Abstract
To determine the potential changes of IL-6, IL-17A and IL-21 levels during induction therapy, and to assess their relationship with disease activity and immunologic features on patients with active lupus nephritis, twenty-eight patients treated with corticosteroid and immunosuppressants were included in this study. Demographic, clinical, serological data and disease activity were assessed. Blood samples were collected at week 0, 12 and 24, and serum concentrations of IL-17A, IL-6 and IL-21 were measured by cytometric bead array. The serum concentrations of IL-6, IL-17A and IL-21 (P<0.001, P<0.01, P=0.001, respectively) decreased progressively during induction therapy. Concentration of IL-6, IL-17A and IL-21 was higher in non-remission group than that in remission group. A positive correlation was established between the concentration of these cytokines and the severity of proteinuria (P<0.001, P=0.020, P=0.045, respectively), ESR (P<0.001), SLEDAI scores (P<0.05), and ANA titers (P=0.018, P=0.048, P<0.05, respectively). Additionally, ROC curve analysis for IL-6, IL-17A and IL-21 was performed to predict the disease activity. The optimal cutoff level was 5.78 pg/ml, 1.98 pg/ml and 8.59 pg/ml, with AUC=0.809, 0.735 and 0.786. The concentration of IL-6 and IL-21 may be regarded as an indicator for the remission of active lupus nephritis, with cutoff value of 9.12 pg/ml and 11.30 pg/ml, while AUC=0.930 and 0.896. The production of serum IL-6, IL-17A and IL-21 in active LN was dramatically declined during induction therapy, which may improve disease activity while delay disease progression of LN.
Keywords: Lupus nephritis, cytokines, Th17, Tfh
Introduction
Systemic lupus erythematosus (SLE) is a chronic heterogeneous inflammatory autoimmune disorder characterized by the deposition of immune complexes in different organs, which may lead to multisystem involvement. It has a progressive and relapsing/remitting nature on many patients, presented as a mixture of mild skin or musculoskeletal and hematological signs and symptoms [1]. Despite extensive research, the detailed mechanism for SLE pathogenesis has not been completely understood. Lupus nephritis (LN) is a common complication, which is a major cause of morbidity and mortality of SLE. Aberrant expansion and dysregulation of several subsets of TH effector, involving T helper 17 (Th17) cells and T follicular helper (Tfh) cells, may be associated with autoimmune diseases, particularly, SLE [1-7]. Previous observations have suggested that the deregulated production of some cytokines from the cells, e.g. IL-6, IL-17A, IL-21, etc, plays a central role in the initiation and progression of SLE [1,5,8,9].
The Th17 profile is mainly characterized by IL-17A and promoted mainly by TGF-β and IL-6 [10,11]. The former is a member in the IL-17 family, which can amplify the immune response by inducing the local production of chemokines and cytokines, recruiting neutrophils and monocytes, augmenting the production of autoantibodies, aggravating the inflammation and damaging target organs, such as the kidney, in SLE [12,13]. Several studies have demonstrated that the serum level of IL-17A was significantly elevated on patients with LN compared with those without nephritis and healthy controls, which positively correlated with disease activity [1,8,9,14-17]. IL-6 is a multifunctional cytokine produced in response to inflammatory stimuli, including IL-1 and TNF-α, with pivotal roles in regulating the host immune response to infection [18]. Thus, IL-6 has been found to be a potent stimulator for the differentiation and activation of lymphoid and myeloid cells and the production of acute phase proteins in liver [19,20]. Moreover, it displayed a synergistic effect with TGF-β to promote the differentiation of Th17 cells [21]. It is now well known that the serum level of the cytokine IL-6 is elevated on patients with SLE, achieving the highest level on those patients with active disease [1,8,9,21,22]. Tfh cells, a recently described novel subset of Th cells, play a critical role in the development of B cell responses, which is a dependent cell of T cell, due to their indispensible role in the pathogenic production of autoantibodies [4,5,23,24]. High level of the immunomodulatory cytokine IL-21 is a hallmark of Tfh cells due to its regulation on the differentiation of CD4+ T cell [25]. It promotes Tfh cell differentiation based on the autocrine mechanism, thus forming a positive feedback loop on naive CD4+ T cells. Additionally, it is critical for the differentiation of Th17 cells and the generation of IL-17 [26].
Even though it has been recognized that the level of IL-6, IL-17A and IL-21 in SLE is higher than that in healthy controls, and higher on patients with active lupus nephritis than those without nephritis, which implies its pathological role in SLE. The potential response of these cytokines in active lupus nephritis to the induction therapy has rarely been elucidated. In this present study, the serum level of IL-6, IL-17A and IL-21 during a 6-month induction therapy was measured so as to assess the correlations between these cytokines and the disease activity of SLE. In addition, the role of these cytokines serving as the biomarkers of disease activity and predictors for the remission of active lupus nephritis were explored.
