Abstract
Fluorescence-guided surgery (FGS) can enhance the surgeon’s ability to achieve a complete oncologic resection. There are a number of tumor-specific probes being developed with many pre-clinical mouse models to evaluate their efficacy. The current review discusses the different pre-clinical mouse models in the setting of probe evaluation and highlights the advantages of patient-derived orthotopic (PDOX) mouse models and genetic reporters to develop FGS.
Keywords: Patient derived orthotopic xenografts (PDOX), surgical orthotopic implantation, pre-clinical mouse models, genetic reporters, fluorescence guided surgery (FGS)
Introduction
The primary goal of curative surgical resection of cancer is complete removal of all neoplastic tissue while limiting damage to normal surrounding structures. The completeness of surgical resection affects outcomes and can provide cures in localized cases. To achieve this goal, clear visualization of the lesion is critical. Traditional techniques of palpation and visual inspection with white light are insufficient[1–3]. Intra-operative navigation using fluorescence guidance is a high-contrast real-time method of visualization that enhances the surgeon’s ability to remove all cancerous tissue[4,5].
While there are several FDA approved fluorescent dyes such as fluorescein, methylene blue, and indocyanine green, they rely on an enhanced permeability and retention effect of tumors and are generally not sufficiently specific for oncologic use[6]. Tumor-specific fluorescence is necessary for complete oncologic resection and this can be delivered thorough fluorophore conjugated probes or viral vector delivery and expression of fluorescent proteins.
A number of pre-clinical animal models have been developed to evaluate tumor specific fluorescence. Here, we address the advantages of patient-derived orthotopic (PDOX) mouse models and genetic reporters to develop fluorescence-guided surgery (FGS).
Background
Subcutaneous mouse models
Since the first description of the ability of nude mice to bear human tumors by Rygaard and Poulsen in 1969, this animal model has been used extensively in oncologic studies[7]. Nude mice bearing subcutaneous tumors derived from patients as cell lines are most commonly used due the ease and uniformity of dissociated cell injection as well as the superficial nature of the lesions that facilitates subsequent monitoring of tumor take and growth. However these models are not representative of the growth and behavior characteristic of most human carcinomas and their malignant phenotype is often underestimated.
The subcutaneous tumor microenvironment is not permissive in development of the appropriate tissue structure or conditions to properly recapitulate a true malignant phenotype. Rather than invasive features, subcutaneously grown tumors have an expansile “pushing” growth pattern and rarely show metastases[8]. These tumors rapidly develop hypoxic and necrotic areas due to their failure to establish appropriate vasculature in an ectopic niche[8,9]. A substantial portion of the tumor volume then consists of necrotic cell debris which can lead to issues, especially when evaluating candidate agents for tumor-specific fluorescence. While the subcutaneous tumor models can be used as a quick screen to grossly evaluate binding of candidate agents for FGS, it is not a platform that can assess the agent in a clinically relevant site. It has limitations in reliably predicting in-vivo efficacy due to the differences tumor microvasculature in and angiogenesis between the subcutaneous pocket and the orthotopic location. This model remains popular in use due to the ease of use and many tumor-specific probes and FGS studies have been performed using this model.
Cell line derived xenografts (CDX) vs. patient derived xenografts (PDX)
Human cancer xenografts into nude mice can be created from established cancer cell lines (CDX) or from fresh fragments of tumors obtained directly from patients at the time of surgical resection (PDX). The use of well established cell lines allows researchers to draw from a body of published literature on the characteristics of these cell lines. The ease of maintenance in cell culture and the uniformity in injecting the same number of cells are advantageous in setting up experimental protocols. However the use of a clonal population of cells with genetic drift due to acclimation in tissue culture can result in a model that has limited clinical application[10]. CDX tumors can be used to show a simplified proof-of-principle, but they may not be able to accurately model the clinical setting[11]. In evaluating FGS probes, the use of CDX tumors may overestimate the fluorescence signal that may be obtained in a given model. PDX tumors on the other hand, can retain the architecture and stromal components of the original tumor better. They more accurately represent the complex biochemical and physical interactions between the cancer cells and their microenvironment[12]. This can especially be important in FGS if targeting a stromal component for fluorescence or accurately evaluating the efficacy of probe penetration into a given tumor. A limitation to keep in mind is that stromal components of PDXs become partially infiltrated with murine cells over prolonged passages and early passages of these xenografts are recommended especially for studies focused on tumor microenvironment and stromal interactions.
Experimental Metastasis Models
Methods such as intravenous, splenic, foot-pad, or intra-cardiac injections of cancer cells have been attempted to model the metastatic cascade, but it is generally accepted that mouse models using these approaches are not physiologic[13]. These experimental metastasis models only demonstrate the last few steps of metastasis: the entry of tumor cells into circulation, arrest in capillary beds, extravasation, survival and proliferation in secondary sites[14]. While these injected tumor cells reach many organs, just the presence of a viable tumor cell within an organ will does not guarantee development of a metastasis[15]. With the exception of intra-cardiac injections that can form widespread metastases, these various approaches usually form metastases at limited sites; such as liver for splenic injection or lung for intravenous injections. They usually require the use of sequentially selected metastatic population of a given cell line to improve efficacy, often overestimating the malignant phenotype[16].
Genetically engineered mouse models (GEMM)
Genetically engineered mouse models with or without induction using organotropic carcinogens are also potential models of tumorigenesis, often showing lesions similar to humans; from adenomas to carcinomas. These mouse models are driven by known promoters, often require a long period of latency before developing tumors and the lesions are often at non-physiologic locations; for example, the small bowel in APC-knockout mice used to study colon cancer[17,18]. However, they can be viable models for evaluating tumor-specific fluorescence agents when thoughtfully paired with the appropriate mouse model. For example, KIT K641E+/− transgenic mice with spontaneous development of cecal GISTs were used to evaluate an anti-c-kit antibody tagged to an AlexaFlour488nm dye[19]. The fluorescent probe was able to detect cecal lesions with a PPV of 85 %, NPV of 100 %, with a specificity of 87%, and a sensitivity of 100%. It is important to note that besides the known driver mutation in GEMMs, the tumors formed are usually missing other key mutations and antigens that are often present in human cancers. They are unable to clearly reflect the diverse spectrum of genetic aberrations found in human tumors, which can be a drawback when these mutations are targets for fluorescence[16,20].
Other applications of genetically engineered mouse models in fluorescence guided surgery are the use of mice expressing fluorescent proteins (GFP, RFP, CFP)[21,22]. These transgenic mice constitutively express fluorescent protein and our laboratory has developed nude fluorescent mice by crossing these fluorescent immunocompetent mice with nu/nu mice, allowing implantation of human cancer xenografts[23,24]. Implantation of xenografts in RFP mice created tumors with red fluorescent stroma and when subsequently passaged into GFP, then CFP mice, the infiltration of secondary and tertiary fluorescence could be evaluated[25]. Color-coding of tumors and their stroma enables improved visualization of the tumor microenvironment.
Orthotopic mouse models
Orthotopic implantation of tumors, placement based on the corresponding site from which the original carcinoma grew in the patient, is based on Paget’s principle that tumor growth is favorable when based in “congenial soil”[26]. Compared to the subcutaneous space, the orthotopic location is able to provide the appropriate microenvironment to realistically model tumor invasiveness and study the metastatic cascade. Wang and Sordat were the first to describe the technique of orthotopic implantation in 1982[27]. They injected colon cancer cells into the cecum of nude mice and saw that the CDX tumors displayed primary and secondary features of invasiveness: they infiltrated layers of the colonic wall, formed peritoneal deposits, and disseminated to mesenteric lymphatics. In contrast, melanoma cell lines implanted in the cecum formed localized nodules, but did not show demonstrate a similar invasive behavior. Since then, orthotopic models have been developed in gastric cancer[28,29], pancreatic cancer[30], lung cancer[31], bladder cancer[32], breast cancer[33,34], head and neck cancer[35], sarcomas, and melanomas. While orthotopically implanted tumors can be challenging to follow, often requiring serial ultrasound studies or laparotomies, they are advantageous in that they more accurately mimic the natural history of cancers. Orthotopically implanted CDX from cell lines tagged with fluorescent proteins can be serially followed with non-invasively with whole body fluorescence imaging and this highly correlates with other imaging approaches such as MRI[36–38].
Humanized mouse models
Humanized mice are severely immunodeficient mice engrafted with human hematopoietic cells to recapitulate a functional human immune system. They are based on NOD/SCID mice, specifically mated and selected for mutation in the interleukin-2 (IL-2) receptor γ-chain locus (Il2rg) leading to impaired development of B and T cells as well as NK cells[39]. These mice could be useful in evaluating for any immune reactions to fluorescent probes, particularly in the case of novel antibody-fluorophore conjugates. They would also be valuable for investigation of theranostic probes where the delivery of both diagnostic and therapeutic molecules could possibly require binding, uptake, and intracellular processing.
