Abstract
Aortic aneurysms are morbid conditions that can lead to rupture or dissection and are categorized as thoracic (TAA) or abdominal aortic aneurysms (AAA) depending on their location. While AAA shares overlapping risk factors with atherosclerotic cardiovascular disease, TAA exhibits strong heritability. Human genetic studies in the past two decades have successfully identified numerous genes involved in both familial and sporadic forms of aortic aneurysm. In this review we will discuss the genetic basis of aortic aneurysm, focusing on the extracellular matrix and how insights from these studies have informed our understanding of human biology and disease pathogenesis.
INTRODUCTION
Aortic aneurysms comprise a heterogeneous set of morbid conditions that can lead to vessel rupture or dissection, contributing to > 16,000 deaths annually in the United States [1] and typically occur in either the abdominal aorta (AAA) or thoracic aorta (TAA). This important dichotomy not only distinguishes the anatomic location where these aneyrusms occur but also provides mechanistic distinctions, as the molecular causes and pathobiology of these conditions differ.
AAA, the more common of these two forms, shares many risk factors with atherosclerotic cardiovascular disease (ASCVD), such as male sex, older age, elevated plasma cholesterol, and smoking [2]. Beyond a significant overlapping risk profile with ASCVD, inherited factors also play a role in the development of AAA. For example, a positive family history doubles the risk of developing AAA [3]. By contrast, TAA is not associated with typical ASCVD risk factors, instead exhibiting much stronger heritability, where risk of disease increases with the number of affected relatives [4]. Many patients with TAA exhibit a pattern of Mendelian inheritance [5], suggesting the presence of a single gene mutation conferring risk of disease in individual pedigrees. Indeed, some of these familial TAA patients harbor established monogenic forms of disease such as Marfan or Loeys-Dietz syndromes. However, up to 20% of individuals with thoracic disease do not have a syndrome but do have related family members with disease (termed Familial Thoracic Aortic Aneurysm and Dissection or FTAAD).
Human genetic studies of individuals and families presenting with aortic aneurysmal disease have been successful in mapping multiple genes underlying these conditions. Subsequent model system and in vitro studies have provided mechanistic insights into how these mutated genes contribute to disease pathogenesis. By identifying the molecular etiology and mechanism of aortic aneurysmal diseases, these studies have highlighted smooth muscle function and components of the extracellular matrix (ECM), including elastic fibers, collagen fibers, and members of the TGF-β signaling pathway that are critical for normal development and structural integrity of the vessel wall. In the following, we discuss syndromic and non-syndromic manifestations of aortic aneurysm, highlighting the genes involved in these processes (with a particular focus on the ECM) and how they have informed our understanding of human biology and disease pathogenesis.
STRUCTURE AND FUNCTION OF THE AORTIC ECM
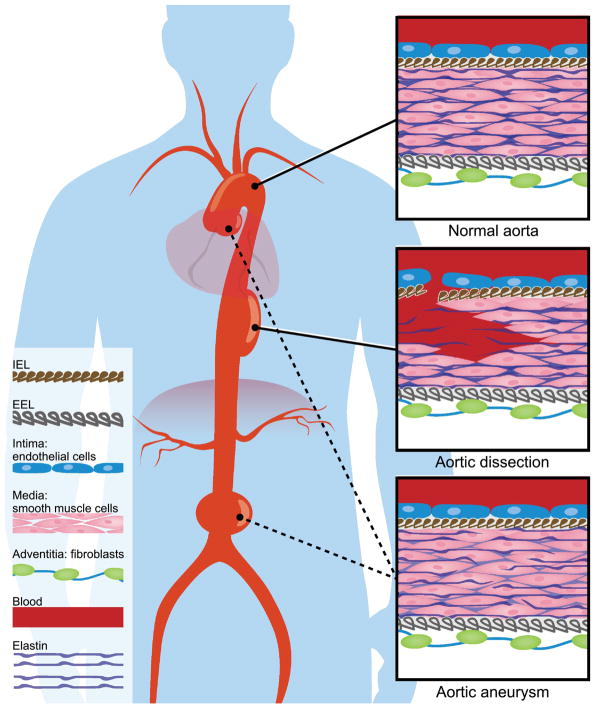
The aorta is composed of three layers: a thin inner layer or tunica intima, a thick middle layer mostly composed of smooth muscle cells (tunica media), and an outer layer called tunica adventitia (Figure 1). The tunica intima consists of a single layer of endothelial cells with an associated sub-endothelial matrix. The tunica media is a thick muscular layer consisting of smooth muscle cells and elastic lamellae, interspersed by collagen fibers and proteoglycan-rich extracellular matrix. The tunica media is bound by the internal elastic lamella (IEL) in the luminal border and external elastic lamella (EEL) in the abluminal border. The outermost layer, tunica adventitia, resides external to the EEL. The tunica adventitia is composed of a collagen-rich ECM and is populated by fibroblast cells. Small arterioles and venules supplying the aorta (vasa vasorum) as well as nerve bundles also reside in this layer.
Figure 1.
The aorta is an elastic reservoir that absorbs the systolic pulsatile blood flow from the heart before distributing that force forward during diastole via the Windkessel effect. The mechanical properties of the arterial wall are mediated by collagen and elastic fibers. These two major constituents of the ECM reside in different yet overlapping anatomic locations and serve distinct functions. The elastic fibers, distensible with a low tensile strength, are woven in an interconnected lamellar network in the tunica media throughout the vessel wall [6]. Collagens are circumferentially aligned fibers that provide tensile strength at higher pressures [6]. The distribution of collagen in the aorta exhibits spatial specificity: collagen types I, III and IV colocalize in the intimal and medial layers in the ascending aorta, while types I and IV do so in the desending segments (type III shows variability in this region [7]). Type III collagen is heavily expressed in the adventitial layer of the entire aorta where they are organized in arrays of fibers [7]. The most common histological finding in TAA, irrespective of its specific etiology, is cystic medial degeneration, characterized by fragmentation and loss of elastic tissue, depletion of vascular smooth muscle cells, defects in collagen microarchitecture, and increased extracellular mucin deposition[8–10], suggesting a final common mechanism centered on ECM disruption.
GENES ENCODING COMPONENTS OF THE AORTIC ECM
The elastic lamellae consist of elastic fibers, formed by polymerized elastin and microfibrils. ELN encodes tropoelastin, characterized by hydrophobic sequences alternating with lysine-containing, cross-linking modules [6]. The lysine residues of tropoelastin are cross-linked by lysyl oxidases [11], forming the molecular basis of its physical elastic properties. Tropoelastin self-assembles into polymer networks of elastin, which then deposits onto microfibrils to form elastic fibers, a process facilitated by fibrillin proteins [12]. Microfibrils are filamentous structures 10–15 nm in diameter, composed of polymerized fibrillins [13]. Fibrillins are large, cysteine-rich glycoproteins that have calcium-binding epidermal growth factor-like (cb-EGF) and 8-cysteine-rich domains. Three fibrillin genes (FBN1, FBN2, and FBN3) exist in the human genome, whereas Fbn3 appears to have been inactivated in the mouse via chromosomal fission [14]. Expression of Fbn2 is seen early in development during early elastogenesis and persists for a shorter period of time while Fbn1 is expressed later, corresponding to the formation of organ structures in a pattern similar to elastin [15]. Eln expression begins during development and increases until early postnatal period, then decreases sharply to low levels in adulthood [16].
Microfibrils can associate with other ECM components, such as fibulins, latent transforming growth factor (TGF)-β-binding proteins (LTBPs), and microfibril-associated glycoproteins (MAGPs). The fibulin family consists of 7 ECM proteins that also contain cb-EGF and unique carboxy-terminal modules. These proteins, evolutionarily conserved as early as C. elegans, have a multitude of protein ligands [17], and hence have been hypothesized to function as molecular connections that stabilize the ECM structures, such as elastic fibers and basement membranes [18]. Four LTBPs exist in the human genome. They, similar to fibrillins, have cb-EGF and 8-cystein domains [19]. While fibrillins are evolutionarily conserved as far back as C. elegans, LTBPs are conserved to deuterostomes, suggesting LTBPs evolved later than fibrillins [20]. LTBPs can bind propeptides of TGF-β, thus helping the assembly and secretion of TGF-β molecules [21] and modulating extracellular TGF-β signaling [22]. MAGP-1 and MAGP-2 are extracellular proteins that can interact with TGF-β factors, NOTCH and their ligands, as well as elastic fiber proteins. They interact with fibrillin to modulate microfibril function [6].
Collagens have a triple helical domain of repeats with glycine occupying every third amino acid residue (Gly-X-Y). Types I, III, IV, V, and VI collagens, encoded by the genes COL1A1, COL1A2, COL3A1, COL3A1, COL4A1, COL4A2, COL5A1, COL5A2, and COL5A3, are expressed in the human aorta, with type I constituting two thirds of the total collagen [23]. Spatial heterogeneity is present in the expression pattern of collagen. Types I, III and V collagen are present in all layers of the aorta with type I most predominant in the intima and adventitia, type III slightly more abundant in the media, and type V greater in the intima. Types IV and VI are found mostly in the intima [23]. The expression pattern of collagen in the aorta might underlie the different phenotypes observed in patients and animal models of different collagen mutations. Collagens type I, II, III and V are fibril- forming collagens that form fibers within tissues to impart structural support. Types IV, VIII and X collagens are network-forming proteins. Type IV collagen is a major structural protein of the basement membrane. As these ECM molecules collectively provide tensile and structural strength to the vessel wall, it is perhaps not surprising that mutations in the genes encoding them may result in aneurysmal diseases.
INHERETED BASIS OF AORTIC ANEURYSM
As more genes whose mutations are involved in genetic aneurysmal diseases are identified, a theme emerges whereby genes involved in elastic fibers, collagen fibers, TGF-β signaling, and smooth muscle function are critical in the formation of aortic aneurysm (Table 1).
Table 1.
Inhereted Basis Of Aortic Aneurysm
| Gene | Location | Phenotype | Inheritance | OMIM | Ref |
|---|---|---|---|---|---|
| Mutations affecting the elastic fiber | |||||
| ELN | 7q11.23 | Cutis laxa/TAA | AD, AR | 123700 | [26] |
| LOX | 5q23.1 | FTAAD | AD | 617168 | [34–36] |
| EFEMP2/FBLN4 | 11q13.1 | Cutis laxa/TAA | AR | 614437 | [37–40, 42] |
| MFAP5 | 12p13.31 | FTAAD | AD | 616166 | [55, 57] |
| Mutations affecting the collagen fiber | |||||
| COL1A1 | 17q21.33 | Classic EDS | AD | 130000 | [58–60] |
| COL1A2 | 7q21.3 | EDS type 7B | AD | 130060 | [58, 59] |
| COL3A1 | 2q32.2 | EDS type 4 | AD | 130050 | [62, 63] |
| COL4A1 | 13q34 | HANAC syndrome | AD | 611773 | [65] |
| COL4A5 | Xp22.3 | Alport syndrome | X-linked | 301050 | [69] |
| PLOD1 | 1p36.22 | EDS type 6 | AR | 225400 | [73–75] |
| BGN | Xq28 | FTAAD | X-linked | 300989 | [79] |
| Mutations affecting the TGF-β signaling | |||||
| FBN1 | 15q21.1 | Marfan syndrome, TAAD, AAA, BAV | AD, sporadic | 154700 | [84, 85, 119, 121, 123] |
| SKI | 1p36.33-32 | SGS | AD | 182212 | [90] |
| TGFBR1 | 9q22.33 | LDS type 1 | AD | 609192 | [92] |
| TGFBR2 | 3p24.1 | LDS type 2, BAV | AD, sporadic | 610168 | [92, 119] |
| SMAD3 | 15q22.33 | LDS type 3 | AD | 613795 | [93] |
| SMAD6 | 15q22.31 | BAV | AD | 614823 | [120] |
| TGFB2 | 1q41 | LDS type 4 | AD | 614816 | [94] |
| TGFB3 | 14q24.3 | LDS type 5 | AD | 615582 | [95, 96] |
| Mutations affecting smooth muscle | |||||
| MYH11 | 16p13.11 | FTAAD, PDA | AD | 132900 | [105, 106] |
| ACTA2 | 10q23.31 | FTAAD, BAV | AD | 611788 | [109] |
| Other loci associated with aortic aneurysm | |||||
| LRP1 | 12q13.3 | TAA, AAA | Sporadic | [123, 124] | |
| ULK4 | 3p22.1 | TAA | Sporadic | [123] | |
| DAB2IP | 9p33.2 | AAA | Sporadic | [125] | |
| LDLR | 19p13.2 | AAA | Sporadic | [126] | |
| SORT1 | 1p13.3 | AAA | Sporadic | [127] | |
| CDKN2BAS1/ANRIL | 9p21.3 | AAA | Sporadic | [128] | |
| SMYD2 | 1q32.3 | AAA | Sporadic | [129] | |
| LINC00540 | 13q12.11 | AAA | Sporadic | [129] | |
| PCIF1 | 20q13.12 | AAA | Sporadic | [129] | |
| ERG | 21q22.2 | AAA | Sporadic | [129] | |
Mutations affecting the elastic fiber
Mutations in ELN
Mutations in ELN can cause autosomal dominant and autosomal recessive forms of cutis laxa, a systemic disorder of connective tissues that manifest as progressive loosening of the skin, as well as disorders of organs containing elastic tissue, such as the pulmonary and cardiovascular systems [24, 25]. Patients with the autosomal dominant form have a normal lifespan, whereas those with the autosomal recessive form are at risk for early morbidity and mortality due to cardiopulmonary conditions, such as emphysema or thoracic aortic aneurysm [25]. Individuals with autosomal dominant cutis laxa may also manifest aortic aneurysm as reported by Szabo et al. who found a dominantly inherited 25-bp ELN deletion and a de novo 1-bp ELN deletion (both of which shifted the open reading frame and induced premature termination codons) in individuals with cutis laxa and aortic aneurysm [26]. Histological analysis of aneurysmal aortas from affected individuals revealed a paucity of elastic fibers and classic characteristics of medial degeneration.
