Abstract
The bed nucleus of the stria terminalis (BNST) is a component of the extended amygdala that shows significant changes in activity and plasticity through chronic exposure to drugs and stress. The region is critical for stress- and cue-induced reinstatement of drug-seeking behaviors and is thus a candidate region for the plastic changes that occur in abstinence that prime addicted patients for reinstatement behaviors. Here, we discuss the various forms of long-term potentiation (LTP) and long-term depression (LTD) in the rodent BNST and highlight the way that these changes in excitatory transmission interact with exposure to alcohol and other drugs of abuse, as well as other stressors. In addition, we highlight potential areas for future research in this area, including investigating input- and cell-specific bidirectional changes in activity. As we continue to accrue foundational knowledge in the mechanisms and effects of plasticity in the BNST, molecular targets and treatment strategies that are relevant to reinstatement behaviors will also begin to emerge. Here, we briefly discuss the effects of catecholamine receptor modulators on synaptic plasticity in the BNST due to the role of norepinephrine in LTD and dopamine on the short-term component of LTP as well as the role that signaling at these receptors plays in reinstatement of drug- and alcohol-seeking behaviors. We hope that insights gained on the specific changes in plasticity that occur within the BNST during abstinence from alcohol and other drugs of abuse will provide insight into the biological underpinnings of relapse behavior in human addicts and inform future treatment modalities for addiction that tackle this complex biological problem.
Keywords: BNST, reinstatement behaviors, synaptic plasticity, addiction, glutamate, catecholamines
■ INTRODUCTION: THE BRAIN DURING PROTRACTED ABSTINENCE
Addiction and alcoholism are chronic diseases characterized by bouts of remission and relapse.1 Only a minority of patients receive treatment; for those that do, relapse to use is a common event; and susceptibility to relapse can last for years into abstinence.2 These high rates of eventual relapse occur in patients with substance use disorders to all drugs of abuse, including cocaine, alcohol, opiates, nicotine, and others, and even in behavioral addictions such as gambling disorder.3 Therefore, we consider that the biology underlying these processes has overlapping neural substrates. During the transition from casual drug use to pathologic addiction, brain changes occur that prime the addict or user to reengage in drug use and relapse to addiction.4,5 It is hypothesized that during this process, as drug use becomes habitual and withdrawal-induced negative affect becomes prominent, the brain recalibrates such that the homeostatic set point of “normalcy” is not reached in the absence of drug and a new allostatic set point is reached only in the presence of drug. This is a useful framework to guide conceptualizing brain changes that occur in the addicted or alcoholic patient. Interestingly, the structural and molecular changes that occur during this transition persist even after the acute physical symptoms of withdrawal have occurred and can last for years after drug use, like susceptibility to relapse. The process of protracted abstinence after extensive drug use has been effectively modeled in rodents as either extinction training or incubation of drug craving after self-administration or other drug conditioning procedures.6,7 In both humans and rodents, relapse can be triggered by stressful life events,8–10 re-exposure to the drug,11 or even exposure to the environment of or cues associated with drug use.12,13 A better understanding of the neural substrates associated with the priming of the brain to respond to these stimuli with drug-seeking will allow for better treatment of addicted patients in the context of relapse prevention.
The effects of abstinence from drug use on structural changes in the brain have been studied in a number of brain regions. The classical neural pathway for these and related studies is the mesolimbic dopamine system consisting of the projection from the ventral tegmental area (VTA) to the nucleus accumbens (NAc). This projection is engaged during use of all drugs of abuse and leads to dopamine release in the NAc that is critical for reinforcement of drug use, reinstatement of drug-seeking, and other drug-related behaviors.14 Compelling evidence supports the notion that drug exposure elicits robust changes in plasticity in projection neurons within the mesocorticolimbic dopamine system.15–17 However, the long-term changes in synaptic plasticity in amygdalar and other regions that project into the mesolimbic dopamine system are likely important in the longer-term effects of protracted use and abstinence-related relapse but much less studied. The extended amygdala is one such collection of nuclei known to play a key role in addiction-related behaviors.18−20 Composed of the anatomically related central nucleus of the amygdala (CeA), the bed nucleus of the stria terminalis (BNST), and the shell of the NAc,21,22 the extended amygdala functions as an integrator of stress and reward information within the brain and is implicated in the withdrawal and negative affect stage of the addictive cycle described above.23–26 Specifically, the BNST has a direct projection to the VTA that is critical for and engaged during drug-seeking behavior as well as withdrawal from drugs of abuse.27 In addition, activity in the BNST is critical for both cue- and stress-induced reinstatement of drug-seeking and has been shown to undergo plastic changes during abstinence from drugs of abuse after extended use.28–31 For this reason, it will be the subject of the remainder of this review, extending prior analyses of this literature.32–34
■ LONG-TERM POTENTIATION OF GLUTAMATERGIC TRANSMISSION IN THE DORSOLATERAL BNST
Early work on synaptic plasticity in the BNST utilized ex vivo brain slices to study changes in neurotransmission after electrical stimulation of glutamatergic afferents within coronal sections. Different stimulation protocols can elicit different changes in activity, with long-term potentiation (LTP) representing enhanced effect to the same stimulation parameters and long-term depression (LTD) diminished effect. Here, we summarize work done on LTP in the BNST before transitioning to LTD. An overview of both LTP and LTD experimental results in the BNST can be seen in Tables 1 and 2, respectively.
Table 1.
Overview of LTP Studies in the BNSTa
| type of LTP | notes | reference |
|---|---|---|
| LTP of excitatory transmission in dlBNST | Induced by HFS (2× 100 Hz for 1 s) | Weitlauf et al. 200435 |
| Recorded by field potentials or sharp electrode recordings in ex vivo BNST slices (mouse) | Weitlauf et al, 200536 | |
| Blocked by NMDAR inhibition, GluN2B KO, GluN2B inhibition | Wills et al. 201237 | |
| Inhibited by prestimulation 100 mM ethanol (only 0–5 min) | ||
| Unaffected by L-type Ca2+ channel inhibition, GABAA inhibition, GluN2A KO, poststimulation 100 mM ethanol | ||
| CIE-induced enhancement of LTP of excitatory transmission in dlBNST | Induced by HFS (2× 100 Hz for 1 s) | Conrad et al. 201140 |
| Recorded by field potentials in ex vivo BNST slices (mouse) | Conrad et al. 201141 | |
| Blocked by GluN2B KO, GluN2B inhibition, chronic or acute social isolation, simultaneous chronic social isolation and chronic unpredictable stress | Wills et al. 201237 | |
| Unaffected by acute corticosterone administration | ||
| DA-induced enhancement of STP of excitatory transmission in dlBNST | Induced by HFS (2× 100 Hz for 1s) | Kash, Nobis et al. 200842 |
| Recorded by field potentials in ex vivo BNST slices (mouse) | ||
| Blocked by NMDAR inhibition, CRFR1 antagonist, pan-dopamine receptor antagonist (flupenthixol), D1R KO | ||
| LTP of excitatory transmission in oval BNST CRF cells | Induced by HFS (5× 100 Hz 1s, interval = 20 s) | Dabrowska et al. 201354 |
| Recorded by whole cell electrophysiology in ex vivo BNST slices (rat) | ||
| Occurs in all CRF+ BNST neurons | ||
| Enhanced by repeated restrain stress (only in Type III) | ||
| Blocked by intracellular STEP (only in Type III) | ||
| LTP of VSub-amBNST projections | Induced and recorded in vivo by HFS (500 pulses at 400 Hz, 250 μs duration) in VSub and electrophysiological recordings in amBNST (rat) | Glangetas et al. 201555 |
| Blocked by NMDAR inhibition | Glangetas et al. 201756 | |
| Induces: potentiation of cocaine-induced locomotor activity (via BNST-VTA projections), anxiolysis (NMDAR-dependent) | ||
| LTP of ILC-BNST projections in nicotine self-administration | Induced and recorded in vivo by LFS (10 Hz for 1 min) in ILC and electrophysiological recordings in BNST (rat) during protracted abstinence | Reisiger et al. 201469 |
| Does not occur in saline self-administration or yoked nicotine controls | ||
| Blocked by NMDAR inhibition, extinction training, CB1R antagonism | ||
| Results in enhanced nicotine seeking | ||
| Increased AMPAR/NMDAR ratio in vlBNST | Observed after cocaine self-administration or with chronic subcutaneous morphine pellet implant in whole-cell recordings from ex vivo rat BNST slices | Dumont et al. 200543 |
| Not seen after: acute cocaine injection, passive administration of cocaine or food | Dumont et al. 200845 | |
| Increased AMPAR/NMDAR ratio in ovBNST | Observed after acquisition in cocaine and sucrose self-administration in whole-cell recordings from ex vivo rat BNST slices. | deBacker et al. 201544 |
| Blocked by in vivo GluN2B inhibition (cocaine only) | ||
| Results in no effects on lever pressing for sucrose or cocaine, potentially disrupts reinstatement behaviors | ||
| HFS LTP of excitatory transmission and intrinsic excitability of jcBNST neurons | Induced by HFS (100 Hz for 1 s at 10 s intervals) in ex vivo BNST slices (rat) | Francesconi et al. 200948 |
| LTP-IE characteristics: reduced inward rectification, depolarized RMP, increased membrane resistance, decreased rheobase, decreased firing threshold, increased temporal fidelity of firing | Francesconi et al. 200953 | |
| Blocked by NMDAR inhibition, mGluR5 inhibition, D1R inhibition, alcohol dependence and 4–6 week protracted withdrawal (see below), long-access cocaine self-administration, long-access heroin self-administration | ||
| Enhanced by GABAA and GABAB inhibition | ||
| Unaffected by D2R inhibition, alcohol dependence and 0–4 week protracted withdrawal, short-access cocaine self-administration, short-access heroin self-administration | ||
| ethanol withdrawal-induced disruption of LTP of excitatory transmission in jcBNST | Induced by HFS (100 Hz for 1 s at 10 s intervals) in ex vivo BNST slices (rat) | Francesconi et al. 200953 |
| Observed in animals with escalated dependent alcohol intake induced by exposure to alcohol vapors Mimicked by chronic ICV CRF (alcohol naivïe animals) | ||
| Blocked by CRFR1 antagonist | ||
| Unaffected by CRFR2 antagonist | ||
| LTP-IE in jcBNST neurons | Observed after self-administration of opioids in ex vivo rat BNST slices (only in Type III jcBNST neurons) | Francesconi et al. 200953 |
| Does not occur in Type I or Type II jcBNST neurons |
amBNST = anteromedial BNST; BNST = bed nucleus of the stria terminalis; CIE = chronic intermittent ethanol; CRF = corticotropin releasing factor; CRFR1 = CRF receptor 1; CRFR2 = CRF receptor 2; DA = dopamine; D1R = DA receptor 1; D2R = DA receptor 2; dlBNST = dorsolateral BNST; GABAA = gamma-aminobutyric acid receptor A; GABAB = GABA receptor B; GluN2A = glutamate NMDAR subunit 2A; GluN2B = glutamate NMDAR subunit 2B; HFS = high frequency stimulation; ICV = intracerebroventricular; KO = knockout; LTP = long-term potentiation; LTP-IE = LTP of intrinsic excitability; mGluR5 = metabotropic glutamate receptor 5; NMDAR = N-methyl-d-aspartate receptor; RMP = resting membrane potential; STEP = striatal enriched protein tyrosine phosphatase; STP = short-term potentiation; vlBNST = ventrolateral BNST; VTA = ventral tegmental area.
Table 2.
