Abstract
Background
Parkinson’s disease patients often have visual alterations, for example loss of visual acuity, contrast sensitivity or motion perception, and diminished electroretinogram responses. Parkinson’s disease pathology is mainly characterized by the accumulation of pathological α-synuclein deposits in the brain, but little is known about how synucleinopathy affects the retina.
Objective
To study the correlation between α-synuclein deposits in the retina and brain of autopsied subjects with Parkinson’s disease and Incidental Lewy Body Disease.
Methods
We evaluated the presence of phosphorylated α-synuclein in the retina of autopsied subjects with Parkinson’s disease (9 subjects), incidental Lewy body disease (4 subjects), and controls (6 subjects) by immunohistochemistry and compared the retinal synucleinopathy with brain disease severity indicators.
Results
While controls did not show any phosphorylated α-synuclein immunoreactivity in their retina, all Parkinson’s disease subjects and 3 of 4 incidental Lewy body disease subjects had phosphorylated α-synuclein deposits in ganglion cell perikarya, dendrites and axons, some of them resembling brain Lewy bodies and Lewy neurites. The Lewy-type synucleinopathy density in the retina significantly correlated with Lewy-type synucleinopathy density in the brain, with the Unified Parkinson’s disease pathology stage and with the motor Unified Parkinson’s Disease Rating Scale.
Conclusion
This data suggests that phosphorylated α-synuclein accumulates in the retina in parallel with that in the brain, including in early stages prior to the development of clinical signs of parkinsonism or dementia. Therefore, the retina may provide an in vivo indicator of brain pathology severity, and its detection could help in the diagnosis and monitoring of disease progression.
Keywords: Parkinson’s disease, human, retina, phosphorylated α-synuclein, vision
Introduction
Parkinson’s disease (PD) is one of the most common neurodegenerative disorders, affecting between seven and ten million people worldwide according to the Parkinson’s Foundation (http://www.parkinson.org/Understanding-Parkinsons/Causes-and-Statistics/Statistics). The most characteristic symptoms are bradykinesia, rest tremor, rigidity, and postural instability (1–3). Non-motor symptoms have also been widely described, including mood disturbance, sleep disorders, cognitive decline and autonomic impairment (2–4). Visual symptoms, including dry eyes, reading difficulties and visual hallucinations, are relatively common. Detailed ophthalmological examinations also suggest a loss of visual acuity, contrast sensitivity, color discrimination and motion perception, and a reduced electroretinogram response (5–11). The cellular and molecular mechanisms that lead to vision impairment in PD are still unclear and little information is known about how PD affects the retina.
The pathology of PD is characterized by the presence of pathological deposits of α-synuclein throughout the central (12,13) and peripheral nervous systems (14–18), causing parkinsonism due to the massive and irreversible loss of dopaminergic neurons in the substantia nigra pars compacta, and eventual cognitive dysfunction due to its effects on the cerebral cortex. The pathological α-synuclein deposits, contained within Lewy bodies and Lewy neurites, are associated with abnormally phosphorylated α-synuclein (p-syn) (19,20). α-synuclein is a small and highly-conserved protein of 140 amino acids that is enriched in presynaptic terminals in different neural regions (21,22). Its physiological functions remain unclear, but some studies suggest a role in the regulation of synaptic vesicle formation and neurotransmitter release (22,23). While the native, unphosphorylated conformation is present in several retinal cell types (21,24), the phosphorylation of α-synuclein at serine 129 can be used as a specific marker of CNS synucleinopathy (25,26).
Because of the importance of p-syn in the possible spreading of the disease and findings of its presence in the peripheral nervous system (PNS) in PD (4,16), this study analyzed Lewy type α-synucleinopathy (LTS) in the retina of autopsied PD subjects. Additionally, subjects that showed no clinical signs of parkinsonism or dementia but had LTS in the brain (incidental Lewy body disease subjects (ILBD), were also studied, as possible prodromal disease. We aimed to characterize which cells and structures accumulate p-syn and to study if the amount of p-syn in the retina was related to p-syn load in the brain. These results could lead to a better understanding of disease spread and help in the search for an accessible diagnostic and progression biomarker for Parkinson’s disease and other synucleinopathies.
Materials and Methods
Source of human subjects
Human retina samples from six controls, four subjects with incidental Lewy body disease (ILBD), and nine PD subjects were obtained postmortem from volunteer donors in the Arizona Study of Aging and Neurodegenerative Disorders (AZSAND)/Banner Sun Health Research Institute Brain and Body Donation Program (BBDP; www.brainandbodydonationprogram.org) (27). All procedures were conducted in accordance with The Code of Ethics of the World Medical Association (Declaration of Helsinki) for experiments involving humans. All subjects provided signed written informed consent approved by an Institutional Review Board.
Clinical and neuropathological characterization of human subjects
Individuals included in the study were clinically characterized using standard tests that analyzed neurological, cognitive and movement disorder components, and private medical records were reviewed and abstracted for each subject as previously described (27). These included the Unified Parkinson Disease Rating Scale (UPDRS). Standardized neuropathological examinations determined the Unified Staging System for Lewy Body disorders histopathological stage as previously described (28). The diagnosis of PD is clinicopathological: the subjects must have had motor parkinsonism as well as Lewy body pathology and pigmented neuron loss in the substantia nigra at autopsy (29).
Immunohistochemistry
After enucleation, eyeballs were immediately fixed in cold neutral-buffered 10% formalin for 48–72 hours. They were washed in 0.1 M sodium phosphate buffer (pH 7.4) and sequentially cryoprotected in 15%, 20% and 30% sucrose. Cornea, lens and vitreous body were removed and eyecups were processed and cut in eight pieces (30). Some portions were employed as wholemount retinas, for which they were subjected to a freeze-thaw cycle to improve antibody penetration. Others were cut on a cryostat to obtain vertical sections of 14 μm.
