Abstract
Objectives:
Donepezil inhibits the acetylcholine degradation molecule acetylcholinesterase (AChE). Clinical studies reported that Alzheimer’s disease (AD) patients with hip fractures had improved bone quality and better fracture healing if they were treated with AD medication donepezil. We asked whether mesenchymal stroma cells (MSC) from an osteoporosis sheep model treated with donepezil increased their proliferation rate and mRNA expression.
Methods:
Sheep were divided into 4 groups: a) untreated control group, b) sheep with bilateral ovariectomy (OVX), c) sheep with OVX and malnutrition, and d) sheep with OVX, malnutrition, and application of corticosteroid. After 8 months MSC were isolated of iliac crest biopsy, treated with donepezil, and AChE activity, proliferation rate, and mRNA expression were analyzed.
Results:
Application of donepezil resulted in a significant decrease of AChE activity. Inhibition of AChE did not lead to a significant increase in proliferation. Expression of the osteogenic marker osteocalcin was not regulated by donepezil while the mRNA concentration of collagen was increased.
Conclusion:
AChE inhibition via donepezil resulted in an increased synthesis of osteoid which consists mainly of collagen. Thus, we suppose that increased acetylcholine levels through AChE inhibition do not support MSC proliferation but osteogenic activity probably combined with osteogenic differentiation.
Keywords: Acetylcholinesterase, Sheep, MSC, Collagen, AChEI
Introduction
One reason for the cognitive dysfunction of Alzheimer’s Disease (AD) patients is a loss of cholinergic neurons in defined brain regions and subsequently a decrease of acetylcholine (ACh) release and transmission in this regions[1]. ACh is a neurotransmitter that is well known in the central and peripheral nervous system as well as a signaling molecule in many non-neuronal cells e.g. osteoblasts, endothelial and immune cells[2]. ACh is enzymatically degraded by its high affinity enzyme acetylcholinesterase (AChE) as well as by the slower, less specific enzyme butyrylcholinesterase (BChE). Inhibition of the cholinesterases slows down the disease progression of AD and leads to an increase in cholinergic signaling. Therefore one therapeutic treatment of AD is the administration of AChE inhibitors (AChEI) e.g. donepezil[3,4]. In a case-control study of 80 AD patients with hip fracture and 2178 AD patients without hip fracture it was shown that the usage of donepezil was associated with a lower hip fracture risk compared to non-usage (0.39 adjusted odds ratio with confidence interval of 95% and p<0.002)[5]. Based on this, Tamimi et al.[6] reported in a second nested case-control study including 1190 cases and 4760 controls that the usage of AChEIs prior to osteoporotic fractures was associated with a reduction in the fracture risk. In line with these studies, Eimar et al. observed in a retrospective cohort study of 46 female AD patients that AChEI users had better bone-quality, better radiographic hip fracture union, and less healing complications[7]. Also the mortality of AD patients after hip fracture decreased in AChEI users[8]. To verify the promising result of AChEI usage, Eimar et al.[9] treated mice with donepezil and reported that they showed an increase in relative bone volume, bone mineral density and trabecular number compared to untreated controls. Therefore, the application of donepezil might also be helpful to increase bone volume, mineral density and trabecular number in osteoporotic bone. Osteoporosis is a systemic disease that is characterized by decreased bone mineral density, reduced bone volume, trabecular number, microarchitectural deteriorations, and accelerated fragility[10]. In 2009, every 4th woman over the age of 50 was suffering from osteoporosis in Germany[11]. The incidence is increasing with age and therefore osteoporosis is listed as one of the global health burdens that concern a growing number of people[12]. It has also been reported that the mesenchymal stem cells/mesenchymal stromal cells (MSC) from osteoporotic patients already show alterations. MSC are the progenitors of the bone forming cells, the osteoblasts. MSC of patients with osteoporosis (oMSC) are reduced in their proliferation, migration and differentiation rate[13]. They synthesize only 50% collagen type 1 and 35-40% tumor growth factor beta (TGFβ) compared to MSC of bone healthy donors (hMSC)[14]. Application of bone morphogenetic protein-2 (BMP-2) stimulates hMSC to increase the osteogenic transcription factor runt-related transcription factor 2 (RUNX2) while oMSC do not change their RUNX2 expression[15]. oMSC have a higher capacity to differentiate into adipocytes than into osteoblasts[16]. oMSC show an overexpression of the osteogenic inhibitor sclerostin[17] which is known as sensor of bone biomechanical stress. In case of tensil strain oMSC do not increase the proliferation rate as strong as hMSC[18].
In the present study we isolated MSC of an animal model with osteoporosis induction through ovariectomy and special diet in sheep[19,20] and treated the cells in vitro with donepezil. Subsequently we analyzed their cellular proliferation capacity as well as the expression of typical osteoblast markers.
Materials and methods
Sheep osteoporosis model
In the present study we isolated mesenchymal stroma cells (MSC) of 20 female skeletally mature merino sheep 8 months after induction of osteoporosis. The animal model as well as the evidence for the osteoporotic phenotype of the source animals was recently described[19,20]. In brief, all animal experiments were conducted in accordance with the German animal welfare law and had been approved by the regional animal council (V54-19c20/15-F31/36). The animals were divided into 4 groups with an average age of 5.5 years: a) non-treated control animals (control), b) sheep with bilateral ovariectomy (OVX), c) sheep with bilateral ovariectomy and special diet with reduced calcium and Vitamin D levels (OVXD, S61809-S010, SNIFF Spezialdiäten GmbH, Germany), and d) animals with bilateral ovariectomy, special diet as above, and a subcutaneous application of a glucocorticoid (OVXDS, 320 mg methylprednisolone acetate, Medrate®, Pfizer, Germany) every 14 days. For ovariectomy the sheep were anesthetized with propofole (2 mg/kg body weight, Fresenius Kabi, Germany) and fentanyl (2 µg/mg body weight, Hameln Pharmaceuticals GmbH, Germany). Additionally animals received a prophylactic administration of penicillin (Vercin® RS, 0.1 mL/kg body weight, Albrecht GmbH, Germany) and buprenophinhydrochlorid for analgesia (Temgesic®, 0.01 mg/kg body weight, RB Pharmaceuticals GmbH, Germany). Sheep were placed in supine position, shaved, covered sterilely. The skin, linea alba and peritoneum were incised and a ligature was performed between fallopian tubes and ovaries. Then the ovaries were removed. Skin and muscle were sutured and the sheep were allowed to regain conscience. Sheep were regularly assessed by veterinaries. Animals of control and OVX groups were released to the pasture and animals of OVXD and OVXDS groups lived in small groups in outside barns of the animal house where they got the special diet (start 2 weeks after ovariectomy). Also two weeks after ovariectomy the administration of methylprednisolone started. After 8 months animals were again anesthetized and then euthanized with pentobarbital (50 mg/kg body weight, Anestesal®, Pfizer, Mexico). Afterwards the skin was incised at the iliac crest and a biopsy of 1 cm in diameter and approximately 2 cm length was taken and transferred to collection medium (F12-K, Gibco, Waltham, MA, USA with 10% fetal bovine serum, FBS, PAN Biotech, Aidenbach, Germany, and 0.2% Gentamicin/Amphothericin, Gibco).
