Abstract
GABAergic interneuron loss, maturational delay, or imbalance of glutamatergic to GABAergic signaling has been implicated in several neuropsychiatric disorders including Tourette syndrome and attention-deficit/hyperactivity disorder (ADHD). In schizophrenia, decreases in Parvalbumin (PV), Somatostatin (Sst), and Glutamic Acid Decarboxylase (GAD) RNA have been observed and seem to indicate a failure in maturation in PV and Sst neurons. In Tourette syndrome, which has a high level of comorbid ADHD, reduced numbers of parvalbumin expressing neurons have been observed in the basal ganglia of affected patients. In addition, polymorphisms in the GAD1 gene that codes for GAD67 protein have been associated with ADHD. We have examined whether mice with a disrupted Gad67 allele, the Gad67 GFP knock-in mice (Gad67-GFP+/−), display abnormal locomotor behavior, or altered anxiety behavior on the elevated plus maze. We found that Gad67-GFP+/− mice displayed a mild hyperactivity compared to control littermates.
Introduction
Glutamic Acid Decarboxylase 67 (GAD67) is encoded by the GAD1 gene, and is one of two GAD proteins responsible for the synthesis of the neurotransmitter gamma aminobutyric acid (GABA) from glutamate (Defelipe et al., 2013). GABA is the main inhibitory neurotransmitter found in the brain. Tamamaki et al. introduced a green fluorescent protein (GFP) transgene into the 5’UTR of the endogenous mouse Gad67 allele in order to genetically tag GABA producing neurons. The GFP insertion disrupts the endogenous Gad67 allele, and therefore results in haploinsufficiency for Gad67 in heterozygous mice (Gad67-GFP+/−) and reduced GABA levels in the brain (Tamamaki et al., 2003). The Gad67-GFP+/− mice have been a valuable tool for studying the development, physiology, and function of GABAergic interneurons (Tamamaki et al., 2003, Cai et al., 2013, Smith et al., 2014). GABAergic interneurons are a diverse class of neurons that, while sharing the use of GABA as a neurotransmitter, display a wide variety of cell morphologies, synaptic targeting specificity, and electrophysiological activity. GABAergic interneurons can be classified according to these characteristics, including their expression of peptides and calcium binding proteins (Howard et al., 2005, Gelman et al., 2012, Defelipe et al., 2013). Three broad classes of interneuron subtypes can be made based on the non-overlapping expression of parvalbumin (PV), somatostatin (Sst) and Calretinin (CR).
Abnormal development, maturation or function of cortical GABAergic interneurons has been implicated in various neuropsychiatric disorders including schizophrenia, depression, and Tourette syndrome (Lewis et al., 1999, Hashimoto et al., 2003, Kalanithi et al., 2005, Kataoka et al., 2010, Gonzalez-Burgos et al., 2011, Bollmann et al., 2015, Bruxel et al., 2016, Naaijen et al., 2017). Decreases in Gad1, parvalbumin (PV) and somatostatin (Sst) have been consistently found in the cerebral cortex of individuals with schizophrenia implicating reduced cortical GABAergic signaling in this disorder (Benes and Berretta, 2001, Howard et al., 2005, Gonzalez-Burgos et al., 2011). Several rodent models of schizophrenia also display decreases in PV+ interneurons and many of these rodent models also share behavioral hyperactivity (Pillai-Nair et al., 2005, Muller Smith et al., 2008, Belforte et al., 2010, Smith et al., 2014). Reduced levels of GABA have been measured in children with attention-deficit/hyperactivity disorder (ADHD) (Edden et al., 2012). Also, the GAD1 gene which encodes the GAD67 protein in humans has been implicated in ADHD (Bruxel et al., 2016). In working with Gad67-GFP+/− mice, we noticed that some mice seemed abnormally active in their home cage, and decided to study this further using an open field locomotor activity test. We found significant differences in locomotor behavior between Gad67-GFP+/− mice and control littermates.