Immunohistochemistry using the di-aminobenzidine method was performed on flat whole mount retinas to specifically stain p-syn, following a previously published protocol (30). A rabbit antibody against α-synuclein phosphorylated at serine 129 was used, kindly provided by Dr. Haruhiko Akiyama, at a 1:1000 dilution. Its specificity has been demonstrated in other studies (14,25,26). Samples were flat-mounted in glycerol:phosphate buffer (PB) 0.1 M (1:1) with the ganglion cell layer side up. Images were taken with a Leica DMR microscope (Leica Microsystems, Wetzlar, Germany). Drawings were made using camera lucida.
Fluorescence immunohistochemistry was performed in vertical sections and in whole mount retinas. First, transverse sections were washed with PB 0.1 M and incubated overnight at room temperature in either the p-syn antibody or a rabbit polyclonal primary antibody against native α-synuclein (Santa Cruz Biotechnology, Dallas, TX, USA, Catalog No. sc-7011) diluted 1:100 in 0.1 M PB plus 0.5% Triton X-100. Next, samples were washed and incubated for 1 h at room temperature with Alexa Fluor 488 donkey anti-rabbit IgG secondary antibody (Life Technologies, Eugene, OR, USA) at a 1:100 dilution. Finally, sections were washed with 0.1 M PB and covered with a coverslip. In whole mount retinas, the incubation times were longer: 3 days for the primary antibodies, which included, for some sections, double-staining with rabbit polyclonal anti-RBPMS (RNA-binding protein with multiple splicing), diluted 1:1000, and 2 days for the secondary antibody (Alexa Fluor 555 donkey anti-rabbit IgG at a 1:100 dilution). The RBPMS antibody was a generous gift from Dr. Nicholas Brecha and specifically recognizes retinal ganglion cells (31). Retinas were flat-mounted in Citifluor® (Citifluor Ltd, London, UK) with the ganglion cell layer side up. Fluorescence images were taken using a TCS SP2 confocal laser-scanning microscope (Leica Microsystems).
Lewy-type synucleinopathy density score in retina and brain
P-syn stained whole mount retinas and brains were semi-quantitatively rated for the density of p-syn immunoreactive cellular structures by reviewers who were blinded to clinical diagnosis. In brain tissue, the load of p-syn immunoreactivity was assessed semi-quantitatively in ten standard brain regions, and their summation represents the final brain p-syn load score (12). In retina, the number of stained neuronal perikarya in the nasal-inferior quadrant was manually counted. The density of stained axons and dendrites was assessed using a semi-quantitative 0–3 scale, where 0 revealed no p-syn and 3 represented high densities of p-syn. The final retina score was calculated as the summation of the separate scores for perikarya as well as axons and dendrites (Table 1).
Table 1.
Age, gender, clinicopathological diagnosis, pathology brain stage of the donors at the moment of dead; brain and retinal LTS density scores, motor unified Parkinson’s disease rating scale, and disease duration of the analyzed subjects.
| Subject | Clinicopathological diagnosis | Age (years) | Gender | Unified LB Brain Stage | LTS density score in brain | LTS density score in retina | nº cells | axons | dendrites | Motor UPDRS score | Years from sign and/or symptom onset |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1C | Control | 92 | Female | 0. No Lewy Bodies | 0 | 0 | 0 | 0 | 0 | 7 | - |
| 2C | Control | 89 | Male | 0. No Lewy Bodies | 0 | 0 | 0 | 0 | 0 | 16 | - |
| 3C | Control | 93 | Male | 0. No Lewy Bodies | 0 | 0 | 0 | 0 | 0 | 17 | - |
| 4C | Control | 92 | Female | 0. No Lewy Bodies | 0 | 0 | 0 | 0 | 0 | 9 | - |
| 5C | Control | 77 | Female | 0. No Lewy Bodies | 0 | 0 | 0 | 0 | 0 | 1 | - |
| 6C | Control | 84 | Female | 0. No Lewy Bodies | 0 | 0 | 0 | 0 | 0 | 2 | - |
|
| |||||||||||
| 1ILBD | ILBD | 90 | Female | lla. Brainstem predominant | 8 | 0 | 0 | 0 | 0 | 0 | - |
| 2ILBD | ILBD | 87 | Male | lll. Brainstem/Limbic | 24 | 2 | 1 | 1 | 0 | 11 | - |
| 3ILBD | ILBD | 97 | Male | lla. Brainstem predominant | 7 | 1 | 1 | 0 | 0 | 16 | - |
| 4ILBD | ILBD | 89 | Male | lll. Brainstem/Limbic | 28 | 2 | 0 | 1 | 1 | 0 | - |
|
| |||||||||||
| 1PD | PD | 88 | Female | lV. Neocortical | 26 | 2 | 1 | 0 | 1 | 45 | 16 |
| 2PD | PD | 73 | Male | lll. Brainstem/Limbic | 18 | 2 | 1 | 1 | 0 | 57 | 3 |
| 3PD | PD | 82 | Female | lV. Neocortical | 34 | 5 | 2 | 1 | 2 | 58 | 22 |
| 4PD | PD | 79 | Female | lV. Neocortical | 36 | 18 | 12 | 3 | 3 | 56 | 10 |
| 5PD | PD | 70 | Female | lll. Brainstem/Limbic | 31 | 20 | 14 | 3 | 3 | * | 26 |
| 6PD | PD | 69 | Female | lll. Brainstem/Limbic | 22 | 6 | 0 | 3 | 3 | 29 | 14 |
| 7PD | PD | 72 | Male | lV. Neocortical | 34 | 12 | 6 | 3 | 3 | 72 | 18 |
| 8PD | PD | 79 | Female | lV. Neocortical | 27 | 3 | 0 | 1 | 2 | * | 13 |
| 9PD | PD | 75 | Male | lV. Neocortical | 28 | 6 | 3 | 0 | 3 | 46 | 15 |
C: Control; ILBD: Incidental Lewy Body Disease; PD: Parkinson Disease; LB: Lewy Bodies; LTS: Lewy-type synucleinopathy; UPDRS: Unified Parkinson’s Disease Rating Scale;
data not available;
-: did not present clinical signs or symptoms
Statistical analysis
All studied subjects were included in correlation analyses to compare retina and brain Lewy-type synucleinopathy density score; retina Lewy-type synucleinopathy density score and brain pathology stage; and retina Lewy-type synucleinopathy density score and motor Unified Parkinson’s Disease Rating Score. The Lewy-type synucleinopathy score was based on the number and amount of p-syn immunoreactive structures in standard regions of the brain and retina. For the retinal analysis, only one eye per subject was employed, using always the nasal inferior quadrant. SigmaPlot (Systat Software, Inc, San Jose, CA, USA) and GraphPad Prism 6 (San Diego, CA, USA) were employed to analyze the data. All the correlations were performed by a two-tailed Spearman correlation test and all the individuals were considered for the study. To compare LTS scores between groups (control, ILBD and PD) the non-parametric Kruskal-Wallis ANOVA was performed and followed by the post-hoc Dunn’s multiple comparison test. The significance level was set at p < 0.05.