Cell culture
After transferring the biopsies into the laboratory they were shredded, put into petri dishes with growing medium (Dulbecco’s Modified Eagle Medium, DMEM, PAN Biotech) with 10% FBS and 0.2% Gentamicin/Amphotericin and cultured in an incubator with an humidified atmosphere of 5% CO2 and 37°C. Medium was changed once a week.
For the experiments in growing medium cells of passage 3 were used, seeded in a density of 15 000 cells/cm2 and the FBS concentration of medium was reduced to 1%.
For one set of experiments we seeded 20 000 cells/cm2 and changed after 24 h the growing medium into osteogenic differentiation medium which consisted of DMEM with 5% FBS, 10-7 M dexamethasone (Sigma, St. Louis, Missouri, USA), 5x10-5 M sodium-L-ascorbate (Sigma), 10-2 M β-glycero phosphate hydrate (Sigma), 5x10-8 M vitamin D3 (Sigma), 1.5x10-3 M calcium chloride (PromoCell, Heidelberg, Germany), and 0.2% Gentamicin/Amphotericin. Additionally we added 15.4 nM of donepezil to the experimental groups and changed the medium after three days. The cells were harvested at day 6 and used for real-time RT-PCR and Picro-Sirius Red staining.
AChE activity assay
The fluorometric AChE activity assay (Fluorometric-Red, Abcam, Cambridge, UK) quantifies the choline production from hydrolysis of acetylcholine by AChE as well as BChE. In our case we used 50 µL of supernatant of the homogenized cells as sample which was added to 50 µL of the ACh probe mixture (kit component), shaken for 30 s, incubated for 29 min in darkness and shaken again for 30 s. Afterwards the fluorescence was measured at Ex/Em=530/590 nm with the plate reader (Synergy HT, BioTek, Winooski, VT, USA).
BrdU assay
To quantify the cell proliferation we used a colorimetric 5-bromo-2’desoxyuridine (BrdU) assay (Roche, Mannheim, Germany) where the determination of proliferation is based on the incorporation of BrdU during DNA synthesis. Therefore 4,800 MSC per well were seeded in a 96 well plate and donepezil was applied. 6 h after donepezil application, 10 µM of BrdU solution was added into the cell culture medium. The cells were again incubated for 6 h and then the proliferation test was performed as described above. Thus, every cell culture was incubated for 12 h with donepezil. This incubation time showed the best results in a pilot series (data not shown). For the proliferation test the cell culture medium was removed and the cells were fixed by addition of 200 µL FixDenat (kit component) for 30 min at room temperature. The solution was removed. Then, peroxidase conjugated BrdU antibody was added in a dilution of 1:100 in antibody dilution solution (both, kit components). After 90 min the cells were carefully washed with washing solution (kit component) and 100 µL of substrate solution (kit component) were added for 30 min. The reaction was stopped with 25 µL of 1 M H2SO4. Afterwards the absorption of the plate was measured in the plate reader at 450 nm with a reference of 690 nm.
Determination of donepezil concentration
Before the experiments were started we determined the most effective concentration (EC) of AChE inhibition by donepezil (Sigma-Aldrich, Taufkirchen, Germany) using the AChE Assay Kit, a sequential series of donepezil (5 µM, 1 µM, 500 nM, 100 nM, 50 nM, 10 nM, 5 nM, 1 nM, 500 pM, 100 pM), and 4,800 MSC per well (96 well plate). After donepezil treatment for 24 h, cells were homogenized and 50 µL of the supernatant was incubated with 50 µL AChE assay mixture according to the protocol of the manufacturer (see above). After incubation in the darkness, samples were measured and the EC values were determined because of the direct relation of measured fluorescence and AChE activity (Figure 1A, EC30 11.1, EC50 24.6, EC70 54.7). The experiment was repeated with a 1:2 dilution of the cells to verify the reproducibility and linearity of the values (Figure 1B, EC30 19.7, EC50 38, EC70 73.5). The means were calculated as final EC values: EC30 15.4, EC50 31.3, EC70 64.1. To determine which EC value was most suitable to stimulate proliferation the BrdU assay was conducted as described above (Figure 1C). Since the highest value could be measured after using EC30 we decided to use 15.4 nM donepezil for the following experiments.
Figure 1.
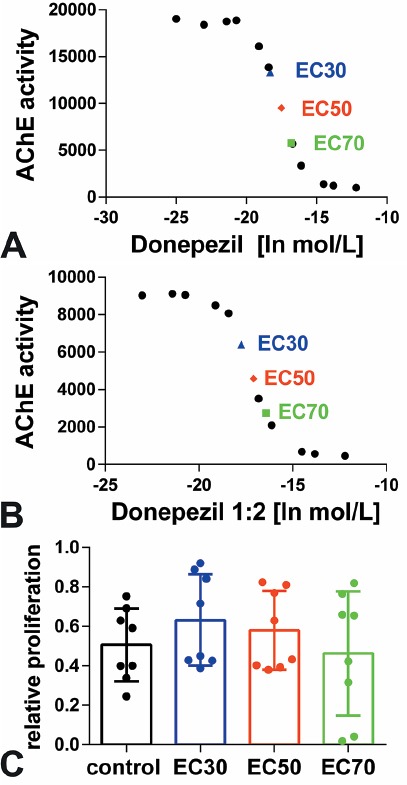
Donepezil concentration. Determination of the effective concentration (EC) of AChE inhibition by (A) undiluted and (B) 1:2 diluted donepezil in a sequential series. (C) The average concentrations of EC30, EC50, and EC70 were used for a pilot series BrdU proliferation assay where the EC30 donepezil concentration was most promising.