Overview of LTD Studies in the BNSTa
| type of LTD | notes | references |
|---|---|---|
| Group I mGluR-LTD in dlBNST | Observed in whole-cell recordings from ex vivo BNST mouse slices | Grueter et al. 200663 |
| Blocked by CB1R inhibition (mGluR1/5), ERK1 inhibition (mGluR5-only), single injection of cocaine (blocked by in vivo mGlu5 antagonism), bath application of cocaine, chronic intermittent ethanol exposure | Grueter et al. 200862 | |
| Unaffected by ERK2 inhibition, GluA1 inhibition, 10 days withdrawal from single cocaine injection, restraint stress, α2A-AR KO | McElligott et al. 201064 | |
| α1-AR-LTD in dlBNST | Observed in whole-cell recordings from ex vivo mouse BNST slices | McElligott et al. 201064 |
| Blocked by clathrin-dependent endocytosis inhibition, GluA1 inhibition, L-type VGCC, restraint stress, continuous or intermittent ethanol exposure, NET KO, α2A-AR KO | McElligott and Winder 200865 | |
| Unaffected by NMDAR inhibition, mGluR5-inhibition, single exposure to cocaine | ||
| Mimicked by 20 min (but not 10 min) 100 uM norepinephrine application | ||
| hM3Dq-LTD in dlBNST | Observed in whole-cell recordings from ex vivo mouse BNST slices | Mazzone et al. 201666 |
| Blocked by PLC inhibition, CB1R inhibition | ||
| Results in anxiogenesis, activation of VTA/PBN/LC | ||
| LFS of ILC-aBNST projection-induced LTD | Induced by 5 min 10 Hz stimulation of prefrontal cortex cell bodies in vivo and recorded in aBNST neurons (mouse) | Glangetas et al. 201368 |
| After stress, switches to LTP (glucocorticoid-independent) | ||
| Blocked by NMDAR inhibition, CB1R antagonism, CB1R KO, glutamatergic neuron-specific CB1R KO (both LTD alone and LTD transition to LTP) | ||
| Occurs in VTA-projection neurons as well as unidentified BNST neurons | ||
| HFS of ILC-BNST projection-induced LTD | Induced and recorded in vivo by HFS (500 pulses at 400 Hz, 250 μs duration pulse) in ILC and electrophysiology recordings in BNST (rat) | Glangetas et al. 201756 |
| Same protocol induces LTP of VSub-BNST projections | ||
| Enhanced by NMDAR inhibition | ||
| LFS LTD in BNST neurons | Induced by LFS (10 min 10 Hz) and recorded by whole cell electrophysiology in ex vivo BNST neurons (rat) | Puente et al. 201171 |
| Blocked by CB1R antagonism, mGluR5 inhibition, TRPV1 inhibition, MAGL inhibition, PLC inhibition, sarcoplasmic/endoplasmic Ca2+ channel blockade | ||
| Occluded by TRPV1 agonism | ||
| Enhanced by FAAH inhibition (subthreshold induction protocol) | ||
| Unaffected by NMDAR inhibition, L-type Ca2+ channel inhibition, DAGL inhibition | ||
| LFS LTD in ovBNST neurons | Induced by LFS (15 min 1 Hz) and recorded by whole cell electrophysiology in ex vivo BNST neurons (rat) | deBacker et al. 201544 |
| Blocked by NMDAR inhibition, GluN2B inhibition, cocaine self-administration (maintenance) | ||
| Rescued by GluN2B inhibition (rescue blocked by NMDAR inhibition) | ||
| Unaffected by sucrose self-administration (maintenance) | ||
| DSE/STD in BNST neurons | Induced by 10 s depolarization in whole cell electrophysiology recordings of ex vivo BNST neurons (rat) | Puente et al. 201171 |
| Blocked by CB1R antagonism, DAGL inhibition, L-type Ca2+ channel inhibition, PLC inhibition, sarcoplasmic/endoplasmic Ca2+ channel blockade | ||
| Enhanced by MAGL inhibition | ||
| Unaffected by FAAH inhibition, TRPV1 inhibition, mGluR5 inhibition, mGluR1 inhibition |
2AG = 2-arachidonyl glycerol; aBNST = anterior BNST; α1-AR = alpha-1-adrenergic receptor; α2A-AR = alpha-2a-adrenergic receptor; BNST = bed nucleus of the stria terminalis; CB1R = cannabinoid receptor 1; DAGL = diacylglyercol lipase; DSE = depolarization-induced suppression of excitation; ERK1 = extracellular related kinase 1; FAAH = fatty acid amide hydrolase; GluA1 = glutamate AMPAR subunit A1; hM3Dq = human M3 muscarinic receptor Gq-coupled DREADD; LC = locus coeruleus; LFS = low frequency stimulation; LTD = long-term depression; KO = knockout; MAGL = monoacylglycerol lipase; mGluR = metabotropic glutamate receptor; NET = norepinephrine transporter; NMDAR = N-methyl-d-aspartate receptor; PBN = parabrachial nucleus; PLC = phospholipase C; STD = short-term depression; TRPV1 = transient receptor potential cation channel subfamily V member 1; VGCC = voltage-gated Ca2+ channel; VSub = ventral subiculum; VTA = ventral tegmental area.
Mechanism(s) of LTP in dlBNST.
LTP can be induced in BNST slices using high frequency stimulation (HFS) protocols, often involving two bouts of 100 Hz stimulation for 1 s each, and recorded either in the synaptic component of field potential responses to stimulation or through intracellular recordings using sharp electrodes. Early studies aimed to uncover the molecular mechanisms underlying this effect in ex vivo mouse BNST slices and showed that this LTP was sensitive to NMDAR (N-methyl-d-aspartate receptor) inhibition by AP5 and insensitive to inhibition of L-type calcium channels by nimodipine or GABAA receptors by picrotoxin.35 Subunit specificity of the effects of NMDAR inhibition was later shown, as GluN2A knockout did not affect LTP,36 but GluN2B knockout and pharmacological inhibition by Ro25-6981 both reduced LTP.37 Interestingly, the early phase of LTP (0–5 min post-HFS) was sensitive to bath application of 100 mM ethanol before but not after HFS through an NMDAR-dependent process.35 The effects of ethanol on NMDAR-dependent transmission were again later shown to be GluN2B-dependent but GluN2A-independent.37,38
Drug Exposure Interactions with dlBNST LTP.
Although ethanol appeared to have an inhibitory effect on LTP in mouse BNST slices when bath applied, chronic exposure to alcohol using the chronic intermittent ethanol (CIE) paradigm was shown to upregulate GluN2B expression39 and enhance LTP induction in the BNST through an GluN2B-dependent process.37 This enhancement was prevented by chronic but not acute corticosterone administration, chronic or acute social isolation,40 and simultaneous chronic social isolation and chronic unpredictable stress.41 The CIE paradigm consists of two cycles of 4 days with 16-h ethanol exposure and 8-h recovery with 3 days in between each cycle. This suggests that repeated exposure to ethanol and withdrawal induces structure and molecular changes in the BNST that increase the potential for LTP induction. Similarly, a single injection of cocaine or bath application of cocaine enhances the early phase of high frequency stimulation-induced LTP, defined here as short-term potentiation (STP), in a dopamine- and CRF-dependent process.42 However, each of these recordings was done on the population level as a field potential and do not give information regarding cell-specific effects on plasticity, which is important in the heterogeneity of the BNST. Parallel cell-specific results were observed, though, in rats undergoing a self-administration procedure for cocaine or food. In both cases, self-administration increased the excitability of anterolateral BNST neurons via an increased AMPAR/NMDAR ratio, an electrophysiological measurement that compares the current passing through the two receptors and is increased upon the synaptic AMPAR insertion that commonly occurs in LTP.43 This increased excitability is not seen after either acute cocaine injection or passive administration of cocaine or food.43 Similar results were also obtained in the oval BNST, where sucrose and cocaine self-administration increased AMPAR/NMDAR ratios during the acquisition phase.44 Interestingly, cocaine-induced excitability changes persisted throughout maintenance and withdrawal and were sensitive to GluN2B blockade, while sucrose-induced changes diminished during maintenance and resulted in increased NMDAR current decay rates, suggesting mechanistic differences. Increased AMPAR/NMDAR ratios were also seen in ventrolateral BNST neurons after chronic treatment with a subcutaneous morphine pellet implant.45 This effect was specific to VTA-projecting BNST neurons and was elicited only by electrical stimulation of afferents in dorsolateral BNST and not medial stimulation, suggesting input and output specificity.
■ LONG-TERM POTENTIATION OF EXCITATORY TRANSMISSION AND INTRINSIC EXCITABILITY IN THE JUXTACAPSULAR BNST
The juxtacapsular nucleus (jcBNST) is a subnucleus of the BNST located on the lateral aspect of the dorsal BNST and runs parallel to the internal capsule that has received specific focus due to its input from the basolateral amygdala alongside lesser input from the CeA.46,47 Upon high frequency stimulation of the stria terminalis in ex vivo rat brain slices, LTP of excitatory transmission is observable within the jcBNST as in the dlBNST.48 This LTP is inhibited by NMDAR, D1R, and mGluR5 antagonism, is enhanced by GABAA and GABAB receptor antagonism, and is unaffected by D2R antagonism. In addition, the same stimulation protocol elicits an alternative form of LTP known as LTP of intrinsic excitability (LTP-IE), which consists of reduced inward rectification, depolarized resting membrane potential, and increased membrane resistance, together decreasing the rheobase and firing threshold and increasing temporal fidelity of firing and overall cellular excitability. The mechanism underlying the changes in cellular excitability were shown to involve the postsynaptic D-type K+ current (ID). Both of these forms of LTP are disrupted by long-term drug exposure in rats during protracted withdrawal after alcohol dependence as well as in rats self-administering cocaine or heroin with long (23 h) access to the self-administration chamber. LTP is unaffected in a variety of control groups including nondependent rats in protracted withdrawal from ethanol as well as self-administering rats on a short-access schedule of drug access. Occlusion of jcBNST LTP induction by withdrawal is suggested by recent data showing that opioid dependence in self-administering rats elicits LTP-IE specifically in the electrophysiologically defined Type III neurons of the jcBNST but not Type I or Type II neurons.49,50 Briefly, in an effort to begin to subcategorize neurons within the heterogeneous BNST, Hammack et al. developed a classification system whereby neurons are subdivided based on their electro-physiological response to positive and negative current injections (Type I: hyperpolarization sag and regular firing pattern; Type II: hyperpolarization sag and burst firing pattern; Type III: no hyperpolarization sag and fast inward rectification).51 This schema was confirmed with follow-up transcriptomic analyses50 and has proven useful in classifying otherwise unidentified BNST neurons and will thus be used here as appropriate.
In contrast to the observed LTP-IE in Type III jcBNST neurons, protracted withdrawal from four weeks of intermittent alcohol vapor exposure reduces excitability of all three types of rat jcBNST neurons,52 suggesting a complex interaction between drug exposure and contingency of administration that needs to be further explored. Interestingly, LTP impairment in protracted abstinence can be mimicked by chronic intracerebroventricular (ICV) administration of corticotrophin releasing factor (CRF) and is blocked by the CRFR1 antagonist R121919 but not the CRFR2 antagonist astressin2-B,53 suggesting a role for this neuropeptide in withdrawal-associated changes in plasticity in the rat BNST. As described above, it was later shown that CRF neurons in the oval subnucleus of the rat BNST can undergo LTP after high frequency stimulation and that this LTP was enhanced by repeated restraint specifically in Type III CRF neurons via a striatal-enriched tyrosine phosphatase (STEP)-dependent mechanism,54 providing a possible source of the CRF for the observed changes in plasticity within the jcBNST. Future work should aim to determine whether or not changes in plasticity observed in the jcBNST are specific to this subnucleus and, if so, how this system interacts with the other subnuclei of the BNST as well as other nuclei of the extended amygdala and the rest of the brain.
■ EXPANDING THE SCOPE OF LTP IN THE BNST: EFFECTS OF IN VIVO INDUCTION ON BEHAVIOR
Two of the limitations of the work on LTP in the BNST described thus far are (1) the lack of input specificity during high frequency stimulation of afferents in ex vivo brain slices and (2) a lack of connection to behavioral outcomes in addition to the well-established connection to behavioral history. In two recent rat studies, high frequency stimulation of the ventricular subiculum and CA1 subregion of the hippocampus were shown to induce LTP in the BNST via an NMDAR-dependent mechanism.55,56 This elicited anxiolysis that was reversed by the NMDAR antagonist AP5 in one study and potentiation of cocaine-induced locomotor activity at a subthreshold dose via LTP in VTA-projecting BNST neurons in the other. Unexpectedly, when the same stimulation protocol was used to stimulate the infralimbic cortex, it induced LTD in the BNST, suggesting that this stimulation protocol differentially affects glutamatergic inputs to the BNST and highlighting the importance of input specificity in studies on BNST plasticity. These two studies provide a framework for future work on BNST LTP in vivo, where the electrophysiological and behavioral effects of high frequency stimulation of cell bodies projecting to the BNST and recording of neuronal effects can inform our understanding of the relevant neural circuitry.