Microdeletions in chromosome 7q11.23, which encompasses a region up to 1.5–1.8 Mb containing 26–28 genes including ELN [27], causes Williams-Beuren syndrome, also known as Williams syndrome. Willams syndrome is a multi-organ disorder including mental retardation, ebullient personality, elf-like facies, failure to thrive, endocrine abnormalities, and supravalvular aortic stenosis (SVAS) [27]. Non-syndromic forms of SVAS have been described and mapped to disruption in ELN [28, 29]. Stenotic changes, found in 79–90% of Williams syndrome patients, may less commonly involve other medium and large arteries, such as pulmonary, intracranial, coronary, mesenteric, and renal arteries [27]. These obstructive lesions are characterized histologically by atherosclerosis- independent medial hypertrophy due to excessive smooth muscle proliferation, as well as fragmentation of elastin in the tunica media [30]. Cardiovascular events are the most common cause of death in Williams syndrome patients [27]. Beyond stenotic lesions, aneurysmal changes of the coronary artery and aorta also have been reported in patients with Williams syndrome [31, 32]. It is interesting to note that these conditions, attributed to alterations in the same locus, manifest non-overlapping symptomatology. A corollary can be found in the blood pressure phenotype in Eln heterozygous mice, which varies with genetic background [33]. It is possible that other genetic modifiers, especially loci involved in smooth muscle function, may interact with elastin to modulate aortic stenosis or aneurysm. Further studies are needed to provide additional insights into the phenotypes of ELN gene alterations.
Mutations in LOX
Lysyl oxidase (LOX) and its related gene family members are a group of copper-dependent oxidodeaminases that cross-link lysyl residues on these structural proteins in the process of forming proper elastic lamellae and collagen fibers. Lox knockout mice die perinatally due to aortic aneurysm associated with aortic regurgitation [34]. However, not until recently did whole genome [35] and exome [36] sequencing identify mutations in LOX as a cause of FTAAD in humans. In one family, a missense mutation in LOX (c.893T > G, p.Met298Arg) was found to segregate with disease. This mutation was “knocked-in” at the orthologous position in the mouse genome using CRISPR/Cas9 technology. Mice homozygous for the mutant allele had markedly reduced Lox activity, discontinuous elastic lamellae in the aortic wall, and died perinatally due to aortic aneurysm and dissection, recapitulating the human phenotype [35]. Pathogenic mutations in LOX appear to result in loss of function [35, 36], supporting a model of haploinsufficiency in humans. Interestingly, the phenotype was not compensated by other Lox family members (Loxl1, Loxl2, Loxl3, and Loxl4), supporting the functional non-redundancy of Lox family proteins.
Mutations in EFEMP2/FBLN4
Autosomal recessive mutations of EFEMP2, also known as FBLN4, which encodes fibulin-4, have been found in human patients with autosomal recessive cutis laxa, arachnodactyly, ascending aortic aneurysm, aortic tortuosity, and hypoplasia of the pulmonary arteries [37–40]. Pathogenic mutations, typically missense or frameshift alterations [37, 40, 41], tend to occur in the cb-EGF domain [41]. Immunohistochemistry of aortic tissue from carriers demonstrated increased TGF-β protein expression and increased downstream pSMAD2 staining [40]. Animal models of fibulin-4 deficiency exhibit phenotypes similar to humans. Mice homozygous with a knocked-in Fbln4 allele corresponding to a recurrent human missense mutation (c.169G > A, p.Glu57Lys) recapitulated findings in autosomal recessive cutis laxa, such as loose skin, aortic aneurysm, arterial tortuosity, emphysema, and skeletal changes [42]. Conditional deletion of Fbln4 in smooth muscle cells caused disorganized thick elastic laminae with aberrant deposition of elastin, increasing arterial stiffness [43]. Fbln4 null mice are not viable and were found to have irregular elastin aggregates instead of intact elastic fibers in the aorta [44]. Functionally, Fbln4 interacts with the pro-peptide of Lox, promoting the assembly of Lox onto tropoelastin during elastogenesis [43]. Therefore, fibulin-4 appears to provide a link between the structural components of the elastic fiber and their crosslinking enzymes.
In contrast to the established role of FBLN4, the role of FBLN5 in aortic aneurysm has not been clearly defined. The expression of fibulin-5 was noted to be decreased in human aneurysmal abdominal aorta [45], but a study of common polymorphisms in FBLN5 failed to find an association with aortic aneurysm [46]. Patients with FBLN5 mutations can present with autosomal recessive or autosomal dominant cutis laxa, typically caused by missense mutations or duplications in the cb-EGF domains [47–49]. While SVAS may be present, aortic aneurysm or dissection has not been reported in patients with FBLN5 mutations [47]. In contrast to the lethality in Fbln4 null mice, Fbln5 null mice enjoy a normal lifespan without evidence of aortic aneurysm or dissection [50, 51]. They develop eastic fiber defects such as an elongated aorta with tortuosity, emphysema, and pelvic organ prolapse [50, 52]. While sharing binding partners in elastogenesis, Fbln4 exhibits strong binding with Lox, whereas Fbln5 binds Lox with less affinity [53]. Fbln5, in contrast, recruits Loxl1 to elastic fibers [54]. The lethal phenotypes in Fbln4 and Lox mutants, compared to the milder elastogenic defects in Fbln5 and Loxl1 mutants, suggest that these phenotypic differences may be explained by their differential affinity to binding partners.
Mutations in MAGP-2
Missense and nonsense mutations in MFAP5 which encodes MAGP-2 have been identified in French families with FTAAD [55]. The aortic tissue of affected individuals had elevated pSmad2/3 expression, attributed to reduced TGF-β sequestration. Mis-regulation of mfap2 expression in zebrafish via either morpholino knockdown or overexpression both leads to dilated blood vessels, indicative of a critical dose of mfap2 in blood vessel formation [56]. Mice lacking either Mfap2 (encoding MAGP-1) or Mfap5 exhibit minimal vascular phenotypes, whereas those lacking both exhibit blood vessel dilation [57]. In contrast, humans with FTAAD caused by MFAP5 do not also harbor mutations in MFAP2.
Mutations affecting the collagen fiber
Mutations in type I collagen
Type I collagen is an abundant protein in many tissues including bone, skin, ligaments, tendons, and blood vessel walls. It consists of a heterotrimer with 2 pro-α1(I)- and 1 pro-α2(I)-collagen chains, encoded by the COL1A1 and COL1A2 genes. The central triple helical domain of these pro-α-chains contains repeating Gly-X-Y triplet amino acids, with hydroxyproline and arginine being the most common amino acids in the Y position. Mutations in type I collagen genes, especially missense mutations affecting the glycine residues, cause osteogenesis imperfecta. Rare non-glycine substitutions may result in connective tissue disorders, such as osteogenesis imperfecta, classic Ehlers-Danlos syndrome, or infantile cortical hyperostosis. Heterozygous missense mutations in COL1A1 or COL1A2 causing substitutions of arginine in the Y position to cysteine or glycine have been described in patients with aneurysms of the aorta, femoral artery, or renal artery [58, 59]. These Arg-to-Cys substitutions lead to a disturbance in collagen fibrillogenesis, resulting in collagen fibrils with variable diameters and irregular interfibrillar spaces [58]. A mouse model of a homozygous 1.3-kb deletion in the first intron of Col1a1 had reduced expression of the type I α1 chain, decreased collagen content in the aorta and developed aortic aneurysm, subsequently dying from aortic dissection [60].
Mutations in type III collagen
Ehlers-Danlos syndrome (EDS) type IV (also known as vascular EDS), is one of the most severe forms of connective tissue disease. Patients with EDS type IV, transmitted in an autosomal dominant fashion, develop easy bruising, skin with visible veins, characteristic facial features, and rupture of arteries, uterus, or intestines [61]. These patients suffer from reduced survival compared to the general population due to arterial dissection or rupture [61]. EDS type IV was first reported due to a deletion in the COL3A1 locus [62] in 2000, the first single-gene linked to aneurysmal disorders [63]. Additional reported pathogenic mutations include exonic deletions, intronic deletions, missense mutations, and splice-site mutations. The type of mutation may associate with disease severity as null mutations seem to portend a more favorable outcome compared with missense substitutions at glycine residues or mutations at the canonical sites of pre-mRNA splicing [61]. Murine mutant alleles of Col3a1 have been generated and most mice with homozygous Col3a1 mutations die perinatally; those surviving die from ruptured large blood vessels [64]. The mutant mice have decreased number of type I collagen fibrils with variable diameter. Type III collagen therefore have an important regulatory role in type I collagen fibrillogenesis.
Mutations in type IV collagen
Mutations in COL4A1 have been identified in patients with Hereditary Angiopathy with Neuropathy, Aneurysms, and Muscle Cramps (HANAC) syndrome, an autosomal dominant disease whose clinical manifestation includes aneurysms of the internal carotid arteries and cerebral arteries [65]. HANAC syndrome arises from missense mutations that cause substitutions of glycine in the Gly-X-Y repeats, although other glycine-substituting missense mutations of COL4A1 were found in patients with small vessel disease involving retinal vessels and cerebral hemorrhage, but not aneurysm [66]. Therefore, mutations in COL4A1 may have multiple phenotypic consequences. In mice, deficiency of Col4a1 causes mid-gestation embryonic lethality associated with structural deficiencies in the basement membrane [67]. By contrast, mice carrying the heterozygous Col4a1Raw (retinal arteriolar wiring) allele harbor detachment of endothelium from the tunica media in the descending aorta. Their vascular smooth muscle cells exhibit reduced contractile strength and their endothelial cells have reduced reaction to NO-mediated vasodilation [68]. These findings may underlie the vascular pathogenesis of HANAC syndrome.
The X-linked Alport Syndrome, characterized by progressive renal dysfunction and abnormalities of the ears and eyes, arise from mutations in COL4A5, which encodes the α5(IV) chain. A small number of cases of TAA and dissection have been reported in male patients with Alport syndrome [69]. A mutant mouse harboring an analogous human nonsense mutation (c.231G > T, p.Glu5X), however, was not found to have aortic aneurysm [70]. While renal dysfunction and chronic hypertension are risk factors for aortic disease in young patients [71], the findings of thoracic rather than abdominal disease, young age at disease onset (13–32 years of age), and short duration between end-stage renal disease and TAAD (3–12 years), suggest that renal disease and hypertension may be facilitators and not primary driving causes of TAAD in patients with Alport syndrome. Supporting this notion is the observation that in the International Registry of Aortic Dissection (IRAD), patients < 40 years of age were less likely to have traditional risk factors (e.g., hypertension) and more likely to have syndromic (e.g., Marfan syndrome) presentation [72]. Therefore, the mechanism and role of COL4A5 in aneurysm remains elusive.
Mutations in lysyl hydroxylase 1
More than 20 mutations in lysyl hydroxylase, encoded by PLOD1, have been linked to the molecular genetics of Ehlers-Danlos syndrome type VI [73]. In rare occurrences these patients have been reported to have spontaneous rupture of the intracranial, vertebral, intrathoracic, or femoral arteries [74]. It is not clear whether these ruptures involve aneurysmal dilation. A small portion of the Plod1 mutant mice died suddenly which was attributed to aortic dissection [75]. While Plod1 mutant mice have no change in the structure or thickness of their aortic walls, microstructural abnormalities including vacuolization and mitochondrial swelling have been observed in the mutant aortic wall smooth muscle cells, corresponding with the site of Plod1 expression.
Mutations in BGN
Biglycan, encoded by BGN, belongs to the small leucine-rich proteoglycan (SLRP) family of secreted proteoglycans. Expressed in all layers of the aorta [76], biglycan interacts with many ECM components including collagen, elastin, and microfibrils [77] and is involved in collagen fibrillogenesis [78]. A recent study identified nonsense and missense BGN mutations (c.5G>A, p.Trp2*; c.908A>C, p.Gln303Pro) in patients with FTAAD who carried a clinical diagnosis of Marfan syndrome without an identifiable molecular cause [79]. Targeted sequencing in another cohort of FTAAD patients revealed additional alterations in BGN predicted to result in loss-of-function, including a splice-site mutation and exonic deletions. Collagen content was reduced in the aorta of some affected individuals, whereas elastin fibers appeared normal. Aortas from these subjects also had increased pSMAD2 staining. Therefore, BGN mutations appear to affect collagen homeostasis in the aortic wall, potentially through the TGF-β pathway. The function of BGN may be modified by other genetic factors. Bgn-null mice in the BALB/c background exhibit aortic rupture [80], whereas those in the C57BL6 background were not found to have a vascular phenotype [81]. The locus responsible for this genetic interaction remains unclear.
