Abstract
Idiopathic pulmonary fibrosis (IPF) is a chronic fibrotic disease of the lung that is marked by progressive decline in pulmonary function and ultimately respiratory failure. Genetic and environmental risk factors have been identified that indicate injury to, and dysfunction of the lung epithelium is central to initiating the pathogenic process. Following injury to the lung epithelium, growth factors, matrikines and extracellular matrix driven signaling together activate a variety of repair pathways that lead to inflammatory cell recruitment, fibroblast proliferation and expansion of the extracellular matrix, culminating in tissue fibrosis. This tissue fibrosis then leads to changes in the biochemical and biomechanical properties of the extracellular matrix, which potentiate profibrotic mechanisms through a “feed-forward cycle.” This review provides an overview of the interactions of the pathogenic mechanisms of IPF with a focus on epithelial-mesenchymal crosstalk and the extracellular matrix as a therapeutic target for idiopathic pulmonary fibrosis.
Keywords: IPF, genetics, interstitial lung disease, matrix, treatment
Introduction
Idiopathic pulmonary fibrosis (IPF) is a chronic progressive fibrotic disease of unknown etiology that is marked by progressive deposition of extracellular matrix proteins and ultimately failure of the respiratory system and death. It is the most common of the idiopathic interstitial pneumonias (IIP’s) accounting for 25–30% of patients diagnosed with interstitial lung disease with an incidence of 3–18/100,000 persons per year in North America and Europe; the incidence and prevalence of IPF appear to be increasing [1–6]. The disease is more common among males [7, 8] and among those with a history of tobacco use [9–12]. IPF is a disease of older adults with a median age of approximately 65 years at the time of diagnosis. In multiple cohorts, median survival averages 3–5 years from the time of diagnosis, though the disease course varies significantly in individuals [2, 13]. Treatment options for patients with IPF remain limited. Lung transplantation remains the only treatment that clearly improves survival in carefully selected patients. After more than a decade of unsuccessful clinical trials, in 2014, the first two pharmacologic treatments for IPF, pirfenidone [14] and nintedanib [15], received FDA approval. These medications were shown to slow the decline in lung function among IPF patients; but did not improve survival or quality of life [14, 15]. Thus, while the success stories of pirfenidone and nintedanib represent important first steps in the care of IPF patients, there is a clear need for new and more efficacious treatments. Through the past decade, there have been substantial insights made into the fundamental pathophysiology of IPF that have revealed promising new therapeutic targets. In this review, we will summarize current understanding of the mechanisms of IPF pathogenesis with a focus on the extracellular matrix as a potential target for disease modifying interventions.
Mechanisms of Disease
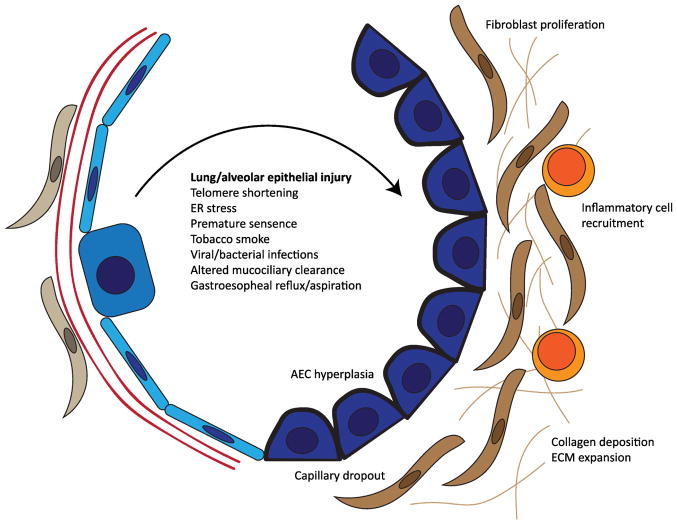
Although reports of fibrotic lung disease date back to the 1800’s, the disease currently classified as IPF was formally established in the late 1960’s by Liebow and Carrington who described “usual interstitial pneumonia” (UIP) as a distinct histopathologic form of diffuse lung disease[16]. The clinical correlate for UIP histopathology was initially termed “cryptogenic fibrosing alveolitis”[17]. Consistent with this terminology, based on early work studying bronchoalveolar lavage fluid from IPF patients, the prevailing paradigm through the 1960’s–1980’s suggested that chronic inflammation in the alveolar compartment was the primary mechanism driving fibrotic remodeling in the lung [18]. In the 1980’–1990’s, there was increased focus on the role of secreted factors in the alveolar compartments (i.e. growth factors) on fibroblast activation and proliferation [18]. In parallel, careful pathologic studies by Katzenstein and others [19, 20] began to question whether inflammatory cells were primary effectors of disease. Elegant histopathologic studies and electron microscopy demonstrated relative paucity of inflammatory cells, but evidence of epithelial damage/injury nearby what were termed “fibroblastic foci”[20]. This led to the modern conceptual framework for IPF pathogenesis (Figure 1), in which epithelial injury and cross-talk between a dysfunctional alveolar epithelium and the adjacent mesenchymal compartment leads to aberrant or persistent activation of tissue repair pathways, akin to a “wound-healing response.”
Figure 1.
Summary of the pathologic features of IPF disease initiation. A variety of genetic and environmental factors have been implicated as sources of injury to the lung and/or alveolar epithelium. Following epithelial injury, activation of canonical injury-repair programs leads to inflammatory cell and fibroblast recruitment, production of collagen and ECM which undergoes pathologic remodeling. This in turn leads to abnormal repair of the alveolar epithelium and failure to coordinate regeneration of functional alveoli.
Disease initiation: A prominent role for the lung epithelium
Several independent lines of evidence support the hypothesis that injury to and/or dysfunction of the alveolar epithelium is central to disease risk initiation. First, established genetic risk factors for pulmonary fibrosis implicate the lung epithelium. Mutations in lung epithelial restricted genes (SFTPC, SFTPA2 and ABCA3) [21–30] have been implicated in familial forms of pulmonary fibrosis (Familial Interstitial Pneumonia, FIP) [31]. Alveolar epithelial dysfunction has been shown to underlie the genetic risk for Hermansky-Pudlak Syndrome[32], a lysosomal trafficking disorder with highly penetrant pulmonary fibrosis in several subtypes[33]. Mutations in genes related to telomere biology [34–41] are found in at least 20% of families with pulmonary fibrosis [31] and perhaps a similar proportion of sporadic IPF [42], and are believed to promote stem cell failure and/or senescence within the alveolar epithelium[43]. In addition, a common polymorphism in the promoter of the gene encoding MUCIN 5B (MUC5B), an airway mucin, is found in more than 2/3 of pulmonary fibrosis patients and carriers of the risk allele have 4–6-fold increased risk for disease [44–48], an unusually large effect size for a common genetic variant. Evidence of alveolar epithelial injury and dysfunction has been reported in asymptomatic family members of patients with pulmonary fibrosis[49]. Further, environmental exposures such as gastroesophageal reflux/chronic aspiration [50], tobacco smoke exposure[9], respiratory viruses [51, 52], wood dust, metal dust, asbestos, and other inhalants [53] have been identified as risk factors for pulmonary fibrosis and may act as sources of recurrent injury to the alveolar epithelium. Finally, studies using transgenic animal models that induce alveolar epithelial cell damage by diphtheria toxin [54] or deletion of shelterin complex components [55–57] develop some degree of spontaneous lung fibrosis. Together, these studies suggest that the lung epithelium plays a prominent role in driving early disease pathogenesis. The mechanisms and mediators linking epithelial cell dysfunction to tissue fibrosis have not yet been comprehensively determined, however it is clear that crosstalk between epithelial cells, the extracellular matrix, and nearby mesenchymal and inflammatory cell populations is central to this process.
The ECM in lung epithelial homeostasis and repair
The pulmonary extracellular matrix is a three-dimensional meshwork consisting of proteins (predominantly collagen and elastin), glycoproteins, and proteoglycans. In addition to these structural elements, there are abundant signaling molecules present within the matrix including transforming growth factor β (TGF-β), fibulin, osteopontin, periostin, connective tissue growth factor (CTGF), and fibronectin [58, 59]. Degradation products of the ECM (i.e. matrikines) also play a signaling role in addition to structural functions. These signaling molecules have been shown to play a central role in the fibrotic response in IPF by modulating epithelial repair, promoting fibroblast recruitment and myofibroblast activation.
As described above, injury to and aberrant repair of the alveolar epithelium is central to the initiation of pulmonary fibrosis. While most work has focused on the role of the ECM and mesenchymal cell interactions in the context of matrix remodeling and disease progression, more recent studies suggests an important role in alveolar homeostasis and coordination of injury-repair. There has been extensive work through the past decade identifying a hierarchy of distinct progenitor (i.e. “stem”) cell niches in the lung epithelium which appear to contribute to epithelial repair in different conditions and experimental models (Reviewed in [60, 61]), including basal cells [62], club/Clara cells [63, 64], bronchoalveolar stem cells (BASCs), lineage-negative epithelial progenitor cells (LNEPs) [65] type II alveolar epithelial cells (AEC2s) [66], and subsets of AEC2s [67].
Work from several groups has underscored the central role the mesenchyme plays in regulating alveologenesis [66, 68]. Recent work from two groups using single-cell transcriptomics and linage tracing mouse models demonstrated that a subset of fibroblasts characterized by PDGFRα+ [68] or Lgr5+ [69] with evidence of active Wnt signaling (Axin2+) localize to the alveolar compartment and are involved in alveolar homeostasis. This Axin2+ mesenchymal population appears to robustly support alveolar growth and maturation in organoid assays [68], and to preferentially expand and differentiate into myofibroblasts following bleomycin injury[68], although other populations contribute as well [63].
Less is known about the roles of specific components of the ECM in alveolar repair. A specific role for the matrix GAG hyaluronan was recently identified in regulating AEC2 progenitor function [70]. In this work, AECs isolated from IPF patients were found to have reduced cell surface hyaluronan. Mice deficient for the innate immune receptor toll-like receptor 4 (TLR4) or hyaluronan synthase 2 in AEC2’s developed worse experimental fibrosis and had impaired AEC renewal capacity in organoid assays [70]. Thus, hyaluronan appears to promote AEC progenitor function in a manner that requires TLR4. As described further below, the role of hyaluronan more broadly in lung fibrosis is complex and appears to involve competing effects in the epithelium [70] versus mesenchymal compartments [71].
Disease Progression: Growth Factors and Matrix Remodeling
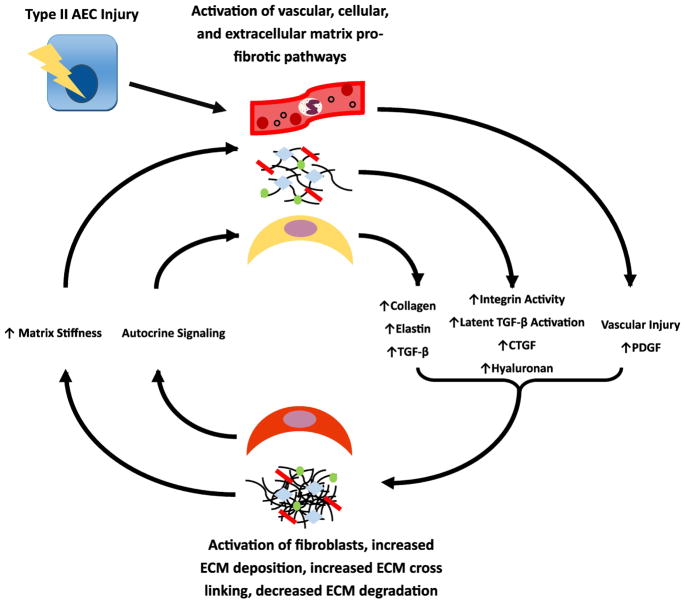
Following injury to the alveolar epithelium, a variety of signaling pathways that must be carefully coordinated to facilitate effective and functional repair. The injured epithelium and recruited inflammatory cells secrete a variety of mediators including transforming growth factor beta (TGF-β), connective tissue growth factor (CTGF), and others that modulate epithelial repair, promote fibroblast recruitment and myofibroblast activation. In IPF, these activated fibroblasts secrete collagen and other ECM components which then undergo pathologic remodeling and abnormal cross-linking, changing the mechanical properties of the pulmonary ECM. Mechanosensing mechanisms in fibroblasts promote further activation and myofibroblast differentiation, establishing a pathologic “feed-forward” cycle of fibrosis begetting more fibrosis. Several steps in this pathologic cycle have emerged as promising novel therapeutic targets (Figure 2).
Figure 2.
Feed-forward profibrotic epithelial-mesenchymal interactions. After activation from injured AECs, there is elaboration and activation of profibrotic mediators including TGF-B, CTGF, and others that lead to recruitment and activation of fibroblasts, increased deposition of ECM and reduced breakdown of the ECM, ultimately leading to a stiffened and poorly organized ECM. The increased matrix stiffness creates a feedback loop along with autocrine signaling from activated fibroblasts to further activate fibrotic signaling.
TGF-β signaling
TGF-β is an integral mediator in the initiation and progression of tissue fibrosis [72]. Within the cell, TGF-β is synthesized non-covalently bound to a latency associated peptide (LAP) to from a complex known as the small latent complex (SLC) [73, 74]. This complex then links to a latent TGF-β-binding protein (LTBP) in the endoplasmic reticulum forming a complex known as the large latent complex (LLC) [73, 74]. The LLC is secreted from the cell into the extracellular matrix where it resides in its inactive form covalently bound to ECM proteins, such as fibronectin and fibrillin-1, and awaits further activation [75–77]. TGF-β can be activated from within the LLC in various ways including variations in temperatures, acidification, oxidation, proteolytic cleavage, or via integrin interactions allowing the TGF-β molecule to interact with the TGF-β Receptors (TGF-βR) and mediate downstream effects [72, 78–81].