Other animal models
Aside from the mouse model, other animals have been used for evaluating advancements in FGS. Animals such as rats, guinea pigs, rabbits, dogs or pigs can be used, but have drawbacks in cancer modeling. Most non-murine models of cancer are induced through chemical carcinogenesis or if xenografted, use syngeneic cell lines. However these larger animal models offer researchers advantages over the limited body size of the mouse model which make developing applicable imaging devices and novel surgical techniques difficult. For example, tissue-conserving surgery cannot be as easily modeled in mouse models. Therefore Mieog et al used an orthotopic syngeneic rat model of breast cancer to show that FGS using a cathepsin protease cleavable fluorescent probe could guide complete resection of the tumor while minimizing resection of non-cancerous breast tissue[40]. When developing clinical fluorescence imaging devices, the size of organ and organ spaces must be closer to humans. Porcine models have been used to evaluate fluorescence enabled laparoscopes, thoracoscopes, endoscopes, with non-specific fluorescent dyes like ICG[41,42]. While these larger animal models cannot effectively model human cancers, the development advanced reproductive technologies and ability to introduce genome modifications through approaches such as CRISPR, larger oncologic animal models such as the oncopig will become available[43]. In evaluating tumor-specific fluorescent probes, these larger animal models may be time and resource limiting and their use carefully considered.
Surgical orthotopic implantation (SOI)
Our laboratory has developed the technique of surgical orthotopic implantation of tumor fragments in a number of cancers. Rather than injection of dissociated cells, which may disrupt tumor integrity, implantation of histologically intact tissue blocks using microsurgical techniques is used[44,45]. Creating these models can be time and resource intensive, as microsurgical skills are necessary and the tumor growth can be difficult to follow non-invasively.
Patient Derived Orthotopic Xenografts (PDOX)
Implantation of patient derived xenografts into their orthotopic location is the approach that best recapitulates the tumor microenvironment and the metastatic cascade. The technique of SOI is used to implant histologically intact fragments of patient tissue into a corresponding location of origin. Our laboratory has developed PDOX models from samples obtained at surgery for patients with colon cancer[44], pancreatic cancer[45], gastric cancer[29], breast cancer[46], lung cancer[47], ovarian cancer[48], mesothelioma[49], melanoma[50], and sarcomas[51]. PDOX models can have limitations in that they are usually implanted in nude mice, therefore evaluation of an immune response is limited. In these cases, xenografts could be implanted into humanized mice.
Orthotopic mouse models to demonstrate outcomes of FGS
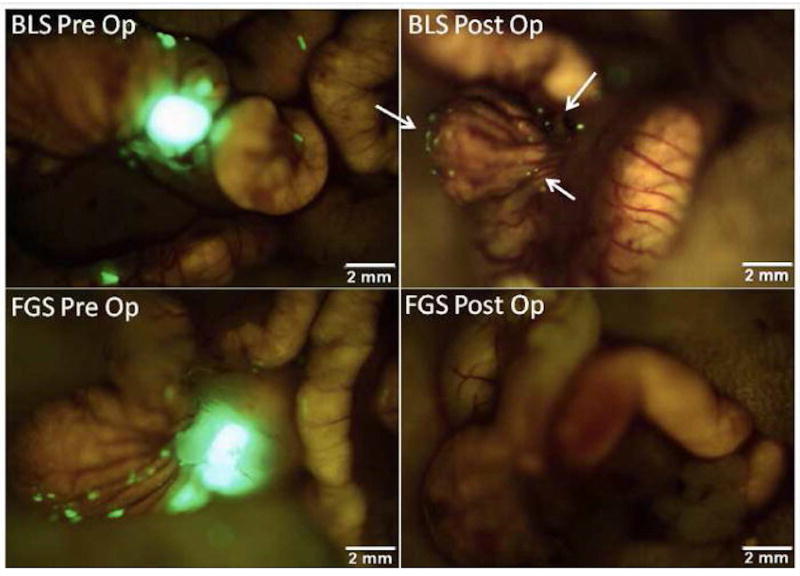
This technique of SOI was used to demonstrate the utility of fluorescence-guidance in improving rates of complete tumor resection and correlated to tumor recurrence and long term out comes. Metildi et al used this in proof-of-concept studies using fluorescently labeled orthotopic tumors[52]. SOI models of human colon cancer were made using HT-29 CDX tagged with a red fluorescent protein (ds-Red) implanted into the cecum of nude mice. The mice were randomized to surgery using BLS or FGS. They showed that visualization with fluorescence allowed a 100% rate of complete R0 resection of the primary colon cancer as well as removal of sub-millimeter metastatic deposits of tumor on the cecum, whereas BLS resulted in an R0 rate in only 58% (Figure 1) (p=0.001). The use of FGS led to decreased recurrence (FGS 33% vs. BLS 62%), lengthened disease-free median survival (BLS 9 weeks vs. FGS >36 weeks) and overall long term survival (BLS 37% vs. FGS 67%). Similar improvements were seen in even more aggressive cancer types such as pancreatic cancer[53]. SOI models were established using a human pancreatic cancer CDX (Bxpc-3) labeled with green fluorescent protein (GFP) was implanted into the pancreatic tail of nude mice and the mice were subjected to either BLS or FGS. Again, the use of FGS led to more complete initial resections (BLS 77.1% vs. FGS 98.9%) and lengthened disease-free interval (BLS 1 week vs. FGS 7 weeks). These experiments successfully established that the use of fluorescence navigation in resection of orthotopic tumors resulted in improvement recurrence rates and extended disease-free survival.
Figure 1.
Pre- and postoperative images under fluorescence-guided surgery (FGS) and bright light surgery (BLS) in an orthotopic mouse model of colon cancer. These are representative pre- and postoperative images of a mouse from the BLS group (top panel) and the FGS group (bottom panel). The enhanced ability to visualize and identify tumor margins under fluorescence-guidance permitted a more complete resection. All mice in the FGS group underwent an R0 resection while only 58% of mice in the BLS group had no evidence of residual fluorescent tumor on postoperative images (arrows in right upper panel) (p=0.001).[52]
Advantages of fluorescent genetic reporters for FGS
While these proof-of-concept studies showed the efficacy of FGS on survival and recurrence, the approach is not clinically applicable as patient tumors do not express fluorescent proteins. Delivery of fluorescent genetic reporters is a potential approach.
Hasegawa et al first showed that intra-peritoneal (IP) administration of viruses containing the GFP transgene could label disseminated cancer in-vivo[54]. Using a retroviral gene transfer approach allowed stable genetic integration of the transgene to rapidly dividing cells such as cancer cells, the fluorescent reporter could be made to selectively express itself in tumors. Viral supernatant of pLEIN retrovirus expressing GFP were injected IP for 7 days into nude mice with peritoneal carcinomatosis using the human gastric cancer cell line NUGC-4. A fluorescent signal could be visualized over tumor deposits on laparotomy 7 days after last viral injection. The fluorescent protein expressed in new peritoneal deposits that developed even after cessation of viral injections and the signal was detectable up to 7 weeks after the last injection.
Fujiwara et al further refined tumor-specificity of fluorescence by engineering a selectively-replicative oncolytic adenovirus virus carrying a GFP transgene[55]. The vector derives its tumor specificity due to selective replication only in cells over-expressing telomerase, which is seen in greater than 85% of cancers[56]. It expresses the E1A and E1B genes under control of the hTERT promoter and GFP under control of the CMV promoter in the E3 region. Fujiwara’s group showed in a number of human cancer cell lines, with or without combination chemotherapy, that the virus OBP-401, had in vitro GFP expression 4 days after infection and cytoxicity 5 days after infection at a low multiplicity of infection (MOI = 0.1–1). OBP-401 also showed efficacy in-vivo after direct intra-tumoral injection into subcutaneous implants of human colon CDX using H1299 cells.
