Abstract
Background:
Whole-body vibration (WBV) is an alternative intervention for patients with diabetic peripheral neuropathy (DPN) but its clinical efficacy is unclear.
Objective:
To summarize the effects of WBV on important outcomes for patients with DPN.
Data Sources:
Medline, PEDro, Cochrane CENTRAL and Google Scholar were searched up to July 2017. Search terms included diabetic neuropathies and WBV.
Study Selection:
Interventional studies that utilized WBV for treating DPN outcomes with at least one-week follow-up were included.
Data Extraction:
Data were independently extracted by two reviewers using a standardized checklist.
Data Synthesis:
Twenty-two registers were identified. Three studies (83 patients) satisfied the selection criteria. Studies assessed the effect of WBV on the glycemic profile, neuropathic pain, and balance. WBV presented positive effects on these outcomes, but a high risk of bias was identified in most studies. No study assessed plantar tactile sensitivity.
Limitations:
Most studies have a high level of bias. No pooling data was possible due to few studies included.
Conclusions:
Very low-quality evidence suggests that WBV has a slight positive effect on glycemic control in patients with DPN, improving neuropathic pain and balance. Future studies may change the WBV estimated effect on DPN outcomes.
Keywords: Glycemic Profile, Plantar Sensitivity, Balance, Physical Therapy, Diabetes
Introduction
Chronic hyperglycemia in individuals with diabetes, independently of type 1 (DM1) or 2 (DM2), leads to several systemic microvascular complications[1]. The most common complication is the diabetic peripheral neuropathy (DPN), which a prevalence up to 50%[1,2] in individuals with diabetes. Sensory and motor impairments are present in DPN such as neuropathic pain, modified sensitivity, lower intensity of proprioceptive and reflex responses, and muscle weakness in the lower limbs, which impacts daily living activities and the quality of life[3-7]. These factors also affect motor coordination of gait performance and, together with the loss of feet protective sensation, increase the risk of falling, foot ulceration, and non-traumatic lower limbs amputations[7,8].
Strict glycemic control and changes in lifestyle are the most effective approach to prevent DPN and its complications[9,10]. However, once developed, there is no appropriate intervention to treat or reverse DPN[6]. Pharmacologic therapies for the management of the neuropathic pain are limited due to frequent side effects such as urinary retention, fatigue, and drowsiness leading some patients to discontinue the treatment[1,2,6]. Therefore, it is recommended a multidisciplinary treatment for diabetic neuropathy, but the identification of safe and effective adjuvant interventions is still required[1].
Physical treatment modalities are options that have few contraindications, rare side effects, and nearly no drug interactions[11]. Examples include electrical stimulation and exercise[12]. Such non-invasive approaches have shown benefits on the management of chronic complications related to DM and DPN, as neuropathic pain, with a positive impact on quality of life[12,13].
Whole-body vibration (WBV) is an alternative intervention that allows using vibration as physical stimuli. In this intervention, individuals usually remain in a standing position over a platform that generates vibrations at certain frequencies and amplitudes. WBV may be applied alone or combined with exercises. Moreover, exercises may be performed over the platform while the individual receives the vibratory stimulus or out of the platform, before or after WBV in this case[14]. A previous systematic review concluded that WBV in addition to exercises performed over or out of the platform improved the glycemic control of individuals with DM2 without DPN in an exposure-dependent way. However, large and well-designed clinical trials are still necessary to understand whether the effects were attributed to vibration, exercise or a combination of both[15].
Besides the glycemic control, the maintenance of feet protective sensation, neuropathic pain management, and fall prevention are patient-important outcomes for individuals with DPN. Most studies that previously investigated the effect of WBV in individuals with DPN exhibited methodological limitations due to lack of control group, randomization process, and small sample size[16-22]. In order to determine the current quality of evidence about the effects of WBV in patients with DPN and whether patient-important outcomes were investigated, this systematic review aimed to summarize the effects of WBV on glycemic profile, neuropathic pain in the lower limbs, plantar tactile sensitivity, or balance in patients with DPN investigated by interventional studies.
Methods
Protocol and registration
This systematic review was performed in accordance with the Preferred Reporting Items for Systematic Review and Meta-Analyses: The PRISMA Statement. The protocol of this systematic review was registered on the International prospective register of systematic reviews, PROSPERO, under the identification CRD42016049585[23] and can be integrally assessed online.