In normal physiology, TGF-β has multiple functions including immune regulation, wound healing, control of cellular proliferation, and as a signal for production of extracellular matrix [82]. TGF-β knockout mice rapidly develop a fatal acute inflammatory response, most prominently in the heart and lungs [83]. This phenotype, with few differences, can be replicated with TGF-βRII knockout and via marrow transplantation from TGF-βRII knockout mice to healthy recipients [84]. Together these studies demonstrate that TGF-β plays an important role in suppressing lung inflammation and promoting lung homeostasis.
There is abundant evidence that TGF-β and related signaling play a central role in IPF pathogenesis [74]. TGF-β levels have been shown to be upregulated in mouse models of experimental pulmonary fibrosis and in BAL fluid of patients with IPF [85, 86]. In addition, TGF-βRI expression is downregulated in the airway cells lining honeycomb cysts of IPF lungs while TGF-βRI and II are upregulated in fibroblasts [87]. It is likely that TGF-β is produced by multiple cell types relevant in the pathogenesis of pulmonary fibrosis. After an injury event, activated TGF-β is released from alveolar epithelial cells, macrophages, platelet granules, and can be activated from LLCs in the extracellular matrix [88, 89]. In addition, TGF-β is also produced by infiltrating regulatory T cells (Tregs) [90] that arrive later in the injury/repair process and have been associated with IPF progression[91].
Studies using transgenic animals and have demonstrated that overexpression of TGF-β1 lead to development of fibrotic lung disease [92, 93]. There are multiple mechanisms through which TGF-β has been shown contribute to lung fibrosis. First, TGF-β has diverging effects on the epithelial and fibroblast proliferative responses; TGF- β generally promotes proliferation of fibroblasts and other mesenchymal cells [73] while suppressing epithelial cell proliferation [72]. TGF- β promotes epithelial cells to undergo a phenotype shift characterized by adoption of mesenchymal features [94, 95]. In addition, active TGF-β signaling plays a central role in mediating myofibroblast activation and subsequent production of collagen and other ECM components [74].
Convincing evidence that TGF-β signaling in the lung epithelium plays a profibrotic role emerged as reports from two groups demonstrated that deletion of TGF-βRII in AECs is protective against bleomycin-induced lung fibrosis [96] despite increased inflammation [97]. TGF-βRII knockout in macrophages was also shown to decrease transition to the M2 phenotype which is associated with wound healing and tissue repair [98]. There has been growing interest in attempting to specifically target mechanisms of TGF- β activation and signaling as a therapeutic strategy.
Integrins and TGF-β activation
Integrins are transmembrane proteins that play a pivotal role in the maintenance of cell-ECM adhesion, cell-cell adhesion, and transduction of signals from the ECM to the intracellular compartment [99]. The integrin family is composed of one of 18 unique α subunits and 8 β subunits that pair to create one of 24 identified integrins [99]. Integrins in the lungs have been associated with asthma, COPD, non-small cell lung cancer, sarcoidosis, bronchopulmonary dysplasia [100] and pulmonary fibrosis [101].
The αvβ6 integrin is the most well-studied in the field of IPF. It is primarily found in the lungs and skin and is upregulated in the lungs of murine bleomycin models of IPF [102–104]. The αvβ6 integrin has been shown to bind to the TGF-β LLC where it leads to extracellular activation of the TGF-β signaling [81]. This occurs via binding of αvβ6to LTBPs where it interacts with an RGD domain on the hinge regions of LTBP-1 and LTBP-3[105]. Through interactions with intracellular actin, αvβ6 exhibits mechanical force on the LLC thus releasing active TGF-β thus propagating its downstream fibrotic potential.
Knockout mouse models of the β6 component have shown similar effects to the TGF -β knockout mouse with inflammation limited to the lung and the skin [104] which can be abrogated by restitution of the β6 gene to type 2 AECs and bronchial epithelial cells [106]. Interestingly, partial inhibition of αvβ6, reduced pulmonary fibrosis in a bleomycin model without increased baseline inflammation in the lungs or skin [107]. Early human studies targeting αvβ6 in IPF are underway. Recently, a monoclonal antibody targeted at the αvβ6 integrin (BG00011, formerly STX-100) was tested in a phase two clinical trial (Clinicaltrials.gov, NCT01371305). Data from this study are anticipated soon.
In addition to αvβ6, other integrins in the αv family have been shown to activate latent TGF-β as well. The αvβ1, αvβ3, αvβ5, αvβ8 integrins have all been shown to contribute to TGF-β activation, activation of fibroblasts and myofibroblasts, and increased ECM production [108–110]. These αv integrin containing complexes have also emerged as potential therapeutic targets [111], in particular αvβ1 integrin. αvβ1 integrin is expressed on fibroblasts, where it has been shown to mediate adhesion of the TGF- β latency associated peptic (LAP) [112]. The LAPs bind secreted TGF- β and maintain it in an inactive state; binding of αvβ1 integrin to LAP leads to TGF- β activation. A small molecule inhibitor of αvβ1 integrin was shown to be protective against bleomycin-induced fibrosis [112].
Growth Factors
A variety of other secreted factors have also been implicated in pulmonary fibrosis pathogenesis, including connective tissue growth factor (CTGF), platelet-derived growth factor (PDGF) and fibroblast growth factors (FGFs).
CTGF is small peptide that has a variety of stimulatory effects on fibroblasts. It is synthesized at low levels in the healthy lung, but has been shown to be upregulated under several models of pulmonary stress including bleomycin and radiation induced fibrosis [113, 114]. CTGF is upregulated in both Type 2 AECs and pulmonary fibroblasts under stress [115–117]. CTGF secreted under stimulation by TGF-β and has downstream effects via the MAP kinase pathway including stimulation of fibroblast migration and increased collagen production via upregulation of the Col1A promoter [116, 118, 119]. In fact, several studies of TGF-β interactions with CTGF suggest that many aspects of the downstream fibrotic activity of TGF-β are dependent on the presence of CTGF [118, 120–123]. Interestingly, CTGF has been shown to increase TGF-β production in fibrotic systems, indicating a potential internal positive feedback loop [124].
PDGF is a common growth factor produced in many cells including macrophages, platelets, endothelial cells, and fibroblasts and is upregulated under stimulation by inflammatory cytokines[125]. PDGF receptors (PDGFRs) are expressed in two forms the α form and the β form which bind to different forms of PDGF and have differed downstream effects [125]. PDGF gene expression is upregulated in bleomycin induced pulmonary fibrosis [126, 127] and introduction of a PDGF-B gene into the mouse lung via adenovirus induces severe lung fibrosis [128]. Inhibition of PDGF via anti-PDGF antibodies or the PDGFR via small molecule inhibition is able to inhibit the progression of lung fibrosis in the murine model [127, 129].
Fibroblast growth factors (FGFs) are a family of 22 proteins with 5 associated FGF-receptors (FGFRs) which have pleiotropic effects on the deposition of fibrotic tissue within the pulmonary parenchyma. Several FGFs have been shown to be upregulated in IPF, making them of interest in the modulation of fibrosis [130, 131], although mechanistic studies to date have not yielded a consensus mechanism. For example, FGF-1 has been shown to have anti-apoptotic and pro-migratory effects on fibroblasts in one study [132], but was shown to have pro-apoptotic effects in another [133]. Interestingly, when fibroblasts harvested from IPF lungs were exposed to FGF-1, there was minimal effect [132]. FGF-2 can be released by macrophages, AECs, fibroblasts, T-lymphocytes and mast cells under signaling from TGF-β [134]. FGF2 levels are upregulated in the IPF lung, and promotes the recruitment of myofibroblasts and stimulation of the production of extracellular matrix proteins [135]. Inhibition of FGF-2 decreased cellular DNA production genes suggesting this is one of the ways in which TGF-β induces the fibrotic response [136]. Importantly, inducible inactivation of FGFRs in murine models attenuated the fibrotic response to bleomycin and reduced fibroblast migration into fibrotic areas suggesting signaling from these receptors is involved in the development of fibrosis [137].
Therapies targeting these molecules and their receptors have been among the most successful antifibrotic treatments to date. Nintedanib, one of the two FDA-approved treatments for IPF, inhibits PDGFRs and FGFRs, in addition to VEGFRs [15, 138]. A monoclonal antibody targeted to CTGF has shown promise in murine and early human studies. Within murine models of fibrosis, including radiation and bleomycin, inhibition of CTGF can both prevent lung fibrosis, as well as, reverse already established lung fibrosis [114, 116, 139–141]. Recent Phase II trials of anti-CTGF monoclonal antibodies in humans showed no particular side effects, and there was no evidence of progression of disease via pulmonary function testing in the patients receiving the drug [142]. Phase III clinical trials of the anti-CTGF monoclonal antibody Pamrevlumab (formerly F-3019) in IPF are currently underway.
Collagen and matrix remodeling
In addition to increased production of ECM components, aberrant matrix remodeling is also a well-recognized feature of pulmonary fibrosis [59]. Collagen is a linear protein formed into cross linked fibrils that is responsible for the strength of the ECM and makes up about 20% of the dry weight of the normal human lung [143]. The predominant type of collagen within both the normal and IPF lung is Type 1 collagen which is deposited in higher amounts within the ECM compared to other collagen subtypes [58, 144]. Collagen is secreted as individual fibers by the fibroblasts and myofibroblasts under signaling primarily from TGF-β, and also stimulated by several other cytokines and via hypoxia [145, 146]. Collagen fibrils are then crosslinked by lysyl oxidase (LOX), lysyl oxidase like proteins (LOXLs), tissue transglutaminase (TTG) and other enzymes to form covalent bonds that solidify the structure of the collagen and increase the tensile strength of the extracellular matrix [147, 148].
Matrix crosslinking appears to be vital to the progression of pulmonary fibrosis. Gene expression of LOX, LOXL and TTG are each upregulated in IPF lungs, and increase the crosslinking between the collagen fibrils. Increase in LOXL2 and TTG activity leads to an overall increased crosslinking of collagen fibrils and increased stability and stiffness of the extracellular matrix [147, 148]. Inhibition of TTG2 was able to increase ECM turnover in vitro and was associated with decreased collagen accumulation in bleomycin treated mice [147, 149]. LOXL2 has been shown to correlate with increased disease progression and mortality in IPF [150]. In addition, LOXL2 was shown along with LOXL3 to be involved in fibroblast transition to myofibroblasts [146]. LOXL2 has the strongest effect on fibrotic tissue remodeling and some data suggests it may play a role in release of TGF-β from the ECM [151]. In addition, inhibition of LOX and the LOXL family has been shown to reduce fibrosis in bleomycin treated mice [152, 153]. Ultimately, increased expression/activity of crosslinking proteins leads to an increase in the thickness and tensile strength of the extracellular matrix and subsequent fibroblast activation [153, 154]. Early phase clinical trials targeting this pathway have been initiated; results to date are limited but one study was terminated at the second interim analysis for lack of efficacy [155].
Matrix metalloproteinases (MMPs) along with their inhibitors, tissue inhibitors of metalloproteinases (TIMPs), are secreted by multiple cell types in the lung including AECs and Fibroblasts. MMPs are responsible for the degradation of collagen and other matrix proteins. MMP-1, MMP-2, MMP-7, MMP-8, and MMP-9 have been shown to be upregulated in IPF [156–160]. MMP-7 has been associated with increasing fibrosis, and is predominantly produced in activated alveolar epithelial cells and bronchiolar cells [160]. Knockout of MMP-7 reduced pulmonary fibrosis in bleomycin treated mice [160]. MMP-9 is also of significant interest. Immunohistochemical staining of human lungs showed evidence of MMP-9 within the ECM in areas of dense scar in UIP, as well as, neutrophils and myofibroblasts [160, 161]. In addition, MMP-9 has been shown to activate TGF-β from the extracellular matrix [78]. In animal models, MMP-9 over-expression within alveolar macrophages attenuated bleomycin induced fibrosis [162], while knockout did not seem to have effect on total fibrosis but did decrease bronchiolization of alveoli [163]. MMP-3 has been shown to have pro-fibrotic effects with overexpression leading to fibrosis and MMP-3 null mice having reduced fibrosis in response to bleomycin [164]. Several other MMPs have been implicated in the pathogenesis of pulmonary fibrosis, and studies are ongoing [165].
TIMPs are also upregulated in the presence of pulmonary fibrosis and spread throughout the pulmonary parenchyma [161]. TIMPs inhibit MMPs in a myriad of ways and are their upregulation is thought to decrease MMP induced degradation of the ECM and thus a pro-fibrotic microenvironment throughout the extracellular matrix [161, 166]. MMP-generated collagen neoepitopes have been identified in serum from IPF patients and have been associated with disease progression, suggesting monitoring this axis may be a promising biomarker strategy [167]. Further investigations are underway to better understand and potentially modulate the MMP/TIMP system [165].
Matrikines
In addition to their structural roles, ECM components or their breakdown products have an increasingly recognized role as signaling mediators known as matrikines. While much work has focused on matrikines in the context of inflammatory signaling, a direct role in regulating epithelial and mesenchymal injury responses is emerging.