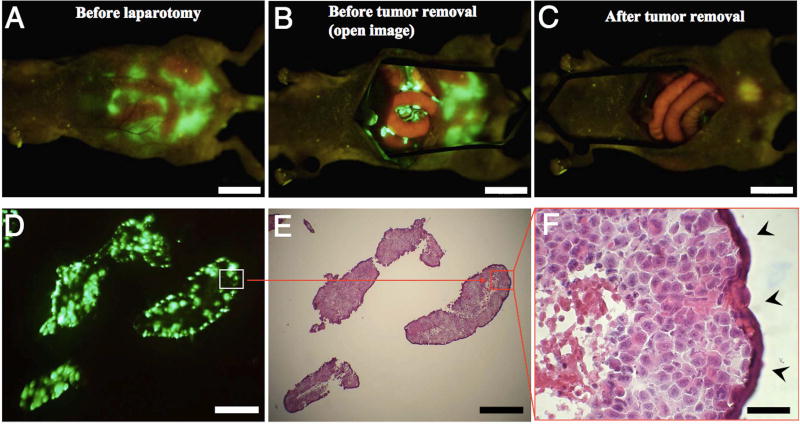
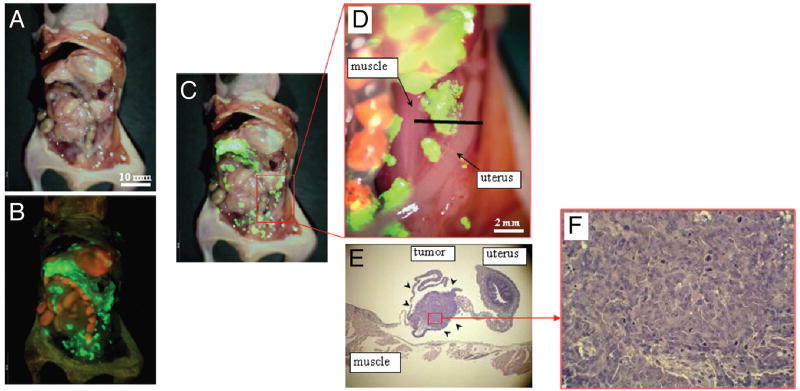
Kishimoto et al first used OBP-401 for in-vivo fluorescence imaging using an orthotopic CDX model of colon cancer using the HT-29 cell line[57]. OBP-401 was injected directly into the orthotopic rectal tumors at 1 × 108 PFU and a fluorescent signal from GFP could be visualized non-invasively at the primary tumor as early as 24 hours after injection. 5 days after intratumoral injections with OBP-401, the mice were subjected laparotomy and fluorescent GFP signals could be detected over para-aortic lymph nodes which were confirmed to be metastases on histology. OBP-401 was then used for FGS in nude mice with peritoneal carcinomatosis using the human colon cancer cell line HCT-116 (Figure 2)[58]. 5 days after IP injection of OBP-401 at 1 × 108 PFU, the peritoneal deposits had a strong GFP signal, allowing for efficacious fluorescence-guided cytoreductive surgery. In addition to efficacy in FGS, Kishimoto et al also emphasized the long term stability of GFP expression in cancer cells that were infected in-vivo and the utility of OBP-401 in detection of tumor recurrence after curative intent surgery[59]. Peritoneal lavage was performed on mice with peritoneal carcinomatosis 5 days after treatment with OBP-401 and cell cultured from the lavage showed GFP expression 8 days after collection and 13 days after viral treatment. Mice with peritoneal carcinomatosis using the human colon cancer cell line HCT-116 underwent curative intent cytoreductive surgery under fluorescence guidance after treatment with OBP-401- at the time of surgery and all fluorescent lesions were removed. However tumors still recurred several weeks after attempted complete resection, and interestingly, these tumors due to proliferating microscopic residual disease continued to express GFP (Figure 3).
Figure 2.
Fluorescence-guided surgical removal of peritoneal disseminated HCT-116 tumors after GFP labeling with OBP-401. Noncolored HCT-116 human colon cancer cells were injected into the abdominal space of nude mice. Ten days later, 1 × 108 PFU of OBP-401 were i.p. injected. (A) Disseminated nodules were efficiently labeled and noninvasively visualized by GFP expression 5 days after virus administration. (B) Under general anesthesia, laparotomy was performed to remove intra-abdominal disease under GFP-guided navigation. (C) Disseminated nodules visualized by GFP-guided navigation were removed. (Scale bars: A–C, 10 mm.) (D) Frozen section of resected HCT-116 disseminated nodules with fluorescence detection. (Scale bar, 500 µm.) (E) H&E section of HCT-116 disseminated nodules shown in D. The box outlines a region of D and E analyzed in F. (Scale bar, 500 µm.) (F) Detail of the boxed region of D and E. (Scale bar, 50 µm.)[58]
Figure 3.
In vivo detection of recurrent tumors after fluorescence-guided surgery. (A) Brightfield observation several weeks after fluorescence-guided surgery of OBP-401 GFP-labeled tumors. Disseminated disease re-emerged. (B) Fluorescence observation of field observed by brightfield in (A). (C) Merge of (A and B). The red box outlines a region of (D) below. (D) Detail of the boxed region of (C). Black line indicates the direction of cross-sections. (E) Histologic sections stained with H&E showing that GFP-labeled lesions are recurrent tumor tissues (arrow heads). ×40 magnification. (F) Detail of the boxed region of (E). ×200 magnification.[59]
This virus has since then been used for FGS of orthotopically implanted CDX’s of glioblastoma[60], soft tissue sarcoma[61], osteosarcoma[62], breast cancer[63,64], lung cancer[65], and melanoma[66]. The approach of adenovirus mediated genetic labeling using a fluorescent protein allowed detection of tumor recurrence and subsequent metastases in SQ and orthotopic tumors.
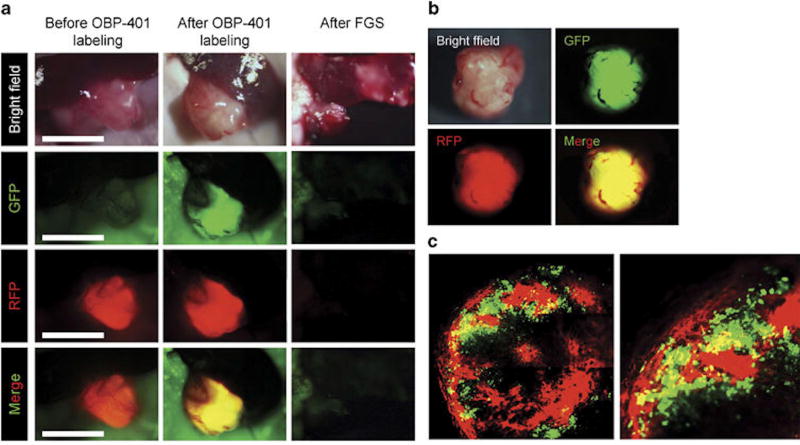
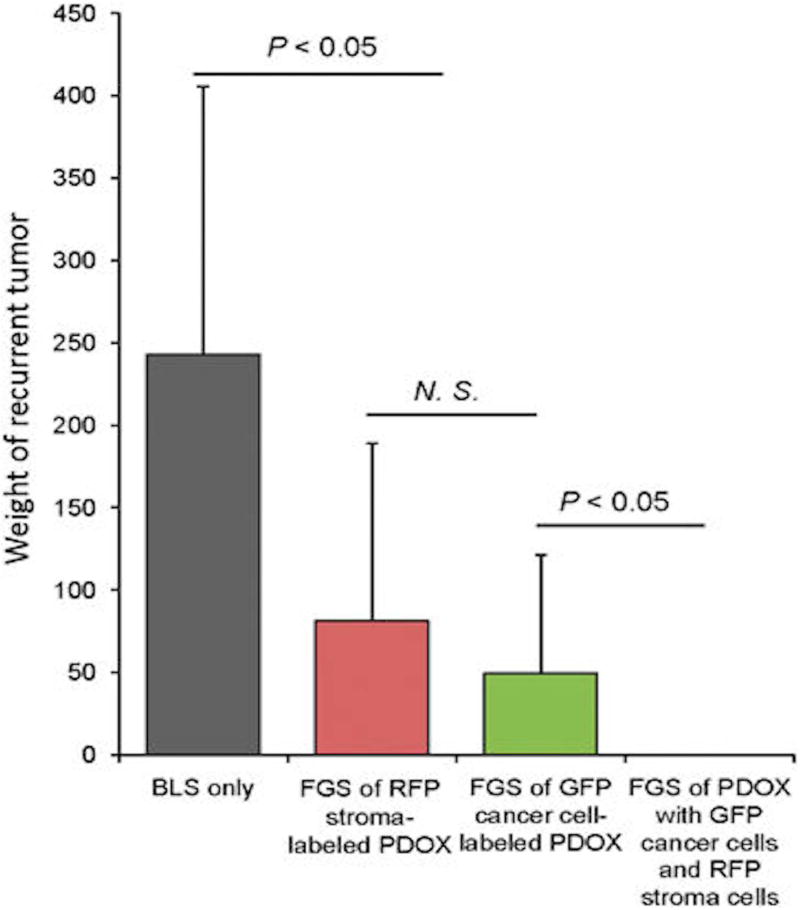
The efficacy of color-coded FGS for in-situ fluorescent genetic labeling in the PDOX platform has also been demonstrated in fluorescent transgenic nude mice. Patient-derived xenografts were implanted into RFP nude mice and the stroma acquired RFP. These tumors were then orthotopically implanted into non-colored nude mice and the tumors were then labeled in-situ with GFP using OBP-401. This approach allowed dual colored FGS, visualizing both the tumor and the stroma. Yano et al was able to use this technique to improve the efficacy of FGS and showed that using both colors significantly prevented local recurrence, which bright-light surgery or single-color FGS could not (Figure 4 and Figure 5)[67].