FBN1, Marfan syndrome, and the TGF-β pathway
The Marfan syndrome is an autosomal dominant disease with a prevalence of 4–6 per 10,000 persons [82]. The classic syndrome involves abnormalities of the eye (ectopia lentis), aorta (ascending aortic aneurysm, aortic regurgitation), skeleton (long limbs, pectus deformity), and fingers (arachnodactyly, joint laxity). The revised Ghent nosology has been established to guide the clinical diagnosis of Marfan syndrome [83], placing an emphasis on findings of aortic dilation, ectopia lentis, and FBN1 mutations. FBN1 was identified as the causative gene for the Marfan syndrome more than 25 years ago [84]. Since then, more than 1800 causal point mutations have been identified [85]. The type and location of mutations appears to correlate with the phenotype as a higher proportion of patients with mutations in exons 24–32 develop ascending aortic aneurysm compared to those with mutations in other exons [86].
As fibrillin-1 associates with TGF-β-binding LTBPs, the molecular pathologenesis of FBN1 mutations has been attributed to upregulation of TGF-β signaling. Increased TGF-β signaling activity has been detected in the lung tissue in mice homozygous for a Fbn1 hypomorphic allele using a GFP reporter [87]. A neutralizing antibody to TGF-β rescues the lung maturation phenotype in the Fbn1 hypomorph mouse [87]. Mice harboring the Fbn1C1039G allele, orthologous to a pathogenic human mutation, exhibit increased nuclear pSmad2, suggestive of increased TGF-β signaling. These mice develop aortic aneurysm, a phenotype rescued by TGF-β-neutralizing antibody or losartan [88], presumably due to TGF-β pathway inhibition. These findings formed the basis for the large Pediatric Heart Network trial, which compared losartan versus atenolol, a β-blocker, in children and young adults with Marfan syndrome and aortic aneurysm [89]. This trial, however, did not demonstrate a superiority of losartan on inhibiting or ameliorating aortic growth compared to atenolol.
Other lines of genetic evidence add to the complexity of TGF-β signaling pathway in aneurysmal diseases. The Shprintzen-Goldberg syndrome (SGS) is characterized by mental retardation, skeletal muscle hypotonia, Marfanoid features, and aortic root aneurysm. SGS has been attributed to mutations in SKI, which encodes a negative regulator of TGF-β signaling [90]. Missense mutations or deletions in SKI disrupt the binding of SKI with SMAD proteins or transcriptional co-repressors, altering TGF-β signaling [91]. The Loeys-Dietz syndrome (LDS), described in 2005, manifests as arterial tortuosity along with aneurysms, craniofacial, neurocognitive and skeletal abnormalities [92]. LDS is caused by mutations in genes encoding proteins involved in the TGF-β pathway, including TGFBR1 [92], TGFBR2 [92], SMAD3 [93], TGFB2 [94], and TGFB3 [95, 96]. Mice with heterozygous knocked-in alleles of pathogenic, missense mutations from either Tfgbr1 or Tfgbr2 recapitulate the LDS phenotype; their vascular smooth muscle cells and those from LDS patients exhibit reduced signaling response to TGF-β ligands, indicating these are loss of function mutations [97]. Similarly, loss of function of TGF-β ligands also leads to an aneurysmal phenotype [95, 96]. Furthermore, heterozygous loss of Smad4 or Tgfb2, smooth muscle-specific deletion of Tgfbr2, or administration of an TGF-β-neutralizing antibody worsen the aortic aneurysm phenotype in the MFS mouse model [94, 98–101]. Although these lines of evidence point to a aneurysmal phenotype from reduced TGF-β signaling, aortic tissues from patients or mice with various genetic etiologies of LDS, including SMAD3, TGFB2, Tgfbr1, Tgfbr2 or Tgfb2, have increased expression of TGF-β ligand or pSmads [93, 94, 97]. Therefore, while it is clear that TGF-β pathway is genetically and mechanistically involved in the pathogenesis of thoracic aortic aneurysms, precisely how TGF-β signaling relates to the development of aneurysms remains unresolved [102].
How does one reconcile these seemingly contradictory findings? Some have proposed that TGF-β signaling may have dynamic and context-dependent effects on aneurysms where reduced TGF-β signaling contributes to the predisposition while increased TGF-β signaling promotes the progression [103]. Others point out the caveat of equating increased pSmad and TGF-β responsive genes as a pathognomonic signature of increased TGF-β signaling [102]. Finally, some suggest that TGF-β signaling is necessary for the proper development and differentiation of aortic smooth muscle cells and associated contractile-elastic units; failure of their appropriate development lead to altered mechanosensing, which in turn causes secondary, compensatory increases in TGF-β signaling [104]. It may be that this seeming contradiction stems from the conceptual disassociation of TGF-β and pSmad. At the ligand and receptor level, both genetic and non-genetic loss of TGF-β signaling causes an aneurysmal phenotype [92–101]. Finding increased expression of pSmad (or downstream genes) in aortic aneurysms does not prove causality [93, 94, 97]. Rather, it may be that increased pSmad is an adaptive, compensatory mechanism for aortic homeostasis in the face of aneurysm, instead of a pathogenic mechanism. Understanding precisely how the TGF-β pathway regulates aortic development, aging, and compensation for mechanical strength in a spatially and temporally controlled manner will require further research.
Mutations affecting the smooth muscle
As smooth muscle cells line the tunica media, it is not surprising that perturbation of smooth muscle cell function may be associated with altered blood vessel mechanics and dilation. A syndrome of TAAD with patent ductus arteriosus (PDA) was mapped to chromosome 16p12.2-p13.13. These patients have low aortic mechanical compliance and distensibility [105]. Their smooth muscle cells do not have an increase in TGF-β or its downstream targets [106]. Among the > 60 genes in this region, MYH11, encoding smooth muscle myosin heavy chain, was found to harbor mutations that correlated with the clinical phenotype [105, 106]. Recently, a moyamoya- like vasculopathy with stenosis of intracranial arteries, aorta and renal arteris, as well as PDA, has been reported in a patient with a MYH11 missense mutation (c.4604G > A, p.Arg1535Gln)[107]. In vitro expression of a mutant protein harboring a pathogenic allele showed co-immunoprecipitation with the tagged mutant protein, suggesting the mutants acts in a dominant- negative fashion [105]. Myh11 knockout mice exhibit perinatal lethality, developing PDA although aortic aneurysm has not been reported [108].
ACTA2 encodes smooth muscle α-actin and missense mutations in ACTA2 have been identified as a cause of FTAAD [109]. The mutations affect actin filament assembly and decrease smooth muscle contraction. Intriguingly, patients with ACTA2 mutations are also at increased risk for premature coronary artery disease, premature ischemic stroke, and a moyamoya-like phenotype [110]. Smooth muscle cells explanted from patients with ACTA2 mutation exhibited increased proliferation in vitro [110]. In animal models, Acta2-null mice were viable but exhibited reduced vascular contractility and blood pressure, although it is not clear whether Acta2 mutants developed aortic aneurysms [111]. These lines of evidence support a role of smooth muscle actin and smooth muscle myosin in occlusive vascular disease.
Vascular smooth muscle cells are known to play an important role in atherosclerosis, a form of occlusive vascular disease. Atherosclerotic plaques contain lipid-laden smooth muscle cells, which undergo phenotypic switching, reducing expression of smooth muscle cell markers (including MYH11 and ACTA2), and acquiring macrophage-associated markers [112]. By contrast, small arteries in MYH11 and ACTA2 mutations exhibit intimal thickening with strong expression of smooth muscle markers, medial fibrosis, and a paucity of lipids [106, 110, 113]. This distinct histopathology suggests that the pathogenic role of smooth muscle cells in patients with MYH11 or ACTA2 mutation may be different from those in atherosclerotic vascular diseases.
Bicuspid aortic valve and aortic aneurysm
Bicuspid aortic valve (BAV), the most common congenital cardiac defect, is found in 1–2% of the general population [1]. Bicuspid aortic valves are associated with ascending aortic aneurysms: about 50% of BAV patients younger than 30 years and about 90% older than 80 years have aortic dilation [114]. These patients have a risk of aortic dissection that is numerically low (3–45 per 10,000 patient-years) but higher than in the general population (8-fold increase in risk)[114]. BAV exhibits an autosomal dominant pattern of heritability with incomplete penetrance [115, 116]. Multiple genes and loci have been associated with BAV, including some in signaling pathways (NOTCH1 [117]) in addition to transcription factors (GATA family and NKX family transcription factors)[118]. Interestingly, mutations causing aortic aneurysmal diseases are also associated with increased risk for BAV. In a case-control study, bicuspid aortic valve was seen in 7% and 4% of patients with mutations in TGFBR2 and FBN1 respectively [119], a prevalence higher than the generally accepted 1–2% rate of BAV in the general population. Other loci involved in both conditions include SMAD6 [120], ACTA2, chromosome 7q11.3 (encompassing ELN), and COL3A1.
Complex genetics of aortic aneurysmal disease
Beyond Mendelian family studies aimed at discovering single gene mutations of large effect, there have been efforts to identify genetic loci underlying complex forms of aneurysmal diseases. The first genome-wide association study (GWAS) in non-syndromic TAA identified 15q21.1, encompassing FBN1, as a susceptibility locus associated with disease at genome-wide significance [121], a finding which was subsequently validated in a separate cohort [122]. Further GWAS of non-familial sporadic thoracic aortic dissection identified FBN1, LRP1, and ULK4 as loci associated with disease [123]. Parallel studies in AAA have now identified loci that are associated with disease at a genome-wide level of significance including LRP1 [124], DAB2IP [125], LDLR [126], SORT1 [127], and CDKN2BAS1/ANRIL [128] loci. Recent meta-analyses have revealed additional loci (SMYD2, LINC00540, PCIF1, ERG)[129] and genetically-determined plasma lipids [130] that associate with AAA. These studies not only highlight the genetic differences between these disorders but interestingly also find overlapping loci for both TAA and AAA with coronary atherosclerosis, myocardial infarction, and intracranial aneurysm [127, 128, 131], implying that common mechanisms may underlie these disparate vascular diseases. These observations may be relevant for informing future mechanistic studies and therapeutic development.
Tying it all together: the extracellular matrix
As reviewed above, genes causing aortic aneurysmal diseases can be loosely categorized into three overlapping functional domains: 1) those providing structural integrity to the ECM; 2) those involved in smooth muscle function; and 3) those involved in the TGF-β pathway. The shared pathological features of ECM disruption across the various genetic forms of aneurysmal disease suggest a final common pathophysiologic mechanism converging on the ECM. How does this all tie together?
The interface between the ECM and smooth muscle cells is an emerging nexus that may provide a unifying mechanism of TAA formation. In the normal aortic media, smooth muscle exhibits a contractile phenotype, whereas those in the aneurysmal aorta show a synthetic phenotype, characterized by increased proliferation and expression of ECM components or matrix metalloproteinases (MMPs), and processes involved in the synthesis, maintenance, and homeostasis of the ECM [104, 132]. Aortic ECM constituents connect to smooth muscle cells and their intracellular contractile filaments via adaptor structures, forming a matrix-cell complex that transduces mechanical properties in the ECM to regulate smooth muscle phenotype [104]. Such a mechanism couples ECM homeostasis with smooth muscle phenotypes. Smooth muscle cells isolated from TAAD showed a synthetic phenotype [133, 134], with increased expression of elastin- and collagen-degrading MMPs enriched at the site of dissection [135]. The smooth muscle phenotype switch precedes disruption of elastic fibers [136]. It is conceivable that mutations in ECM structural proteins also misregulate smooth muscle behavior via altered mechanics. Indeed, disrupted collagen or elastin in the ECM could promote smooth muscle cell proliferation and migration [137, 138]. MYH11 and ACTA2 encode proteins that form the contractile machinery of smooth muscle cells and are themselves markers of a contractile phenotype [132]. It is therefore plausible that mutations in these genes could lead to a synthetic phenotype. The TGF-β/Smad pathway is well-described to stimulate smooth muscle differentiation and maintain a contractile phenotype [132, 139]. TGF-β inhibits MMP expression and enhances the expression of tissue inhibitor of metalloproteinase (TIMP) proteins in smooth muscle [140]. Together, these lines of evidence support a critical role of a TGF-β signaling dependent, matrix-smooth muscle complex that regulates smooth muscle phenotype and the balance between MMP/TIMP, thereby regulating ECM homeostasis.
