Abstract
Omega-3 polyunsaturated fatty acids (n-3 PUFAs) are associated with a variety of cellular alterations that mitigate cardiovascular disease. However, pinpointing the positive therapeutic effects is challenging due to inconsistent clinical trial results and overly simplistic in vitro studies. Here we aimed to develop realistic models of n-3 PUFA effects on platelet function so that preclinical results can better align with and predict clinical outcomes. Human platelets incubated with the n-3 PUFAs docosahexaenoic acid and eicosapentaenoic acid were stimulated with agonist combinations mirroring distinct regions of a growing thrombus. Platelet responses were then monitored in a number of ex-vivo functional assays. Furthermore, intravital microscopy was used to monitor arterial thrombosis and fibrin deposition in mice fed an n-3 PUFA-enriched diet. We found that n-3 PUFA treatment had minimal effects on many basic ex-vivo measures of platelet function using agonist combinations. However, n-3 PUFA treatment delayed platelet-derived thrombin generation in both humans and mice. This impaired thrombin production paralleled a reduced platelet accumulation within thrombi formed in either small arterioles or larger arteries of mice fed an n-3 PUFA-enriched diet, without impacting P-selectin exposure. Despite an apparent lack of robust effects in many ex-vivo assays of platelet function, increased exposure to n-3 PUFAs reduces platelet-mediated thrombin generation and attenuates elements of thrombus formation. These data support the cardioprotective value of-3 PUFAs and strongly suggest that they modify elements of platelet function in vivo.
Keywords: Intravital microscopy, omega-3 fatty acids, platelet activation, platelets, thrombosis
Introduction
Long-chain polyunsaturated omega-3 fatty acids (n-3 PUFAs) have acquired a reputation for providing cardioprotective benefits. The evidence for this cardioprotection is derived from many sources, including epidemiological studies analyzing populations with high dietary n-3 PUFA intake (1–6) and promising placebo-controlled clinical trials (7–9). However, a clear shift in thinking about the efficacy of n-3 PUFAs has occurred in the last decade, sparked by recent large clinical trials that predominantly demonstrated neutral effects of n-3 PUFAs in placebo-controlled studies. These include the OPERA trial, which showed no alterations in the number or severity of atrial fibrillation events or incidents of myocardial infarction or stroke (10). Furthermore, the Risk and Prevention Study (11) and the Alpha Omega trials (12) showed no alterations in cardiovascular-derived incidents of mortality, and the OMEGA-PAD I trial (13) showed no alterations in vascular endothelial cell functions. Several interventional studies were reviewed by Begg et al. and largely concluded that n-3 PUFAs may provide a clinical benefit, but the degree and nature of n-3 PUFA cardioprotection remains unclear (14). Recent American Heart Association guidelines mirror these findings, recommending n-3 PUFA intake in some clinical conditions but with insufficient evidence to recommend wider use (15).
Thrombosis, like many of the other endpoints examined in n-3 PUFA-oriented clinical trials, also shows mixed results (10,16–19), but in general has not been a primary focus of recent clinical trials other than in the context of subject recruitment after an adverse thrombotic event. Furthermore, patients recruited in clinical trials are often on a regimen of anti-clotting, anti-arrhythmia, anti-platelet, and/or anti-cholesterol medications that could readily mask any effects of n-3 PUFAs. This masking may obfuscate the positive outcomes arising from moderate anti-thrombotic effects provided by n-3 PUFAs that could limit adverse thrombotic events while maintaining normal hemostasis, unlike other more potent therapies that exhibit significant bleeding side effects (20).
Despite the difficulties of studying the anti-thrombotic benefits in response to n-3 PUFAs within the clinical setting, there is significant promise in investigating whether n-3 PUFAs provide cardioprotection via this avenue. Studies have shown anti-thrombotic effects in human cells and tissues sampled after either post-dietary intervention (21–24) or exogenous n-3 PUFA incubation (25). Additionally, platelets show substantial reductions in activation following enrichment of n-3 PUFA content (19). Consequently, reduced platelet activation could account for many of the improved outcomes of enhanced n-3 PUFA consumption noted in epidemio-logical studies, including limiting the formation of platelet thrombi at the site of ruptured atherosclerotic lesions (26), reduced release of pro-inflammatory mediators contained in platelets (27,28), and reduced thrombin generation on the anionic lipid surface of activated platelets (29) or in pooled blood during arrhythmias (30). Indeed, even the overall neutral OPERA clinical trial demonstrated a significant reduction in arterial thromboembolism within 30 days of treatment (10), a result that could be mediated by diminished platelet function.
What remains is a way to reconcile the n-3 PUFA-mediated inhibitory platelet effects observed in vitro and the swell of clinical data (perhaps confounded by concomitant therapies) that paints a more neutral and/or unclear picture of n-3 PUFA treatment, particularly with regard to thrombosis. In this study, we aimed to utilize a more relevant in vitro platelet activation model based on the spatial and activation-state heterogeneity of a developing clot as described by Stalker et al. (31). Our model attempts to more closely replicate damaged vasculature in vivo by using multiple agonists encountered by platelets in distinct areas of the forming thrombus. This model was then used to measure the effects of the n-3 PUFAs eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) on ex-vivo platelet function assays. To augment the modeled in vitro data, we also used two well-established intravital murine models of thrombosis, one in large arteries and one in small arterioles, to more accurately gauge the effects of n-3 PUFA supplementation on platelet function in vivo for the first time.
Methods
Human subjects
All procedures using human blood from healthy males and females (in relatively even numbers) were approved by the Augustana University and University of Kentucky Institutional Review Boards. Informed consent was obtained from all subjects.
Animal use and welfare
All experimental procedures on animals in this study were approved by the Institutional Animal Care and Use Committee at the University of Michigan. C57BL/6 WT mice were purchased from Jackson Laboratories (Bar Harbor, ME, USA). Mice were fed a modified AIN-93G diet for 6 weeks with or without the addition of menhaden oil, providing DHA (~3.5–3.9 g/kg) and EPA (~4.6–5.6 g/kg) (Envigo Teklad, Madison, WI, USA). Soybean oil was used in place of menhaden oil in the control diet.
Human platelet isolation and treatment
Platelets were isolated largely as described by Walsh et al. (32), with citrated whole blood rested for 1 hr at room temperature with gentle agitation (33). To obtain platelets with ex-vivo-enhanced levels of DHA and EPA comparable to levels found after dietary supplementation (25), platelet-rich plasma was incubated for 1 h at 37° with 750 μM of both DHA and EPA (25 mM stocks, Nu-Chek Prep, Elysian, MN, USA) prepared under argon or vehicle control (1% fatty acid-free bovine serum albumin (BSA) in HEPES-Tyrodes buffer) (25), and either used directly in plasma or spun for 10 min at 550 g with 0.02 U/mL grade VII apyrase (Sigma, St. Louis, MO, USA) and 50 ng/mL PGI2 (Cayman Chemical, Ann Arbor, MI, USA) to obtain washed platelets.
Flow cytometry
DHA- and EPA-treated platelet-rich plasma was added various concentrations of agonists adenosine diphosphate (ADP, Chronolog, Havertown, PA, USA) and the thromboxane A2 receptor agonist U46619 (Cayman) (shell agonists) or convulxin (CVX, Santa Cruz Biotechnology, Santa Cruz, CA, USA) and thrombin receptor agonist peptide SFLLRN (TRAP, Tocris, Bristol, UK) (core agonists), and either FITC-PAC-1 (active GPIIb/IIIa) or FITC-anti-P-selectin (BD Biosciences, San Jose, CA, USA) antibodies. Samples were incubated at room temperature in the dark for 10 min, fixed with 0.2% paraformaldehyde and analyzed via flow cytometry (Accuri C6, BD Biosciences). Data were evaluated for significance with a one-way ANOVA using JMP software (SAS, Cary, NC, USA).