Methods
Animals
This study was carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health as listed in the Compliance with Ethical Standards Section. All mice were provided food ad libitum and were housed in a mouse vivarium with a 12 hour light-12 hour dark cycle. Gad67-GFP+/− and control mice were obtained from breeding Gad67-GFP+/− males or females to a GFP negative mate, yielding 50% offspring with the Gad67-GFP allele and 50% control offspring. Animals were genotyped within 3 days of birth using GFP epifluorescent microscopy, or GFP googles (BLS). GFP positive pups were marked by making a 0.5–1 mm tail clipping followed by cauterization. When mice were not genotyped between postnatal day P0–P3, tail clipping, DNA isolation and PCR for GFP was performed to identify GFP+ animals.
Behavioral Tests
Seven month old Gad67-GFP+/− (n=8) and GFP negative control littermates (n=10) were tested on an open field for locomotor behavior, and on the elevated plus maze for anxiety on separate days. All animals were males. Animals were tested for locomotor behavior on day 1 and the elevated plus maze on day 2. For both tests, animals were brought to the animal behavior room and allowed to acclimate 30 min–1 hour to the room prior to behavioral testing.
For locomotor behavior, mice were placed in a chamber measuring 49.5 cm × 34 cm illuminated with a ceiling light from above. Mice were placed in the chamber one at a time, in the same corner each time. Mice were monitored for 30 minutes. The two groups were compared for the following measures: distance traveled, average speed, and total time mobile. Testing for anxiety like behaviors was done on an elevated plus maze apparatus (Stoelting). The apparatus height is 50 cm, and it is placed on a flat raised platform. The apparatus arm dimensions are 5 cm wide arm width, 35 cm open arm length, and 15 cm high closed arm walls. Mice were placed in the center of the maze facing an open arm and monitored for 5 minutes. The two groups were compared for the following measures: time in closed arm, time in open arm, latency to enter open arm. The locomotor activity chamber and elevated plus maze apparatus were wiped down with 70% ethanol between animals in order to clean droppings and urine, and to remove odorants. Data was acquired with a logitec web camera mounted over the apparatus and connected to a computer with a USB cable and analyzed with the ANYMAZE program (Stoelting) using t-tests.
Results
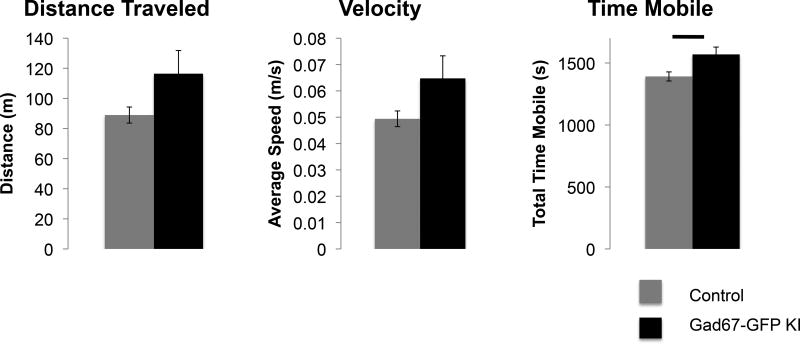
During routine handling of mice, we noticed that Gad67-GFP+/− mice seemed to be more active than their GFP negative littermates. In order to test whether this observation was significant, we tested the locomotor behavior in an open field in Gad67-GFP+/− mice and their control wild type littermates. Over a 30-minute period, Gad67-GFP+/− mice showed a significant higher time spent mobile than control GFP− littermates (p=0.017, Figure 1). Gad67-GFP+/− mice also displayed non-significant trends towards increase distance traveled and average speed (p=0.084 and p=0.083) (Figure 1).
Figure 1. Locomotor analysis of Gad67-GFP+/− and control littermates.
Mean distance traveled, velocity, and time mobile over 30 minutes in an open field were compared between Gad67-GFP+/− (n=8) and control littermates lacking the GFP knock in allele (n=10). Time Mobile was significantly increased in Gad67-GFP+/− mice.