Conclusions and future perspectives
Research progress in the past few decades has yielded a plethora of genes involved in vascular aneurysmal diseases. The methodology has evolved from traditional Mendelian observations, linkage analysis, and site-directed cloning to high-throughput sequencing [118]. The next phase of human genetic studies and “big data” analyses are poised to identify additional causal genes and pathways for aortic aneurysmal disease, with the promise of providing further biological and clinical insights into these important disorders.
Highlights.
The extracellular matrix plays a critical role in determining the mechanical integrity of the aortic wall.
Mutations affecting the normal structure and function of the extracellular matrix can affect the stability of the aortic wall resulting in aneurysmal disease.
We provide an overview of the genes encoding the components of the aortic extracellular matrix and discuss syndromic and non-syndromic forms of aortic disease resulting from mutations in these genes.
Acknowledgments
NOS is supported in part by grants from the National Heart, Lung, and Blood Institute (R01HL131961), the National Human Genome Research Institute (UM1HG008853), the National Center for Advancing Translational Sciences (UL1TR002345) and by The Foundation for Barnes-Jewish Hospital.
Footnotes
Funding sources/Disclosures/Conflicts of interest: none
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Benjamin EJ, Blaha MJ, Chiuve SE, Cushman M, Das SR, Deo R, de Ferranti SD, Floyd J, Fornage M, Gillespie C, Isasi CR, Jimenez MC, Jordan LC, Judd SE, Lackland D, Lichtman JH, Lisabeth L, Liu S, Longenecker CT, Mackey RH, Matsushita K, Mozaffarian D, Mussolino ME, Nasir K, Neumar RW, Palaniappan L, Pandey DK, Thiagarajan RR, Reeves MJ, Ritchey M, Rodriguez CJ, Roth GA, Rosamond WD, Sasson C, Towfighi A, Tsao CW, Turner MB, Virani SS, Voeks JH, Willey JZ, Wilkins JT, Wu JH, Alger HM, Wong SS, Muntner P C American Heart Association Statistics, S. Stroke Statistics, Heart Disease and Stroke Statistics-2017 Update: A Report From the American Heart Association. Circulation. 2017;135(10):e146–e603. doi: 10.1161/CIR.0000000000000485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Forsdahl SH, Singh K, Solberg S, Jacobsen BK. Risk factors for abdominal aortic aneurysms: a 7-year prospective study: the Tromso Study, 1994–2001. Circulation. 2009;119(16):2202–8. doi: 10.1161/CIRCULATIONAHA.108.817619. [DOI] [PubMed] [Google Scholar]
- 3.Larsson E, Granath F, Swedenborg J, Hultgren R. A population-based case-control study of the familial risk of abdominal aortic aneurysm. J Vasc Surg. 2009;49(1):47–50. doi: 10.1016/j.jvs.2008.08.012. discussion 51. [DOI] [PubMed] [Google Scholar]
- 4.Olsson C, Granath F, Stahle E. Family history, comorbidity and risk of thoracic aortic disease: a population-based case-control study. Heart. 2013;99(14):1030–3. doi: 10.1136/heartjnl-2013-303654. [DOI] [PubMed] [Google Scholar]
- 5.Albornoz G, Coady MA, Roberts M, Davies RR, Tranquilli M, Rizzo JA, Elefteriades JA. Familial thoracic aortic aneurysms and dissections--incidence, modes of inheritance, and phenotypic patterns. Ann Thorac Surg. 2006;82(4):1400–5. doi: 10.1016/j.athoracsur.2006.04.098. [DOI] [PubMed] [Google Scholar]
- 6.Wagenseil JE, Mecham RP. Vascular extracellular matrix and arterial mechanics. Physiol Rev. 2009;89(3):957–89. doi: 10.1152/physrev.00041.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Howard PS, Macarak EJ. Localization of collagen types in regional segments of the fetal bovine aorta. Lab Invest. 1989;61(5):548–55. [PubMed] [Google Scholar]
- 8.Pomerance A, Yacoub MH, Gula G. The surgical pathology of thoracic aortic aneurysms. Histopathology. 1977;1(4):257–76. doi: 10.1111/j.1365-2559.1977.tb01665.x. [DOI] [PubMed] [Google Scholar]
- 9.Jain D, Dietz HC, Oswald GL, Maleszewski JJ, Halushka MK. Causes and histopathology of ascending aortic disease in children and young adults. Cardiovasc Pathol. 2011;20(1):15–25. doi: 10.1016/j.carpath.2009.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lindeman JH, Ashcroft BA, Beenakker JW, van Es M, Koekkoek NB, Prins FA, Tielemans JF, Abdul-Hussien H, Bank RA, Oosterkamp TH. Distinct defects in collagen microarchitecture underlie vessel-wall failure in advanced abdominal aneurysms and aneurysms in Marfan syndrome. Proc Natl Acad Sci U S A. 2010;107(2):862–5. doi: 10.1073/pnas.0910312107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lucero HA, Kagan HM. Lysyl oxidase: an oxidative enzyme and effector of cell function. Cell Mol Life Sci. 2006;63(19–20):2304–16. doi: 10.1007/s00018-006-6149-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Muiznieks LD, Cirulis JT, van der Horst A, Reinhardt DP, Wuite GJ, Pomes R, Keeley FW. Modulated growth, stability and interactions of liquid- like coacervate assemblies of elastin. Matrix Biol. 2014;36:39–50. doi: 10.1016/j.matbio.2014.03.008. [DOI] [PubMed] [Google Scholar]
- 13.Ramirez F, Sakai LY, Rifkin DB, Dietz HC. Extracellular microfibrils in development and disease. Cell Mol Life Sci. 2007;64(18):2437–46. doi: 10.1007/s00018-007-7166-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Corson GM, Charbonneau NL, Keene DR, Sakai LY. Differential expression of fibrillin-3 adds to microfibril variety in human and avian, but not rodent, connective tissues. Genomics. 2004;83(3):461–72. doi: 10.1016/j.ygeno.2003.08.023. [DOI] [PubMed] [Google Scholar]
- 15.Zhang H, Hu W, Ramirez F. Developmental expression of fibrillin genes suggests heterogeneity of extracellular microfibrils. J Cell Biol. 1995;129(4):1165–76. doi: 10.1083/jcb.129.4.1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kelleher CM, McLean SE, Mecham RP. Vascular extracellular matrix and aortic development. Curr Top Dev Biol. 2004;62:153–88. doi: 10.1016/S0070-2153(04)62006-0. [DOI] [PubMed] [Google Scholar]
- 17.Chu ML, Tsuda T. Fibulins in development and heritable disease. Birth Defects Res C Embryo Today. 2004;72(1):25–36. doi: 10.1002/bdrc.20003. [DOI] [PubMed] [Google Scholar]
- 18.Argraves WS, Greene LM, Cooley MA, Gallagher WM. Fibulins: physiological and disease perspectives. EMBO Rep. 2003;4(12):1127–31. doi: 10.1038/sj.embor.7400033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Robertson IB, Horiguchi M, Zilberberg L, Dabovic B, Hadjiolova K, Rifkin DB. Latent TGF-beta-binding proteins. Matrix Biol. 2015;47:44–53. doi: 10.1016/j.matbio.2015.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Robertson IB, Rifkin DB. Unchaining the beast; insights from structural and evolutionary studies on TGFbeta secretion, sequestration, and activation. Cytokine Growth Factor Rev. 2013;24(4):355–72. doi: 10.1016/j.cytogfr.2013.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Miyazono K, Olofsson A, Colosetti P, Heldin CH. A role of the latent TGF-beta 1-binding protein in the assembly and secretion of TGF-beta 1. EMBO J. 1991;10(5):1091–101. doi: 10.1002/j.1460-2075.1991.tb08049.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yoshinaga K, Obata H, Jurukovski V, Mazzieri R, Chen Y, Zilberberg L, Huso D, Melamed J, Prijatelj P, Todorovic V, Dabovic B, Rifkin DB. Perturbation of transforming growth factor (TGF)-beta1 association with latent TGF-beta binding protein yields inflammation and tumors. Proc Natl Acad Sci U S A. 2008;105(48):18758–63. doi: 10.1073/pnas.0805411105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Murata K, Motayama T, Kotake C. Collagen types in various layers of the human aorta and their changes with the atherosclerotic process. Atherosclerosis. 1986;60(3):251–62. doi: 10.1016/0021-9150(86)90172-3. [DOI] [PubMed] [Google Scholar]
- 24.Harris RB, Heaphy MR, Perry HO. Generalized elastolysis (cutis laxa) Am J Med. 1978;65(5):815–22. doi: 10.1016/0002-9343(78)90801-x. [DOI] [PubMed] [Google Scholar]
- 25.Beighton P. The dominant and recessive forms of cutis laxa. J Med Genet. 1972;9(2):216–21. doi: 10.1136/jmg.9.2.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Szabo Z, Crepeau MW, Mitchell AL, Stephan MJ, Puntel RA, Yin Loke K, Kirk RC, Urban Z. Aortic aneurysmal disease and cutis laxa caused by defects in the elastin gene. J Med Genet. 2006;43(3):255–8. doi: 10.1136/jmg.2005.034157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pober BR. Williams-Beuren syndrome. N Engl J Med. 2010;362(3):239–52. doi: 10.1056/NEJMra0903074. [DOI] [PubMed] [Google Scholar]
- 28.Ewart AK, Morris CA, Ensing GJ, Loker J, Moore C, Leppert M, Keating M. A human vascular disorder, supravalvular aortic stenosis, maps to chromosome 7. Proc Natl Acad Sci U S A. 1993;90(8):3226–30. doi: 10.1073/pnas.90.8.3226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Olson TM, Michels VV, Lindor NM, Pastores GM, Weber JL, Schaid DJ, Driscoll DJ, Feldt RH, Thibodeau SN. Autosomal dominant supravalvular aortic stenosis: localization to chromosome 7. Hum Mol Genet. 1993;2(7):869–73. doi: 10.1093/hmg/2.7.869. [DOI] [PubMed] [Google Scholar]
- 30.Urban Z, Riazi S, Seidl TL, Katahira J, Smoot LB, Chitayat D, Boyd CD, Hinek A. Connection between elastin haploinsufficiency and increased cell proliferation in patients with supravalvular aortic stenosis and Williams-Beuren syndrome. Am J Hum Genet. 2002;71(1):30–44. doi: 10.1086/341035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gupta MP, Zoneraich S, Aintablain A, Mehta J. Congenital aneurysm of the left ventricle associated with supravalvular aortic stenosis and aneurysm of the left main coronary artery: case report and review of the literature. Angiology. 1975;26(3):269–75. doi: 10.1177/000331977502600304. [DOI] [PubMed] [Google Scholar]
- 32.McKenna AJ, Craig B, Graham AN. Williams syndrome in an adult. J Card Surg. 2010;25(3):339–42. doi: 10.1111/j.1540-8191.2010.01020.x. [DOI] [PubMed] [Google Scholar]
- 33.Kozel BA, Knutsen RH, Ye L, Ciliberto CH, Broekelmann TJ, Mecham RP. Genetic modifiers of cardiovascular phenotype caused by elastin haploinsufficiency act by extrinsic noncomplementation. J Biol Chem. 2011;286(52):44926–36. doi: 10.1074/jbc.M111.274779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Maki JM, Rasanen J, Tikkanen H, Sormunen R, Makikallio K, Kivirikko KI, Soininen R. Inactivation of the lysyl oxidase gene Lox leads to aortic aneurysms, cardiovascular dysfunction, and perinatal death in mice. Circulation. 2002;106(19):2503–9. doi: 10.1161/01.cir.0000038109.84500.1e. [DOI] [PubMed] [Google Scholar]