Thrombin generation assay
Thrombin production (using ex-vivo-treated human platelets or murine platelets following increased dietary DHA and EPA intake) was measured using Factor Xa (2.5 nM for humans, 0.024 nM for mice), prothrombin (0.7 mM, Haematologic Technologies, Essex Junction, VT, USA), and SpectrozymeTH thrombin substrate (Sekisui Diagnostics, Lexington, MA, USA) combined with Ca2+ (7.4 mM) and CVX and/or TRAP (PAR-1 specific in humans, PAR-4 specific in mice). Washed platelets were then added and the absorbance at 405 nm was measured every 60 s for 1 hr (SpectraMax M2, Molecular Devices, Sunnyvale, CA). Data were evaluated for significance with a one-way ANOVA using JMP software.
Aggregation and ATP granule release
ATP-dependent luciferase luminescence reagent (Chronolog) was incubated with DHA- and EPA-treated platelet-rich plasma for 1 min with stirring at 37° in a lumi-aggregometer (Chonolog) followed by the addition of medium (EC50) concentrations of shell or core agonists. Absorbance and luminescence was then continuously measured for 4–5 min to determine the level of platelet aggregation and dense granule secretion. Data were evaluated for significance with a one-way ANOVA using JMP software.
Platelet spreading
Coverslips were coated in 100-μg/mL fibrinogen (Sigma) for 1 h and blocked with 2 mg/mL denatured fatty acid-free BSA for 30 min. Washed platelets were then added and allowed to adhere for 1 h followed by fixation with 10% neutral buffered formalin (Sigma) and mounted using Fluoromunt-G (Southern Biotechnology, Birmingham, AL, USA). Platelet spreading area was determined using an Olympus FV1200 automated confocal microscope with a 60× oil (1.42 NA) objective and Olympus Fluoview 10 imaging software. Data were evaluated for significance with a one-way ANOVA using JMP software.
Laser-induced cremaster arteriole thrombosis model
Laser-induced cremaster arteriole thrombosis intravital microscopy was performed as described (34–36) in 12-week-old C57BL/6 WT male mice. The cremaster muscle was prepared for imaging under dissecting microscope and constantly super-perfused with preheated bicarbonate-buffered saline throughout the experiments. Platelet and fibrin labeling was achieved by injecting anti-platelet (DyLight 488 anti-GPIb, 1 μg/g; Emfret, EIbelstadt, Germany) and anti-fibrin (a kind gift from Dr. R. Camire from Children’s Hospital of Philadelphia, labeled with Alexa Fluor 647, 0.3 μg/g) antibodies via jugular vein catheter prior to intravital microscopy imaging. Multiple independent thrombi in arterioles (30–50-μm diameter) in each mouse were induced by a laser ablation system (Ablate! Photoablation System; Intelligent Imaging Innovations, Denver, CO, USA). Images of thrombus formation were acquired in real-time under 63× water-immersion objective with a Zeiss Axio Examiner Z1 fluorescent microscope equipped with solid laser launch system (LaserStack; Intelligent Imaging Innovations) and high-speed sCMOS camera. Images of thrombi were analyzed for the dynamics mean florescent intensity over the course of thrombus growth after subtracting the florescent background each thrombus using Slidebook 6.0 (Intelligent Imaging Innovations). The curves were averaged over 8–10 independent injuries from at least three mice. P-selectin expression on platelets accumulating into growing thrombi in mice were studied by injecting mice with Alexa Flour 647-rat anti-mouse CD62P antibody (BD Biosciences; 3 μg/per mouse). To determine the platelets’ P-selectin positive area at the thrombus core in growing platelet thrombi (2–4 independent injuries from at least three mice), thrombus growth was recorded on a single plain at the center of vessel using confocal (CSU-X1 A1) spinning disk mounted to Zeiss Axio Examiner Z1 fluorescent microscope system. Data were evaluated for significance with two-way ANOVA and Mann-Whitney test for nonparametric data using Prism 6 software (Graphpad, La Jolla, CA, USA).
Feci3-induced carotid artery thrombosis model
Mice were anesthetized and via tail vein injected with calcein-AM fluorescently labeled gel-filtered platelets (3 × 105 platelets/kg) prepared as described (34,37) from donor mice with same diet treatment. The right common carotid artery was prepared under the dissecting microscope and placed on the microscopic stage of a Zeiss Axio Examiner Z1 upright fluorescent microscope. Blood flow was visualized under 5× air objective and injured by applying 7.5% FeCI3-saturated Whatman paper for 2 min. Images of platelet adhesion and thrombus formation were recorded through a high-speed sCMOS camera using Slidebook 6.0.
Tail bleeding assay
Mice were anesthetized and placed on a heating pad, and 5 mm of tail tip in was excised and the tails were immersed into saline solution at 37°C. Bleeding time was recorded as the cessation of blood flow from the tail for 1 min.
Results
DHA and EPA platelet incorporation shows relatively minimal effects on agonist-induced secretion, aggregation, and adhesion
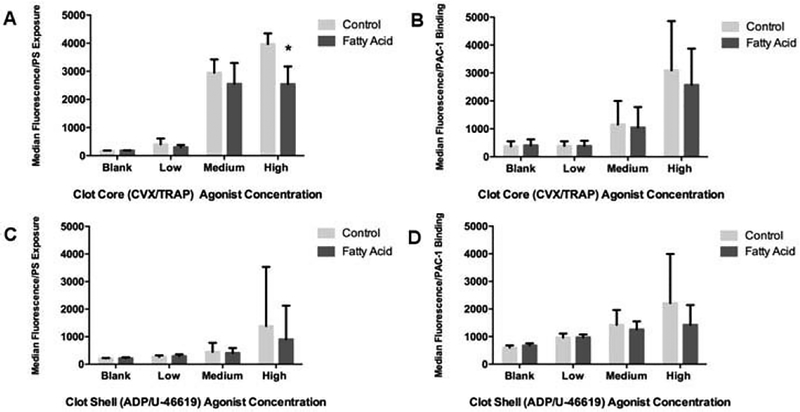
Previous studies have focused on the effects of DHA and EPA on platelets, but few, if any have examined their effects in response to the heterogeneous chemical conditions found in the arterial clot environment. Thus, we compared the activation responses of DHA- and EPA-treated platelets to control platelets in the presence of multiple agonists corresponding to distinct spatial regions of the thrombus. Platelets were exposed to combined agonists added at individual agonist EC10 (low dose), EC50 (medium dose), and EC90 (high dose) levels in a basic measure of platelet activity. The combinations added were either CVX and TRAP, agonists that simulate the collagen and thrombin found in the highly activated and minimally permeable thrombus core, or ADP and a TxA2 analog, agonists found in the minimally and transiently active thrombus shell (31). Flow cytometric analysis of α-granule release via P-selectin exposure (Figure 1A,C) and GPIIb/IIIa activation (Figure 1B,D) indicated a trend of mild DHA/EPA-mediated inhibition when compared to control platelets for all three agonist dosages from the clot core (Figure 1A,B) or shell (Figure 1C,B) agonists. However, only P-selectin exposure in response to a high dose of core agonists was significantly inhibited by DHA and EPA (Figure 1A). In general, inhibitory trends observed in the n-3 PUFA-treated platelets were subtle, in marked contrast to earlier studies using monoagonist conditions (23,24).