Eligibility criteria
Inclusion criteria were prospective interventional studies randomized or not, controlled or uncontrolled, that assessed the effects of WBV in patients with DPN, independently of type 1 or 2 diabetes (DM1 or DM2), in at least one of the following outcomes: glycemic profile, neuropathic pain in the lower limbs, plantar tactile sensitivity, or balance. The exclusion criteria were case studies, studies with follow-up fewer than one-week or/and no peer-reviewed publication.
Search strategy
Literature searches were conducted in the following electronic databases (from inception to July 2017): MEDLINE (accessed by PubMed), PEDro, Cochrane Central Register of Controlled Trials (Cochrane CENTRAL) and Google Scholar. The search terms used individually or combined included ‘diabetic neuropathies’ (mesh term and entry terms) and ‘whole body vibration’ (and synonyms). To enhance the sensibility of our search, we did not include words related to the outcomes of interest or type of study. There were no restrictions regarding language. The references included in the published articles identified in these searches were used as an additional source to identify other studies. The full search strategy used for the PubMed database is available online (www.crd.york.ac.uk/PROSPEROFILES/49585_STRATEGY_20160916.pdf)[23]. Terms were adjusted to fit the requirements of each electronic database.
Study selection and data extraction
Two reviewers (CCR, RPGB) separately and independently screened the titles and abstracts of studies identified from initial searches. A standard screening checklist based on the eligibility criteria was employed for each study. Studies that did not meet the criteria according to titles or abstracts were excluded. Full-text versions of the remaining studies were retrieved for a second independent review by the two reviewers to assure the eligibility. There were no disagreements regarding the study eligibility between authors. When studies reported results from the same population in more than one publication, the article with the largest sample size was chosen.
The following data were extracted from the included studies: methodological design, number of participants, type of diabetes, comparison groups, intervention protocol and outcome results. The primary outcome was glycemic profile, assessed by 12-hours fasting blood glucose (12-h FBG) or glycated hemoglobin (HbA1c). Secondary outcomes were: neuropathic pain in the lower limbs assessed by pain scales; plantar tactile sensitivity assessed with Semmes-Weinstein monofilament; balance assessed by dynamic or/and static balance tests.
The review authors (CCR, RPGB) separately and independently extracted the data from the eligible studies. There were no disagreements regarding data extraction between the authors. When data were missing for synthesis or assessment of the study quality, we contacted the study authors at least twice. The study was excluded if there were still insufficient data following this process.
Risk of bias and quality assessment
Risk of bias was independently assessed considering the study design by the two authors. Randomized-controlled trial (RCT) studies were assessed by using the Cochrane Risk of Bias Tool[24]. Other interventional study designs were classified as high risk of bias. Furthermore, the quality of each article was evaluated based on the recommendation of the International Society of Musculoskeletal and Neuronal Interactions (ISMNI)[25] for reporting WBV intervention studies, which suggests 13 minimal items reporting about WBV parameters and participant positioning. The instruments were independently applied by the two reviewers and no disagreements were observed.
Data analysis
After data extraction, a descriptive synthesis was performed considering study characteristics and outcomes assessed. No pooling data was performed due to a small number of included studies, high variability in the interventions and outcome measurements. The Grading of Recommendation, Assessment, Development and Evaluation (GRADE) system[26] was used to evaluate the quality of evidence.
Results
Description of studies
The search strategy yielded 22 articles, seven[16-22] of them were considered potentially relevant and retrieved for detailed analysis. After full-text reading, four articles were excluded: two case-studies[16,17], one study did not assess the stated outcomes[20], one study with less than one-week follow-up[21]. Finally, three studies[18,19,22] were included in this systematic review. [Figure 1] shows the flow diagram of studies included.
Figure 1.