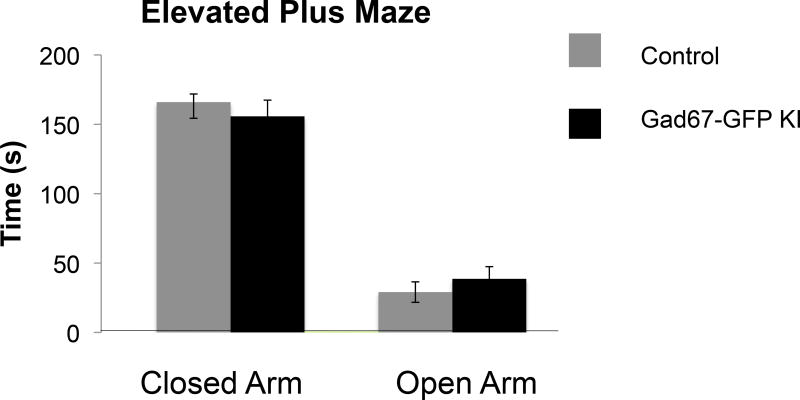
We also tested whether there were differences in anxiety like behaviors on the elevated plus maze test. Mice from both groups were tested for 5-min trials on an EPM apparatus. We found no significant differences between groups when we examined time spent in open arms, times spent in closed arms, or latency to enter open arms (Figure 2).
Figure 2. Elevated plus maze testing of Gad67-GFP+/− and control littermates.
Average time in closed and open arms of the elevated plus maze test Gad67-GFP+/− (n=8) and control littermates lacking the GFP knock in allele (n=10). No differences in time spent in the closed, or open, arms of the elevated plus maze were observed between groups, indicating no difference in baseline anxiety.
Conclusions
We found that Gad67-GFP+/− mice have a mild hyperactive phenotype characterized by more time spent mobile than control littermates. This occurred in the absence of an anxiety phenotype as assessed by the elevated plus maze test. This finding is of interest given the ample literature implicating GABAergic neuron involvement in neuropsychiatric disorders with impaired executive function and impulse control such as Schizophrenia, Bipolar disorder, ADHD, and Tourette Syndrome. Previous studies of Fibroblast growth factor receptor 1 (Fgfr1) disruption in the brain implicated the loss of PV+ neuron maturation in the hyperactive behavior of mice lacking Fgfr1 in the dorsal telencephalon (Muller Smith et al., 2008, Smith et al., 2014). Mooney et al. 2016, also found evidence implicating Fgfr1 signaling in ADHD in human genetic studies by employing pathway analyses of two genome wide association study datasets. These studies identified both the Fgfr1 and Fgfr2 binding and activation pathway gene sets as significantly associated with ADHD (Mooney et al., 2016). Other rodent models have also observed decreased interneuron number and hyperactive behavior (Pillai-Nair et al., 2005, Cunningham et al., 2006, Penschuck et al., 2006, Rujescu et al., 2006, Braun et al., 2007, Xenos et al., 2017). As reported in the original description of this genetically modified mouse line, mice with the GFP insertion into the gene for Gad67 (Gad1) had demonstrably lower levels of GABA in the brain since they lack one functioning copy of this gene (Tamamaki et al., 2003). They are therefore expected to have a lowered inhibitory tone. Our current evidence suggests that this reduced GABA dosage leads to increased locomotion and a hyperactive phenotype. This evidence helps support previous findings implicating GABA signaling, and imbalances in excitatory/inhibitory tone in ADHD (Edden et al., 2012, Bruxel et al., 2016, Naaijen et al., 2017). Of note, Zhang et al. reported a striatal specific knockout of the gene for Gad67 by mating Gad1lox/lox mice to GPR88cre mice (Zhang et al., 2015). These mice displayed an increase in locomotor behavior. Therefore, the hyperactive behavior observed in the Gad67-GFP+/− mice may not be exclusively attributed to changes in cortical GABAergic tone.