Results
The age, clinical diagnosis, neuropathological diagnosis, Unified LTS stage and LTS density scores in brain and retina, as well as the motor UPDRS scores of analyzed subjects are shown in Table 1.
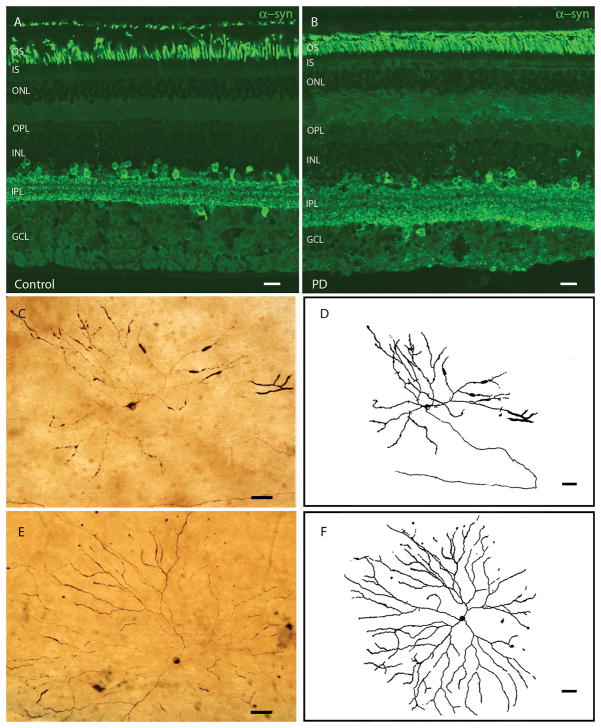
Native α-syn is ubiquitous in the CNS and it is present in all retinal layers and cells, although predominantly in photoreceptor outer segments, amacrine cells and the inner plexiform layer. No immunostaining differences were found between PD and control subjects: α-syn was present in the same cell types and with a similar intensity in both groups (Fig. 1A–B). By contrast, p-syn, a specific pathological marker of synucleinopathies, is present in the retinas of PD subjects and 3 of 4 ILBD subjects compared to controls. Figs. 1–3 show representative photomicrographs of immunohistochemical staining for p-syn in the retina of PD and ILBD subjects. P-syn deposits were found as axonal fibers and dendrites and/or neuronal perikarya (Fig. 1, Fig. 2, Fig. 3). Cells containing p-syn had different morphologies, soma sizes (ranging from 15 to 30 μm), dendritic lengths (ranging from 570 μm to 1620 μm) and receptive fields. They had their cell bodies located in the ganglion cell layer, near the inner surface of the retina, with major dendritic ramifications in retinal strata S3 and S4 of the inner plexiform layer (Fig. 1C–F).
Fig. 1. Immunohistochemical staining pattern of α-syn and p-syn.
A–B: α-syn staining (green) of a control (left) and a PD (right) retinal transversal cut. No differences in immunostaining pattern or intensity are found between controls and PD. C–F: Ganglion cells from PD retinas accumulating p-syn. D and F are drawings of C and E, respectively, made with camera lucida. Control retinas did not have any stained p-syn structures or cells (data not shown). Scale bars A–B= 20 μm; C–F=50 μm.
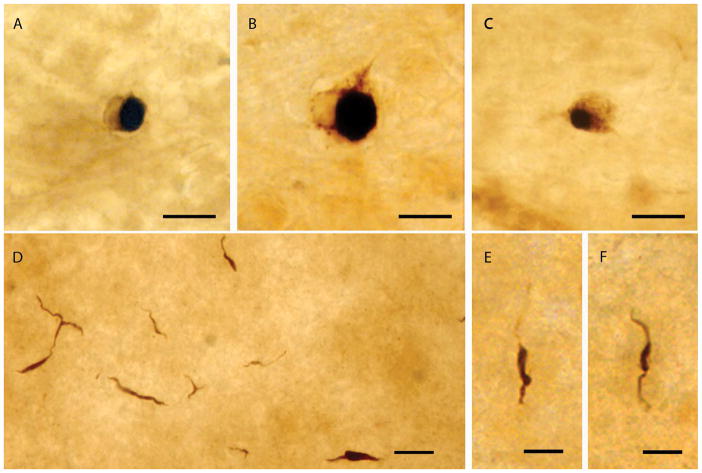
Fig. 3. Lewy-like bodies and neurites in PD.