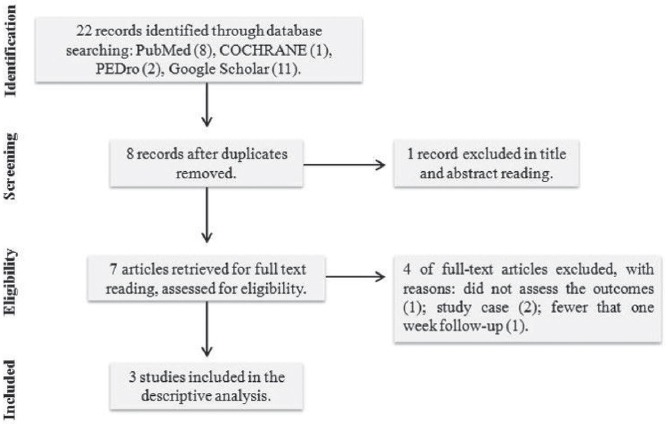
Flow diagram of studies included.
[Table 1] summarizes the characteristics of these studies. The year of publication of the included studies ranged from 2013 to 2015. Studies assessed individuals with DM1 or DM2, totaling 83 participants with DPN. Age ranged from 57 to 76 years and participants of both genders were assessed. RCT[19], uncontrolled[18] and controlled[22] prospective interventional studies were the study designs. Follow-up ranged from 4 to 6 weeks. Regarding WBV intervention, all studies applied intermittent vibratory protocols with total time exposure fewer than 15 minutes. Frequencies ranged from 15 to 30 Hz, peak-to-peak amplitude ranged from 1 to 5 mm. [Table 2] shows the quality of each study based on the recommendation of the ISMNI[25] for reporting WBV intervention studies. Regarding risk of bias, two studies presented overall high risk of bias and one study, a RCT, presented overall low risk of bias.
Table 1.
Characteristics of the included studies.
| Author, year | Study design | Participants | Age | Gender (male/female) | Intervention | Comparison | Outcomes | Follow-up | Conclusion |
|---|---|---|---|---|---|---|---|---|---|
| Kessler and Hong, 201318 | Uncontrolled prospective interventional | DM 1 and 2, with DPN | 56.12 (6.78) | 6/2 | WBV:4 bouts of 3-min WBV (25 Hz; 5 mm); 30-s of rest between bouts; three times a week. | Not applicable | Pain intensity: NPS each week and VAS each pre- and post-session and duration (in hours). | 4 weeks | A 4-weeks WBV intervention reduced acute and long-term neuropathic pain |
| Lee, Lee, Song 201319 | RCT | DM 2, with DPN ≥ 65 years, either two or more falls during the previous 12 months or one fall plus a TUG test > 15 sec or recurrent unexplained falls. | WBV+BE: 76.31 (4.78) BE: 74.05 (5.42) CG: 75.77 (5.69) | WBV+BE: 9/10 BE: 7/11 CG: 8/10 |
WBV+BE:BE (as the comparison group) and WBV: 3 bouts of 3-min with 1-min of rest between bouts; three times a week. - 1st week: 15 Hz and 2 mm, - 2nd and 3rd weeks: 20 Hz and 1 mm, - 4th and 5th weeks: 25 Hz and 2 mm, - 6th week. 30 Hz and 3 mm |
CG: without intervention BE: 60-min balance exercise twice a week, which progressive strength, balance, and functional mobility training, for 6 weeks. | Glycemic profile: HbA1c Balance: Postural stability (CoP sway and velocity moment at force plate). Dynamic stability: OLST, BBS, FRT, and TUG. | 6 weeks | A 6-week WBV+BE significantly improved HbA1c levels and balance, in comparison with CG and BE groups. |
| Kordi Yoosefinejad et al., 201522 | Controlled prospective interventional | DM 1 or 2 with DPN; HbA1C < 8.5 %; BMI between 25; age between 50 and 70 years | WBV: 57 (1.8) CG: 57 (1.5) | WBV: 6/4 CG: 6/4 | WBV: synchronous plate, two times a week (30 Hz, 2 mm). Application time increased every 2 weeks: 30-s/ 45-s/ 1- min. | CG: without intervention | Balance: OLST, TUG, eight different positions to perform on the force plate. | 6 weeks | A 6-weeks WBV intervention significantly improved TUG time in comparison with CG. |
RCT: randomized controlled trial. DM: diabetes. DPN: diabetic peripheral neuropathy. TUG: timed up and go. BMI: body-mass index; WBV: whole-body vibration. BE: balance exercises. CG: control group. VAS: visual analogue scale. NPS: neuropathic pain scale. CoP: center of pressure. OLST: one leg stance test. BBS: Berg balance scale. FRT: Functional reach test.