Figure 4.
FGS of PDOX with GFP-labeled cancer cells and RFP-labeled stroma. (a) Representative intravital images of a PDOX with RFP stroma before labeling with OBP-401 (left panels); after 0BP-401-GFP labeling (middle panels); and after FGS (right panels). Images were acquired with the OV100 before FGS and after FGS. (b) Representative images of resected dual-color tumor after FGS. Images were acquired with the OV100. (c) Representative images of a cross-section of the resected dual-color tumor. Images were acquired with the FV1000 confocal laser imaging system. FGS, fluorescence-guided surgery; GFP, green fluorescent protein; PDOX, patient-derived orthotopic xenograft; RFP, red fluorescent protein.[67]
Figure 5.
Comparison of the extent of tumor recurrence after different modalities of surgery. Tumors were resected by either BLS or FGS with tumors labeled either with RFP stroma only; GFP cancer cells only; or with both RFP-labeled stroma and GFP-labeled cancer cells. Please see Materials and Methods for details. BLS, bright-light surgery; FGS, fluorescence-guided surgery; GFP, green fluorescent protein; NS, not significant; PDOX, patient-derived orthotopic xenograft; RFP, red fluorescent protein.[67]
Phase I trials evaluating OBP-301, the base vector without GFP expression were conducted with intra-tumoral injections in a variety of GI malignancies. The study showed safety at the three evaluated dosages and efficacy in viral replication as well as the ability to induce disease stability in 7/12 patients at 56 days post-treatment[68]. OBP-401 has been modified to increase tumor-specificity[69]. It is manufactured as TelomeScan® by Oncolys Bio Pharma and is being evaluated as an agent to improve detection of circulating tumor cells ex-vivo.
The approach of viral genetic labeling has also been used by Wong et al with NV1066, a tumor-specific, replication-competent, herpes virus carrying an eGFP transgene under the control of a CMV promoter[70]. This virus has been shown to effectively deliver fluorescence and cause oncolysis in subcutaneous and intraperitoneal CDX models of esophageal cancer, gastric cancer, colon cancer, pancreatic cancer, peritoneal mesothelioma, orthotopic CDX model of lung cancer, gastric cancer, as well as lymph node metastases [71,72]. The group has shown efficacy of in-situ fluorescence imaging with NV1066 using a 3mm modified Olympus laparoscope[71,73]. NV1066 has also been used to detect circulating tumor cells and peritoneal washings ex-vivo with a sensitivity of detecting 1 cancer cell within in 1 million normal cells[74,75].
Advantages of fluorescent antibodies for FGS
These SOI models of CDX and PDX described above have been used to evaluate fluorophore labeled antibodies in nude mice. Subcutaneous, orthotopic primary, metastatic and peritoneal dissemination models of pancreatic and colon tumors derived from both cell lines and patients were able to be visualized after intravenous administration of anti-CEA antibody conjugated to fluorophores[76–81]. A fluorescent anti-CA19-9 was also able to visualize primary and metastatic pancreatic cell-line based and patient-derived tumors[82–84]. Fluorescent antibody labeling led to a detectable signal as early as 30 minutes after intravenous injection and remained for as long as 2 weeks, with optimal signal obtained around 24–48 hours. Fluorescent anti-EGFR antibodies have been studied in orthotopic models of head and neck cancers. The squamous cancer cell lines (SCC1, CAL27, FaDu, or OSC-19) were injected into the base of the mouth or the tongue of nude mice to create the orthotopic model. Chimeric anti-EGFR antibodies initially conjugated with Cy5.5 were studied[85]. This has since then advanced into studies of fully human anti-EGFR antibody conjugated with the NIR fluorophore IRDye800[86]. The results of the studies in orthotopic mouse models have been translated into a number of clinical trials using these probes for FGS in in GI cancers and head and neck cancers (NCT02784028, NCT02973672, NCT01987375, NCT02736578, NCT03384238) [87,88]. These approaches require imaging past several days required repeat administration of the agent for a strong fluorescence signal.
Compared to genetic labeling with viral vector which retains a persistent signal, this approach is not able to detect tumor recurrences or future metastases. An advantage is that a number of fluorophore and antibody combinations could potentially be utilized, including near-infrared wavelength dyes that can penetrate at increased tissue depth. Fluorescent genetic labeling is limited to the wavelengths of fluorescent protein cassettes that are in the visible wavelengths. However the visible wavelengths are detectable by the human eye and do not need a dedicated detector to capture the fluorescence signal which can be an issue with near-infrared dyes.
Other approaches to tumor-specific fluorophore delivery
Delivery of tumor-specific fluorescence is not limited to viral reporters and antibody conjugates. While it is beyond the scope of this review on pre-clinical mouse models to discuss the available probe platforms, a summary is included with recommended references in table 2. These probes have been evaluated for in-vivo fluorescence in both subcutaneous and orthotopic nude mouse xenograft models, with some are progressing into clinical trials.
Table 2.
Approaches to delivery of in-situ tumor-specific fluorescence for FGS
| Technique | Probe | Mechanism & clinical application | Clinical Trial | References |
|---|---|---|---|---|
|
| ||||
| Activatable probes | γ-glutamyl hydroxymethyl rhodamine green (gGlu-HMRG) | Fluorophore quenched by spirocyclic caging is released after proteolysis by γ –glutamyltransferase. Evaluated clinically for fluorescent in ex-vivo specimen of hepatobiliary and pancreatic cancers. | UMIN000003655 | [91,93] |
|
| ||||
| Ratiometric activatable cell penetrating peptides (RACPP’s) | Cleavable linker undergoes proteolysis by matrix metalloprotease followed by polycationic peptide linked to fluorophore uptake into cells. Evaluated clinically for FGS in breast cancers. | NCT02391194, NCT03113825 | [89,94,95] | |
|
| ||||
| Small molecule/Peptide binding | Folate-FITC | Folate conjugated with fluorescein isothiocyanate (FITC) binds to folate receptor overexpressing cells. Evaluated clinically for FGS in ovarian cancers. | EudraCT 2009-010559-29 | [96,97] |
|
| ||||
| Cyclic arginine-glycine-aspartic acid (cRGD) peptides | Peptide binds to integrins overexpressed in a number of malignancies. Evaluated clinically in immunoimaging of GI, lung, and gyn malignancies. | NCT03384511* | [98] | |
|
| ||||
| Cystiene knot peptide | Peptide binds to integrin overexpressing tumors. Evaluated clinically in immunoimaging of pancreatic cancers. | NCT02683824* | [99] | |
|
| ||||
| Urokinase-plasminogen activator (uPA) | Fragment of uPA binds to uPA receptor overexpressed in malignancies. Evaluated clinically in immunoimaging of brain, head and neck, neuroendocrine, breast, prostate, bladder, and lung cancers. | NCT02945826* | [100] | |
| NCT02965001* | ||||
| NCT03278275* | ||||
| NCT02681640* | ||||
| NCT02964988* | ||||
| NCT02805608* | ||||
| NCT02755675* | ||||
|
| ||||
| Fluorescent antibodies | Epidermal growth factor receptor (EGFR) antibodies Cetuximab, Panitumamab | Antibodies target EGFR overexpressing tumors. Evaluated clinically for FGS in head and neck, brain and pancreatic cancers. | NCT01987375 | [86,88] |
| NCT03405142 | ||||
| NCT03384238 | ||||
| NCT01987375 | ||||
| NCT02855086 | ||||
| NCT02415881 | ||||
| NCT03510208 | ||||
| NCT02736578 | ||||
|
| ||||
| Vascular endothelial growth factor receptor (VEGFR) antibodies Bevacizumab | Antibodies target VEGFR overexpressing tumors. Evaluated clinically for FGS in esophageal, breast, and pancreatic cancer. | NTR4632, NCT02743975 | [101–103] | |
| NCT02583568 | ||||
| NCT01508572 | ||||
| NCT03205501 | ||||
|
| ||||
| Carcino-embryonic antigen (CEA) antibodies | Antibodies target CEA overexpressing tumors. Evaluated clinically for FGS in colon and pancreatic cancers. | NCT02784028, NCT02973672 | [81] | |
|
| ||||
| Sialyl-Lewis antigen A also known as cancer antigen 19-1 (CA19-9) antibodies | Antibodies target CA19-9 overexpressing tumors. Evaluated clinically for immunoimaging in pancreatic cancers and other CA19-9 expressing tumors. | NCT02687230 | [82] | |
|
| ||||
| Cell surface associated mucin 1 (MUC1) antibodies | Antibodies target MUC1 overexpressing tumors. Not yet evaluated for clinical FGS. | [104,105,106] | ||
|
| ||||
| Viral reporters | OBP-401 | Selectively-replicative oncolytic adenovirus virus carrying a GFP transgene under the control of CMV promoter. Not yet evaluated for clinical FGS. | ||
|
| ||||
| NV1066 | Selectively-replicative herpes virus carrying an eGFP transgene under the control of a CMV promoter. Evaluated clinically for fluorescent detection of pancreatic cancer in peritoneal cytology. | [73–75,107] | ||
Activatable probes such as γ-glutamyl hydroxymethyl rhodamine green (gGlu-HMRG) or Ratiometric activatable cell penetrating peptides (RACPP’s) carry quenched fluorophores that are released upon encountering tumor-specific enzymes. gGlu-HMRG has been evaluated in ex-vivo patient samples (UMIN000003655) while RACPP’s have been translated from animal studies to a phase I breast cancer clinical trial(NCT02391194, NCT03113825)[89–91].