■ LTP IN THE BNST: A SUMMARY
LTP has been shown to occur in BNST neurons after either high frequency stimulation (HFS) of afferent populations or after long-term drug exposures. In both the dorsolateral and juxtacapsular subnuclei of the BNST, HFS-induced LTP is sensitive to NMDAR inhibition. However, the two types of LTP differ in that dlBNST LTP is enhanced by exposure to alcohol in a CIE paradigm and cocaine in vivo or ex vivo, while jcBNST LTP is disrupted by long-term exposures to alcohol, cocaine, and heroin, suggesting regional differences. In the dlBNST, LTP is disrupted by chronic stressors including corticosterone, social isolation, and combined isolation with unpredictable stress, while the effects of these stressors have not been evaluated in the jcBNST. In the jcBNST, LTP of intrinsic excitability is also observed after HFS and during protracted abstinence in opioid withdrawal specifically in Type III neurons, driven by changes in the D-type K+ current ID. LTP-IE has not been studied in dlBNST neurons, but anterolateral and ventrolateral BNST neurons show enhanced AMPA/NMDA ratios after cocaine self-administration or chronic morphine pellet implant, which suggest increased excitability to synaptic inputs through a parallel mechanism. Future work should aim to further compare these types of LTP as well as continue to uncover molecular correlates underlying this change in synaptic plasticity. In addition, a future focus on species differences in these forms of LTP would inform this literature due to a lack of direct comparison and substantial points of divergence between rat and mouse BNST.57,58
■ METABOTROPIC RECEPTOR-INDUCED LONG-TERM DEPRESSION IN THE BNST: HETEROGENEITY OF STIMULI
Plasticity is bidirectional, with LTP resulting in increased activity and LTD resulting in decreased activity. Classically, the intracellular levels of Ca2+ that initiate LTD are lower than the high levels required to initiate LTP.59 The two most common means of LTD induction are low frequency stimulation protocols or activation of metabotropic signaling cascades, the latter of which has been more extensively studied in the BNST again in ex vivo brain slices. Specifically, LTD of excitatory transmission in the BNST can be initiated by Gq-coupled GPCR signaling cascades downstream of metabotropic glutamate receptors (mGluR),60–63 the α1-adrenergic receptor (α1-AR),64,65 and the chemogenetic receptor hM3Dq.66
Metabotropic glutamate receptors can be functionally split into three classes: (1) Group I consists of mGluR1 and mGluR5, which are predominantly postsynaptic and stimulate Gq-GPCR signaling cascades; (2) Group II consists of mGluR2 and mGluR3, which are both presynaptic and postsynaptic and stimulate Gi-GPCR signaling cascades; and (3) Group III consists of mGluR4, mGluR6, mGluR7, and mGluR8, all of which are both presynaptic and postsynaptic and also stimulate Gi-GPCR signaling cascades.67 Both Group I mGluRs and α1-ARs are Gq-coupled GPCRs. The processes by which they elicit LTD share some features but differ in others. In the mouse BNST, Group I mGluR-dependent LTD was shown to occur via two distinct pathways: (1) an mGluR1 and mGluR5-dependent process that involves presynaptic cannabinoid receptor 1 (CB1R) signaling, and (2) an mGluR5-dependent pathway involving postsynaptic extracellular related kinase 1 (ERK1) but not ERK2 activation.63 mGluR5-LTD was unaffected by GluA1 inhibition and did not result in a change in calcium-permeable AMPA receptor expression.64 On the other hand, α1-AR-dependent LTD was shown to be postsynaptic and dependent on clathrin-dependent endocytosis, GluA1 activity, and L-type voltage-gated calcium channel (VGCC) activation.64,65 α1-AR-LTD was independent of NMDAR and mGluR5 activation65 and resulted in loss of sensitivity to calcium-permeable AMPA receptors.64 Norepinephrine (100 uM) initiates α1-AR-dependent LTD after 20 min but not 10 min bath application.
To determine if Gq-GPCR-initiated LTD was translatable beyond the mGluR5 and α1-AR, hM3Dq-dependent LTD was tested and confirmed in mouse VGAT-expressing BNST neurons.66 hM3Dq is a modified form of the M3 muscarinic receptor that does not respond to its endogenous ligand acetylcholine but instead responds to the otherwise inert ligand clozapine-N-oxide (CNO). In this study, hM3Dq-LTD was sensitive to phospholipase C (PLC) inhibition and CB1R antagonism, induced anxiogenesis not seen with activation of the chemogenetic Gi-coupled hM4Di receptor, and led to downstream recruitment of the VTA, parabrachial nucleus (PBN), and locus coeruleus (LC), as seen by DREADD-associated metabolic mapping (DREAMM). In addition to extending mGluR5- and α1-AR-LTD to hM3Dq, this work also highlighted the potential for alternative Gq-GPCR-LTD pathways as VGAT+ BNST neurons also expressed the acetylcholine M1 muscarinic receptor and the serotonin 5-HT2C receptor.
■ METABOTROPIC RECEPTOR-INDUCED LTD IN THE BNST: INTERACTIONS WITH BEHAVIORAL HISTORY
Analogous to BNST HFS-induced LTP, LTD initiated by Gq-GPCR agonism is also highly sensitive to behavioral and pharmacological history of the animal. mGluR5/ERK1-dependent LTD in mouse BNST is sensitive to a single injection of cocaine, bath application of cocaine, and CIE exposure.62,63 The duration of disruption of mGluR5/ERK1-LTD is extended with repeated injections of cocaine but is not permanent, as LTD is observable 10 days after final cocaine injection.62 Cocaine-induced disruption of mGluR5/ERK1-LTD can also be prevented by in vivo mGluR5 antagonism.62 α1-AR-dependent LTD is absent after restraint stress or exposure to either continuous or intermittent alcohol and in various genetic models of affective disorders, including the norepinephrine transporter (NET) and α2A-adrenergeric receptor (α2A-AR) knockout mice.64,65 While there are many similarities in processes that affect these types of LTD, there are differences. For example, mGluR5-dependent LTD is not affected by restraint stress or by α2A-AR knockout, while α1-AR-dependent LTD is not affected by a single exposure to cocaine.64,65
■ LOW FREQUENCY STIMULATION-INDUCED LTD IN THE BNST
Low frequency stimulation (LFS; 15 min 10 Hz stimulation) of glutamatergic afferents in ex vivo rat BNST slices is also capable of inducing LTD of excitatory transmission.44 This LTD is NMDAR- and GluN2B-dependent and is unaffected by sucrose self-administration but occluded by cocaine self-administration in a GluN2B-dependent process, again connecting plasticity changes with drug history. Honing in on a specific population of afferents, a similar stimulation protocol (5 min 10 Hz stimulation) within prefrontal cortex in vivo has also been shown to lead to LTD of firing in mouse BNST neurons.68 After stress, though, this LTD switches to LTP in a glucocorticoid receptor-independent fashion.68 In both cases, CB1R antagonism, full knockout, or glutamatergic neuron-specific knockout blocks both the LTD and the stress-induced transition to LTP. Similarly, after extended nicotine self-administration in rats, LTP is observed broadly in BNST neurons and specifically in VTA-projecting BNST neurons after stimulation of the infralimbic cortex.69,70 This LTP was NMDAR- and CB1R-dependent and was absent in extinguished but not abstinent mice and induced nicotine-seeking in trained animals. Prior rat ex vivo work highlighted the role of CB1R agonism in the BNST in both depolarization-induced suppression of excitation (DSE) and LTD, dissociating the 2-arachidonylglycerol-mediated L-type calcium channel-dependent short-term depression (STD) and the mGluR5-initiated anandamide-mediated TRPV1-dependent LFS LTD.71 More mechanistic work on in vivo endocannabinoid modulation of BNST plasticity will inform our understanding of endocannabinoid mediated LTD and the molecular correlates underlying the transition from LTD to LTP.
As can be seen here, BNST neurons are highly susceptible to long-term depression after activation of subtypes of metabotropic receptors to glutamate and norepinephrine or low frequency stimulation of afferent populations, and that these forms of plasticity are differentially affected by stress and drug exposure. The behavioral correlates and molecular under-pinnings, in addition to species differences, alternative targets, and effect specificity, remain to be fully elucidated.
■ INPUT SPECIFICITY: WHERE TO LOOK AND WHY
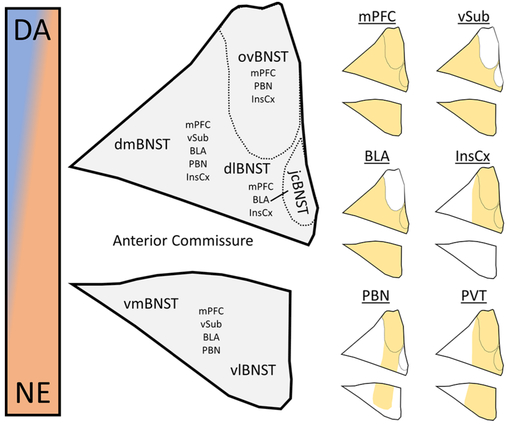
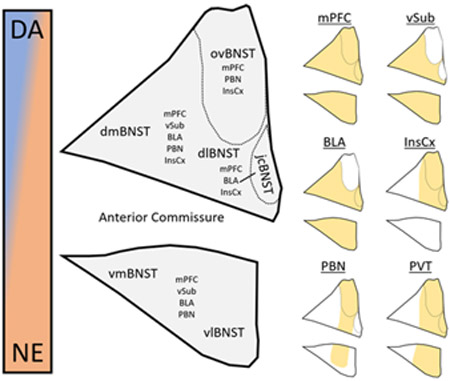
Through the processes of LTP and LTD detailed above, BNST neurons increase or decrease their response to glutamatergic input. However, in most cases, it is unclear whether these effects occur in response to all glutamatergic afferents or whether it is input- and synapse-specific. In the basolateral nucleus of the amygdala (BLA), for example, auditory fear conditioning initiates changes in plasticity in an input-specific manner.72 In auditory fear conditioning, an auditory stimulus functions as the conditioned stimulus (CS) that is eventually learned to be a signal of a forthcoming footshock, which is the aversive unconditioned stimulus (US). After learning has taken place, LTP of input from the auditory thalamus and auditory cortex to the BLA is specifically observed, consistent with the strengthened connection between the CS and US-mediated behaviors such as freezing.73 In the context of protracted abstinence-induced plasticity in the BNST, the corollary of these inputs is unclear, as the source of withdrawal stimuli-associated activity has not been clearly elucidated. In addition, one of the key points of differentiation between the amygdala and the BNST in stress and anxiety is that the former is engaged during acute, predictable stressors while the latter is engaged during chronic, diffuse stressors that may lack a specific unitary source.24 However, with projection-specific optogenetic technology and other recently developed tools that can differentiate specific inputs to or populations of neurons of interest, this is an experimentally testable hypothesis. Here, we provide rationale for investigations of plasticity at specific glutamatergic inputs to the BNST that play a role in reinstatement behaviors and attempt to connect these inputs to the LTP and LTD experiments described above. For an overview of the anatomy of these inputs with respect to subnucleus divisions of the BNST as well as relationships to catecholamine input (discussed below), see Figure 1.
Figure 1.
Anatomy of glutamatergic and catecholaminergic input to the bed nucleus of the stria terminalis. (Left) Anatomical depiction of BNST subnuclei shows location of the oval (ovBNST), juxtacapsular (jcBNST), dorsomedial (dmBNST), dorsolateral (dlBNST), ventromedial (vmBNST), and ventrolateral (vlBNST) subnuclei (Allen Reference Atlas). In addition, glutamatergic afferent populations are noted within the subnuclei, and catecholaminergic input is represented as a gradient from high prevalence of dopaminergic (blue) to noradrenergic terminals (orange). (Right) Terminal field afferent populations from each of the six glutamatergic inputs, including the ventral subiculum (VSub), medial prefrontal cortex (mPFC), para-brachial nucleus (PBN), basolateral amygdala (BLA), insular cortex (InsCx), and paraventricular nucleus of the thalamus (PVT). The topography of afferent population terminal fields are adapted from McDonald et al. 199687 for the mPFC, Dong et al. 2001104 for the VSub, Kim et al. 2013103 for the BLA, Yasui et al. 1991116 for the InsCx, Saper and Loewy 1980131 for the PBN, and Li and Kirouac 2008.154
Medial Prefrontal Cortex.