Patients and methods
Patient selection
This study was performed at the Division of Rheumatology, the First Affiliated Hospital of Zhengzhou University. All the participants signed the informed consent forms. The study was approved by the Ethic Committee of Zhengzhou University [2016-LW-124]. Demographic and clinical data were collected. Twenty-eight patients with active lupus nephritis (SLEDAI≥5) were recruited. All of them met the classification criteria from the American College of Rheumatology [27] and the International Society of Nephrology/Renal Pathology Society (ISN/RPS) [28]. Patients were excluded in the study if they met the following criteria: (1) <18 years old or >60 years old; (2) in pregnancy or lactation; (3) treated with immunosuppressive medicine; (4) coexistence of other autoimmune diseases, such as rheumatoid arthritis and systemtic sclerosis, or chronic diseases affecting cytokines levels, such as systemic infection. Histological analysis of renal biopsies showed that the categories of nephritis covering from class III to V. All the patients were treated with pulse methylprednisolone and with subsequent oral prednisone (0.8 mg/kg/day) and tacrolimus or intravenous cyclophosphamide. The efficacy evaluation was conducted at week 24. Complete remission (CR) was defined as proteinuria <0.5 g/24 hr, serum albumin ≥3.5 g/L, while creatinine increased 15% less than the baseline level. Partial remission (PR) was defined as 0.5 g/24 hr≤ proteinuria <3.5 g/24 hr, proteinuria decreased >50% baseline, while creatinine increased ≤15% of baseline. Patients who met none of the criteria mentioned above were designated as the non-remission ones.
Clinical follow-up
After baseline assessment, all patients were treated with oral corticosteroid and immunosuppressants. The treatment regimens were individualized by the attending physicians without any interference from the current study designs. Clinical and laboratory data were collected at week 0, 12, and 24, which included the routine tests of blood and urine, serum albumin, creatinine, complements, erythrocyte sedimentation rate (ESR), the titers of antinuclear antibody (ANA), the serum level of IL-6, IL-17A and IL-21, and the total protein amount of 24-hour urine (24 hTP). Lupus disease activity was assessed by the SLEDAI scores [29].
Cytokine assay
Serum was separated from blood samples via density gradient centrifugation by using the Ficoll-Paques reagent. Aliquots were stored at -80°C. The concentration of IL-6, IL-17A and IL-21 was determined by the Cytometric Bead Array Kit (Biolegend, America). Quantification was performed by the BD calibur flow cytometer (BD Biosciences). The corresponding results were analyzed by using LEGENDplex Data Analysis software (Biolegend). The relevant experiments were performed by following the manufacturer’s protocol with the corresponding results presented as pg/ml.
Statistical analysis
Statistical analysis was performed by using SPSS statistics software (version 21). Variables were summarized by using the mean and standard deviation (SD). Person’s rank correlation analysis was used for assessing the relationship of the serum concentration of IL-6, IL-17A and IL-21, the SLEDAI score and laboratory parameters of LN patients. One-way ANOVA was used for analyzing the serum level of IL-6, IL-17A and IL-21 in different treatment groups (NR, PR and CR). The validity of IL-6, IL-17A and IL-21 as a predictor of disease activity and remission was estimated by Receiver Operator Characteristics (ROC) curves, area under curve (AUC), cutoff point sensitivity, and specificity with 95% confidence intervals (CIs). The statistical differences were considered as significance when P<0.05.
Results
Baseline characteristics
The baseline characteristics of 28 LN patients and the change of clinical parameters during the study were summarized in Table 1. Among these patients, 27 (96.43%) were female, with the mean age at the time of the study of 33.50±7.47 years and the mean disease duration of 68.04±56.61 months. All of them were treated with prednisone; among them, 9 (32.14%) combined with tacrolimus, while 19 (67.86%) combined with cyclophosphamide. Renal biopsy was performed in all patients and pathological classes of lupus nephritis included III (n=2), IV (n=9), V (n=2), III+ V (n=4), and IV +V (n=11). The treatment outcomes included CR (n=14), PR (n=9) and NR (n=5).
Table 1.
Baseline characteristics of LN patients
| Characteristics | Visit time | ||
|---|---|---|---|
|
| |||
| 0 week | 12 week | 24 week | |
| Female sex. n (%) | 27 (96.43) | NS | NS |
| Age (years)※ | 33.50±7.47 | NS | NS |
| Diseaseduration (month)※ | 68.04±56.61 | NS | NS |
| Immunologic features※ | |||
| ANA titers (≥1:320). n (%) | 17 (60.71) | 15 (53.57) | 14 (50) |
| C3 (g/L)※ | 0.56±0.26 | 1.00±0.22 | 1.03±0.24 |
| C4 (g/L)※ | 0.36±0.35 | 0.66±0.47 | 0.70±0.50 |
| Serum albumin (g/L)※ | 22.89±6.35 | 35.00±5.86 | 38.96±4.83 |
| Proteinuria (g/24 hr)※ | 5.35±3.18 | 2.01±2.12 | 0.98±1.49 |
| SLEDAI Scores※ | 11.68±4.40 | 6.46±2.57 | 4.96±3.31 |
LN: Lupus nephritis; ANA: antinuclear antibodies; SLEDAI: Systemic lupus erythematosus disease activity index; C3: complement 3; C4: complement 4;
mean ± SD.
Alterations of serum cytokines in LN patients during induction therapy
The changes in the serum concentration of IL-6, IL-17A and IL-21 in LN patients receiving induction therapy were shown in Figure 1. A significant decrease in the serum concentration of IL-6 was observed after induction treatment (Figure 1A). The mean level of serum IL-6 was significantly higher (P=0.001) at week 0 (23.78±17.86 pg/ml) compared with that at week 12 (12.16±10.88 pg/ml) and week 24 (7.78±5.37 pg/ml) (P<0.05). The mean level of serum IL-17A at week 12 (2.90 pg/ml) was lower than that at week 0 (5.03±4.90 pg/ml), but with no statistical significance observed (P=0.077). However, it was significantly lower at week 24 (1.56±1.85 pg/ml) compared with that at week 0 (P=0.006) (Figure 1B). Similar to IL-6, there was a dramatic decline in the serum level of IL-21 during Induction therapy (Figure 1C).
