Abstract
Prostate cancer (PCa) is one of the most common malignancies in men and is a major contributor to cancer related deaths worldwide. Metastatic spread and disease progression under androgen deprivation therapy signify the onset of metastatic castration resistant prostate cancer (mCRPCa)-the lethal form of the disease, which severely deteriorates the quality of life of patients. Over the last decade, tremendous progress has been made toward identifying appropriate molecular targets that could enable efficient in vivo targeting for non-invasive imaging and therapy of mCPRCa. In this context, a promising enzymatic target is prostate specific membrane antigen (PSMA), which is overexpressed on PCa cells, in proportion to the stage and grade of the tumor progression. This is especially relevant for mCRPCa, which has significant overexpression of PSMA. For therapy of mCRPCa, several nuclear medicine clinics all over the world have confirmed that 177Lu-labeled-PSMA enzyme inhibitors (177Lu-PSMA-617 and 177Lu-PSMA I&T) have a favorable dosimetry and convincing therapeutic response. However, ~30% of patients were found to be short or non-responders and dose escalation was severely limited by chronic hematological toxicity. Such limitations could be better overcome by targeted alpha therapy (TAT) which has the potential to bring a paradigm shift in treatment of mCRPCa patients. This concise review presents an overview of the successes and challenges currently faced in TAT of mCRPCa using radiolabeled PSMA inhibitors. The preclinical and clinical data reported to date are quite promising, and it is expected that this therapeutic modality will play a pivotal role in advanced stage PCa management in the foreseeable future.
Keywords: 225Ac, 211At, 213Bi, metastasis, prostate cancer, PSMA, targeted alpha therapy
Introduction
Prostate cancer (PCa) is one of the most common non-cutaneous malignancies in men worldwide, and its incidence has increased substantially in recent years [1-6]. In the United States alone, more than 40,000 men die from PCa every year [7]. The incidence of prostate cancer increases proportionally with age: published post-autopsy data illustrate an incidence of ~12% in the 60-69 year old male age group, which increases to a value of ~48% in 80-89 year old men [8-10]. There is a high probability that cases may be under diagnosed, especially in developing countries, and the numbers are likely to be much higher than reported. Interestingly, incidence of prostate cancer also seems to be dependent on demographics and racial prevalence, highlighting the influence of environmental factors on occurrence of the disease [11]. PCa has a low incidence in Asia (3-8 per 100,000 men per year), an intermediate incidence in Africa and Eastern Europe, and higher incidence in Western Europe and North America [11,12]. In many countries, there are clinical recommendations for carrying out PCa screening by using the prostate specific antigen (PSA) test [8]. However, the harms caused by overdiagnosis and overtreatment must also be considered while adhering to these recommendations.
Conventional treatment options for localized PCa include surgery, chemotherapy, cryotherapy, brachytherapy with radioactive seeds, and the use of external radiation which is now being aggressively promoted with the use of proton therapy machines using conformal targeting technologies [2,10,13-18]. The choice of therapeutic modality is based on the individual cancer characteristics, evaluating histopathology, serum PSA, Gleason score, comorbidity and life expectancy. As such, PCa is a highly assorted disease and it can be heterogeneous in the prostate of the same patient [19,20]. To optimize therapeutic outcome, especially in high-risk PCa patients, treatment for PCa is moving rapidly toward personalization. Molecular imaging plays an important role in personalized cancer management, as it aims to deliver patient-specific, targeted treatment at the appropriate time [3,21-25]. Thanks to recent developments in radiopharmaceuticals chemistry and imaging technologies, the role of molecular nuclear medicine for diagnosis, as well as therapy, of prostate cancer is expected to increase significantly in the future [26-33].
There are several mechanisms by which a radiopharmaceutical may accumulate in cancerous lesions. An enzyme-substrate reaction taking place at the cellular level with prostate specific membrane antigen (PSMA) targeting enzyme inhibitors is a recently explored strategy that can be utilized for the development of radiopharmaceuticals for imaging and therapy of PCa [10,34]. PSMA is highly expressed in all types of PCa, and the expression increases with tumor aggressiveness, metastatic disease, and recurrence [34]. Using suitably radiolabeled PSMA inhibitors, PCa can be accurately visualized, characterized and optimally treated according to tumor biology, patient preferences, and survivorship goals [35,36]. Radiolabeled PSMA inhibitor is the first class of radiopharmaceutical based on an enzyme inhibitor as the targeting agent, with a cellular enzyme target [10]. The name PSMA is somewhat of a misnomer as it is also expressed in the vasculature of other tumors including, bladder, pancreas, lung, and kidney carcinomas [10,37]. Nevertheless, there has been overwhelming interest in use of radiolabeled analogues of several PSMA inhibitors for their high potential in diagnosis and therapy of PCa, and studies indicate that such agents are quite effective as new clinical options in PCa management [36]. The aim of this review is to provide an overview of the recent advances in targeted α-therapy of PCa using radiolabeled PSMA inhibitors which is poised to be a game changer in therapeutic nuclear medicine.
PSMA as an enzymatic target for developing radiopharmaceuticals for prostate cancer
PSMA, also known as folate hydrolase I, glutamate carboxypeptidase II, N-acetyl-L-aspartyl-L-glutamate peptidase I (NAALDase I) or N-acetyl-L-aspartyl-L-glutamate (NAAG) peptidase, shows several biological features which make it an ideal target structure for radiopharmaceutical development [10,38]. Basically, PSMA is a zinc containing metalloenzyme, having a molecular weight of 100-104 kDa [10]. The PSMA structure reveals a symmetric dimer with each polypeptide chain containing three domains: a protease domain (residues 56-116 and 352-591), an apical domain (residues 117-351), and a helical domain (residues 592-750) (Figure 1A) [39]. A large cavity (~1,100 Å2) exists at the periphery of the three domains and this includes a binuclear zinc site (Figure 1B) [39]. Two zinc ions have been observed in this cavity, which has been identified as the substrate binding site (Figure 1C). As mentioned, PSMA shows significant overexpression on most prostate cancer cells, especially those which are in advanced stage, such as mCPRCa [10]. Several clinical studies have revealed a correlation between PSMA and the stage and grade of the cancer, showing enhanced PSMA levels with higher stages and grading [40-44].
Figure 1.
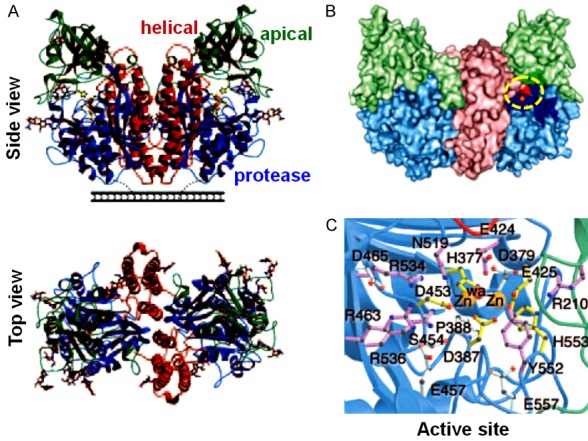
Structure of PSMA. A. Ribbon diagrams of side and top view of PSMA. B. A surface rendering in which the apical domain is light green, the helical domain is light red, the protease domain is light blue, and zinc ions are orange. The residues facing the substrate-binding cavity are indicated in a darker version of the color matching to the domain from which the residue derives. PSMA active site is encircled. C. Stereoview of the PSMA active site. Zinc ions are orange spheres, and a water molecule is shown as a red sphere. Zinc binding residues are yellow sticks, water- or substrate-binding ligands are purple sticks, and residues with structural roles are light blue sticks. Adapted from Ref. [39] with permission. Copyright 2005 National Academy of Sciences.
In nuclear medicine practices, two different approaches have been used for targeting PSMA. The first approach takes advantage of the macromolecular protein structure of PSMA to provide specific monoclonal antibodies as targeting vectors [45]. The second approach relies on the enzymatic activity of PSMA and uses radiolabeled enzyme inhibitors or binding agents as target seeking agents [46]. Though both these approaches have been successfully demonstrated for the development of diagnostic and therapeutic radiopharmaceuticals for targeting prostate cancer, the second approach, which utilizes radiolabeled small molecules targeting the enzyme activity of PSMA, is gaining overwhelming popularity recently. This is because smaller molecules show rapid blood clearance compared to monoclonal antibodies. This leads to a desirable higher target-to-nontarget ratio. Additionally, with use of radiolabelled small molecules, after the ligand binds to its membrane-anchored target, internalization occurs via clathrin-coated pits and endocytosis [10]. This results in effective transportation of the bound molecule into the cells, ending up in the late endosomes, leading to increased tumor uptake and retention.
PSMA has two distinct enzyme activities: (a) folate hydrolase and (b) NAALDase, and in both cases the enzymatic role is to release the terminal glutamate residue from the substrate molecules (Figure 2) [10]. The NAALDase activity of PSMA (i.e. hydrolysis of the NAAG substrate to yield aspartate and glutamate) has been explored for the development of radiopharmaceuticals for imaging and therapy of PCa [10]. The NAAG substrate binds to PSMA in the extracellular portion of the enzyme [10]. However, NAAG substrate cannot be used as the targeting molecule for PCa because the substrate molecule gets converted into the product and will not be retained within the cells. Additionally, being small residues, NAAG substrates are likely to get released from the cells and hence will not concentrate in the cancerous lesions. Therefore, modified forms of NAALDase inhibitors mimicking the NAAG substrate were designed and used as PSMA targeting agents for PCa imaging and therapy [47,48].
Figure 2.
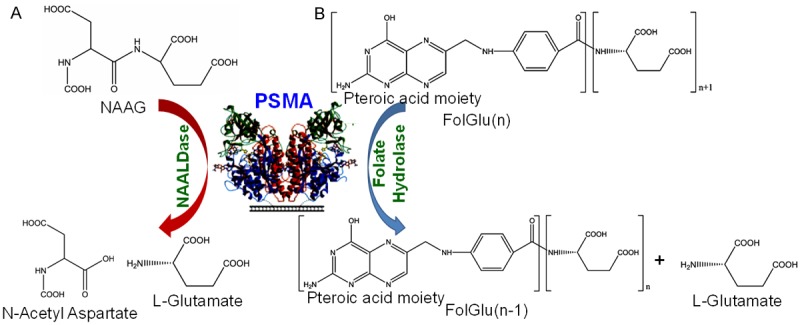
The enzymatic action of PSMA. A. N-Acetyl-L-aspartyl-L-glutamate (NAAG) is hydrolyzed to aspartate and glutamate. B. Glutamic acid is released from folate polyglutamate resulting in the release of folic acid. After successive release of glutamate, folate is released. Adapted from Ref. [10] with permission. Copyright 2016 Elsevier.
The PSMA ligands based on NAALDase inhibitors are classified into three groups: (a) phosphorous-based compounds (including phosphonate, phosphate, and phosphoramidate), (b) thiol, indole-thiol, hydroxamate and sulfonamide derivatives, and (c) urea based compounds [47,48]. The phosphorus-based compounds were the first high affinity PSMA ligands with nanomolar inhibitory potency [10]. However, these compounds are highly polar and have a relatively poor pharmacokinetic profile, which limits their clinical application [10,49]. Subsequently, thiol-based agents were considered a good alternative to phosphorus-containing molecules as they demonstrated enhanced membrane permeability and oral bioavailability [10,49]. However, metabolic stability and selectivity of these compounds are not adequate to advance them into clinics. In order to overcome these limitations, a series of novel urea-based PSMA ligands has been developed which finds relevance in clinical context [47].
Generally, PSMA ligands consist of three components: (a) the binding motif, (b) a radiolabel bearing moiety which can be a chelator or a prosthetic group and (c) a linker molecule that connects both binding motif and radiolabel bearing moiety and adjusts the lipophilicity of the agent [50-52]. Among various ligands reported to date, the class consisting of peptidomimetic, urea-based PSMA inhibitors has been most widely studied from clinical perspective (Figure 3) [27,36,47]. Over the last few years, the number of clinical studies using urea-based PSMA ligands, such as 123/124/131I-MIP-1072/-1095, [53,54] 99mTc-MIP-1404/-1405, [55] 68Ga-PSMA-11, [56] [18F]-DCFBC, [57] [18F]-DCFPyl, [58] 177Lu-PSMA617, [59] and 177Lu-PSMA I&T [60] has exponentially increased. Among these agents, today, 68Ga-PSMA-11 is the most prominent positron emission tomography (PET) radioligand for PET imaging of PCa [61-66]. Aside from use in diagnostic imaging, radiolabeled PSMA ligands also have potential for use in radionuclide therapy of PCa. Radiolabeling of PSMA ligands with the 131I, 177Lu, 90Y and 188Re therapeutic radionuclides has also been attempted and results of clinical studies with 177Lu and 131I have been reported [10,67-71]. From a clinical perspective, 177Lu offers several advantages over 131I, such as easier synthesis protocol and a lower proportion of γ-radiation, which can potentially result in reduced hospital admission times and lower toxicity compared to 131I [50]. Therefore, the majority of recent studies have focused on the use of 177Lu for PSMA-based radioligand therapies, concentrating mainly on efficacy evaluations of 177Lu-PSMA-617 and 177Lu-PSMA I&T [60,72-75]. Since the focus of the present review is on targeted α-therapy of PCa, for comprehensive discussion on positron emission tomography-computed tomography (PET-CT) imaging of PCa and targeted β- therapy using 177Lu labeled PSMA inhibitors, the readers are referred to recent reviews on these topics [24,43,44,46,76-84].