Table 2.
Assessment of minimum items reported for whole-body vibration interventions.
| Author | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | TOTAL |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Kessler and Hong, 2013[18] | - | - | 25 Hz | 5 mm | - | - | - | Parameters did not change | - | - | - | Stood on the platform (knees bent at 20°) | Maintaining posture: 4 bouts of 3-min WBV with 30-s of rest between bout; thrice per week | 5/13 |
| Lee, Lee, Song 2013[19] | Galileo 2000, Novotec Medical GmBH, Germany | - | 15 to 30 Hz | 1 to 3 mm | - | - | - | Frequency and amplitude increased every two weeks | To reproduce Von Stengel et al. 2011 results | Handrail for support if required | Normal footwear | Stood on the platform (in a 110° squatting position) | Maintaining posture: 3 bouts of 3-min WBV with 1-min of rest between bouts; thrice per week | 9/13 |
| Kordi Yoosefinejad et al., 2015[22] | Power-Plate, Next Generation, USA | Sync. | 30 Hz | 2 mm | 3,61 g | - | Assessed during intervention | Time increased every two weeks | - | No support was allowed | - | Stood on the platform (knees bent at 30°) | Maintaining posture: 30-s/ 45-s / 1-min of WBV; twice per week | 10/13 |
Sync: synchronous. WBV: whole-body vibration. 1, Brand name of the vibration platform; 2, Type of vibration; 3, Vibration frequency; 4, Vibration amplitude; 5, Peak acceleration; 6, Accuracy of the vibration parameter; 7, Evaluation of skidding of the feet; 8, Changes of vibration parameters; 9, Rationale for choosing vibration parameters; 10, Supported devices during vibration exposure; 11, Type of footwear; 12, Body position; 13, Description of exercise.
Glycemic profile
HbA1c was assessed in only one RCT[19]. In that study, WBV in addition to balance exercises (WBV+BE) performed out of the platform were compared with BE alone and a control group without intervention. After six weeks of intervention, HbA1c levels showed a statistically significant within-group decrease only for the WBV+BE group. Between-group comparison showed no statistically significant difference across groups[19]. Quality of evidence regarding this outcome is low, as described in [Table 3].
Table 3.
Quality of the evidence.
| Quality assessment | No of patients | Effect | Quality | Comment | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| No of studies | Study design | Risk of bias | Inconsistency | Indirectness | Imprecision | WBV | Control | |||
| Glycemic profile (follow-up range: 6 weeks; assessed with HbA1c) | ||||||||||
| 1 | RCT | Not serious1 | Serious3 | Not serious | Serious4 | 19 | 36 | -0.8% (p>0.005) | ⨁⨁◯◯LOW | Effect found after 6 weeks intervention in comparison whit both the active and inactive control group |
| Neuropathic pain (follow-up range: 4 weeks; assessed with: VAS scale) | ||||||||||
| 1 | Uncontrolled pre-test, post- test | Serious2 | Serious3 | Not serious | Serious4 | 9 | 0 | -50% (p>0.005) | ⨁◯◯◯VERY LOW | Effect found after 4 weeks intervention |
| Balance (follow-up range: 4 weeks to 6 weeks; assessed with: TUG test) | ||||||||||
| 2 | RCT / Controlled pre-test, post- test | Serious2 | Serious3 | Not serious | Serious4 | 29 | 46 | -7% (p>0.005) -0.83 (p=0.002) seconds | ⨁◯◯◯VERY LOW | We considered only TUG test as measure of balance |
WBV: whole-body vibration. RCT: randomized controlled trail. VAS: visual analogue scale. TUG: timed up and go. 1: Quality of the evidence was not downgraded for risk of bias since most of studies were blinded and no major problems with randomization were detected; concerns about length of follow-up and method of the measurement were considered in other domains. 2: Quality of the evidence was downgraded for risk of bias since most of studies were no randomized controlled trials; concerns about length of follow-up and method of the measurement were considered in other domains. 3: Few patients were evaluated. 4: Few studies and few assessed patients to evaluate real heterogeneity.