Lewy body- and Lewy neurite-like structures in PD retinas stained for p-syn. A–C: Lewy body-like structures. D–F: Lewy neurite-like structures; E and F are higher magnifications of Lewy neurite-like structures. Scale bars A–D = 20 μm; E–F = 10 μm
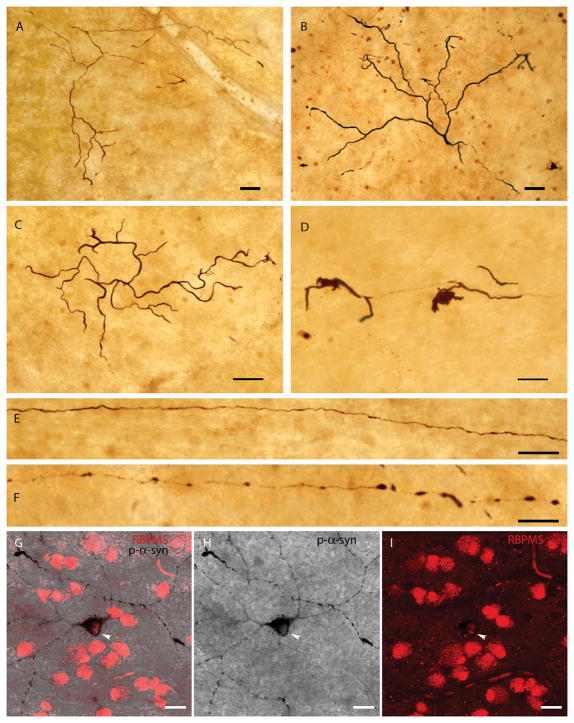
Fig. 2. Other p-syn-immunoreactive structures in PD retinas.
A–B: Normal-appearing dendrites in the ganglion cell layer that contain p-syn. C–D: Dendrites accumulating p-syn that display an abnormal and aberrant morphology, typical of degenerative processes. E–F: Long axons stained with p-syn in PD retinas. G–I: Double staining of RBPMS (red) and p-syn (black) in PD retinas. Arrows show the soma of p-syn-containing ganglion cells stained with RBPMS. Scale bars A–F: 50 μm; G–I: 20 μm.
Along with normal-appearing dendrites and cell bodies, some aberrant structures were also detected in the ganglion cell layer of PD subjects. In Fig. 2 curly dendrites, abnormal and twisted structures, swollen dendrites and intracytoplasmic accumulations of p-syn can be observed. These dendritic alterations are a characteristic mark of cell pathology, degeneration or dysfunction, including synucleinopathy. Some of the immunoreactive cell bodies clearly were associated with immunoreactive axons (Fig. 1C–D). Other long fibers, putatively axons, that crossed the retina but did not visibly emerge from any cell body were also found and can be seen in Fig. 2. Some of these axons had normal morphology (Fig. 2E), but others had abnormal beading and swollen segments (Fig. 2F). All of these p-syn immuoreactive morphological alterations were always found within the ganglion cell layer and the immunoreactive perikarya were all ganglion cells, as shown by double staining with RBPMS, a ganglion cell marker (Fig. 2G–I).
Retinas with positive staining for p-syn had either all or several types of these stained structures present, at relatively sparse densities from the center to periphery. The neural perikaryal staining shown in Fig. 3 is condensed into defined inclusions in the cell cytoplasm, resembling classic brain Lewy bodies. P-syn positive Lewy body-like structures in the PD retinas were more frequent and prevalent than p-syn positive complete perikarya or neurites. We also observed p-syn immunoreactive dotted neurites with typical dystrophic Lewy neurite morphology. This is the first time that p-syn Lewy-like bodies and neurites have been described in the retina of PD subjects.
The p-syn positive structures described were observed in the retinas of all nine PD subjects and in three of four subjects with incidental Lewy body disease (ILBD). P-syn immunoreactivity was absent in the brain and retina of all six clinicopathologically diagnosed controls.
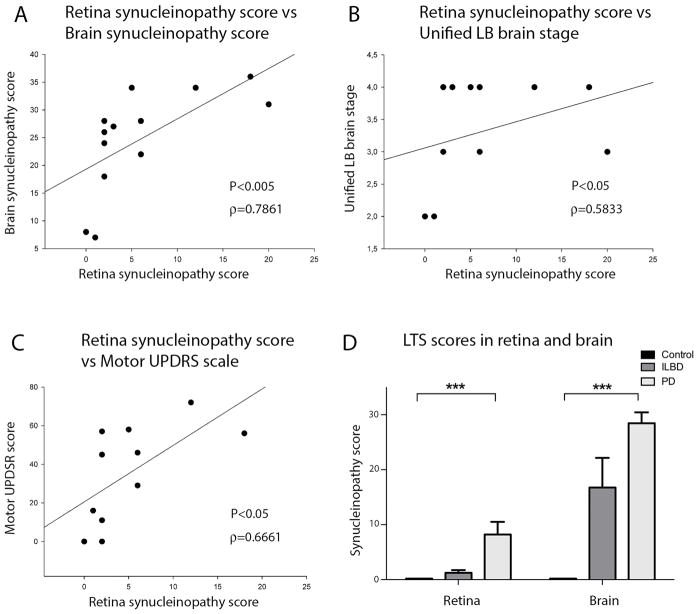
Retina and brain LTS scores differed between the three clinicopathological groups, being statistically significant between controls and PD (p < 0.001). The Spearman’s correlation test, done considering only the affected groups (ILBD and PD), revealed a strong positive correlation between LTS density score in brain and retina (Spearman’s ρ = 0.7861; p < 0.005) (Fig. 4). Retinal LTS density score also correlated with the brain pathology stage (Spearman’s ρ = 0.5833; p < 0.05) and with the motor UPDRS score (Spearman’s ρ = 0.6661; p < 0.05), suggesting that the pathology progression is related in both tissues and that retinal analysis may give information about the brain disease stage and severity.
Fig. 4. Correlation of retinal Lewy-type synucleinopathy score with indicators of PD brain pathology.
A: Correlation plot between retinal and brain LTS density score in all subjects, Spearman correlation ρ = 0.7861; p < 0.005. B: Correlation plot between retinal LTS density score and Unified LTS brain stage in all subjects, Spearman correlation ρ = 0.5833; p < 0.05. C: Correlation plot between retinal LTS density score and motor Unified Parkinson’s Disease Rating Scale (UPDRS) score in all subjects, Spearman correlation ρ = 0.6661; p < 0.05. D: LTS density score comparison between control, ILBD and PD groups in retina and brain. LTS scores differ between the three clinicopathological groups and are significantly different (P<0.001) between controls and PD subjects both in retina and brain.