- 35.Lee VS, Halabi CM, Hoffman EP, Carmichael N, Leshchiner I, Lian CG, Bierhals AJ, Vuzman D, Brigham Genomic M, Mecham RP, Frank NY, Stitziel NO. Loss of function mutation in LOX causes thoracic aortic aneurysm and dissection in humans. Proc Natl Acad Sci U S A. 2016;113(31):8759–64. doi: 10.1073/pnas.1601442113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Guo DC, Regalado ES, Gong L, Duan X, Santos-Cortez RL, Arnaud P, Ren Z, Cai B, Hostetler EM, Moran R, Liang D, Estrera A, Safi HJ, Leal SM, Bamshad MJ, Shendure J, Nickerson DA, Jondeau G, Boileau C, Milewicz DM University of Washington Center for Mendelian G. LOX Mutations Predispose to Thoracic Aortic Aneurysms and Dissections. Circ Res. 2016;118(6):928–34. doi: 10.1161/CIRCRESAHA.115.307130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dasouki M, Markova D, Garola R, Sasaki T, Charbonneau NL, Sakai LY, Chu ML. Compound heterozygous mutations in fibulin-4 causing neonatal lethal pulmonary artery occlusion, aortic aneurysm, arachnodactyly, and mild cutis laxa. Am J Med Genet A. 2007;143A(22):2635–41. doi: 10.1002/ajmg.a.31980. [DOI] [PubMed] [Google Scholar]
- 38.Hucthagowder V, Sausgruber N, Kim KH, Angle B, Marmorstein LY, Urban Z. Fibulin-4: a novel gene for an autosomal recessive cutis laxa syndrome. Am J Hum Genet. 2006;78(6):1075–80. doi: 10.1086/504304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Al-Hassnan ZN, Almesned AR, Tulbah S, Hakami A, Al-Omrani A, Al Sehly A, Mohammed S, Majid S, Meyer B, Al-Fayyadh M. Recessively inherited severe aortic aneurysm caused by mutated EFEMP2. Am J Cardiol. 2012;109(11):1677–80. doi: 10.1016/j.amjcard.2012.01.394. [DOI] [PubMed] [Google Scholar]
- 40.Renard M, Holm T, Veith R, Callewaert BL, Ades LC, Baspinar O, Pickart A, Dasouki M, Hoyer J, Rauch A, Trapane P, Earing MG, Coucke PJ, Sakai LY, Dietz HC, De Paepe AM, Loeys BL. Altered TGFbeta signaling and cardiovascular manifestations in patients with autosomal recessive cutis laxa type I caused by fibulin-4 deficiency. Eur J Hum Genet. 2010;18(8):895–901. doi: 10.1038/ejhg.2010.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kappanayil M, Nampoothiri S, Kannan R, Renard M, Coucke P, Malfait F, Menon S, Ravindran HK, Kurup R, Faiyaz-Ul-Haque M, Kumar K, De Paepe A. Characterization of a distinct lethal arteriopathy syndrome in twenty-two infants associated with an identical, novel mutation in FBLN4 gene, confirms fibulin-4 as a critical determinant of human vascular elastogenesis. Orphanet J Rare Dis. 2012;7:61. doi: 10.1186/1750-1172-7-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Igoucheva O, Alexeev V, Halabi CM, Adams SM, Stoilov I, Sasaki T, Arita M, Donahue A, Mecham RP, Birk DE, Chu ML. Fibulin-4 E57K Knock-in Mice Recapitulate Cutaneous, Vascular and Skeletal Defects of Recessive Cutis Laxa 1B with both Elastic Fiber and Collagen Fibril Abnormalities. J Biol Chem. 2015;290(35):21443–59. doi: 10.1074/jbc.M115.640425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Horiguchi M, Inoue T, Ohbayashi T, Hirai M, Noda K, Marmorstein LY, Yabe D, Takagi K, Akama TO, Kita T, Kimura T, Nakamura T. Fibulin-4 conducts proper elastogenesis via interaction with cross-linking enzyme lysyl oxidase. Proc Natl Acad Sci U S A. 2009;106(45):19029–34. doi: 10.1073/pnas.0908268106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.McLaughlin PJ, Chen Q, Horiguchi M, Starcher BC, Stanton JB, Broekelmann TJ, Marmorstein AD, McKay B, Mecham R, Nakamura T, Marmorstein LY. Targeted disruption of fibulin-4 abolishes elastogenesis and causes perinatal lethality in mice. Mol Cell Biol. 2006;26(5):1700–9. doi: 10.1128/MCB.26.5.1700-1709.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Orriols M, Varona S, Marti-Pamies I, Galan M, Guadall A, Escudero JR, Martin-Ventura JL, Camacho M, Vila L, Martinez-Gonzalez J, Rodriguez C. Down-regulation of Fibulin-5 is associated with aortic dilation: role of inflammation and epigenetics. Cardiovasc Res. 2016;110(3):431–42. doi: 10.1093/cvr/cvw082. [DOI] [PubMed] [Google Scholar]
- 46.Badger SA, Soong CV, O’Donnell ME, Sharif MA, Makar RR, Hughes AE. Common polymorphisms of Fibulin-5 and the risk of abdominal aortic aneurysm development. Vasc Med. 2010;15(2):113–7. doi: 10.1177/1358863X09355667. [DOI] [PubMed] [Google Scholar]
- 47.Loeys B, Van Maldergem L, Mortier G, Coucke P, Gerniers S, Naeyaert JM, De Paepe A. Homozygosity for a missense mutation in fibulin-5 (FBLN5) results in a severe form of cutis laxa. Hum Mol Genet. 2002;11(18):2113–8. doi: 10.1093/hmg/11.18.2113. [DOI] [PubMed] [Google Scholar]
- 48.Hu Q, Loeys BL, Coucke PJ, De Paepe A, Mecham RP, Choi J, Davis EC, Urban Z. Fibulin-5 mutations: mechanisms of impaired elastic fiber formation in recessive cutis laxa. Hum Mol Genet. 2006;15(23):3379–86. doi: 10.1093/hmg/ddl414. [DOI] [PubMed] [Google Scholar]
- 49.Markova D, Zou Y, Ringpfeil F, Sasaki T, Kostka G, Timpl R, Uitto J, Chu ML. Genetic heterogeneity of cutis laxa: a heterozygous tandem duplication within the fibulin-5 (FBLN5) gene. Am J Hum Genet. 2003;72(4):998–1004. doi: 10.1086/373940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yanagisawa H, Davis EC, Starcher BC, Ouchi T, Yanagisawa M, Richardson JA, Olson EN. Fibulin-5 is an elastin-binding protein essential for elastic fibre development in vivo. Nature. 2002;415(6868):168–71. doi: 10.1038/415168a. [DOI] [PubMed] [Google Scholar]
- 51.Nakamura T, Lozano PR, Ikeda Y, Iwanaga Y, Hinek A, Minamisawa S, Cheng CF, Kobuke K, Dalton N, Takada Y, Tashiro K, Ross J, Jr, Honjo T, Chien KR. Fibulin-5/DANCE is essential for elastogenesis in vivo. Nature. 2002;415(6868):171–5. doi: 10.1038/415171a. [DOI] [PubMed] [Google Scholar]
- 52.Drewes PG, Yanagisawa H, Starcher B, Hornstra I, Csiszar K, Marinis SI, Keller P, Word RA. Pelvic organ prolapse in fibulin-5 knockout mice: pregnancy-induced changes in elastic fiber homeostasis in mouse vagina. Am J Pathol. 2007;170(2):578–89. doi: 10.2353/ajpath.2007.060662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Choudhury R, McGovern A, Ridley C, Cain SA, Baldwin A, Wang MC, Guo C, Mironov A, Jr, Drymoussi Z, Trump D, Shuttleworth A, Baldock C, Kielty CM. Differential regulation of elastic fiber formation by fibulin-4 and -5. J Biol Chem. 2009;284(36):24553–67. doi: 10.1074/jbc.M109.019364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Liu X, Zhao Y, Gao J, Pawlyk B, Starcher B, Spencer JA, Yanagisawa H, Zuo J, Li T. Elastic fiber homeostasis requires lysyl oxidase-like 1 protein. Nat Genet. 2004;36(2):178–82. doi: 10.1038/ng1297. [DOI] [PubMed] [Google Scholar]
- 55.Barbier M, Gross MS, Aubart M, Hanna N, Kessler K, Guo DC, Tosolini L, Ho-Tin-Noe B, Regalado E, Varret M, Abifadel M, Milleron O, Odent S, Dupuis-Girod S, Faivre L, Edouard T, Dulac Y, Busa T, Gouya L, Milewicz DM, Jondeau G, Boileau C. MFAP5 loss-of-function mutations underscore the involvement of matrix alteration in the pathogenesis of familial thoracic aortic aneurysms and dissections. Am J Hum Genet. 2014;95(6):736–43. doi: 10.1016/j.ajhg.2014.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chen E, Larson JD, Ekker SC. Functional analysis of zebrafish microfibril-associated glycoprotein-1 (Magp1) in vivo reveals roles for microfibrils in vascular development and function. Blood. 2006;107(11):4364–74. doi: 10.1182/blood-2005-02-0789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mecham RP, Gibson MA. The microfibril-associated glycoproteins (MAGPs) and the microfibrillar niche. Matrix Biol. 2015;47:13–33. doi: 10.1016/j.matbio.2015.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Malfait F, Symoens S, De Backer J, Hermanns-Le T, Sakalihasan N, Lapiere CM, Coucke P, De Paepe A. Three arginine to cysteine substitutions in the pro-alpha (I)-collagen chain cause Ehlers-Danlos syndrome with a propensity to arterial rupture in early adulthood. Hum Mutat. 2007;28(4):387–95. doi: 10.1002/humu.20455. [DOI] [PubMed] [Google Scholar]
- 59.Phillips CL, Shrago-Howe AW, Pinnell SR, Wenstrup RJ. A substitution at a non-glycine position in the triple-helical domain of pro alpha 2(I) collagen chains present in an individual with a variant of the Marfan syndrome. J Clin Invest. 1990;86(5):1723–8. doi: 10.1172/JCI114897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rahkonen O, Su M, Hakovirta H, Koskivirta I, Hormuzdi SG, Vuorio E, Bornstein P, Penttinen R. Mice with a deletion in the first intron of the Col1a1 gene develop age-dependent aortic dissection and rupture. Circ Res. 2004;94(1):83–90. doi: 10.1161/01.RES.0000108263.74520.15. [DOI] [PubMed] [Google Scholar]
- 61.Pepin MG, Schwarze U, Rice KM, Liu M, Leistritz D, Byers PH. Survival is affected by mutation type and molecular mechanism in vascular Ehlers-Danlos syndrome (EDS type IV) Genet Med. 2014;16(12):881–8. doi: 10.1038/gim.2014.72. [DOI] [PubMed] [Google Scholar]
- 62.Pepin M, Schwarze U, Superti-Furga A, Byers PH. Clinical and genetic features of Ehlers-Danlos syndrome type IV, the vascular type. N Engl J Med. 2000;342(10):673–80. doi: 10.1056/NEJM200003093421001. [DOI] [PubMed] [Google Scholar]
- 63.Superti-Furga A, Gugler E, Gitzelmann R, Steinmann B. Ehlers-Danlos syndrome type IV: a multi-exon deletion in one of the two COL3A1 alleles affecting structure, stability, and processing of type III procollagen. J Biol Chem. 1988;263(13):6226–32. [PubMed] [Google Scholar]
- 64.Liu X, Wu H, Byrne M, Krane S, Jaenisch R. Type III collagen is crucial for collagen I fibrillogenesis and for normal cardiovascular development. Proc Natl Acad Sci U S A. 1997;94(5):1852–6. doi: 10.1073/pnas.94.5.1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Plaisier E, Gribouval O, Alamowitch S, Mougenot B, Prost C, Verpont MC, Marro B, Desmettre T, Cohen SY, Roullet E, Dracon M, Fardeau M, Van Agtmael T, Kerjaschki D, Antignac C, Ronco P. COL4A1 mutations and hereditary angiopathy, nephropathy, aneurysms, and muscle cramps. N Engl J Med. 2007;357(26):2687–95. doi: 10.1056/NEJMoa071906. [DOI] [PubMed] [Google Scholar]
- 66.Gould DB, Phalan FC, van Mil SE, Sundberg JP, Vahedi K, Massin P, Bousser MG, Heutink P, Miner JH, Tournier-Lasserve E, John SW. Role of COL4A1 in small-vessel disease and hemorrhagic stroke. N Engl J Med. 2006;354(14):1489–96. doi: 10.1056/NEJMoa053727. [DOI] [PubMed] [Google Scholar]
- 67.Poschl E, Schlotzer-Schrehardt U, Brachvogel B, Saito K, Ninomiya Y, Mayer U. Collagen IV is essential for basement membrane stability but dispensable for initiation of its assembly during early development. Development. 2004;131(7):1619–28. doi: 10.1242/dev.01037. [DOI] [PubMed] [Google Scholar]