Figure 1.
DHA- and EPA-treated platelets show mild inhibition of a-granule release and integrin activation in response to multiple agonists. Clot core agonist conditions with DHA/EPA-treated platelets were modeled using low (EC10; 0.24 pM CVX and 2.7 nM TRAP), medium (EC50; 2.4 pM CVX and 27 nM TRAP), and high (EC90; 24 pM CVX and 270 nM TRAP) concentrations of convulxin and TRAP activation conditions followed by platelet binding to a FITC-anti-P-Selectin antibody (A) or the GPIIb/IIIa active-conformation epitope antibody PAC-1 (B). Clot shell agonist conditions with DHA/EPA-treated platelets were modeled using low (2 μM ADP and 10 nM U-46619), medium (20 μM ADP and 100 nM U-46619), and high (200 μM ADP and 1.1 μM U-46619) concentrations of U46619 (a TxA2 analog) and ADP followed by binding to the P-Selectin antibody (C) or PAC-1 (D). *p < 0.01 via one-way ANOVA, n = 4, error bars represent SD. The label “Blank” refers to no agonist stimulation, showing that DHA/EPA incubation alone does not alter the platelet activation profile of these two markers.
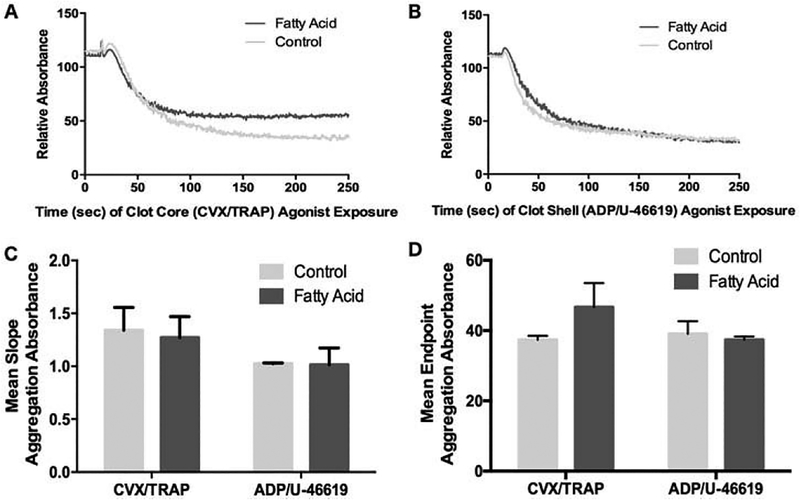
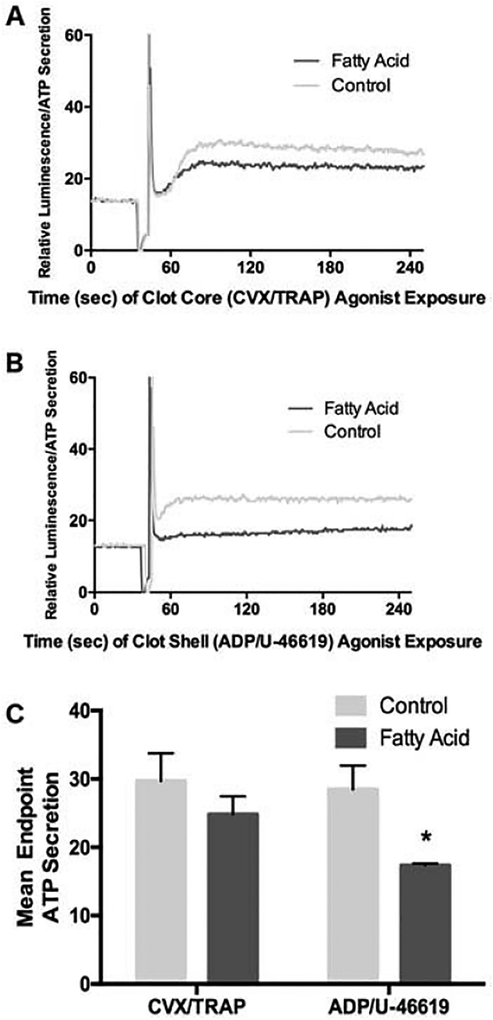
A similar diminutive effect of DHA and EPA in response to agonist combinations was also seen in platelet assays of agonist-induced platelet aggregation (Figure 2) and ATP release from dense granules (Figure 3). DHA- and EPA-treated platelets showed no significant change in aggregation parameters in response to combined core agonists or shell agonists. This included no observed reduction in the initiation of aggregation (the downward slope of the aggregation curve in Figure 2A and 2B, summated in Figure 2C), the initial platelet shape change (the initial upward absorbance of light in Figure 2A and 2B) or the maximum level of platelet aggregation (Figure 2D). A greater inhibitory effect of DHA and EPA was seen in the extent of dense granule secretion in response to both core (Figure 3A,C) and shell (Figure 3B,C) agonist combinations, but the overall effect could still be described as mild.
Figure 2.
DHA- and EPA-treated platelets show minimal inhibition of platelet aggregation in response to multiple agonists. Washed platelets treated with or without DHA/EPA were stimulated with EC50 doses of clot core agonists (A) or clot shell agonists (B) and measured for decreased light absorbance as a measure of platelet aggregation. A representative example is shown for each agonist condition, and (C) and (D) represent the mean of slope change and maximum aggregation, respectively, from n = 4 experiments, error bars represent SD.
Figure 3.
Agonist-induced secretion of platelet dense granules is inhibited by DHA and EPA modification in response to the shell agonists, but not core agonists. Washed platelets treated with or without DHA/EPA were stimulated with EC50 doses of clot core agonists (A) or clot shell agonists (B) and measured for ATP- induced luminescence as a measure of dense granule release. A representative example is shown for (A) and (B), with (C) as a mean raw fluorescence emission from n = 4 experiments, *p = 0.001 via one-way ANOVA, error bars represent SD. The spike in (A) and (B) represents the point of agonist addition to the platelets.
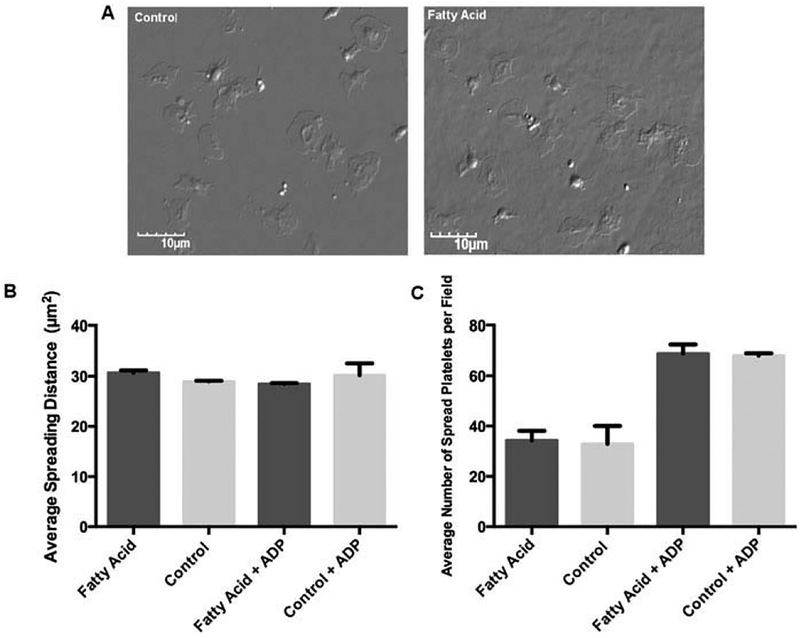
As an alternative assay of platelet activation, we added either DHA- and EPA-treated or control-treated platelets to fibrinogen-coated coverslips in a static assay of platelet adhesion. Platelet adhesion was unaffected after DHA- and EPA-treatment, as equal number of platelets adhering to fibrinogen over the course of 1 h and equal amounts of platelet spreading area were observed in the two treatment groups (Figure 4). The addition of ADP as a co-agonist in this spreading model increased the number of platelets adhering to fibrinogen, but did not show a differential effect between treatment groups (Figure 4B,C).