Figure 1.
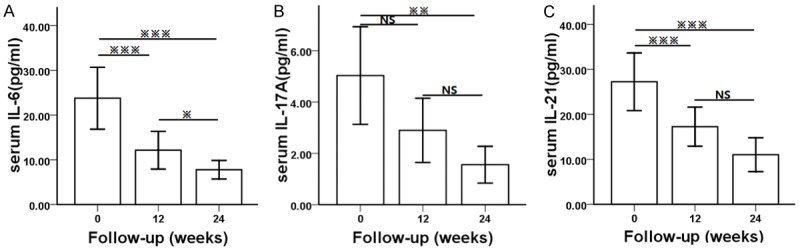
Alterations of serum cytokines on LN patients during induction therapy: A. Alteration of serum IL-6 level on LN patients during induction therapy. B. Alteration of serum IL-17A level on LN patients during induction therapy. C. Alteration of serum IL-21 level on LN patients during induction therapy. P values depict comparison by Broforroni. ※ is significance at the 0.05 level, ※※ is significance at the 0.01 level, ※※※ is significance at the 0.001 level, NS is no significance.
The level of IL-6, IL-17A and IL-21 in NR, PR and CR groups
Furthermore, the level of IL-6, IL-17A and IL-21 in NR, PR and CR groups at week 24 was analyzed with the corresponding results summarized in Table 2. The results showed significantly higher serum level of IL-6 and IL-21 in NR groups at week 24 than that in PR and CR groups (12.90±5.49 versus 6.63±4.18 and 6.14±3.64 pg/ml, 21.72±8.57 versus 9.51±8.98 and 8.70±8.43 pg/ml, respectively). A decline trend for IL-17A was found but no statistical significant difference was observed.
Table 2.
The levels of IL-6, IL-17A and IL-21 in NR, PR and CR groups
| NR | PR | CR | NR vs PR | PR vs CR | NR vs CR | |
|---|---|---|---|---|---|---|
|
| ||||||
| P | P | P | ||||
| IL-6 (pg/ml) | 12.90±5.49 | 6.63±4.18 | 6.14±3.64 | 0.031 | 0.959 | 0.012 |
| IL-17A (pg/ml) | 2.29±2.05 | 1.80±2.32 | 0.99±0.88 | 0.863 | 0.553 | 0.348 |
| IL-21 (pg/ml) | 21.72±8.57 | 9.51±8.98 | 8.70±8.43 | 0.042 | 0.972 | 0.018 |
NR: non-remission; PR: partial remission; CR: complete remission. Data are expressed as mean ± SD.
Correlations between cytokines level and laboratorial parameters
The correlations between the serum level of cytokines and clinical parameters were further explored with the relevant results showed in Table 3. Serum level of IL-6 was positively correlated with SLEDAI score (r=0.248, P=0.023), proteinuria (r=0.400, P<0.001), ANA titers (r=0.259, P=0.018), serum C3 (r=-0.270, P=0.013), and ESR (r=0.409, P<0.001). Similarly, serum level of IL-17A was correlated with SLEDAI score (r=0.261, P=0.016), proteinuria (r=0.254, p=0.020), ANA titers (r=0.210, P=0.048) and ESR (r=0.388, P<0.001). Such correlations were also found among the serum level of IL-21, SLEDAI score (r=0.252, P=0.021), proteinuria (r=0.220, P=0.045), ANA titers (r=0.334, P<0.05) and ESR (r=0.526, P<0.001). Somewhat surprisingly, no significant correlation was detected between these cytokines and the serum level of C4.
Table 3.
Correlations between Cytokines levels and Laboratorial parameters
| IL-6 (pg/ml) | 0.248※ | 0.400※※※ | -0.270※ | -0.052 | 0.259※ | 0.409※※※ |
| IL-17A (pg/ml) | 0.261※ | 0.254※ | -0.084 | -0.056 | 0.210※ | 0.388※※※ |
| IL-21 (pg/ml) | 0.252※ | 0.220※ | -0.209 | -0.184 | 0.334※※ | 0.526※※※ |
Correlation is significance at the 0.05 level.
Correlation is significance at the 0.01 level.
Correlation is significance at the 0.001 level.
NS correlation is no significance.
Correlation among the serum concentrations of IL-17A and IL-6, IL-21 on lupus nephritis patients
Interestingly, the correlation among the serum concentrations of IL-17A, IL-6 and IL-21 was also analyzed on LN patients with the relevant results summarized in Figure 2. There was a positive correlation among different concentrations of IL-17A and IL-6, IL-21 on patients with LN (r=0.454, P<0.001; Figure 2A) and (r=0.440, P<0.001; Figure 2B). A strong correlation between the serum concentrations of IL-21 and IL-6 on patients with LN was also identified (r=0.342, P=0.001; Figure 2C).
Figure 2.