One caveat to the application of these studies towards understanding the neurobiology of hyperactivity, is the degree to which hyperactivity in a mouse model, as measured by locomotor recordings in an open field, reflect upon the symptoms and underlying causes of ADHD. Locomotor hyperactivity in ADHD is conceptualized to reflect defects in behavioral inhibition, and can be contextual such as remaining seated in situations where being seated is expected (Barkley, 1997). Other hyperactivity symptoms include “often acts as if driven by a motor” or “often on the go”. The validity of laboratory measurements of hyperactivity in humans is limited, and is not relied upon for diagnosis (Barkley, 1991). Wickens et al. 2011 reviewed the literature concerning animal models of ADHD, as well as the validity of open field tests compared to cognitive tasks that are designed to assess behavioral inhibition such as the Delay Discounting paradigm and the Five Choice Serial Reaction Time test (Wickens et al., 2011). While these tests are designed to assess behavioral inhibition through measures of impulsivity, they require longer entrainment of the animal to the test, and have generally been performed with rats, which are more adept at learning complicated tasks, compared to mice. Of note, our decision to test the behavior of the Gad67-GFP+/− mice was based on our observation that they appeared to have more activity within their home cages, to which they are habituated. This increased activity was also reflected in open field tests in a novel environment, where the mice spent more time mobile than control littermates.
Acknowledgments
The author wishes to thank Dr. Flora Vaccarino for support and editorial review, and Dr. Yanagawa for sharing the Gad67-GFP+/− mouse line, and Dr. Russ Barkley for helpful discussion. Support was provided by National Institutes of Health, NIH: K01MH087845 and Brain and Behavior Research Foundation NARSAD Young Investigator Award. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Conflict of Interest: The author declares that she has no conflict of interest.
Compliance with Ethical Standards
Ethical Approval: This study was carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. The protocol was approved by the Yale University Institutional Animal Care and Use Committee (protocol number 2012-07621).
Informed Consent: This article does not contain studies with human participants performed by the author.
References
- Barkley RA. The ecological validity of laboratory and analogue assessment methods of ADHD symptoms. J Abnorm Child Psychol. 1991;19:149–178. doi: 10.1007/BF00909976. [DOI] [PubMed] [Google Scholar]
- Barkley RA. Attention-deficit/hyperactivity disorder, self-regulation, and time: toward a more comprehensive theory. J Dev Behav Pediatr. 1997;18:271–279. [PubMed] [Google Scholar]
- Belforte JE, Zsiros V, Sklar ER, Jiang Z, Yu G, Li Y, Quinlan EM, Nakazawa K. Postnatal NMDA receptor ablation in corticolimbic interneurons confers schizophrenia-like phenotypes. Nat Neurosci. 2010;13:76–83. doi: 10.1038/nn.2447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benes FM, Berretta S. GABAergic interneurons: implications for understanding schizophrenia and bipolar disorder. Neuropsychopharmacology. 2001;25:1–27. doi: 10.1016/S0893-133X(01)00225-1. [DOI] [PubMed] [Google Scholar]
- Bollmann S, Ghisleni C, Poil SS, Martin E, Ball J, Eich-Hochli D, Edden RA, Klaver P, Michels L, Brandeis D, O'Gorman RL. Developmental changes in gamma-aminobutyric acid levels in attention-deficit/hyperactivity disorder. Transl Psychiatry. 2015;5:e589. doi: 10.1038/tp.2015.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun I, Genius J, Grunze H, Bender A, Moller HJ, Rujescu D. Alterations of hippocampal and prefrontal GABAergic interneurons in an animal model of psychosis induced by NMDA receptor antagonism. Schizophr Res. 2007;97:254–263. doi: 10.1016/j.schres.2007.05.005. [DOI] [PubMed] [Google Scholar]