Small molecules or peptides conjugated to fluorophores can be designed to target specific pockets or motifs that are overexpressed in specific malignancies. EGFR is over expressed in head and neck cancers and Keereweer et al used a recombinant epidermal growth factor molecule conjugated to IRDye800 to target these receptors in an orthotopic oral cancer model[92]. The OSC-19-luc CDX was used and when implanted in the orthotopic location, capable of lymph node metastases. The CW800-EGF probe was able to highlight not only the primary tumor on the tongue but also the draining lymph nodes, confirmed by histology. Folate, cyclic-arginine-glycine-aspartic acid (cRGD), cysteine knots, and urokinase-plasminogen activator (uPA) are additional examples of fluorescent peptide probes. These probes have been combined with radio-tracers for immunoimaging clinical trials, but need further optimization for dosing and pharmacokinetics before progressing into clinical trials. Small probes such as fragmented antibodies, nanobodies, alpha bodies, centyrins, and aptamers are also being investigated in mouse models for in-situ tumor-specific fluorescence signal delivery.
Conclusion
Fluorescence-guided surgery (FGS) enhances the surgeon’s ability to achieve a complete oncologic resection. A number of modalities to deliver tumor-specific fluorescence including genetic reporters and fluorescent antibodies are currently being evaluated in pre-clinical mouse models. PDOX mouse models are most cost-effective and efficient avenue for evaluating FGS in a realistic platform that mimics the patient’s tumor microenvironment and metastatic cascade.
Table 1.
Murine mouse models
| Advantages | Disadvantages | |
|---|---|---|
| Subcutaneous models |
|
|
| Orthotopic models |
|
|
| Experimental Metastasis models |
|
|
| Genetically engineered models |
|
|
Synopsis.
A number of pre-clinical mouse models are available to evaluate tumor specific fluorescence. The current review addresses the advantages of patient-derived orthotopic (PDOX) mouse models and genetic reporters to develop fluorescence-guided surgery (FGS).
Acknowledgments
Sources of Support: US National Cancer Institute Grant numbers: CA126023, CA142669 (MB); NIH/NCI T32CA121938 (TL).
Footnotes
Disclosures: The authors do not have any disclosures.
References
- 1.Verbeke CS, Menon KV. Redefining resection margin status in pancreatic cancer. HPB. 2009;11:282–289. doi: 10.1111/j.1477-2574.2009.00055.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ethun CG, Kooby DA. The importance of surgical margins in pancreatic cancer. J. Surg. Oncol. 2016;113:283–288. doi: 10.1002/jso.24092. [DOI] [PubMed] [Google Scholar]
- 3.Pawlik TM, Scoggins CR, Zorzi D, et al. Effect of Surgical Margin Status on Survival and Site of Recurrence After Hepatic Resection for Colorectal Metastases. Ann. Surg. 2005;241:715–724. doi: 10.1097/01.sla.0000160703.75808.7d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rosenthal EL, Warram JM, de Boer E, et al. Successful Translation of Fluorescence Navigation During Oncologic Surgery: A Consensus Report. J. Nucl. Med. Off. Publ. Soc. Nucl. Med. 2016;57:144–150. doi: 10.2967/jnumed.115.158915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.de Boer E, Harlaar NJ, Taruttis A, et al. Optical innovations in surgery. Br. J. Surg. 2015;102:e56–72. doi: 10.1002/bjs.9713. [DOI] [PubMed] [Google Scholar]
- 6.DeLong JC, Hoffman RM, Bouvet M. Current status and future perspectives of fluorescence-guided surgery for cancer. Expert Rev. Anticancer Ther. 2016;16:71–81. doi: 10.1586/14737140.2016.1121109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rygaard J, Poulsen CO. Heterotransplantation of a Human Malignant Tumour to “Nude” Mice. Acta Pathol. Microbiol. Scand. 1969;77:758–760. doi: 10.1111/j.1699-0463.1969.tb04520.x. [DOI] [PubMed] [Google Scholar]
- 8.Sordat BCM. Patient-Deriv. Mouse Models Cancer [Internet] Humana Press; Cham: 2017. From Ectopic to Orthotopic Tumor Grafting Sites: Evidence for a Critical Role of the Host Tissue Microenvironment for the Actual Expression of the Malignant Phenotype; pp. 43–53. [cited 2018 Feb 14]. Available from: https://link.springer.com/chapter/10.1007/978-3-319-57424-0_4. [Google Scholar]
- 9.Hoffman RM. Patient-derived orthotopic xenografts: better mimic of metastasis than subcutaneous xenografts. Nat. Rev. Cancer. 2015;15:451–452. doi: 10.1038/nrc3972. [DOI] [PubMed] [Google Scholar]
- 10.Rosfjord E, Lucas J, Li G, et al. Advances in patient-derived tumor xenografts: from target identification to predicting clinical response rates in oncology. Biochem. Pharmacol. 2014;91:135–143. doi: 10.1016/j.bcp.2014.06.008. [DOI] [PubMed] [Google Scholar]
- 11.Garber K. From Human to Mouse and Back: “Tumorgraft” Models Surge in Popularity. JNCI J. Natl. Cancer Inst. 2009;101:6–8. doi: 10.1093/jnci/djn481. [DOI] [PubMed] [Google Scholar]
- 12.Tentler JJ, Tan AC, Weekes CD, et al. Patient-derived tumour xenografts as models for oncology drug development. Nat. Rev. Clin. Oncol. 2012;9:338–350. doi: 10.1038/nrclinonc.2012.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Manzotti C, Audisio RA, Pratesi G. Importance of orthotopic implantation for human tumors as model systems: relevance to metastasis and invasion. Clin. Exp. Metastasis. 1993;11:5–14. doi: 10.1007/BF00880061. [DOI] [PubMed] [Google Scholar]
- 14.Watson SA, Kumari R. Metastasis Res. Protoc. [Internet] Humana Press; New York, NY: 2014. Theoretical Considerations in Using Animal Models of Metastasis and Brief Methodology for In Vivo Colorectal Cancer Models in SCID and Nude Mice; pp. 117–129. [cited 2018 Feb 14]. Available from: https://link.springer.com/protocol/10.1007/978-1-4614-8244-4_9. [DOI] [PubMed] [Google Scholar]
- 15.Fidler IJ. Metastasis: Quantitative Analysis of Distribution and Fate of Tumor Emboli Labeled With 125I-5-Iodo-2′-deoxyuridine. JNCI J. Natl. Cancer Inst. 1970;45:773–782. [PubMed] [Google Scholar]
- 16.Kobaek-Larsen M, Thorup I, Diederichsen A, et al. Review of colorectal cancer and its metastases in rodent models: comparative aspects with those in humans. Comp. Med. 2000;50:16–26. [PubMed] [Google Scholar]
- 17.Tong Y, Yang W, Koeffler HP. Mouse models of colorectal cancer. Chin. J. Cancer. 2011;30:450–462. doi: 10.5732/cjc.011.10041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Talmadge JE, Singh RK, Fidler IJ, et al. Murine Models to Evaluate Novel and Conventional Therapeutic Strategies for Cancer. Am. J. Pathol. 2007;170:793–804. doi: 10.2353/ajpath.2007.060929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Metildi CA, Tang C-M, Kaushal S, et al. In Vivo Fluorescence Imaging of Gastrointestinal Stromal Tumors Using Fluorophore-Conjugated Anti-KIT Antibody. Ann. Surg. Oncol. 2013;20:S693–S700. doi: 10.1245/s10434-013-3172-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Heyer J, Yang K, Lipkin M, et al. Mouse models for colorectal cancer. Oncogene. 1999;18:5325–5333. doi: 10.1038/sj.onc.1203036. [DOI] [PubMed] [Google Scholar]
- 21.Okabe M, Ikawa M, Kominami K, et al. “Green mice” as a source of ubiquitous green cells. FEBS Lett. 1997;407:313–319. doi: 10.1016/s0014-5793(97)00313-x. [DOI] [PubMed] [Google Scholar]
- 22.Hadjantonakis A-K, Macmaster S, Nagy A. Embryonic stem cells and mice expressing different GFP variants for multiple non-invasive reporter usage within a single animal. BMC Biotechnol. 2002;2:11. doi: 10.1186/1472-6750-2-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yang M, Reynoso J, Jiang P, et al. Transgenic Nude Mouse with Ubiquitous Green Fluorescent Protein Expression as a Host for Human Tumors. Cancer Res. 2004;64:8651–8656. doi: 10.1158/0008-5472.CAN-04-3118. [DOI] [PubMed] [Google Scholar]