Discussion
Possibly due to the difficulty in obtaining high quality postmortem human retinas, there are very few studies about retinal changes at a cellular level in PD subjects. The aim of the study was to analyze the presence of p-syn, one of the main hallmarks of PD, in postmortem retinal tissue of control and PD donors and to compare it with clinical and brain neuropathological features.
While Parkinson’s disease can be clinically diagnosed with reasonable accuracy in subjects with longstanding disease, in those with clinical symptoms of less than 5 years duration, diagnostic accuracy may be as low as 53% (32). The importance of early diagnosis, and the need to monitor the effects of therapy, makes necessary the identification of new biomarkers for PD. Due to the close relationship of the eye with the brain, their common embryonic nature and the ability to examine the eyes and retina of living subjects with imaging techniques, the retina could be a candidate biomarker tissue for neurodegenerative diseases. As a part of the CNS, the retina reflects some of the pathological alterations of brain-predominant neurodegenerative diseases like Alzheimer’s disease, Parkinson’s disease, and Huntington’s disease (33).
Visual dysfunction and retinal changes in PD have been widely reported (5,6). Patients suffering from PD have functional visual alterations such as reduced electroretinogram (ERG) responses and prolonged latency in visual evoked potentials (33–36). They also show a loss in contrast sensitivity and color perception abnormalities (11,33,34,37,38). In PD animal models, loss of dopaminergic amacrine cells together with reduced ERG scotopic a- and b-wave amplitudes, have been demonstrated (33,39,40). In addition, using the optical coherence tomography (OCT) imaging technique in patients in vivo, some authors have shown a thinning of the inner retinal layers: the ganglion cell layer, inner plexiform layer and inner nuclear layer (41–43), although there is some controversy about this issue and other studies show no difference in this aspect (44). All these studies seem to indicate that the retina becomes involved in PD, although it remains unknown to what extent.
This study establishes the presence of p-syn within retinal ganglion cells, the major retinal projection neurons, as demonstrated by double-staining with RBPMS. This accumulation is relatively sparse, with relatively few ganglion cells affected. The exact type of ganglion cell affected is still undetermined but they seem to be different ganglion cell types based on their different morphologies. This suggests that the p-syn accumulation may not be cell-type-specific. Supporting a localization exclusively to ganglion cells, retinal amacrine cells, including dopaminergic amacrine cells, did not have any p-syn immunoreactivity.
This study is the first to demonstrate p-syn immunoreactive retinal structures similar to brain Lewy bodies and neurites. Previous research using antibodies against α-syn in thin paraffin sections stated that no pathological α-syn immunoreactivity could be found in the retina and lens of PD patients (45) or in any part of the ocular globe in AD (46). Differences with our study may be due to our use of antibodies against p-syn rather than unmodified α-syn, and our use of retinal whole mounts rather than thin paraffin sections. The relatively small number of p-syn positive structures may be difficult to detect in the small tissue volumes available in paraffin sections. Despite these differences between studies, further investigations of the eye in PD are desirable, as it is known that ocular structures are involved in the pathology of several neurodegenerative diseases (33,47). For example, tears (48,49), lens (50,51), cornea (52) and retina (53) have already been investigated and proposed as sources for possible PD biomarkers.
Additionally, in this study it was demonstrated that the accumulation of p-syn in the retina specifically co-segregated with subjects that had LTS in the brain. This included all 9 PD subjects as well as 3 of the 4 ILBD subjects. No study has previously found p-syn accumulation in ILBD and its presence, even prior to clinical signs of parkinsonism or dementia, could be extremely important as a potential biomarker for neuroprotective prevention trials. Specificity was excellent as none of the 6 controls had p-syn in the retina. Additionally, there was a strong correlation between brain and retina LTS density scores and between retinal LTS density and clinical disease. The major limitation of this study is the small number of subjects in each group. However, this is offset, to some degree, by the fact that all subjects in the study had autopsy confirmation of disease. The fact that all 9 PD subjects, and 3 of 4 ILBD subjects had retinal LTS, and that none of the six controls had retinal LTS, suggests sensitivity and specificity may be very high, even prior to clinical signs of PD become present.
The positive correlation between LTS density in the retina and the brains of PD subjects and its correlation with motor scores and disease stage suggests that the progression of the disease is related in both tissues. Because of that, the retina could act as a window into the brain pathology and serve as a biomarker of brain PD pathology. In fact, researchers have been able to detect p-syn:GFP aggregates in the retina of a PD mouse model (transgenic mice expressing a fused α-syn:GFP gene under the PDGFβ promoter (PDNG78 line)) using a non-invasive in vivo retinal imaging microscope (54). This technique allowed longitudinal evaluation of the same retinal areas over time.
We suggest that a methodology similar to that employed by Price et al. could be used to evaluate the in vivo presence of synucleinopathy in the retinas of prodromal and symptomatic PD patients. As Price at al. have done in the mouse, the retinas of living individuals could potentially be assessed using available and routine ophthalmological non-invasive imaging techniques like OCT, eye fundus, angiography, etc. These techniques allow to visualize the whole retina and to see retinal changes. To specifically mark LTS, development of specific fluorescent dyes and its delivery to the retina by intravitreal injection could be used. Intravitreal injections have an extremely low rate of complications or adverse effects, and are widely used in clinical ophthalmology, especially for the treatment of glaucoma, macular degeneration, or other retinal diseases. The development of fluorescent ligands specific for p-syn, along with intraocular injection and retinal imaging analysis (as fluorescent OCT or eye fundus), could theoretically be used to detect and monitor the progression of Parkinson’s disease in living subjects based on the retinal LTS density. The findings of this research invite the development of future applications leading to the utilization of retinal LTS as a PD biomarker.