Figure 3.
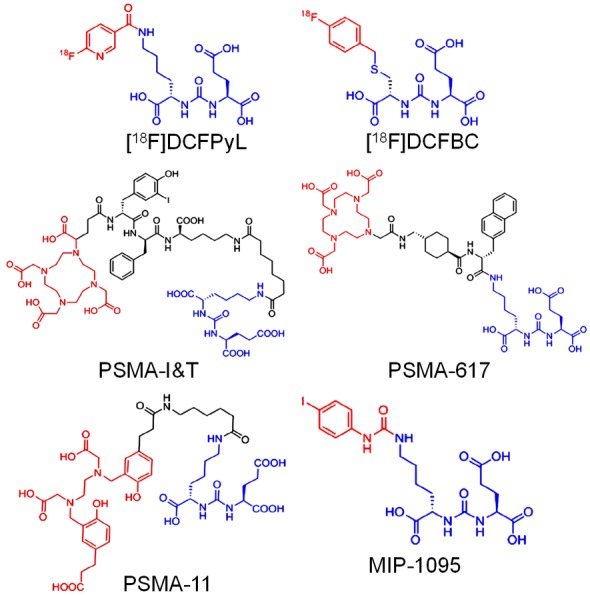
Structures of representative urea-based PSMA ligands used in clinical context. Adapted from Ref. [50] with permission. Copyright 2017 Elsevier.
Targeted α-therapy of PCa
Androgen deprivation is the mainstay of therapy for advanced PCa, and this treatment leads to PSA responses and clinical improvements in > 90% of patients [85]. However, this treatment is not curative, and despite initial responses, almost all patients progress to castration-resistant prostate cancer (CRPCa), which keeps growing even when testosterone body levels are very low [86]. Many early-stage prostate cancers need normal levels of testosterone to grow, but CRPCa does not [86]. In many cases, CRPCa demonstrates clinical metastases in bone and other organs of the body, significantly deteriorating the quality of life of patients. This condition is known as metastatic castration-resistant prostate cancer (mCRPCa) [87-89].
Several international nuclear medicine centers have used 177Lu-PSMA-617 as a potential radiopharmaceutical for therapy of mCRPCa [72,79,90-93]. These centers confirmed that 177Lu-PSMA-617 possesses a favorable dosimetry and shows convincing therapeutic response in terms of both serum PSA level and radiologic findings. However, ~30% of patients were found to be short or non-responders and dose escalation was severely limited by chronic hematological toxicity [94,95]. In this premise, it has recently been demonstrated that targeted α-therapy (TAT) with radiolabeled PSMA inhibitors could prevent radioresistance to β-emitters while simultaneously reducing hematological toxicity in PCa patients [96]. After binding at the tumor cell surface, radiolabeled PSMA inhibitors are internalized. This is particularly beneficial for short-range α-particle radiation, and this is also important for radionuclides that decay into unstable daughter nuclides [97,98]. In addition, the short tissue range of α-radiation offers the prospective of targeting tumor cells which are infiltrating bone marrow, with reduced toxicity compared to β-emitters [97,98].
Production of α-emitting radioisotopes for TAT of mCRPCa
The α-emitting radioisotopes which have been utilized for radiolabeling PSMA inhibitors for TAT of mCRPCa are 211At, 225Ac and 213Bi. The physical characteristics and production methodology of each of these radioisotopes are discussed in the following sections.
211At
Astatine-211 is an ideal radionuclide for TAT due to its favorable nuclear decay characteristics [99]. The decay of 211At follows a branched decay scheme with a half-life of 7.21 hours (Figure 4). One branch leads to 207Bi by emission of an α-particle. The radioisotope, 207Bi, decays with a half-life of 33.9 year to 207Pb via electron capture. The second decay branch occurs via electron capture and leads to formation of 211Po, which has a half life of 516 milliseconds. Polonium-211 in turn decays to stable 207Pb by emission of an α-particle. The result of these two decay pathways is 100% α-particle emission during the decay of 211At (5.87 and 7.45 MeV in 42% and 58% of the decays, respectively). A major concern with the clinical utilization of 211At is the presence of long-lived 207Bi (t½ = 32.9 years), and potential for future health issues due to the uptake of this radionuclidic impurity in the bone, liver, and kidneys [100]. However, 347 MBq of 211At, which is the highest dose that has been administered to a human, [100] leads to only 310 kBq of 207Bi, making its potential toxicity negligible.
Figure 4.
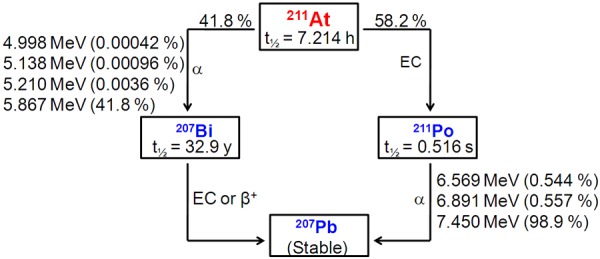
Simplified decay scheme of 211At.
The most common method of 211At production is by irradiation of natural bismuth (209Bi) target following the nuclear reaction: 209Bi (α, 2n) 211At [99,101,102]. Other production methods such as 209Bi (7Li, 5n) 211Rn → 211At, 209Bi (3He, n) 211At, natU (p, x) 211At and 234Th (p, x) 211Rn → 211At have also been investigated [99,103]. However, these production routes are inefficient and require particle energies in the range of 160-660 MeV and extensive separation procedures. Therefore, these alternative methods are unreliable with respect to clinical-scale production of 211At and have very limited scope.
Adopting 209Bi (α, 2n) 211At nuclear reaction, 211At production is possible for α-beam energies ranging from 21 to greater than 40 MeV, with a maximum cross-section observed for 31 MeV (Figure 5A) [104]. However, one cannot take advantage of the full breadth of the cross section for this nuclear reaction because of concerns regarding co-production of 210At (t½ = 8.3 h) by the 209Bi (α, 3n) 210At nuclear reaction (Figure 5B) [104]. This radioisotope is especially problematic because greater than 99% of its decays results in the production of α-emitting 210Po (t½ = 138.4 d) that can be extremely toxic to the bone marrow [99,100]. Therefore, the α-beam energy is generally controlled to 28-29 MeV to limit formation of 210At while maintaining appreciable yields of 211At.
Figure 5.
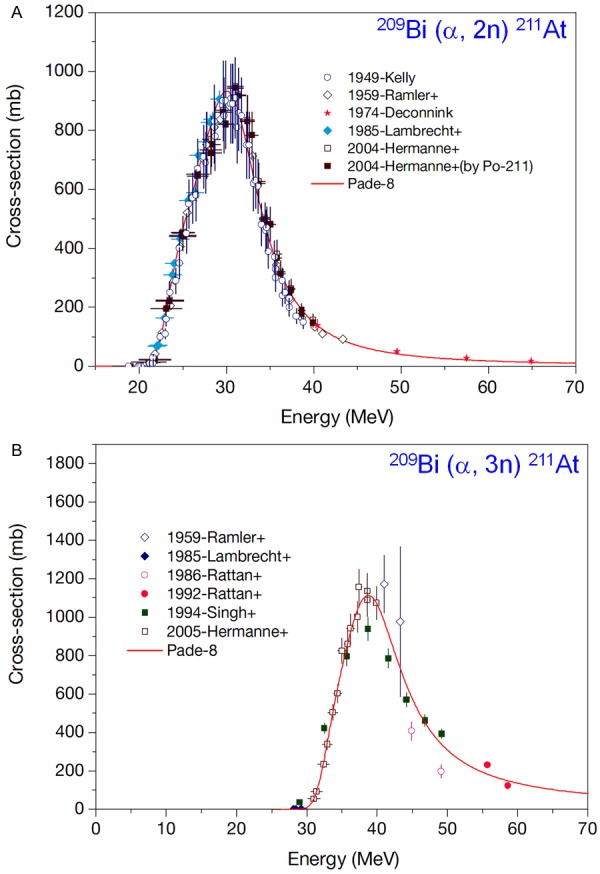
(A) Excitation function for the 209Bi (a, 2n) 211At reaction, (B) Excitation function for the 209Bi (a, 3n) 211At reaction. Adapted from Ref. [104] with permission. Copyright 2009 International Atomic Energy Agency.
In terms of cost, use of natural bismuth is an ideal target material as it precludes the need for procedures to recover and purify the target material. However, thermal properties of bismuth are not ideal, as it has poor thermal conductivity (7.97 W.K-1.m-1) and a low melting point (272°C) [100]. Owing to the relatively high volatility of astatine (337°C), this could lead to vaporization of the produced activity, due to overheating of the target during the irradiation. This overheating has been addressed by target cooling systems using cold gas and water during irradiation. Also, in certain cases, a suitable irradiation angle has been chosen that spreads the beam over a larger impact surface on the bismuth target. For target preparation, metallic bismuth is generally fused or vaporized onto an aluminium backing plate (k = 250 W m-1 K-1), which then may be machined to provide a smooth surface [99,104]. Backing plates made from copper (k = 390 W m-1 K-1) have also been investigated in order to improve the thermal conductivity [99]. However, this has led to lower 211At isolation yields and also resulted in the co-production of several radionuclidic impurities including 67Ga, 66Ga, 65Zn and 69Ge [99].
After irradiation, 211At produced must be separated from the bulk target material (209Bi) and traces of co-produced 210Po. Generally, two methods have been utilized for the separation of 211At from the irradiated target: (a) dry distillation [105] and (b) liquid-liquid extraction that requires dissolution of the target in an acidic solution [106]. In dry distillation method, the target is placed in a furnace and heated above the boiling point of astatine [105]. Since, the boiling points of bismuth and polonium are 1564°C and 962°C, respectively; the furnace temperature is generally set to 650-900°C. During this process, while the bismuth and the polonium melt and stay on the support, the volatile astatine is carried away by a stream of gas (generally nitrogen or argon) and trapped at the outlet. The astatine activity is finally obtained by bubbling the stream of gas directly into the solvent of choice, though it can also be captured in capillary tubing cooled in dry ice/ethanol placed at the outlet. In the wet distillation procedure, the irradiated target is dissolved in concentrated nitric acid followed by extraction with di-isopropyl ether [106,107]. Although the wet distillation method is easy to perform in a hot cell, the choice of the extraction solvent is limited to di-isopropyl ether or analogous solvent, which is an issue because of the associated hazards. Furthermore, some nitric acid is extracted into the organic phase in notable concentrations, which can lead to side reactions during the radiolabeling chemistry [100]. Therefore, the dry distillation procedure is more widely used for radiochemical processing of 211At.
225Ac
Actinium-225 (t½ = 10 d) is another α-emitting radioisotope with immense potential in TAT of mCRPCa [98,108-110]. The decay of 225Ac results in 6 daughter products (221Fr, 217At, 213Bi, 213Po, 209Pb, and 209Tl) with several α and β- decays (Figure 6). Despite its excellent nuclear decay characteristics, the widespread use of 225Ac in TAT has been restricted due to unavailability of the radioisotope. Presently, 225Ac can be obtained in limited quantities (~37 GBq/y) by radiochemical separation from two 229Th sources, one located at Oak Ridge National Laboratory (ORNL), USA and the other at the Institute for Transuranium Elements in Karlsruhe (ITU), Germany (Figure 6) [111,112]. The 229Th available at both sites was recovered from 233U, which has been in long-term storage at ORNL (Figure 6). This 233U was produced in kilogram quantities during the 1960s by neutron irradiation of 232Th in molten salt breeder reactors. The radiochemical separation procedure involves two steps: (a) ion exchange separation of 225Ac/225Ra from 229Th, and (b) extraction chromatography separation of 225Ac from 225Ra [111,112]. After the radiochemical separation, clinical grade no-carrier-added (NCA) 225Ac could be obtained with >95% yield.
Figure 6.
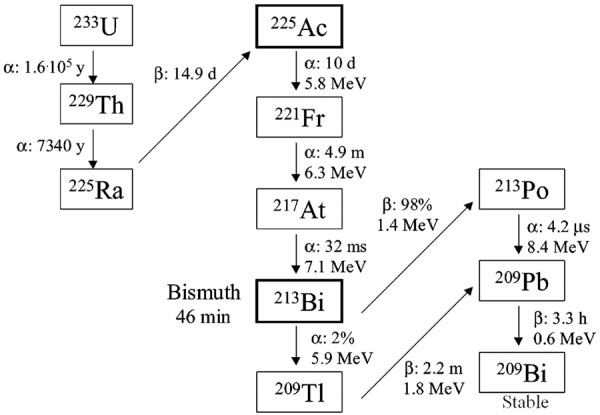
Decay chain of 233U indicating production and decay of 225Ac and 213Bi. Adapted from Ref. [111] with permission. Copyright 2005 American Chemical Society.