Real-time reverse transcriptase polymerase chain reaction (real-time RT-PCR)
Also for RNA isolation the cells of the experimental group were treated with 15.4 nM (EC30) donepezil for 12 h. Total RNA was isolated with the RNeasy Mini Kit (peqlab, VWR, Darmstadt, Germany) according to the manufacturer’s protocol. In brief, after washing the cells, they were homogenized by 600 µL of Lysis Buffer (kit component). The genomic DNA was removed by loading the solution on DNA removing column and subsequent centrifugation. Then, the through flow was added to Perfect Bind RNA column (kit component) and carefully washed. Finally RNA was eluted with RNase-free water and the RNA concentration was determined by NanoDrop spectrometry (ND-1000, Thermo Fisher Scientific, Waltham, MA, USA). Afterwards cDNA was synthesized using QuantiTect Kit (Qiagen, Hilden, Germany) which begins with elimination of genomic DNA by incubation with Wipeout-Buffer (Kit component). Then, reverse transcriptase and buffer (both Kit components) were added and the solution was incubated at 42° for 30 min for cDNA synthesis. Reverse transcriptase was inactivated by heating to 95°C for 3 min. Primers for β2-microglobulin (B2M), bglap (osteocalcin=OCN), collagen type I alpha 1 (Col1a1) were designed using the Primer Blast (www.ncbi.nlm.nih.gov/tools/primer-blast/) software and RefSeq gene database (www.ncbi.nlm.nih.gov/refseq/) and finally the primer generation was ordered by MWG Eurofins (Ebersberg, Germany, Table 1). Since for sheep AChE and BChE no detailed sequence was found in the databank, standard primers for a long DNA fragment were established. The PCR product was sequenced (MWG Eurofins) and using these new sequences primers for PCR were designed by Primer-BLAST and RefSeq gene database. The efficiency of primers was controlled by serial dilutions and standard curve. Purity of the PCR product was evaluated by melting curve and controls were performed with templates omitting reverse transcriptase during cDNA synthesis. Real-time RT-PCR was run in a LightCycler 2.0 (Roche, Basel, Switzerland) using the QuantiFast SYBR Green (Qiagen) and the following program: 5 min 95°C initial denaturation, 40 cycles with 10 s 95°C for denaturation, 30s 60°C for annealing, subsequent melting curve, and final cool down to 4°C. The values were calculated according to the ΔΔCT method[21] and B2M was used as housekeeping gene.
Table 1.
Primer pairs used for real-time RT-PCR.
| Primer | Sequence | Length [bph] | Effi | GenBank ID (accession) |
|---|---|---|---|---|
| AChEa forb revc | CCCTCGCTCAACTACACCAT CGAAGTTGGCCCAGTATCTC |
70 | 1.97 | Sequence analysis |
| BChEd for rev | AGGTGCCCTTGGATTCTTAGC TAGACCCACATTCCCTGGTG |
61 | 2.0 | Sequence analysis |
| B2Me for rev | CCAGAAGATGGAAAGCCAAA AGCGTGGGACAGAAGCTAGA |
159 | 1.98 | NM_001009284.1 |
| Col1a1f for rev | CCAGTCACCTGCGTACAGAACG GCCAGTGTCTCCTTTGGGTCC |
246 | 1.9 | XM_012185918.1 |
| OCNg for rev | CAGCGAGGTGGTGAAGAGAC GCTCATCACAGTCAGGGTTG |
122 | 1.86 | NM_00104009.1 |
AChE: acetylcholinesterase,
for: forward,
rev: reverse,
BChE: butyrylcholinesterase,
B2M: beta-2-microglobulin, housekeeping gene,
Col1a1: Collagen 1a1,
OCN: bglap, osteocalcin,
bp: base pairs,
Eff: efficiency.
Picro-Sirius Red staining and histomorphometrical analysis
Collagen 1 and 3 can be visualized by Picro-Sirius Red staining. Therefor cells were fixed with 4% phosphate buffered paraformaldehyde for 10 min on ice. After washing with water Picro-Sirius Red stain (Abcam, Cambridge, UK) was added for 60 min at room temperature. Cells were washed in acetic acid solution (kit component), incubated 2x 1 min in 100% ethanol and coverslipped with Depex (Serva, Heidelberg, Germany). Staining was observed with a light microscope (DM5500B, Leica, Wetzlar, Germany) equipped with a digital camera (DCF 7000 T, Leica) and polarization filter (Leica). Per well 5 randomized pictures were taken (each 1.28 mm x 0.96 mm) and converted into black and white. The threshold was set and the collagen type 1 covered area was measured by ImageJ software[22].
Statistical tests
The paired two-sided t-test was used to determine effects of donepezil on AChE, proliferation (BrdU), histomorphometry (Picro-Sirius Red staining) as well as means of real-time RT-PCR of BChE. Independent means of the remaining real-time RT-PCR were compared by the non-parametric Wilcoxon signed-rank test as well as by Kruskal-Wallis-Test followed by the non-parametric Mann-Whitney-Test. Differences of p≤0.05 were considered as statistically significant. The graphs were generated with GraphPad Prism Version 6.02 and p≤0.05 was labeled by *, p≤0.01 by **, and p≤0.001 by ***.
Results
AChE activity
Donepezil is a well-known inhibitor of AChE and should down-regulate the AChE activity. In the present study we added a concentration of donepezil (15.4 nM) that we determined as the EC30 value as described above (Figure 1). After an incubation time of 12 h the cells were harvested and the AChE activity measured. MSC of control sheep showed a significant down-regulation of AChE activity in the treatment group (donepezil) compared to control cells (p<0.05, Figure 2A) whereas MSC of sheep with OVX showed no significant decrease in AChE activity compared to cells without donepezil treatment (Figure 2B). MSC of the OD group were significantly down-regulated after donepezil application (p<0.001, Figure 2C) and also MSC of ODS sheep showed a significant decline compared to cells without donepezil treatment (p<0.05, Figure 2D). Finally we summed up all sheep and found a significant decrease in the donepezil treated MSC compared to controls without donepezil application (p<0.001, Figure 2E).
Figure 2.
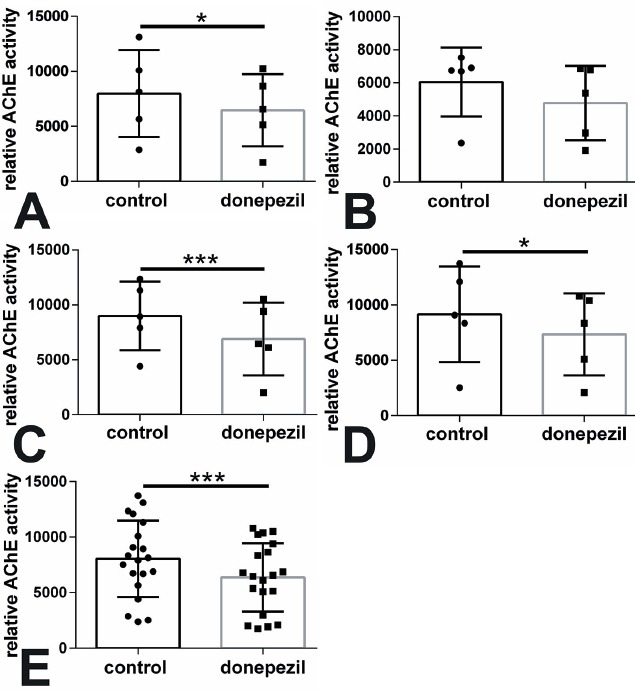
Relative AChE activity was down-regulated by donepezil treatment (concentration of EC30=15.4 nM) in MSC of the (A) control, (B) OVX, (C) OD, and (D) ODS group in comparison to control cells. In (E) all sheep groups were summed up. *p<0.05, ***p<0.001.
Proliferation
The BrdU assay was used to determine the proliferation of MSC after application of Donepezil. MSC of the control, OVX, and ODS group showed no difference in proliferation after application of donepezil (Figure 3A, B, D). Only MSC of the OD group were significantly reduced in their proliferation capacity compared to MSC without donepezil treatment (p<0.05, Figure 3C). Summing up all sheep did not result in significant changes (Figure 3E). Moreover light microscopical evaluation did not show remarkable alterations in cell growth (Figure 4).