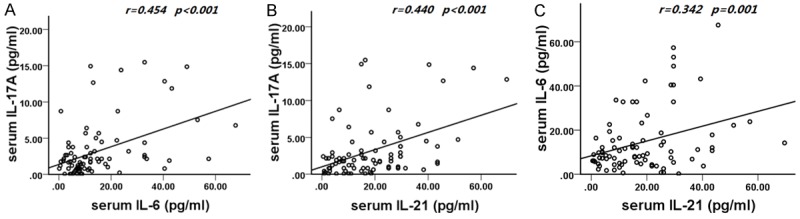
Correlations among the serum concentrations of IL-17A and IL-6, IL-21 on lupus nephritis patients. A. Correlation between the serum concentrations of IL-17A and IL-6 on lupus nephritis patients. B. Correlation between the serum concentrations of IL-17A and IL-21 on lupus nephritis patients. C. Correlation between the serum concentrations of IL-6 and IL-21 on lupus nephritis patients.
ROC curve analysis
Based on the strong correlation between cytokines and SLEDAI scores, the validity of these cytokines as predictors of disease activity was subsequently investigated. ROC curve analysis showed that the optimal cutoff level of IL-6 was 5.78 pg/ml, which had 90.2% sensitivity, 70.0% specificity, and AUC of 0.809 (95% CI of 0.704-0.915). Whereas, the optimal cutoff level of IL-17A was 1.98 pg/ml, with 67.2% sensitivity, 82.9% specificity, and AUC of 0.735 (95% CI of 0.615-0.856). The optimal cutoff level of IL-21 was 8.59 pg/ml, with 88.5% sensitivity, 82.6% specificity, and AUC of 0.786 (95% CI of 0.655-0.916). The study showed the NR group had higher level of IL-6 and IL-21 compared with the remission groups (PR and CR group) (Figure 3A). Furthermore, ROC curve analysis was used for evaluating the role of both cytokines as predictors for the outcome of active LN during induction therapy. ROC curve showed that, the most appropriate cutoff value of IL-6 was 9.12 pg/ml, which had 100.0% sensitivity, 91.3% specificity, and AUC of 0.930 (95% CI of 0.00-1.00). The appropriate cutoff value of IL-21 was 11.30 pg/ml, which had 100.0% sensitivity, 73.9% specificity, and AUC of 0.896 (95% CI of 0.712-1.000) (Figure 3B).
Figure 3.
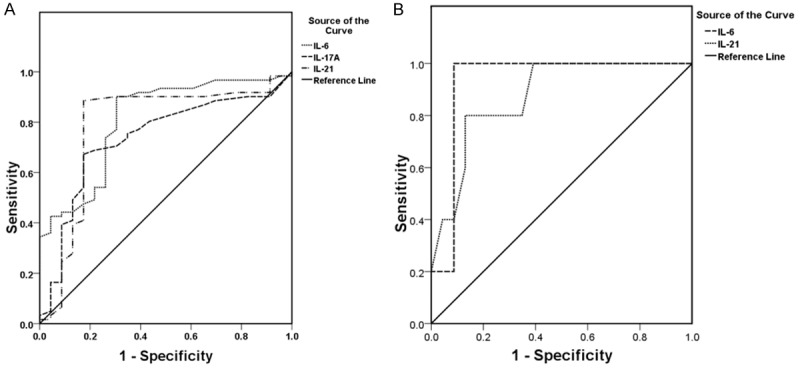
A. Receiver operating characteristic (ROC) curve of IL-6, IL-17A and IL-21 for the prediction of SLE diseases activity. The cutoff value of IL-6 was 5.78 pg/ml, 1.98 pg/ml of IL-17A and 8.59 pg/ml of IL-21, with sensitivity of 90.2%, 67.2% and 88.5%, specificity of 70.0%, 82.9% and 82.6% and AUC of 0.809, 0.735 and 0.786 (95% CI of 0.704-0.915, 0.615-0.856, 0.655-0.916 respectively). B. ROC curve of IL-6 and IL-21 for prediction of remission of active lupus nephritis. The cutoff value of IL-6 was 9.12 pg/ml versus 11.03 pg/ml of IL-21, with sensitivity of 100% versus 100%, specificity of 91.3% versus 73.9% and AUC of 0.930 versus 0.896 (95% CI of 0.000-1.000 versus 0.712-1.000), respectively.
Discussion
SLE is an autoimmune disease with multiple organ involvement. Compared with healthy individuals, patients with SLE have more autoantibody-producing Tfh cells [4,5,23,24] and more Th17 cells, thus creating a pro-inflammatory milieu in which innate immune cells would cause tissue damage [2,3,12]. Although previous studies have suggested pathogenic roles for the raised level of IL6, IL-17A and IL-21 in SLE [1-9], the change of serum cytokines during induction therapy is first proposed herein.
In a previous study involving the SLE Disease Activity Index (SLEDAI) and the SLE Activity Measure (SLAM), a lack of correlation between the level of IL-6 and overall disease activity was reported [30]. However, in other studies, which measured level of IL-6 both before and after disease exacerbations or at various stages of disease activity, the level of IL-6 correlated well with overall disease activity [8]. In addition, an enhanced expression of IL-6 was detected in renal glomeruli and tubules with its excretion increased in the urine of lupus patients with renal disorders [21]. Besides, Maier-Moore JS showed that the deficiency of IL-6 not only decreased autoantibody production and renal disease in lupus mice model, but also abrogated the differentiation of Th1 and extra follicular T helper cells, germinal center B cells, and plasma cells in the spleen and eliminated renal T cells with IL-17, and IL-21 production potential [20]. Moreover, in a murine SLE model, blocking the IL-6R alleviated skin lesions [31]. Additional role of IL-6 in prometing autoimmune inflammation is related to its role in interfering the functional activities of T regulatory cells. It has been well recognized that T regulatory cells play an important role in controlling autoimmune response [32,33], it is clear that IL-6 can reduce the phenotype and function of T regulatory cells and then worsen autoimmune diseases [34,35].