In order to meet the increasing demand of 225Ac for clinical studies, alternative ways of production of 225Ac are being discussed. Among them, high energy proton spallation reaction on natural thorium metal targets has been utilized to produce 225Ac adopting 232Th (p, 2p, 6n) 225Ac nuclear reaction [113,114]. In this route, the cross sections for production of 225Ac range from 3.6-16.7 mb in the incident proton energy range of 78-192 MeV. Accordingly, production of curie quantities of 225Ac is possible by irradiating a 232Th target (5.0 g cm-2) for 10 days. Although appreciable activity of 225Ac can be produced in a single batch, the major limitation of this approach is the unavailability of high energy proton accelerators which must be operated continuously for a prolonged period of 10 days.
In order to circumvent this limitation, it has been proposed to produce 225Ac via 226Ra (p, 2n) 225Ac reaction in a cyclotron [104,115,116]. Adopting this reaction, maximum yield of 225Ac was reached at incident proton energies of 16.8 MeV (Figure 7) and therefore it is possible to produce 225Ac at many cyclotron facilities in the world by this route. Another alternative method for production of 255Ac is by the use of linear accelerator (LINAC) by 226Ra (γ, n) 225Ra → 225Ac nuclear reaction [116]. Though this route is inefficient, it can produce required quantities of 225Ac by irradiating a large amount of target in the LINAC. In both these methods, separation of 225Ac from the 226Ra target material could be performed using lanthanide extraction resin chromatography [115,116]. Since the target material used in both these production routes is radioactive and long-lived, additional safety precautions must be taken into consideration during the irradiation process. This may pose regulatory issues, especially in facilities which do not allow use of radioactive material as targets for radioisotope production.
Figure 7.
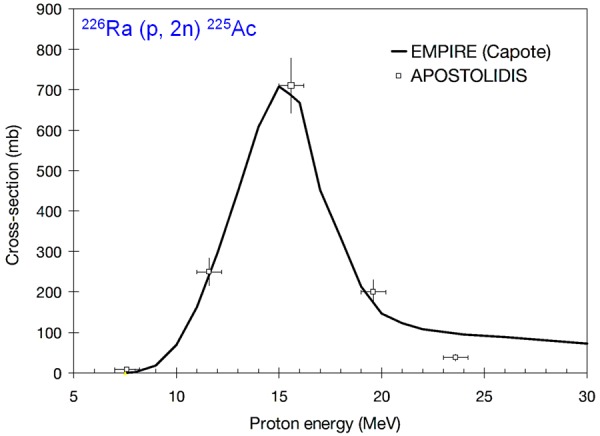
Excitation function for the 226Ra (p, 2n) 225Ac reaction. Adapted from Ref. [104] with permission. Copyright 2009 International Atomic Energy Agency.
213Bi
Among the daughter products of 225Ac, 213Bi is well suited for TAT of mCRPCa. Bismuth-213 decays with a t½ of 45.6 min and emits an 8.4 MeV α-particle with a branching ratio of 97.8% (Figure 6) [98,117,118]. The decay of 213Bi follows with the emission of rather low intensity γ-rays [440 keV (26%) and 1566 keV (2%)] which make it an ideal theranostic radioisotope. NCA 213Bi can be obtained from 225Ac/213Bi generator [119,120]. Due to the 10-day half life of 225Ac, the useful life time of 225Ac/213Bi generators is several weeks. Owing to the 45.6 min half life of 213Bi, the 225Ac/213Bi generator can be eluted several times in a day. Over the last several years, various types of 225Ac/213Bi generators have been reported, based on cation and anion exchange, or extraction chromatography [119,121,122].
Among the 225Ac/213Bi generators reported, the generators based on AG MP-50 cation exchange resin are the most widely used and have been applied for all patient studies with 213Bi to date [121,122]. For preparation of the generator, both trivalent cations (Ac3+ and Bi3+) are efficiently sorbed to AG MP-50 cation exchange resin. As hard Lewis acid, the Bi3+ cation has a strong affinity to form complexes with sulfur and halogens, especially iodide. The strong affinity of Bi3+ for complexation with iodide is used for selective elution of 213Bi from the cation exchange resin as anionic BiI4 -/BiI5 2- species using a solution of 0.1 M HCl/0.1 M NaI as the eluent. This procedure provided a high yield of 213Bi elution, low breakthrough of the 225Ac, and the radioactivity was obtained in a medium amenable for subsequent radiopharmaceutical preparation.
Radiolabeling techniques
The radiolabeling of biological molecules with radiometals for preparation of radiopharmaceuticals involves an interdisciplinary approach requiring knowledge of coordination chemistry, kinetics and thermodynamics, radiochemistry, synthetic chemistry, and biology/physiology [123-126]. Generally, radiolabeling of PSMA inhibitors with 211At is performed by adopting conventional radioiodination chemistry, since astatine belongs to the halogen family and has similar chemical properties to iodine. Tin precursors and prosthetic groups have been used to label PSMA inhibitor with 211At as shown in Figure 8 [127]. It is pertinent that the carbon-astatine bond in the radiolabeled agent is relatively weak, and the release of free astatine can result in uptake of radioactivity in non-targeted organs. Like iodine, free astatine is taken up in the thyroid, stomach, and macrophage bearing organs such as lung and spleen [127]. Therefore, it is more prudent to radiolabel PSMA inhibitors with α-emitting radiometals such as 225Ac and 213Bi, involving the use of bifunctional chelators (BFCs) for preparation of radiopharmaceuticals for TAT of PCa.
Figure 8.
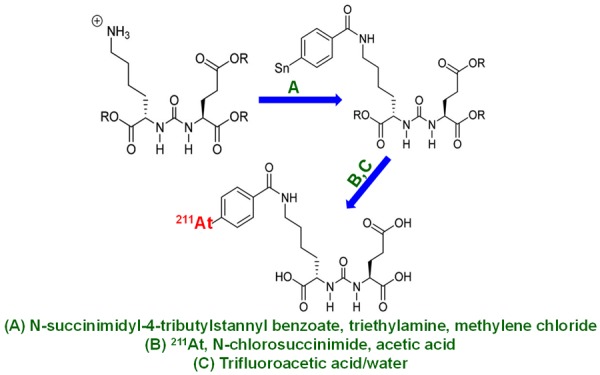
Synthesis of 211At-labeled PSMA inhibitor. R = 5 para-methoxybenzyl. Adapted from Ref. [127] with permission. Copyright 2016 Society of Nuclear Medicine and Molecular Imaging.
The popular tetraaza amino carboxylatebased macrocyclic chelator, DOTA (1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid), along with its bifunctional derivatives, form a class of ‘gold standards’ that have been extensively used for radiolabeling biomolecules with 225Ac and 213Bi [123]. Other macrocyclic chelators which have been used for this purpose include {4-[2-(bis-carboxymethylamino)-ethyl]-7-carboxymethyl-[1,4,7]triazonan-1-yl}-acetic acid (NETA), 1,4,7,10-tetrakis(carbamoylmethyl)-l,4,7,10-tetraazacyclododecane (TCMC), 2-[(carboxymethyl)]-[5-(4-nitrophenyl-1-[4,7,10-tris-(carboxymethyl)-1,4,7,10-tetraazacyclododecan-1-yl]pentan-2-yl)-amino]acetic acid (3p-C-DEPA) and their derivatives [123]. The macrocyclic chelators require minimal physical manipulation during coordination with radiometal ions, as they possess inherently constrained geometries and partially pre-organized metal ion binding sites, thereby decreasing the entropic loss experienced upon metal ion coordination [123]. Complex formation using macrocyclic chelators requires heating at elevated temperatures (80-95°C), however this is not an issue for small molecular weight ligands such as PSMA inhibitors, which remain stable in this temperature range [128]. For all clinical studies reported to date with 225Ac and 213Bi for TAT of PCa, [96,129] DOTA derivatives have been used as the BFCs of choice because of their excellent thermodynamic stability and kinetic rigidity in vivo.
TAT of mCRPCa using radiolabeled PSMA inhibitors
The preclinical and clinical studies related to TAT of mCRPCa with PSMA inhibitors radiolabeled with different α-emitting radioisotopes are summarized in Table 1 and described in the following text.
Table 1.
Representative examples of radiolabeled PSMA inhibitors used for TAT of mCRPCa in preclinical and clinical settings
| Radioisotope | Radiolabeled agent used | Type of study (Preclinical/clinical) | Prostate cancer type | Treatment response | Reference |
|---|---|---|---|---|---|
| 211At | (2S)-2-(3-(1-carboxy-5-(4-211At-astatobenzamido) pentyl)ureido)-pentanedioic acid | Preclinical | (PSMA+) PC3 PIP xenograft | Caused significant delay in tumor growth. | [127] |
| 225Ac | 225Ac-PSMA-617 | Clinical | mCRPCa | Experienced PSA decline to below the measurable level and showed complete response on PET imaging. | [96] |
| 213Bi | 213Bi-PSMA I&T | Preclinical | PSMA+ LNCaP xenografts | Caused DNA double strand breaks in tumors. | [132] |
| 213Bi | 213Bi-PSMA-617 | Clinical | mCRPCa | Experienced decrease in PSA level from 237 μg/L to 43 μg/L and showed complete response on PET imaging. | [133] |
211At-labeled PSMA inhibitor
In an attempt to explore the utility of 211At-labeled PSMA inhibitor for the treatment of mCRPCa, Kiess et. al. synthesized (2S)-2-(3-(1-carboxy-5-(4-211At-astatobenzamido) pentyl)ureido)-pentanedioic acid and evaluated its efficacy in preclinical settings [127]. The radiolabeled agent could be synthesized with > 60% yield and > 98% radiochemical purity. The authors evaluated the cellular uptake and clonogenic survival in PSMA-positive (PSMA+) PC3 PIP and PSMA-negative (PSMA-) PC3 flu human PCa cells after treatment with (2S)-2-(3-(1-carboxy-5-(4-211At-astatobenzamido) pentyl)ureido)-pentanedioic acid. From this study, it was observed that the uptake in (PSMA+) PC3 PIP cells increased from 4.9 ± 0.3% at 0.5 h to 19.3 ± 1.0 at 4 h [127]. When co-incubated with the PSMA inhibitor, the radiotracer uptake was reduced to < 2% of the initial dose, indicating PSMA specificity of (2S)-2-(3-(1-carboxy-5-(4-211At-astatobenzamido) pentyl)ureido)-pentanedioic acid. Also, it was observed that the uptake in PSMA- PC3 flu cells was significantly lower than in PSMA+ PC3 PIP cells, thus further corroborating the PSMA specificity of the radiotracer synthesized. Additionally, decreased clonogenic survival of (PSMA+) PC3 PIP cells after incubation with (2S)-2-(3-(1-carboxy-5-(4-211At-astatobenzamido) pentyl)ureido)-pentanedioic acid radiotracer was observed.
Biodistribution studies showed significant uptake of (2S)-2-(3-(1-carboxy-5-(4-211At-astatobenzamido) pentyl)ureido)-pentanedioic acid radiotracer in (PSMA+) PC3 PIP tumors (maximum uptake 28.2% ID/g) and in kidneys (> 90% ID/g) [127]. For the PSMA- PC3 flu tumors, maximum uptake was 2.1 ± 1.4% ID/g, indicating PSMA specificity of the radiotracer in vivo (Figure 9A). Microscale kidney dosimetry was done based on α-camera imaging and a nephron model was developed, which revealed hot spots in the proximal renal tubules (Figure 9A). The long-term toxicity studies confirmed that the dose-limiting toxicity was late radiation nephropathy with loss of the proximal tubules. This was demonstrated by both histopathology at necropsy and serial laboratory studies (Figure 9B). However, (2S)-2-(3-(1-carboxy-5-(4-211At-astatobenzamido) pentyl)ureido)-pentanedioic acid did not show significant dose-limiting hematologic toxicity.
Figure 9.