Figure 4.
Platelet spreading on insoluble fibrinogen is unimpaired following DHA/EPA incorporation. (A) Representative photos of platelets spread on fibrinogen, either control (left panel) or DHA/EPA-treated (right panel).(B) Quantitation of n = 50 spread platelets area using ImageJ, with or without the addition of ADP as a co- agonist. (C) Total number of platelets spread per 60× microscope field, with or without the addition of ADP as a co-agonist, n = 10 fields counted. Error bars represent SD.
Platelet-mediated thrombin generation kinetics are impaired after DHA and EPA incorporation both exogenously and in vivo
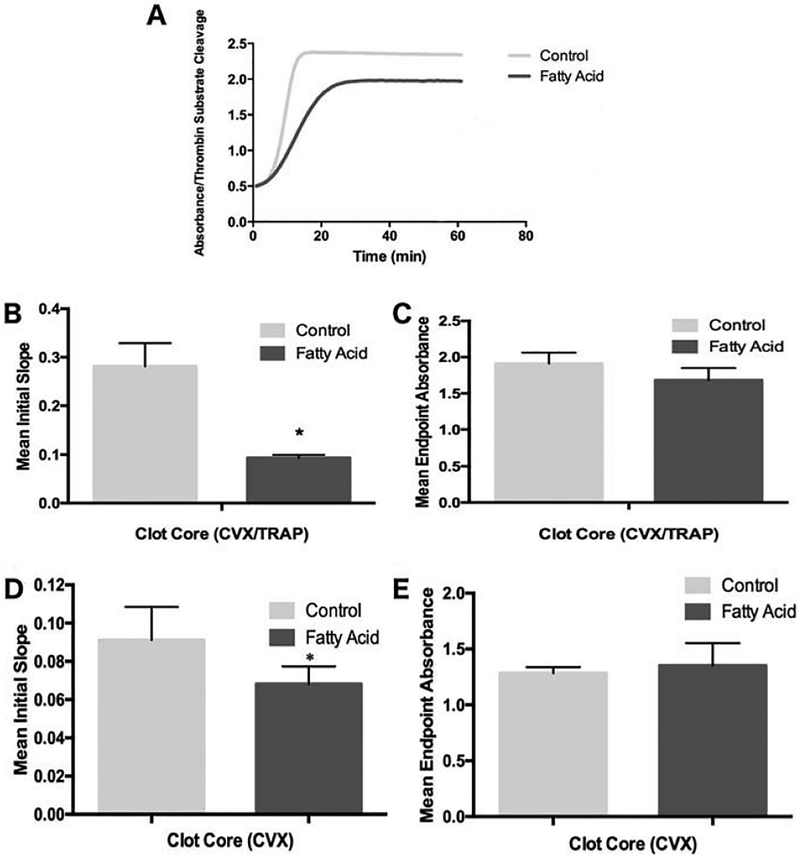
Despite the apparent lack of DHA- and EPA-mediated platelet inhibition in more complex agonist activation assays, n-3 PUFA-induced alterations to the platelet membrane can drastically affect hemostatic events external to the platelet itself. As such, we measured the capacity of human platelets with or without exogenously added DHA and EPA to catalyze thrombin generation at the membrane. We consistently saw higher rates of thrombin generation (Figure 5A, 5B), though not maximal thrombin generation (Figure 5B) in control platelets compared to platelets treated with DHA and EPA stimulated with core agonists. The same effect was also evident in washed murine platelets after six weeks of enhanced dietary DHA/EPA intake in response to CVX (Figure 5D,E) or TRAP (data not shown). Notably, the time for DHA and EPA to incorporate into human platelet membranes was critical, as no difference was seen in thrombin generation kinetics immediately after the addition of exogenous DHA and EPA to the human platelets compared to control platelets (data not shown). These results suggest increased DHA and EPA levels via either exogenous addition or dietary modification can consistently impair platelet-derived thrombin production kinetics, similar to earlier studies using monoagonist conditions (25).
Figure 5.
Thrombin generation rate kinetics are impaired after DHA and EPA modification. Platelets treated with or without added DHA and EPA were stimulated with an EC50 dose of TRAP and convulxin and added to a chromogenic thrombin substrate in the presence of exogenous factor Xa and prothrombin. Increased absorbance corresponds to increased substrate cleavage by thrombin, and slope of the curves reflects thrombin generation rate kinetics. (A) A representative example of thrombin generation in human platelets treated ex vivo with DHA and EPA followed by CVX and TRAP stimulation. Averages of the mean slope (Abs/min, obtained from the steepest part of initial slop prior to leveling off) of thrombin formation (B) and maximum thrombin generation (C) for washed human platelets, n = 5. Averages of the mean slope of thrombin formation (D) and maximum thrombin generation (E) for washed murine platelets treated with 75 μg/mL CVX, n = 5. *p < 0.001, +p < 0.05 via one-way ANOVA, error bars represent SD.
Increased DHA and EPA intake in mice resulted in decrease of platelet accumulation and fibrin deposition without altering P-selectin expression in thrombi in vivo
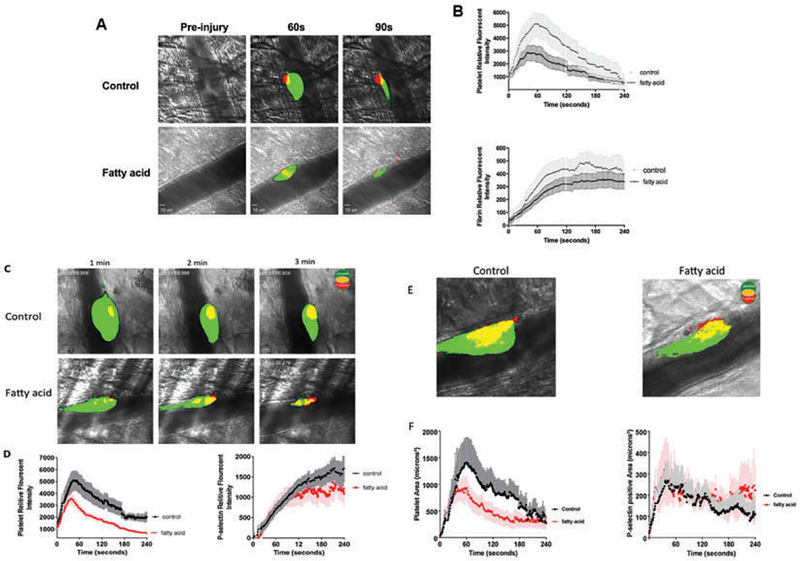
A major shortcoming of exogenous n-3 PUFA addition and in vitro platelet assays is the relative isolation of these events from intact physiological systems. To provide a more physiological approach to augment the in vitro models of the clot, we turned to intravital thrombosis models to visualize the effects of DHA and EPA in real-time. Specifically, we measured dynamic platelet accumulation, fibrin formation, and P-selectin expression within growing arterial thrombi following laser-induced cremaster muscle arteriole damage in mice consuming a DHA- and EPA-fortified or control diet for 6 weeks. A total of 9–13 independent thrombi in each mouse on the DHA/EPA diet and control diet were compared (three mice in each group). In control mice, platelets rapidly deposited to the site of vascular injury and continuously grew to maximal size resulting in a stable thrombus at the site of injury. Along with platelet deposition, fibrin formation and P-selectin exposure at the base and core region of thrombi was detected at the growing thrombi.