- 68.Van Agtmael T, Bailey MA, Schlotzer-Schrehardt U, Craigie E, Jackson IJ, Brownstein DG, Megson IL, Mullins JJ. Col4a1 mutation in mice causes defects in vascular function and low blood pressure associated with reduced red blood cell volume. Hum Mol Genet. 2010;19(6):1119–28. doi: 10.1093/hmg/ddp584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kashtan CE, Segal Y, Flinter F, Makanjuola D, Gan JS, Watnick T. Aortic abnormalities in males with Alport syndrome. Nephrol Dial Transplant. 2010;25(11):3554–60. doi: 10.1093/ndt/gfq271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Rheault MN, Kren SM, Thielen BK, Mesa HA, Crosson JT, Thomas W, Sado Y, Kashtan CE, Segal Y. Mouse model of X-linked Alport syndrome. J Am Soc Nephrol. 2004;15(6):1466–74. doi: 10.1097/01.asn.0000130562.90255.8f. [DOI] [PubMed] [Google Scholar]
- 71.Vogt BA, Birk PE, Panzarino V, Hite SH, Kashtan CE. Aortic dissection in young patients with chronic hypertension. Am J Kidney Dis. 1999;33(2):374–8. doi: 10.1016/s0272-6386(99)70315-x. [DOI] [PubMed] [Google Scholar]
- 72.Januzzi JL, Isselbacher EM, Fattori R, Cooper JV, Smith DE, Fang J, Eagle KA, Mehta RH, Nienaber CA, Pape LA D. International Registry of Aortic. Characterizing the young patient with aortic dissection: results from the International Registry of Aortic Dissection (IRAD) J Am Coll Cardiol. 2004;43(4):665–9. doi: 10.1016/j.jacc.2003.08.054. [DOI] [PubMed] [Google Scholar]
- 73.Yeowell HN, Walker LC. Mutations in the lysyl hydroxylase 1 gene that result in enzyme deficiency and the clinical phenotype of Ehlers-Danlos syndrome type VI. Mol Genet Metab. 2000;71(1–2):212–24. doi: 10.1006/mgme.2000.3076. [DOI] [PubMed] [Google Scholar]
- 74.Wenstrup RJ, Murad S, Pinnell SR. Ehlers-Danlos syndrome type VI: clinical manifestations of collagen lysyl hydroxylase deficiency. J Pediatr. 1989;115(3):405–9. doi: 10.1016/s0022-3476(89)80839-x. [DOI] [PubMed] [Google Scholar]
- 75.Takaluoma K, Hyry M, Lantto J, Sormunen R, Bank RA, Kivirikko KI, Myllyharju J, Soininen R. Tissue-specific changes in the hydroxylysine content and cross-links of collagens and alterations in fibril morphology in lysyl hydroxylase 1 knock-out mice. J Biol Chem. 2007;282(9):6588–96. doi: 10.1074/jbc.M608830200. [DOI] [PubMed] [Google Scholar]
- 76.Theocharis AD, Karamanos NK. Decreased biglycan expression and differential decorin localization in human abdominal aortic aneurysms. Atherosclerosis. 2002;165(2):221–30. doi: 10.1016/s0021-9150(02)00231-9. [DOI] [PubMed] [Google Scholar]
- 77.Reinboth B, Hanssen E, Cleary EG, Gibson MA. Molecular interactions of biglycan and decorin with elastic fiber components: biglycan forms a ternary complex with tropoelastin and microfibril-associated glycoprotein 1. J Biol Chem. 2002;277(6):3950–7. doi: 10.1074/jbc.M109540200. [DOI] [PubMed] [Google Scholar]
- 78.Hocking AM, Shinomura T, McQuillan DJ. Leucine-rich repeat glycoproteins of the extracellular matrix. Matrix Biol. 1998;17(1):1–19. doi: 10.1016/s0945-053x(98)90121-4. [DOI] [PubMed] [Google Scholar]
- 79.Meester JA, Vandeweyer G, Pintelon I, Lammens M, Van Hoorick L, De Belder S, Waitzman K, Young L, Markham LW, Vogt J, Richer J, Beauchesne LM, Unger S, Superti-Furga A, Prsa M, Dhillon R, Reyniers E, Dietz HC, Wuyts W, Mortier G, Verstraeten A, Van Laer L, Loeys BL. Loss-of-function mutations in the X-linked biglycan gene cause a severe syndromic form of thoracic aortic aneurysms and dissections. Genet Med. 2017;19(4):386–395. doi: 10.1038/gim.2016.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Heegaard AM, Corsi A, Danielsen CC, Nielsen KL, Jorgensen HL, Riminucci M, Young MF, Bianco P. Biglycan deficiency causes spontaneous aortic dissection and rupture in mice. Circulation. 2007;115(21):2731–8. doi: 10.1161/CIRCULATIONAHA.106.653980. [DOI] [PubMed] [Google Scholar]
- 81.Xu T, Bianco P, Fisher LW, Longenecker G, Smith E, Goldstein S, Bonadio J, Boskey A, Heegaard AM, Sommer B, Satomura K, Dominguez P, Zhao C, Kulkarni AB, Robey PG, Young MF. Targeted disruption of the biglycan gene leads to an osteoporosis-like phenotype in mice. Nat Genet. 1998;20(1):78–82. doi: 10.1038/1746. [DOI] [PubMed] [Google Scholar]
- 82.Pyeritz RE, McKusick VA. The Marfan syndrome: diagnosis and management. N Engl J Med. 1979;300(14):772–7. doi: 10.1056/NEJM197904053001406. [DOI] [PubMed] [Google Scholar]
- 83.Loeys BL, Dietz HC, Braverman AC, Callewaert BL, De Backer J, Devereux RB, Hilhorst-Hofstee Y, Jondeau G, Faivre L, Milewicz DM, Pyeritz RE, Sponseller PD, Wordsworth P, De Paepe AM. The revised Ghent nosology for the Marfan syndrome. J Med Genet. 2010;47(7):476–85. doi: 10.1136/jmg.2009.072785. [DOI] [PubMed] [Google Scholar]
- 84.Dietz HC, Cutting GR, Pyeritz RE, Maslen CL, Sakai LY, Corson GM, Puffenberger EG, Hamosh A, Nanthakumar EJ, Curristin SM, et al. Marfan syndrome caused by a recurrent de novo missense mutation in the fibrillin gene. Nature. 1991;352(6333):337–9. doi: 10.1038/352337a0. [DOI] [PubMed] [Google Scholar]
- 85.Collod-Beroud G, Le Bourdelles S, Ades L, Ala-Kokko L, Booms P, Boxer M, Child A, Comeglio P, De Paepe A, Hyland JC, Holman K, Kaitila I, Loeys B, Matyas G, Nuytinck L, Peltonen L, Rantamaki T, Robinson P, Steinmann B, Junien C, Beroud C, Boileau C. Update of the UMD-FBN1 mutation database and creation of an FBN1 polymorphism database. Hum Mutat. 2003;22(3):199–208. doi: 10.1002/humu.10249. [DOI] [PubMed] [Google Scholar]
- 86.Faivre L, Collod-Beroud G, Loeys BL, Child A, Binquet C, Gautier E, Callewaert B, Arbustini E, Mayer K, Arslan-Kirchner M, Kiotsekoglou A, Comeglio P, Marziliano N, Dietz HC, Halliday D, Beroud C, Bonithon-Kopp C, Claustres M, Muti C, Plauchu H, Robinson PN, Ades LC, Biggin A, Benetts B, Brett M, Holman KJ, De Backer J, Coucke P, Francke U, De Paepe A, Jondeau G, Boileau C. Effect of mutation type and location on clinical outcome in 1,013 probands with Marfan syndrome or related phenotypes and FBN1 mutations: an international study. Am J Hum Genet. 2007;81(3):454–66. doi: 10.1086/520125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Neptune ER, Frischmeyer PA, Arking DE, Myers L, Bunton TE, Gayraud B, Ramirez F, Sakai LY, Dietz HC. Dysregulation of TGF-beta activation contributes to pathogenesis in Marfan syndrome. Nat Genet. 2003;33(3):407–11. doi: 10.1038/ng1116. [DOI] [PubMed] [Google Scholar]
- 88.Habashi JP, Judge DP, Holm TM, Cohn RD, Loeys BL, Cooper TK, Myers L, Klein EC, Liu G, Calvi C, Podowski M, Neptune ER, Halushka MK, Bedja D, Gabrielson K, Rifkin DB, Carta L, Ramirez F, Huso DL, Dietz HC. Losartan, an AT1 antagonist, prevents aortic aneurysm in a mouse model of Marfan syndrome. Science. 2006;312(5770):117–21. doi: 10.1126/science.1124287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lacro RV, Dietz HC, Sleeper LA, Yetman AT, Bradley TJ, Colan SD, Pearson GD, Selamet Tierney ES, Levine JC, Atz AM, Benson DW, Braverman AC, Chen S, De Backer J, Gelb BD, Grossfeld PD, Klein GL, Lai WW, Liou A, Loeys BL, Markham LW, Olson AK, Paridon SM, Pemberton VL, Pierpont ME, Pyeritz RE, Radojewski E, Roman MJ, Sharkey AM, Stylianou MP, Wechsler SB, Young LT, Mahony L I. Pediatric Heart Network. Atenolol versus losartan in children and young adults with Marfan’s syndrome. N Engl J Med. 2014;371(22):2061–71. doi: 10.1056/NEJMoa1404731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Doyle AJ, Doyle JJ, Bessling SL, Maragh S, Lindsay ME, Schepers D, Gillis E, Mortier G, Homfray T, Sauls K, Norris RA, Huso ND, Leahy D, Mohr DW, Caulfield MJ, Scott AF, Destree A, Hennekam RC, Arn PH, Curry CJ, Van Laer L, McCallion AS, Loeys BL, Dietz HC. Mutations in the TGF-beta repressor SKI cause Shprintzen-Goldberg syndrome with aortic aneurysm. Nat Genet. 2012;44(11):1249–54. doi: 10.1038/ng.2421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Schepers D, Doyle AJ, Oswald G, Sparks E, Myers L, Willems PJ, Mansour S, Simpson MA, Frysira H, Maat-Kievit A, Van Minkelen R, Hoogeboom JM, Mortier GR, Titheradge H, Brueton L, Starr L, Stark Z, Ockeloen C, Lourenco CM, Blair E, Hobson E, Hurst J, Maystadt I, Destree A, Girisha KM, Miller M, Dietz HC, Loeys B, Van Laer L. The SMAD-binding domain of SKI: a hotspot for de novo mutations causing Shprintzen-Goldberg syndrome. Eur J Hum Genet. 2015;23(2):224–8. doi: 10.1038/ejhg.2014.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Loeys BL, Chen J, Neptune ER, Judge DP, Podowski M, Holm T, Meyers J, Leitch CC, Katsanis N, Sharifi N, Xu FL, Myers LA, Spevak PJ, Cameron DE, De Backer J, Hellemans J, Chen Y, Davis EC, Webb CL, Kress W, Coucke P, Rifkin DB, De Paepe AM, Dietz HC. A syndrome of altered cardiovascular, craniofacial, neurocognitive and skeletal development caused by mutations in TGFBR1 or TGFBR2. Nat Genet. 2005;37(3):275–81. doi: 10.1038/ng1511. [DOI] [PubMed] [Google Scholar]
- 93.van de Laar IM, Oldenburg RA, Pals G, Roos-Hesselink JW, de Graaf BM, Verhagen JM, Hoedemaekers YM, Willemsen R, Severijnen LA, Venselaar H, Vriend G, Pattynama PM, Collee M, Majoor-Krakauer D, Poldermans D, Frohn-Mulder IM, Micha D, Timmermans J, Hilhorst-Hofstee Y, Bierma-Zeinstra SM, Willems PJ, Kros JM, Oei EH, Oostra BA, Wessels MW, Bertoli-Avella AM. Mutations in SMAD3 cause a syndromic form of aortic aneurysms and dissections with early-onset osteoarthritis. Nat Genet. 2011;43(2):121–6. doi: 10.1038/ng.744. [DOI] [PubMed] [Google Scholar]
- 94.Lindsay ME, Schepers D, Bolar NA, Doyle JJ, Gallo E, Fert-Bober J, Kempers MJ, Fishman EK, Chen Y, Myers L, Bjeda D, Oswald G, Elias AF, Levy HP, Anderlid BM, Yang MH, Bongers EM, Timmermans J, Braverman AC, Canham N, Mortier GR, Brunner HG, Byers PH, Van Eyk J, Van Laer L, Dietz HC, Loeys BL. Loss-of-function mutations in TGFB2 cause a syndromic presentation of thoracic aortic aneurysm. Nat Genet. 2012;44(8):922–7. doi: 10.1038/ng.2349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Rienhoff HY, Jr, Yeo CY, Morissette R, Khrebtukova I, Melnick J, Luo S, Leng N, Kim YJ, Schroth G, Westwick J, Vogel H, McDonnell N, Hall JG, Whitman M. A mutation in TGFB3 associated with a syndrome of low muscle mass, growth retardation, distal arthrogryposis and clinical features overlapping with Marfan and Loeys-Dietz syndrome. Am J Med Genet A. 2013;161A(8):2040–6. doi: 10.1002/ajmg.a.36056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Matyas G, Naef P, Tollens M, Oexle K. De novo mutation of the latency-associated peptide domain of TGFB3 in a patient with overgrowth and Loeys-Dietz syndrome features. Am J Med Genet A. 2014;164A(8):2141–3. doi: 10.1002/ajmg.a.36593. [DOI] [PubMed] [Google Scholar]