- Bruxel EM, Akutagava-Martins GC, Salatino-Oliveira A, Genro JP, Zeni CP, Polanczyk GV, Chazan R, Schmitz M, Rohde LA, Hutz MH. GAD1 gene polymorphisms are associated with hyperactivity in Attention-Deficit/Hyperactivity Disorder. Am J Med Genet B Neuropsychiatr Genet. 2016;171:1099–1104. doi: 10.1002/ajmg.b.32489. [DOI] [PubMed] [Google Scholar]
- Cai Y, Zhang Y, Shen Q, Rubenstein JL, Yang Z. A Subpopulation of Individual Neural Progenitors in the Mammalian Dorsal Pallium Generates Both Projection Neurons and Interneurons vitro. Stem Cells. 2013 doi: 10.1002/stem.1363. [DOI] [PubMed] [Google Scholar]
- Cunningham MO, Hunt J, Middleton S, LeBeau FE, Gillies MJ, Davies CH, Maycox PR, Whittington MA, Racca C. Region-specific reduction in entorhinal gamma oscillations and parvalbumin-immunoreactive neurons in animal models of psychiatric illness. J Neurosci. 2006;26:2767–2776. doi: 10.1523/JNEUROSCI.5054-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Defelipe J, Lopez-Cruz PL, Benavides-Piccione R, Bielza C, Larranaga P, Anderson S, Burkhalter A, Cauli B, Fairen A, Feldmeyer D, Fishell G, Fitzpatrick D, Freund TF, Gonzalez-Burgos G, Hestrin S, Hill S, Hof PR, Huang J, Jones EG, Kawaguchi Y, Kisvarday Z, Kubota Y, Lewis DA, Marin O, Markram H, McBain CJ, Meyer HS, Monyer H, Nelson SB, Rockland K, Rossier J, Rubenstein JL, Rudy B, Scanziani M, Shepherd GM, Sherwood CC, Staiger JF, Tamas G, Thomson A, Wang Y, Yuste R, Ascoli GA. New insights into the classification and nomenclature of cortical GABAergic interneurons. Nat Rev Neurosci. 2013;14:202–216. doi: 10.1038/nrn3444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edden RA, Crocetti D, Zhu H, Gilbert DL, Mostofsky SH. Reduced GABA concentration in attention-deficit/hyperactivity disorder. Arch Gen Psychiatry. 2012;69:750–753. doi: 10.1001/archgenpsychiatry.2011.2280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelman DM, Marin, Rubenstein JLR. The Generation of Cortical Interneurons. 2012 [PubMed] [Google Scholar]
- Gonzalez-Burgos G, Fish KN, Lewis DA. GABA neuron alterations, cortical circuit dysfunction and cognitive deficits in schizophrenia. Neural Plast. 2011;2011:723184. doi: 10.1155/2011/723184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto T, Volk DW, Eggan SM, Mirnics K, Pierri JN, Sun Z, Sampson AR, Lewis DA. Gene expression deficits in a subclass of GABA neurons in the prefrontal cortex of subjects with schizophrenia. J Neurosci. 2003;23:6315–6326. doi: 10.1523/JNEUROSCI.23-15-06315.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard A, Tamas G, Soltesz I. Lighting the chandelier: new vistas for axo-axonic cells. Trends Neurosci. 2005;28:310–316. doi: 10.1016/j.tins.2005.04.004. [DOI] [PubMed] [Google Scholar]
- Kalanithi PS, Zheng W, Kataoka Y, DiFiglia M, Grantz H, Saper CB, Schwartz ML, Leckman JF, Vaccarino FM. Altered parvalbumin-positive neuron distribution in basal ganglia of individuals with Tourette syndrome. Proc Natl Acad Sci U S A. 2005;102:13307–13312. doi: 10.1073/pnas.0502624102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kataoka Y, Kalanithi PS, Grantz H, Schwartz ML, Saper C, Leckman JF, Vaccarino FM. Decreased number of parvalbumin and cholinergic interneurons in the striatum of individuals with Tourette syndrome. J Comp Neurol. 2010;518:277–291. doi: 10.1002/cne.22206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis DA, Pierri JN, Volk DW, Melchitzky DS, Woo TU. Altered GABA neurotransmission and prefrontal cortical dysfunction in schizophrenia. Biol Psychiatry. 1999;46:616–626. doi: 10.1016/s0006-3223(99)00061-x. [DOI] [PubMed] [Google Scholar]