Neuropathic pain
Neuropathic pain was investigated by only one uncontrolled interventional prospective study in which eight patients with DPN performed WBV sessions three times a week during four weeks[18]. Changes in pain were assessed using a 0 to 10 Visual Analogue Scale (VAS) of Pain and the Neuropathic Pain Scale (NPS). After four weeks, WBV showed 50% reduction in self-reported VAS. It also showed a statistically significant reduction in the following NPS variables: intensity (78% reduction), sharpness (100% reduction), unpleasantness (81% reduction) and deep pain (100% reduction). Evidence of WBV in neuropathic pain improvement is very low (Table 1).
Plantar sensitivity
None of the included studies assessed plantar sensitivity.
Balance
Two studies investigated WBV effects on balance[19,22]. Balance was assessed with several different methods such as postural sway measured by force plate, or validated balance tests as Timed up and go (TUG), One Leg Stance Test (OLST) or Unilateral Stance Test (UST), Berg Balance Scale (BBS), Functional Reach Test (FRT) and Five-Time-Sit-to-Stand (FTST). TUG was the only balance measurement that improved in both studies in the groups receiving WBV[19,22]. One study[19] showed between-group statistically significant improvement in postural sway, BBS and FTSTS in the WBV+BE and BE groups. As the only consistent test among studies was the TUG test, quality of the evidence for balance was evaluated considering this measure (Table 1).
Discussion
Our systematic review showed that among the stated important outcomes for individuals with DPN, glycemic profile[19], neuropathic pain[18] and balance were assessed in primary studies[19,22]. None of the included studies assessed plantar sensitivity, which is a safety-related outcome. Despite the potential benefits of WBV in this population, current quality of the evidence is low or very low depending on the outcome, most of the studies were methodologically weak regarding its design and, with a small number of patients assessed.
The effect of WBV on the glycemic profile was investigated with HbA1c measurements in one RCT[19]. Despite a decrease in HbA1c for the group performing WBV was reported, it did not reach the 1% reduction considered as clinically relevant[27]. HbA1c is usually utilized as a predictor for the progression of diabetes and its complications in DPN and must be a mandatory outcome in interventional studies with individuals with diabetes[28]. A previous meta-analysis concluded that WBV in addition to exercises improved blood glucose in patients with DM2 without DPN or other complications[15]. Considering this indirect evidence and that results of glycemic profile in individuals with DPN were provided from one RCT[19], it is possible to affirm that WBV have a slight but not clinical effect on the glycemic profile of individuals with DPN, although quality of the evidence is low.
Neuropathic pain is the most disabling symptom in patients with DPN, related to the impairment of quality of life[1]. In addition, neuropathic pain has a difficult management as patients often fail to adhere to typical drug treatments[1]. In fact, participants included in the study that investigated the effect of WBV in neuropathic pain were taking gabapentin, opiate analgesics and/or non-steroids anti-inflammatory to control their pain symptoms, but no participants reported satisfaction from these treatments[18]. In this primary study, WBV reduced both acute and long-term pain in patients with DPN reaching the minimal clinically important difference[29,30]. It is supposed that vibration reduces pain through the gate control theory[31] and diffuse central noxious inhibitory control[32]. Unfortunately, it is not possible to affirm that WBV decreases neuropathic pain as the quality of the evidence is very low and placebo effects cannot be completely excluded.
Previous studies assessing healthy individuals have shown that continuous vibration protocols reduced the plantar tactile sensitivity in a short-term follow-up[33,34]. All studies assessing individuals with DPN used intermittent vibration protocols but none of the studies investigated the effects on plantar tactile sensitivity[16-22]. Furthermore, there is no previous direct or indirect information about the impact of intermittent vibration protocol on this outcome. Since plantar tactile sensitivity is a predictor of foot ulceration and amputation in individuals with DPN[35], further studies should assess this outcome to assure the safety of this intervention.
Direct and indirect measurements were used to determine improvement in balance. Postural sway, which is a direct measurement, presented inconsistent results across the studies included in this review. It is supposed that direct measurements of balance were only improved when a specific balance intervention was addressed[19] rather than only WBV[22]. TUG was the most utilized indirect tool for assessing balance and it showed statistically significant improvement after WBV intervention despite the study design[19,22]. Balance improvement can be attributable to a postural control strategy that is adopted during WBV and improvement in muscle function[36]. There is strong evidence that WBV improves balance in frail populations, for instance, elderly individuals[36,37]. Given this indirect evidence and the results provided by an RCT, it is advocated that WBV should be extensively investigated as a potential intervention for balance improvement in individuals with DPN. Additionally, further studies are needed to determine whether balance improvement is enough to prevent falls in that population.
