501–509. doi: 10.1016/j.biopsych.2012.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chan WY, McKinzie DL, Bose S, Mitchell SN, Witkin JM, Thompson RC, Christopoulos A, Lazareno S, Birdsall NJM, Bymaster FP, Felder CC. Allosteric modulation of the muscarinic M4 receptor as an approach to treating schizophrenia. Proc Natl Acad Sci U S A. 2008;105:10978–10983. doi: 10.1073/pnas.0800567105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wood MR, Noetzel MJ, Poslusney MS, Melancon BJ, Tarr JC, Lamsal A, Chang S, Luscombe VB, Weiner RL, Cho HP, Bubser M, Jones CK, Niswender CM, Wood MW, Engers DW, Brandon NJ, Duggan ME, Conn PJ, Bridges TM, Lindsley CW. Challenges in the development of an M4 PAM in vivo tool compound: The discovery of VU0467154 and unexpected DMPK profiles of close analogs. Bioorg Med Chem Lett. 2017;27:171–175. doi: 10.1016/j.bmcl.2016.11.086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Digby GJ, Noetzel MJ, Bubser M, Utley TJ, Walker AG, Byun NE, Lebois EP, Xiang Z, Sheffler DJ, Cho HP, Davis AA, Nemirovsky NE, Mennenga SE, Camp BW, Bimonte-Nelson HA, Bode J, Italiano K, Morrison R, Daniels JS, Niswender CM, Olive MF, Lindsley CW, Jones CK, Conn PJ. Novel allosteric agonists of M1 muscarinic acetylcholine receptors induce brain region-specific responses that correspond with behavioral effects in animal models. J Neurosci. 2012;32:8532–8544. doi: 10.1523/JNEUROSCI.0337-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Digby GJ, Utley TJ, Lamsal A, Sevel C, Sheffler DJ, Lebois EP, Bridges TM, Wood MR, Niswender CM, Lindsley CW, Conn PJ. Chemical modification of the M(1) agonist VU0364572 reveals molecular switches in pharmacology and a bitopic binding mode. ACS Chem Neurosci. 2012;3:1025–1036. doi: 10.1021/cn300103e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Porter AC, Bymaster FP, DeLapp NW, Yamada M, Wess J, Hamilton SE, Nathanson NM, Felder CC. M1 muscarinic receptor signaling in mouse hippocampus and cortex. Brain Res. 2002;944:82–89. doi: 10.1016/s0006-8993(02)02721-x. [DOI] [PubMed] [Google Scholar]
- 24.Martin HGS, Bernabeu A, Lassalle O, Bouille C, Beurrier C, Pelissier-Alicot AL, Manzoni OJ. Endocannabinoids Mediate Muscarinic Acetylcholine Receptor-Dependent Long-Term Depression in the Adult Medial Prefrontal Cortex. Front Cell Neurosci. 2015;9:457. doi: 10.3389/fncel.2015.00457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sheffler DJ, Williams R, Bridges TM, Xiang Z, Kane AS, Byun NE, Jadhav S, Mock MM, Zheng F, Lewis LM, Jones CK, Niswender CM, Weaver CD, Lindsley CW, Conn PJ. A novel selective muscarinic acetylcholine receptor subtype 1 antagonist reduces seizures without impairing hippocampus-dependent learning. Mol Pharmacol. 2009;76:356–368. doi: 10.1124/mol.109.056531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rook JM, Abe M, Cho HP, Nance KD, Luscombe VB, Adams JJ, Dickerson JW, Remke DH, Garcia-Barrantes PM, Engers DW, Engers JL, Chang S, Foster JJ, Blobaum AL, Niswender CM, Jones CK, Conn PJ, Lindsley CW. Diverse Effects on M1 Signaling and Adverse Effect Liability within a Series of M1 Ago-PAMs. ACS Chem Neurosci. 2017;8:866–883. doi: 10.1021/acschemneuro.6b00429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bridges TM, Rook JM, Noetzel MJ, Morrison RD, Zhou Y, Gogliotti RD, Vinson PN, Xiang Z, Jones CK, Niswender CM, Lindsley CW, Stauffer SR, Conn PJ, Daniels JS. Biotransformation of a novel positive allosteric modulator of metabotropic glutamate receptor subtype 5 contributes to seizure-like adverse events in rats involving a receptor agonism-dependent mechanism. Drug Metab Dispos. 2013;41:1703–1714. doi: 10.1124/dmd.113.052084. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