Input-specificity of abstinence-associated changes in synaptic plasticity has been performed on infralimbic cortical input to the BNST, as previously described. LTP of this input occurs in nicotine self-administration in rats, and low frequency stimulation in drug-naïve mice elicits LTD of this input that transitions to LTP with stress exposure in a CB1R-dependent fashion.68,69 This suggests that drug exposure correlates with a strengthening of this input and thus may underlie some of the behavioral changes associated with increased drug exposure. Indeed, in humans, increased glucose metabolism is observed in the related orbitofrontal cortex (OFC) one week into withdrawal, and metabolism within the OFC and the prefrontal cortex (PFC) correlates with drug craving in abstinent patients.74 In the context of stress, stress-induced changes in PFC activity by functional magnetic resonance imaging (fMRI) was associated with greater number of days since last cocaine exposure and shorter time to relapse.75 In rodent models, prefrontal cortex activity has been shown a number of times to be critical for stress-induced reinstatement of drug-seeking and incubation of drug craving.76–78 PFC neurons have also been shown to undergo changes in plasticity and enhancement of activity during either protracted abstinence from drugs of abuse or after acute or chronic stress exposure.77,79–82 The BNST mediates an interaction between medial PFC activity and recruitment of the paraventricular nucleus of the hypothalamus (PVN) during psychological stressors, suggesting a role of this nucleus as a relay between cortical input and behavioral output that may translate from stress circuitry to drug and reward circuitry.83–86 This collection of work highlights the potential for a PFC-BNST circuit to mediate aspects of stress-induced reinstatement of drug- and alcohol-seeking behaviors. However, the PFC is a heterogeneous collection of nuclei consisting of at least the infralimbic cortex, prelimbic cortex, and orbitofrontal cortex, each of which project to the BNST87 and are known to engage differently in addiction-related and other behaviors. It will be important to parse out these differences as plasticity in this circuit is investigated.
Ventral Subiculum.
Long-term potentiation induced by in vivo high frequency stimulation of the ventral subicular (VSub) input to the BNST was determined to be anxiolytic in mice and potentiating of the locomotor response to cocaine via BNST-VTA projections in rats.55,68 Similar to the input to the BNST from the PFC, this projection is known to act as an inhibitory relay between the ventral hippocampus and the PVN to dampen the stress response.88,89 This suggests that engagement of this pathway, as occurs during stressful stimuli, would attempt to counteract the effects of stress. Chronic engagement of this pathway, though, could lead to loss of potential for plasticity. However, with chronic or juvenile exposure to stress, we see enhanced LTP within the ventral hippocampus, suggesting otherwise.90–93 The cocaine-potentiating effects of high frequency stimulation of the ventral subicular input to the BNST via projections to the VTA suggests that interactions between stress and cocaine may also occur via this circuit. Supporting this hypothesis, cocaine conditioning also increased90 and induced structural changes that support increased activity in the region.94,95 These data suggest that protracted abstinence may lead to increased plasticity at VSub-BNST projections, and that this projection may contribute to susceptibility to reinstatement. Specifically, the ventral subiculum is critical for cocaine-, cue-, and context-induced reinstatement of cocaine-seeking behaviors.96–99 Interestingly, electrical stimulation of the ventral subiculum itself is capable of reinstating drug-seeking behaviors.100 In human patients, though, we see decreased hippocampal volume in alcoholics101 and after childhood maltreatment,102 suggesting a loss of activity with these chronic stressors. Future studies will inform whether or not changes in plasticity occur at VSub-BNST projections during protracted abstinence and, if they do, whether the projection still elicits anxiolysis as it does in naïve animals.
Basolateral Amygdala.
The BLA sends a glutamatergic projection to the BNST that has also previously been shown to induce anxiolysis and decrease respiratory rate.103 Although this projection has not been directly investigated in the context of abstinence-induced changes in plasticity, the BLA sends a portion of its BNST projections to the juxtacapsular subnucleus (jcBNST),104 where high frequency stimulation of the stria terminalis induces LTP-IE that is occluded by prolonged drug exposure. Consistent with a potential role in abstinence-induced changes in plasticity, extensive evidence has linked the BLA with reinstatement of drug-seeking behaviors. Specifically, the BLA is shown to be critical for context-, cue-, and drug-induced reinstatement of drug-seeking behaviors in some but not all studies.105–108 Furthermore, a history of drug exposure and re-exposure to drug-associated stimuli leads to robust activation and activity-dependent changes in the BLA in both rodent models109,110 and humans,111,112 supporting the hypothesis that BLA-BNST connectivity may be enhanced in protracted abstinence. However, given the anxiolysis induced by stimulation of this pathway and that withdrawal induces anxiogenesis in addicted patients, it is also a candidate pathway for input-specific LTD of glutamatergic transmission in the BNST. Consistent with this hypothesis, BLA lesions did not affect footshock-induced reinstatement of cocaine-seeking behavior dependent on BNST activity,29 and direct administration of NMDAR antagonists into the BLA to block development of NMDAR-dependent plasticity did not affect conditioned reward or reinstatement of cocaine self-administration.113 In addition, human imaging studies show that a reduced amygdalar volume is consistent with high risk for the development of alcoholism and other neuropsychiatric disorders.114,115 It will be important for future studies to determine the presence, directionality, behavioral relevance, and subnucleus specificity of plasticity at glutamatergic synapses originating in the BLA projecting to the BNST.
Insular Cortex.
The insular cortex is another glutamatergic input to the BNST, including but not limited to the jcBNST,116 that has the potential to either enhance or inhibit reinstatement of drug-seeking behaviors and thus could undergo bidirectional changes in plasticity with increased drug exposure and protracted abstinence. Broadly, the insular cortex functions in integrating interoceptive processes and translating that information into conscious feelings and behavioral decisions regarding risk and reward.117 The insula has received substantial interest since the observation that nicotine-dependent patients who had stroke-associated damage to the insula reported substantially decreased craving and high rates of success in quitting smoking.117 This clinical finding has been well-replicated in humans118,119 and modeled in rodents.120,121 Insular activity is also critical for addiction-like behaviors to other drugs of abuse, as inactivation blocks conditioned place preference (CPP) for amphetamine,122,123 cue-induced reinstatement to cocaine-seeking,124 and operant responding for alcohol.125 Consistent with a potentiating role in the development and maintenance of addiction-like behaviors during abstinence, the insula is engaged during cue or context presentations in humans126 and rodents122 and shows increased dendritic complexity after chronic nicotine exposure and withdrawal in rodents.127 Although the case for a hyperactive insula in addiction is compelling, there is also a case for loss of insular activity with chronic drug exposure and abstinence. Reduced gray matter volume is observed in the insula cortex of drug users as well as decreased regional activity during decision-making processes,128 and stimulation of the insula in rodent models decreases nicotine self-administration.129 The only study to evaluate the potential function of insula—BNST connectivity shows that inactivation of either population blocks the ability of safety signals to decrease stress-associated decrements in social interaction,130 although the authors do not test the projection directly and posit that it occurs via a relay in the BLA. This hypothesis will need to be tested in future studies.
Parabrachial Nucleus.
The parabrachial nucleus (PBN) provides a dense glutamatergic input to the BNST that extends from the oval to the ventrolateral subnuclei but remains largely unexplored.131 These synapses tend to be axosomatic in structure and likely instructive in functionality,132,133 as introduction of channelrhodopsin and elicitation of light-evoked currents is capable of initiating action potentials or hyperpolarizing BNST neurons recorded in whole-cell electro-physiology.134 The behavioral relevance of PBN-BNST projections has yet to be evaluated, yet PBN neurons and specifically those projection neurons positive for the neuropeptide calcitonin-gene related peptide (CGRP) have been implicated in a number of behaviors, including pain, thirst, taste, and other physiologic processes.135 Specifically, CGRP+ PBN neurons are activated by diverse events such as noxious stimulation (both internal and external), satiation, consumption of novel foods, and auditory cues in a fear conditioning paradigm, and are critical for food neophobia and conditioned fear responses.136 Together, these data suggest that the PBN plays a role both in the processing of danger signals and engaging those adaptive responses that will limit resultant harm.136 CGRP corelease with glutamate from PBN afferents in the BNST mimics the actions of intracerebroventricular (ICV) administration of CGRP in the induction of anxiety and leads to recruitment of other brain regions involved in the stress response,137 suggesting relevance to abstinence-induced negative affect and stress-induced reinstatement. The parallel CGRP+ projection from the PBN to the CeA encodes the affective components of pain138 and is critically involved in pain-associated plasticity and excitatory transmission in this region,139–142 providing a framework for PBN circuits in the extended amygdala interacting with chronic stressors. Interestingly, though, CGRP appears to have excitatory actions in the CeA but potentiate GABAA-mediated currents in the BNST, potentially leading to increased inhibition, highlighting potential regional differences.143
Paraventricular Nucleus of the Thalamus.
The paraventricular nucleus of the thalamus (PVT) also provides a dense glutamatergic input to the lateral BNST that has been extensively studied by anatomical methods but minimally by functional methods.144 Preclinical data support a role for the PVT in drug-seeking behaviors, as activity within the subnucleus has been shown to be critical for expression of cocaine conditioned place preference as well as cue- and drug-induced reinstatement of cocaine-seeking.145–148 In humans, the thalamus is activated by drug cues but shows reduced activity during response inhibition,149 suggesting a role in addiction-related processes. The projection from the PVT to the NAc has been heavily investigated as the nexus for connections between PVT activity and drug-seeking behavior. Consistent with this notion, drug exposure increases excitability and induces synaptic plasticity in neurons projecting from PVT to NAc,150–152 and in vivo depotentiation of this pathway by an LTD protocol can limit the expression of withdrawal-associated symptomatology.153 Interestingly, there is heavy collateralization between PVT projections to the NAc, the BNST, and the central nucleus of the amygdala (CeA), suggesting that the same synaptic plasticity that is occurring in projections from the PVT to the NAc might also be occurring in projections to the BNST.144 Furthermore, PVT fibers appose with extended amygdala neurons positive for the neuropeptide corticotropin releasing factor (CRF), suggesting an interaction that may underlie abstinence-induced changes in plasticity and behavior.154 Future studies should aim to explore the function of this projection in the context of reinstatement behaviors.
Together, the glutamatergic inputs from the prefrontal cortex, ventral subiculum, basolateral amygdala, insular cortex, parabrachial nucleus, and paraventricular nucleus of the thalamus are potential candidates for mediating the changes in input-specific synaptic plasticity in the BNST that occur with protracted abstinence from drugs of abuse that prime the brain for relapse to drug-seeking behaviors. Future experiments should aim to connect changes in the strength of these inputs to the different types of plasticity observed in the BNST and determine the directionality (i.e., LTP or LTD) and behavioral relevance of these effects. This increased understanding of the input specificity underlying abstinence-induced changes in plasticity will open the door to more focused pharmacological studies aimed at protecting against these maladaptive changes.
■ CELL-TYPE SPECIFICITY: PROJECTION TARGETS AND GENETIC MARKERS
Studies of plasticity in the BNST have mostly focused on effects within neuronal populations through field potential recordings or unidentified neurons through in vivo or ex vivo electro-physiology. Although determining the source of input to a defined neuronal population will be important for circuit-based interventions, the outcome of LTP or LTD will converge on increased or decreased likelihood of neuronal firing and neurotransmitter release in the BNST neurons themselves. Then, depending on the projection target and the neuro-transmitter packaged for release, this change in activity will be translated into increased or decreased likelihood of reinstatement of drug-seeking or related behaviors. Differentiating cell-specific mechanisms of plasticity in the BNST will be especially important due to the known heterogeneity of and complex inter-relationships among cells within the BNST.155,156
Within the rat BNST, neurons positive for the neuropeptide CRF undergo HFS-induced LTP that is further potentiated by repeated restraint stress specifically in Type III CRF neurons that are defined by their electrophysiological response to positive and negative current injection.54 This process was determined to be dependent on a lack of expression of striatal-enriched tyrosine phosphatase (STEP), an enzyme that shows downregulation with repeated restraint stress. LTP in CRF neurons would thus be expected to increase CRF release either in the BNST from interneurons or in projection targets. As CRF signaling is critical for cocaine-induced enhancement of short-term potentiation,42 stressful stimuli that engage LTP of this population may exacerbate changes in excitatory transmission within the region as a result of extended drug exposure and have long-term effects on BNST plasticity. Extrahypothalamic CRF is critical for stress-induced reinstatement of drug-seeking behavior, as CRF itself can act as a stimulus for reinstatement, and CRFR1 antagonists reduce stress-induced reinstatement when injected systemically or directly to the BNST.157,158 The role of CRF is independent of the drug of abuse, the type of stressor, or the experimental procedure used.159 CRF levels in the BNST are elevated during withdrawal from alcohol and normalize after re-exposure,31 a process that will lead to increased engagement of a BNST-VTA projection population via enhancement of excitatory transmission.160 Thus, LTP of BNST CRF neurons and enhancement of this process by chronic stressors may drive reinstatement behaviors. Both the connection between CRF and LTP as well as the role of other neuropeptides and genetic markers expressed in the BNST, including but not limited to neuropeptide Y (NPY), somatostatin (SST), and protein kinase Cδ (PKCδ), remain to be further explored (for a review of neuropeptide signaling in the BNST, see Kash et al.161 and Daniel and Rainnie162).