- Mooney MA, McWeeney SK, Faraone SV, Hinney A, Hebebrand J, Nigg JT, Wilmot B. Pathway analysis in attention deficit hyperactivity disorder: An ensemble approach. Am J Med Genet B Neuropsychiatr Genet. 2016;171:815–826. doi: 10.1002/ajmg.b.32446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller Smith K, Fagel DM, Stevens HE, Rabenstein RL, Maragnoli ME, Ohkubo Y, Picciotto MR, Schwartz ML, Vaccarino FM. Deficiency in inhibitory cortical interneurons associates with hyperactivity in fibroblast growth factor receptor 1 mutant mice. Biol Psychiatry. 2008;63:953–962. doi: 10.1016/j.biopsych.2007.09.020. [DOI] [PubMed] [Google Scholar]
- Naaijen J, Bralten J, Poelmans G, Glennon JC, Franke B, Buitelaar JK. Glutamatergic and GABAergic gene sets in attention-deficit/hyperactivity disorder: association to overlapping traits in ADHD and autism. Transl Psychiatry. 2017;7:e999. doi: 10.1038/tp.2016.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penschuck S, Flagstad P, Didriksen M, Leist M, Michael-Titus AT. Decrease in parvalbumin-expressing neurons in the hippocampus and increased phencyclidine-induced locomotor activity in the rat methylazoxymethanol (MAM) model of schizophrenia. Eur J Neurosci. 2006;23:279–284. doi: 10.1111/j.1460-9568.2005.04536.x. [DOI] [PubMed] [Google Scholar]
- Pillai-Nair N, Panicker AK, Rodriguiz RM, Gilmore KL, Demyanenko GP, Huang JZ, Wetsel WC, Maness PF. Neural cell adhesion molecule-secreting transgenic mice display abnormalities in GABAergic interneurons and alterations in behavior. J Neurosci. 2005;25:4659–4671. doi: 10.1523/JNEUROSCI.0565-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rujescu D, Bender A, Keck M, Hartmann AM, Ohl F, Raeder H, Giegling I, Genius J, McCarley RW, Moller HJ, Grunze H. A pharmacological model for psychosis based on N-methyl-D-aspartate receptor hypofunction: molecular, cellular, functional and behavioral abnormalities. Biol Psychiatry. 2006;59:721–729. doi: 10.1016/j.biopsych.2005.08.029. [DOI] [PubMed] [Google Scholar]
- Smith KM, Maragnoli ME, Phull PM, Tran KM, Choubey L, Vaccarino FM. Fgfr1 inactivation in the mouse telencephalon results in impaired maturation of interneurons expressing parvalbumin. PLoS One. 2014;9:e103696. doi: 10.1371/journal.pone.0103696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamamaki N, Yanagawa Y, Tomioka R, Miyazaki J, Obata K, Kaneko T. Green fluorescent protein expression and colocalization with calretinin, parvalbumin, and somatostatin in the GAD67-GFP knock-in mouse. J Comp Neurol. 2003;467:60–79. doi: 10.1002/cne.10905. [DOI] [PubMed] [Google Scholar]
- Wickens JR, Hyland BI, Tripp G. Animal models to guide clinical drug development in ADHD: lost in translation? Br J Pharmacol. 2011;164:1107–1128. doi: 10.1111/j.1476-5381.2011.01412.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xenos D, Kamceva M, Tomasi S, Cardin JA, Schwartz ML, Vaccarino FM. Loss of TrkB Signaling in Parvalbumin-Expressing Basket Cells Results in Network Activity Disruption and Abnormal Behavior. Cereb Cortex. 2017:1–15. doi: 10.1093/cercor/bhx173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang K, Chammas C, Soghomonian JJ. Loss of glutamic acid decarboxylase (Gad67) in striatal neurons expressing the Drdr1a dopamine receptor prevents L-DOPA-induced dyskinesia in 6-hydroxydopamine-lesioned mice. Neuroscience. 2015;303:586–594. doi: 10.1016/j.neuroscience.2015.07.032. [DOI] [PubMed] [Google Scholar]