Hyaluronan (HA) is a non-sulfated glycosaminoglycan that is formed as a linear chain of repeated sugars, specifically D-glucuronic acid and N-acetylglucosamine [168]. The long chain allows for binding of salts and water and creates a visco-elastic substance that provides structure to the ECM [169, 170]. Within the pulmonary system, HA is primarily localized to the airways and interstitial spaces and has been shown to be induced by inflammatory signaling from cytokines including IL-1β and TNF-α [171, 172].
The role of HA has proven to be complex. As discussed above, in the alveolar epithelium HA promotes AEC homeostasis and progenitor cell function through an interaction with TLR4 [70]. Interestingly, HA secretion has been shown to be induced by endoplasmic reticulum stress in AECs[173]; whether this is adaptive or pathologic is less clear. In the mesenchymal compartment, HA confers a profibrotic phenotype [71]. HA is produced primarily by myofibroblasts and has been shown to be increased within the lungs of patients with IPF compared to normal controls [174]. In mouse models of bleomycin injury, HA has been shown to accumulate in the fibrosing lung. Further analysis of HA action in the IPF lung suggest that it contributes to inflammation, mediated by interaction with CD44 on a variety of cells. The CD44/HA axis has also been linked to mesothelial cell EMT[175], which has been implicated as a potential source of myofibroblasts in IPF[176, 177].
CD44/HA interactions have been shown to be important in fibroblast recruitment and upregulation, recruitment of inflammatory cells from the peripheral blood, and signaling for its own degradation. Knockout of the hyaluronan 2 synthetase (HAS2) gene was embryonic lethal secondary to cardiac malformations [178]. Conversely, overexpression of HAS2 within myofibroblasts was shown to increase these cells invasiveness into the ECM and augmented their fibrotic potential [71], mediated in part through HAS2 regulation of fibroblast senescence[179]. Conditional deletion of HAS2 in mesenchymal cells, as well as a more specific loss in fibroblasts, lead to decreased hyaluronan and collagen deposition and decreased accumulation of myofibroblasts within sites of lung remodeling [71]. The CD44 receptor has been shown to be upregulated in the IPF lung and in the lungs of bleomycin challenged mice [180]. CD44 is involved in the wound resolution response by inducing macrophage clearance of ECM proteins, especially hyaluronan, and is under the control of several cytokines including IL-1, TNF-α, and LPS [181–183]. CD44 knockout mice are not resistant to bleomycin induced fibrosis, however, they are resistant to development of fibrosis induced by overexpression of hyaluronan. Similarly, inhibition of CD44 by a monoclonal antibody abrogated the fibrotic response to hyaluronan overexpression, but not bleomycin induced fibrosis in the murine model. Evaluation of fibroblasts treated with anti-CD44 monoclonal antibody in vitro and in vivo models showed that inhibition of CD44 reduced myofibroblast invasiveness [71, 184].
The collagen breakdown proline-glycine-proline (PGP) motif has been well established as a neutrophil chemoattractant and driver of COPD pathogenesis [185] and regulator of acute airway inflammation[186]. While acetylated PGP is resistant to degradation and promotes sustained neutrophilic inflammation, it surprisingly appears to protect against fibrosis [187] in a manner dependent on angiotensin-converting enzyme (ACE)-mediated degradation. This provides at least one potential mechanism explaining the paradox of why some smokers develop emphysema while others develop pulmonary fibrosis. Conversely, studies suggest that collagen V, tenascin C, and elastin deposited into the ECM under fibrotic conditions can stimulate fibroblasts to increase ECM remodeling, thus creating a positive feedback loop that increases pulmonary fibrosis [188]. With further study, it seems likely that increasingly complex signaling roles for ECM components and matrikines will be discovered and present new therapeutic targets.
Matrix Stiffness and Mechanosensing in disease progression
The injury-repair mechanisms described above lead to a variety of alterations in the composition and organization of the pulmonary extracellular matrix. This remodeling leads to changes in the mechanical properties of the ECM, particularly an increase in matrix stiffness[189]. It has become increasingly evident that in the setting of increased matrix stiffness, mechanosensing mechanisms in fibroblasts potentiate profibrotic cellular phenotypes [190]. Normal human lung fibroblasts grown on decellularized lung matrices from IPF lungs show evidence of enhanced TGF-β signaling and differentiate into myofibroblasts [191], and ex-vivo culture experiments have indicated this process is mediated through Rho/ROCK signaling and megakaryoblastic leukemia factor-1 (MKL1) [192].
Several potential sensors of matrix stiffness have been implicated, including the transient receptor potential vanilloid 4 (TRPV4) channel [193] and α6 integrin [194]. The signaling events driving stiffness-mediated fibroblast activation involve accumulation of phosphorylated myosin-2 [195]. The transcriptional control of stiffness-driven myofibroblast differentiation is mediated through the yes-associated protein (YAP) and transcriptional coactivator with PDZ-binding motif (TAZ) [196]. Knockdown of YAP and TAZ specifically abrogate matrix-stiffness driven fibroblast activation, and expression of active YAP or TAZ in normal fibroblasts grown on soft matrix promotes invasiveness and myofibroblast differentiation [196].
Intriguingly, myofibroblast differentiation does not appear to be a terminal cell fate. Mice treated with a Rho kinase inhibitor during the recovery phase of bleomycin injury were protected from lung fibrosis [197]. Further, when grown on physiologic (i.e. normal) stiffness matrix, primary IPF fibroblasts lose their contractile phenotype; this can be recapitulated by Rho kinase inhibition [198].
The role of ECM stiffness on epithelial cells has been less studied, although there are suggestions ECM stiffness regulates epithelial cell phenotypes. Data from isolated rat AECs suggested that matrix stiffness did not alter AEC differentiation or promote adoption of mesenchymal features, but did increase laminin and fibronectin expression [199]. The Hippo-Yap-Taz axis has been implicated in regulating lung epithelial development [200], and in regulating basal cell migration and differentiation following injury[201]; additional study is needed to better elucidate the role of matrix stiffness and mechanosensing on epithelial repair more broadly.
Together, these data indicate that the mechanical properties of the ECM drive a feed-forward cycle in which matrix remodeling drives further fibroblast activation, myofibroblast activation, and additional matrix remodeling both directly and through paracrine signaling [188, 202]. There is extensive ongoing investigation in multiple fields looking to target aspects of mechanosensitive signaling as an antifibrotic strategy [190]. Further work is needed to better understand the endogenous “checks” on this feed-forward system as this may also hold promise as a therapeutic target.
Conclusions
Advances in understanding of the pathogenesis of idiopathic pulmonary fibrosis have highlighted the many roles the extracellular matrix plays in coordinating normal lung homeostasis and mediating pathologic lung remodeling. Available evidence suggests that the ECM plays a central role in driving feed-forward cycles of pathogenic disease signaling mediating by integrins, growth factors, matrikines, and matrix metalloproteinases. In addition, the matrix structure and biomechanical properties have emerged as key drivers of the fibrotic process. A variety of exciting novel therapeutic strategies targeting these ECM-driven processes hold promise as novel treatments for IPF and other chronic fibrotic disorders.
Highlights.
Lung epithelial injury-related signaling is involved in initiating IPF pathogenesis
The ECM plays a central role in coordinating injury-repair signaling
Aberrant and persistent activation of injury-repair signaling may be mediated through changes in the biomechanical properties of the ECM, driving a feed-forward profibrotic cycle
Acknowledgments
Funding Sources: This worked was supported by NIH NHLBI K08HL130595 (JAK), P01HL92870 (TSB), R01HL085317 (TSB), Department of Veterans Affairs (TSB), Francis Family Foundation (JAK), Pulmonary Fibrosis Foundation (JAK). The funding sources had no involvement in the design or content of this manuscript.
Footnotes
Contributors: JCH, JAK, TSB designed wrote and revised the manuscripts.
Declarations of interest: The authors have declared that no conflict of interest exists. JAK and TSB have received nonfinancial research support from Genentech. TSB has research grants from Boehringer Ingelheim and Celgene.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Coultas DB, Zumwalt RE, Black WC, Sobonya RE. The epidemiology of interstitial lung diseases. Am J Respir Crit Care Med. 1994;150(4):967–72. doi: 10.1164/ajrccm.150.4.7921471. [DOI] [PubMed] [Google Scholar]
- 2.Raghu G, Weycker D, Edelsberg J, Bradford WZ, Oster G. Incidence and prevalence of idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2006;174(7):810–6. doi: 10.1164/rccm.200602-163OC. [DOI] [PubMed] [Google Scholar]
- 3.Hutchinson J, Fogarty A, Hubbard R, McKeever T. Global incidence and mortality of idiopathic pulmonary fibrosis: a systematic review. 2015 doi: 10.1183/09031936.00185114. [DOI] [PubMed] [Google Scholar]
- 4.Raghu G, Chen SY, Yeh WS, Maroni B, Li Q, Lee YC, Collard HR. Idiopathic pulmonary fibrosis in US Medicare beneficiaries aged 65 years and older: incidence, prevalence, and survival, 2001–11. The Lancet. Respiratory medicine. 2014;2(7):566–72. doi: 10.1016/S2213-2600(14)70101-8. [DOI] [PubMed] [Google Scholar]
- 5.Raghu G, Collard HR, Egan JJ, Martinez FJ, Behr J, Brown KK, Colby TV, Cordier JF, Flaherty KR, Lasky JA, Lynch DA, Ryu JH, Swigris JJ, Wells AU, Ancochea J, Bouros D, Carvalho C, Costabel U, Ebina M, Hansell DM, Johkoh T, Kim DS, King TE, Jr, Kondoh Y, Myers J, Muller NL, Nicholson AG, Richeldi L, Selman M, Dudden RF, Griss BS, Protzko SL, Schunemann HJ. An official ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis: evidence-based guidelines for diagnosis and management. Am J Respir Crit Care Med. 2011;183(6):788–824. doi: 10.1164/rccm.2009-040GL. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ley B, Collard HR. Epidemiology of idiopathic pulmonary fibrosis. Clin Epidemiol. 2013:483–92. doi: 10.2147/CLEP.S54815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Olson AL, Swigris JJ. Idiopathic pulmonary fibrosis: diagnosis and epidemiology. Clin Chest Med. 2012;33(1):41–50. doi: 10.1016/j.ccm.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 8.Schwartz DA, Helmers RA, Galvin JR, Van Fossen DS, Frees KL, Dayton CS, Burmeister LF, Hunninghake GW. Determinants of survival in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 1994;149(2 Pt 1):450–4. doi: 10.1164/ajrccm.149.2.8306044. [DOI] [PubMed] [Google Scholar]
- 9.Baumgartner KB, Samet JM, Stidley CA, Colby TV, Waldron JA. Cigarette smoking: a risk factor for idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 1997;155(1):242–8. doi: 10.1164/ajrccm.155.1.9001319. [DOI] [PubMed] [Google Scholar]
- 10.Garcia-Sancho C, Buendia-Roldan I, Fernandez-Plata MR, Navarro C, Perez-Padilla R, Vargas MH, Loyd JE, Selman M. Familial pulmonary fibrosis is the strongest risk factor for idiopathic pulmonary fibrosis. Respir Med. 2011;105(12):1902–7. doi: 10.1016/j.rmed.2011.08.022. [DOI] [PubMed] [Google Scholar]
- 11.Taskar VS, Coultas DB. Is idiopathic pulmonary fibrosis an environmental disease? Proc Am Thorac Soc. 2006;3(4):293–8. doi: 10.1513/pats.200512-131TK. [DOI] [PubMed] [Google Scholar]