In addition to defining neuronal populations by their expression pattern of genetic markers, BNST neurons can also be defined based on their projection target. Although not directly applied to plasticity changes in the BNST or with abstinence and reinstatement, this approach allowed for distinction of projection-specific functions that contribute to the various phenotypes associated with anxiety-like behavior in rodent models. Specifically, optogenetic excitation of BNST afferents in the lateral hypothalamus (LH) elicits reduced risk avoidance in the elevated plus maze, while those in the PBN elicits reduced respiratory rate and those in the VTA elicits increased positive valence.103 Although these responses have not been fully translated into the behaviors seen with reinstatement of drug-seeking, nor have the behaviors seen with reinstatement of drug-seeking been elegantly dissected in this manner, one could imagine parallels of these effects in the rodent undergoing protracted abstinence from drugs of abuse.
Finally, the data presented thus far suggest that experience-dependent or stimulation-induced LTP in either CRF+ neurons or anxiogenesis-inducing projections from the BNST to the VTA, LH, and PBN would likely increase the probability of reinstatement. This begs the question of whether the LTD observed through a variety of mechanisms described above could represent a therapeutic end-goal for reducing the ability of stress or other factors to re-engage the circuitry necessary for reinitiating drug-seeking behaviors. However, Gq-mediated LTD initiated by the hM3Dq DREADD in VGAT+ BNST neurons elicited anxiety-like behaviors and lead to increased engagement of downstream neuronal activity in the VTA, the PBN, and the locus coeruleus (LC), suggesting that either LTD occurs in interneurons in the BNST and leads to disinhibition of BNST projection neurons, or that decreased firing of BNST neurons leads to disinhibition of downstream target neurons in the VTA and other regions of interest. New genetic tools like INTRSECT (Intronic Recombinase Sites Enabling Combinatorial Targeting) will allow for populations like interneurons to be isolated with or without the use of a genetic marker and studied to answer questions such as these.163
■ MODULATION OF PLASTICITY: CATECHOLAMINE RECEPTOR MODULATION
As more information is gained on the mechanism and specificity of LTP and LTD, molecular targets for modulation of these processes are likely to emerge. Currently, much focus has been placed on NMDAR antagonists such as Ro25-6981, as they have been shown to attenuate ex vivo and in vivo LTP in the BNST.37,55,56 This work has promising applications related to the clinical use of ketamine and other similar drugs but may be limited due to off-target as well as undesired on-target effects. Due to the complex and bidirectional actions on BNST synaptic plasticity and neurotransmission in general, targeting of the receptors for the catecholamines dopamine and norepinephrine represents a potential therapeutic modality for these synaptic changes as well as their resultant behavioral outcomes. Dopamine and norepinephrine input to the BNST spans the dorsoventral extent of the nucleus with a patterned distribution where dopamine input is more prominent in the dorsal aspect and norepinephrine in the ventral (Figure 1).164
Dopamine.
Release of dopamine in the BNST through stimulant action or exogenous drug actions at dopamine receptors is permissive and enhancing of the molecular changes that underlie STP and LTP in the region. Many drugs of abuse, including morphine, nicotine, cocaine, and alcohol, lead to increased extracellular levels of dopamine in the BNST as they do in the nucleus accumbens.19 Palatable substances like sucrose also increase dopamine, while aversive stimuli such as quinine reduce these levels, a profile opposite that of norepinephrine.165 Once released, dopamine initiates excitatory actions in the BNST, enhancing glutamatergic transmission via signaling at D1 and D2 receptors in an activity- and CRFR1-dependent manner in the mouse42 and inhibiting GABAergic transmission via D2Rs in the rat oval subnucleus.166 Potentiating these excitatory actions, dopamine enhances the short-term component of high frequency stimulation-induced LTP in the mouse BNST.42 Upon contingent drug exposure paradigms, however, these excitatory actions become inhibitory, with upregulation of the D1R leading to enhancement and LTP of GABAa inhibitory postsynaptic currents (IPSCs) not seen in control, sucrose self-administering, cocaine self-administration acquiring, and cocaine-yoked rats.167 This GABAA LTP involves c-Srk tyrosine kinase and neurotensin receptor signaling pathways and is independent of canonical G-protein and other tyrosine kinase signaling pathways. Further, the magnitude GABAA LTP is proportional to the break-point on the progressive ratio schedule of cocaine reinforcement, a measure that defines motivation for reward-seeking behaviors. Inhibitory actions of dopamine can also be seen on NMDAR currents in ex vivo brain slices from cocaine self-administering rats via D1Rs and D2Rs via activation of PLC and protein phosphatases168 as well as on LTP of excitatory transmission in the rat jcBNST in a D1-dependent manner.48 Regardless of the directionality of effects on neurotransmission in the region, blockade of dopamine signaling at D1Rs in the BNST alters drug-seeking behaviors as evidenced by decreased alcohol-motivated responding20 and decreased cocaine reinforcement,18 suggesting that targeting dopamine-mediated transmission and its downstream effects on plasticity represents a therapeutic modality for drug- and alcohol-seeking behaviors.
Norepinephrine.
Norepinephrine receptor modulators are specifically known to modulate stress-induced reinstatement of drug-seeking behavior and also to affect BNST plasticity. Specifically, disruption of the norepinephrine input to the BNST as well as inhibition of β- and α1-adrenergic receptor signaling and agonism of α2-adrenergic receptors inhibits stress-induced reinstatement of drug-seeking behaviors.28,169,170 In ex vivo brain slices, 20 but not 10 min application of norepinephrine (100 μM) initiates LTD mimicking α1-AR-LTD,65 and α1-AR-LTD is disrupted as a result of aberrant norepinephrine signaling in the norepinephrine transporter (NET) and α2A-AR knockout mouse model.65 In the BLA, norepinephrine is known to gate LTP via decreased inhibitory tone on pyramidal projection neurons likely via actions on local inhibitory interneurons, enabling the plastic changes that occur with fear conditioning paradigms.73 If norepinephrine-mediated α1-AR-LTD were to occur in BNST inhibitory interneurons and decrease their inhibition of BNST-VTA projection neurons, for example, a similar process may control BNST-dependent reinstatement behaviors.
Modulation of norepinephrine action and potential effects of norepinephrine on abstinence-induced changes in plasticity represents a promising therapeutic strategy for addiction. These effects could be due to direct inhibition of norepinephrine release via autoreceptor α2-ARs or via blockade of norepinephrine actions at α1-ARs, β-ARs, or heteroceptor α2-ARs. Direct administration of β1- and β2-AR antagonists or α2-AR agonists to the BNST blocks stress-induced reinstatement of cocaine- and morphine-seeking behaviors,171–174 highlighting a role for these receptors in the process that we hypothesize is connected to abstinence-induced changes in plasticity. Similarly, direct administration of α1-, β1-, and β2-AR antagonists to the BNST elicits anxiolysis.175 With respect to changes in activity, briefly, β-ARs broadly enhance BNST neuronal activity via a postsynaptic mechanism, while α1-ARs elicit LTD and α2-ARs inhibit both norepinephrine and glutamate release (for a more detailed review, see Flavin and Winder176). β-AR activity specifically enhances BNST activity through a microcircuit involving CRF signaling, while BNST α2A-ARs inhibit glutamate release in an input-specific manner at PBN but not BLA terminals,134,177 highlighting the potential for actions that can be targeted to specific BNST inputs and outputs as more information is gleaned from work on the specificity of changes in plasticity as described above. In addition, Gq-coupled LTD-mediated activation of downstream components of addiction-related neurocircuitry178 and potential excitatory actions of α2A-ARs within the BNST134,179 highlight the possibility that activation of inhibitory signaling cascades and their effects on neuronal activity, and thus neuronal plasticity, are not always direct and thus may inform future work in the area of plasticity.
■ CONCLUDING REMARKS AND FUTURE DIRECTIONS
Chronic exposure to drugs and stress leads to changes in neurotransmission and synaptic plasticity in the bed nucleus of the stria terminalis, a component of the extended amygdala critical for reinstatement of drug- and alcohol-seeking behaviors. We hypothesize that long-term changes in activity in this region prime the addict or alcoholic for relapse during protracted abstinence. Here, we discuss the mechanisms underlying long-term potentiation and depression in the region and highlight how these changes interact with a history of stress and drug exposure in rodent models. In addition, we highlight important future directions including input- and cell-specific bidirectional changes in activity. A better understanding of the molecular correlates and mechanisms underlying synaptic plasticity will lead to more effective treatment strategies that have the ultimate goal of decreasing the emergence of reinstatement behaviors during abstinence. Here, we discuss the role of catecholamine receptor modulators in the process, as inhibition of both dopamine and norepinephrine receptors affect reinstatement behaviors, while activation enhances or initiates LTP and LTD, respectively. We hope that the insights gained from studying the specific changes that underlie synaptic plasticity in the BNST during protracted abstinence from alcohol and other drugs of abuse will provide insight into the biology underlying relapse behavior in human addicts and alcoholics and inform future treatment modalities for this complex biological problem.
Acknowledgments
Funding
This work was supported by National Institutes of Health Grants R01DA042475 (D.G.W.), R37AA019455 (D.G.W.), F30DA042501 (N.A.H.), and T32GM07347 (N.A.H.).
Footnotes
Notes
The authors declare no competing financial interest.