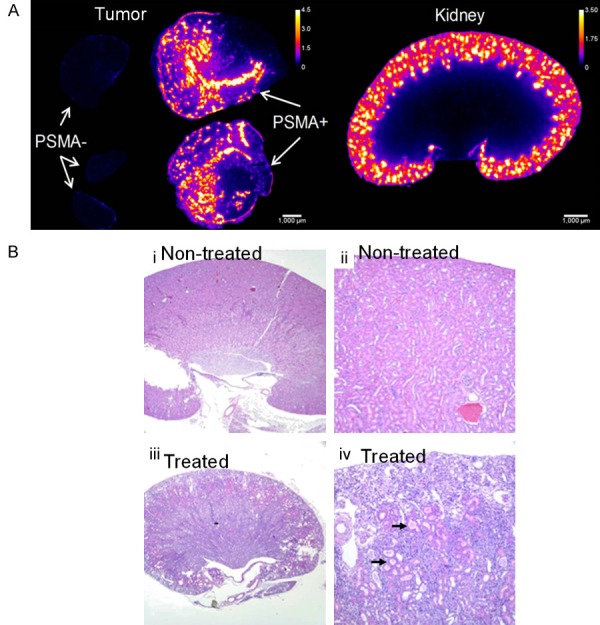
(A) α-camera images at 1 h showing relative 211At-labeled PSMA inhibitor activity concentrations for PSMA+ and PSMA- tumors and kidneys. Scale shows activity concentration relative to whole tumor/kidney average concentration. (B) Renal histopathology from non-treated mouse (i and ii) and mouse treated with 1.5 MBq of 211At-labeled PSMA inhibitor (iii and iv). Treated kidney showed subcortical atrophy and degenerative loss of proximal tubules (arrows) consistent with late nephropathy due to α-particle irradiation. Adapted from Ref. [127] with permission. Copyright 2016 Society of Nuclear Medicine and Molecular Imaging.
Another cause of concern with this radiotracer is in vivo deastatination as indicated by uptake of radioactivity in thyroid, stomach, spleen, and lungs, in the biodistribution studies [127]. Therefore, from the perspective of clinical translation of this class of radiolabeled agents for TAT of mCRPCa, urea-based PSMA inhibitors with more favorable tumor-kidney dose ratios must be identified, and improved radiolabeling techniques must be developed to achieve enhanced in vivo stability of the radiolabeled agent. Nevertheless, the present study could set the stage for development of new 211At-based agents for TAT of PCa, and it also highlighted the importance of long-term toxicity studies and microscale dosimetry in this context.
225Ac-labeled PSMA inhibitor
The first-in-human treatment with an α-emitting radionuclide (225Ac) labeled PSMA inhibitor was reported by Kratochwil et al [96]. For this purpose, PSMA-617 was radiolabeled with 225Ac with > 98% radiochemical purity and with specific activity of 0.17 ± 0.05 MBq/nmol. In this study, 2 patients in highly challenging clinical situations were subjected to targeted 225Ac-PSMA-617 therapy. Among these 2 patients, treatment with β-emitters had contraindicated in one patient (patient A) while the other patient (patient B) was resistant to 177Lu-PSMA-617. In both the patients, 68Ga-PSMA-11 PET/CT scans validated the presence of the PSMA+ tumor phenotype (Figure 10). For TAT, a 100-kBq activity dose of 225Ac-PSMA-617 per kilogram of body weight was administered bimonthly and the PSA response and hematologic toxicity were determined at minimum every 4 weeks. Restaging was done with 68Ga-PSMA-11 PET/CT (Figure 10). Both patients demonstrated a PSA decline to below the measurable level and showed a complete response on imaging. Also, no significant hematological toxicity was experienced. However, xerostomia was observed as the clinical side effect of this therapy. Despite the impressive therapeutic responses observed in 2 PCa patients in clinically critical situations, investigation of therapeutic modality in larger cohort of PCa patients is warranted to arrive at a definite conclusion.
Figure 10.
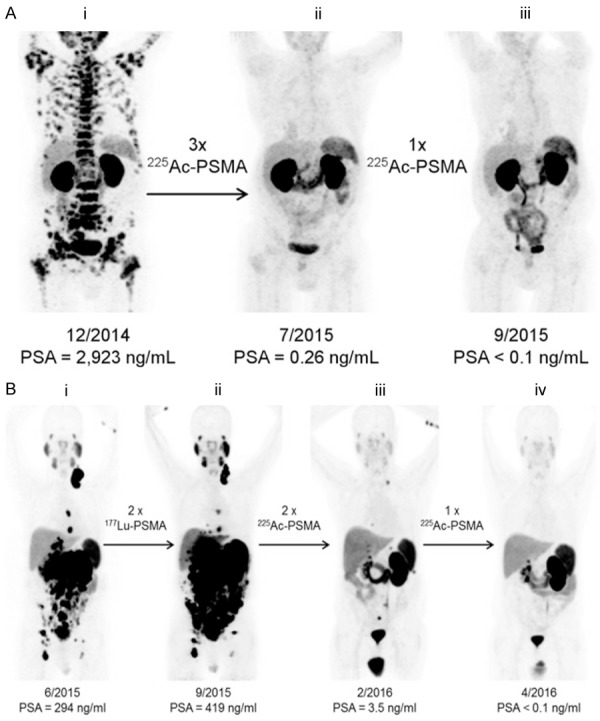
(A) 68Ga-PSMA-11 PET/CT scans of patient A. Pretherapeutic tumor spread (i), restaging 2 months after third cycle of 225Ac-PSMA-617 (ii), and restaging 2 months after one additional consolidation therapy (iii). (B) 68Ga-PSMA-11 PET/CT scans of patient B. In comparison to initial tumor spread (i), restaging after 2 cycles of β-emitting 177Lu-PSMA-617 presented progression (ii). In contrast, restaging after second (iii) and third (iv) cycles of 225Ac-PSMA-617 therapy presented impressive response. Adapted from Ref. [96] with permission. Copyright 2016 Society of Nuclear Medicine and Molecular Imaging.
In a more extensive study, the same group of authors developed a treatment protocol for 225Ac-PSMA-617 α-radiation therapy in advanced-stage, mCRPCa patients with PSMA positive tumor phenotype [130]. Mainly, end-stage patients who had already exhausted the approved therapeutic options were admitted for the study. The authors calculated a dosimetry estimate on the basis of time-activity curves derived from serially obtained 177Lu-PSMA-617 scans extrapolated to the physical half-life of 225Ac. For these calculations, instant decay of unstable daughter nuclides of 225Ac was assumed. This study revealed mean doses of 2.3 Sv for salivary glands, 0.7 Sv for kidneys, and 0.05 Sv for red marrow that are composed of 99.4% α, 0.5% β, and 0.1% γ radiation, respectively. Salvage therapies were empirically conducted with 50, 100, 150, 200 kBq/kg dose of 225Ac-PSMA-617, and treatment and toxicity responses were retrospectively evaluated. It was observed that severe xerostomia became the dose-limiting toxicity if treatment dose of 225Ac-PSMA-617 exceeded 100 kBq/kg per cycle. At 100 kBq/kg, the duration of PSA decline was < 4 months. However, if TAT was repeated every 2 months, patients experienced additive antitumor effects. Treatment doses of 50 kBq/kg were nontoxic, but the antitumor response was insufficient in these high-tumor-burden patients. Based on these clinical results, the authors concluded that for mCRPCa patients, a treatment dose of 100 kBq/kg of 225Ac-PSMA-617 per cycle, repeated every 8 weeks, presents a reasonable balance between toxicity and biochemical response. This therapeutic regimen was used by the same group of authors in a larger cohort of patients, wherein remarkable anti-tumor activity of 225Ac-PSMA-617 was demonstrated [131]. In this study, swimmer-plot analysis provided first longitudinal indicators that TAT with 225Ac-PSMA-617 presented clinical efficacy with regard to duration of tumor control. As in the previous study, xerostomia was the main cause to stop the therapy or to reject additional administrations and was in the same facet as non-response. Despite promising attributes, this study indicated that further modifications of the therapeutic protocol with regard to side effects might be essential in order to further improve the therapeutic range.
213Bi-labeled PSMA inhibitor
In a first preclinical study on TAT of PCa using 213Bi-labeled PSMA inhibitor (213Bi-PSMA I&T), Nonnekens et. al. demonstrated that the radiolabeled agent induced DNA double strand breaks in PCa xenografts [132]. The authors prepared 213Bi-PSMA I&T with > 95% radiolabeling yield with a specific activity of 58 MBq/nmol. In vitro studies conducted in PSMA+ LNCaP cells indicated that 213Bi-PSMA I&T led to increased number of DNA double-strand breaks, detected as 53BP1 and γH2AX nuclear foci. The results of the biodistribution studies in mice bearing LNCaP xenografts showed significant tumor uptake of the radiotracer at 1 h p.i., with accumulation in the kidneys. Additionally, 213Bi-PSMA I&T induced in vivo DNA double strand breaks in the tumors, which were detected between 1 hour and 24 hours p.i. The results of this preliminary study set the stage for further evaluation of 213Bi-labeled PSMA inhibitors with regard to their therapeutic efficacy and toxicity for PCa management.
The first-in-human treatment with 213Bi-PSMA-617 in a patient with mCRPCa was reported by Sathekge et. al. [133]. In this study, the lone patient was treated with two cycles of 213Bi-PSMA-617 with a cumulative activity of 592 MBq. Restaging was done with 68Ga-PSMA PET/CT after 11 months, which showed a remarkable molecular imaging response (Figure 11). Also, the patient had demonstrated a biochemical response (decrease in PSA level from 237 to 43 μg/L). Despite promising results, detailed clinical evaluation of 213Bi-PSMA-617 in a large cohort of PCa patients is warranted to arrive at any conclusion. In another recent clinical study, Kratochwil et. al. estimated the radiation dosimetry of 213Bi-PSMA-617 in PCa patients [129]. The authors used 68Ga (t½ = 68 min) as a surrogate nuclide for 213Bi, enabling high-resolution quantitative 68Ga-PSMA-617 PET-imaging. Based on this, the extrapolated radiation dosimetry for 213Bi-PSMA-617 was estimated and its therapeutic index was compared with findings for 225Ac-PSMA-617. The authors found that the dosimetry of 213Bi-PSMA-617 was in a range usually considered suitable for clinical use. However, as compared to 225Ac-PSMA-617 dosimetry, it suffered from higher perfusion-dependent off-target radiation, therefore its therapeutic index for PCa therapy was considered to be inferior. Moreover, due to longer biological half-life of PSMA-617 in dose-limiting organs compared to the physical half-life of 213Bi, this radionuclide becomes a second choice (after 225Ac) for radiolabeling PSMA inhibitors for TAT of PCa [129]. A major limitation of this study is that a different complex (68Ga-PSMA-617) was used to estimate the dosimetry of 213Bi-PSMA-617 in PCa patients. It is worth mentioning that the radiometal in the chelator complex could affect the pharmacokinetics of the radiotracer and these effects can be significant [134]. Nevertheless, the results of this study amply demonstrated that 213Bi-PSMA-617 is suitable for clinical translation and more detailed studies in larger cohort of patients are warranted.
Figure 11.
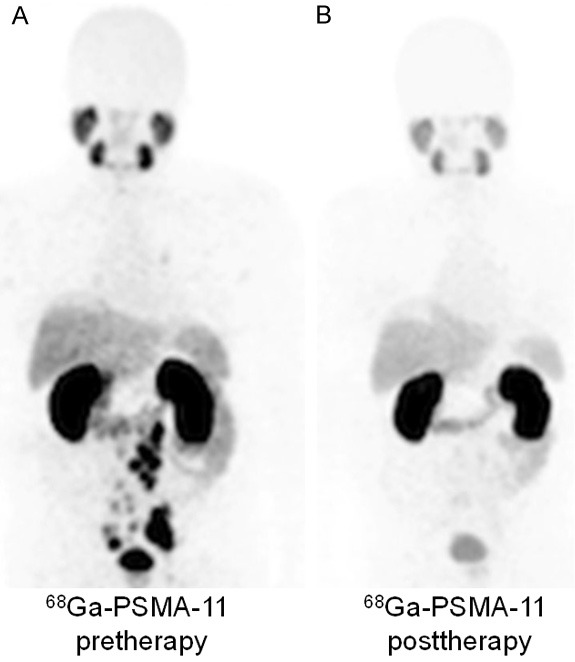
68Ga-PSMA-11 PET/CT scans of patient. (A) Pretherapeutic tumor spread, (B) restaging 11 months after therapy with 213Bi-PSMA-617. Adapted from Ref. [133] with permission. Copyright 2017 Springer.
Conclusions and future perspectives
TAT using radiolabeled PSMA inhibitors is emerging as a promising new modality for the treatment of mCRPCa. Significant research activity and resulting outstanding progress in production of clinically safe, radionuclidically pure, α-emitters, design and synthesis of PSMA inhibitors, and suitable linkers forming radiolabeled constructs with appreciable in vitro and in vivo stability, make it very likely that this modality will become a new line of therapy protocol for advanced stage PCa management. It is worth mentioning that the onset of early clinical trials with PSMA inhibitors radiolabeled with α-emitters will definitely enable the nuclear medicine practitioners to come up with extremely effective and highly specific radiopharmaceuticals to target micro metastases in advanced stage PCa patients. With regard to the safety, convenience of handling, transportation logistics and clinical efficacy, 225Ac is the most advantageous among the radionuclides studied for TAT of PCa. Additionally, the radiolabeling chemistry for preparation of 225Ac-based radiopharmaceuticals is well established and clinically proven. Nevertheless, production of this radioisotope is still limited to very few countries in the world, and therefore its availability at a reasonable cost is an issue for widespread clinical use.