Figure 3.
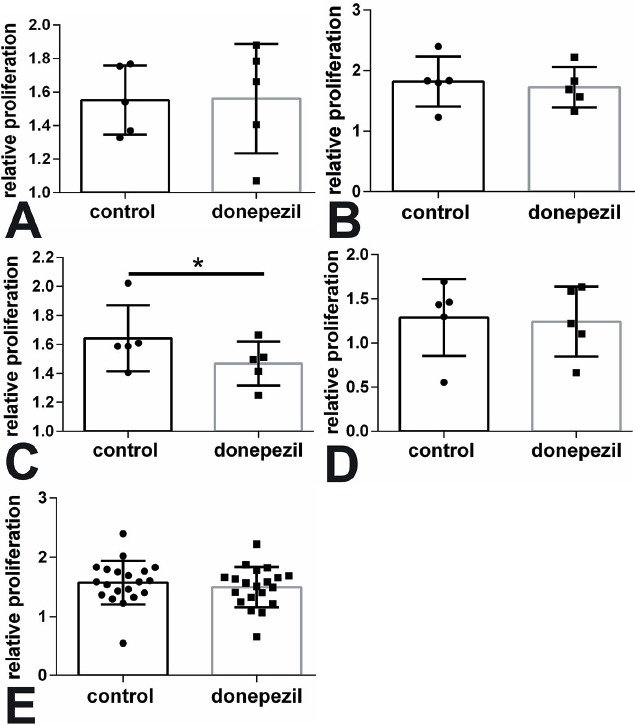
Proliferation was mainly not altered by donepezil treatment. MSC of (A) control, (B) OVX, (C) OD, and (D) ODS groups were analyzed by BrdU and summed up in (E). *p<0.05.
Figure 4.
No significant changes were determined by light microscopy of (A-B) control, (C-D) OVX, (E-F) OD, and (G-H) ODS MSC after application of donepezil (B, D, F, H) compared to untreated cells (A, C, E, G). Bar: 100 µm.
Gene expression
Real-time RT-PCR was used to determine changes in gene expression. Using gene-specific primers for AChE and BChE we could not determine significant differences between MSC that were treated with donepezil and the untreated control cells (Figure 5). In addition we analyzed the expression of osteocalcin in growing as well as in osteogenic differentiation medium. For both conditions no significant alterations were found (Figure 6A-E). In growing medium we only found a significant increase in collagen 1α1 mRNA expression when all sheep were summed up (p=0.033, Figure 6E). MSC of groups OVX, OD, ODS, and control did not result in a significant regulation of gene expression (Figure 6A-D). In osteogenic differentiation medium we measured a significant increase in collagen 1α1 expression in cells of the ODS group treated with donepezil compared to untreated cells (p=0.029, Figure 7A).
Figure 5.
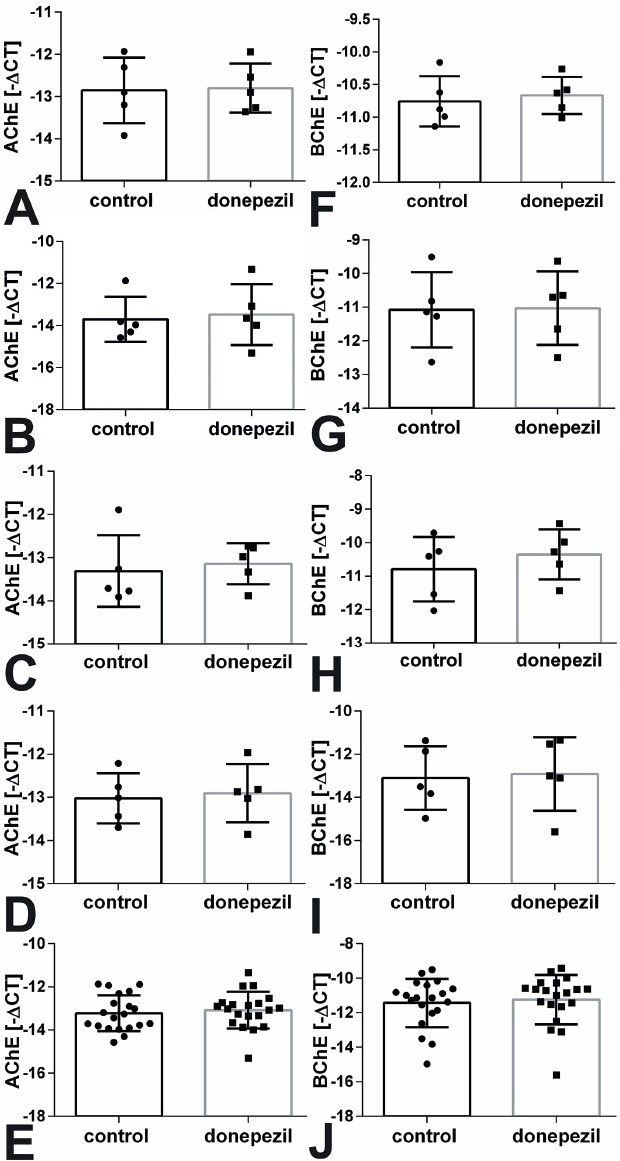
AChE (A-E) and BChE (F-J) mRNA expression was not changed after treatment with donepezil compared to untreated MSC of (A, F) control, (B, G) OVX, (C, H) OD, and (D, I) ODS groups by real-time RT-PCR. (E, J) demonstrate the sum of all animals. CT: cycle threshold.
Figure 6.
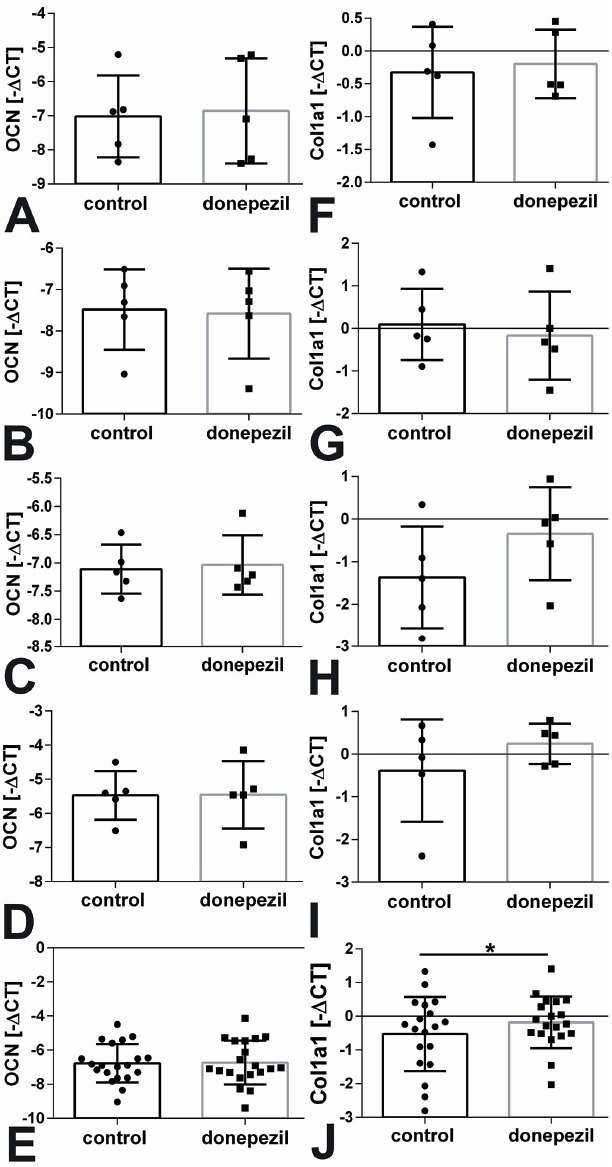
RNA expression of osteocalcin (OCN, A-E) was not regulated by donepezil treatment while Col1a1 (F-J) was significantly increased. CT: cycle threshold. A, F: MSC of control, B, G: of OVX, C, H: of OD, and D, I of ODS group. (E, J) show the summed results of all animals. *p<0.05.