REFERENCES
- (1).Lopez-Quintero C, et al. (2011) Probability and predictors of remission from life-time nicotine, alcohol, cannabis or cocaine dependence: Results from the National Epidemiologic Survey on Alcohol and Related Conditions. Addiction 106, 657–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (2).Degenhardt L, et al. (2013) Global burden of disease attributable to illicit drug use and dependence: findings from the Global Burden of Disease Study 2010. Lancet 382, 1564–1574. [DOI] [PubMed] [Google Scholar]
- (3).Ledgerwood DM, and Petry NM (2006) What do we know about relapse in pathological gambling? Clin. Psychol. Rev 26, 216–228. [DOI] [PubMed] [Google Scholar]
- (4).George O, Le Moal M, and Koob GF (2012) Allostasis and addiction: Role of the dopamine and corticotropin-releasing factor systems. Physiol. Behav 106, 58–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).Koob GF, and Volkow ND (2010) Neurocircuitry of addiction. Neuropsychopharmacology 35, 217–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).Lu L, Grimm JW, Hope BT, and Shaham Y (2004) Incubation of cocaine craving after withdrawal: A review of preclinical data. Neuropharmacology 47, 214–226. [DOI] [PubMed] [Google Scholar]
- (7).Shalev U (2002) Neurobiology of Relapse to Heroin and Cocaine Seeking: A Review. Pharmacol. Rev 54, 1–42. [DOI] [PubMed] [Google Scholar]
- (8).Brown SA, Vik PW, Patterson TL, Grant I, and Schuckit MA (1995) Stress, vulnerability and adult alcohol relapse. J. Stud. Alcohol 56, 538–545. [DOI] [PubMed] [Google Scholar]
- (9).Sinha R, Shaham Y, and Heilig M (2011) Translational and reverse translational research on the role of stress in drug craving and relapse. Psychopharmacology 218, 69–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).Shaham Y, Erb S, and Stewart J (2000) Stress-induced relapse to heroin and cocaine seeking in rats: A review. Brain Res. Rev 33, 13–33. [DOI] [PubMed] [Google Scholar]
- (11).De Vries TJ, Binnekade R, Mulder AH, and Vanderschuren LJMJ (1998) Drug - induced reinstatement of heroin - and cocaine - seeking behaviour following long - term extinction is associated with expression of behavioural sensitization. Eur. J. Neurosci 10, 3565–3571. [DOI] [PubMed] [Google Scholar]
- (12).Shaham Y, Shalev U, Lu L, De Wit H, and Stewart J (2003) The reinstatement model of drug relapse: History, methodology and major findings. Psychopharmacology 168, 3–20. [DOI] [PubMed] [Google Scholar]
- (13).Crombag HS, Bossert JM, Koya E, and Shaham Y (2008) Context-induced relapse to drug seeking: a review. Philos. Trans. R. Soc, B 363, 3233–3243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Pierce RC, and Kumaresan V (2006) The mesolimbic dopamine system: The final common pathway for the reinforcing effect of drugs of abuse? Neurosci. Biobehav. Rev 30, 215–238. [DOI] [PubMed] [Google Scholar]
- (15).Kauer JA, and Malenka RC (2007) Synaptic plasticity and addiction. Nat. Rev. Neurosci 8, 844–858. [DOI] [PubMed] [Google Scholar]
- (16).Nestler EJ, Hope BT, and Widnell KL (1993) Drug addiction: A model for the molecular basis of neural plasticity. Neuron 11, 995–1006. [DOI] [PubMed] [Google Scholar]
- (17).Lüscher C, and Malenka RC (2011) Drug-Evoked Synaptic Plasticity in Addiction: From Molecular Changes to Circuit Remodeling. Neuron 69, 650–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (18).Epping-Jordan MP, Markou A, and Koob GF (1998) The dopamine D-1 receptor antagonist SCH 23390 injected into the dorsolateral bed nucleus of the stria terminalis decreased cocaine reinforcement in the rat. Brain Res 784, 105–115. [DOI] [PubMed] [Google Scholar]
- (19).Carboni E, Silvagni a., Rolando MT, and Di Chiara G (2000) Stimulation of in vivo dopamine transmission in the bed nucleus of stria terminalis by reinforcing drugs. J. Neurosci 20, RC102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).Eiler WJA, Seyoum R, Foster KL, Mailey C, and June HL (2003) D1 dopamine receptor regulates alcohol-motivated behaviors in the bed nucleus of the stria terminalis in alcohol-preferring (P) rats. Synapse 48, 45–56. [DOI] [PubMed] [Google Scholar]
- (21).Alheid GF, and Heimer L (1988) New perspectives in basal forebrain organization of special relevance for neuropsychiatric disorders: The striatopallidal, amygdaloid, and corticopetal components of substantia innominata. Neuroscience 27, 1–39. [DOI] [PubMed] [Google Scholar]
- (22).Alheid GF (2003) Extended amygdala and basal forebrain. Ann. N. Y. Acad. Sci 985, 185–205. [DOI] [PubMed] [Google Scholar]
- (23).Koob GF (2009) Brain stress systems in the amygdala and addiction. Brain Res 1293, 61–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (24).Walker DL, and Davis M (2008) Role of the extended amygdala in short-duration versus sustained fear: A tribute to Dr. Lennart Heimer. Brain Struct. Funct 213, 29–42. [DOI] [PubMed] [Google Scholar]
- (25).Jennings JH, et al. (2013) Distinct extended amygdala circuits for divergent motivational states. Nature 496, 224–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (26).Adhikari A (2014) Distributed circuits underlying anxiety. Front. Behav. Neurosci 8, 112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (27).Sartor GC, and Aston-Jones G (2012) Regulation of the ventral tegmental area by the bed nucleus of the stria terminalis is required for expression of cocaine preference. Eur. J. Neurosci 36, 3549–3558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (28).Shalev U, Morales M, Hope BT, Yap J, and Shaham Y (2001) Time-dependent changes in extinction behavior and stress-induced reinstatement of drug seeking following withdrawal from heroin in rats. Psychopharmacology 156, 98–107. [DOI] [PubMed] [Google Scholar]
- (29).McFarland K, Davidge SB, Lapish CC, and Kalivas PW (2004) Limbic and motor circuitry underlying footshock-induced reinstatement of cocaine-seeking behavior. J. Neurosci 24, 1551–1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (30).Delfs J, Zhu Y, Druhan JP, and Aston-Jones G (2000) Noradrenaline in the ventral forebrain is critical for opiate withdrawal-induced aversion. Nature 403, 430–434. [DOI] [PubMed] [Google Scholar]
- (31).Olive MF, Koenig HN, Nannini MA, and Hodge CW (2002) Elevated extracellular CRF levels in the bed nucleus of the stria terminalis during ethanol withdrawal and reduction by subsequent ethanol intake. Pharmacol., Biochem. Behav 72, 213–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (32).Lovinger DM, and Kash TL (2015) Mechanisms of Neuroplasticity and Ethanol’s Effects on Plasticity in the Striatum and Bed Nucleus of the Stria Terminalis. Alcohol Res 37, 109–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (33).Normandeau CP, and Dumont EC (2017) Cocaine and Dysregulated Synaptic Transmission in the Bed Nucleus of the Stria Terminalis. Neurosci. Cocaine, 537–543. [Google Scholar]
- (34).McElligott ZA, and Winder DG (2009) Modulation of glutamatergic synaptic transmission in the bed nucleus of the stria terminalis. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 33, 1329–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (35).Weitlauf C, Egli RE, Grueter BA, and Winder DG (2004) High-Frequency Stimulation Induces Ethanol-Sensitive Long-Term Potentiation at Glutamatergic Synapses in the Dorsolateral Bed Nucleus of the Stria Terminalis. J. Neurosci 24, 5741–5747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (36).Weitlauf C, et al. (2005) Activation of NR2A-Containing NMDA Receptors Is Not Obligatory for NMDA Receptor-Dependent Long-Term Potentiation. J. Neurosci 25, 8386–8390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (37).Wills TA, et al. (2012) GluN2B subunit deletion reveals key role in acute and chronic ethanol sensitivity of glutamate synapses in bed nucleus of the stria terminalis. Proc. Natl. Acad. Sci. U. S. A 109, E278–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (38).Kash TL, Matthews RT, and Winder DG (2008) Alcohol inhibits NR2B-containing NMDA receptors in the ventral bed nucleus of the stria terminalis. Neuropsychopharmacology 33, 1379–1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (39).Kash TL, Baucum AJ, Conrad KL, Colbran RJ, and Winder DG (2009) Alcohol exposure alters NMDAR function in the bed nucleus of the stria terminalis. Neuropsychopharmacology 34, 2420–2429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (40).Conrad KL, Louderback KM, Gessner CP, and Winder DG (2011) Stress-induced alterations in anxiety-like behavior and adaptations in plasticity in the bed nucleus of the stria terminalis. Physiol. Behav 104, 248–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (41).Conrad KL, and Winder DG (2011) Altered anxiety-like behavior and long-term potentiation in the bed nucleus of the stria terminalis in adult mice exposed to chronic social isolation, unpredictable stress, and ethanol beginning in adolescence. Alcohol 45, 585–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (42).Kash TL, Nobis WP, Matthews RT, and Winder DG (2008) Dopamine Enhances Fast Excitatory Synaptic Transmission in the Extended Amygdala by a CRF-R1-Dependent Process. J. Neurosci 28, 13856–13865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (43).Dumont EC, Mark GP, Mader S, and Williams JT (2005) Self-administration enhances excitatory synaptic transmission in the bed nucleus of the stria terminalis. Nat. Neurosci 8, 413–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (44).Debacker J, et al. (2015) GluN2B-containing NMDA receptors blockade rescues bidirectional synaptic plasticity in the bed nucleus of the stria terminalis of cocaine self-administering rats. Neuropsychopharmacology 40, 394–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (45).Dumont EC, Rycroft BK, Maiz J, and Williams JT (2008) Morphine produces circuit-specific neuroplasticity in the bed nucleus of the stria terminalis. Neuroscience 153, 232–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (46).Dong HW, Petrovich GD, and Swanson LW (2000) Organization of projections from the juxtacapsular nucleus of the BST: A PHAL study in the rat. Brain Res 859, 1–14. [DOI] [PubMed] [Google Scholar]
- (47).Larriva-Sahd J (2004) Juxtacapsular nucleus of the stria terminalis of the adult rat: Extrinsic inputs, cell types, and neuronal modules: A combined golgi and electron microscopic study. J. Comp. Neurol 475, 220–237. [DOI] [PubMed] [Google Scholar]
- (48).Francesconi W, Berton F, Koob GF, and Sanna PP (2009) Intrinsic neuronal plasticity in the juxtacapsular nucleus of the bed nuclei of the stria terminalis (jcBNST). Prog. Neuro-Psychopharmacol. Biol. Psychiatry 33, 1347–1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (49).Francesconi W, et al. (2017) Opiate dependence induces cell type-specific plasticity of intrinsic membrane properties in the rat juxtacapsular bed nucleus of stria terminalis (jcBNST). Psychopharmacology 234, 3485–3498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (50).Hazra R, et al. (2011) A transcriptomic analysis of type I-III neurons in the bed nucleus of the stria terminalis. Mol. Cell. Neurosci 46, 699–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (51).Hammack SE, Mania I, and Rainnie DG (2007) Differential expression of intrinsic membrane currents in defined cell types of the anterolateral bed nucleus of the stria terminalis. J. Neurophysiol 98, 638–56. [DOI] [PubMed] [Google Scholar]
- (52).Szücs A, Berton F, Sanna PP, and Francesconi W (2012) Excitability of jcBNST neurons is reduced in alcohol-dependent animals during protracted alcohol withdrawal. PLoS One 7, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (53).Francesconi W, et al. (2009) Protracted withdrawal from alcohol and drugs of abuse impairs long-term potentiation of intrinsic excitability in the juxtacapsular bed nucleus of the stria terminalis. J. Neurosci 29, 5389–5401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (54).Dabrowska J, et al. (2013) Striatal-enriched protein tyrosine phosphatase - STEPs toward understanding chronic stress-induced activation of corticotrophin releasing factor neurons in the rat bed nucleus of the stri aterminalis. Biol. Psychiatry 74, 817–826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (55).Glangetas C, et al. (2015) Ventral Subiculum Stimulation Promotes Persistent Hyperactivity of Dopamine Neurons and Facilitates Behavioral Effects of Cocaine. Cell Rep 13, 2287–2296. [DOI] [PubMed] [Google Scholar]
- (56).Glangetas C, et al. (2017) In vivo single-cell homeostatic plasticity in the Bed Nucleus of the Stria terminalis triggers long-term anxiolysis. Nat. Commun 8, 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (57).Daniel SE, Guo J, and Rainnie DG (2017) A comparative analysis of the physiological properties of neurons in the anterolateral bed nucleus of the stria terminalis in the Mus musculus, Rattus norvegicus, and Macaca mulatta. J. Comp. Neurol 525, 2235–2248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (58).Kaufling J, Girard D, Maitre M, Leste-Lasserre T, and Georges F (2017) Species-specific diversity in the anatomical and physiological organisation of the BNST-VTA pathway. Eur. J. Neurosci 45, 1230–1240. [DOI] [PubMed] [Google Scholar]
- (59).Winder DG, and Sweatt J (2001) Roles of serine/threonine phosphatases in hippocampel synaptic plasticity. Nat. Rev. Neurosci 2, 461–474. [DOI] [PubMed] [Google Scholar]
- (60).Grueter BA, and Winder DG (2005) Group II and III metabotropic glutamate receptors suppress excitatory synaptic transmission in the dorsolateral bed nucleus of the stria terminalis. Neuropsychopharmacology 30, 1302–11. [DOI] [PubMed] [Google Scholar]