- 24.Yang M, Reynoso J, Bouvet M, et al. A TRANSGENIC RED FLUORESCENT PROTEIN-EXPRESSING NUDE MOUSE FOR COLOR-CODED IMAGING OF THE TUMOR MICROENVIRONMENT. J. Cell. Biochem. 2009;106:279–284. doi: 10.1002/jcb.21999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Suetsugu A, Katz M, Fleming J, et al. MULTI-COLOR PALLET OF FLUORESCENT PROTEINS FOR IMAGING THE TUMOR MICROENVIRONMENT OF ORTHOTOPIC TUMORGRAFT MOUSE MODELS OF CLINICAL PANCREATIC CANCER SPECIMENS. J. Cell. Biochem. 2012;113:2290–2295. doi: 10.1002/jcb.24099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Paget S. THE DISTRIBUTION OF SECONDARY GROWTHS IN CANCER OF THE BREAST. The Lancet. 1889;133:571–573. [PubMed] [Google Scholar]
- 27.Sordat B, Wang WR. Human colorectal tumor xenografts in nude mice: expression of malignancy. Behring Inst. Mitt. 1984:291–300. [PubMed] [Google Scholar]
- 28.Yamashita T. Manifestation of Metastatic Potential in Human Gastric Cancer Implanted into the Stomach Wall of Nude Mice. Jpn. J. Cancer Res. 1988;79:945–951. doi: 10.1111/j.1349-7006.1988.tb00059.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Furukawa T, Fu X, Kubota T, et al. Nude mouse metastatic models of human stomach cancer constructed using orthotopic implantation of histologically intact tissue. Cancer Res. 1993;53:1204–1208. [PubMed] [Google Scholar]
- 30.Vezeridis MP, Doremus CM, Tibbetts LM, et al. Invasion and metastasis following orthotopic transplantation of human pancreatic cancer in the nude mouse. J. Surg. Oncol. 1989;40:261–265. doi: 10.1002/jso.2930400412. [DOI] [PubMed] [Google Scholar]
- 31.McLemore TL, Liu MC, Blacker PC, et al. Novel intrapulmonary model for orthotopic propagation of human lung cancers in athymic nude mice. Cancer Res. 1987;47:5132–5140. [PubMed] [Google Scholar]
- 32.Ahlering TE, Dubeau L, Jones PA. A New in Vivo Model to Study Invasion and Metastasis of Human Bladder Carcinoma. Cancer Res. 1987;47:6660–6665. [PubMed] [Google Scholar]
- 33.Miller FR, McInerney D. Epithelial Component of Host-Tumor Interactions in the Orthotopic Site Preference of a Mouse Mammary Tumor. Cancer Res. 1988;48:3698–3701. [PubMed] [Google Scholar]
- 34.White AC, Levy JA, McGrath CM. Site-selective Growth of a Hormone-responsive Human Breast Carcinoma in Athymic Mice. Cancer Res. 1982;42:906–912. [PubMed] [Google Scholar]
- 35.Dinesman A, Haughey B, Gates GA, et al. Development of a New in Vivo Model for Head and Neck Cancer. Otolaryngol.-Head Neck Surg. 1990;103:766–774. doi: 10.1177/019459989010300517. [DOI] [PubMed] [Google Scholar]
- 36.Bhattacharya A, Turowski SG, San Martin ID, et al. Magnetic resonance and fluorescence-protein imaging of the anti-angiogenic and anti-tumor efficacy of selenium in an orthotopic model of human colon cancer. Anticancer Res. 2011;31:387–393. [PMC free article] [PubMed] [Google Scholar]
- 37.Bouvet M, Spernyak J, Katz MH, et al. High Correlation of Whole-Body Red Fluorescent Protein Imaging and Magnetic Resonance Imaging on an Orthotopic Model of Pancreatic Cancer. Cancer Res. 2005;65:9829–9833. doi: 10.1158/0008-5472.CAN-05-1548. [DOI] [PubMed] [Google Scholar]
- 38.Hoffman RM. The multiple uses of fluorescent proteins to visualize cancer in vivo. Nat. Rev. Cancer. 2005;5:796–806. doi: 10.1038/nrc1717. [DOI] [PubMed] [Google Scholar]
- 39.Ito M, Hiramatsu H, Kobayashi K, et al. NOD/SCID/gamma(c)(null) mouse: an excellent recipient mouse model for engraftment of human cells. Blood. 2002;100:3175–3182. doi: 10.1182/blood-2001-12-0207. [DOI] [PubMed] [Google Scholar]
- 40.Mieog JSD, Hutteman M, van der Vorst JR, et al. Image-guided tumor resection using real-time near-infrared fluorescence in a syngeneic rat model of primary breast cancer. Breast Cancer Res. Treat. 2011;128:679–689. doi: 10.1007/s10549-010-1130-6. [DOI] [PubMed] [Google Scholar]
- 41.Mao Y, Wang K, He K, et al. Development and application of the near-infrared and white-light thoracoscope system for minimally invasive lung cancer surgery. J. Biomed. Opt. 2017;22:66002. doi: 10.1117/1.JBO.22.6.066002. [DOI] [PubMed] [Google Scholar]
- 42.Oh G, Park Y, Yoo SW, et al. Clinically compatible flexible wide-field multi-color fluorescence endoscopy with a porcine colon model. Biomed. Opt. Express. 2017;8:764–775. doi: 10.1364/BOE.8.000764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schachtschneider KM, Schwind RM, Newson J, et al. The Oncopig Cancer Model: An Innovative Large Animal Translational Oncology Platform. Front. Oncol. [Internet] 2017:7. doi: 10.3389/fonc.2017.00190. [cited 2018 May 13]. Available from: http://journal.frontiersin.org/article/10.3389/fonc.2017.00190/full. [DOI] [PMC free article] [PubMed]
- 44.Fu XY, Besterman JM, Monosov A, et al. Models of human metastatic colon cancer in nude mice orthotopically constructed by using histologically intact patient specimens. Proc. Natl. Acad. Sci. U. S. A. 1991;88:9345–9349. doi: 10.1073/pnas.88.20.9345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fu X, Guadagni F, Hoffman RM. A metastatic nude-mouse model of human pancreatic cancer constructed orthotopically with histologically intact patient specimens. Proc. Natl. Acad. Sci. U. S. A. 1992;89:5645–5649. doi: 10.1073/pnas.89.12.5645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fu X, Le P, Hoffman RM. A metastatic orthotopic-transplant nude-mouse model of human patient breast cancer. Anticancer Res. 1993;13:901–904. [PubMed] [Google Scholar]
- 47.Wang X, Fu X, Hoffman RM. A new patient-like metastatic model of human lung cancer constructed orthotopically with intact tissue via thoracotomy in immunodeficient mice. Int. J. Cancer. 1992;51:992–995. doi: 10.1002/ijc.2910510626. [DOI] [PubMed] [Google Scholar]
- 48.Astoul P, Colt HG, Wang X, et al. Metastatic human pleural ovarian cancer model constructed by orthotopic implantation of fresh histologically-intact patient carcinoma in nude mice. Anticancer Res. 1993;13:1999–2002. [PubMed] [Google Scholar]
- 49.Astoul P, Wang X, Colt H, et al. A patient-like human malignant pleural mesothelioma nude-mouse model. Oncol. Rep. 1996;3:483–487. [PubMed] [Google Scholar]
- 50.Yamamoto M, Zhao M, Hiroshima Y, et al. Efficacy of Tumor-Targeting Salmonella A1-R on a Melanoma Patient-Derived Orthotopic Xenograft (PDOX) Nude-Mouse Model. PloS One. 2016;11:e0160882. doi: 10.1371/journal.pone.0160882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hiroshima Y, Zhang Y, Zhang N, et al. Patient-derived orthotopic xenograft (PDOX) nude mouse model of soft-tissue sarcoma more closely mimics the patient behavior in contrast to the subcutaneous ectopic model. Anticancer Res. 2015;35:697–701. [PubMed] [Google Scholar]
- 52.Metildi CA, Kaushal S, Snyder CS, et al. Fluorescence-guided surgery of human colon cancer increases complete resection resulting in cures in an orthotopic nude mouse model. J. Surg. Res. 2013;179:87–93. doi: 10.1016/j.jss.2012.08.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Metildi CA, Kaushal S, Hardamon C, et al. Fluorescence-Guided Surgery Allows for More Complete Resection of Pancreatic Cancer Resulting in Longer Disease-Free Survival Compared to Standard Surgery in Orthotopic Mouse Models. J. Am. Coll. Surg. 2012;215:126–135. doi: 10.1016/j.jamcollsurg.2012.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hasegawa S, Yang M, Chishima T, et al. In vivo tumor delivery of the green fluorescent protein gene to report future occurrence of metastasis. Cancer Gene Ther. 2000;7:1336–1340. doi: 10.1038/sj.cgt.7700244. [DOI] [PubMed] [Google Scholar]