Figure 7.
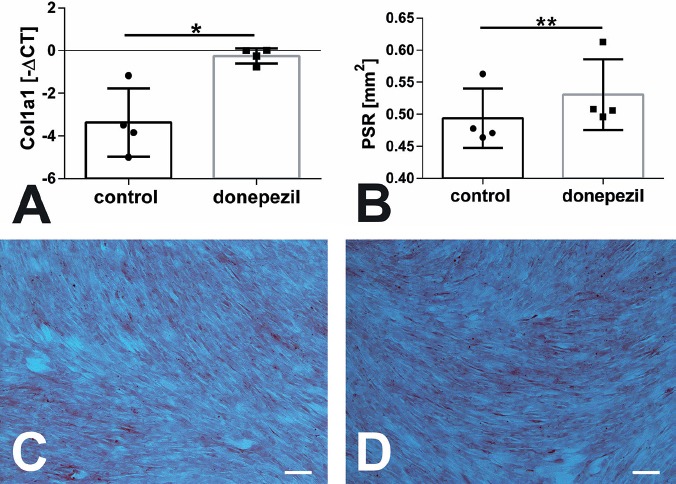
Application of donepezil in osteogenic differentiation medium resulted in a significant increase in Collagen 1a1 mRNA expression in ODS group (A, p=0.029). On protein level Collagen type 1 was determined by Picro-Sirius Red (PSR) staining followed by histomorphometrical measurement which determined a significant increase in the OVX group compared to untreated cells (B, p=0.009). (C, D) showed images of PSR staining of MSC from OVX group with (D) and without donepezil application (C). Scale bar: 100 µm.
Collagen type 1 staining and histomorphometry
Picro-Sirius Red staining was used for visualization of collagen type 1 by light microscopy equipped with a polarization filter. A significant increase of collagen type 1 was measured in cell of OVX sheep group cultured in proliferation medium treated with donepezil compared to cells without donepezil treatment (p=0.009, Figure 7B). No significant differences were detected in cells in the osteogenic differentiation medium as well as in cells of the other sheep groups in proliferation medium (Figure 7C-D).
Discussion
Donepezil is a derivate of piperidine that was specially designed for systemic AD treatment. Patients with AD usually take in a once-daily dosage of 5 to 10 mg of donepezil (donepezil hydrochloride, E2020) that have a half-life of approximately 70 h in human plasma[23]. In the present study we used the EC30 concentration of 15.4 nM (6.68 µg/L) donepezil which was established in our pilot series as described in the material and methods section. The EC30 showed the most promising effect regarding proliferation of ovine MSC in vitro by means of BrdU. Sugimoto et al.[24] used a lower concentration of IC50 at 5.7±0.2 nM for treatment of rat brain homogenates. Sato et al.[25] utilized a much higher concentration of 2 mg/kg body weight in an animal model for analyzing the effect of donepezil on osteoclasts. Thus, we feel that our donepezil concentration of 15.4 nM might be suitable since it is situated in between the generally used range of donepezil.
The relative AChE activity of MSC treated with donepezil was significantly reduced in most of the treatment groups. Only the OVX group showed no significant results (p=0.1). The reason for this is highly speculative. All MSC were identically isolated of iliac crest biopsies of a recently established ovine osteoporosis model[19,20]. The bone mineral density (BMD) by means of DXA-scan of proximal femur was used to calculate a clinically relevant T-score: 0.3744 for the control group, 0.9148 for OVX, 0.3412 for OD, and -3.3127 for ODS[20]. Thus, regarding the BMD only the ODS group showed an osteoporosis phenotype. Therefore, we expected to measure the highest effects on inhibition of AChE activity in the ODS group and afterwards a graded series of ODS > OD > OVX > control sheep. However, AChE activity was significantly down-regulated in all sheep groups except OVX. Interestingly, El Khassawna et al.[19] determined a higher relative expression of carbonic anhydrase 2 (CA2) in iliac crest biopsies of the ovine OVX group. CA2 is one of the characteristic enzymes of the bone breaking osteoclasts. Thus, we suggest that more osteoclasts or osteoclasts with a higher activity of degrading enzymes are localized in the samples of the OVX group and this might have impact on the MSC culture. Osteoclasts, osteoblasts as well as osteocytes are cross linked through several mechanisms e.g. the RANK/RANKL/OPG axis and through these the activity of the cells is regulated[26]. Thus, the osteoclast might also influence the MSC and the ability to isolate and culture them. The MSC of iliac crest biopsies were isolated according to a protocol established for human MSC from bone debris that were collected during routine clinical fracture treatment[27].