Studies were consistent regarding WBV parameters. All the protocols were intermittent with at least 30 seconds of rest between vibration expositions[18,19,22]. Regarding the type of vibration, only one study reported this information[22]. Furthermore, studies failed in reporting minimal technical parameters required for interventions with WBV[25]. Few outcomes have shown improvement associated with the type of vibration: oscillatory or synchronous. For example, previous studies have shown that oscillatory platforms have better effects on balance[36,37]. In fact, vibration from oscillatory platforms is generated from a side-alternated movement like a teeter-totter with larger amplitudes that requires wider lower-limbs ranges of motion when compared to synchronous platforms[38].
This is the first systematic review aiming to summarize the current evidence regarding effects of WBV in patients with DPN. We performed an extensive database search without limitation of language and time of publication. In addition, we incorporated a comprehensive assessment of the quality of the evidence for each outcome using GRADE and we also evaluated if the reporting of minimal items for studies utilizing WBV as intervention occurred. Such information will allow quality improvement of future studies. Although we followed methodological standards, we did not use specific gray literature databases to search studies beyond Google Scholar, that might affect the inclusion of all existent studies available.
Conclusion
WBV was associated with a slight improvement of the glycemic profile but the quality of the evidence is low. Neuropathic pain and balance seem to improve in patients with DPN after WBV intervention, but evidence for these outcomes is very low. Further studies are likely to change the estimated effect, not supporting the current use of WBV in patients with DPN for relieving neuropathic pain, improving balance, and plantar tactile sensitivity. Randomized controlled trials investigating the effects of WBV in glycemic profile, neuropathic pain, plantar sensitivity, and balance are still required to improve quality of evidence and understand limitations of the potential benefits of this intervention in patients with DPN.
Funding support
This study received no funding support.
Footnotes
The authors have no conflict of interest.
Edited by: G. Lyritis
References
- 1.Tesfaye S, Boulton AJM, Dickenson AH. Mechanisms and management of diabetic painful distal symmetrical polyneuropathy. Diabetes Care. 2013;36(9):2456–2465. doi: 10.2337/dc12-1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boulton AJM, Malik RA, Arezzo JC, Sosenko JM. Diabetic somatic neuropathies. Diabetes Care. 2004;27(6):1458–1486. doi: 10.2337/diacare.27.6.1458. [DOI] [PubMed] [Google Scholar]
- 3.Cavanagh PR, Derr JA, Ulbrecht JS, Maser RE, Orchard TJ. Problems with gait and posture in neuropathic patients with insulin-dependent diabetes mellitus. Diabet Med J Br Diabet Assoc. 1992;9(5):469–474. doi: 10.1111/j.1464-5491.1992.tb01819.x. [DOI] [PubMed] [Google Scholar]
- 4.Payne C, Turner D, Miller K. Determinants of plantar pressures in the diabetic foot. J Diabetes Complications. 2002;16(4):277–283. doi: 10.1016/s1056-8727(01)00187-8. [DOI] [PubMed] [Google Scholar]
- 5.Petrofsky J, Macnider BS, Navarro BS, Lee S. Motor Control and Gait Characteristics in Patients with Type 1 and Type 2 Diabetes without Sensory. Impairment in the Foot. 2005;15(2):75–86. [Google Scholar]
- 6.Boulton AJM, Vinik AI, Arezzo JC, et al. Diabetic neuropathies: a statement by the American Diabetes Association. Diabetes Care. 2005;28(4):956–962. doi: 10.2337/diacare.28.4.956. [DOI] [PubMed] [Google Scholar]
- 7.Rao S, Saltzman CL, Yack HJ. Relationships between segmental foot mobility and plantar loading in individuals with and without diabetes and neuropathy. Gait Posture. 2010;31(2):251–255. doi: 10.1016/j.gaitpost.2009.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Allet L, Armand S, Golay A, Monnin D, de Bie RA, de Bruin ED. Gait characteristics of diabetic patients: a systematic review. Diabetes Metab Res Rev. 2008;24(3):173–191. doi: 10.1002/dmrr.809. [DOI] [PubMed] [Google Scholar]