- 12.Steele MP, Speer MC, Loyd JE, Brown KK, Herron A, Slifer SH, Burch LH, Wahidi MM, Phillips JA, 3rd, Sporn TA, McAdams HP, Schwarz MI, Schwartz DA. Clinical and pathologic features of familial interstitial pneumonia. Am J Respir Crit Care Med. 2005;172(9):1146–52. doi: 10.1164/rccm.200408-1104OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pérez ERF, Daniels CE, Schroeder DR, St Sauver J, Hartman TE, Bartholmai BJ, Yi ES, Ryu JH. Incidence, Prevalence, and Clinical Course of Idiopathic Pulmonary Fibrosis: A Population-Based Study. Chest. 2010:129–37. doi: 10.1378/chest.09-1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.King TE, Jr, Bradford WZ, Castro-Bernardini S, Fagan EA, Glaspole I, Glassberg MK, Gorina E, Hopkins PM, Kardatzke D, Lancaster L, Lederer DJ, Nathan SD, Pereira CA, Sahn SA, Sussman R, Swigris JJ, Noble PW. A phase 3 trial of pirfenidone in patients with idiopathic pulmonary fibrosis. N Engl J Med. 2014;370(22):2083–92. doi: 10.1056/NEJMoa1402582. [DOI] [PubMed] [Google Scholar]
- 15.Richeldi L, du Bois RM, Raghu G, Azuma A, Brown KK, Costabel U, Cottin V, Flaherty KR, Hansell DM, Inoue Y, Kim DS, Kolb M, Nicholson AG, Noble PW, Selman M, Taniguchi H, Brun M, Le Maulf F, Girard M, Stowasser S, Schlenker-Herceg R, Disse B, Collard HR. Efficacy and safety of nintedanib in idiopathic pulmonary fibrosis. N Engl J Med. 2014;370(22):2071–82. doi: 10.1056/NEJMoa1402584. [DOI] [PubMed] [Google Scholar]
- 16.Liebow A, CCB . The interstitial pneumonias, Frontiers of Pulmonary Radiology. Grune and Stratton; New York: 1969. [Google Scholar]
- 17.Scadding JG, Hinson KF. Diffuse fibrosing alveolitis (diffuse interstitial fibrosis of the lungs). Correlation of histology at biopsy with prognosis. Thorax. 1967;22(4):291–304. doi: 10.1136/thx.22.4.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Noble PW, Homer RJ. Back to the future: historical perspective on the pathogenesis of idiopathic pulmonary fibrosis. Am J Respir Cell Mol Biol. 2005;33(2):113–20. doi: 10.1165/rcmb.F301. [DOI] [PubMed] [Google Scholar]
- 19.Katzenstein AL. Pathogenesis of "fibrosis" in interstitial pneumonia: an electron microscopic study. Human pathology. 1985;16(10):1015–24. doi: 10.1016/s0046-8177(85)80279-3. [DOI] [PubMed] [Google Scholar]
- 20.Myers JL, Katzenstein AL. Epithelial necrosis and alveolar collapse in the pathogenesis of usual interstitial pneumonia. Chest. 1988;94(6):1309–11. doi: 10.1378/chest.94.6.1309. [DOI] [PubMed] [Google Scholar]
- 21.Cottin V, Reix P, Khouatra C, Thivolet-Bejui F, Feldmann D, Cordier JF. Combined pulmonary fibrosis and emphysema syndrome associated with familial SFTPC mutation. Thorax. 2011;66(10):918–9. doi: 10.1136/thx.2010.151407. [DOI] [PubMed] [Google Scholar]
- 22.Fernandez BA, Fox G, Bhatia R, Sala E, Noble B, Denic N, Fernandez D, Duguid N, Dohey A, Kamel F, Edwards L, Mahoney K, Stuckless S, Parfrey PS, Woods MO. A Newfoundland cohort of familial and sporadic idiopathic pulmonary fibrosis patients: clinical and genetic features. Respir Res. 2012;13:64. doi: 10.1186/1465-9921-13-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ono S, Tanaka T, Ishida M, Kinoshita A, Fukuoka J, Takaki M, Sakamoto N, Ishimatsu Y, Kohno S, Hayashi T, Senba M, Yasunami M, Kubo Y, Yoshida LM, Kubo H, Ariyoshi K, Yoshiura K, Morimoto K. Surfactant protein C G100S mutation causes familial pulmonary fibrosis in Japanese kindred. Eur Respir J. 2011;38(4):861–9. doi: 10.1183/09031936.00143610. [DOI] [PubMed] [Google Scholar]
- 24.Thomas AQ, Lane K, Phillips J, 3rd, Prince M, Markin C, Speer M, Schwartz DA, Gaddipati R, Marney A, Johnson J, Roberts R, Haines J, Stahlman M, Loyd JE. Heterozygosity for a surfactant protein C gene mutation associated with usual interstitial pneumonitis and cellular nonspecific interstitial pneumonitis in one kindred. Am J Respir Crit Care Med. 2002;165(9):1322–8. doi: 10.1164/rccm.200112-123OC. [DOI] [PubMed] [Google Scholar]
- 25.van Moorsel CH, van Oosterhout MF, Barlo NP, de Jong PA, van der Vis JJ, Ruven HJ, van Es HW, van den Bosch JM, Grutters JC. Surfactant protein C mutations are the basis of a significant portion of adult familial pulmonary fibrosis in a dutch cohort. Am J Respir Crit Care Med. 2010;182(11):1419–25. doi: 10.1164/rccm.200906-0953OC. [DOI] [PubMed] [Google Scholar]
- 26.Moorsel CHMv, Klooster Lt, Oosterhout MFMv, Jong PAd, Adams H, Es HWv, Ruven HJT, Vis JJvd, Grutters JC. SFTPA2 Mutations in Familial and Sporadic Idiopathic Interstitial Pneumonia. American Journal of Respiratory and Critical Care Medicine. 2015;192(10):1249–1252. doi: 10.1164/rccm.201504-0675LE. [DOI] [PubMed] [Google Scholar]
- 27.Wang Y, Kuan PJ, Xing C, Cronkhite JT, Torres F, Rosenblatt RL, DiMaio JM, Kinch LN, Grishin NV, Garcia CK. Genetic defects in surfactant protein A2 are associated with pulmonary fibrosis and lung cancer. Am J Hum Genet. 2009;84(1):52–9. doi: 10.1016/j.ajhg.2008.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Campo I, Zorzetto M, Mariani F, Kadija Z, Morbini P, Dore R, Kaltenborn E, Frixel S, Zarbock R, Liebisch G, Hegermann J, Wrede C, Griese M, Luisetti M. A large kindred of pulmonary fibrosis associated with a novel ABCA3 gene variant. Respir Res. 2014;15:43. doi: 10.1186/1465-9921-15-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Epaud R, Delestrain C, Louha M, Simon S, Fanen P, Tazi A. Combined pulmonary fibrosis and emphysema syndrome associated with ABCA3 mutations. Eur Respir J. 2014;43(2):638–41. doi: 10.1183/09031936.00145213. [DOI] [PubMed] [Google Scholar]
- 30.Young LR, Nogee LM, Barnett B, Panos RJ, Colby TV, Deutsch GH. Usual interstitial pneumonia in an adolescent with ABCA3 mutations. Chest. 2008;134(1):192–5. doi: 10.1378/chest.07-2652. [DOI] [PubMed] [Google Scholar]
- 31.Kropski JA, Lawson WE, Young LR, Blackwell TS. Genetic studies provide clues on the pathogenesis of idiopathic pulmonary fibrosis. Dis Model Mech. 2013;6(1):9–17. doi: 10.1242/dmm.010736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Young LR, Gulleman PM, Bridges JP, Weaver TE, Deutsch GH, Blackwell TS, McCormack FX. The Alveolar Epithelium Determines Susceptibility to Lung Fibrosis in Hermansky-Pudlak Syndrome. Am J Respir Crit Care Med. 2012 doi: 10.1164/rccm.201207-1206OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.El-Chemaly S, Young LR. Hermansky-Pudlak Syndrome. Clin Chest Med. 2016;37(3):505–11. doi: 10.1016/j.ccm.2016.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Armanios MY, Chen JJ, Cogan JD, Alder JK, Ingersoll RG, Markin C, Lawson WE, Xie M, Vulto I, Phillips JA, 3rd, Lansdorp PM, Greider CW, Loyd JE. Telomerase mutations in families with idiopathic pulmonary fibrosis. N Engl J Med. 2007;356(13):1317–26. doi: 10.1056/NEJMoa066157. [DOI] [PubMed] [Google Scholar]
- 35.Tsakiri KD, Cronkhite JT, Kuan PJ, Xing C, Raghu G, Weissler JC, Rosenblatt RL, Shay JW, Garcia CK. Adult-onset pulmonary fibrosis caused by mutations in telomerase. Proc Natl Acad Sci U S A. 2007;104(18):7552–7. doi: 10.1073/pnas.0701009104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cogan JD, Kropski JA, Zhao M, Mitchell DB, Rives L, Markin C, Garnett ET, Montgomery KH, Mason WR, McKean DF, Powers J, Murphy E, Olson LM, Choi L, Cheng DS, Blue EM, Young LR, Lancaster LH, Steele MP, Brown KK, Schwarz MI, Fingerlin TE, Schwartz DA, Lawson WE, Loyd JE, Zhao Z, Phillips JA, III, Blackwell TS G. University of Washington Center for Mendelian. Rare Variants in RTEL1 are Associated with Familial Interstitial Pneumonia. Am J Respir Crit Care Med. 2015 doi: 10.1164/rccm.201408-1510OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kannengiesser C, Borie R, Menard C, Reocreux M, Nietschke P, Gazal S, Taile C, Cadranel J, Nunes H, Cordier J-F, Callebaut I, Bioleau C, Cottin V, Claude B, Grandchamp H, Revy P, Crestani B. Heterozygous RTEL1 mutations are associated with familial pulmonary fibrosis. European Respiratory Journal. 2015 doi: 10.1183/09031936.00040115. [DOI] [PubMed] [Google Scholar]
- 38.Stuart BD, Choi J, Zaidi S, Xing C, Holohan B, Chen R, Choi M, Dharwadkar P, Torres F, Girod CE, Weissler J, Fitzgerald J, Kershaw C, Klesney-Tait J, Mageto Y, Shay JW, Ji W, Bilguvar K, Mane S, Lifton RP, Garcia CK. Exome sequencing links mutations in PARN and RTEL1 with familial pulmonary fibrosis and telomere shortening. Nat Genet. 2015;47(5):512–7. doi: 10.1038/ng.3278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kropski JA, Mitchell DB, Markin C, Polosukhin VV, Choi LA, Johnson JE, Lawson WE, Phillips JA, 3rd, Cogan JD, Blackwell TS, Loyd JE. A novel dyskerin (DKC1) mutation is associated with Familial Interstitial Pneumonia. Chest. 2014 doi: 10.1378/chest.13-2224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Alder JK, Stanley SE, Wagner CL, Hamilton M, Hanumanthu VS, Armanios M. Exome sequencing identifies mutant TINF2 in a family with pulmonary fibrosis. Chest. 2014 doi: 10.1378/chest.14-1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stanley SE, Gable DL, Wagner CL, Carlile TM, Hanumanthu VS, Podlevsky JD, Khalil SE, DeZern AE, Rojas-Duran MF, Applegate CD, Alder JK, Parry EM, Gilbert WV, Armanios M. Loss-of-function mutations in the RNA biogenesis factor NAF1 predispose to pulmonary fibrosis-emphysema. Sci Transl Med. 2016;8(351):351ra107. doi: 10.1126/scitranslmed.aaf7837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Petrovski S, Todd JL, Durheim MT, Wang Q, Chien JW, Kelly FL, Frankel C, Mebane CM, Ren Z, Bridgers J, Urban TJ, Malone CD, Finlen Copeland A, Brinkley C, Allen AS, O'Riordan T, McHutchison JG, Palmer SM, Goldstein DB. An Exome Sequencing Study to Assess the Role of Rare Genetic Variation in Pulmonary Fibrosis. Am J Respir Crit Care Med. 2017 doi: 10.1164/rccm.201610-2088OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Armanios M. Telomerase and idiopathic pulmonary fibrosis. Mutat Res. 2012;730(1–2):52–8. doi: 10.1016/j.mrfmmm.2011.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Seibold MA, Wise AL, Speer MC, Steele MP, Brown KK, Loyd JE, Fingerlin TE, Zhang W, Gudmundsson G, Groshong SD, Evans CM, Garantziotis S, Adler KB, Dickey BF, du Bois RM, Yang IV, Herron A, Kervitsky D, Talbert JL, Markin C, Park J, Crews AL, Slifer SH, Auerbach S, Roy MG, Lin J, Hennessy CE, Schwarz MI, Schwartz DA. A common MUC5B promoter polymorphism and pulmonary fibrosis. N Engl J Med. 2011;364(16):1503–12. doi: 10.1056/NEJMoa1013660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Borie R, Crestani B, Dieude P, Nunes H, Allanore Y, Kannengiesser C, Airo P, Matucci-Cerinic M, Wallaert B, Israel-Biet D, Cadranel J, Cottin V, Gazal S, Peljto AL, Varga J, Schwartz DA, Valeyre D, Grandchamp B. The MUC5B variant is associated with idiopathic pulmonary fibrosis but not with systemic sclerosis interstitial lung disease in the European Caucasian population. PLoS One. 2013;8(8):e70621. doi: 10.1371/journal.pone.0070621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Horimasu Y, Ohshimo S, Bonella F, Tanaka S, Ishikawa N, Hattori N, Kohno N, Guzman J, Costabel U. MUC5B promoter polymorphism in Japanese patients with idiopathic pulmonary fibrosis. Respirology. 2015 doi: 10.1111/resp.12466. [DOI] [PubMed] [Google Scholar]
- 47.Stock CJ, Sato H, Fonseca C, Banya WA, Molyneaux PL, Adamali H, Russell AM, Denton CP, Abraham DJ, Hansell DM, Nicholson AG, Maher TM, Wells AU, Lindahl GE, Renzoni EA. Mucin 5B promoter polymorphism is associated with idiopathic pulmonary fibrosis but not with development of lung fibrosis in systemic sclerosis or sarcoidosis. Thorax. 2013;68(5):436–41. doi: 10.1136/thoraxjnl-2012-201786. [DOI] [PubMed] [Google Scholar]