Native MSC have a higher proliferation capacity than osteogenic differentiated osteoblasts. After application of donepezil we measured no increase in proliferation in any animal group. In the OD group we found a decrease of proliferation after donepezil treatment. In an in vivo study with donepezil application during mice bone development an increase in the RANKL/OPG ratio was found but no change in number of osteoblasts and bone formation rate[9]. It seems that the number and activity of osteoclasts was down-regulated by the increase in RANKL/OPG ratio via osteoblast-osteoclast cross talk. And indeed, Eimar et al.[9] observed a reduced osteoclast number and therefore an increased bone mass, bone volume, and trabecular volume in the mouse model with donepezil treatment. Sato et al.[25] demonstrated in vitro as well as in vivo in a mouse model that donepezil treatment inhibited the differentiation of osteoclast but not proliferation of bone marrow macrophages, the precursors of osteoclasts. Thus, inhibition of AChE seems to stimulate differentiation but not proliferation of bone cells. However, since inhibition of AChE leads to an increase in ACh we were astonished that we did not find an up-regulation in proliferation in our study, because Sato et al.[28,29] showed, that administration of ACh, carbachol (ACh analogon), and nicotine (ACh agonist) to primary murine osteoblasts as well as to the murine osteoblast cell line MC3T3 resulted in (i) an increase in proliferation by means of BrdU assay and (ii) increase in expression of cyclin D1 and (iii) a reduced ALP activity. Additionally, Liu et al.[30] reported that metacholine (ACh agonist for muscarinic acetylcholine receptors) also stimulated proliferation of human osteosarcoma cell line HOS by acetylcholine. Rothem et al.[31] showed enhanced proliferation in the osteosarcoma cell line MG-63 by stimulation of nicotinic acetylcholine receptors with nicotine and Walker et al.[32] verified the result for application of ACh. These contrary effects of application of ACh and inhibition of AChE might be explained by the non-enzymatic functions of AChE. Recently, a study indicated that AChE stimulated osteoblast differentiation by RUNX2 and Wnt3a signaling and can be blocked by the Wnt/β-catenin inhibitor Dickkopf-1 (DKK1)[33]. Furthermore, several data indicate that limb differentiation is also regulated by non-enzymatic as well as by enzymatic AChE effects[34,35]. Thus we suppose that AChE of ovine MSC is involved in non-enzymatic functions and therefore does not enhance the proliferation.
To learn more about the effects of donepezil on ovine MSC differentiation we used real-time RT-PCR of osteoblast markers. To be sure that regulation of AChE gene expression does not hide a regulation of osteoblast markers on mRNA level we also performed real-time RT-PCR for AChE where we found no regulation. Since donepezil is also able to inhibit BChE we also analyzed the mRNA expression of BChE and did not find any significant regulation. For BChE we did not expect changes because of the very low affinity of donepezil to BChE. Sugimoto et al.[24] described a 1250 times lower effect for BChE than for AChE.
Regarding osteoblast differentiation markers we decided to analyze Col1a1 and osteocalcin. The differentiation fate of osteoblasts starts with MSC as precursors that differentiated first into immature osteoblasts which are characterized by collagen type 1, ALP and osteopontin. Afterwards the cells differentiate into mature osteoblasts characterized by osteocalcin which is the most abundant non-collagenous organic component of bone matrix. After synthesis by mature osteoblasts osteocalcin is released into the circulation where the undercarboxylated isoform of osteocalcin acts as hormone and is enhanced by increasing bone turn-over[36]. The carboxylated osteocalcin is entrapped in the mineralized bone matrix and is negatively involved in bone formation. Nevertheless the exact function of osteocalcin remains still not fully understood. In the present study we measured the mRNA expression of the osteocalcin gene, bglap, and did not find any regulation even if we used osteogenic differentiation medium.
Col1a1 was used as marker for immature osteoblasts and was significantly up-regulated on mRNA level in the ODS group in osteogenic differentiation medium and in proliferation medium when all sheep were summed up. Protein level was analyzed by Picro-Sirius Red staining that labels collagen type 1 fibers and showed a significant increase in collagen type 1 in OVX group after application of donepezil. Bone matrix is composed of approximately 90% collagenous proteins of which collagen type 1 is most abundant[37]. Collagen type 1 provides elasticity and flexibility of bone because of its fibrillary structure[38]. Silva et al.[39] described that in a murine model of senile osteoporosis the collagen fiber orientation was the main difference of the test group compared to the healthy control mice. In line with this we measured in rat ovariectomy model an inhomogeneous distribution of collagen fibers and Cauble et al. found additionally a decrease in collagen type 1 fibrils with parallel orientation in ovariectomized rats compared to sham operated animals[40,41]. Thus, estrogen deficiency seems to affect collagen type 1 synthesis. Alterations of collagen type 1 crosslinking and urinary excretion of the C-terminal telopeptide of collagen type 1 (CTX) were described in women with postmenopausal osteoporosis[42]. Mann et al.[43] demonstrated a relation of osteoporotic fractures and a polymorphism in the Col1a1 Sp1 binding site. Furthermore, collagen type 1 formation (crosslinking, racemization, and isomerization) is modified by advanced glycation end products that are accumulated in age-related diseases like osteoporosis[44,45]. Moreover, several studies reported that ACh, stimulation of nicotinic acetylcholine receptors (nAChR), and nicotine (ligand of the nAChR) enhanced the collagen type 1 expression[46] mainly via the nAChR alpha7[47,48].
In conclusion our results demonstrated that inhibition of AChE in cell cultures of ovine MSC lead to an increase in Col1a1 mRNA expression. Since posttranslational processes are strongly involved in formation of collagen type 1 fibers we suppose that estrogen, age- and osteoporosis related effects might be detectable at the protein level which should be analyzed in detail in future studies.
Acknowledgements
Authors’ roles: Study conception: KSL, TEK, CH. Study design: RJN, VK, KT, KSL. Study conduct: RJN, VK, KT, TEK, CH, KSL. Data acquisition: RJN, VK, KT. Data analysis: RJN, VK, KT, KSL. Interpretation of data: RJN, VK, KT, KSL. Drafting the work: KSL, RJN. Revising manuscript critically for important intellectual content: RJN, VK, KT, TEK, CH. Approving final version of manuscript: RJN, VK, KT, TEK, CH, KSL; Agreement to be accountable for all aspects of the work: RJN, VK, KT, TEK, CH, KSL.
RJN and KSL accept responsibility for the integrity of data analysis.
The authors thank Ivonne Bergen, Olga Dakischew and Annette Stengel for skillful technical assistance. This study was supported by the German Research Society (DFG SFB/TRR 79 projects B7 and T1).
Footnotes
The authors have no conflict of interest.