Over the last decade, a variety of PSMA inhibitors have been synthesized which can be used for preparation of radiopharmaceuticals. For effective clinical utilization, PSMA inhibitor selection should be based on rapid uptake and persistent localization at the target site, with negligible retention in non-targeted tissues. In addition to the normal prostate, low levels of endogenous PSMA expression have also been found in many organs, including the proximal tubules of the kidneys, the lacrimal and salivary glands, the spleen, the liver, the intestinal membranes, the testes, the ovaries, and the brain [135,136]. Therefore, upon administration of radiolabeled PSMA inhibitor, uptake of small amount of radioactivity also occurs in these normal tissues in addition to cancerous lesions, which might cause unnecessary side effects, especially while using α-emitting radioisotopes. Based on the results of clinical trials reported to date, the most significant adverse side effect observed with 225Ac-PSMA-617 is xerostomia, which as such could be avoided using radiolabeled monoclonal antibodies such as J591 (which targets a different epitope) [137-140] instead of PSMA ligands for targeting PCa. However, small molecular agents such as PSMA inhibitors have a critical advantage over much larger constructs, as they clear faster from the blood, and demonstrate increased tumor permeability, allowing them to escape physiological barriers met by larger molecules, such as monoclonal antibodies. Therefore, it is prudent to design and synthesize a new class of PSMA ligands wherein the uptake in these organs can be minimized for enhanced clinical benefits.
While numerous complications face all new technologies, materializing the opportunities presented by TAT of mCRPCa requires addressing several interdisciplinary challenges. Additionally, there are other complex factors, which include considerable regulatory hurdles in handling α-emitters in hospital radiopharmacies, a limited potential market (at least initially), lobbying by the manufacturers of conventional PCa therapy agents, lack of reimbursement strategies by the insurance agencies for such novel strategies, and socio-economic factors, which might obstruct widespread translation of this novel therapeutic modality in nuclear medicine clinics. In view of all these challenges, concerted efforts of all stakeholders, which include radiopharmaceutical scientists, nuclear medicine physicians, radiochemists, medical physicists, radiologists, program advisory boards, and regulatory authorities would be required, both to create enthusiasm for developing this new concept and to prevent undesirable messaging based on myths, speculations, exaggeration, and prejudice. This in turn would provide impetus to further clinical research, which might aid toward use of TAT in routine clinical practices for advanced stage PCa management.
Acknowledgements
This work is supported, in part, by the Bhabha Atomic Research Centre, University of Wisconsin-Madison, and the National Institutes of Health (NIBIB/NCI 1R01CA169365, P30CA014520). The authors from Bhabha Atomic Research Centre are grateful to Dr. P. K. Pujari, Associate Director, Radiochemistry and Isotope Group, Bhabha Atomic Research Centre for his constant encouragement and support and to their colleague Dr. Sudipta Chakraborty for fruitful discussions which helped in preparing this manuscript.
References
- 1.Domachevsky L, Goldberg N, Bernstine H, Nidam M, Groshar D. Quantitative characterisation of clinically significant intra-prostatic cancer by prostate-specific membrane antigen (PSMA) expression and cell density on PSMA-11. Eur Radiol. 2018 doi: 10.1007/s00330-018-5484-1. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 2.Goolam AS, la Rosa AH, Manoharan M. Surgical management of organ-confined prostate cancer with review of literature and evolving evidence. Indian J Surg Oncol. 2018;9:225–231. doi: 10.1007/s13193-016-0594-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rahbar K, Afshar-Oromieh A, Jadvar H, Ahmadzadehfar H. PSMA theranostics: current status and future directions. Mol Imaging. 2018;17:1536012118776068. doi: 10.1177/1536012118776068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ruggero K, Farran-Matas S, Martinez-Tebar A, Aytes A. Epigenetic regulation in prostate cancer progression. Curr Mol Biol Rep. 2018;4:101–115. doi: 10.1007/s40610-018-0095-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Smith RA, Andrews KS, Brooks D, Fedewa SA, Manassaram-Baptiste D, Saslow D, Brawley OW, Wender RC. Cancer screening in the United States, 2018: a review of current American cancer society guidelines and current issues in cancer screening. CA Cancer J Clin. 2018;68:297–316. doi: 10.3322/caac.21446. [DOI] [PubMed] [Google Scholar]
- 6.Wang K, Ruan H, Xu T, Liu L, Liu D, Yang H, Zhang X, Chen K. Recent advances on the progressive mechanism and therapy in castration-resistant prostate cancer. Onco Targets Ther. 2018;11:3167–3178. doi: 10.2147/OTT.S159777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68:7–30. doi: 10.3322/caac.21442. [DOI] [PubMed] [Google Scholar]
- 8.Litwin MS, Tan HJ. The diagnosis and treatment of prostate cancer: a review. JAMA. 2017;317:2532–2542. doi: 10.1001/jama.2017.7248. [DOI] [PubMed] [Google Scholar]
- 9.Spencer BA, Steinberg M, Malin J, Adams J, Litwin MS. Quality-of-care indicators for earlystage prostate cancer. J. Clin. Oncol. 2003;21:1928–1936. doi: 10.1200/JCO.2003.05.157. [DOI] [PubMed] [Google Scholar]
- 10.Pillai MRA, Nanabala R, Joy A, Sasikumar A, Russ Knapp FF. Radiolabeled enzyme inhibitors and binding agents targeting PSMA: effective theranostic tools for imaging and therapy of prostate cancer. Nucl Med Biol. 2016;43:692–720. doi: 10.1016/j.nucmedbio.2016.08.006. [DOI] [PubMed] [Google Scholar]
- 11.Kehinde EO, Mojiminiyi OA, Sheikh M, Al-Awadi KA, Daar AS, Al-Hunayan A, Anim JT, Al-Sumait AA. Age-specific reference levels of serum prostate-specific antigen and prostate volume in healthy Arab men. BJU Int. 2005;96:308–312. doi: 10.1111/j.1464-410X.2005.05620.x. [DOI] [PubMed] [Google Scholar]
- 12.Abedi AR, Fallah-Karkan M, Allameh F, Ranjbar A, Shadmehr A. Incidental prostate cancer: a 10-year review of a tertiary center, Tehran, Iran. Res Rep Urol. 2018;10:1–6. doi: 10.2147/RRU.S146159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Song P, Huang C, Wang Y. The efficacy and safety comparison of docetaxel, cabazitaxel, estramustine, and mitoxantrone for castrationresistant prostate cancer: a network metaanalysis. Int J Surg. 2018;56:133–140. doi: 10.1016/j.ijsu.2018.06.010. [DOI] [PubMed] [Google Scholar]
- 14.Linares-Espinos E, Carneiro A, Martinez-Salamanca JI, Bianco F, Castro-Alfaro A, Cathelineau X, Valerio M, Sanchez-Salas R. New technologies and techniques for prostate cancer focal therapy: a review of the current literature. Minerva Urol Nefrol. 2018;70:252–263. doi: 10.23736/S0393-2249.18.03094-1. [DOI] [PubMed] [Google Scholar]
- 15.Shah S, Young HN, Cobran EK. Comparative effectiveness of conservative management compared to cryotherapy in localized prostate cancer patients. Am J Mens Health. 2018;12:1681–1691. doi: 10.1177/1557988318781731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Quivrin M, Peignaux-Casasnovas K, Martin E, Rouffiac M, Thibouw D, Chevalier C, Vulquin N, Aubignac L, Truc G, Crehange G. Salvage brachytherapy as a modern reirradiation technique for local cancer failure: the phoenix is reborn from its ashes. Cancer Radiother. 2018;22:372–381. doi: 10.1016/j.canrad.2018.01.005. [DOI] [PubMed] [Google Scholar]
- 17.Arimura T, Yoshiura T, Matsukawa K, Kondo N, Kitano I, Ogino T. Proton beam therapy alone for intermediate- or high-risk prostate cancer: an institutional prospective cohort study. Cancers (Basel) 2018;10:116. doi: 10.3390/cancers10040116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ojerholm E, Bekelman JE. Finding value for protons: the case of prostate cancer? Semin Radiat Oncol. 2018;28:131–137. doi: 10.1016/j.semradonc.2017.11.003. [DOI] [PubMed] [Google Scholar]
- 19.Yadav SS, Stockert JA, Hackert V, Yadav KK, Tewari AK. Intratumor heterogeneity in prostate cancer. Urol Oncol. 2018;36:349–360. doi: 10.1016/j.urolonc.2018.05.008. [DOI] [PubMed] [Google Scholar]
- 20.Santamaria L, Ingelmo I, Sinues B, Martinez L, Teba F. Quantification of the heterogeneity of cytokeratin 18 immunoexpression in prostate adenocarcinoma and normal prostate: global and local features. Histol Histopathol. 2018:18009. doi: 10.14670/HH-18-009. [DOI] [PubMed] [Google Scholar]
- 21.Kulkarni HR, Singh A, Langbein T, Schuchardt C, Mueller D, Zhang J, Lehmann C, Baum RP. Theranostics of prostate cancer: from molecular imaging to precision molecular radiotherapy targeting the prostate specific membrane antigen. Br J Radiol. 2018:20180308. doi: 10.1259/bjr.20180308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chatalic KL, Heskamp S, Konijnenberg M, Molkenboer-Kuenen JD, Franssen GM, Clahsenvan Groningen MC, Schottelius M, Wester HJ, van Weerden WM, Boerman OC, de Jong M. Towards personalized treatment of prostate cancer: PSMA I&T, a promising prostate-specific membrane antigen-targeted theranostic agent. Theranostics. 2016;6:849–861. doi: 10.7150/thno.14744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bouchelouche K, Choyke PL. Prostatespecific membrane antigen positron emission tomography in prostate cancer: a step toward personalized medicine. Curr Opin Oncol. 2016;28:216–221. doi: 10.1097/CCO.0000000000000277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zschaeck S, Lohaus F, Beck M, Habl G, Kroeze S, Zamboglou C, Koerber SA, Debus J, Holscher T, Wust P, Ganswindt U, Baur ADJ, Zophel K, Cihoric N, Guckenberger M, Combs SE, Grosu AL, Ghadjar P, Belka C. PSMA-PET based radiotherapy: a review of initial experiences, survey on current practice and future perspectives. Radiat Oncol. 2018;13:90. doi: 10.1186/s13014-018-1047-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yu CY, Desai B, Ji L, Groshen S, Jadvar H. Comparative performance of PET tracers in biochemical recurrence of prostate cancer: a critical analysis of literature. Am J Nucl Med Mol Imaging. 2014;4:580–601. [PMC free article] [PubMed] [Google Scholar]
- 26.Almeida FD, Yen CK, Scholz MC, Lam RY, Turner J, Bans LL, Lipson R. Performance characteristics and relationship of PSA value/kinetics on carbon-11 acetate PET/CT imaging in biochemical relapse of prostate cancer. Am J Nucl Med Mol Imaging. 2017;7:1–11. [PMC free article] [PubMed] [Google Scholar]
- 27.Cui C, Hanyu M, Hatori A, Zhang Y, Xie L, Ohya T, Fukada M, Suzuki H, Nagatsu K, Jiang C, Luo R, Shao G, Zhang M, Wang F. Synthesis and evaluation of [64Cu] PSMA-617 targeted for prostate-specific membrane antigen in prostate cancer. Am J Nucl Med Mol Imaging. 2017;7:40–52. [PMC free article] [PubMed] [Google Scholar]