In contrast, thrombi in the DHA/EPA-fed mice were smaller in size and rapidly resolve toward the core. Platelet accumulation and fibrin formation (Figure 6A,B) was attenuated as analyzed by dynamics of platelet and fibrin fluorescence intensity in growing thrombi. Intriguingly, the level of P-selectin exposure of platelets at the site of injury was not significantly altered between the two groups of mice (Figure 6C,D) despite the notable reduction of total platelets accumulated into thrombi in the DHA/EPA group. A confocal analysis of the induced thrombi also showed a decrease in total area of platelet coverage but no decrease in the P-selectin positive area of thrombi at the site of injury in the DHA/EPA-fed mice (Figure 6E, F), suggesting platelet activation in the core of the thrombus was largely unchanged by DHA/EPA incorporation.
Figure 6.
DHA and EPA modification attenuated laser induced-thrombus formation in vivo. Thrombus formation in response to laser-induced injury on cremaster arterioles in mice fed with a control diet or DHA/EPA-rich diet. Thrombus formation at the site of vascular was monitored in real-time under intravital microscopy for 4 min. (A) Representative images of platelet (green) accumulation and fibrin formation (red) injury in mice fed with a control diet (upper panel) or DHA/EPA-rich diet (lower panel). (B) Dynamics of platelet accumulation and fibrin formation in thrombi assessed by relative mean fluorescence intensity of platelet and fibrin (P < 0.001) averaged by 10–15 thrombi per mouse three mice in each group. Data represent mean ±SEM. (C) Representative images of total platelet (green) accumulation and platelets P-selectin expression (red) within thrombi in mice fed with a control diet (upper panel) or DHA/EPA-rich diet (lower panel). (D) Dynamics of total platelet accumulation (P < 0.001) and platelet P-selectin expression (P > 0.05) in thrombi assessed by relative mean fluorescence intensity averaged by 8–10 thrombi per mouse three mice in each group. (E) Representative two-dimensional confocal images of platelet area (green) and P-selectin positive area (red) in stable thrombi recorded under confocal intravital microscopy. (F) Dynamics of total platelet accumulated area (P < 0.001) and platelet P-selectin positive area (P > 0.05) in thrombi assessed by under confocal intravital microscopy 3–4 thrombi in each mouse three mice per group. Dynamics of fluorescent intensity and surface area were analyzed Slidebook 6.0 program and compared by two-way ANOVA analysis for statistical difference.
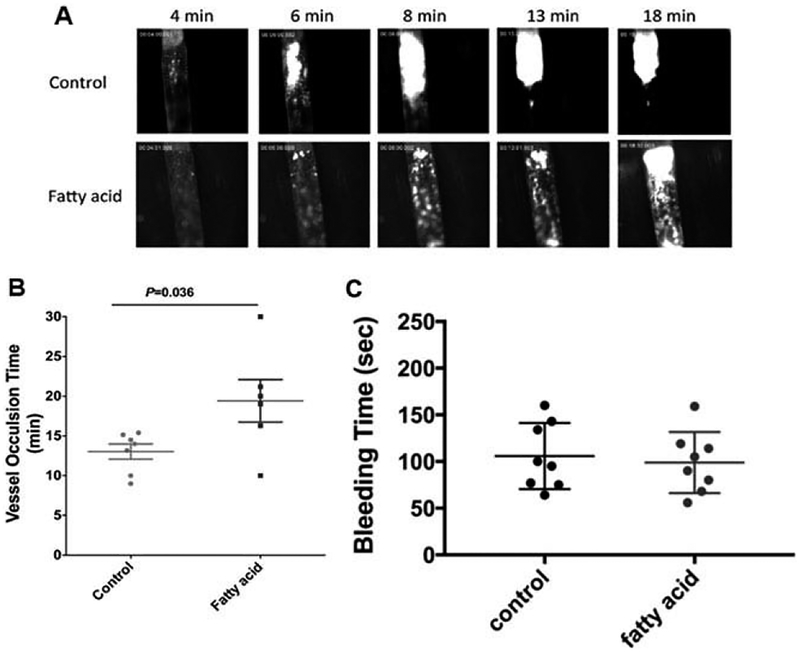
Furthermore, a FeCI3-induced thrombosis model of vascular damage in larger arteries demonstrated delayed vessel occlusion in the carotid artery of mice on the DHA/EPA diet. Platelets started adhering to the injury site and formed visible platelet aggregates resulting in the formation of stable thrombi shortly after FeCI3 application (Figure 7A). The thrombi continuously grew and eventually reached occlusive size and resulted in complete vessel occlusion and secession of blood flow, with an average vessel occlusion time in the control group of113.1 ± 0.9 min (n = 7). Conversely, the accumulation of platelets was impaired in the DHA/EPA-fed mice, with an average vessel occlusion time of 19.4 ± 2.7min (n = 6, Figure 7B). Together, the two intravital models show a clear inhibitory effect of DHA and EPA incorporation on the hemo-dynamics of platelet accumulation at sites of vascular injury that aligns with the in vitro model that shows minimal platelet activation effects but impaired thrombin kinetics. In contrast, we compared hemostatic tail-bleeding time in mice with control or fatty acid diet and no detectable difference was found between the two groups (Figure 7C).
Figure 7.
DHA and EPA modification delayed the vessel occlusion in FeCI3- induced carotid artery thrombosis model. (A) Representative images of thrombosis in carotid artery in respond to FeCl3 injury. 7.5% FeCl3 was topically applied on the right carotid artery for2 min and images of thrombosis were recorded in real-time and vessel occlusion was determined by the secession of blood flow. (B) Carotid artery vessel occlusion time in control or DHA/EPA-fed mice. The mean vessel occlusion time in the control mice is 13.1 ± 0.9 min and 19.4 ± 2.7min (p = 0.036) in DHA/EPA group. Data represent mean±SEM, 6–7 mice in each group and analyzed by student’s t-test. (C) The DHA/EPA diet did not alter tail bleeding time in mice. Tail bleeding time was compared in mice with control diet and DHA/DPA diet (eight mice in each group). No significant differences were found in tail bleeding time between two groups. Data represent mean ±SEM; two-tailed unpaired t-test.
Discussion
Numerous studies where platelets have been altered with elevated DHA and EPA levels have described a general platelet inhibition. However, these studies almost exclusively focus on platelet responses to one agonist. This mode of testing fails to account for the greater environmental complexity that platelets encounter after vascular damage in the growing thrombus. As such, the ability of DHA and EPA to alter platelet function in vivo was uncertain. In this study we applied a more complex agonistic approach to analyze the effects of DHA and EPA on platelet function ex vivo, and we find that most common measures of standard platelet activation show at most a mild inhibition following platelet membrane alterations with DHA and EPA. Granule secretion was most affected, with both dense and α-granule secretion assays demonstrating some degree of impaired response to multiple agonists after n-3 PUFA enrichment. However, aggregation, spreading, and GPIIb/IIIa integrin activation were largely unaffected. The mild platelet response in our model requires a reconciliation with the number of clinical studies that suggest n-3 PUFA supplementation significantly reduces the likelihood of adverse cardiovascular events (38), particularly if platelets contribute to n-3 PUFA cardioprotection.