- 55.Fujiwara T, Kagawa S, Kishimoto H, et al. Enhanced antitumor efficacy of telomerase-selective oncolytic adenoviral agent OBP-401 with docetaxel: preclinical evaluation of chemovirotherapy. Int. J. Cancer. 2006;119:432–440. doi: 10.1002/ijc.21846. [DOI] [PubMed] [Google Scholar]
- 56.Shay JW, Wright WE. Telomerase activity in human cancer. Curr. Opin. Oncol. 1996;8:66–71. doi: 10.1097/00001622-199601000-00012. [DOI] [PubMed] [Google Scholar]
- 57.Kishimoto H, Kojima T, Watanabe Y, et al. In vivo imaging of lymph node metastasis with telomerase-specific replication-selective adenovirus. Nat. Med. 2006;12:1213–1219. doi: 10.1038/nm1404. [DOI] [PubMed] [Google Scholar]
- 58.Kishimoto H, Zhao M, Hayashi K, et al. In vivo internal tumor illumination by telomerase-dependent adenoviral GFP for precise surgical navigation. Proc. Natl. Acad. Sci. U. S. A. 2009;106:14514–14517. doi: 10.1073/pnas.0906388106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kishimoto H, Aki R, Urata Y, et al. Tumor-selective, adenoviral-mediated GFP genetic labeling of human cancer in the live mouse reports future recurrence after resection. Cell Cycle Georget. Tex. 2011;10:2737–2741. doi: 10.4161/cc.10.16.16756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yano S, Miwa S, Kishimoto H, et al. Experimental Curative Fluorescence-guided Surgery of Highly Invasive Glioblastoma Multiforme Selectively Labeled With a Killer-reporter Adenovirus. Mol. Ther. J. Am. Soc. Gene Ther. 2015;23:1182–1188. doi: 10.1038/mt.2015.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yano S, Miwa S, Kishimoto H, et al. Targeting tumors with a killer-reporter adenovirus for curative fluorescence-guided surgery of soft-tissue sarcoma. Oncotarget. 2015;6:13133–13148. doi: 10.18632/oncotarget.3811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yano S, Miwa S, Kishimoto H, et al. Eradication of osteosarcoma by fluorescence-guided surgery with tumor labeling by a killer-reporter adenovirus. J. Orthop. Res. Off. Publ. Orthop. Res. Soc. 2016;34:836–844. doi: 10.1002/jor.23073. [DOI] [PubMed] [Google Scholar]
- 63.Yano S, Takehara K, Miwa S, et al. Fluorescence-guided surgery of a highly-metastatic variant of human triple-negative breast cancer targeted with a cancer-specific GFP adenovirus prevents recurrence. Oncotarget. 2016;7:75635–75647. doi: 10.18632/oncotarget.12314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yano S, Takehara K, Kishimoto H, et al. Tumor-targeting adenovirus OBP-401 inhibits primary and metastatic tumor growth of triple-negative breast cancer in orthotopic nude-mouse models. Oncotarget. 2016;7:85273–85282. doi: 10.18632/oncotarget.13296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yano S, Zhang Y, Miwa S, et al. Precise navigation surgery of tumours in the lung in mouse models enabled by in situ fluorescence labelling with a killer-reporter adenovirus. BMJ Open Respir. Res. [Internet] 2015:2. doi: 10.1136/bmjresp-2015-000096. [cited 2016 Jul 12]. Available from: http://www.ncbi.nlm.nih.gov/pmc/articles/PMC4567685/ [DOI] [PMC free article] [PubMed]
- 66.Yano S, Takehara K, Kishimoto H, et al. Adenoviral targeting of malignant melanoma for fluorescence-guided surgery prevents recurrence in orthotopic nude-mouse models. Oncotarget. 2015;7:18558–18572. doi: 10.18632/oncotarget.6670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yano S, Hiroshima Y, Maawy A, et al. Color-coding cancer and stromal cells with genetic reporters in a patient-derived orthotopic xenograft (PDOX) model of pancreatic cancer enhances fluorescence-guided surgery. Cancer Gene Ther. 2015;22:344–350. doi: 10.1038/cgt.2015.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Nemunaitis J, Tong AW, Nemunaitis M, et al. A phase I study of telomerase-specific replication competent oncolytic adenovirus (telomelysin) for various solid tumors. Mol. Ther. J. Am. Soc. Gene Ther. 2010;18:429–434. doi: 10.1038/mt.2009.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sakurai F, Narii N, Tomita K, et al. Efficient detection of human circulating tumor cells without significant production of false-positive cells by a novel conditionally replicating adenovirus. Mol. Ther. Methods Clin. Dev. 2016;3:16001. doi: 10.1038/mtm.2016.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wong RJ, Joe JK, Kim S-H, et al. Oncolytic Herpesvirus Effectively Treats Murine Squamous Cell Carcinoma and Spreads by Natural Lymphatics to Treat Sites of Lymphatic Metastases. Hum. Gene Ther. 2002;13:1213–1223. doi: 10.1089/104303402320138998. [DOI] [PubMed] [Google Scholar]
- 71.Adusumilli PS, Stiles BM, Chan M-K, et al. Real-time diagnostic imaging of tumors and metastases by use of a replication-competent herpes vector to facilitate minimally invasive oncological surgery. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2006;20:726–728. doi: 10.1096/fj.05-5316fje. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Warner SG, Haddad D, Au J, et al. Oncolytic herpes simplex virus kills stem-like tumor-initiating colon cancer cells. Mol. Ther. Oncolytics. 2016;3:16013. doi: 10.1038/mto.2016.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Stiles BM, Adusumilli PS, Bhargava A, et al. Minimally invasive localization of oncolytic herpes simplex viral therapy of metastatic pleural cancer. Cancer Gene Ther. 2006;13:53–64. doi: 10.1038/sj.cgt.7700860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Adusumilli PS, Gholami S, Chun YS, et al. Fluorescence-assisted cytological testing (FACT): Ex Vivo viral method for enhancing detection of rare cancer cells in body fluids. Mol. Med. Camb. Mass. 2011;17:628–634. doi: 10.2119/molmed.2011.00078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kelly KJ, Wong J, Gönen M, et al. Human Trial of a Genetically Modified Herpes Simplex Virus for Rapid Detection of Positive Peritoneal Cytology in the Staging of Pancreatic Cancer. EBioMedicine. 2016;7:94–99. doi: 10.1016/j.ebiom.2016.03.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Metildi CA, Kaushal S, Luiken GA, et al. Fluorescently-labeled chimeric anti-CEA antibody improves detection and resection of human colon cancer in an orthotopic nude mouse model. J. Surg. Oncol. 2014;109:451–458. doi: 10.1002/jso.23507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hiroshima Y, Maawy A, Metildi CA, et al. Successful fluorescence-guided surgery on human colon cancer patient-derived orthotopic xenograft mouse models using a fluorophore-conjugated anti-CEA antibody and a portable imaging system. J. Laparoendosc. Adv. Surg. Tech. A. 2014;24:241–247. doi: 10.1089/lap.2013.0418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hiroshima Y, Maawy A, Zhang Y, et al. Fluorescence-guided surgery, but not bright-light surgery, prevents local recurrence in a pancreatic cancer patient derived orthotopic xenograft (PDOX) model resistant to neoadjuvant chemotherapy (NAC) Pancreatol. Off. J. Int. Assoc. Pancreatol. IAP Al. 2015;15:295–301. doi: 10.1016/j.pan.2015.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Murakami T, Hiroshima Y, Zhang Y, et al. Fluorescence-Guided Surgery of Liver Metastasis in Orthotopic Nude-Mouse Models. PloS One. 2015;10:e0138752. doi: 10.1371/journal.pone.0138752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hiroshima Y, Lwin TM, Murakami T, et al. Effective fluorescence-guided surgery of liver metastasis using a fluorescent anti-CEA antibody. J. Surg. Oncol. 2016;114:951–958. doi: 10.1002/jso.24462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Gutowski M, Framery B, Boonstra MC, et al. SGM-101: An innovative near-infrared dye-antibody conjugate that targets CEA for fluorescence-guided surgery. Surg. Oncol. 2017;26:153–162. doi: 10.1016/j.suronc.2017.03.002. [DOI] [PubMed] [Google Scholar]