- 28.Fonager RF, Zacho HD, Langkilde NC, Fledelius J, Ejlersen JA, Haarmark C, Hendel HW, Lange MB, Jochumsen MR, Mortensen JC, Petersen LJ. Diagnostic test accuracy study of 18F-sodium fluoride PET/CT, 99mTc-labelled diphosphonate SPECT/CT, and planar bone scintigraphy for diagnosis of bone metastases in newly diagnosed, high-risk prostate cancer. Am J Nucl Med Mol Imaging. 2017;7:218–227. [PMC free article] [PubMed] [Google Scholar]
- 29.Gerke O, Poulsen MH, Hoilund-Carlsen PF. Added value of cost-utility analysis in simple diagnostic studies of accuracy: 18Ffluoromethylcholine PET/CT in prostate cancer staging. Am J Nucl Med Mol Imaging. 2015;5:183–194. [PMC free article] [PubMed] [Google Scholar]
- 30.Muzahir S, Jeraj R, Liu G, Hall LT, Rio AM, Perk T, Jaskowiak C, Perlman SB. Differentiation of metastatic vs degenerative joint disease using semi-quantitative analysis with 18F-NaF PET/CT in castrate resistant prostate cancer patients. Am J Nucl Med Mol Imaging. 2015;5:162–168. [PMC free article] [PubMed] [Google Scholar]
- 31.Vali R, Loidl W, Pirich C, Langesteger W, Beheshti M. Imaging of prostate cancer with PET/CT using 18F-Fluorocholine. Am J Nucl Med Mol Imaging. 2015;5:96–108. [PMC free article] [PubMed] [Google Scholar]
- 32.Aparici CM, Carlson D, Nguyen N, Hawkins RA, Seo Y. Combined SPECT and multidetector CT for prostate cancer evaluations. Am J Nucl Med Mol Imaging. 2012;2:48–54. [PMC free article] [PubMed] [Google Scholar]
- 33.Cai W, Hong H. Peptoid and positron emission tomography: an appealing combination. Am J Nucl Med Mol Imaging. 2011;1:76–79. [PMC free article] [PubMed] [Google Scholar]
- 34.Kratochwil C, Afshar-Oromieh A, Kopka K, Haberkorn U, Giesel FL. Current status of prostate-specific membrane antigen targeting in nuclear medicine: clinical translation of chelator containing prostate-specific membrane antigen ligands into diagnostics and therapy for prostate cancer. Semin Nucl Med. 2016;46:405–418. doi: 10.1053/j.semnuclmed.2016.04.004. [DOI] [PubMed] [Google Scholar]
- 35.Ceci F, Castellucci P, Cerci JJ, Fanti S. New aspects of molecular imaging in prostate cancer. Methods. 2017;130:36–41. doi: 10.1016/j.ymeth.2017.07.009. [DOI] [PubMed] [Google Scholar]
- 36.Will L, Sonni I, Kopka K, Kratochwil C, Giesel FL, Haberkorn U. Radiolabeled prostate-specific membrane antigen small-molecule inhibitors. Q J Nucl Med Mol Imaging. 2017;61:168–180. doi: 10.23736/S1824-4785.17.02977-6. [DOI] [PubMed] [Google Scholar]
- 37.Chang SS. Overview of prostate-specific membrane antigen. Rev Urol. 2004;6(Suppl 10):S13–18. [PMC free article] [PubMed] [Google Scholar]
- 38.Bouchelouche K, Turkbey B, Choyke PL. PSMA PET and padionuclide therapy in prostate cancer. Semin Nucl Med. 2016;46:522–535. doi: 10.1053/j.semnuclmed.2016.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Davis MI, Bennett MJ, Thomas LM, Bjorkman PJ. Crystal structure of prostate-specific membrane antigen, a tumor marker and peptidase. Proc Natl Acad Sci U S A. 2005;102:5981–5986. doi: 10.1073/pnas.0502101102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Berger I, Annabattula C, Lewis J, Shetty DV, Kam J, Maclean F, Arianayagam M, Canagasingham B, Ferguson R, Khadra M, Ko R, Winter M, Loh H, Varol C. 68Ga-PSMA PET/CT vs. mpMRI for locoregional prostate cancer staging: correlation with final histopathology. Prostate Cancer Prostatic Dis. 2018;21:204–211. doi: 10.1038/s41391-018-0048-7. [DOI] [PubMed] [Google Scholar]
- 41.Bouchelouche K, Choyke PL. Advances in prostate-specific membrane antigen PET of prostate cancer. Curr Opin Oncol. 2018;30:189–196. doi: 10.1097/CCO.0000000000000439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Corfield J, Perera M, Bolton D, Lawrentschuk N. 68Ga-prostate specific membrane antigen (PSMA) positron emission tomography (PET) for primary staging of high-risk prostate cancer: a systematic review. World J Urol. 2018;36:519–527. doi: 10.1007/s00345-018-2182-1. [DOI] [PubMed] [Google Scholar]
- 43.Cuccurullo V, Di Stasio GD, Mansi L. Nuclear medicine in prostate cancer: a new era for radiotracers. World J Nucl Med. 2018;17:70–78. doi: 10.4103/wjnm.WJNM_54_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li R, Ravizzini GC, Gorin MA, Maurer T, Eiber M, Cooperberg MR, Alemozzaffar M, Tollefson MK, Delacroix SE, Chapin BF. The use of PET/CT in prostate cancer. Prostate Cancer Prostatic Dis. 2018;21:4–21. doi: 10.1038/s41391-017-0007-8. [DOI] [PubMed] [Google Scholar]
- 45.Jindal V. Immunotherapy: a glimmer of hope for metastatic prostate cancer. Chin Clin Oncol. 2018 doi: 10.21037/cco.2018.02.01. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 46.Udovicich C, Perera M, Hofman MS, Siva S, Del Rio A, Murphy DG, Lawrentschuk N. 68Gaprostate-specific membrane antigen-positron emission tomography/computed tomography in advanced prostate cancer: current state and future trends. Prostate Int. 2017;5:125–129. doi: 10.1016/j.prnil.2017.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lutje S, Heskamp S, Cornelissen AS, Poeppel TD, van den Broek SA, Rosenbaum-Krumme S, Bockisch A, Gotthardt M, Rijpkema M, Boerman OC. PSMA ligands for radionuclide imaging and therapy of prostate cancer: clinical status. Theranostics. 2015;5:1388–1401. doi: 10.7150/thno.13348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schwarzenboeck SM, Rauscher I, Bluemel C, Fendler WP, Rowe SP, Pomper MG, Asfhar-Oromieh A, Herrmann K, Eiber M. PSMA ligands for PET imaging of prostate cancer. J Nucl Med. 2017;58:1545–1552. doi: 10.2967/jnumed.117.191031. [DOI] [PubMed] [Google Scholar]
- 49.Machulkin AE, Ivanenkov YA, Aladinskaya AV, Veselov MS, Aladinskiy VA, Beloglazkina EK, Koteliansky VE, Shakhbazyan AG, Sandulenko YB, Majouga AG. Small-molecule PSMA ligands. Current state, SAR and perspectives. J Drug Target. 2016;24:679–693. doi: 10.3109/1061186X.2016.1154564. [DOI] [PubMed] [Google Scholar]
- 50.Lutje S, Slavik R, Fendler W, Herrmann K, Eiber M. PSMA ligands in prostate cancer-Probe optimization and theranostic applications. Methods. 2017;130:42–50. doi: 10.1016/j.ymeth.2017.06.026. [DOI] [PubMed] [Google Scholar]
- 51.Ray Banerjee S, Chen Z, Pullambhatla M, Lisok A, Chen J, Mease RC, Pomper MG. Preclinical comparative study of 68Ga-Labeled DOTA, NOTA, and HBED-CC chelated radiotracers for targeting PSMA. Bioconjug Chem. 2016;27:1447–1455. doi: 10.1021/acs.bioconjchem.5b00679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Benesova M, Bauder-Wust U, Schafer M, Klika KD, Mier W, Haberkorn U, Kopka K, Eder M. Linker modification strategies to control the prostate-specific membrane antigen (PSMA)-targeting and pharmacokinetic properties of DOTA-conjugated PSMA inhibitors. J Med Chem. 2016;59:1761–1775. doi: 10.1021/acs.jmedchem.5b01210. [DOI] [PubMed] [Google Scholar]
- 53.Barrett JA, Coleman RE, Goldsmith SJ, Vallabhajosula S, Petry NA, Cho S, Armor T, Stubbs JB, Maresca KP, Stabin MG, Joyal JL, Eckelman WC, Babich JW. First-in-man evaluation of 2 high-affinity PSMA-avid small molecules for imaging prostate cancer. J Nucl Med. 2013;54:380–387. doi: 10.2967/jnumed.112.111203. [DOI] [PubMed] [Google Scholar]
- 54.Zechmann CM, Afshar-Oromieh A, Armor T, Stubbs JB, Mier W, Hadaschik B, Joyal J, Kopka K, Debus J, Babich JW, Haberkorn U. Radiation dosimetry and first therapy results with a 124I/131I-labeled small molecule (MIP-1095) targeting PSMA for prostate cancer therapy. Eur J Nucl Med Mol Imaging. 2014;41:1280–1292. doi: 10.1007/s00259-014-2713-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Vallabhajosula S, Nikolopoulou A, Babich JW, Osborne JR, Tagawa ST, Lipai I, Solnes L, Maresca KP, Armor T, Joyal JL, Crummet R, Stubbs JB, Goldsmith SJ. 99mTc-labeled small-molecule inhibitors of prostate-specific membrane antigen: pharmacokinetics and biodistribution studies in healthy subjects and patients with metastatic prostate cancer. J Nucl Med. 2014;55:1791–1798. doi: 10.2967/jnumed.114.140426. [DOI] [PubMed] [Google Scholar]
- 56.Eder M, Neels O, Muller M, Bauder-Wust U, Remde Y, Schafer M, Hennrich U, Eisenhut M, Afshar-Oromieh A, Haberkorn U, Kopka K. Novel preclinical and radiopharmaceutical aspects of [68Ga] Ga-PSMA-HBED-CC: a new PET tracer for imaging of prostate cancer. Pharmaceuticals (Basel) 2014;7:779–796. doi: 10.3390/ph7070779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cho SY, Gage KL, Mease RC, Senthamizhchelvan S, Holt DP, Jeffrey-Kwanisai A, Endres CJ, Dannals RF, Sgouros G, Lodge M, Eisenberger MA, Rodriguez R, Carducci MA, Rojas C, Slusher BS, Kozikowski AP, Pomper MG. Biodistribution, tumor detection, and radiation dosimetry of 18F-DCFBC, a low-molecularweight inhibitor of prostate-specific membrane antigen, in patients with metastatic prostate cancer. J Nucl Med. 2012;53:1883–1891. doi: 10.2967/jnumed.112.104661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Szabo Z, Mena E, Rowe SP, Plyku D, Nidal R, Eisenberger MA, Antonarakis ES, Fan H, Dannals RF, Chen Y, Mease RC, Vranesic M, Bhatnagar A, Sgouros G, Cho SY, Pomper MG. Initial evaluation of [18F] DCFPyL for prostate-specific membrane antigen (PSMA)-targeted PET imaging of prostate cancer. Mol Imaging Biol. 2015;17:565–574. doi: 10.1007/s11307-015-0850-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Afshar-Oromieh A, Hetzheim H, Kratochwil C, Benesova M, Eder M, Neels OC, Eisenhut M, Kubler W, Holland-Letz T, Giesel FL, Mier W, Kopka K, Haberkorn U. The theranostic PSMA ligand PSMA-617 in the diagnosis of prostate cancer by PET/CT: biodistribution in humans, radiation dosimetry, and first evaluation of tumor lesions. J Nucl Med. 2015;56:1697–1705. doi: 10.2967/jnumed.115.161299. [DOI] [PubMed] [Google Scholar]
- 60.Weineisen M, Schottelius M, Simecek J, Baum RP, Yildiz A, Beykan S, Kulkarni HR, Lassmann M, Klette I, Eiber M, Schwaiger M, Wester HJ. 68Ga- and 177Lu-labeled PSMA I&T: optimization of a PSMA-targeted theranostic concept and first proof-of-concept human studies. J Nucl Med. 2015;56:1169–1176. doi: 10.2967/jnumed.115.158550. [DOI] [PubMed] [Google Scholar]
- 61.Calais J, Kishan AU, Cao M, Fendler WP, Eiber M, Herrmann K, Ceci F, Reiter RE, Rettig MB, Hegde JV, Shaverdian N, King CR, Steinberg ML, Czernin J, Nickols NG. Potential impact of 68Ga-PSMA-11 PET/CT on prostate cancer definitive radiation therapy planning. J Nucl Med. 2018 doi: 10.2967/jnumed.118.209387. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Farolfi A, Ceci F, Castellucci P, Graziani T, Siepe G, Lambertini A, Schiavina R, Lodi F, Morganti AG, Fanti S. 68Ga-PSMA-11 PET/CT in prostate cancer patients with biochemical recurrence after radical prostatectomy and PSA <0.5 ng/ml. Efficacy and impact on treatment strategy. Eur J Nucl Med Mol Imaging. 2018 doi: 10.1007/s00259-018-4066-4. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 63.Pizzuto DA, Muller J, Muhlematter U, Rupp NJ, Topfer A, Mortezavi A, Nagel H, Kranzbuhler B, Eberli D, Burger IA. The central zone has increased 68Ga-PSMA-11 uptake: “mickey mouse ears” can be hot on 68Ga-PSMA-11 PET. Eur J Nucl Med Mol Imaging. 2018;45:1335–1343. doi: 10.1007/s00259-018-3979-2. [DOI] [PubMed] [Google Scholar]