One avenue for this reconciliation is that the observed clinical effects of n-3 PUFAs may be driven more by extracellular events that occur at the platelet surface after the initial activation. One such event is thrombin generation on the platelet surface, where acidic phospholipids exposed upon platelet activation recruit thrombogenic factors (namely factor Va and factor Xa) in the presence of Ca2+ to convert prothrombin to thrombin. The thrombin can in turn lead to fibrin formation in the proximal area of platelet deposition (39), furthering the return to normal hemostasis. Indeed, we observed impaired kinetics of platelet-derived thrombin production using more realistic agonist combinations to stimulate DHA- and EPA-treated platelets. The same effect was observed in murine platelets after increased dietary intake of DHA and EPA. These current findings align with our previous studies where we observed diminished phosphatidylserine exposure after increased n-3 PUFA dietary intake (24) and impaired platelet prothrombin processing after DHA/EPA incubation (25). The persistent n-3 PUFA-driven impairment of thrombin generation in vitro also aligns elegantly with the observations of real-time thrombus formation in vivo, models that allow an even more complete recapitulation of the thrombotic environment. The reduced recruitment of platelets to the thrombus observed in damaged DHA- and EPA-enriched murine cremaster arterioles or carotid arteries and the reduction in fibrin formation in the cremaster arterioles strongly implicates an attenuation of occlusive platelet thrombosis without impacting formation of stable hemostatic clot consisted by P-selectin-positive platelets at the core of thrombi after n-3 PUFA treatment.
It should be noted that we did not detect any alterations to factor Xa binding to platelets ex vivo after DHA and EPA enrichment (data not shown). This suggests that thrombin activation or activity, rather than the formation of the catalytic complex at the platelet surface, may be the cause of thrombin deficit. As a result, thrombin function could be impaired in the following ways: (1) prothrombin is not recruited to the activation complex, (2) prothrombin is not efficiently converted to thrombin, and/or (3) thrombin proteolytic activity is impaired after activation from prothrombin. While all three mechanisms are possible, the absence of free DHA and EPA in the ex-vivo assays after washout suggests that thrombin catalytic activity towards its substrates is not directly inhibited by n-3 PUFAs. Further mechanistic studies on the mechanism of thrombin impairment are clearly warranted based on these results. Additionally, the application of FeCl3 on vessels is known to induce oxidative damage (40) and DHA/EPA may have antioxidant effects on platelets as reported (41). However, our observed effect of the DHA/EPA diet on thrombosis in mice seems unlikely due to the artifact of the FeCl3 model, as the findings are consistent between the laser-induced cremaster arteriole thrombosis model, a different type of vascular injury in different vascular bed. However, in our current study we cannot exclude the possibility of the in vivo alterations in thrombus growth and composition arising partially from enhanced plasminogen binding to platelets following the modification of platelet membrane composition in DHA/EPA-fed mice. Characterization of the ability of DHA/EPA to regulate the plasminogen activation system in vivo warrants future exploration.
Overly simplistic agonist models may account for some of the differences between this study and others. However, another source of inter-study variability, particularly between in vivo and in vitro n-3 PUFA modification, is the potential requirement of DHA- and EPA-derived metabolites to provide observable efficacy. Cellular oxidation of DHA and EPA produces physiologically active metabolites that show anti-platelet function (42), and loss of some of these oxidative enzymes has been shown to diminish fatty acid-derived platelet inhibition (43). Our past in vitro modification studies suggest that it is possible to recapitulate the quantitative change in membrane DHA and EPA phospholipid composition found after increased dietary composition (25), but it is highly unlikely that this short-term method of alteration (on the scale of hours) can encompass the whole of lipid metabolism found in long-term DHA and EPA dietary enrichment. As such, studies that use in vivo models that allow for the longer-term metabolic events provide a broader view of DHA and EPA effects that can perhaps better demonstrate how these fatty acids alter cellular function. It is also important to consider that DHA and EPA may alter physiological characteristics of other cell types that influence thrombus formation, such as endothelial cells (44,45) and immune cells (46,47). Furthermore, DHA and EPA can potentially influence cell function by altering post-translational lipid modification of proteins that contribute to vascular function (Zhang and Whiteheart, unpublished data).
While the murine models used in this study demonstrate a reduced capacity to recruit platelets and generate fibrin at sites of vascular damage after DHA- and EPA-enrichment, it is critical to note that both events are not entirely abrogated and that tail vein bleeding cessation remains unchanged. This is in contrast with current anti-thrombotic therapies that drastically impair the ability of platelets to adhere at sites of injury and/or decrease the capacity for clot formation. The subsequent side effect of increased bleeding is unsurprisingly one of their most predominant adverse effects (20). Conversely, most studies that analyzed bleeding defects in human and animal models found little to no notable increase in bleeding after increased n-3 PUFA intake, including studies with significant vascular trauma as a background event (e.g., major surgery) (19). Similarly, our results here indicate that there is impairment, but not ablation of hemostatic response elements following DHA and EPA enrichment. This may be of critical importance in lengthening the time to occlusion in a diseased or stenosed blood vessel, while simultaneously maintaining a relatively minimal effect toward the normal vasculature.
The ability to walk the knife-edge between reduced, but not drastically impaired thrombotic potential may also explain the relatively neutral effects that recent n-3 PUFA-driven clinical trials have observed. In the clinical setting, many of the study patients are on anti-thrombotic medications. A drastic impairment of clotting function, whether via pharmacological platelet or thrombin inhibition, will make it exceedingly difficult for the subtler effects of n-3 PUFAs to be observed at all. In other words, if the main effect of DHA and EPA is to reduce (but not eliminate) the ability of platelets to catalyze thrombin generation, it should come as no surprise that a patient on warfarin would not benefit as much or at all from enhanced DHA and EPA uptake.
While existing pharmacological profiles may overshadow the benefits of DHA and EPA, a more tantalizing therapeutic use for these n-3 PUFAs may lie in their use as prophylactics. The increased intake of n-3 PUFAs by otherwise healthy individuals prior to the diagnosis or recurrence of cardiovascular conditions and subsequent prescription of substantial medication may be a promising route of therapeutic use. Furthermore, this use would be supported by the large body of ecological evidence of the reduced incidence of cardiovascular disease in high fatty fish oil-consuming countries (48,49). Though such studies are always confounded by the significant variability of human populations, even mild therapeutic effects of DHA and EPA could have an ability to combat adverse vascular effects. Along these lines, it should be noted that a long-term placebo-controlled trial of n-3 PUFA supplementation in a large population of apparently healthy individuals over 50 years of age is ongoing (50). That said, our results here suggest that n-3 PUFAs still hold promise as an anti-thrombotic treatment, as their inhibitory capacity still persists in more complex experimental environments.
Acknowledgments
The authors would like to thank Lance Shaull, Betsy McCue, Anna Kollasch, and Dr. Jinchao Zhang for critical research support.
Declaration of Interest Statement
This work was supported in part by an Institutional Development Award (IDeA) from the National Institute of General Medical Sciences of the National Institutes of Health [grant number P20GM103443 to ML, LH, and EM]; the National Institutes of Health Office of Dietary Supplement [grant number GM105671 to MH], [grant number HL114405 to MH] as well as funding from the National Science Foundation/EPSCoR program [grant number IIA-1355423]; the state of South Dakota (to ML, EV, KF, AV); and an undergraduate fellowship from the American Physiological Society (to JB). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or the National Science Foundation.