In the present study, it found that the serum concentration of IL-6 was positively correlated with 24 hTP, SLEDAI scores, ANA titers, ESR while negatively correlated with the level of C3. In addition, it found that the concentration of IL-6 was higher on the non-remission patients (NR group) than that on patients in the remission groups (PR and CR group). Possibly, therefore, any relationship between IL-6 and disease activity in SLE may be most appropriately assessed by the evaluation of changes in these variables in the same patients over time.
In agreement with other previous studies [1,8,9,14,16,17], this study demonstrated that the serum concentration of IL-17A was positively correlated with 24 hTP, SLEDAI scores, ANA titers and ESR. Surprisingly, low level of C3 and C4 is one of the signs of lupus nephritis. However, there was no correlation between IL-17A with the level of C3 and C4 found. Although IL-17A has been implicated in the pathogenesis of SLE, in the study, only the serum level of IL-6 and IL-21 showed significant difference among NR, PR and CR group. The relevant result was inconsistent with the finding of Comte and his colleagues, showing that the production of IL-17A was not significantly different between SLE patients and healthy subjects [36]. In addition, Sigdel did not detect significant correlation between the serum level of IL-17A and SLEDAI in LN patients [15]. These discrepancies may due to multiple factors, such as the heterogeneity of SLE, measuring differences in ELISA sensitivity, effect of different immunosuppressive medications [37], and more localized production of IL-17 in the affected tissues than in plasma [38].
IL-21 is a member of the type-I cytokine family with pleiotropic activities. It regulates B cell differentiation and function, promotes Tfh cell and Th17 cell differentiation, and downregulates the induction of T regulatory cells, which suggests that IL-21 may play an important role in the development and pathogenesis of autoimmune diseases, such as SLE [39]. Increased expression of IL-21 has been detected in BXSB-Yaa+/J mice, which would develop into severe SLE-related symptoms, such as lymphadenopathy and hypergammaglobulinemia, and severe immune-complex mediated glomerulonephritis. The development of the severe SLE-like disease characteristic of BXSB-Yaa mice was critically dependent on IL-21 signaling. IL-21 plays a role in the accumulation of plasma cells and the production of autoantibodies observed in SLE [40]. Inhibition of IL-21 expression and Tfh cell differentiation effectively suppressed the expansion of Tfh cell and the production of IL-21, thereby alleviating lupus nephritis and prolonging the survival rate of lupus-prone mice [41]. Similarly, the blockade of IL-21R in BWF1 mice completely prevented the onset of nephritis, which was associated with the dramatic reduction in splenomegaly and the activation of B and T cells [42]. Consistent with other studies, similar to IL-6, this study indicated that the serum concentration of IL-21 was positively correlated with 24 hTP, SLEDAI scores, ANA titers and ESR. In addition, IL-21 showed a significant difference between NR vs PR and NR vs CR groups. There was no correlation between the level of IL-17A and low level of C3 and C4 found. Moreover, Pan HF showed that the serum level of IL-21 decreased on patients with SLE compared with those in control group. In addition, no significant difference was found in the serum level of IL-21 between SLE patients with nephritis and those without nephritis. Furthermore, no significant difference was found in patients with different disease activity [43]. Nevertheless, up to now, studies on the serum expression of IL-21 and the association with disease activity in SLE patients are very limited. Therefore, future studies are needed to examine the role of IL-21 in chronic inflammation and the development of SLE.
In summary, the relevant results confirmed a strong correlation between the serum level of IL-6, IL-17A, IL-21 and the related pathogenic role in lupus nephritis. IL-6 together with TGF-β can promote the differentiation of Th17 cells and the production of IL-17A [21]. IL-17A in turn upregulates the production of IL-6 [44]. The recent study has discovered that the cytokine IL-21 may incite T-cell responses towards IL-17 producing cells [26]. Compared with healthy individuals, the increased number of the IL-21-expressing CD4+ T was observed in SLE patients with a concomitant increase in the number of Th17 cells [45], leading to overproduction of IL-17A and IL-21. Moreover, IL-6 promotes the differentiation of the subset of naive CD8+ T cells into IL-21-producing B helper CD8+ T cells [46]. Collectively, the corresponding results indicated that there is a positive feedback loop between Th17 and Tfh cells, and the cytokines produced by these cells are closely involved in the formation and perpetuation of lupus nephritis.
In this study, a significant decrease of IL-6, IL-17A and IL-21 was observed during induction therapy on patients with active lupus nephritis, accompanied with the decline of SLEDAI, the reduction of 24 hTP, the decrease of ANA titers, and the drop of ESR. Therefore, it denotes a significant correlation with SLE disease activity and exacerbation. In addition, ROC curve analysis of IL-6, IL-17A and IL-21 suggested that the cytokines can serve as sensitive and specific biomarkers for disease activity. Moreover, the concentration of IL-6 and IL-21 can serve as a predictor for lupus remission.
Conclusion
The production of serum IL-6, IL-17A and IL-21 in active LN was dramatically declined during induction therapy, which may improve disease activity and delay disease progression of LN.
Acknowledgements
This study was supported by the grant from the nature science fund project of Henan Province 162300410273. We thank the Ethic Committee of Zhengzhou University [2016-LW-124] and the grant from the nature science fund project of Henan Province [162300410273] for their support. All authors have contributed sufficiently to the project.