Acknowledgments
Funding: This work was supported by the Michael J Fox Foundation for Parkinson’s research. IOL acknowledges financial support from the Ministerio de Educación, Spain (FPU 14/03166). NC acknowledges financial support from the Ministerio de Economía y Competitividad, Spain (MINECO-FEDER-BFU2015-67139-R), Generalitat Valenciana (Prometeo 2016/158) and Instituto Carlos III (ISCIII RETICS-FEDER RD12/0034/0010). The Brain and Body Donation Program has been supported by the National Institute of Neurological Disorders and Stroke (U24 NS072026), the National Institute on Aging (P30 AG19610), the Arizona Department of Health Services, the Arizona Biomedical Research Commission and the Michael J. Fox Foundation for Parkinson’s Research.
Abbreviations
- GFP
Green Fluorescent Protein
- LTS
Lewy-type synucleinopathy
- p-syn
Phosphorylated-α-synuclein
- PD
Parkinson’s Disease
- RBPMS
RNA-binding protein with multiple splicing
- UPDRS
Unified Parkinson’s disease rating scale
Footnotes
Financial Disclosure: The authors declare they have no conflicts of interest.
Authors’ roles
Conception and design: Cuenca, Beach, Adler, Walker
Analysis and interpretation: Ortuño-Lizarán, Cuenca, Beach, Serrano, Walker, Adler
Data collection: Ortuño-Lizarán, Serrano,
Manuscript draft and revision: Ortuño-Lizarán, Cuenca, Beach, Serrano, Walker, Adler
Obtained funding: Cuenca, Beach, Adler, Walker
Overall responsibility: Cuenca, Ortuño-Lizarán, Beach, Serrano, Walker, Adler
References
- 1.Ferreira M, Massano J. An updated review of Parkinson’s disease genetics and clinicopathological correlations. Acta Neurol Scand. 2016 May;:1–12. doi: 10.1111/ane.12616. [DOI] [PubMed] [Google Scholar]
- 2.Postuma RB, Berg D, Stern M, Poewe W, Olanow CW, Oertel W, et al. MDS clinical diagnostic criteria for Parkinson’s disease. Mov Disord. 2015 Oct;30(12):1591–601. doi: 10.1002/mds.26424. [DOI] [PubMed] [Google Scholar]
- 3.Fahn S. Description of Parkinson’s Disease as a Clinical Syndrome. Ann N Y Acad Sci. 2006 Jan 24;991(1):1–14. doi: 10.1111/j.1749-6632.2003.tb07458.x. [DOI] [PubMed] [Google Scholar]
- 4.Licker V, Burkhard PR. Proteomics as a new paradigm to tackle Parkinson’s disease research challenges. Transl Proteomics. 2014:4–5. [Google Scholar]
- 5.Archibald NK, Clarke MP, Mosimann UP, Burn DJ. The retina in Parkinsons disease. Brain. 2009;132(5):1128–45. doi: 10.1093/brain/awp068. [DOI] [PubMed] [Google Scholar]
- 6.Rodnitzky RL. Visual dysfunction in Parkinson’s disease. Clin Neurosci. 1998;5(2):102–6. [PubMed] [Google Scholar]
- 7.Uc EY, Rizzo M, Anderson SW, Qian S, Rodnitzky RL, Dawson JD. Visual dysfunction in Parkinson disease without dementia. Neurology. 2005 Dec;65(12):1907–13. doi: 10.1212/01.wnl.0000191565.11065.11. [DOI] [PubMed] [Google Scholar]
- 8.Büttner T, Kuhn W, Klotz P, Steinberg R, Voss L, Bulgaru D, et al. Disturbance of colour perception in Parkinson’s disease. J Neural Transm - Park Dis Dement Sect. 1993 Feb;6(1):11–5. doi: 10.1007/BF02252618. [DOI] [PubMed] [Google Scholar]
- 9.Archibald NK, Clarke MP, Mosimann UP, Burn DJ. Visual symptoms in Parkinson’s disease and Parkinson’s disease dementia. Mov Disord. 2011 Nov;26(13):2387–95. doi: 10.1002/mds.23891. [DOI] [PubMed] [Google Scholar]
- 10.Nowacka B, Lubinski W, Honczarenko K, Potemkowski A, Safranow K. Ophthalmological Features of Parkinson Disease. Med Sci Monit. 2014;20:2243–9. doi: 10.12659/MSM.890861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lin TP, Rigby H, Adler JS, Hentz JG, Balcer LJ, Galetta SL, et al. Abnormal visual contrast acuity in Parkinson’s disease. J Parkinsons Dis. 2015;5(1):125–30. doi: 10.3233/JPD-140470. [DOI] [PubMed] [Google Scholar]
- 12.Beach TG, Adler CH, Lue L, Sue LI, Bachalakuri J, Henry-Watson J, et al. Unified staging system for Lewy body disorders: correlation with nigrostriatal degeneration, cognitive impairment and motor dysfunction. Acta Neuropathol. 2009 Jun;117(6):613–34. doi: 10.1007/s00401-009-0538-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Campello L, Esteve-Rudd J, Cuenca N, Martín-Nieto J. The Ubiquitin-Proteasome System in Retinal Health and Disease. Mol Neurobiol. 2013:1–21. doi: 10.1007/s12035-012-8391-5. [DOI] [PubMed] [Google Scholar]
- 14.Beach TG, Carew J, Serrano G, Adler CH, Shill Ha, Sue LI, et al. Phosphorylated α-synuclein-immunoreactive retinal neuronal elements in Parkinson’s disease subjects. Neurosci Lett. 2014;571:34–8. doi: 10.1016/j.neulet.2014.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Waxman EA, Giasson BI. Specificity and Regulation of Casein Kinase-Mediated Phosphorylation of α-Synuclein. J Neuropathol Exp Neurol. 2008 May 1;67(5):402–16. doi: 10.1097/NEN.0b013e3186fc995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee JM, Derkinderen P, Kordower JH, Freeman R, Munoz DG, Kremer T, et al. The Search for a Peripheral Biopsy Indicator of α-Synuclein Pathology for Parkinson Disease. J Neuropathol Exp Neurol. 2017;76(1):nlw103. doi: 10.1093/jnen/nlw103. [DOI] [PubMed] [Google Scholar]