- 64.Schmidkonz C, Cordes M, Schmidt D, Bauerle T, Goetz TI, Beck M, Prante O, Cavallaro A, Uder M, Wullich B, Goebell P, Kuwert T, Ritt P. 68Ga-PSMA-11 PET/CT-derived metabolic parameters for determination of whole-body tumor burden and treatment response in prostate cancer. Eur J Nucl Med Mol Imaging. 2018 doi: 10.1007/s00259-018-4042-z. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 65.Thalgott M, Duwel C, Rauscher I, Heck MM, Haller B, Gafita A, Gschwend JE, Schwaiger M, Maurer T, Eiber M. One-stop shop wholebody 68Ga-PSMA-11 PET/MRI compared to clinical Nomograms for preoperative T- and N-staging of high-risk prostate cancer. J Nucl Med. 2018 doi: 10.2967/jnumed.117.207696. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 66.Wang Y, Shao G, Wu J, Cui C, Zang S, Qiu F, Jia R, Wang Z, Wang F. Preparation of 68Ga-PSMA-11 with a synthesis module for micro PET-CT imaging of PSMA expression during prostate cancer progression. Contrast Media Mol Imaging. 2018;2018:8046541. doi: 10.1155/2018/8046541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Afshar-Oromieh A, Haberkorn U, Zechmann C, Armor T, Mier W, Spohn F, Debus N, Holland-Letz T, Babich J, Kratochwil C. Repeated PSMA-targeting radioligand therapy of metastatic prostate cancer with 131I-MIP-1095. Eur J Nucl Med Mol Imaging. 2017;44:950–959. doi: 10.1007/s00259-017-3665-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wang Z, Jacobson O, Tian R, Mease RC, Kiesewetter DO, Niu G, Pomper MG, Chen X. Radioligand therapy of prostate cancer with a long-lasting prostate-specific membrane antigen targeting agent 90Y-DOTA-EB-MCG. Bioconjug Chem. 2018;29:2309–2315. doi: 10.1021/acs.bioconjchem.8b00292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bradley CA. [177Lu] PSMA-617 radionuclide therapy shows promise. Nat Rev Urol. 2018;15:468. doi: 10.1038/s41585-018-0029-6. [DOI] [PubMed] [Google Scholar]
- 70.Chakraborty S, Vimalnath KV, Chakravarty R, Sarma HD, Dash A. Multidose formulation of ready-to-use 177Lu-PSMA-617 in a centralized radiopharmacy set-up. Appl Radiat Isot. 2018;139:91–97. doi: 10.1016/j.apradiso.2018.04.033. [DOI] [PubMed] [Google Scholar]
- 71.Paganelli G, De Giorgi U. [177Lu] -PSMA-617 for targeted prostate cancer treatment: a magic bullet? Lancet Oncol. 2018;19:725–726. doi: 10.1016/S1470-2045(18)30268-7. [DOI] [PubMed] [Google Scholar]
- 72.Hofman MS, Violet J, Hicks RJ, Ferdinandus J, Thang SP, Akhurst T, Iravani A, Kong G, Ravi Kumar A, Murphy DG, Eu P, Jackson P, Scalzo M, Williams SG, Sandhu S. [177Lu] -PSMA-617 radionuclide treatment in patients with metastatic castration-resistant prostate cancer (LuPSMA trial): a single-centre, singlearm, phase 2 study. Lancet Oncol. 2018;19:825–833. doi: 10.1016/S1470-2045(18)30198-0. [DOI] [PubMed] [Google Scholar]
- 73.Rahbar K, Ahmadzadehfar H, Boegemann M. 177Lu-PSMA-617 radioligand therapy in mCRPC: ready for phase III trial? Eur J Nucl Med Mol Imaging. 2018;45:513–514. doi: 10.1007/s00259-017-3892-0. [DOI] [PubMed] [Google Scholar]
- 74.Rahbar K, Bogeman M, Yordanova A, Eveslage M, Schafers M, Essler M, Ahmadzadehfar H. Delayed response after repeated 177Lu-PSMA-617 radioligand therapy in patients with metastatic castration resistant prostate cancer. Eur J Nucl Med Mol Imaging. 2018;45:243–246. doi: 10.1007/s00259-017-3877-z. [DOI] [PubMed] [Google Scholar]
- 75.von Eyben FE, Kiljunen T, Joensuu T, Kairemo K, Uprimny C, Virgolini I. 177Lu-PSMA-617 radioligand therapy for a patient with lymph node metastatic prostate cancer. Oncotarget. 2017;8:66112–66116. doi: 10.18632/oncotarget.19805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Boegemann M, Schrader AJ, Rahbar K. 177Lu-PSMA therapy: current evidence for use in the treatment of patients with metastatic prostate cancer. Urologe A. 2017;56:1440–1444. doi: 10.1007/s00120-017-0510-5. [DOI] [PubMed] [Google Scholar]
- 77.Fendler WP, Rahbar K, Herrmann K, Kratochwil C, Eiber M. 177Lu-PSMA radioligand therapy for prostate cancer. J Nucl Med. 2017;58:1196–1200. doi: 10.2967/jnumed.117.191023. [DOI] [PubMed] [Google Scholar]
- 78.Giovacchini G, Giovannini E, Riondato M, Ciarmiello A. PET/CT with 68Ga-PSMA in prostate cancer: radiopharmaceutical background and clinical implications. Curr Radiopharm. 2018;11:4–13. doi: 10.2174/1874471010666171101121803. [DOI] [PubMed] [Google Scholar]
- 79.von Eyben FE, Roviello G, Kiljunen T, Uprimny C, Virgolini I, Kairemo K, Joensuu T. Third-line treatment and 177Lu-PSMA radioligand therapy of metastatic castration-resistant prostate cancer: a systematic review. Eur J Nucl Med Mol Imaging. 2018;45:496–508. doi: 10.1007/s00259-017-3895-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ceci F, Castellucci P, Fanti S. Current application and future perspectives of PSMA PET imaging in prostate cancer. Q J Nucl Med Mol Imaging. 2018 doi: 10.23736/S1824-4785.18.03059-5. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 81.Chaloupka M, Herlemann A, D’Anastasi M, Cyran CC, Ilhan H, Gratzke C, Stief CG. 68Gallium-prostate-specific membrane antigen PET/computed tomography for primary and secondary staging in prostate cancer. Urol Clin North Am. 2017;44:557–563. doi: 10.1016/j.ucl.2017.07.004. [DOI] [PubMed] [Google Scholar]
- 82.Han S, Woo S, Kim YJ, Suh CH. Impact of 68Ga-PSMA PET on the management of patients with prostate cancer: a systematic review and meta-analysis. Eur Urol. 2018;74:179–190. doi: 10.1016/j.eururo.2018.03.030. [DOI] [PubMed] [Google Scholar]
- 83.Lenzo NP, Meyrick D, Turner JH. Review of Gallium-68 PSMA PET/CT imaging in the management of prostate cancer. Diagnostics (Basel) 2018;8:E16. doi: 10.3390/diagnostics8010016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zacho HD, Nielsen JB, Haberkorn U, Stenholt L, Petersen LJ. 68Ga-PSMA PET/CT for the detection of bone metastases in prostate cancer: a systematic review of the published literature. Clin Physiol Funct Imaging. 2017 doi: 10.1111/cpf.12480. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 85.Afriansyah A, Hamid A, Mochtar CA, Umbas R. Prostate specific antigen (PSA) kinetic as a prognostic factor in metastatic prostate cancer receiving androgen deprivation therapy: systematic review and meta-analysis. F1000Res. 2018;7:246. doi: 10.12688/f1000research.14026.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Mizokami A, Namiki M. Reconsideration of progression to CRPC during androgen deprivation therapy. J Steroid Biochem Mol Biol. 2015;145:164–171. doi: 10.1016/j.jsbmb.2014.03.015. [DOI] [PubMed] [Google Scholar]
- 87.Heidenreich A, Bastian PJ, Bellmunt J, Bolla M, Joniau S, van der Kwast T, Mason M, Matveev V, Wiegel T, Zattoni F, Mottet N. EAU guidelines on prostate cancer. Part II: treatment of advanced, relapsing, and castration-resistant prostate cancer. Eur Urol. 2014;65:467–479. doi: 10.1016/j.eururo.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 88.Rajpar S, Fizazi K. Bone targeted therapies in metastatic castration-resistant prostate cancer. Cancer J. 2013;19:66–70. doi: 10.1097/PPO.0b013e31827f123e. [DOI] [PubMed] [Google Scholar]
- 89.Osvaldo GF, Salvador MS, Zael SR, Nora SM. Radium-223 in metastatic hormone-sensitive high-grade prostate cancer: initial experience. Am J Nucl Med Mol Imaging. 2017;7:236–245. [PMC free article] [PubMed] [Google Scholar]
- 90.Ahmadzadehfar H, Albers P, Bockisch A, Boegemann M, Bohme C, Burchert W, Dietlein M, Drzezga A, Fabry U, Feldmann G, Heidenreich A, Heinzel A, Herrmann K, Heyll A, Hohling C, Kreuzer C, Laufer D, Mengel R, Mottaghy FM, Muller HW, Muller SC, Ost E, Rahbar K, Reifenhauser W, Schafers M, Schlenkhoff C, Schmidt M, Schmidt-Wolf I, Wildenhain C, Zimmer B, Essler M. Lutetium-177-PSMA radioligand therapy: consensus within the framework of GKV-funded care between the university hospitals in Aachen, Bonn, Dusseldorf, Essen, and Cologne and the MDK Nordrhein. Urologe A. 2018;57:709–713. doi: 10.1007/s00120-018-0642-2. [DOI] [PubMed] [Google Scholar]
- 91.Ahmadzadehfar H, Essler M. Predictive factors of response and overall survival in patients with castration-resistant metastatic prostate cancer undergoing 177Lu-PSMA therapy. J Nucl Med. 2018;59:1033–1034. doi: 10.2967/jnumed.118.209270. [DOI] [PubMed] [Google Scholar]
- 92.Ahmadzadehfar H, Schlolaut S, Fimmers R, Yordanova A, Hirzebruch S, Schlenkhoff C, Gaertner FC, Awang ZH, Hauser S, Essler M. Predictors of overall survival in metastatic castration-resistant prostate cancer patients receiving [177Lu] Lu-PSMA-617 radioligand therapy. Oncotarget. 2017;8:103108–103116. doi: 10.18632/oncotarget.21600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kurth J, Krause BJ, Schwarzenbock SM, Stegger L, Schafers M, Rahbar K. External radiation exposure, excretion, and effective halflife in 177Lu-PSMA-targeted therapies. EJNMMI Res. 2018;8:32. doi: 10.1186/s13550-018-0386-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Yadav MP, Ballal S, Tripathi M, Damle NA, Sahoo RK, Seth A, Bal C. Post-therapeutic dosimetry of 177Lu-DKFZ-PSMA-617 in the treatment of patients with metastatic castration-resistant prostate cancer. Nucl Med Commun. 2017;38:91–98. doi: 10.1097/MNM.0000000000000606. [DOI] [PubMed] [Google Scholar]
- 95.Rahbar K, Ahmadzadehfar H, Kratochwil C, Haberkorn U, Schafers M, Essler M, Baum RP, Kulkarni HR, Schmidt M, Drzezga A, Bartenstein P, Pfestroff A, Luster M, Lutzen U, Marx M, Prasad V, Brenner W, Heinzel A, Mottaghy FM, Ruf J, Meyer PT, Heuschkel M, Eveslage M, Bogemann M, Fendler WP, Krause BJ. German multicenter study investigating 177Lu-PSMA-617 radioligand therapy in advanced prostate cancer patients. J Nucl Med. 2017;58:85–90. doi: 10.2967/jnumed.116.183194. [DOI] [PubMed] [Google Scholar]
- 96.Kratochwil C, Bruchertseifer F, Giesel FL, Weis M, Verburg FA, Mottaghy F, Kopka K, Apostolidis C, Haberkorn U, Morgenstern A. 225Ac-PSMA-617 for PSMA-targeted alpharadiation therapy of metastatic castration-resistant prostate cancer. J Nucl Med. 2016;57:1941–1944. doi: 10.2967/jnumed.116.178673. [DOI] [PubMed] [Google Scholar]
- 97.Kojima S, Cuttler JM, Shimura N, Koga H, Murata A, Kawashima A. Present and future prospects of radiation therapy using alpha-emitting nuclides. Dose Response. 2018;16:1559325817747387. doi: 10.1177/1559325817747387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Morgenstern A, Apostolidis C, Kratochwil C, Sathekge M, Krolicki L, Bruchertseifer F. An overview of targeted alpha therapy with 225Actinium and 213Bismuth. Curr Radiopharm. 2018 doi: 10.2174/1874471011666180502104524. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Zalutsky MR, Pruszynski M. Astatine-211: production and availability. Curr Radiopharm. 2011;4:177–185. doi: 10.2174/1874471011104030177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Guerard F, Gestin JF, Brechbiel MW. Production of [211At] -astatinated radiopharmaceuticals and applications in targeted alphaparticle therapy. Cancer Biother Radiopharm. 2013;28:1–20. doi: 10.1089/cbr.2012.1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Larsen RH, Wieland BW, Zalutsky MR. Evaluation of an internal cyclotron target for the production of 211At via the 209Bi (α, 2n) 211At reaction. Appl Radiat Isot. 1996;47:135–143. doi: 10.1016/0969-8043(95)00285-5. [DOI] [PubMed] [Google Scholar]