- 9.Loprinzi PD, Hager KK, Ramulu PY. Physical activity, glycemic control, and diabetic peripheral neuropathy: a national sample. J Diabetes Complications. 2014;28(1):17–21. doi: 10.1016/j.jdiacomp.2013.08.008. [DOI] [PubMed] [Google Scholar]
- 10.Andersen H, Nielsen S, Mogensen CE, Jakobsen J. Muscle strength in type 2 diabetes. Diabetes. 2004;53(6):1543–1548. doi: 10.2337/diabetes.53.6.1543. [DOI] [PubMed] [Google Scholar]
- 11.Sluka KA, Walsh D. Transcutaneous electrical nerve stimulation: basic science mechanisms and clinical effectiveness. J Pain Off J Am Pain Soc. 2003;4(3):109–121. doi: 10.1054/jpai.2003.434. [DOI] [PubMed] [Google Scholar]
- 12.Dornelas de Andrade A, Dean E. Aligning Physical Therapy practice with Brazil's leading Health priorities: a “call to action” in the 21st century. Braz J Phys Ther. 2008;12(4):260–267. [Google Scholar]
- 13.Hayashino Y, Jackson JL, Fukumori N, Nakamura F, Fukuhara S. Effects of supervised exercise on lipid profiles and blood pressure control in people with type 2 diabetes mellitus: a meta-analysis of randomized controlled trials. Diabetes Res Clin Pract. 2012;98(3):349–360. doi: 10.1016/j.diabres.2012.10.004. [DOI] [PubMed] [Google Scholar]
- 14.Cochrane DJ. Vibration exercise: the potential benefits. Int J Sports Med. 2011;32(2):75–99. doi: 10.1055/s-0030-1268010. [DOI] [PubMed] [Google Scholar]
- 15.Robinson CC, Barreto RPG, Sbruzzi G, Plentz RDM. The effects of whole body vibration in patients with type 2 diabetes: a systematic review and meta-analysis of randomized controlled trials. Braz J Phys Ther. 2016;20(1):4–14. doi: 10.1590/bjpt-rbf.2014.0133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hong J. Whole body vibration therapy for diabetic peripheral neuropathic pain: a case report. [Accessed December 18 2017];Health Sci J. 2011 5(1) http://www.hsj.gr/abstract/whole-body-vibration-therapy-for-diabetic-peripheral-neuropathic-pain-a-case-report-3472.html . [Google Scholar]
- 17.Hong J, Barnes M, Kessler N. Case study: use of vibration therapy in the treatment of diabetic peripheral small fiber neuropathy. J Bodyw Mov Ther. 2013;17(2):235–238. doi: 10.1016/j.jbmt.2012.08.007. [DOI] [PubMed] [Google Scholar]
- 18.Kessler NJ, Hong J. Whole body vibration therapy for painful diabetic peripheral neuropathy: a pilot study. J Bodyw Mov Ther. 2013;17(4):518–522. doi: 10.1016/j.jbmt.2013.03.001. [DOI] [PubMed] [Google Scholar]
- 19.Lee K, Lee S, Song C. Whole-body vibration training improves balance, muscle strength and glycosylated hemoglobin in elderly patients with diabetic neuropathy. Tohoku J Exp Med. 2013;231(4):305–314. doi: 10.1620/tjem.231.305. [DOI] [PubMed] [Google Scholar]
- 20.Johnson PK, Feland JB, Johnson AW, Mack GW, Mitchell UH. Effect of whole body vibration on skin blood flow and nitric oxide production. J Diabetes Sci Technol. 2014;8(4):889–894. doi: 10.1177/1932296814536289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kordi Yoosefinejad A, Shadmehr A, Olyaei G, Talebian S, Bagheri H. The effectiveness of a single session of Whole-Body Vibration in improving the balance and the strength in type 2 diabetic patients with mild to moderate degree of peripheral neuropathy: a pilot study. J Bodyw Mov Ther. 2014;18(1):82–86. doi: 10.1016/j.jbmt.2013.10.007. [DOI] [PubMed] [Google Scholar]
- 22.Kordi Yoosefinejad A, Shadmehr A, Olyaei G, Talebian S, Bagheri H, Mohajeri-Tehrani MR. Short-term effects of the whole-body vibration on the balance and muscle strength of type 2 diabetic patients with peripheral neuropathy: a quasi-randomized-controlled trial study. J Diabetes Metab Disord. 2015;14:45. doi: 10.1186/s40200-015-0173-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Robinson CC, Barreto RPG, Sbruzzi G, Plentz RDM. Effects of whole body vibration in patients with diabetic peripheral neuropathy: a systematic review. Centre for Reviews and Dissemination, University of York. PROSPERO. [Accessed December 18 2017]. http://www.crd.york.ac.uk/PROSPERO/display_record.php?ID=CRD42016049585 .