- 17.Nakamura K, Mori F, Tanji K, Miki Y, Toyoshima Y, Kakita A, et al. α-Synuclein pathology in the cranial and spinal nerves in Lewy body disease. Neuropathology. 2016;36(3):262–9. doi: 10.1111/neup.12269. [DOI] [PubMed] [Google Scholar]
- 18.Beach TG, Adler CH, Sue LI, Vedders L, Lue L, White CL, III, et al. Multi-organ distribution of phosphorylated alpha-synuclein histopathology in subjects with Lewy body disorders. Acta Neuropathol. 2010 Jun;119(6):689–702. doi: 10.1007/s00401-010-0664-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee H-J, Bae E-J, Lee S-J. Extracellular α--synuclein-a novel and crucial factor in Lewy body diseases. Nat Rev Neurol. 2014;10(2):92–8. doi: 10.1038/nrneurol.2013.275. [DOI] [PubMed] [Google Scholar]
- 20.Spillantini MG, Schmidt ML, Lee VM, Trojanowski JQ, Jakes R, Goedert M. Alpha-synuclein in Lewy bodies. Nature. 1997;388(6645):839–40. doi: 10.1038/42166. [DOI] [PubMed] [Google Scholar]
- 21.Martínez-Navarrete GC, Martín-Nieto J, Esteve-Rudd J, Angulo A, Cuenca N. Alpha synuclein gene expression profile in the retina of vertebrates. Mol Vis. 2007 Oct;13:949–61. 2006. [PMC free article] [PubMed] [Google Scholar]
- 22.Gallegos S, Pacheco C, Peters C, Opazo C, Aguayo LG. Features of alpha-synuclein that could explain the progression and irreversibility of Parkinson’s disease. Front Neurosci. 2015 Feb;9:1–11. doi: 10.3389/fnins.2015.00059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lotharius J, Brundin P. Pathogenesis of Parkinson’s disease: dopamine, vesicles and alpha-synuclein. Nat Rev Neurosci. 2002;3(12):932–42. doi: 10.1038/nrn983. [DOI] [PubMed] [Google Scholar]
- 24.Bodis-Wollner I, Kozlowski PB, Glazman S, Miri S. A-Synuclein in the Inner Retina in Parkinson Disease. Ann Neurol. 2014;75:964–6. doi: 10.1002/ana.24182. [DOI] [PubMed] [Google Scholar]
- 25.Walker DG, Lue L-F, Adler CH, Shill HA, Caviness JN, Sabbagh MN, et al. Changes in properties of serine 129 phosphorylated α-synuclein with progression of Lewy-type histopathology in human brains. Experimental Neurology. 2013;240:190–204. doi: 10.1016/j.expneurol.2012.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fujiwara H, Hasegawa M, Dohmae N, Kawashima A, Masliah E, Goldberg MS, et al. alpha-Synuclein is phosphorylated in synucleinopathy lesions. Nat Cell Biol. 2002 Feb;4(2):160–4. doi: 10.1038/ncb748. [DOI] [PubMed] [Google Scholar]
- 27.Beach TG, Adler CH, Sue LI, Serrano G, Shill HA, Walker DG, et al. Arizona Study of Aging and Neurodegenerative Disorders and Brain and Body Donation Program. Neuropathology. 2015 Aug;35(4):354–89. doi: 10.1111/neup.12189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Beach TG, Sue LI, Walker DG, Roher AE, Lue L, Vedders L, et al. The Sun Health Research Institute Brain Donation Program: Description and Eexperience, 1987–2007. Cell Tissue Bank. 2008;9(3):229–45. doi: 10.1007/s10561-008-9067-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gelb D, Oliver E, Gilman S. Diagnostic criteria for parkinson disease. Arch Neurol. 1999 Jan 1;56(1):33–9. doi: 10.1001/archneur.56.1.33. [DOI] [PubMed] [Google Scholar]
- 30.Esquiva G, Lax P, Pérez-Santonja JJ, García-Fernández JM, Cuenca N. Loss of Melanopsin-Expressing Ganglion Cell Subtypes and Dendritic Degeneration in the Aging Human Retina. Front Aging Neurosci. 2017 Apr 4;Apr 4;9:79. doi: 10.3389/fnagi.2017.00079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gómez-Vicente V, Lax P, Fernández-Sánchez L, Rondón N, Esquiva G, Germain F, et al. Neuroprotective effect of tauroursodeoxycholic acid on n-methyl-daspartate-induced retinal ganglion cell degeneration. PLoS One. 2015;10(9):1–14. doi: 10.1371/journal.pone.0137826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Adler CH, Beach TG, Hentz JG, Shill HA, Caviness JN, Driver-Dunckley E, et al. Low clinical diagnostic accuracy of early vs advanced Parkinson disease: clinicopathologic study. Neurology. 2014 Jul;83(5):406–12. doi: 10.1212/WNL.0000000000000641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cuenca N, Fernández-Sánchez L, Campello L, Maneu V, De la Villa P, Lax P, et al. Cellular responses following retinal injuries and therapeutic approaches for neurodegenerative diseases. Prog Retin Eye Res. 2014;43:17–75. doi: 10.1016/j.preteyeres.2014.07.001. [DOI] [PubMed] [Google Scholar]
- 34.Bodis-Wollner I. Retinopathy in Parkinson disease. J Neural Transm. 2009;116(11):1493–501. doi: 10.1007/s00702-009-0292-z. [DOI] [PubMed] [Google Scholar]
- 35.Bodis-Wollner I, Yahr MD. Measurements of visual evoked potentials in Parkinson’s disease. Brain. 1978 Dec;101(4):661–71. doi: 10.1093/brain/101.4.661. [DOI] [PubMed] [Google Scholar]
- 36.Miri S, Glazman S, Mylin L, Bodis-Wollner I. A combination of retinal morphology and visual electrophysiology testing increases diagnostic yield in Parkinson’s disease. Park Relat Disord. 2016;22:S134–7. doi: 10.1016/j.parkreldis.2015.09.015. [DOI] [PubMed] [Google Scholar]