- 48.Zhang Y, Noth I, Garcia JG, Kaminski N. A variant in the promoter of MUC5B and idiopathic pulmonary fibrosis. N Engl J Med. 2011;364(16):1576–7. doi: 10.1056/NEJMc1013504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kropski JA, Pritchett JM, Zoz DF, Crossno PF, Markin C, Garnett ET, Degryse AL, Mitchell DB, Polosukhin VV, Rickman OB, Choi L, Cheng DS, McConaha ME, Jones BR, Gleaves LA, McMahon FB, Worrell JA, Solus JF, Ware LB, Lee JW, Massion PP, Zaynagetdinov R, White ES, Kurtis JD, Johnson JE, Groshong SD, Lancaster LH, Young LR, Steele MP, Phillips JA, Iii, Cogan JD, Loyd JE, Lawson WE, Blackwell TS. Extensive phenotyping of individuals at risk for familial interstitial pneumonia reveals clues to the pathogenesis of interstitial lung disease. Am J Respir Crit Care Med. 2015;191(4):417–26. doi: 10.1164/rccm.201406-1162OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tobin RW, Pope CE, 2nd, Pellegrini CA, Emond MJ, Sillery J, Raghu G. Increased prevalence of gastroesophageal reflux in patients with idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 1998;158(6):1804–8. doi: 10.1164/ajrccm.158.6.9804105. [DOI] [PubMed] [Google Scholar]
- 51.Lawson WE, Crossno PF, Polosukhin VV, Roldan J, Cheng DS, Lane KB, Blackwell TR, Xu C, Markin C, Ware LB, Miller GG, Loyd JE, Blackwell TS. Endoplasmic reticulum stress in alveolar epithelial cells is prominent in IPF: association with altered surfactant protein processing and herpesvirus infection. Am J Physiol Lung Cell Mol Physiol. 2008;294(6):L1119–26. doi: 10.1152/ajplung.00382.2007. [DOI] [PubMed] [Google Scholar]
- 52.Naik PK, Moore BB. Viral infection and aging as cofactors for the development of pulmonary fibrosis. Expert Rev Respir Med. 2010;4(6):759–71. doi: 10.1586/ers.10.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Baumgartner KB, Samet JM, Coultas DB, Stidley CA, Hunt WC, Colby TV, Waldron JA. Occupational and environmental risk factors for idiopathic pulmonary fibrosis: a multicenter case-control study. Collaborating Centers. Am J Epidemiol. 2000;152(4):307–15. doi: 10.1093/aje/152.4.307. [DOI] [PubMed] [Google Scholar]
- 54.Sisson TH, Mendez M, Choi K, Subbotina N, Courey A, Cunningham A, Dave A, Engelhardt JF, Liu X, White ES, Thannickal VJ, Moore BB, Christensen PJ, Simon RH. Targeted injury of type II alveolar epithelial cells induces pulmonary fibrosis. Am J Respir Crit Care Med. 2010;181(3):254–63. doi: 10.1164/rccm.200810-1615OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Alder JK, Barkauskas CE, Limjunyawong N, Stanley SE, Kembou F, Tuder RM, Hogan BL, Mitzner W, Armanios M. Telomere dysfunction causes alveolar stem cell failure. Proc Natl Acad Sci U S A, United States. 2015:5099–104. doi: 10.1073/pnas.1504780112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Naikawadi RP, Disayabutr S, Mallavia B, Donne ML, Green G, La JL, Rock JR, Looney MR, Wolters PJ. Telomere dysfunction in alveolar epithelial cells causes lung remodeling and fibrosis. JCI insight. 2016;1(14):e86704. doi: 10.1172/jci.insight.86704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Povedano JM, Martinez P, Flores JM, Mulero F, Blasco MA. Mice with Pulmonary Fibrosis Driven by Telomere Dysfunction. Cell reports. 2015;12(2):286–99. doi: 10.1016/j.celrep.2015.06.028. [DOI] [PubMed] [Google Scholar]
- 58.Raghu G, Striker LJ, Hudson LD, Striker GE. Extracellular matrix in normal and fibrotic human lungs. The American review of respiratory disease. 1985;131(2):281–9. doi: 10.1164/arrd.1985.131.2.281. [DOI] [PubMed] [Google Scholar]
- 59.Thannickal VJ, Henke CA, Horowitz JC, Noble PW, Roman J, Sime PJ, Zhou Y, Wells RG, White ES, Tschumperlin DJ. Matrix biology of idiopathic pulmonary fibrosis: a workshop report of the national heart, lung, and blood institute. Am J Pathol. 2014;184(6):1643–51. doi: 10.1016/j.ajpath.2014.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tata PR, Rajagopal J. Plasticity in the lung: making and breaking cell identity. Development (Cambridge, England) 2017;144(5):755–766. doi: 10.1242/dev.143784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chen F, Fine A. Stem Cells in Lung Injury and Repair. Am J Pathol. 2016;186(10):2544–50. doi: 10.1016/j.ajpath.2016.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zuo W, Zhang T, Wu DZ, Guan SP, Liew AA, Yamamoto Y, Wang X, Lim SJ, Vincent M, Lessard M, Crum CP, Xian W, McKeon F. p63(+)Krt5(+) distal airway stem cells are essential for lung regeneration. Nature. 2015;517(7536):616–20. doi: 10.1038/nature13903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rock JR, Barkauskas CE, Cronce MJ, Xue Y, Harris JR, Liang J, Noble PW, Hogan BL. Multiple stromal populations contribute to pulmonary fibrosis without evidence for epithelial to mesenchymal transition. Proc Natl Acad Sci U S A. 2011;108(52):E1475–83. doi: 10.1073/pnas.1117988108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rawlins EL, Okubo T, Xue Y, Brass DM, Auten RL, Hasegawa H, Wang F, Hogan BL. The role of Scgb1a1+ Clara cells in the long-term maintenance and repair of lung airway, but not alveolar, epithelium. Cell stem cell. 2009;4(6):525–34. doi: 10.1016/j.stem.2009.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Vaughan AE, Brumwell AN, Xi Y, Gotts JE, Brownfield DG, Treutlein B, Tan K, Tan V, Liu FC, Looney MR, Matthay MA, Rock JR, Chapman HA. Lineage-negative progenitors mobilize to regenerate lung epithelium after major injury. Nature. 2015;517(7536):621–5. doi: 10.1038/nature14112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Barkauskas CE, Cronce MJ, Rackley CR, Bowie EJ, Keene DR, Stripp BR, Randell SH, Noble PW, Hogan BL. Type 2 alveolar cells are stem cells in adult lung. J Clin Invest. 2013;123(7):3025–36. doi: 10.1172/JCI68782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Frank DB, Peng T, Zepp JA, Snitow M, Vincent TL, Penkala IJ, Cui Z, Herriges MJ, Morley MP, Zhou S, Lu MM, Morrisey EE. Emergence of a Wave of Wnt Signaling that Regulates Lung Alveologenesis by Controlling Epithelial Self-Renewal and Differentiation. Cell reports. 2016;17(9):2312–2325. doi: 10.1016/j.celrep.2016.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zepp JA, Zacharias WJ, Frank DB, Cavanaugh CA, Zhou S, Morley MP, Morrisey EE. Distinct Mesenchymal Lineages and Niches Promote Epithelial Self-Renewal and Myofibrogenesis in the Lung. Cell. 2017;170(6):1134–1148.e10. doi: 10.1016/j.cell.2017.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lee JH, Tammela T, Hofree M, Choi J, Marjanovic ND, Han S, Canner D, Wu K, Paschini M, Bhang DH, Jacks T, Regev A, Kim CF. Anatomically and Functionally Distinct Lung Mesenchymal Populations Marked by Lgr5 and Lgr6. Cell. 2017;170(6):1149–1163.e12. doi: 10.1016/j.cell.2017.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Liang J, Zhang Y, Xie T, Liu N, Chen H, Geng Y, Kurkciyan A, Mena JM, Stripp BR, Jiang D, Noble PW. Hyaluronan and TLR4 promote surfactant-protein-C-positive alveolar progenitor cell renewal and prevent severe pulmonary fibrosis in mice. Nat Med. 2016;22(11):1285–1293. doi: 10.1038/nm.4192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Li Y, Jiang D, Liang J, Meltzer EB, Gray A, Miura R, Wogensen L, Yamaguchi Y, Noble PW. Severe lung fibrosis requires an invasive fibroblast phenotype regulated by hyaluronan and CD44. J Exp Med. 2011;208(7):1459–71. doi: 10.1084/jem.20102510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Aschner Y, Downey GP. Transforming Growth Factor-beta: Master Regulator of the Respiratory System in Health and Disease. Am J Respir Cell Mol Biol. 2016;54(5):647–55. doi: 10.1165/rcmb.2015-0391TR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tatler AL, Jenkins G. TGF-beta activation and lung fibrosis. Proc Am Thorac Soc. 2012;9(3):130–6. doi: 10.1513/pats.201201-003AW. [DOI] [PubMed] [Google Scholar]
- 74.Sheppard D. Transforming Growth Factor β. Proceedings of the American Thoracic Society. 2006;3(5):413–417. doi: 10.1513/pats.200601-008AW. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Unsold C, Hyytiainen M, Bruckner-Tuderman L, Keski-Oja J. Latent TGF-beta binding protein LTBP-1 contains three potential extracellular matrix interacting domains. Journal of cell science. 2001;114(Pt 1):187–197. doi: 10.1242/jcs.114.1.187. [DOI] [PubMed] [Google Scholar]
- 76.Annes JP, Munger JS, Rifkin DB. Making sense of latent TGFbeta activation. Journal of cell science. 2003;116(Pt 2):217–24. doi: 10.1242/jcs.00229. [DOI] [PubMed] [Google Scholar]
- 77.Hyytiainen M, Penttinen C, Keski-Oja J. Latent TGF-beta binding proteins: extracellular matrix association and roles in TGF-beta activation. Critical reviews in clinical laboratory sciences. 2004;41(3):233–64. doi: 10.1080/10408360490460933. [DOI] [PubMed] [Google Scholar]
- 78.Yu Q, Stamenkovic I. Cell surface-localized matrix metalloproteinase-9 proteolytically activates TGF-beta and promotes tumor invasion and angiogenesis. Genes & development. 2000;14(2):163–76. [PMC free article] [PubMed] [Google Scholar]
- 79.Schultz-Cherry S, Murphy-Ullrich JE. Thrombospondin causes activation of latent transforming growth factor-beta secreted by endothelial cells by a novel mechanism. The Journal of cell biology. 1993;122(4):923–32. doi: 10.1083/jcb.122.4.923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Crawford SE, Stellmach V, Murphy-Ullrich JE, Ribeiro SM, Lawler J, Hynes RO, Boivin GP, Bouck N. Thrombospondin-1 is a major activator of TGF-beta1 in vivo. Cell. 1998;93(7):1159–70. doi: 10.1016/s0092-8674(00)81460-9. [DOI] [PubMed] [Google Scholar]
- 81.Munger JS, Huang X, Kawakatsu H, Griffiths MJ, Dalton SL, Wu J, Pittet JF, Kaminski N, Garat C, Matthay MA, Rifkin DB, Sheppard D. The integrin alpha v beta 6 binds and activates latent TGF beta 1: a mechanism for regulating pulmonary inflammation and fibrosis. Cell. 1999;96(3):319–28. doi: 10.1016/s0092-8674(00)80545-0. [DOI] [PubMed] [Google Scholar]
- 82.Blobe GC, Schiemann WP, Lodish HF. Role of transforming growth factor beta in human disease. N Engl J Med. 2000;342(18):1350–8. doi: 10.1056/NEJM200005043421807. [DOI] [PubMed] [Google Scholar]
- 83.Kulkarni AB, Huh CG, Becker D, Geiser A, Lyght M, Flanders KC, Roberts AB, Sporn MB, Ward JM, Karlsson S. Transforming growth factor beta 1 null mutation in mice causes excessive inflammatory response and early death. Proc Natl Acad Sci U S A. 1993;90(2):770–4. doi: 10.1073/pnas.90.2.770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Karlsson PL, Jonas L, Mats E, Corrado MC, Martin S, Lottie Jansson S, Rikard H, Stefan Induced disruption of the transforming growth factor beta type II receptor gene in mice causes a lethal inflammatory disorder that is transplantable. 2002 doi: 10.1182/blood.v100.2.560. [DOI] [PubMed] [Google Scholar]
- 85.Coker RK, Laurent GJ, Shahzeidi S, Lympany PA, du Bois RM, Jeffery PK, McAnulty RJ. Transforming growth factors-beta 1, -beta 2, and -beta 3 stimulate fibroblast procollagen production in vitro but are differentially expressed during bleomycin-induced lung fibrosis. Am J Pathol. 1997;150(3):981–91. [PMC free article] [PubMed] [Google Scholar]
- 86.Coker RK, Laurent GJ, Jeffery PK, du Bois RM, Black CM, McAnulty RJ. Localisation of transforming growth factor beta1 and beta3 mRNA transcripts in normal and fibrotic human lung. Thorax. 2001;56(7):549–56. doi: 10.1136/thorax.56.7.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Khalil N, O'Connor RN, Unruh HW, Warren PW, Flanders KC, Kemp A, Bereznay OH, Greenberg AH. Increased production and immunohistochemical localization of transforming growth factor-beta in idiopathic pulmonary fibrosis. Am J Respir Cell Mol Biol. 1991;5(2):155–62. doi: 10.1165/ajrcmb/5.2.155. [DOI] [PubMed] [Google Scholar]
- 88.Xu YD, Hua J, Mui A, O'Connor R, Grotendorst G, Khalil N. Release of biologically active TGF-beta1 by alveolar epithelial cells results in pulmonary fibrosis. Am J Physiol Lung Cell Mol Physiol. 2003;285(3):L527–39. doi: 10.1152/ajplung.00298.2002. [DOI] [PubMed] [Google Scholar]