- (61).Grueter BA, McElligott ZA, Robison AJ, Mathews GC, and Winder DG (2008) In vivo metabotropic glutamate receptor 5 (mGluR5) antagonism prevents cocaine-induced disruption of postsynaptically maintained mGluR5-dependent long-term depression. J. Neurosci 28, 9261–9270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (62).Grueter BA, Mcelligott ZA, and Robison AJ (2008) In vivo mGluR5 antagonism prevents cocaine-induced disruption of postsynaptically-maintained mGluR5-dependent long-term depression. J. Neurosci 28, 9261–9270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (63).Grueter BA, et al. (2006) Extracellular-signal regulated kinase 1-dependent metabotropic glutamate receptor 5-induced long-term depression in the bed nucleus of the stria terminalis is disrupted by cocaine administration. J. Neurosci 26, 3210–3219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (64).McElligott ZA, et al. (2010) Distinct forms of Gq-receptor-dependent plasticity of excitatory transmission in the BNST are differentially affected by stress. Proc. Natl. Acad. Sci. U. S. A 107, 2271–2276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (65).McElligott ZA, and Winder DG (2008) Alpha1-adrenergic receptor-induced heterosynaptic long-term depression in the bed nucleus of the stria terminalis is disrupted in mouse models of affective disorders. Neuropsychopharmacology 33, 2313–2323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (66).Mazzone CM, et al. (2016) Acute engagement of Gq-mediated signaling in the bed nucleus of the stria terminalis induces anxiety-like behavior. Mol. Psychiatry 23, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (67).Niswender CM, and Conn PJ (2010) Metabotropic Glutamate Receptors: Physiology, Pharmacology, and Disease. Annu. Rev. Pharmacol. Toxicol 50, 295–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (68).Glangetas C, et al. (2013) Stress Switches Cannabinoid Type-1 (CB1) Receptor-Dependent Plasticity from LTD to LTP in the Bed Nucleus of the Stria Terminalis. J. Neurosci 33, 19657–19663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (69).Reisiger A-R, et al. (2014) Nicotine Self-Administration Induces CB1-Dependent LTP in the Bed Nucleus of the Stria Terminalis. J. Neurosci 34, 4285–4292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (70).Caille S, Guillem K, Cador M, Manzoni OJ, and Georges F (2009) Voluntary nicotine consumption triggers in vivo potentiation of cortical excitatory drives to midbrain dopaminergic neurons. J. Neurosci 29, 10410–10415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (71).Puente N, et al. (2011) Polymodal activation of the endocannabinoid system in the extended amygdala. Nat. Neurosci 14, 1542–1547. [DOI] [PubMed] [Google Scholar]
- (72).Sigurdsson T, Doyere V, Cain CK, and LeDoux JE (2007) Long-term potentiation in the amygdala: A cellular mechanism of fear learning and memory. Neuropharmacology 52, 215–227. [DOI] [PubMed] [Google Scholar]
- (73).Tully K, Li Y, Tsvetkov E, and Bolshakov VY (2007) Norepinephrine enables the induction of associative long-term potentiation at thalamo-amygdala synapses. Proc. Natl. Acad. Sci. U. S. A 104, 14146–14150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (74).Volkow ND, et al. (1991) Changes in brain glucose metabolism in cocaine dependence and withdrawal. Am. J. Psychiatry 148, 621–626. [DOI] [PubMed] [Google Scholar]
- (75).Sinha R, and Li CSR (2007) Imaging stress- and cue-induced drug and alcohol craving: Association with relapse and clinical implications. Drug Alcohol Rev 26, 25–31. [DOI] [PubMed] [Google Scholar]
- (76).Capriles N, Rodaros D, Sorge RE, and Stewart J (2003) A role for the prefrontal cortex in stress- and cocaine-induced reinstatement of cocaine seeking in rats. Psychopharmacology 168, 66–74. [DOI] [PubMed] [Google Scholar]
- (77).Koya E, et al. (2009) Role of ventral medial prefrontal cortex in incubation of cocaine craving. Neuropharmacology 56, 177–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (78).Willcocks AL, and Mcnally GP (2013) The role of medial prefrontal cortex in extinction and reinstatement of alcohol-seeking in rats. Eur. J. Neurosci 37, 259–268. [DOI] [PubMed] [Google Scholar]
- (79).Lu H, Cheng PL, Lim BK, Khoshnevisrad N, and Poo MM (2010) Elevated BDNF after Cocaine Withdrawal Facilitates LTP in Medial Prefrontal Cortex by Suppressing GABA Inhibition. Neuron 67, 821–833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (80).Ostrander MM, Richtand NM, and Herman JP (2003) Stress and amphetamine induce Fos expression in medial prefrontal cortex neurons containing glucocorticoid receptors. Brain Res 990, 209–214. [DOI] [PubMed] [Google Scholar]
- (81).Flak JN, Solomon MB, Jankord R, Krause EG, and Herman JP (2012) Identification of chronic stress-activated regions reveals a potential recruited circuit in rat brain. Eur. J. Neurosci 36, 2547–2555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (82).Pleil KE (2015) Effects of chronic ethanol exposure on neuronal function in the prefrontal cortex and extended amygdala. Neuropharmacology 99, 735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (83).Spencer SJ, Buller KM, and Day TA (2005) Medial prefrontal cortex control of the paraventricular hypothalamic nucleus response to psychological stress: Possible role of the bed nucleus of the stria terminalis. J. Comp. Neurol 481, 363–376. [DOI] [PubMed] [Google Scholar]
- (84).Johnson SB, et al. (2016) A Basal Forebrain Site Coordinates the Modulation of Endocrine and Behavioral Stress Responses via Divergent Neural Pathways. J. Neurosci 36, 8687–8699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (85).Radley JJ, Anderson RM, Hamilton BA, Alcock JA, and Romig-Martin SA (2013) Chronic Stress-Induced Alterations of Dendritic Spine Subtypes Predict Functional Decrements in an Hypothalamo-Pituitary-Adrenal-Inhibitory Prefrontal Circuit. J. Neuro-sci 33, 14379–14391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (86).Radley JJ, Gosselink KL, and Sawchenko PEA (2009) Discrete GABAergic Relay Mediates Medial Prefrontal Cortical Inhibition of the Neuroendocrine Stress Response. J. Neurosci 29, 7330–7340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (87).Mcdonald AJ, Mascagni F, and Guo L (1996) Projections of the medial and lateral prefrontal cortices to the amygdala: A Phaseolus vulgaris leucoagglutinin study in the rat. Neuroscience 71, 55–75. [DOI] [PubMed] [Google Scholar]
- (88).Cullinan WE, Herman JP, and Watson SJ (1993) Ventral subicular interactions with the hypothalamic paraventricular nucleus: evidence for a relay in the bed nucleus of the stria terminalis. J. Comp. Neurol 332, 1–20. [DOI] [PubMed] [Google Scholar]
- (89).Radley JJ, and Sawchenko PEA (2011) Common Substrate for Prefrontal and Hippocampal Inhibition of the Neuroendocrine Stress Response. J. Neurosci 31, 9683–9695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (90).Keralapurath MM, Clark JK, Hammond S, and Wagner JJ (2014) Cocaine- or stress-induced metaplasticity of LTP in the dorsal and ventral hippocampus. Hippocampus 24, 577–590. [DOI] [PubMed] [Google Scholar]
- (91).Grigoryan G, Ardi Z, Albrecht A, Richter-Levin G, and Segal M (2015) Juvenile stress alters LTP in ventral hippocampal slices: Involvement of noradrenergic mechanisms. Behav. Brain Res 278, 559–562. [DOI] [PubMed] [Google Scholar]
- (92).Thompson AM, Swant J, Gosnell BA, and Wagner JJ (2004) Modulation of long-term potentiation in the rat hippocampus following cocaine self-administration. Neuroscience 127, 177–185. [DOI] [PubMed] [Google Scholar]
- (93).del Olmo N, et al. (2006) Enhancement of hippocampal long-term potentiation induced by cocaine self-administration is maintained during the extinction of this behavior. Brain Res 1116, 120–126. [DOI] [PubMed] [Google Scholar]
- (94).Caffino L, et al. (2014) Cocaine-induced glutamate receptor trafficking is abrogated by extinction training in the rat hippocampus. Pharmacol. Rep 66, 198–204. [DOI] [PubMed] [Google Scholar]
- (95).Keralapurath MM, Briggs SB, and Wagner JJ (2017) Cocaine self-administration induces changes in synaptic transmission and plasticity in ventral hippocampus. Addict. Biol 22, 446–456. [DOI] [PubMed] [Google Scholar]
- (96).Sun W, and Rebec GV (2003) Lidocaine inactivation of ventral subiculum attenuates cocaine-seeking behavior in rats. J. Neurosci 23, 10258–10264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (97).Rogers JL, and See RE (2007) Selective inactivation of the ventral hippocampus attenuates cue-induced and cocaine-primed reinstatement of drug-seeking in rats. Neurobiol Learn. Mem 87, 688–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (98).Lasseter HC, Xie X, Ramirez DR, and Fuchs R A. (2010) Sub-region specific contribution of the ventral hippocampus to drug context-induced reinstatement of cocaine-seeking behavior in rats. Neuroscience 171, 830–839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (99).Atkins AL, Mashhoon Y, and Kantak KM (2008) Hippocampal regulation of contextual cue-induced reinstatement of cocaine-seeking behavior. Pharmacol., Biochem. Behav 90, 481–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (100).Taepavarapruk P, and Phillips AG (2003) Neurochemical correlates of relapse to D-amphetamine self-administration by rats induced by stimulation of the ventral subiculum. Psychopharmacology 168, 99–108. [DOI] [PubMed] [Google Scholar]
- (101).De Bellis MD, et al. (2000) Hippocampal volume in adolescent-onset alcohol use disorders. Am. J. Psychiatry 157, 737–744. [DOI] [PubMed] [Google Scholar]
- (102).Teicher MH, Anderson CM, and Polcari A (2012) Childhood maltreatment is associated with reduced volume in the hippocampal subfields CA3, dentate gyrus, and subiculum. Proc. Natl. Acad. Sci. U. S. A 109, E563–E572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (103).Kim S-Y, et al. (2013) Diverging neural pathways assemble a behavioural state from separable features in anxiety. Nature 496, 219–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (104).Dong HW, Petrovich GD, and Swanson LW (2001) Topography of projections from amygdala to bed nuclei of the stria terminalis. Brain Res. Rev 38, 192–246. [DOI] [PubMed] [Google Scholar]
- (105).Fuchs RA, et al. (2005) The role of the dorsomedial prefrontal cortex, basolateral amygdala, and dorsal hippocampus in contextual reinstatement of cocaine seeking in rats. Neuropsychopharmacology 30, 296–309. [DOI] [PubMed] [Google Scholar]
- (106).Meil WM, and See RE (1997) Lesions of the basolateral amygdala abolish the ability of drug associated cues to reinstate responding during withdrawal from self- administered cocaine. Behav. Brain Res 87, 139–148. [DOI] [PubMed] [Google Scholar]
- (107).Grimm JW, and See RE (2000) Dissociation of primary and secondary reward-relevant limbic nuclei in an animal model of relapse. Neuropsychopharmacology 22, 473–479. [DOI] [PubMed] [Google Scholar]
- (108).Kantak KM, Black Y, Valencia E, Green-Jordan K, and Eichenbaum HB (2002) Dissociable effects of lidocaine inactivation of the rostral and caudal basolateral amygdala on the maintenance and reinstatement of cocaine-seeking behavior in rats. J. Neurosci 22, 1126–1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (109).Meredith GE, Callen S, and Scheuer DA (2002) Brain-derived neurotrophic factor expression is increased in the rat amygdala, piriform cortex and hypothalamus following repeated amphetamine administration. Brain Res 949, 218–227. [DOI] [PubMed] [Google Scholar]
- (110).Neisewander JL, et al. (2000) Fos protein expression and cocaine-seeking behavior in rats after exposure to a cocaine self-administration environment. J. Neurosci 20, 798–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (111).Bonson KR, et al. (2002) Neural Systems and Cue-Induced Cocaine Craving. Neuropsychopharmacology 26, 376–386. [DOI] [PubMed] [Google Scholar]
- (112).Moaddab M, Mangone E, Ray MH, and McDannald MA (2017) Adolescent alcohol drinking renders adult drinking BLA-dependent: BLA hyper-activity as contributor to comorbid alcohol use disorder and anxiety disorders. Brain Sci 7, 151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (113).See RE, Kruzich PJ, and Grimm JW (2001) Dopamine, but not glutamate, receptor blockade in the basolateral amygdala attenuates conditioned reward in a rat model of relapse to cocaine-seeking behavior. Psychopharmacology 154, 301–310. [DOI] [PubMed] [Google Scholar]
- (114).Hill SY, et al. (2001) Right amygdala volume in adolescent and young adult offspring from families at high risk for developing alcoholism. Biol. Psychiatry 49, 894–905. [DOI] [PubMed] [Google Scholar]
- (115).Benegal V, Antony G, Venkatasubramanian G, and Jayakumar PN (2007) Gray matter volume abnormalities and externalizing symptoms in subjects at high risk for alcohol dependence. Addict. Biol 12, 122–132. [DOI] [PubMed] [Google Scholar]
- (116).Yasui Y, Breder CD, Safer CB, and Cechetto DF (1991) Autonomic Responses and Efferent Pathway From the Insular Cortex in the Rat. J. Comp. Neurol 303, 355–374. [DOI] [PubMed] [Google Scholar]
- (117).Naqvi N, Rudrauf D, Damasio H, and Bechara A (2007) Damage to the Insula Disrupts Addition to Cigarette Smoking. Science 315, 531–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (118).Abdolahi A, et al. (2015) Smoking cessation behaviors three months following acute insular damage from stroke. Addict. Behav 51, 24–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (119).Abdolahi A, et al. (2015) Damage to the insula leads to decreased nicotine withdrawal during abstinence. Addiction 110, 1994–2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (120).Forget B, Pushparaj A, and Le Foll B (2010) Granular insular cortex inactivation as a novel therapeutic strategy for nicotine addiction. Biol. Psychiatry 68, 265–271. [DOI] [PubMed] [Google Scholar]