References
- 1.Von Schacky C, Harris WS. Cardiovascular benefits of omega-3 fatty acids. Cardiovasc Res. 2007;73(2):310–315. [DOI] [PubMed] [Google Scholar]
- 2.Breslow JL. n-3 fatty acids and cardiovascular disease. Am J Clin Nutr. 2006;83(6 Suppl):1477S–1482S. [DOI] [PubMed] [Google Scholar]
- 3.Harris WS, Kennedy KF, O’Keefe JH Jr, Spertus JA. Red blood cell fatty acid levels improve GRACE score prediction of 2-yr mortality in patients with myocardial infarction. Int J Cardiol. 2013;168(1):53–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Farzaneh-Far R, Harris WS, Garg S, Na B, Whooley MA. Inverse association of erythrocyte n-3 fatty acid levels with inflammatory bio-markers in patients with stable coronary artery disease: the heart and soul study. Atherosclerosis. 2009;205(2):538–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Siscovick DS, Raghunathan TE, King I, Weinmann S, Wicklund KG, Albright J, Bovbjerg V, Arbogast P, Smith H, Kushi LH, et al. Dietary intake and cell membrane levels of long-chain n-3 polyun-saturated fatty acids and the risk of primary cardiac arrest. JAMA. 1995;274(17):1363–1367. [DOI] [PubMed] [Google Scholar]
- 6.Guasch-Ferré M, Babio N, MartÍnez-González MA, Corella D, Ros E, MartÍn-Peláez S, Estruch R, ArÓs F, Guasch-Ferré M, Gómez-Gracia E, Fiol M, et al. Dietary fat intake and risk of cardiovascular disease and all-cause mortality in a population at high risk of cardiovascular disease. Am J Clin Nutr. 2015;102(6):1563–1573. [DOI] [PubMed] [Google Scholar]
- 7.Dietary supplementation with n-3 polyunsaturated fatty acids and vitamin E after myocardial infarction: results of the GISSI-prevenzione trial. Gruppo italiano per lo studio della sopravvivenza nell’Infarto miocardico. Lancet. 1999;354(9177):447–455. [PubMed] [Google Scholar]
- 8.Tavazzi L, Maggioni AP, Marchioli R, Barlera S, Franzosi MG, Latini R, Lucci D, Nicolosi GL, Porcu M, Tognoni G; Gissi-HF Investigators. Effect of n-3 polyunsaturated fatty acids in patients with chronic heart failure (the GISSI-HF trial): a randomised, double-blind, placebo-controlled trial. Lancet. 2008;372(9645):1223–1230. [DOI] [PubMed] [Google Scholar]
- 9.Yokoyama M, Origasa H, Matsuzaki M, Matsuzawa Y, Saito Y, Ishikawa Y, Oikawa S, Sasaki J, Hishida H, Itakura H, et al. Effects of eicosapentaenoic acid on major coronary events in hypercholesterolaemic patients (JELIS): a randomised open-label, blinded endpoint analysis. Lancet. 2007;369(9567):1090–1098. [DOI] [PubMed] [Google Scholar]
- 10.Mozaffarian D, Marchioli R, Macchia A, Silletta MG, Ferrazzi P, Gardner TJ, Latini R, Libby P, Lombardi F, O’Gara PT, et al. Fish oil and postoperative atrial fibrillation: the omega-3 fatty acids for prevention of post-operative atrial fibrillation (OPERA) randomized trial. JAMA. 2012;308(19):2001–2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Group RAPSC. n-3 fatty acids in patients with multiple cardiovascular risk factors. N Engl J Med. 2013;368(19):1800–1808. [DOI] [PubMed] [Google Scholar]
- 12.Kromhout D, Giltay EJ, Geleijnse JM; Alpha Omega Trial Group. n-3 fatty acids and cardiovascular events after myocardial infarction. N Engl J Med. 2010;363(21):2015–2026. [DOI] [PubMed] [Google Scholar]
- 13.Grenon SM, Owens CD, Nosova EV, Hughes-Fulford M, Alley HF, Chong K, Perez S, Yen PK, Boscardin J, Hellmann J, et al. Short-term, high-dose fish oil supplementation increases the production of omega-3 fatty acid-derived mediators in patients with peripheral artery disease (the OMEGA-PAD I trial). J Am Heart Assoc. 2015;4(8):e002034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Begg A, Connolly S, Halcox J, Kaba A, Main L, Ray K, Purcell H, Williams H, Yellon D. Omega-3 fatty acids in cardiovascular disease: re-assessing the evidence. Br J Cardiol. 2012;19:79–84. [Google Scholar]
- 15.Siscovick DS, Fretts AM, Wu JH, Lichtenstein AH, Costello RB, Kris-Etherton PM, Engler MB, Alger HM, et al. Omega-3 polyun-saturated fatty acid (fish oil) supplementation and the prevention of clinical cardiovascular disease: A science advisory from the american heart association. Circulation. 2017;135(15):e867–e884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thorngren M, Gustafson A. Effects of 11-week increases in dietary eicosapentaenoic acid on bleeding time, lipids, and platelet aggregation. Lancet. 1981;2(8257):1190–1193. [DOI] [PubMed] [Google Scholar]
- 17.Lorenz R, Spenglerr U, Fischer S, Duhm J, Weber PC. Platelet function, thromboxane formation and blood pressure control during supplementation of the Western diet with cod liver oil. Circulation. 1983;67(3):504–511. [DOI] [PubMed] [Google Scholar]
- 18.Gibney MJ, Bolton-Smith C. The effect of a dietary supplement of n-3 polyunsaturated fat on platelet lipid composition, platelet function and platelet plasma membrane fluidity in healthy volunteers. Br J Nutr. 1988;60(1):5–12. [DOI] [PubMed] [Google Scholar]
- 19.Wachira JK, Larson MK, Harris WS. n-3 fatty acids affect haemostasis but do not increase the risk of bleeding: clinical observations and mechanistic insights. Br J Nutr. 2014;111(9):1652–1662. [DOI] [PubMed] [Google Scholar]
- 20.Mega JL, Simon T. Pharmacology of antithrombotic drugs: an assessment of oral antiplatelet and anticoagulant treatments. Lancet. 2015;386 (9990):281–291. [DOI] [PubMed] [Google Scholar]
- 21.Driss F, Vericel E, Lagarde M, Dechavanne M, Darcet P. Inhibition of platelet aggregation and thromboxane synthesis after intake of small amount of icosapentaenoic acid. Thromb Res. 1984;36(5):389–396. [DOI] [PubMed] [Google Scholar]
- 22.Vanschoonbeek K, Feijge MA, Paquay M, Rosing J, Saris W, Kluft C, Giesen PL, de Maat MP, Heemskerk JW. Variable hypocoagulant effect of fish oil intake in humans: modulation of fibrinogen level and thrombin generation. Arterioscler Thromb Vasc Biol. 2004;24(9):1734–1740. [DOI] [PubMed] [Google Scholar]
- 23.Larson MK, Ashmorel JH, Harris KA, Vogelaar JL, Pottala JV, Sprehe M, Harris WS. Effects of omega-3 acid ethyl esters and aspirin, alone and in combination, on platelet function in healthy subjects. Thromb Haemost. 2008;100(4):634–641. [PubMed] [Google Scholar]