- 89.Assoian RK, Sporn MB. Type beta transforming growth factor in human platelets: release during platelet degranulation and action on vascular smooth muscle cells. The Journal of cell biology. 1986;102(4):1217–23. doi: 10.1083/jcb.102.4.1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ostroukhova M, Qi Z, Oriss TB, Dixon-McCarthy B, Ray P, Ray A. Treg-mediated immunosuppression involves activation of the Notch-HES1 axis by membrane-bound TGF-beta. J Clin Invest. 2006;116(4):996–1004. doi: 10.1172/JCI26490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hou Z, Ye Q, Qiu M, Hao Y, Han J, Zeng H. Increased activated regulatory T cells proportion correlate with the severity of idiopathic pulmonary fibrosis. Respir Res. 2017;18(1):170. doi: 10.1186/s12931-017-0653-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Sime PJ, Xing Z, Graham FL, Csaky KG, Gauldie J. Adenovector-mediated gene transfer of active transforming growth factor-beta1 induces prolonged severe fibrosis in rat lung. J Clin Invest. 1997;100(4):768–76. doi: 10.1172/JCI119590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Liu JY, Sime PJ, Wu T, Warshamana GS, Pociask D, Tsai SY, Brody AR. Transforming growth factor-beta(1) overexpression in tumor necrosis factor-alpha receptor knockout mice induces fibroproliferative lung disease. Am J Respir Cell Mol Biol. 2001;25(1):3–7. doi: 10.1165/ajrcmb.25.1.4481. [DOI] [PubMed] [Google Scholar]
- 94.Kim KK, Kugler MC, Wolters PJ, Robillard L, Galvez MG, Brumwell AN, Sheppard D, Chapman HA. Alveolar epithelial cell mesenchymal transition develops in vivo during pulmonary fibrosis and is regulated by the extracellular matrix. Proc Natl Acad Sci U S A. 2006;103(35):13180–5. doi: 10.1073/pnas.0605669103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Tanjore H, Cheng DS, Degryse AL, Zoz DF, Abdolrasulnia R, Lawson WE, Blackwell TS. Alveolar epithelial cells undergo epithelial-to-mesenchymal transition in response to endoplasmic reticulum stress. J Biol Chem. 2011;286(35):30972–80. doi: 10.1074/jbc.M110.181164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Li M, Krishnaveni MS, Li C, Zhou B, Xing Y, Banfalvi A, Li A, Lombardi V, Akbari O, Borok Z, Minoo P. Epithelium-specific deletion of TGF-beta receptor type II protects mice from bleomycin-induced pulmonary fibrosis. J Clin Invest. 2011;121(1):277–87. doi: 10.1172/JCI42090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Degryse AL, Tanjore H, Xu XC, Polosukhin VV, Jones BR, Boomershine CS, Ortiz C, Sherrill TP, McMahon FB, Gleaves LA, Blackwell TS, Lawson WE. TGFbeta signaling in lung epithelium regulates bleomycin-induced alveolar injury and fibroblast recruitment. Am J Physiol Lung Cell Mol Physiol. 2011;300(6):L887–97. doi: 10.1152/ajplung.00397.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Gong D, Shi W, Yi SJ, Chen H, Groffen J, Heisterkamp N. TGFbeta signaling plays a critical role in promoting alternative macrophage activation. BMC immunology. 2012;13:31. doi: 10.1186/1471-2172-13-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hynes RO. Integrins: bidirectional, allosteric signaling machines. Cell. 2002;110(6):673–87. doi: 10.1016/s0092-8674(02)00971-6. [DOI] [PubMed] [Google Scholar]
- 100.Plosa EJ, Young LR, Gulleman PM, Polosukhin VV, Zaynagetdinov R, Benjamin JT, Im AM, van der Meer R, Gleaves LA, Bulus N, Han W, Prince LS, Blackwell TS, Zent R. Epithelial beta1 integrin is required for lung branching morphogenesis and alveolarization. Development (Cambridge, England) 2014;141(24):4751–62. doi: 10.1242/dev.117200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Teoh CM, Tan SS, Tran T. Integrins as Therapeutic Targets for Respiratory Diseases. Current molecular medicine. 2015;15(8):714–34. doi: 10.2174/1566524015666150921105339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.John AE, Luckett JC, Tatler AL, Awais RO, Desai A, Habgood A, Ludbrook S, Blanchard AD, Perkins AC, Jenkins RG, Marshall JF. Preclinical SPECT/CT imaging of alphavbeta6 integrins for molecular stratification of idiopathic pulmonary fibrosis. Journal of nuclear medicine : official publication, Society of Nuclear Medicine. 2013;54(12):2146–52. doi: 10.2967/jnumed.113.120592. [DOI] [PubMed] [Google Scholar]
- 103.Breuss JM, Gallo J, DeLisser HM, Klimanskaya IV, Folkesson HG, Pittet JF, Nishimura SL, Aldape K, Landers DV, Carpenter W, et al. Expression of the beta 6 integrin subunit in development, neoplasia and tissue repair suggests a role in epithelial remodeling. Journal of cell science. 1995;108(Pt 6):2241–51. doi: 10.1242/jcs.108.6.2241. [DOI] [PubMed] [Google Scholar]
- 104.Huang XZ, Wu JF, Cass D, Erle DJ, Corry D, Young SG, Farese RV, Jr, Sheppard D. Inactivation of the integrin beta 6 subunit gene reveals a role of epithelial integrins in regulating inflammation in the lung and skin. The Journal of cell biology. 1996;133(4):921–8. doi: 10.1083/jcb.133.4.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Annes JP, Chen Y, Munger JS, Rifkin DB. Integrin alphaVbeta6-mediated activation of latent TGF-beta requires the latent TGF-beta binding protein-1. The Journal of cell biology. 2004;165(5):723–34. doi: 10.1083/jcb.200312172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Huang X, Wu J, Zhu W, Pytela R, Sheppard D. Expression of the human integrin beta6 subunit in alveolar type II cells and bronchiolar epithelial cells reverses lung inflammation in beta6 knockout mice. Am J Respir Cell Mol Biol. 1998;19(4):636–42. doi: 10.1165/ajrcmb.19.4.3293. [DOI] [PubMed] [Google Scholar]
- 107.Horan GS, Wood S, Ona V, Li DJ, Lukashev ME, Weinreb PH, Simon KJ, Hahm K, Allaire NE, Rinaldi NJ, Goyal J, Feghali-Bostwick CA, Matteson EL, O'Hara C, Lafyatis R, Davis GS, Huang X, Sheppard D, Violette SM. Partial inhibition of integrin alpha(v)beta6 prevents pulmonary fibrosis without exacerbating inflammation. Am J Respir Crit Care Med. 2008;177(1):56–65. doi: 10.1164/rccm.200706-805OC. [DOI] [PubMed] [Google Scholar]
- 108.Wipff PJ, Rifkin DB, Meister JJ, Hinz B. Myofibroblast contraction activates latent TGF-beta1 from the extracellular matrix. The Journal of cell biology. 2007;179(6):1311–23. doi: 10.1083/jcb.200704042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Tatler AL, Alison EJ, Lisa J, Anthony H, Jo P, Chris B, Alan JK, Linhua P, Dean S, Xiaozhu H, Jenkins G. Integrin αvβ5-Mediated TGF-β Activation by Airway Smooth Muscle Cells in Asthma. 2011 [Google Scholar]
- 110.Kitamura H, Cambier S, Somanath S, Barker T, Minagawa S, Markovics J, Goodsell A, Publicover J, Reichardt L, Jablons D, Wolters P, Hill A, Marks JD, Lou J, Pittet JF, Gauldie J, Baron JL, Nishimura SL. Mouse and human lung fibroblasts regulate dendritic cell trafficking, airway inflammation, and fibrosis through integrin alphavbeta8-mediated activation of TGF-beta. J Clin Invest. 2011;121(7):2863–75. doi: 10.1172/JCI45589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Henderson NC, Arnold TD, Katamura Y, Giacomini MM, Rodriguez JD, McCarty JH, Pellicoro A, Raschperger E, Betsholtz C, Ruminski PG, Griggs DW, Prinsen MJ, Maher JJ, Iredale JP, Lacy-Hulbert A, Adams RH, Sheppard D. Targeting of alphav integrin identifies a core molecular pathway that regulates fibrosis in several organs. Nat Med. 2013;19(12):1617–24. doi: 10.1038/nm.3282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Reed NI, Jo H, Chen C, Tsujino K, Arnold TD, DeGrado WF, Sheppard D. The alphavbeta1 integrin plays a critical in vivo role in tissue fibrosis. Sci Transl Med. 2015;7(288):288ra79. doi: 10.1126/scitranslmed.aaa5094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Lasky JA, Ortiz LA, Tonthat B, Hoyle GW, Corti M, Athas G, Lungarella G, Brody A, Friedman M. Connective tissue growth factor mRNA expression is upregulated in bleomycin-induced lung fibrosis. The American journal of physiology. 1998;275(2 Pt 1):L365–71. doi: 10.1152/ajplung.1998.275.2.L365. [DOI] [PubMed] [Google Scholar]
- 114.Lipson KE, Wong C, Teng Y, Spong S. CTGF is a central mediator of tissue remodeling and fibrosis and its inhibition can reverse the process of fibrosis. Fibrogenesis & tissue repair. 2012;5(Suppl 1):S24. doi: 10.1186/1755-1536-5-S1-S24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Pan LH, Yamauchi K, Uzuki M, Nakanishi T, Takigawa M, Inoue H, Sawai T. Type II alveolar epithelial cells and interstitial fibroblasts express connective tissue growth factor in IPF. Eur Respir J. 2001;17(6):1220–7. doi: 10.1183/09031936.01.00074101. [DOI] [PubMed] [Google Scholar]
- 116.Ponticos M, Holmes AM, Shi-wen X, Leoni P, Khan K, Rajkumar VS, Hoyles RK, Bou-Gharios G, Black CM, Denton CP, Abraham DJ, Leask A, Lindahl GE. Pivotal role of connective tissue growth factor in lung fibrosis: MAPK-dependent transcriptional activation of type I collagen. Arthritis and rheumatism. 2009;60(7):2142–55. doi: 10.1002/art.24620. [DOI] [PubMed] [Google Scholar]
- 117.Grotendorst GR. Connective tissue growth factor: a mediator of TGF-beta action on fibroblasts. Cytokine & growth factor reviews. 1997;8(3):171–9. doi: 10.1016/s1359-6101(97)00010-5. [DOI] [PubMed] [Google Scholar]
- 118.Duncan MR, Frazier KS, Abramson S, Williams S, Klapper H, Huang X, Grotendorst GR. Connective tissue growth factor mediates transforming growth factor beta-induced collagen synthesis: down-regulation by cAMP. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 1999;13(13):1774–86. [PubMed] [Google Scholar]
- 119.Bogatkevich GS, Ludwicka-Bradley A, Singleton CB, Bethard JR, Silver RM. Proteomic analysis of CTGF-activated lung fibroblasts: identification of IQGAP1 as a key player in lung fibroblast migration. Am J Physiol Lung Cell Mol Physiol. 2008;295(4):L603–11. doi: 10.1152/ajplung.00530.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Kothapalli D, Frazier KS, Welply A, Segarini PR, Grotendorst GR. Transforming growth factor beta induces anchorage-independent growth of NRK fibroblasts via a connective tissue growth factor-dependent signaling pathway. Cell growth & differentiation : the molecular biology journal of the American Association for Cancer Research. 1997;8(1):61–8. [PubMed] [Google Scholar]
- 121.Kothapalli D, Hayashi N, Grotendorst GR. Inhibition of TGF-beta-stimulated CTGF gene expression and anchorage-independent growth by cAMP identifies a CTGF-dependent restriction point in the cell cycle. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 1998;12(12):1151–61. doi: 10.1096/fasebj.12.12.1151. [DOI] [PubMed] [Google Scholar]
- 122.Wang Q, Usinger W, Nichols B, Gray J, Xu L, Seeley TW, Brenner M, Guo G, Zhang W, Oliver N, Lin A, Yeowell D. Cooperative interaction of CTGF and TGF-beta in animal models of fibrotic disease. Fibrogenesis & tissue repair. 2011;4(1):4. doi: 10.1186/1755-1536-4-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Grotendorst GR, Okochi H, Hayashi N. A novel transforming growth factor beta response element controls the expression of the connective tissue growth factor gene. Cell growth & differentiation : the molecular biology journal of the American Association for Cancer Research. 1996;7(4):469–80. [PubMed] [Google Scholar]
- 124.Mori T, Kawara S, Shinozaki M, Hayashi N, Kakinuma T, Igarashi A, Takigawa M, Nakanishi T, Takehara K. Role and interaction of connective tissue growth factor with transforming growth factor-beta in persistent fibrosis: A mouse fibrosis model. Journal of cellular physiology. 1999;181(1):153–9. doi: 10.1002/(SICI)1097-4652(199910)181:1<153::AID-JCP16>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 125.Heldin CH, Westermark B. Mechanism of action and in vivo role of platelet-derived growth factor. Physiological reviews. 1999;79(4):1283–316. doi: 10.1152/physrev.1999.79.4.1283. [DOI] [PubMed] [Google Scholar]
- 126.Maeda A, Hiyama K, Yamakido H, Ishioka S, Yamakido M. Increased expression of platelet-derived growth factor A and insulin-like growth factor-I in BAL cells during the development of bleomycin-induced pulmonary fibrosis in mice. Chest. 1996;109(3):780–6. doi: 10.1378/chest.109.3.780. [DOI] [PubMed] [Google Scholar]