Disclosure of conflict of interest
None.
References
- 1.Talaat RM, Mohamed SF, Bassyouni IH, Raouf AA. Th1/Th2/Th17/Treg cytokine imbalance in systemic lupus erythematosus (SLE) patients: Correlation with disease activity. Cytokine. 2015;72:146–153. doi: 10.1016/j.cyto.2014.12.027. [DOI] [PubMed] [Google Scholar]
- 2.Krebs CF, Schmidt T, Riedel JH, Panzer U. T helper type 17 cells in immune-mediated glomerular disease. Nat Rev Nephrol. 2017;13:647–659. doi: 10.1038/nrneph.2017.112. [DOI] [PubMed] [Google Scholar]
- 3.Krebs CF, Turner JE, Paust HJ, Kapffer S, Koyro T, Krohn S, Ufer F, Friese MA, Flavell RA, Stockinger B, Steinmetz OM, Stahl RA, Huber S, Panzer U. Plasticity of Th17 cells in autoimmune kidney diseases. J Immunol. 2016;197:449–457. doi: 10.4049/jimmunol.1501831. [DOI] [PubMed] [Google Scholar]
- 4.Zhang X, Lindwall E, Gauthier C, Lyman J, Spencer N, Alarakhia A, Fraser A, Ing S, Chen M, Webb-Detiege T, Zakem J, Davis W, Choi YS, Quinet R. Circulating CXCR5+CD4+helper T cells in systemic lupus erythematosus patients share phenotypic properties with germinal center follicular helper T cells and promote antibody production. Lupus. 2015;24:909–917. doi: 10.1177/0961203314567750. [DOI] [PubMed] [Google Scholar]
- 5.Szabó K, Papp G, Szántó A, Tarr T, Zeher M. A comprehensive investigation on the distribution of circulating follicular T helper cells and B cell subsets in primary Sjögren’s syndrome and systemic lupus erythematosus. Clin Exp Immunol. 2016;183:76–89. doi: 10.1111/cei.12703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen M, Guo Z, Ju W, Ryffel B, He X, Zheng SG. The development and function of follicular helper T cells in immune responses. Cell Mol Immunol. 2012;9:375–379. doi: 10.1038/cmi.2012.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen M, Lin X, Liu Y, Li Q, Deng Y, Liu Z, Brand D, Guo Z, He X, Ryffel B, Zheng SG. The function of BAFF on T helper cells in autoimmunity. Cytokine Growth Factor Rev. 2014;25:301–305. doi: 10.1016/j.cytogfr.2013.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Abdel Galil SM, Ezzeldin N, El-Boshy ME. The role of serum IL-17 and IL-6 as biomarkers of disease activity and predictors of remission in patients with lupus nephritis. Cytokine. 2015;76:280–287. doi: 10.1016/j.cyto.2015.05.007. [DOI] [PubMed] [Google Scholar]
- 9.Ballantine LE, Ong J, Midgley A, Watson L, Flanagan BF, Beresford MW. The pro-inflammatory potential of T cells in juvenile-onset systemic lupus erythematosus. Pediatr Rheumatol Online J. 2014;12:4. doi: 10.1186/1546-0096-12-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zheng SG, Wang J, Horwitz DA. Cutting edge: Foxp3+CD4+CD25+ regulatory T cells induced by IL-2 and TGF-beta are resistant to Th17 conversion by IL-6. J Immunol. 2008;180:7112–7116. doi: 10.4049/jimmunol.180.11.7112. [DOI] [PubMed] [Google Scholar]
- 11.Lu L, Wang J, Zhang F, Chai Y, Brand D, Wang X, Horwitz DA, Shi W, Zheng SG. Role of SMAD and non-SMAD signals in the development of Th17 and regulatory T cells. J Immunol. 2010;184:4295–4306. doi: 10.4049/jimmunol.0903418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Apostolidis SA, Crispín JC, Tsokos GC. IL-17-producing T cells in lupus nephritis. Lupus. 2011;20:120–124. doi: 10.1177/0961203310389100. [DOI] [PubMed] [Google Scholar]
- 13.Ma J, Yu J, Tao X, Cai L, Wang J, Zheng SG. The imbalance between regulatory and IL-17-secreting CD4+ T cells in lupus patients. Clin Rheumatol. 2010;29:1251–1258. doi: 10.1007/s10067-010-1510-7. [DOI] [PubMed] [Google Scholar]
- 14.Chen XQ, Yu YC, Deng HH, Sun JZ, Dai Z, Wu YW, Yang M. Plasma IL-17A is increased in new-onset SLE patients and associated with disease activity. J Clin Immunol. 2010;30:221–225. doi: 10.1007/s10875-009-9365-x. [DOI] [PubMed] [Google Scholar]
- 15.Sigdel KR, Duan L, Wang Y, Hu W, Wang N, Sun Q, Liu Q, Liu X, Hou X, Cheng A, Shi G, Zhang Y. Serum cytokines Th1, Th2, and Th17 expression profiling in active lupus nephritis-IV: from a Southern Chinese Han population. Mediators Inflamm. 2016;2016:4927530. doi: 10.1155/2016/4927530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jakiela B, Iwaniec T, Plutecka H, Celinska-Lowenhoff M, Dziedzina S, Musial J. Signs of impaired immunoregulation and enhanced effector T-cell responses in the primary antiphospholipid syndrome. Lupus. 2016;25:389–398. doi: 10.1177/0961203315618267. [DOI] [PubMed] [Google Scholar]