- 97.Gallo EM, Loch DC, Habashi JP, Calderon JF, Chen Y, Bedja D, van Erp C, Gerber EE, Parker SJ, Sauls K, Judge DP, Cooke SK, Lindsay ME, Rouf R, Myers L, ap Rhys CM, Kent KC, Norris RA, Huso DL, Dietz HC. Angiotensin II-dependent TGF-beta signaling contributes to Loeys-Dietz syndrome vascular pathogenesis. J Clin Invest. 2014;124(1):448–60. doi: 10.1172/JCI69666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Holm TM, Habashi JP, Doyle JJ, Bedja D, Chen Y, van Erp C, Lindsay ME, Kim D, Schoenhoff F, Cohn RD, Loeys BL, Thomas CJ, Patnaik S, Marugan JJ, Judge DP, Dietz HC. Noncanonical TGFbeta signaling contributes to aortic aneurysm progression in Marfan syndrome mice. Science. 2011;332(6027):358–61. doi: 10.1126/science.1192149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Li W, Li Q, Jiao Y, Qin L, Ali R, Zhou J, Ferruzzi J, Kim RW, Geirsson A, Dietz HC, Offermanns S, Humphrey JD, Tellides G. Tgfbr2 disruption in postnatal smooth muscle impairs aortic wall homeostasis. J Clin Invest. 2014;124(2):755–67. doi: 10.1172/JCI69942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Cook JR, Clayton NP, Carta L, Galatioto J, Chiu E, Smaldone S, Nelson CA, Cheng SH, Wentworth BM, Ramirez F. Dimorphic effects of transforming growth factor-beta signaling during aortic aneurysm progression in mice suggest a combinatorial therapy for Marfan syndrome. Arterioscler Thromb Vasc Biol. 2015;35(4):911–7. doi: 10.1161/ATVBAHA.114.305150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Wei H, Hu JH, Angelov SN, Fox K, Yan J, Enstrom R, Smith A, Dichek DA. Aortopathy in a Mouse Model of Marfan Syndrome Is Not Mediated by Altered Transforming Growth Factor beta Signaling. J Am Heart Assoc. 2017;6(1) doi: 10.1161/JAHA.116.004968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Mallat Z, Ait-Oufella H, Tedgui A. The Pathogenic Transforming Growth Factor-beta Overdrive Hypothesis in Aortic Aneurysms and Dissections: A Mirage? Circ Res. 2017;120(11):1718–1720. doi: 10.1161/CIRCRESAHA.116.310371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Dietz HC. TGF-beta in the pathogenesis and prevention of disease: a matter of aneurysmic proportions. J Clin Invest. 2010;120(2):403–7. doi: 10.1172/JCI42014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Humphrey JD, Schwartz MA, Tellides G, Milewicz DM. Role of mechanotransduction in vascular biology: focus on thoracic aortic aneurysms and dissections. Circ Res. 2015;116(8):1448–61. doi: 10.1161/CIRCRESAHA.114.304936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Zhu L, Vranckx R, Khau Van Kien P, Lalande A, Boisset N, Mathieu F, Wegman M, Glancy L, Gasc JM, Brunotte F, Bruneval P, Wolf JE, Michel JB, Jeunemaitre X. Mutations in myosin heavy chain 11 cause a syndrome associating thoracic aortic aneurysm/aortic dissection and patent ductus arteriosus. Nat Genet. 2006;38(3):343–9. doi: 10.1038/ng1721. [DOI] [PubMed] [Google Scholar]
- 106.Pannu H, Tran-Fadulu V, Papke CL, Scherer S, Liu Y, Presley C, Guo D, Estrera AL, Safi HJ, Brasier AR, Vick GW, Marian AJ, Raman CS, Buja LM, Milewicz DM. MYH11 mutations result in a distinct vascular pathology driven by insulin- like growth factor 1 and angiotensin II. Hum Mol Genet. 2007;16(20):2453–62. doi: 10.1093/hmg/ddm201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Keylock A, Hong Y, Saunders D, Omoyinmi E, Mulhern C, Roebuck D, Brogan P, Ganesan V, Eleftheriou D. Moyamoya-like cerebrovascular disease in a child with a novel mutation in myosin heavy chain 11. Neurology. 2018;90(3):136–138. doi: 10.1212/WNL.0000000000004828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Morano I, Chai GX, Baltas LG, Lamounier-Zepter V, Lutsch G, Kott M, Haase H, Bader M. Smooth-muscle contraction without smooth-muscle myosin. Nat Cell Biol. 2000;2(6):371–5. doi: 10.1038/35014065. [DOI] [PubMed] [Google Scholar]
- 109.Guo DC, Pannu H, Tran-Fadulu V, Papke CL, Yu RK, Avidan N, Bourgeois S, Estrera AL, Safi HJ, Sparks E, Amor D, Ades L, McConnell V, Willoughby CE, Abuelo D, Willing M, Lewis RA, Kim DH, Scherer S, Tung PP, Ahn C, Buja LM, Raman CS, Shete SS, Milewicz DM. Mutations in smooth muscle alpha-actin (ACTA2) lead to thoracic aortic aneurysms and dissections. Nat Genet. 2007;39(12):1488–93. doi: 10.1038/ng.2007.6. [DOI] [PubMed] [Google Scholar]
- 110.Guo DC, Papke CL, Tran-Fadulu V, Regalado ES, Avidan N, Johnson RJ, Kim DH, Pannu H, Willing MC, Sparks E, Pyeritz RE, Singh MN, Dalman RL, Grotta JC, Marian AJ, Boerwinkle EA, Frazier LQ, LeMaire SA, Coselli JS, Estrera AL, Safi HJ, Veeraraghavan S, Muzny DM, Wheeler DA, Willerson JT, Yu RK, Shete SS, Scherer SE, Raman CS, Buja LM, Milewicz DM. Mutations in smooth muscle alpha-actin (ACTA2) cause coronary artery disease, stroke, and Moyamoya disease, along with thoracic aortic disease. Am J Hum Genet. 2009;84(5):617–27. doi: 10.1016/j.ajhg.2009.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Schildmeyer LA, Braun R, Taffet G, Debiasi M, Burns AE, Bradley A, Schwartz RJ. Impaired vascular contractility and blood pressure homeostasis in the smooth muscle alpha-actin null mouse. FASEB J. 2000;14(14):2213–20. doi: 10.1096/fj.99-0927com. [DOI] [PubMed] [Google Scholar]
- 112.Bennett MR, Sinha S, Owens GK. Vascular Smooth Muscle Cells in Atherosclerosis. Circ Res. 2016;118(4):692–702. doi: 10.1161/CIRCRESAHA.115.306361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Georgescu MM, da Pinho MC, Richardson TE, Torrealba J, Buja LM, Milewicz DM, Raisanen JM, Burns DK. The defining pathology of the new clinical and histopathologic entity ACTA2-related cerebrovascular disease. Acta Neuropathol Commun. 2015;3:81. doi: 10.1186/s40478-015-0262-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Michelena HI, Khanna AD, Mahoney D, Margaryan E, Topilsky Y, Suri RM, Eidem B, Edwards WD, Sundt TM, 3rd, Enriquez-Sarano M. Incidence of aortic complications in patients with bicuspid aortic valves. JAMA. 2011;306(10):1104–12. doi: 10.1001/jama.2011.1286. [DOI] [PubMed] [Google Scholar]
- 115.Cripe L, Andelfinger G, Martin LJ, Shooner K, Benson DW. Bicuspid aortic valve is heritable. J Am Coll Cardiol. 2004;44(1):138–43. doi: 10.1016/j.jacc.2004.03.050. [DOI] [PubMed] [Google Scholar]
- 116.Loscalzo ML, Goh DL, Loeys B, Kent KC, Spevak PJ, Dietz HC. Familial thoracic aortic dilation and bicommissural aortic valve: a prospective analysis of natural history and inheritance. Am J Med Genet A. 2007;143A(17):1960–7. doi: 10.1002/ajmg.a.31872. [DOI] [PubMed] [Google Scholar]
- 117.Garg V, Muth AN, Ransom JF, Schluterman MK, Barnes R, King IN, Grossfeld PD, Srivastava D. Mutations in NOTCH1 cause aortic valve disease. Nature. 2005;437(7056):270–4. doi: 10.1038/nature03940. [DOI] [PubMed] [Google Scholar]
- 118.Giusti B, Sticchi E, De Cario R, Magi A, Nistri S, Pepe G. Genetic Bases of Bicuspid Aortic Valve: The Contribution of Traditional and High-Throughput Sequencing Approaches on Research and Diagnosis. Front Physiol. 2017;8:612. doi: 10.3389/fphys.2017.00612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Attias D, Stheneur C, Roy C, Collod-Beroud G, Detaint D, Faivre L, Delrue MA, Cohen L, Francannet C, Beroud C, Claustres M, Iserin F, Khau Van Kien P, Lacombe D, Le Merrer M, Lyonnet S, Odent S, Plauchu H, Rio M, Rossi A, Sidi D, Steg PG, Ravaud P, Boileau C, Jondeau G. Comparison of clinical presentations and outcomes between patients with TGFBR2 and FBN1 mutations in Marfan syndrome and related disorders. Circulation. 2009;120(25):2541–9. doi: 10.1161/CIRCULATIONAHA.109.887042. [DOI] [PubMed] [Google Scholar]
- 120.Gillis E, Kumar AA, Luyckx I, Preuss C, Cannaerts E, van de Beek G, Wieschendorf B, Alaerts M, Bolar N, Vandeweyer G, Meester J, Wunnemann F, Gould RA, Zhurayev R, Zerbino D, Mohamed SA, Mital S, Mertens L, Bjorck HM, Franco-Cereceda A, McCallion AS, Van Laer L, Verhagen JMA, van de Laar I, Wessels MW, Messas E, Goudot G, Nemcikova M, Krebsova A, Kempers M, Salemink S, Duijnhouwer T, Jeunemaitre X, Albuisson J, Eriksson P, Andelfinger G, Dietz HC, Verstraeten A, Loeys BL, Mibava Leducq C. Candidate Gene Resequencing in a Large Bicuspid Aortic Valve-Associated Thoracic Aortic Aneurysm Cohort: SMAD6 as an Important Contributor. Front Physiol. 2017;8:400. doi: 10.3389/fphys.2017.00400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.LeMaire SA, McDonald ML, Guo DC, Russell L, Miller CC, 3rd, Johnson RJ, Bekheirnia MR, Franco LM, Nguyen M, Pyeritz RE, Bavaria JE, Devereux R, Maslen C, Holmes KW, Eagle K, Body SC, Seidman C, Seidman JG, Isselbacher EM, Bray M, Coselli JS, Estrera AL, Safi HJ, Belmont JW, Leal SM, Milewicz DM. Genome-wide association study identifies a susceptibility locus for thoracic aortic aneurysms and aortic dissections spanning FBN1 at 15q21.1. Nat Genet. 2011;43(10):996–1000. doi: 10.1038/ng.934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Iakoubova OA, Tong CH, Rowland CM, Luke MM, Garcia VE, Catanese JJ, Moomiaie RM, Sotonyi P, Ascady G, Nikas D, Dedelias P, Tranquilli M, Elefteriades JA. Genetic variants in FBN-1 and risk for thoracic aortic aneurysm and dissection. PLoS One. 2014;9(4):e91437. doi: 10.1371/journal.pone.0091437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Guo DC, Grove ML, Prakash SK, Eriksson P, Hostetler EM, LeMaire SA, Body SC, Shalhub S, Estrera AL, Safi HJ, Regalado ES, Zhou W, Mathis MR, Gen TACI, Eagle KA, Yang B, Willer CJ, Boerwinkle E, Milewicz DM Investigators BA. Genetic Variants in LRP1 and ULK4 Are Associated with Acute Aortic Dissections. Am J Hum Genet. 2016;99(3):762–769. doi: 10.1016/j.ajhg.2016.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Bown MJ, Jones GT, Harrison SC, Wright BJ, Bumpstead S, Baas AF, Gretarsdottir S, Badger SA, Bradley DT, Burnand K, Child AH, Clough RE, Cockerill G, Hafez H, Scott DJ, Futers S, Johnson A, Sohrabi S, Smith A, Thompson MM, van Bockxmeer FM, Waltham M, Matthiasson SE, Thorleifsson G, Thorsteinsdottir U, Blankensteijn JD, Teijink JA, Wijmenga C, de Graaf J, Kiemeney LA, Assimes TL, McPherson R, Global BC, Folkersen L, Franco-Cereceda A, Palmen J, Smith AJ, Sylvius N, Wild JB, Refstrup M, Edkins S, Gwilliam R, Hunt SE, Potter S, Lindholt JS, Frikke-Schmidt R, Tybjaerg-Hansen A, Hughes AE, Golledge J, Norman PE, van Rij A, Powell JT, Eriksson P, Stefansson K, Thompson JR, Humphries SE, Sayers RD, Deloukas P, Samani NJ Consortium CA, Consortium D, Consortium V. Abdominal aortic aneurysm is associated with a variant in low-density lipoprotein receptor-related protein 1. Am J Hum Genet. 2011;89(5):619–27. doi: 10.1016/j.ajhg.2011.