- 127.Walsh J, Absher M, Kelley J. Variable expression of platelet-derived growth factor family proteins in acute lung injury. Am J Respir Cell Mol Biol. 1993;9(6):637–44. doi: 10.1165/ajrcmb/9.6.637. [DOI] [PubMed] [Google Scholar]
- 128.Yoshida M, Sakuma J, Hayashi S, Abe K, Saito I, Harada S, Sakatani M, Yamamoto S, Matsumoto N, Kaneda Y, et al. A histologically distinctive interstitial pneumonia induced by overexpression of the interleukin 6, transforming growth factor beta 1, or platelet-derived growth factor B gene. Proc Natl Acad Sci U S A. 1995;92(21):9570–4. doi: 10.1073/pnas.92.21.9570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Abdollahi A, Li M, Ping G, Plathow C, Domhan S, Kiessling F, Lee LB, McMahon G, Grone HJ, Lipson KE, Huber PE. Inhibition of platelet-derived growth factor signaling attenuates pulmonary fibrosis. The Journal of experimental medicine. 2005;201(6):925–35. doi: 10.1084/jem.20041393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Coffey E, Newman DR, Sannes PL. Expression of fibroblast growth factor 9 in normal human lung and idiopathic pulmonary fibrosis. The journal of histochemistry and cytochemistry : official journal of the Histochemistry Society. 2013;61(9):671–9. doi: 10.1369/0022155413497366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.MacKenzie B, Korfei M, Henneke I, Sibinska Z, Tian X, Hezel S, Dilai S, Wasnick R, Schneider B, Wilhelm J, El Agha E, Klepetko W, Seeger W, Schermuly R, Gunther A, Bellusci S. Increased FGF1-FGFRc expression in idiopathic pulmonary fibrosis. Respir Res. 2015;16:83. doi: 10.1186/s12931-015-0242-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Joannes A, Brayer S, Besnard V, Marchal-Somme J, Jaillet M, Mordant P, Mal H, Borie R, Crestani B, Mailleux AA. FGF9 and FGF18 in idiopathic pulmonary fibrosis promote survival and migration and inhibit myofibroblast differentiation of human lung fibroblasts in vitro. Am J Physiol Lung Cell Mol Physiol. 2016;310(7):L615–29. doi: 10.1152/ajplung.00185.2015. [DOI] [PubMed] [Google Scholar]
- 133.Ramos C, Montano M, Becerril C, Cisneros-Lira J, Barrera L, Ruiz V, Pardo A, Selman M. Acidic fibroblast growth factor decreases alpha-smooth muscle actin expression and induces apoptosis in human normal lung fibroblasts. Am J Physiol Lung Cell Mol Physiol. 2006;291(5):L871–9. doi: 10.1152/ajplung.00019.2006. [DOI] [PubMed] [Google Scholar]
- 134.Wollin L, Wex E, Pautsch A, Schnapp G, Hostettler KE, Stowasser S, Kolb M. Mode of action of nintedanib in the treatment of idiopathic pulmonary fibrosis. Eur Respir J. 2015;45(5):1434–45. doi: 10.1183/09031936.00174914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Hetzel M, Bachem M, Anders D, Trischler G, Faehling M. Different effects of growth factors on proliferation and matrix production of normal and fibrotic human lung fibroblasts. Lung. 2005;183(4):225–37. doi: 10.1007/s00408-004-2534-z. [DOI] [PubMed] [Google Scholar]
- 136.Khalil N, Xu YD, O'Connor R, Duronio V. Proliferation of pulmonary interstitial fibroblasts is mediated by transforming growth factor-beta1-induced release of extracellular fibroblast growth factor-2 and phosphorylation of p38 MAPK and JNK. J Biol Chem. 2005;280(52):43000–9. doi: 10.1074/jbc.M510441200. [DOI] [PubMed] [Google Scholar]
- 137.Guzy RD, Li L, Smith C, Dorry SJ, Koo HY, Chen L, Ornitz DM. Pulmonary fibrosis requires cell-autonomous mesenchymal fibroblast growth factor (FGF) signaling. J Biol Chem. 2017;292(25):10364–10378. doi: 10.1074/jbc.M117.791764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Richeldi L, Costabel U, Selman M, Kim DS, Hansell DM, Nicholson AG, Brown KK, Flaherty KR, Noble PW, Raghu G, Brun M, Gupta A, Juhel N, Kluglich M, du Bois RM. Efficacy of a tyrosine kinase inhibitor in idiopathic pulmonary fibrosis. N Engl J Med. 2011;365(12):1079–87. doi: 10.1056/NEJMoa1103690. [DOI] [PubMed] [Google Scholar]
- 139.Makino K, Makino T, Stawski L, Lipson KE, Leask A, Trojanowska M. Anti-connective tissue growth factor (CTGF/CCN2) monoclonal antibody attenuates skin fibrosis in mice models of systemic sclerosis. Arthritis research & therapy. 2017;19(1):134. doi: 10.1186/s13075-017-1356-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Bickelhaupt S, Erbel C, Timke C, Wirkner U, Dadrich M, Flechsig P, Tietz A, Pfohler J, Gross W, Peschke P, Hoeltgen L, Katus HA, Grone HJ, Nicolay NH, Saffrich R, Debus J, Sternlicht MD, Seeley TW, Lipson KE, Huber PE. Effects of CTGF Blockade on Attenuation and Reversal of Radiation-Induced Pulmonary Fibrosis. Journal of the National Cancer Institute. 2017;109(8) doi: 10.1093/jnci/djw339. [DOI] [PubMed] [Google Scholar]
- 141.Yang Z, Sun Z, Liu H, Ren Y, Shao D, Zhang W, Lin J, Wolfram J, Wang F, Nie S. Connective tissue growth factor stimulates the proliferation, migration and differentiation of lung fibroblasts during paraquat-induced pulmonary fibrosis. Molecular medicine reports. 2015;12(1):1091–7. doi: 10.3892/mmr.2015.3537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Raghu GSM, De Andrade J, Lancaster L, Mageto Y, Goldin J, Porter S, Neff T, Valone F, Stauffer J. Safety And Efficacy Of Anti-CTGF Monoclonal Antibody FG-3019 For Treatment Of Idiopathic Pulmonary Fibrosis (IPF): Results Of Phase 2 Clinical Trial Two Years After Initiation | A38. DIAMONDS ARE FOREVER BUT NEW TREATMENTS FOR INTERSTITIAL LUNG DISEASE CAN'T WAIT. 2014 [Google Scholar]
- 143.Bradley K, McConnell-Breul S, Crystal RG. Collagen in the human lung. Quantitation of rates of synthesis and partial characterization of composition. J Clin Invest. 1975;55(3):543–50. doi: 10.1172/JCI107961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Madri JA, Furthmayr H. Collagen polymorphism in the lung. An immunochemical study of pulmonary fibrosis. Human pathology. 1980;11(4):353–66. doi: 10.1016/s0046-8177(80)80031-1. [DOI] [PubMed] [Google Scholar]
- 145.Bellaye PS, Shimbori C, Upagupta C, Sato S, Shi W, Gauldie J, Ask K, Kolb M. Lysyl Oxidase-like 1 Protein Deficiency Protects Mice from AdTGF-beta1 Induced Pulmonary Fibrosis. Am J Respir Cell Mol Biol. 2017 doi: 10.1165/rcmb.2017-0252OC. [DOI] [PubMed] [Google Scholar]
- 146.Aumiller V, Strobel B, Romeike M, Schuler M, Stierstorfer BE, Kreuz S. Comparative analysis of lysyl oxidase (like) family members in pulmonary fibrosis. Scientific reports. 2017;7(1):149. doi: 10.1038/s41598-017-00270-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Philp CJ, Siebeke I, Clements D, Miller S, Habgood A, John AE, Navaratnam V, Hubbard RB, Jenkins G, Johnson SR. ECM Crosslinking Enhances Fibroblast Growth and Protects Against Matrix Proteolysis in Lung Fibrosis. Am J Respir Cell Mol Biol. 2017 doi: 10.1165/rcmb.2016-0379OC. [DOI] [PubMed] [Google Scholar]
- 148.Olsen KC, Sapinoro RE, Kottmann RM, Kulkarni AA, Iismaa SE, Johnson GV, Thatcher TH, Phipps RP, Sime PJ. Transglutaminase 2 and its role in pulmonary fibrosis. Am J Respir Crit Care Med. 2011;184(6):699–707. doi: 10.1164/rccm.201101-0013OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Oh K, Park HB, Byoun OJ, Shin DM, Jeong EM, Kim YW, Kim YS, Melino G, Kim IG, Lee DS. Epithelial transglutaminase 2 is needed for T cell interleukin-17 production and subsequent pulmonary inflammation and fibrosis in bleomycin-treated mice. The Journal of experimental medicine. 2011;208(8):1707–19. doi: 10.1084/jem.20101457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Chien JW, Richards TJ, Gibson KF, Zhang Y, Lindell KO, Shao L, Lyman SK, Adamkewicz JI, Smith V, Kaminski N, O'Riordan T. Serum lysyl oxidase-like 2 levels and idiopathic pulmonary fibrosis disease progression. Eur Respir J. 2014;43(5):1430–8. doi: 10.1183/09031936.00141013. [DOI] [PubMed] [Google Scholar]
- 151.Barry-Hamilton V, Spangler R, Marshall D, McCauley S, Rodriguez HM, Oyasu M, Mikels A, Vaysberg M, Ghermazien H, Wai C, Garcia CA, Velayo AC, Jorgensen B, Biermann D, Tsai D, Green J, Zaffryar-Eilot S, Holzer A, Ogg S, Thai D, Neufeld G, Van Vlasselaer P, Smith V. Allosteric inhibition of lysyl oxidase-like-2 impedes the development of a pathologic microenvironment. Nature medicine. 2010;16(9):1009–17. doi: 10.1038/nm.2208. [DOI] [PubMed] [Google Scholar]
- 152.Cheng T, Liu Q, Zhang R, Zhang Y, Chen J, Yu R, Ge G. Lysyl oxidase promotes bleomycin-induced lung fibrosis through modulating inflammation. Journal of molecular cell biology. 2014;6(6):506–15. doi: 10.1093/jmcb/mju039. [DOI] [PubMed] [Google Scholar]
- 153.Tjin G, White ES, Faiz A, Sicard D, Tschumperlin DJ, Mahar A, Kable EPW, Burgess JK. Lysyl oxidases regulate fibrillar collagen remodelling in idiopathic pulmonary fibrosis. Dis Model Mech. 2017;10(11):1301–1312. doi: 10.1242/dmm.030114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Asano S, Ito S, Takahashi K, Furuya K, Kondo M, Sokabe M, Hasegawa Y. Matrix stiffness regulates migration of human lung fibroblasts. Physiol Rep. 2017;5(9) doi: 10.14814/phy2.13281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Raghu G, Brown KK, Collard HR, Cottin V, Gibson KF, Kaner RJ, Lederer DJ, Martinez FJ, Noble PW, Song JW, Wells AU, Whelan TPM, Wuyts W, Moreau E, Patterson SD, Smith V, Bayly S, Chien JW, Gong Q, Zhang JJ, O'Riordan TG. Efficacy of simtuzumab versus placebo in patients with idiopathic pulmonary fibrosis: a randomised, double-blind, controlled, phase 2 trial. The Lancet Respiratory Medicine. 2017;5(1):22–32. doi: 10.1016/S2213-2600(16)30421-0. [DOI] [PubMed] [Google Scholar]
- 156.McKeown S, Richter AG, O'Kane C, McAuley DF, Thickett DR. MMP expression and abnormal lung permeability are important determinants of outcome in IPF. Eur Respir J. 2009;33(1):77–84. doi: 10.1183/09031936.00060708. [DOI] [PubMed] [Google Scholar]
- 157.Zhou LF, Jiang L, Li ZH, Kang J. Change of matrix metalloproteinase-1 and matrix metalloproteinase-7 in serum and bronchoalveolar lavage fluid of patients with idiopathic pulmonary fibrosis and sarcoidosis. Zhonghua jie he he hu xi za zhi = Zhonghua jiehe he huxi zazhi = Chinese journal of tuberculosis and respiratory diseases. 2010;33(6):441–4. [PubMed] [Google Scholar]
- 158.Rosas IO, Richards TJ, Konishi K, Zhang Y, Gibson K, Lokshin AE, Lindell KO, Cisneros J, MacDonald SD, Pardo A, Sclurba F, Dauber J, Selman M, Gochuico BR, Kaminski N. MMP1 and MMP7 as Potential Peripheral Blood Biomarkers in Idiopathic Pulmonary Fibrosis. PLoS Medicine. 2008;5(4):623–633. doi: 10.1371/journal.pmed.0050093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Pardo A, Cabrera S, Maldonado M, Selman M. Role of matrix metalloproteinases in the pathogenesis of idiopathic pulmonary fibrosis. Respir Res. 2016;17:23. doi: 10.1186/s12931-016-0343-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Zuo F, Kaminski N, Eugui E, Allard J, Yakhini Z, Ben-Dor A, Lollini L, Morris D, Kim Y, DeLustro B, Sheppard D, Pardo A, Selman M, Heller RA. Gene expression analysis reveals matrilysin as a key regulator of pulmonary fibrosis in mice and humans. Proc Natl Acad Sci U S A. 2002;99(9):6292–7. doi: 10.1073/pnas.092134099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Selman M, Ruiz V, Cabrera S, Segura L, Ramirez R, Barrios R, Pardo A. TIMP-1, -2, -3, and -4 in idiopathic pulmonary fibrosis. A prevailing nondegradative lung microenvironment? Am J Physiol Lung Cell Mol Physiol. 2000;279(3):L562–74. doi: 10.1152/ajplung.2000.279.3.L562. [DOI] [PubMed] [Google Scholar]
- 162.Cabrera S, Gaxiola M, Arreola JL, Ramirez R, Jara P, D'Armiento J, Richards T, Selman M, Pardo A. Overexpression of MMP9 in macrophages attenuates pulmonary fibrosis induced by bleomycin. The international journal of biochemistry & cell biology. 2007;39(12):2324–38. doi: 10.1016/j.biocel.2007.06.022. [DOI] [PubMed] [Google Scholar]