- 17.Alfadhli S, Alfailakawi A, Ghanem AA. Th-17 related regulatory network in the pathogenesis of Arab patients with systemic lupus erythematosus and lupus nephritis. Int J Rheum Dis. 2016;19:512–520. doi: 10.1111/1756-185X.12393. [DOI] [PubMed] [Google Scholar]
- 18.Luo Y, Zheng SG. Hall of Fame among Pro-inflammatory cytokines: interleukin-6 gene and its transcriptional regulation mechanisms. Front Immunol. 2016;19:604. doi: 10.3389/fimmu.2016.00604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ripley BJ, Goncalves B, Isenberg DA, Latchman DS, Rahman A. Raised levels of interleukin 6 in systemic lupus erythematosus correlate with anaemia. Ann Rheum Dis. 2005;64:849–853. doi: 10.1136/ard.2004.022681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Maier-Moore JS, Horton CG, Mathews SA, Confer AW, Lawrence C, Pan Z, Coggeshall KM, Farris AD. IL-6 deficiency corrects nephritis, lymphocyte abnormalities, and secondary Sjogren’s syndrome features in lupus-prone Sle1. Yaa mice. Arthritis Rheumatol. 2014;66:2521–2531. doi: 10.1002/art.38716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Horii Y, Iwano M, Hirata E, Shiiki M, Fujii Y, Dohi K, Ishikawa H. Role of interleukin-6 in the progression of mesangial proliferative glomerulonephritis. Kidney Int Suppl. 1993;39:S71–75. [PubMed] [Google Scholar]
- 22.Resende AL, Elias RM, Wolf M, Dos Reis LM, Graciolli FG, Santos GD, Dias CB, Jorgetti V, Woronik V, Moysés RM. Serum levels of fibroblast growth factor 23 are elevated in patients with active Lupus nephritis. Cytokine. 2017;91:124–127. doi: 10.1016/j.cyto.2016.12.022. [DOI] [PubMed] [Google Scholar]
- 23.Nakou M, Papadimitraki ED, Fanouriakis A, Bertsias GK, Choulaki C, Goulidaki N, Sidiropoulos P, Boumpas DT. Interleukin-21 is increased in active systemic lupus erythematosus patients and contributes to the generation of plasma B cells. Clin Exp Rheumatol. 2013;31:172–179. [PubMed] [Google Scholar]
- 24.Xu H, Liu J, Cui X, Zuo Y, Zhang Z, Li Y, Tao R, Li Y, Pang J. Increased frequency of circulating follicular helper T cells in lupus patients is associated with autoantibody production in a CD40L-dependent manner. Cell Immunol. 2015;29:46–51. doi: 10.1016/j.cellimm.2015.01.014. [DOI] [PubMed] [Google Scholar]
- 25.Terrier B, Costedoat-Chalumeau N, Garrido M, Geri G, Rosenzwajg M, Musset L, Klatzmann D, Saadoun D, Cacoub P. Interleukin 21 correlates with T cell and B cell subset alterations in systemic lupus erythematosus. J Rheumatol. 2012;39:1819–1828. doi: 10.3899/jrheum.120468. [DOI] [PubMed] [Google Scholar]
- 26.Ohl K, Wiener A, Lippe R, Schippers A, Zorn C, Roth J, Wagner N, Tenbrock K. CREM Alpha enhances IL-21 production in T Cells in vivo and in vitro. Front Immunol. 2016;19:618. doi: 10.3389/fimmu.2016.00618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hochberg MC. Updating the American college of rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1997;40:1725. doi: 10.1002/art.1780400928. [DOI] [PubMed] [Google Scholar]
- 28.Weening JJ, D’Agati VD, Schwartz MM, Seshan SV, Alpers CE, Appel GB, Balow JE, Bruijn JA, Cook T, Ferrario F, Fogo AB, Ginzler EM, Hebert L, Hill G, Hill P, Jennette JC, Kong NC, Lesavre P, Lockshin M, Looi LM, Makino H, Moura LA, Nagata M International Society of Nephrology Working Group on the Classification of Lupus Nephritis; Renal Pathology Society Working Group on the Classification of Lupus Nephritis. The classification of glomerulonephritis in systemic lupus erythematosus revisited. Kidney Int. 2004;65:521–530. doi: 10.1111/j.1523-1755.2004.00443.x. [DOI] [PubMed] [Google Scholar]
- 29.Ward MM, Marx AS, Barry NN. Comparison of the validity and sensitivity to change of 5 activity indices in systemic lupus erythematosus. J Rheumatol. 2000;27:664–670. [PubMed] [Google Scholar]
- 30.Grondal G, Gunnarsson I, Ronnelid J, Rogberg S, Klareskog L, Lundberg I. Cytokine production, serum levels and disease activity in systemic lupus erythematosus. Clin Exp Rheumatol. 2000;18:565–570. [PubMed] [Google Scholar]
- 31.Birner P, Heider S, Petzelbauer P, Wolf P, Kornauth C, Kuroll M, Merkel O, Steiner G, Kishimoto T, Rose-John S, Soleiman A, Moriggl R, Kenner L. Interleukin-6 receptor alpha blockade improves skin lesions in a murine model of systemic lupus erythematosus. Exp Dermatol. 2016;25:305–310. doi: 10.1111/exd.12934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sakaguchi S. Naturally arising Foxp3-expressing CD25+CD4+ regulatory T cells in immunological tolerance to self and non-self. Nat Immunol. 2005;6:345–352. doi: 10.1038/ni1178. [DOI] [PubMed] [Google Scholar]