Edited by: E. Paschalis
References
- 1.Roberson MR, Harrell LE. Cholinergic activity and amyloid precursor protein metabolism. Brain Res Brain Res Rev. 1997;25(1):50–69. doi: 10.1016/s0165-0173(97)00016-7. [DOI] [PubMed] [Google Scholar]
- 2.Wessler I, Kirkpatrick CJ. Acetylcholine beyond neurons: the non-neuronal cholinergic system in humans. Br J Pharmacol. 2008;154(8):1558–71. doi: 10.1038/bjp.2008.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Westman E, Spenger C, Oberg J, Reyer H, Pahnke J, Wahlund LO. In vivo 1H-magnetic resonance spectroscopy can detect metabolic changes in APP/PS1 mice after donepezil treatment. BMC Neurosci. 2009;10:33. doi: 10.1186/1471-2202-10-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Van Dam D, Abramowski D, Staufenbiel M, De Deyn PP. Symptomatic effect of donepezil, rivastigmine, galantamine and memantine on cognitive deficits in the APP23 model. Psychopharmacology (Berl) 2005;180(1):177–90. doi: 10.1007/s00213-004-2132-z. [DOI] [PubMed] [Google Scholar]
- 5.Tamimi I, Ojea T, Sanchez-Siles JM, Rojas F, Martin I, Gormaz I, Perez A, Dawid-Milner MS, Mendez L, Tamimi F. Acetylcholinesterase inhibitors and the risk of hip fracture in Alzheimer's disease patients: a case-control study. J Bone Miner Res. 2012;27(7):1518–27. doi: 10.1002/jbmr.1616. [DOI] [PubMed] [Google Scholar]
- 6.Tamimi I, Nicolau B, Eimar H, Arekunnath Madathil S, Kezouh A, Karp I, Tamimi F. Acetylcholinesterase inhibitors and the risk of osteoporotic fractures: nested case-control study. Osteoporos Int. 2017 doi: 10.1007/s00198-017-4346-z. doi:10.1007/s00198-017-4346-z. [DOI] [PubMed] [Google Scholar]
- 7.Eimar H, Perez Lara A, Tamimi I, Marquez Sanchez P, Gormaz Talavera I, Rojas Tomba F, Garcia de la Oliva T, Tamimi F. Acetylcholinesterase inhibitors and healing of hip fracture in Alzheimer's disease patients: a retrospective cohort study. J Musculoskeletal Neuronal Interact. 2013;13(4):454–63. [PubMed] [Google Scholar]
- 8.Tamimi I, Madathil SA, Kezouh A, Nicolau B, Karp I, Tamimi F. Effect of acetylcholinesterase inhibitors on post-surgical complications and mortality following a hip fracture: a cohort study. J Musculoskeletal Neuronal Interact. 2017;17(2):69–77. [PMC free article] [PubMed] [Google Scholar]
- 9.Eimar H, Alebrahim S, Manickam G, Al-Subaie A, Abu-Nada L, Murshed M, Tamimi F. Donepezil regulates energy metabolism and favors bone mass accrual. Bone. 2016;84:131–8. doi: 10.1016/j.bone.2015.12.009. [DOI] [PubMed] [Google Scholar]
- 10.Nih Consensus Development Panel on Osteoporosis Prevention D Therapy. Osteoporosis prevention, diagnosis, and therapy. JAMA. 2001;285(6):785–95. doi: 10.1001/jama.285.6.785. [DOI] [PubMed] [Google Scholar]
- 11.Hadji P, Klein S, Gothe H, Haussler B, Kless T, Schmidt T, Steinle T, Verheyen F, Linder R. The epidemiology of osteoporosis - Bone Evaluation Study (BEST): an analysis of routine health insurance data. Dtsch Arztebl Int. 2013;110(4):52–7. doi: 10.3238/arztebl.2013.0052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li G, Thabane L, Papaioannou A, Ioannidis G, Levine MA, Adachi JD. An overview of osteoporosis and frailty in the elderly. BMC Musculoskelet Disord. 2017;18(1):46. doi: 10.1186/s12891-017-1403-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rodriguez JP, Garat S, Gajardo H, Pino AM, Seitz G. Abnormal osteogenesis in osteoporotic patients is reflected by altered mesenchymal stem cells dynamics. J Cell Biochem. 1999;75:414–23. doi: 10.1002/(sici)1097-4644(19991201)75:3<414::aid-jcb7>3.3.co;2-3. [DOI] [PubMed] [Google Scholar]
- 14.Rodriguez JP, Montecinos L, Rios S, Reyes P, Martinez J. Mesenchymal stem cells from osteoporotic patients produce a type I collagen-deficient extracellular matrix favoring adipogenic differentiation. J Cell Biochem. 2000;79(4):557–65. doi: 10.1002/1097-4644(20001215)79:4<557::aid-jcb40>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 15.Donoso O, Pino AM, Seitz G, Osses N, Rodriguez JP. Osteoporosis-associated alteration in the signalling status of BMP-2 in human MSCs under adipogenic conditions. J Cell Biochem. 2015;116(7):1267–77. doi: 10.1002/jcb.25082. [DOI] [PubMed] [Google Scholar]
- 16.Pino AM, Rosen CJ, Rodriguez JP. In osteoporosis, differentiation of mesenchymal stem cells (MSCs) improves bone marrow adipogenesis. Biol Res. 2012;45(3):279–87. doi: 10.4067/S0716-97602012000300009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Benisch P, Schilling T, Klein-Hitpass L, Frey SP, Seefried L, Raaijmakers N, Krug M, Regensburger M, Zeck S, Schinke T, Amling M, Ebert R, Jakob F. The transcriptional profile of mesenchymal stem cell populations in primary osteoporosis is distinct and shows overexpression of osteogenic inhibitors. PloS One. 2012;7(9):e45142. doi: 10.1371/journal.pone.0045142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Charoenpanich A, Wall ME, Tucker CJ, Andrews DM, Lalush DS, Dirschl DR, Loboa EG. Cyclic tensile strain enhances osteogenesis and angiogenesis in mesenchymal stem cells from osteoporotic donors. Tissue Eng Part A. 2014;20(1-2):67–78. doi: 10.1089/ten.tea.2013.0006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.El Khassawna T, Merboth F, Malhan D, Bocker W, Daghma DES, Stoetzel S, Kern S, Hassan F, Rosenbaum D, Langenstein J, Bauer N, Schlagenhauf A, Rösen-Wolff A, Schulze F, Rupp M, Hose D, Seckinger A, Ignatius A, Wilke HJ, Lips KS, Heiss C. Osteocyte Regulation of Receptor Activator of NF-kappaB Ligand/Osteoprotegerin in a Sheep Model of Osteoporosis. Am J Pathol. 2017;187(8):1686–99. doi: 10.1016/j.ajpath.2017.04.005. [DOI] [PubMed] [Google Scholar]
- 20.Heiss C, Kern S, Malhan D, Bocker W, Engelhardt M, Daghma DES, Stoetzel S, Schmitt J, Ivo M, Kauschke V, Lips KS, Tushtev K, Rezwan K, El Khassawna T. A New Clinically Relevant T-Score Standard to Interpret Bone Status in a Sheep Model. Med Sci Monit Basic Res. 2017;23:326–35. doi: 10.12659/MSMBR.905561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29(9):e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Egan KP, Brennan TA, Pignolo RJ. Bone histomorphometry using free and commonly available software. Histopathology. 2012;61(6):1168–73. doi: 10.1111/j.1365-2559.2012.04333.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rogers SL, Friedhoff LT. Pharmacokinetic and pharmacodynamic profile of donepezil HCl following single oral doses. Br J Clin Pharmacol. 1998;46(Suppl1):1–6. doi: 10.1046/j.1365-2125.1998.0460s1001.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sugimoto H, Iimura Y, Yamanishi Y, Yamatsu K. Synthesis and structure-activity relationships of acetylcholinesterase inhibitors:1-benzyl-4- [(5,6-dimethoxy-1-oxoindan-2-yl)methyl] piperidine hydrochloride and related compounds. J Med Chem. 1995;38(24):4821–9. doi: 10.1021/jm00024a009. [DOI] [PubMed] [Google Scholar]