- 82.McElroy M, Kaushal S, Luiken GA, et al. Imaging of Primary and Metastatic Pancreatic Cancer Using a Fluorophore-Conjugated Anti-CA19-9 Antibody for Surgical Navigation. World J. Surg. 2008;32:1057–1066. doi: 10.1007/s00268-007-9452-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hiroshima Y, Maawy A, Sato S, et al. Hand-Held High-Resolution Fluorescence Imaging System for Fluorescence-Guided Surgery of Patient and Cell-Line Pancreatic Tumors Growing Orthotopically in Nude Mice. J. Surg. Res. 2014;187:510–517. doi: 10.1016/j.jss.2013.11.1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hiroshima Y, Maawy A, Zhang Y, et al. Metastatic Recurrence in a Pancreatic Cancer Patient Derived Orthotopic Xenograft (PDOX) Nude Mouse Model Is Inhibited by Neoadjuvant Chemotherapy in Combination with Fluorescence-Guided Surgery with an Anti-CA 19-9-Conjugated Fluorophore. PLoS ONE [Internet] 2014:9. doi: 10.1371/journal.pone.0114310. [cited 2016 Jul 12]. Available from: http://www.ncbi.nlm.nih.gov/pmc/articles/PMC4252107/. [DOI] [PMC free article] [PubMed]
- 85.Rosenthal EL, Kulbersh BD, Duncan RD, et al. In Vivo Detection of Head and Neck Cancer Orthotopic Xenografts by Immunofluorescence. The Laryngoscope. 2006;116:1636–1641. doi: 10.1097/01.mlg.0000232513.19873.da. [DOI] [PubMed] [Google Scholar]
- 86.Heath CH, Deep NL, Sweeny L, et al. Use of Panitumumab-IRDye800 to Image Microscopic Head and Neck Cancer in an Orthotopic Surgical Model. Ann. Surg. Oncol. 2012;19:3879–3887. doi: 10.1245/s10434-012-2435-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Rosenthal EL, Warram JM, de Boer E, et al. Safety and Tumor Specificity of Cetuximab-IRDye800 for Surgical Navigation in Head and Neck Cancer. Clin. Cancer. Res. Off. J. Am. Assoc. Cancer Res. 2015;21:3658–3666. doi: 10.1158/1078-0432.CCR-14-3284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Tummers WS, Miller SE, Teraphongphom NT, et al. Intraoperative Pancreatic Cancer Detection using Tumor-Specific Multimodality Molecular Imaging. Ann. Surg. Oncol. 2018 doi: 10.1245/s10434-018-6453-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Unkart JT, Chen SL, Wapnir IL, et al. Intraoperative Tumor Detection Using a Ratiometric Activatable Fluorescent Peptide: A First-in-Human Phase 1 Study. Ann. Surg. Oncol. 2017 doi: 10.1245/s10434-017-5991-3. [DOI] [PubMed] [Google Scholar]
- 90.Olson ES, Aguilera TA, Jiang T, et al. In vivo characterization of activatable cell penetrating peptides for targeting protease activity in cancer. Integr. Biol. Quant. Biosci. Nano Macro. 2009;1:382–393. doi: 10.1039/b904890a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Miyata Y, Ishizawa T, Kamiya M, et al. Intraoperative imaging of hepatic cancers using γ-glutamyltranspeptidase-specific fluorophore enabling real-time identification and estimation of recurrence. Sci. Rep. 2017;7:3542. doi: 10.1038/s41598-017-03760-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Keereweer S, Kerrebijn JDF, Mol IM, et al. Optical imaging of oral squamous cell carcinoma and cervical lymph node metastasis. Head Neck. 2012;34:1002–1008. doi: 10.1002/hed.21861. [DOI] [PubMed] [Google Scholar]
- 93.Mori K, Ishizawa T, Yamashita S, et al. Intraoperative visualization of pancreatic juice leaking from the pancreatic stump in a swine model. Gastroenterology. 2015;149:1334–1336. doi: 10.1053/j.gastro.2015.07.068. [DOI] [PubMed] [Google Scholar]
- 94.Bloomston M, Zervos EE, Rosemurgy AS. Matrix metalloproteinases and their role in pancreatic cancer: a review of preclinical studies and clinical trials. Ann. Surg. Oncol. 2002;9:668–674. doi: 10.1007/BF02574483. [DOI] [PubMed] [Google Scholar]
- 95.Metildi CA, Felsen CN, Savariar EN, et al. Ratiometric Activatable Cell-Penetrating Peptides Label Pancreatic Cancer, Enabling Fluorescence-Guided Surgery, Which Reduces Metastases and Recurrence in Orthotopic Mouse Models. Ann. Surg. Oncol. 2015;22:2082–2087. doi: 10.1245/s10434-014-4144-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.van Dam GM, Themelis G, Crane LMA, et al. Intraoperative tumor-specific fluorescence imaging in ovarian cancer by folate receptor-α targeting: first in-human results. Nat. Med. 2011;17:1315–1319. doi: 10.1038/nm.2472. [DOI] [PubMed] [Google Scholar]
- 97.Tummers QRJG, Hoogstins CES, Gaarenstroom KN, et al. Intraoperative imaging of folate receptor alpha positive ovarian and breast cancer using the tumor specific agent EC17. Oncotarget. 2016;7:32144–32155. doi: 10.18632/oncotarget.8282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Handgraaf HJM, Boonstra MC, Prevoo HAJM, et al. Real-time near-infrared fluorescence imaging using cRGD-ZW800-1 for intraoperative visualization of multiple cancer types. Oncotarget. 2017;8:21054–21066. doi: 10.18632/oncotarget.15486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Tummers WS, Kimura RH, Abou-Elkacem L, et al. Development and preclinical validation of a cysteine knottin peptide targeting Integrin αvβ6 for near-infrared fluorescent-guided surgery in pancreatic cancer. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2018 doi: 10.1158/1078-0432.CCR-17-2491. [DOI] [PubMed] [Google Scholar]
- 100.Yang L, Sajja HK, Cao Z, et al. uPAR-targeted optical imaging contrasts as theranostic agents for tumor margin detection. Theranostics. 2013;4:106–118. doi: 10.7150/thno.7409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Harlaar NJ, Koller M, de Jongh SJ, et al. Molecular fluorescence-guided surgery of peritoneal carcinomatosis of colorectal origin: a single-centre feasibility study. Lancet Gastroenterol. Hepatol. 2016;1:283–290. doi: 10.1016/S2468-1253(16)30082-6. [DOI] [PubMed] [Google Scholar]
- 102.Scheltinga AGTTvan, Dam GMvan, Nagengast WB, et al. Intraoperative Near-Infrared Fluorescence Tumor Imaging with Vascular Endothelial Growth Factor and Human Epidermal Growth Factor Receptor 2 Targeting Antibodies. J. Nucl. Med. 2011;52:1778–1785. doi: 10.2967/jnumed.111.092833. [DOI] [PubMed] [Google Scholar]
- 103.Tjalma JJ, Garcia-Allende PB, Hartmans E, et al. Molecular Fluorescence Endoscopy Targeting Vascular Endothelial Growth Factor A for Improved Colorectal Polyp Detection. J. Nucl. Med. Off. Publ. Soc. Nucl. Med. 2016;57:480–485. doi: 10.2967/jnumed.115.166975. [DOI] [PubMed] [Google Scholar]
- 104.Park JY, Hiroshima Y, Lee JY, et al. MUC1 selectively targets human pancreatic cancer in orthotopic nude mouse models. PloS One. 2015;10:e0122100. doi: 10.1371/journal.pone.0122100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Qu CF, Li Y, Song YJ, et al. MUC1 expression in primary and metastatic pancreatic cancer cells for in vitro treatment by (213)Bi-C595 radioimmunoconjugate. Br. J. Cancer. 2004;91:2086–2093. doi: 10.1038/sj.bjc.6602232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Fan X-N, Karsten U, Goletz S, et al. Reactivity of a humanized antibody (hPankoMab) towards a tumor-related MUC1 epitope (TA-MUC1) with various human carcinomas. Pathol. - Res. Pract. 2010;206:585–589. doi: 10.1016/j.prp.2010.03.006. [DOI] [PubMed] [Google Scholar]
- 107.Fong SMB, Lee MK, Adusumilli PS, et al. Fluorescence-expressing viruses allow rapid identification and separation of rare tumor cells in spiked samples of human whole blood. Surgery. 2009;146:498–505. doi: 10.1016/j.surg.2008.12.007. [DOI] [PubMed] [Google Scholar]