- (121).Pushparaj A, Kim AS, Musiol M, Trigo JM, and Le Foll B (2015) Involvement of the rostral agranular insular cortex in nicotine self-administration in rats. Behav. Brain Res 290, 77–83. [DOI] [PubMed] [Google Scholar]
- (122).Contreras M, Ceric F, and Torrealba F (2007) Inactivation of the interoceptive insula disrupts drug craving and malaise induced by lithium. Science 318, 655–658. [DOI] [PubMed] [Google Scholar]
- (123).Contreras M, et al. (2012) A role for the insular cortex in long-term memory for context-evoked drug craving in rats. Neuropsychopharmacology 37, 2101–2108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (124).Cosme CV, Gutman AL, and LaLumiere R T. (2015) The Dorsal Agranular Insular Cortex Regulates the Cued Reinstatement of Cocaine-Seeking, but not Food-Seeking, Behavior in Rats. Neuropsychopharmacology 40, 2425–2433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (125).Pushparaj A, and Le Foll B (2015) Involvement of the caudal granular insular cortex in alcohol self-administration in rats. Behav. Brain Res 293, 203–207. [DOI] [PubMed] [Google Scholar]
- (126).Janes AC, et al. (2017) Revisiting the role of the insula and smoking cue-reactivity in relapse: A replication and extension of neuroimaging findings. Drug Alcohol Depend 179, 8–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (127).Ehlinger DG, Bergstrom HC, McDonald CG, and Smith RF (2012) Nicotine-induced dendritic remodeling in the insular cortex. Neurosci. Lett 516, 89–93. [DOI] [PubMed] [Google Scholar]
- (128).Droutman V, Read SJ, and Bechara A (2015) Revisiting the role of the insula in addiction. Trends Cognit. Sci 19, 414–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (129).Pushparaj A, et al. (2013) Electrical stimulation of the insular region attenuates nicotine-taking and nicotine-seeking behaviors. Neuropsychopharmacology 38, 690–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (130).Christianson JP, et al. (2011) Safety signals mitigate the consequences of uncontrollable stress via a circuit involving the sensory insular cortex and bed nucleus of the stria terminalis. Biol. Psychiatry 70, 458–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (131).Saper CB, and Loewy AD (1980) Efferent connections of the parabrachial nucleus in the rat. Brain Res 197, 291–317. [DOI] [PubMed] [Google Scholar]
- (132).Dobolyi A, Irwin S, Makara G, Usdin TB, and Palkovits M (2005) Calcitonin gene-related peptide-containing pathways in the rat forebrain. J. Comp. Neurol 489, 92–119. [DOI] [PubMed] [Google Scholar]
- (133).Shimada S, et al. (1989) Light and electron microscopic studies of calcitonin gene-related peptide-like immunoreactive terminals in the central nucleus of the amygdala and the bed nucleus of the stria terminalis of the rat. Exp. Brain Res 77, 217–220. [DOI] [PubMed] [Google Scholar]
- (134).Flavin SA, Matthews RT, Wang Q, Muly EC, and Winder DG (2014) α2A-Adrenergic Receptors Filter Parabrachial Inputs to the Bed Nucleus of the Stria Terminalis. J. Neurosci 34, 9319–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (135).Palmiter RD (2018) The Parabrachial Nucleus: CGRP Neurons Function as a General Alarm. Trends Neurosci 41, 280–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (136).Campos CA, Bowen AJ, Roman CW, and Palmiter RD (2018) Encoding of danger by parabrachial CGRP neurons. Nature 555, 617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (137).Sink KS, Walker DL, Yang Y, and Davis M (2011) Calcitonin gene-related peptide in the bed nucleus of the stria terminalis produces an anxiety-like pattern of behavior and increases neural activation in anxiety-related structures. J. Neurosci 31, 1802–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (138).Han S, Soleiman M, Soden M, Zweifel LS, and Palmiter RD (2015) Elucidating an Affective Pain Circuit that Creates a Threat Memory. Cell 162, 363–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (139).Okutsu Y, et al. (2017) Potentiation of NMDA receptor-mediated synaptic transmission at the parabrachial-central amygdala synapses by CGRP in mice. Mol. Pain 13, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (140).Shinohara K, et al. (2017) Essential role of endogenous calcitonin gene-related peptide in pain-associated plasticity in the central amygdala. Eur. J. Neurosci 46, 2149–2160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (141).Han JS (2005) Critical Role of Calcitonin Gene-Related Peptide 1 Receptors in the Amygdala in Synaptic Plasticity and Pain Behavior. J. Neurosci 25, 10717–10728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (142).Han JS, Adwanikar H, Li Z, Ji G, and Neugebauer V (2010) Facilitation of synaptic transmission and pain responses by CGRP in the amygdala of normal rats. Mol. Pain 6, 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (143).Gungor NZ, and Pare D (2014) CGRP Inhibits Neurons of the Bed Nucleus of the Stria Terminalis: Implications for the Regulation of Fear and Anxiety. J. Neurosci 34, 60–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (144).Dong X, Li S, and Kirouac GJ (2017) Collateralization of projections from the paraventricular nucleus of the thalamus to the nucleus accumbens, bed nucleus of the stria terminalis, and central nucleus of the amygdala. Brain Struct. Funct 222, 3927–3943. [DOI] [PubMed] [Google Scholar]
- (145).Browning JR, Jansen HT, and Sorg BA (2014) Inactivation of the paraventricular thalamus abolishes the expression of cocaine conditioned place preference in rats. Drug Alcohol Depend 134, 387–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (146).Matzeu A, Weiss F, and Martin-Fardon R (2015) Transient inactivation of the posterior paraventricular nucleus of the thalamus blocks cocaine-seeking behavior. Neurosci. Lett 608, 34–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (147).Kuhn BN, Klumpner MS, Covelo IR, Campus P, and Flagel SB (2018) Transient inactivation of the paraventricular nucleus of the thalamus enhances cue-induced reinstatement in goal-trackers, but not sign-trackers. Psychopharmacology 235, 999–1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (148).Wunsch AM, et al. (2017) Chemogenetic inhibition reveals midline thalamic nuclei and thalamo-accumbens projections mediate cocaine-seeking in rats. Eur. J. Neurosci 46, 1850–1862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (149).Huang AS, Mitchell JA, Haber SN, Alia-Klein N, and Goldstein RZ (2018) The thalamus in drug addiction: from rodents to humans. Philos. Trans. R. Soc., B 373, 20170028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (150).James MH (2010) Cocaine- and Amphetamine-Regulated Transcript (CART) signaling within the paraventricular thalamus modulates cocaine-seeking behaviour. PLoS One 5, e12980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (151).Joffe ME, and Grueter BA (2016) Cocaine Experience Enhances Thalamo-Accumbens N-Methyl-D-Aspartate Receptor Function. Biol. Biol. Psychiatry 80, 671–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (152).Neumann PA, et al. (2016) Cocaine-induced synaptic alterations in thalamus to nucleus accumbens projection. Neuropsychopharmacology 41, 2399–2410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (153).Zhu Y, Wienecke CFR, Nachtrab G, and Chen X (2016) A thalamic input to the nucleus accumbens mediates opiate dependence. Nature 530, 219–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (154).Li S, et al. (2008) Projections From the Paraventricular Nucleus of the Thalamus to the Forebrain, With Special Emphasis on the Extended Amygdala. J. Comp. Neurol 506, 275–290. [DOI] [PubMed] [Google Scholar]
- (155).Gungor NZ, and Pare D (2016) Functional Heterogeneity in the Bed Nucleus of the Stria Terminalis. J. Neurosci 36, 8038–8049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (156).Lebow MA, and Chen A (2016) Overshadowed by the amygdala: The bed nucleus of the stria terminalis emerges as key to psychiatric disorders. Mol. Psychiatry 21, 450–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (157).Wang J, Fang Q Liu Z, and Lu L (2006) Region-specific effects of brain corticotropin-releasing factor receptor type 1 blockade on footshock-stress-or drug-priming-induced reinstatement of morphine conditioned place preference in rats. Psychopharmacology 185, 19–28. [DOI] [PubMed] [Google Scholar]
- (158).Zislis G, Desai TV, Prado M, Shah HP, and Bruijnzeel AW (2007) Effects of the CRF receptor antagonist d-Phe CRF(12–41) and the α2-adrenergic receptor agonist clonidine on stress-induced reinstatement of nicotine-seeking behavior in rats. Neuropharmacology 53, 958–966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (159).Shalev U, Erb S, and Shaham Y (2010) Role of CRF and other neuropeptides in stress-induced reinstatement of drug seeking. Brain Res 1314, 15–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (160).Silberman Y, Matthews RT, and Winder DG (2013) A corticotropin releasing factor pathway for ethanol regulation of the ventral tegmental area in the bed nucleus of of the stria terminalis. J. Neurosci 33, 950–960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (161).Kash TL (2015) Neuropeptide Regulation of Signaling and Behavior in the BNST. Mol. Cells 38, 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (162).Daniel SE, and Rainnie DG (2016) Stress Modulation of Opposing Circuits in the Bed Nucleus of the Stria Terminalis. Neuropsychopharmacology 41, 103–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (163).Marcinkiewcz CA, et al. (2016) Serotonin engages an anxiety and fear-promoting circuit in the extended amygdala. Nature 537, 1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (164).Miles PR, Mundorf ML, and Wightman R M. (2002) Release and uptake of catecholamines in the bed nucleus of the stria terminalis measured in the mouse brain slice. Synapse 44, 188–197. [DOI] [PubMed] [Google Scholar]
- (165).Park J, et al. (2012) Catecholamines in the bed nucleus of the stria terminalis reciprocally respond to reward and aversion. Biol. Psychiatry 71, 327–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (166).Krawczyk M, et al. (2011) Double-Dissociation of the Catecholaminergic Modulation of Synaptic Transmission in the Oval Bed Nucleus of the Stria Terminalis. J. Neurophysiol 105, 145–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (167).Krawczyk M, et al. (2011) A Switch in the Neuromodulatory Effects of Dopamine in the Oval Bed Nucleus of the Stria Terminalis Associated with Cocaine Self-Administration in Rats. J. Neurosci 31, 8928–8935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (168).Krawczyk M, DeBacker J, Mason X, Jones AA, and Dumont EC (2014) Dopamine decreases NMDA currents in the oval bed nucleus of the stria terminalis of cocaine self-administering rats. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 51, 83–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (169).Erb S, et al. (2000) Alpha-2 Adrenergic Receptor Agonists Block Stress-Induced Reinstatement of Cocaine Seeking. Neuropsychopharmacology 23, 138–150. [DOI] [PubMed] [Google Scholar]
- (170).Shaham Y, Highfield D, Delfs J, Leung S, and Stewart J (2000) Clonidine blocks stress-induced reinstatement of heroin seeking in rats: An effect independent of locus coeruleus noradrenergic neurons. Eur. J. Neurosci 12, 292–302. [DOI] [PubMed] [Google Scholar]
- (171).Leri F, Flores J, Rodaros D, and Stewart J (2002) Blockade of stress-induced but not cocaine-induced reinstatement by infusion of noradrenergic antagonists into the bed nucleus of the stria terminalis or the central nucleus of the amygdala. J. Neurosci 22, 5713–5718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (172).Mantsch JR, et al. (2010) Involvement of noradrenergic neurotransmission in the stress- but not cocaine-induced reinstatement of extinguished cocaine-induced conditioned place preference in mice: role for β-2 adrenergic receptors. Neuropsychopharmacology 35, 2165–2178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (173).Vranjkovic O, Gasser PJ, Gerndt CH, Baker D. a., and Mantsch JR (2014) Stress-induced cocaine seeking requires a beta-2 adrenergic receptor-regulated pathway from the ventral bed nucleus of the stria terminalis that regulates CRF actions in the ventral tegmental area. J. Neurosci 34, 12504–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (174).Wang X, Cen X, and Lu L (2001) Noradrenaline in the bed nucleus of the stria terminalis is critical for stress-induced reactivation of morphine-conditioned place preference in rats. Eur. J. Pharmacol 432, 153–161. [DOI] [PubMed] [Google Scholar]
- (175).Khoshbouei H, Cecchi M, and Morilak D. a. (2002) Modulatory effects of galanin in the lateral bed nucleus of the stria terminalis on behavioral and neuroendocrine responses to acute stress. Neuropsychopharmacology 27, 25–34. [DOI] [PubMed] [Google Scholar]
- (176).Flavin SA, and Winder DG (2013) Noradrenergic control of the bed nucleus of the stria terminalis in stress and reward. Neuropharmacology 70, 324–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (177).Nobis WP, Kash TL, Silberman Y, and Winder DG (2011) Beta-Adrenergic receptors enhance excitatory transmission in the bed nucleus of the stria terminalis through a corticotrophin-releasing factor receptor dependent and cocaine-regulated mechanism. Biol. Psychiatry 69, 1083–1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (178).Rinker JA, et al. (2017) Extended Amygdala to Ventral Tegmental Area Corticotropin-Releasing Factor Circuit Controls Binge Ethanol Intake. Biol. Psychiatry 81, 930–940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (179).Savchenko VL, and Boughter JD (2011) Regulation of neuronal activation by alpha2A adrenergic receptor agonist. Neurotoxic. Res 20, 226–239. [DOI] [PubMed] [Google Scholar]