- 33.Zheng SG, Wang JH, Gray JD, Soucier H, Horwitz DA. Natural and induced CD4+CD25+ cells educate CD4+CD25- cells to develop suppressive activity: the role of IL-2, TGF-beta, and IL-10. J Immunol. 2004;172:5213–5221. doi: 10.4049/jimmunol.172.9.5213. [DOI] [PubMed] [Google Scholar]
- 34.Zhou X, Kong N, Wang J, Fan H, Zou H, Horwitz D, Brand D, Liu Z, Zheng SG. Cutting edge: all-trans retinoic acid sustains the stability and function of natural regulatory T cells in an inflammatory milieu. J Immunol. 2010;185:2675–2679. doi: 10.4049/jimmunol.1000598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lu L, Lan Q, Li Z, Zhou X, Gu J, Li Q, Wang J, Chen M, Liu Y, Shen Y, Brand DD, Ryffel B, Horwitz DA, Quismorio FP, Liu Z, Li B, Olsen NJ, Zheng SG. Critical role of all-trans retinoic acid in stabilizing human natural regulatory T cells under inflammatory conditions. Proc Natl Acad Sci U S A. 2014;111:E3432–3440. doi: 10.1073/pnas.1408780111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Comte D, Karampetsou MP, Kis-Toth K, Yoshida N, Bradley SJ, Kyttaris VC, Tsokos GC. CD4+ T cells from SLE patients respond poorly to exogenous IL-2. Arthritis Rheumatol. 2016;69:808–813. doi: 10.1002/art.40014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vincent FB, Northcott M, Hoi A, Mackay F, Morand EF. Clinical associations of serum interleukin-17 in systemic lupus erythematosus. Arthritis Res Ther. 2013;15:R97. doi: 10.1186/ar4277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Crispin JC, Oukka M, Bayliss G, Cohen RA, Van-Beek CA, Stillman IE. Expanded double negative T cells in patients with systemic lupus erythematosus produce IL-17 and infiltrate the kidneys. J Immunol. 2008;181:8761–8766. doi: 10.4049/jimmunol.181.12.8761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nguyen V, Luzina I, Rus H, Tegla C, Chen C, Ruset V. IL-21 promotes lupus-like disease in chronic graft-versus-host disease through both CD4 T cell- and B cell-intrinsic mechanisms. J Immunol. 2012;189:1081–1093. doi: 10.4049/jimmunol.1200318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bubier JA, Sproule TJ, Foreman O, Spolski R, Shaffer DJ, Morse HC. A critical role for IL-21 receptor signaling in the pathogenesis of systemic lupus erythematosus in BXSB-Yaa mice. Proc Natl Acad Sci U S A. 2009;106:1518–1523. doi: 10.1073/pnas.0807309106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yang X, Yang J, Li X, Ma W, Zou H. Bone marrow-derived mesenchymal stem cells inhibit T follicular helper cell in lupus-prone mice. Lupus. 2018;27:49–59. doi: 10.1177/0961203317711013. [DOI] [PubMed] [Google Scholar]
- 42.Zhang M, Yu G, Chan B, Pearson JT, Rathanaswami P, Delaney J, Ching Lim A, Babcook J, Hsu H, Gavin MA. IL-21R blockade inhibits secondary humoral responses and halts the progression of pre-established disease in NZB/W F1 systemic lupus erythematosus. Arthritis Rheumatol. 2015;67:2723–2731. doi: 10.1002/art.39233. [DOI] [PubMed] [Google Scholar]
- 43.Pan HF, Wu GC, Fan YG, Leng RX, Peng H, Zhou M, Li BZ, Zhu Y, Tao JH, Li XP, Ye DQ. Decreased serum level of IL-21 in new-onset systemic lupus erythematosus patients. Rheumatol Int. 2013;33:2337–2342. doi: 10.1007/s00296-013-2724-1. [DOI] [PubMed] [Google Scholar]
- 44.Ogura H, Murakami M, Okuyama Y, Tsuruoka M, Kitabayashi C, Kanamoto M, Nishihara M, Iwakura Y, Hirano T. Interleukin-17 promotes autoimmunity by triggering a positive-feedback loop via interleukin-6 induction. Immunity. 2008;29:628–636. doi: 10.1016/j.immuni.2008.07.018. [DOI] [PubMed] [Google Scholar]
- 45.Wang HX, Chu S, Li J, Lai WN, Wang HX, Wu XJ, Kang X, Qiu YR. Increased IL-17 and IL-21 producing TCRαβ+CD4-CD8- T cells in Chinese systemic lupus erythematosus patients. Lupus. 2014;23:643–654. doi: 10.1177/0961203314524467. [DOI] [PubMed] [Google Scholar]
- 46.Yang R, Masters AR, Fortner KA, Champagne DP, Yanguas-Casás N, Silberger DJ, Weaver CT, Haynes L, Rincon M. IL-6 promotes the differentiation of a subset of naive CD8+ T cells into IL-21-producing B helper CD8+ T cells. J Exp Med. 2016;213:2281–2291. doi: 10.1084/jem.20160417. [DOI] [PMC free article] [PubMed] [Google Scholar]