- 24.Larson MK, Shearer GC, Ashmore JH, Anderson-Daniels JM, Graslie EL, Tholen JT, Vogelaar JL, Korth AJ, Nareddy V, Sprehe M, Harris WS. Omega-3 fatty acids modulate collagen signaling in human platelets. Prostaglandins Leukot Essent Fatty Acids. 2011;84(3–4):93–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Larson MK, Tormoen GW, Weaver LJ, Luepke KJ, Patel IA, Hjelmen CE, Ensz NM, McComas LS, McCarty OJ. Exogenous modification of platelet membranes with the omega-3 fatty acids EPA and DHA reduces platelet procoagulant activity and thrombus formation. Am J Physiol Cell Physiol. 2013;304(3):C273–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Massberg S, Brand K, Grüner S, Page S, Müller E, Müller I, Bergmeier W, Richter T, Lorenz M, Konrad I, et al. A critical role of platelet adhesion in the initiation of atherosclerotic lesion formation. J Exp Med. 2002;196(7):887–896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tousoulis D, Plastiras A, Siasos G, Oikonomou E, Verveniotis A, Kokkou E, Maniatis K, Gouliopoulos N, Miliou A, Paraskevopoulos T, Stefanadis C. Omega-3 PUFAs improved endothelial function and arterial stiffness with a parallel antiinflammatory effect in adults with metabolic syndrome. Atherosclerosis. 2014;232(1):10–16. [DOI] [PubMed] [Google Scholar]
- 28.Golebiewska EM, Poole AW. Platelet secretion: from haemostasis to wound healing and beyond. Blood Rev. 2015;29(3):153–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Clark SR, Thomas CP, Hammond VJ, Aldrovandi M, Wilkinson GW, Hart KW, Murphy RC, Collins PW, O’Donnell VB. Characterization of platelet aminophospholipid externalization reveals fatty acids as molecular determinants that regulate coagulation. Proc Natl Acad Sci U S A. 2013;110(15):5875–5880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Drabik L, Wołkow P, Undas A. Denser plasma clot formation and impaired fibrinolysis in paroxysmal and persistent atrial fibrillation while on sinus rhythm: association with thrombin generation, endothelial injury and platelet activation. Thromb Res. 2015;136(2):408–414. [DOI] [PubMed] [Google Scholar]
- 31.Stalker TJ, Traxler EA, Wu J, Wannemacher KM, Cermignano SL, Voronov R, Diamond SL, Brass LF. Hierarchical organization in the hemostatic response and its relationship to the platelet-signaling network. Blood. 2013;121(10):1875–1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Walsh TG, Harper MT, Poole AW. SDF-1α is a novel autocrine activator of platelets operating through its receptor CXCR4. Cell Signal. 2015;27(1):37–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Diagouraga B, Grichine A, Fertin A, Wang J, Khochbin S, SadoulK. Motor-driven marginal band coiling promotes cell shape change during platelet activation. J Cell Biol. 2014;204(2):177–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Reheman A, Yang H, Zhu G, Jin W, He F, Spring CM, Bai X, Gross PL, Freedman J, Ni H. Plasma fibronectin depletion enhances platelet aggregation and thrombus formation in mice lacking fibrinogen and von Willebrand factor. Blood. 2009;113(8):1809–1817. [DOI] [PubMed] [Google Scholar]
- 35.Wang Y, Reheman A, Spring CM, Kalantari J, Marshall AH, Wolberg AS, Gross PL, Weitz JI, Rand ML, Mosher DF, et al. Plasma fibronectin supports hemostasis and regulates thrombosis. J Clin Invest. 2014;124(10):4281–4293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yeung J, Tourdot BE, Adili R, Green AR, Freedman CJ, Fernandez-Perez P, Yu J, Holman TR, Holinstat M. 12(S)-HETrE, a 12-lipoxygenase oxylipin of dihomo-γ-linolenic acid, inhibits thrombosis via Gαs signaling in platelets. Arterioscler Thromb Vasc Biol. 2016;36(10):2068–2077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Reheman A, Gross P, Yang H, Chen P, Allen D, Leytin V, Freedman J, Ni H. Vitronectin stabilizes thrombi and vessel occlusion but plays a dual role in platelet aggregation. J Thromb Haemost. 2005;3(5):875–883. [DOI] [PubMed] [Google Scholar]
- 38.Von Schacky C Omega-3 index and cardiovascular health. Nutrients. 2014;6(2):799–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Falati S, Gross P, Merrill-Skoloff G, Furie BC, Furie B. Real-time in vivo imaging of platelets, tissue factor and fibrin during arterial thrombus formation in the mouse. Nat Med. 2002;8(10):1175–1181. [DOI] [PubMed] [Google Scholar]
- 40.Li W, McIntyre TM, Silverstein RL. Ferric chloride-induced murine carotid arterial injury: A model of redox pathology. Redox Biol. 2013;1:50–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Guillot N, Caillet E, Laville M, Calzada C, Lagarde M, Véricel E. Increasing intakes of the long-chain omega-3 docosahexaenoic acid: effects on platelet functions and redox status in healthy men. Faseb J. 2009;23(9):2909–2916. [DOI] [PubMed] [Google Scholar]
- 42.Fredman G, Van Dyke TE, Serhan CN. Resolvin E1 regulates adenosine diphosphate activation of human platelets. Arterioscler Thromb Vasc Biol. 2010;30(10):2005–2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yeung J, Tourdot BE, Fernandez-Perez P, Vesci J, Ren J, Smyrniotis CJ, Luci DK, Jadhav A, Simeonov A, Maloney DJ, et al. Platelet 12-LOX is essential for FcγRIIa-mediated platelet activation. Blood. 2014;124(14):2271–2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Harvey KA, Xu Z, Pavlina TM, Zaloga GP, Siddiqui RA. Modulation of endothelial cell integrity and inflammatory activation by commercial lipid emulsions. Lipids Health Dis. 2015;14:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bo L, Jiang S, Xie Y, Kan H, Song W, Zhao J. Effect of vitamin E and omega-3 fatty acids on protecting ambient PM2.5-induced inflammatory response and oxidative stress in vascular endothelial cells. PLoS One. 2016;11(3):e0152216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.De Caterina R, Liao JK, Libby P. Fatty acid modulation of endothelial activation. Am J Clin Nutr. 2000;71(1 Suppl):213S–23S. [DOI] [PubMed] [Google Scholar]
- 47.Fenton JI, Hord NG, Ghosh S, Gurzell EA. Immunomodulation by dietary long chain omega-3 fatty acids and the potential for adverse health outcomes. Prostaglandins Leukot Essent Fatty Acids. 2013;89(6):379–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Oomen CM, Feskens EJ, Räsänen L, Fidanza F, Nissinen AM, Menotti A, Kok FJ, Kromhout D. Fish consumption and coronary heart disease mortality in Finland, Italy, and the Netherlands. Am J Epidemiol. 2000;151(10):999–1006. [DOI] [PubMed] [Google Scholar]
- 49.Kromhout D. N-3 fatty acids and coronary heart disease: epidemiology from Eskimos to western populations. J Intern Med Suppl. 1989;731:47–51. [DOI] [PubMed] [Google Scholar]
- 50.Bassuk SS, Manson JE, Lee IM, Cook NR, Christen WG, Bubes VY, Gordon DS, Copeland T, Friedenberg G, D’Agostino DM, et al. Baseline characteristics of participants in the vitamin D and omega-3 trial (VITAL). Contemp Clin Trials. 2016;47:235–243. [DOI] [PMC free article] [PubMed] [Google Scholar]