- 163.Betsuyaku T, Fukuda Y, Parks WC, Shipley JM, Senior RM. Gelatinase B is required for alveolar bronchiolization after intratracheal bleomycin. Am J Pathol. 2000;157(2):525–35. doi: 10.1016/S0002-9440(10)64563-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Yamashita CM, Dolgonos L, Zemans RL, Young SK, Robertson J, Briones N, Suzuki T, Campbell MN, Gauldie J, Radisky DC, Riches DW, Yu G, Kaminski N, McCulloch CA, Downey GP. Matrix metalloproteinase 3 is a mediator of pulmonary fibrosis. Am J Pathol. 2011;179(4):1733–45. doi: 10.1016/j.ajpath.2011.06.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Craig VJ, Zhang L, Hagood JS, Owen CA. Matrix Metalloproteinases as Therapeutic Targets for Idiopathic Pulmonary Fibrosis. Am J Respir Cell Mol Biol. 2015;53(5):585–600. doi: 10.1165/rcmb.2015-0020TR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Giannandrea M, Parks WC. Diverse functions of matrix metalloproteinases during fibrosis. Dis Model Mech. 2014;7(2):193–203. doi: 10.1242/dmm.012062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Jenkins RG, Simpson JK, Saini G, Bentley JH, Russell AM, Braybrooke R, Molyneaux PL, McKeever TM, Wells AU, Flynn A, Hubbard RB, Leeming DJ, Marshall RP, Karsdal MA, Lukey PT, Maher TM. Longitudinal change in collagen degradation biomarkers in idiopathic pulmonary fibrosis: an analysis from the prospective, multicentre PROFILE study. Lancet Respir Med. 2015;3(6):462–72. doi: 10.1016/S2213-2600(15)00048-X. [DOI] [PubMed] [Google Scholar]
- 168.Lennon FE, Singleton PA. Role of hyaluronan and hyaluronan-binding proteins in lung pathobiology. Am J Physiol Lung Cell Mol Physiol. 2011:L137–47. doi: 10.1152/ajplung.00071.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Furlan S, La Penna G, Perico A, Cesaro A. Hyaluronan chain conformation and dynamics. Carbohydrate research. 2005;340(5):959–70. doi: 10.1016/j.carres.2005.01.030. [DOI] [PubMed] [Google Scholar]
- 170.Donati A, Magnani A, Bonechi C, Barbucci R, Rossi C. Solution structure of hyaluronic acid oligomers by experimental and theoretical NMR, and molecular dynamics simulation. Biopolymers. 2001;59(6):434–45. doi: 10.1002/1097-0282(200111)59:6<434::AID-BIP1048>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 171.Wilkinson TS, Potter-Perigo S, Tsoi C, Altman LC, Wight TN. Pro- and anti-inflammatory factors cooperate to control hyaluronan synthesis in lung fibroblasts. Am J Respir Cell Mol Biol. 2004;31(1):92–9. doi: 10.1165/rcmb.2003-0380OC. [DOI] [PubMed] [Google Scholar]
- 172.Mohamadzadeh M, DeGrendele H, Arizpe H, Estess P, Siegelman M. Proinflammatory stimuli regulate endothelial hyaluronan expression and CD44/HA-dependent primary adhesion. J Clin Invest. 1998;101(1):97–108. doi: 10.1172/JCI1604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Lauer ME, Erzurum SC, Mukhopadhyay D, Vasanji A, Drazba J, Wang A, Fulop C, Hascall VC. Differentiated Murine Airway Epithelial Cells Synthesize a Leukocyte-adhesive Hyaluronan Matrix in Response to Endoplasmic Reticulum Stress. J Biol Chem. 2008:26283–96. doi: 10.1074/jbc.M803350200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Bjermer L, Lundgren R, Hallgren R. Hyaluronan and type III procollagen peptide concentrations in bronchoalveolar lavage fluid in idiopathic pulmonary fibrosis. Thorax. 1989;44(2):126–31. doi: 10.1136/thx.44.2.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Koistinen V, Härkönen K, Kärnä R, Arasu UT, Oikari S, Rilla K. EMT induced by EGF and wounding activates hyaluronan synthesis machinery and EV shedding in rat primary mesothelial cells. Matrix Biology. 2017;63:38–54. doi: 10.1016/j.matbio.2016.12.007. [DOI] [PubMed] [Google Scholar]
- 176.Mubarak KK, Montes-Worboys A, Regev D, Nasreen N, Mohammed KA, Faruqi I, Hensel E, Baz MA, Akindipe OA, Fernandez-Bussy S, Nathan SD, Antony VB. Parenchymal trafficking of pleural mesothelial cells in idiopathic pulmonary fibrosis. Eur Respir J. 2012;39(1):133–40. doi: 10.1183/09031936.00141010. [DOI] [PubMed] [Google Scholar]
- 177.Zolak JS, Jagirdar R, Surolia R, Karki S, Oliva O, Hock T, Guroji P, Ding Q, Liu RM, Bolisetty S, Agarwal A, Thannickal VJ, Antony VB. Pleural mesothelial cell differentiation and invasion in fibrogenic lung injury. Am J Pathol. 2013;182(4):1239–47. doi: 10.1016/j.ajpath.2012.12.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Camenisch TD, Spicer AP, Brehm-Gibson T, Biesterfeldt J, Augustine ML, Calabro A, Kubalak S, Klewer SE, McDonald JA. Disruption of hyaluronan synthase-2 abrogates normal cardiac morphogenesis and hyaluronan-mediated transformation of epithelium to mesenchyme. J Clin Invest. 2000:349–60. doi: 10.1172/JCI10272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Li Y, Liang J, Yang T, Monterrosa Mena J, Huan C, Xie T, Kurkciyan A, Liu N, Jiang D, Noble PW. Hyaluronan synthase 2 regulates fibroblast senescence in pulmonary fibrosis. Matrix Biology. 2016;55:35–48. doi: 10.1016/j.matbio.2016.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Teder P, Heldin P. Mechanism of impaired local hyaluronan turnover in bleomycin-induced lung injury in rat. Am J Respir Cell Mol Biol. 1997;17(3):376–85. doi: 10.1165/ajrcmb.17.3.2698. [DOI] [PubMed] [Google Scholar]
- 181.Fitzgerald KA, O'Neill LA. Characterization of CD44 induction by IL-1: a critical role for Egr-1. Journal of immunology (Baltimore, Md : 1950) 1999;162(8):4920–7. [PubMed] [Google Scholar]
- 182.Gee K, Lim W, Ma W, Nandan D, Diaz-Mitoma F, Kozlowski M, Kumar A. Differential regulation of CD44 expression by lipopolysaccharide (LPS) and TNF-alpha in human monocytic cells: distinct involvement of c-Jun N-terminal kinase in LPS-induced CD44 expression. Journal of immunology (Baltimore, Md : 1950) 2002;169(10):5660–72. doi: 10.4049/jimmunol.169.10.5660. [DOI] [PubMed] [Google Scholar]
- 183.Foster LC, Arkonac BM, Sibinga NE, Shi C, Perrella MA, Haber E. Regulation of CD44 gene expression by the proinflammatory cytokine interleukin-1beta in vascular smooth muscle cells. J Biol Chem. 1998;273(32):20341–6. doi: 10.1074/jbc.273.32.20341. [DOI] [PubMed] [Google Scholar]
- 184.Svee K, White J, Vaillant P, Jessurun J, Roongta U, Krumwiede M, Johnson D, Henke C. Acute lung injury fibroblast migration and invasion of a fibrin matrix is mediated by CD44. J Clin Invest. 1996;98(8):1713–27. doi: 10.1172/JCI118970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Abdul Roda M, Fernstrand AM, Redegeld FA, Blalock JE, Gaggar A, Folkerts G. The matrikine PGP as a potential biomarker in COPD. Am J Physiol Lung Cell Mol Physiol. 2015;308(11):L1095–101. doi: 10.1152/ajplung.00040.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Akthar S, Patel DF, Beale RC, Peiro T, Xu X, Gaggar A, Jackson PL, Blalock JE, Lloyd CM, Snelgrove RJ. Matrikines are key regulators in modulating the amplitude of lung inflammation in acute pulmonary infection. Nature communications. 2015;6:8423. doi: 10.1038/ncomms9423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.O'Reilly PJ, Ding Q, Akthar S, Cai G, Genschmer KR, Patel DF, Jackson PL, Viera L, Roda M, Locy ML, Bernstein EA, Lloyd CM, Bernstein KE, Snelgrove RJ, Blalock JE. Angiotensin-converting enzyme defines matrikine-regulated inflammation and fibrosis. JCI insight. 2017;2(22) doi: 10.1172/jci.insight.91923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Blaauboer ME, Boeijen FR, Emson CL, Turner SM, Zandieh-Doulabi B, Hanemaaijer R, Smit TH, Stoop R, Everts V. Extracellular matrix proteins: A positive feedback loop in lung fibrosis? Matrix Biology. 2014;34:170–178. doi: 10.1016/j.matbio.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 189.Haak AJ, Tan Q, Tschumperlin DJ. Matrix biomechanics and dynamics in pulmonary fibrosis. Matrix biology : journal of the International Society for Matrix Biology. 2017 doi: 10.1016/j.matbio.2017.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Tschumperlin DJ, Ligresti G, Hilscher MB, Shah VH. Mechanosensing and fibrosis. J Clin Invest. 2018;128(1):74–84. doi: 10.1172/JCI93561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Booth AJ, Hadley R, Cornett AM, Dreffs AA, Matthes SA, Tsui JL, Weiss K, Horowitz JC, Fiore VF, Barker TH, Moore BB, Martinez FJ, Niklason LE, White ES. Acellular normal and fibrotic human lung matrices as a culture system for in vitro investigation. Am J Respir Crit Care Med. 2012;186(9):866–76. doi: 10.1164/rccm.201204-0754OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Huang X, Yang N, Fiore VF, Barker TH, Sun Y, Morris SW, Ding Q, Thannickal VJ, Zhou Y. Matrix stiffness-induced myofibroblast differentiation is mediated by intrinsic mechanotransduction. Am J Respir Cell Mol Biol. 2012;47(3):340–8. doi: 10.1165/rcmb.2012-0050OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Rahaman SO, Grove LM, Paruchuri S, Southern BD, Abraham S, Niese KA, Scheraga RG, Ghosh S, Thodeti CK, Zhang DX, Moran MM, Schilling WP, Tschumperlin DJ, Olman MA. TRPV4 mediates myofibroblast differentiation and pulmonary fibrosis in mice. J Clin Invest. 2014;124(12):5225–38. doi: 10.1172/JCI75331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Chen H, Qu J, Huang X, Kurundkar A, Zhu L, Yang N, Venado A, Ding Q, Liu G, Antony VB, Thannickal VJ, Zhou Y. Mechanosensing by the alpha6-integrin confers an invasive fibroblast phenotype and mediates lung fibrosis. Nature communications. 2016;7:12564. doi: 10.1038/ncomms12564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Southern BD, Grove LM, Rahaman SO, Abraham S, Scheraga RG, Niese KA, Sun H, Herzog EL, Liu F, Tschumperlin DJ, Egelhoff TT, Rosenfeld SS, Olman MA. Matrix-driven Myosin II Mediates the Pro-fibrotic Fibroblast Phenotype. J Biol Chem. 2016;291(12):6083–95. doi: 10.1074/jbc.M115.712380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Liu F, Lagares D, Choi KM, Stopfer L, Marinkovic A, Vrbanac V, Probst CK, Hiemer SE, Sisson TH, Horowitz JC, Rosas IO, Fredenburgh LE, Feghali-Bostwick C, Varelas X, Tager AM, Tschumperlin DJ. Mechanosignaling through YAP and TAZ drives fibroblast activation and fibrosis. Am J Physiol Lung Cell Mol Physiol. 2015;308(4):L344–57. doi: 10.1152/ajplung.00300.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Zhou Y, Huang X, Hecker L, Kurundkar D, Kurundkar A, Liu H, Jin TH, Desai L, Bernard K, Thannickal VJ. Inhibition of mechanosensitive signaling in myofibroblasts ameliorates experimental pulmonary fibrosis. J Clin Invest. 2013;123(3):1096–108. doi: 10.1172/JCI66700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Marinkovic A, Liu F, Tschumperlin DJ. Matrices of physiologic stiffness potently inactivate idiopathic pulmonary fibrosis fibroblasts. Am J Respir Cell Mol Biol. 2013;48(4):422–30. doi: 10.1165/rcmb.2012-0335OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Eisenberg JL, Safi A, Wei X, Espinosa HD, Budinger GS, Takawira D, Hopkinson SB, Jones JC. Substrate stiffness regulates extracellular matrix deposition by alveolar epithelial cells. Research and reports in biology. 2011;2011(2):1–12. doi: 10.2147/RRB.S13178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Lange AW, Sridharan A, Xu Y, Stripp BR, Perl AK, Whitsett JA. Hippo/Yap signaling controls epithelial progenitor cell proliferation and differentiation in the embryonic and adult lung. Journal of molecular cell biology. 2015;7(1):35–47. doi: 10.1093/jmcb/mju046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Volckaert T, Yuan T, Chao CM, Bell H, Sitaula A, Szimmtenings L, El Agha E, Chanda D, Majka S, Bellusci S, Thannickal VJ, Fassler R, De Langhe SP. Fgf10-Hippo Epithelial-Mesenchymal Crosstalk Maintains and Recruits Lung Basal Stem Cells. Developmental cell. 2017;43(1):48–59.e5. doi: 10.1016/j.devcel.2017.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Jorgenson AJ, Choi KM, Sicard D, Smith KM, Hiemer SE, Varelas X, Tschumperlin DJ. TAZ activation drives fibroblast spheroid growth, expression of profibrotic paracrine signals, and context-dependent ECM gene expression. Am J Physiol Cell Physiol. 2017;312(3):C277–c285. doi: 10.1152/ajpcell.00205.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]