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Gretarsdottir S, Baas AF, Thorleifsson G, Holm H, den Heijer M, de Vries JP, Kranendonk SE, Zeebregts CJ, van Sterkenburg SM, Geelkerken RH, van Rij AM, Williams MJ, Boll AP, Kostic JP, Jonasdottir A, Jonasdottir A, Walters GB, Masson G, Sulem P, Saemundsdottir J, Mouy M, Magnusson KP, Tromp G, Elmore JR, Sakalihasan N, Limet R, Defraigne JO, Ferrell RE, Ronkainen A, Ruigrok YM, Wijmenga C, Grobbee DE, Shah SH, Granger CB, Quyyumi AA, Vaccarino V, Patel RS, Zafari AM, Levey AI, Austin H, Girelli D, Pignatti PF, Olivieri O, Martinelli N, Malerba G, Trabetti E, Becker LC, Becker DM, Reilly MP, Rader DJ, Mueller T, Dieplinger B, Haltmayer M, Urbonavicius S, Lindblad B, Gottsater A, Gaetani E, Pola R, Wells P, Rodger M, Forgie M, Langlois N, Corral J, Vicente V, Fontcuberta J, Espana F, Grarup N, Jorgensen T, Witte DR, Hansen T, Pedersen O, Aben KK, de Graaf J, Holewijn S, Folkersen L, Franco-Cereceda A, Eriksson P, Collier DA, Stefansson H, Steinthorsdottir V, Rafnar T, Valdimarsson EM, Magnadottir HB, Sveinbjornsdottir S, Olafsson I, Magnusson MK, Palmason R, Haraldsdottir V, Andersen K, Onundarson PT, Thorgeirsson G, Kiemeney LA, Powell JT, Carey DJ, Kuivaniemi H, Lindholt JS, Jones GT, Kong A, Blankensteijn JD, Matthiasson SE, Thorsteinsdottir U, Stefansson K. Genome-wide association study identifies a sequence variant within the DAB2IP gene conferring susceptibility to abdominal aortic aneurysm. Nat Genet. 2010;42(8):692–7. doi: 10.1038/ng.622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Bradley DT, Hughes AE, Badger SA, Jones GT, Harrison SC, Wright BJ, Bumpstead S, Baas AF, Gretarsdottir S, Burnand K, Child AH, Clough RE, Cockerill G, Hafez H, Scott DJ, Ariens RA, Johnson A, Sohrabi S, Smith A, Thompson MM, van Bockxmeer FM, Waltham M, Matthiasson SE, Thorleifsson G, Thorsteinsdottir U, Blankensteijn JD, Teijink JA, Wijmenga C, de Graaf J, Kiemeney LA, Wild JB, Edkins S, Gwilliam R, Hunt SE, Potter S, Lindholt JS, Golledge J, Norman PE, van Rij A, Powell JT, Eriksson P, Stefansson K, Thompson JR, Humphries SE, Sayers RD, Deloukas P, Samani NJ, Bown MJ. A variant in LDLR is associated with abdominal aortic aneurysm. Circ Cardiovasc Genet. 2013;6(5):498–504. doi: 10.1161/CIRCGENETICS.113.000165. [DOI] [PubMed] [Google Scholar]
- 127.Jones GT, Bown MJ, Gretarsdottir S, Romaine SP, Helgadottir A, Yu G, Tromp G, Norman PE, Jin C, Baas AF, Blankensteijn JD, Kullo IJ, Phillips LV, Williams MJ, Topless R, Merriman TR, Vasudevan TM, Lewis DR, Blair RD, Hill AA, Sayers RD, Powell JT, Deloukas P, Thorleifsson G, Matthiasson SE, Thorsteinsdottir U, Golledge J, Ariens RA, Johnson A, Sohrabi S, Scott DJ, Carey DJ, Erdman R, Elmore JR, Kuivaniemi H, Samani NJ, Stefansson K, van Rij AM. A sequence variant associated with sortilin-1 (SORT1) on 1p13.3 is independently associated with abdominal aortic aneurysm. Hum Mol Genet. 2013;22(14):2941–7. doi: 10.1093/hmg/ddt141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Helgadottir A, Thorleifsson G, Magnusson KP, Gretarsdottir S, Steinthorsdottir V, Manolescu A, Jones GT, Rinkel GJ, Blankensteijn JD, Ronkainen A, Jaaskelainen JE, Kyo Y, Lenk GM, Sakalihasan N, Kostulas K, Gottsater A, Flex A, Stefansson H, Hansen T, Andersen G, Weinsheimer S, Borch-Johnsen K, Jorgensen T, Shah SH, Quyyumi AA, Granger CB, Reilly MP, Austin H, Levey AI, Vaccarino V, Palsdottir E, Walters GB, Jonsdottir T, Snorradottir S, Magnusdottir D, Gudmundsson G, Ferrell RE, Sveinbjornsdottir S, Hernesniemi J, Niemela M, Limet R, Andersen K, Sigurdsson G, Benediktsson R, Verhoeven EL, Teijink JA, Grobbee DE, Rader DJ, Collier DA, Pedersen O, Pola R, Hillert J, Lindblad B, Valdimarsson EM, Magnadottir HB, Wijmenga C, Tromp G, Baas AF, Ruigrok YM, van Rij AM, Kuivaniemi H, Powell JT, Matthiasson SE, Gulcher JR, Thorgeirsson G, Kong A, Thorsteinsdottir U, Stefansson K. The same sequence variant on 9p21 associates with myocardial infarction, abdominal aortic aneurysm and intracranial aneurysm. Nat Genet. 2008;40(2):217–24. doi: 10.1038/ng.72. [DOI] [PubMed] [Google Scholar]
- 129.Jones GT, Tromp G, Kuivaniemi H, Gretarsdottir S, Baas AF, Giusti B, Strauss E, Van’t Hof FN, Webb TR, Erdman R, Ritchie MD, Elmore JR, Verma A, Pendergrass S, Kullo IJ, Ye Z, Peissig PL, Gottesman O, Verma SS, Malinowski J, Rasmussen-Torvik LJ, Borthwick KM, Smelser DT, Crosslin DR, de Andrade M, Ryer EJ, McCarty CA, Bottinger EP, Pacheco JA, Crawford DC, Carrell DS, Gerhard GS, Franklin DP, Carey DJ, Phillips VL, Williams MJ, Wei W, Blair R, Hill AA, Vasudevan TM, Lewis DR, Thomson IA, Krysa J, Hill GB, Roake J, Merriman TR, Oszkinis G, Galora S, Saracini C, Abbate R, Pulli R, Pratesi C, Saratzis A, Verissimo AR, Bumpstead S, Badger SA, Clough RE, Cockerill G, Hafez H, Scott DJ, Futers TS, Romaine SP, Bridge K, Griffin KJ, Bailey MA, Smith A, Thompson MM, van Bockxmeer FM, Matthiasson SE, Thorleifsson G, Thorsteinsdottir U, Blankensteijn JD, Teijink JA, Wijmenga C, de Graaf J, Kiemeney LA, Lindholt JS, Hughes A, Bradley DT, Stirrups K, Golledge J, Norman PE, Powell JT, Humphries SE, Hamby SE, Goodall AH, Nelson CP, Sakalihasan N, Courtois A, Ferrell RE, Eriksson P, Folkersen L, Franco-Cereceda A, Eicher JD, Johnson AD, Betsholtz C, Ruusalepp A, Franzen O, Schadt EE, Bjorkegren JL, Lipovich L, Drolet AM, Verhoeven EL, Zeebregts CJ, Geelkerken RH, van Sambeek MR, van Sterkenburg SM, de Vries JP, Stefansson K, Thompson JR, de Bakker PI, Deloukas P, Sayers RD, Harrison SC, van Rij AM, Samani NJ, Bown MJ. Meta-Analysis of Genome-Wide Association Studies for Abdominal Aortic Aneurysm Identifies Four New Disease-Specific Risk Loci. Circ Res. 2017;120(2):341–353. doi: 10.1161/CIRCRESAHA.116.308765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Harrison SC, Holmes MV, Burgess S, Asselbergs FW, Jones GT, Baas AF, van ’t Hof FN, de Bakker PIW, Blankensteijn JD, Powell JT, Saratzis A, de Borst GJ, Swerdlow DI, van der Graaf Y, van Rij AM, Carey DJ, Elmore JR, Tromp G, Kuivaniemi H, Sayers RD, Samani NJ, Bown MJSE. Humphries, Genetic Association of Lipids and Lipid Drug Targets With Abdominal Aortic Aneurysm: A Meta-analysis. JAMA Cardiol. 2017 doi: 10.1001/jamacardio.2017.4293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Webb TR, Erdmann J, Stirrups KE, Stitziel NO, Masca NG, Jansen H, Kanoni S, Nelson CP, Ferrario PG, Konig IR, Eicher JD, Johnson AD, Hamby SE, Betsholtz C, Ruusalepp A, Franzen O, Schadt EE, Bjorkegren JL, Weeke PE, Auer PL, Schick UM, Lu Y, Zhang H, Dube MP, Goel A, Farrall M, Peloso GM, Won HH, Do R, van Iperen E, Kruppa J, Mahajan A, Scott RA, Willenborg C, Braund PS, van Capelleveen JC, Doney AS, Donnelly LA, Asselta R, Merlini PA, Duga S, Marziliano N, Denny JC, Shaffer C, El-Mokhtari NE, Franke A, Heilmann S, Hengstenberg C, Hoffmann P, Holmen OL, Hveem K, Jansson JH, Jockel KH, Kessler T, Kriebel J, Laugwitz KL, Marouli E, Martinelli N, McCarthy MI, Van Zuydam NR, Meisinger C, Esko T, Mihailov E, Escher SA, Alver M, Moebus S, Morris AD, Virtamo J, Nikpay M, Olivieri O, Provost S, AlQarawi A, Robertson NR, Akinsansya KO, Reilly DF, Vogt TF, Yin W, Asselbergs FW, Kooperberg C, Jackson RD, Stahl E, Muller-Nurasyid M, Strauch K, Varga TV, Waldenberger M, Zeng L, Chowdhury R, Salomaa V, Ford I, Jukema JW, Amouyel P, Kontto J, Nordestgaard BG, Ferrieres J, Saleheen D, Sattar N, Surendran P, Wagner A, Young R, Howson JM, Butterworth AS, Danesh J, Ardissino D, Bottinger EP, Erbel R, Franks PW, Girelli D, Hall AS, Hovingh GK, Kastrati A, Lieb W, Meitinger T, Kraus WE, Shah SH, McPherson R, Orho-Melander M, Melander O, Metspalu A, Palmer CN, Peters A, Rader DJ, Reilly MP, Loos RJ, Reiner AP, Roden DM, Tardif JC, Thompson JR, Wareham NJ, Watkins H, Willer CJ, Samani NJ, Schunkert H, Deloukas P, Kathiresan SG Investigators M. Myocardial Infarction, C.A.E.C. Investigators, Systematic Evaluation of Pleiotropy Identifies 6 Further Loci Associated With Coronary Artery Disease. J Am Coll Cardiol. 2017;69(7):823–836. doi: 10.1016/j.jacc.2016.11.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Owens GK, Kumar MS, Wamhoff BR Wellcome Trust Case Control C. Molecular regulation of vascular smooth muscle cell differentiation in development and disease. Physiol Rev. 2004;84(3):767–801. doi: 10.1152/physrev.00041.2003. [DOI] [PubMed] [Google Scholar]
- 133.Wang L, Zhang J, Fu W, Guo D, Jiang J, Wang Y. Association of smooth muscle cell phenotypes with extracellular matrix disorders in thoracic aortic dissection. J Vasc Surg. 2012;56(6):1698–709. 1709 e1. doi: 10.1016/j.jvs.2012.05.084. [DOI] [PubMed] [Google Scholar]
- 134.Crosas-Molist E, Meirelles T, Lopez-Luque J, Serra-Peinado C, Selva J, Caja L, Gorbenko Del Blanco D, Uriarte JJ, Bertran E, Mendizabal Y, Hernandez V, Garcia-Calero C, Busnadiego O, Condom E, Toral D, Castella M, Forteza A, Navajas D, Sarri E, Rodriguez-Pascual F, Dietz HC, Fabregat I, Egea G. Vascular smooth muscle cell phenotypic changes in patients with Marfan syndrome. Arterioscler Thromb Vasc Biol. 2015;35(4):960–72. doi: 10.1161/ATVBAHA.114.304412. [DOI] [PubMed] [Google Scholar]
- 135.Ishii T, Asuwa N. Collagen and elastin degradation by matrix metalloproteinases and tissue inhibitors of matrix metalloproteinase in aortic dissection. Hum Pathol. 2000;31(6):640–6. doi: 10.1053/hupa.2000.7642. [DOI] [PubMed] [Google Scholar]
- 136.Bunton TE, Biery NJ, Myers L, Gayraud B, Ramirez F, Dietz HC. Phenotypic alteration of vascular smooth muscle cells precedes elastolysis in a mouse model of Marfan syndrome. Circ Res. 2001;88(1):37–43. doi: 10.1161/01.res.88.1.37. [DOI] [PubMed] [Google Scholar]
- 137.Koyama H, Raines EW, Bornfeldt KE, Roberts JM, Ross R. Fibrillar collagen inhibits arterial smooth muscle proliferation through regulation of Cdk2 inhibitors. Cell. 1996;87(6):1069–78. doi: 10.1016/s0092-8674(00)81801-2. [DOI] [PubMed] [Google Scholar]
- 138.Li DY, Brooke B, Davis EC, Mecham RP, Sorensen LK, Boak BB, Eichwald E, Keating MT. Elastin is an essential determinant of arterial morphogenesis. Nature. 1998;393(6682):276–80. doi: 10.1038/30522. [DOI] [PubMed] [Google Scholar]
- 139.Mack CP. Signaling mechanisms that regulate smooth muscle cell differentiation. Arterioscler Thromb Vasc Biol. 2011;31(7):1495–505. doi: 10.1161/ATVBAHA.110.221135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Ma C, Chegini N. Regulation of matrix metalloproteinases (MMPs) and their tissue inhibitors in human myometrial smooth muscle cells by TGF-beta1. Mol Hum Reprod. 1999;5(10):950–4. doi: 10.1093/molehr/5.10.950. [DOI] [PubMed] [Google Scholar]