- 25.Sato T, Enoki Y, Sakamoto Y, et al. Donepezil prevents RANK-induced bone loss via inhibition of osteoclast differentiation by downregulating acetylcholinesterase. Heliyon. 2015;1(1):e00013. doi: 10.1016/j.heliyon.2015.e00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Florencio-Silva R, Sasso GR, Sasso-Cerri E, Simoes MJ, Cerri PS. Biology of Bone Tissue: Structure, Function, and Factors That Influence Bone Cells. Bio Med Res Int. 2015;2015:421746. doi: 10.1155/2015/421746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wenisch S, Trinkaus K, Hild A, et al. Human reaming debris: a source of multipotent stem cells. Bone. 2005;36(1):74–83. doi: 10.1016/j.bone.2004.09.019. [DOI] [PubMed] [Google Scholar]
- 28.Sato T, Abe T, Nakamoto N, et al. Nicotine induces cell proliferation in association with cyclin D1 up-regulation and inhibits cell differentiation in association with p53 regulation in a murine pre-osteoblastic cell line. Biochem Biophys Res Commun. 2008;377:126–30. doi: 10.1016/j.bbrc.2008.09.114. [DOI] [PubMed] [Google Scholar]
- 29.Sato T, Abe T, Chida D, et al. Functional role of acetylcholine and the expression of cholinergic receptors and components in osteoblasts. FEBS letters. 2010;584(4):817–24. doi: 10.1016/j.febslet.2010.01.001. [DOI] [PubMed] [Google Scholar]
- 30.Liu PS, Chen YY, Feng CK, Lin YH, Yu TC. Muscarinic acetylcholine receptors present in human osteoblast and bone tissue. Eur J Pharmacol. 2011;650(1):34–40. doi: 10.1016/j.ejphar.2010.09.031. [DOI] [PubMed] [Google Scholar]
- 31.Rothem DE, Rothem L, Dahan A, Eliakim R, Soudry M. Nicotinic modulation of gene expression in osteoblast cells, MG-63. Bone. 2011;48(4):903–9. doi: 10.1016/j.bone.2010.12.007. [DOI] [PubMed] [Google Scholar]
- 32.Walker LM, Preston MR, Magnay JL, Thomas PB, El Haj AJ. Nicotinic regulation of c-fos and osteopontin expression in human-derived osteoblast-like cells and human trabecular bone organ culture. Bone. 2001;28(6):603–8. doi: 10.1016/s8756-3282(01)00427-6. [DOI] [PubMed] [Google Scholar]
- 33.Xu ML, Bi CWC, Liu EYL, Dong TTX, Tsim KWK. Wnt3a induces the expression of acetylcholinesterase during osteoblast differentiation via the Runx2 transcription factor. J Biol Chem. 2017;292(30):12667–78. doi: 10.1074/jbc.M117.777581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Spieker J, Mudersbach T, Vogel-Hopker A, Layer PG. Endochondral Ossification Is Accelerated in Cholinesterase-Deficient Mice and in Avian Mesenchymal Micromass Cultures. PloS one. 2017;12(1):e0170252. doi: 10.1371/journal.pone.0170252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Spieker J, Ackermann A, Salfelder A, Vogel-Hopker A, Layer PG. Acetylcholinesterase Regulates Skeletal In Ovo Development of Chicken Limbs by ACh-Dependent and -Independent Mechanisms. PloS one. 2016;11(8):e0161675. doi: 10.1371/journal.pone.0161675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li J, Zhang H, Yang C, Li Y, Dai Z. An overview of osteocalcin progress. J Bone Miner Metab. 2016;34(4):367–79. doi: 10.1007/s00774-015-0734-7. [DOI] [PubMed] [Google Scholar]
- 37.Clarke B. Normal bone anatomy and physiology. Clin J Am Soc Nephrol. 2008;3(Suppl3):131–9. doi: 10.2215/CJN.04151206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nair AK, Gautieri A, Chang SW, Buehler MJ. Molecular mechanics of mineralized collagen fibrils in bone. Nature communications. 2013;4:1724. doi: 10.1038/ncomms2720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Silva MJ, Brodt MD, Wopenka B, et al. Decreased collagen organization and content are associated with reduced strength of demineralized and intact bone in the SAMP6 mouse. J Bone Miner Res. 2006;21(1):78–88. doi: 10.1359/JBMR.050909. [DOI] [PubMed] [Google Scholar]
- 40.Daghma DES, Malhan D, Simon P, et al. Computational segmentation of collagen fibers in bone matrix indicates bone quality in ovariectomized rat spine. J Bone Mineral Metab. 2017 doi: 10.1007/s00774-017-0844-5. doi:10.1007/s00774-017-0844-5. [DOI] [PubMed] [Google Scholar]
- 41.Cauble MA, Muckley MJ, Fang M, et al. Estrogen depletion and drug treatment alter the microstructure of type I collagen in bone. Bone Rep. 2016;5:243–51. doi: 10.1016/j.bonr.2016.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Garnero P, Cloos P, Sornay-Rendu E, Qvist P, Delmas PD. Type I collagen racemization and isomerization and the risk of fracture in postmenopausal women: the OFELY prospective study. J Bone Miner Res. 2002;17(5):826–33. doi: 10.1359/jbmr.2002.17.5.826. [DOI] [PubMed] [Google Scholar]
- 43.Mann V, Hobson EE, Li B, et al. A COL1A1 Sp1 binding site polymorphism predisposes to osteoporotic fracture by affecting bone density and quality. J Clin Invest. 2001;107(7):899–907. doi: 10.1172/JCI10347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Viguet-Carrin S, Garnero P, Delmas PD. The role of collagen in bone strength. Osteoporos Int. 2006;17(3):319–36. doi: 10.1007/s00198-005-2035-9. [DOI] [PubMed] [Google Scholar]
- 45.Saito M, Marumo K. Effects of Collagen Crosslinking on Bone Material Properties in Health and Disease. Calcif Tissue Int. 2015;97(3):242–61. doi: 10.1007/s00223-015-9985-5. [DOI] [PubMed] [Google Scholar]
- 46.Arredondo J, Hall LL, Ndoye A, et al. Central role of fibroblast alpha3 nicotinic acetylcholine receptor in mediating cutaneous effects of nicotine. Lab Invest. 2003;83(2):207–25. doi: 10.1097/01.lab.0000053917.46614.12. [DOI] [PubMed] [Google Scholar]
- 47.Sekhon HS, Keller JA, Proskocil BJ, Martin EL, Spindel ER. Maternal nicotine exposure upregulates collagen gene expression in fetal monkey lung. Association with alpha7 nicotinic acetylcholine receptors. Am J Respir Cell Mol Biol. 2002;26(1):31–41. doi: 10.1165/ajrcmb.26.1.4170. [DOI] [PubMed] [Google Scholar]
- 48.Vicary GW, Ritzenthaler JD, Panchabhai TS, Torres-Gonzalez E, Roman J. Nicotine stimulates collagen type I expression in lung via alpha7 nicotinic acetylcholine receptors. Respir Res. 2017;18(1):115. doi: 10.1186/s12931-017-0596-8. [DOI] [PMC free article] [PubMed] [Google Scholar]