- 102.Henriksen G, Messelt S, Olsen E, Larsen RH. Optimisation of cyclotron production parameters for the 209Bi (alpha, 2n) 211At reaction related to biomedical use of 211At. Appl Radiat Isot. 2001;54:839–844. doi: 10.1016/s0969-8043(00)00346-8. [DOI] [PubMed] [Google Scholar]
- 103.Crawford JR, Yang H, Kunz P, Wilbur DS, Schaffer P, Ruth TJ. Development of a preclinical 211Rn/211At generator system for targeted alpha therapy research with 211At. Nucl Med Biol. 2017;48:31–35. doi: 10.1016/j.nucmedbio.2017.01.011. [DOI] [PubMed] [Google Scholar]
- 104.Technical report series No. 468. Austria: International Atomic Energy Agency; 2009. Cyclotron produced radionuclides: physical characteristics and production methods. [Google Scholar]
- 105.Lindegren S, Back T, Jensen HJ. Drydistillation of astatine-211 from irradiated bismuth targets: a time-saving procedure with high recovery yields. Appl Radiat Isot. 2001;55:157–160. doi: 10.1016/s0969-8043(01)00044-6. [DOI] [PubMed] [Google Scholar]
- 106.Yordanov AT, Pozzi O, Carlin S, Akabani G, Wieland B, Zalutsky MR. Wet harvesting of no-carrier-added 211At from an irradiated 209Bi target for radiopharmaceutical applications. J Radioanal Nucl Chem. 2004;262:593. [Google Scholar]
- 107.Alliot C, Chérel M, Barbet J, Sauvage T, Montavon G. Extraction of astatine-211 in diisopropylether (DIPE) Radiochim Acta. 2009;97:161–165. [Google Scholar]
- 108.Engle JW. The Production of Ac-225. Curr Radiopharm. 2018 doi: 10.2174/1874471011666180418141357. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 109.Robertson AK, Ramogida CF, Schaffer P, Radchenko V. Development of 225Ac radiopharmaceuticals: TRIUMF perspectives and experiences. Curr Radiopharm. 2018 doi: 10.2174/1874471011666180416161908. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Scheinberg DA, McDevitt MR. Actinium-225 in targeted alpha-particle therapeutic applications. Curr Radiopharm. 2011;4:306–320. doi: 10.2174/1874471011104040306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Apostolidis C, Molinet R, Rasmussen G, Morgenstern A. Production of Ac-225 from Th-229 for targeted alpha therapy. Anal Chem. 2005;77:6288–6291. doi: 10.1021/ac0580114. [DOI] [PubMed] [Google Scholar]
- 112.Boll RA, Malkemus D, Mirzadeh S. Production of actinium-225 for alpha particle mediated radioimmunotherapy. Appl Radiat Isot. 2005;62:667–679. doi: 10.1016/j.apradiso.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 113.Griswold JR, Medvedev DG, Engle JW, Copping R, Fitzsimmons JM, Radchenko V, Cooley JC, Fassbender ME, Denton DL, Murphy KE, Owens AC, Birnbaum ER, John KD, Nortier FM, Stracener DW, Heilbronn LH, Mausner LF, Mirzadeh S. Large scale accelerator production of 225Ac: effective cross sections for 78-192 MeV protons incident on 232Th targets. Appl Radiat Isot. 2016;118:366–374. doi: 10.1016/j.apradiso.2016.09.026. [DOI] [PubMed] [Google Scholar]
- 114.Weidner JW, Mashnik SG, John KD, Ballard B, Birnbaum ER, Bitteker LJ, Couture A, Fassbender ME, Goff GS, Gritzo R, Hemez FM, Runde W, Ullmann JL, Wolfsberg LE, Nortier FM. 225Ac and 223Ra production via 800 MeV proton irradiation of natural thorium targets. Appl Radiat Isot. 2012;70:2590–2595. doi: 10.1016/j.apradiso.2012.07.003. [DOI] [PubMed] [Google Scholar]
- 115.Apostolidis C, Molinet R, McGinley J, Abbas K, Mollenbeck J, Morgenstern A. Cyclotron production of Ac-225 for targeted alpha therapy. Appl Radiat Isot. 2005;62:383–387. doi: 10.1016/j.apradiso.2004.06.013. [DOI] [PubMed] [Google Scholar]
- 116.Melville G, Allen BJ. Cyclotron and linac production of Ac-225. Appl Radiat Isot. 2009;67:549–555. doi: 10.1016/j.apradiso.2008.11.012. [DOI] [PubMed] [Google Scholar]
- 117.Morgenstern A, Bruchertseifer F, Apostolidis C. Bismuth-213 and actinium-225 -- generator performance and evolving therapeutic applications of two generator-derived alphaemitting radioisotopes. Curr Radiopharm. 2012;5:221–227. doi: 10.2174/1874471011205030221. [DOI] [PubMed] [Google Scholar]
- 118.Morgenstern A, Bruchertseifer F, Apostolidis C. Targeted alpha therapy with 213Bi. Curr Radiopharm. 2011;4:295–305. doi: 10.2174/1874471011104040295. [DOI] [PubMed] [Google Scholar]
- 119.Wu C, Brechbiel MW, Gansow OA. An improved generator for the production of 213Bi from 225Ac. Radiochim Acta. 1997;79:141–144. [Google Scholar]
- 120.Guseva LI. Production of high-purity α-emitting 225Ac and 213Bi radionuclides for use in nuclear medicine. Radiochemistry. 2013;55:317–323. [Google Scholar]
- 121.Ma D, McDevitt MR, Finn RD, Scheinberg DA. Breakthrough of 225Ac and its radionuclide daughters from an 225Ac/213Bi generator: development of new methods, quantitative characterization, and implications for clinical use. Appl Radiat Isot. 2001;55:667–678. doi: 10.1016/s0969-8043(01)00062-8. [DOI] [PubMed] [Google Scholar]
- 122.McDevitt MR, Finn RD, Sgouros G, Ma D, Scheinberg DA. An 225Ac/213Bi generator system for therapeutic clinical applications: construction and operation. Appl Radiat Isot. 1999;50:895–904. doi: 10.1016/s0969-8043(98)00151-1. [DOI] [PubMed] [Google Scholar]
- 123.Price EW, Orvig C. Matching chelators to radiometals for radiopharmaceuticals. Chem Soc Rev. 2014;43:260–290. doi: 10.1039/c3cs60304k. [DOI] [PubMed] [Google Scholar]
- 124.Zeglis BM, Houghton JL, Evans MJ, Viola-Villegas N, Lewis JS. Underscoring the influence of inorganic chemistry on nuclear imaging with radiometals. Inorg Chem. 2014;53:1880–1899. doi: 10.1021/ic401607z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Ramogida CF, Orvig C. Tumour targeting with radiometals for diagnosis and therapy. Chem Commun (Camb) 2013;49:4720–4739. doi: 10.1039/c3cc41554f. [DOI] [PubMed] [Google Scholar]
- 126.Cutler CS, Hennkens HM, Sisay N, Huclier-Markai S, Jurisson SS. Radiometals for combined imaging and therapy. Chem Rev. 2013;113:858–883. doi: 10.1021/cr3003104. [DOI] [PubMed] [Google Scholar]
- 127.Kiess AP, Minn I, Vaidyanathan G, Hobbs RF, Josefsson A, Shen C, Brummet M, Chen Y, Choi J, Koumarianou E, Baidoo K, Brechbiel MW, Mease RC, Sgouros G, Zalutsky MR, Pomper MG. (2S)-2-(3-(1-carboxy-5-(4-211Atastatobenzamido) pentyl)ureido)-pentanedioic acid for PSMA-targeted alpha-particle radiopharmaceutical therapy. J Nucl Med. 2016;57:1569–1575. doi: 10.2967/jnumed.116.174300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Kelly JM, Amor-Coarasa A, Nikolopoulou A, Kim D, Williams C Jr, Vallabhajosula S, Babich JW. Assessment of PSMA targeting ligands bearing novel chelates with application to theranostics: stability and complexation kinetics of 68Ga3+, 111In3+, 177Lu3+ and 225Ac3+ . Nucl Med Biol. 2017;55:38–46. doi: 10.1016/j.nucmedbio.2017.10.001. [DOI] [PubMed] [Google Scholar]
- 129.Kratochwil C, Schmidt K, Afshar-Oromieh A, Bruchertseifer F, Rathke H, Morgenstern A, Haberkorn U, Giesel FL. Targeted alpha therapy of mCRPC: dosimetry estimate of 213Bismuth-PSMA-617. Eur J Nucl Med Mol Imaging. 2018;45:31–37. doi: 10.1007/s00259-017-3817-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Kratochwil C, Bruchertseifer F, Rathke H, Bronzel M, Apostolidis C, Weichert W, Haberkorn U, Giesel FL, Morgenstern A. Targeted alpha-therapy of metastatic castration-resistant prostate cancer with 225Ac-PSMA-617: dosimetry estimate and empiric dose finding. J Nucl Med. 2017;58:1624–1631. doi: 10.2967/jnumed.117.191395. [DOI] [PubMed] [Google Scholar]
- 131.Kratochwil C, Bruchertseifer F, Rathke H, Hohenfellner M, Giesel FL, Haberkorn U, Morgenstern A. Targeted alpha-therapy of metastatic castration-resistant prostate cancer with 225Ac-PSMA-617: swimmer-plot analysis suggests efficacy regarding duration of tumor control. J Nucl Med. 2018;59:795–802. doi: 10.2967/jnumed.117.203539. [DOI] [PubMed] [Google Scholar]
- 132.Nonnekens J, Chatalic KL, Molkenboer-Kuenen JD, Beerens CE, Bruchertseifer F, Morgenstern A, Veldhoven-Zweistra J, Schottelius M, Wester HJ, van Gent DC, van Weerden WM, Boerman OC, de Jong M, Heskamp S. 213Bi-labeled prostate-specific membrane antigen-targeting agents induce DNA double-strand breaks in prostate cancer xenografts. Cancer Biother Radiopharm. 2017;32:67–73. doi: 10.1089/cbr.2016.2155. [DOI] [PubMed] [Google Scholar]
- 133.Sathekge M, Knoesen O, Meckel M, Modiselle M, Vorster M, Marx S. 213Bi-PSMA-617 targeted alpha-radionuclide therapy in metastatic castration-resistant prostate cancer. Eur J Nucl Med Mol Imaging. 2017;44:1099–1100. doi: 10.1007/s00259-017-3657-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Deshmukh MV, Voll G, Kuhlewein A, Macke H, Schmitt J, Kessler H, Gemmecker G. NMR studies reveal structural differences between the gallium and yttrium complexes of DOTA-DPhe1-Tyr3-octreotide. J Med Chem. 2005;48:1506–1514. doi: 10.1021/jm0496335. [DOI] [PubMed] [Google Scholar]
- 135.Gaertner FC, Halabi K, Ahmadzadehfar H, Kurpig S, Eppard E, Kotsikopoulos C, Liakos N, Bundschuh RA, Strunk H, Essler M. Uptake of PSMA-ligands in normal tissues is dependent on tumor load in patients with prostate cancer. Oncotarget. 2017;8:55094–55103. doi: 10.18632/oncotarget.19049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Kabasakal L, AbuQbeitah M, Aygun A, Yeyin N, Ocak M, Demirci E, Toklu T. Pre-therapeutic dosimetry of normal organs and tissues of 177Lu-PSMA-617 prostate-specific membrane antigen (PSMA) inhibitor in patients with castration-resistant prostate cancer. Eur J Nucl Med Mol Imaging. 2015;42:1976–1983. doi: 10.1007/s00259-015-3125-3. [DOI] [PubMed] [Google Scholar]
- 137.Vallabhajosula S, Goldsmith SJ, Kostakoglu L, Milowsky MI, Nanus DM, Bander NH. Radioimmunotherapy of prostate cancer using 90Y- and 177Lu-labeled J591 monoclonal antibodies: effect of multiple treatments on myelotoxicity. Clin Cancer Res. 2005;11:7195s–7200s. doi: 10.1158/1078-0432.CCR-1004-0023. [DOI] [PubMed] [Google Scholar]
- 138.Vallabhajosula S, Kuji I, Hamacher KA, Konishi S, Kostakoglu L, Kothari PA, Milowski MI, Nanus DM, Bander NH, Goldsmith SJ. Pharmacokinetics and biodistribution of 111Inand 177Lu-labeled J591 antibody specific for prostate-specific membrane antigen: prediction of 90Y-J591 radiation dosimetry based on 111In or 117Lu? J Nucl Med. 2005;46:634–641. [PubMed] [Google Scholar]
- 139.Vallabhajosula S, Nikolopoulou A, Jhanwar YS, Kaur G, Tagawa ST, Nanus DM, Bander NH, Goldsmith SJ. Radioimmunotherapy of metastatic prostate cancer with 117Lu-DOTAhuJ591 anti prostate specific membrane antigen specific monoclonal antibody. Curr Radiopharm. 2016;9:44–53. doi: 10.2174/1874471008666150313114005. [DOI] [PubMed] [Google Scholar]
- 140.Bandekar A, Zhu C, Jindal R, Bruchertseifer F, Morgenstern A, Sofou S. Anti-prostatespecific membrane antigen liposomes loaded with 225Ac for potential targeted antivascular alpha-particle therapy of cancer. J Nucl Med. 2014;55:107–114. doi: 10.2967/jnumed.113.125476. [DOI] [PubMed] [Google Scholar]