- 37.Price MJ, Feldman RG, Adelberg D, Kayne H. Abnormalities in color vision and contrast sensitivity in Parkinson’s disease. Neurology. 1992 Apr;42(4):887–90. doi: 10.1212/wnl.42.4.887. [DOI] [PubMed] [Google Scholar]
- 38.Regan D, Neima D. Low-contrast letter charts in early diabetic retinopathy, ocular hypertension, glaucoma, and Parkinson’s disease. Br J Ophthalmol. 1984;68:885–9. doi: 10.1136/bjo.68.12.885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Esteve-Rudd J, Fernández-Sánchez L, Lax P, De Juan E, Martín-Nieto J, Cuenca N. Rotenone induces degeneration of photoreceptors and impairs the dopaminergic system in the rat retina. Neurobiol Dis. 2011;44(1):102–15. doi: 10.1016/j.nbd.2011.06.009. [DOI] [PubMed] [Google Scholar]
- 40.Cuenca N, Herrero MT, Angulo A, De Juan E, Martínez-Navarrete GC, López S, et al. Morphological impairments in retinal neurons of the scotopic visual pathway in a monkey model of Parkinson’s disease. J Comp Neurol. 2005 Aug;493:261–73. doi: 10.1002/cne.20761. 2004. [DOI] [PubMed] [Google Scholar]
- 41.Bodis-Wollner I, Miri S, Glazman S. Venturing into the no-man’s land of the retina in Parkinson’s disease. Mov Disord. 2014;29(1):15–22. doi: 10.1002/mds.25741. [DOI] [PubMed] [Google Scholar]
- 42.Satue M, Rodrigo MJ, Obis J, Vilades E, Gracia H, Otin S, et al. Evaluation of progressive visual dysfunction and retinal degeneration in patients with parkinson’s disease. Investig Ophthalmol Vis Sci. 2017;58(2):1151–7. doi: 10.1167/iovs.16-20460. [DOI] [PubMed] [Google Scholar]
- 43.Garcia-Martin E, Rodriguez-Mena D, Satue M, Almarcegui C, Dolz I, Alarcia R, et al. Electrophysiology and Optical Coherence Tomography to Evaluate Parkinson Disease Severity. Investig Opthalmology Vis Sci. 2014;55(2):696. doi: 10.1167/iovs.13-13062. [DOI] [PubMed] [Google Scholar]
- 44.Miri S, Glazman S, Bodis-Wollner I. OCT and Parkinson’s Disease. In: Grzybowski A, Barboni P, editors. OCT in Central Nervous System Diseases. Cham: Springer International Publishing; 2016. pp. 105–21. [Google Scholar]
- 45.Ho CY, Troncoso JC, Knox D, Stark W, Eberhart CG. Beta-amyloid, phospho-tau and alpha-synuclein deposits similar to those in the brain are not identified in the eyes of Alzheimer’s and Parkinson’s disease patients. Brain Pathol. 2014;24(1):25–32. doi: 10.1111/bpa.12070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Williams EA, McGuone D, Frosch MP, Hyman BT, Laver N, Stemmer-Rachamimov A. Absence of Alzheimer disease neuropathologic changes in eyes of subjects with Alzheimer disease. J Neuropathol Exp Neurol. 2017;76(5):376–83. doi: 10.1093/jnen/nlx020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.La Morgia C, Ross-Cisneros FN, Sadun AA, Carelli V. Retinal ganglion cells and circadian rhythms in Alzheimer’s disease, Parkinson’s disease, and beyond. Front Neurol. 2017 May;8:1–8. doi: 10.3389/fneur.2017.00162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kalló G, Emri M, Varga Z, Ujhelyi B, Tőzsér J, Csutak A, et al. Changes in the Chemical Barrier Composition of Tears in Alzheimer’s Disease Reveal Potential Tear Diagnostic Biomarkers. In: Jacobs JM, editor. PLoS One. 6. Vol. 11. 2016. Jun 21, p. e0158000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Csősz É, Kalló G, Márkus B, Deák E, Csutak A, Tőzsér J. Quantitative body fluid proteomics in medicine — A focus on minimal invasiveness. J Proteomics. 2017 Feb;153:30–43. doi: 10.1016/j.jprot.2016.08.009. [DOI] [PubMed] [Google Scholar]
- 50.Klettner A, Richert E, Kuhlenbäumer G, Nölle B, Bhatia KP, Deuschl G, et al. Alpha synuclein and crystallin expression in human lens in Parkinson’s disease. Mov Disord. 2016;31(4):600–1. doi: 10.1002/mds.26557. [DOI] [PubMed] [Google Scholar]
- 51.Klettner A, Tholey A, Wiegandt A, Richert E, Nölle B, Deuschl G, et al. Reduction of GAPDH in lenses of Parkinson’s disease patients: A possible new biomarker. Mov Disord. 2017;32(3):459–62. doi: 10.1002/mds.26863. [DOI] [PubMed] [Google Scholar]
- 52.Arrigo A, Rania L, Calamuneri A, Postorino EI, Mormina E, Gaeta M, et al. Early Corneal Innervation and Trigeminal Alterations in Parkinson Disease. Cornea. 2018;0(0):1. doi: 10.1097/ICO.0000000000001517. [DOI] [PubMed] [Google Scholar]
- 53.Slotnick S, Ding Y, Glazman S, Durbin M, Miri S, Selesnick I, et al. A novel retinal biomarker for Parkinson’s disease: Quantifying the foveal pit with optical coherence tomography. Mov Disord. 2015;30(12):1692–5. doi: 10.1002/mds.26411. [DOI] [PubMed] [Google Scholar]
- 54.Price DL, Rockenstein E, Mante M, Adame A, Overk C, Spencer B, et al. Longitudinal live imaging of retinal α-synuclein::GFP deposits in a transgenic mouse model of Parkinson’s Disease/Dementia with Lewy Bodies. Sci Rep. 2016 Feb;6:29523. doi: 10.1038/srep29523. [DOI] [PMC free article] [PubMed] [Google Scholar]