- 24.Higgins J, Sally G. Cochrane Handbook for Systematic Reviews of Interventions |Cochrane Training. [Accessed December 18 2017]. http://training.cochrane.org/handbook .
- 25.Rauch F, Sievanen H, Boonen S, et al. Reporting whole-body vibration intervention studies: recommendations of the International Society of Musculoskeletal and Neuronal Interactions. J Musculoskelet Neuronal Interact. 2010;10(3):193–198. [PubMed] [Google Scholar]
- 26.Balshem H, Helfand M, Schünemann HJ, et al. GRADE guidelines:3. Rating the quality of evidence. J Clin Epidemiol. 2011;64(4):401–406. doi: 10.1016/j.jclinepi.2010.07.015. [DOI] [PubMed] [Google Scholar]
- 27.Stratton IM, Adler AI, Neil HA, et al. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ. 2000;321(7258):405–412. doi: 10.1136/bmj.321.7258.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Goldstein DE, Little RR, Lorenz RA, et al. Tests of glycemia in diabetes. Diabetes Care. 2004;27(7):1761–1773. doi: 10.2337/diacare.27.7.1761. [DOI] [PubMed] [Google Scholar]
- 29.Dworkin RH, Turk DC, Wyrwich KW, et al. Interpreting the clinical importance of treatment outcomes in chronic pain clinical trials: IMMPACT recommendations. J Pain Off J Am Pain Soc. 2008;9(2):105–121. doi: 10.1016/j.jpain.2007.09.005. [DOI] [PubMed] [Google Scholar]
- 30.Haanpää M, Attal N, Backonja M, et al. NeuPSIG guidelines on neuropathic pain assessment. Pain. 2011;152(1):14–27. doi: 10.1016/j.pain.2010.07.031. [DOI] [PubMed] [Google Scholar]
- 31.Melzack R, Wall PD. Pain mechanisms: a new theory. Science. 1965;150(3699):971–979. doi: 10.1126/science.150.3699.971. [DOI] [PubMed] [Google Scholar]
- 32.Coghill RC, Talbot JD, Evans AC, et al. Distributed processing of pain and vibration by the human brain. J Neurosci Off J Soc Neurosci. 1994;14(7):4095–4108. doi: 10.1523/JNEUROSCI.14-07-04095.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schlee G, Reckmann D, Milani TL. Whole body vibration training reduces plantar foot sensitivity but improves balance control of healthy subjects. Neurosci Lett. 2012;506(1):70–73. doi: 10.1016/j.neulet.2011.10.051. [DOI] [PubMed] [Google Scholar]
- 34.Sonza A, Robinson CC, Achaval M, Zaro MA. Whole Body Vibration at Different Exposure Frequencies: Infrared Thermography and Physiological Effects. Sci World J. 2015;ID 452657:1–10. doi: 10.1155/2015/452657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dyck PJ, Herrmann DN, Staff NP, Dyck PJB. Assessing decreased sensation and increased sensory phenomena in diabetic polyneuropathies. Diabetes. 2013;62(11):3677–3686. doi: 10.2337/db13-0352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Orr R. The effect of whole body vibration exposure on balance and functional mobility in older adults: a systematic review and meta-analysis. Maturitas. 2015;80(4):342–358. doi: 10.1016/j.maturitas.2014.12.020. [DOI] [PubMed] [Google Scholar]
- 37.Lam FMH, Lau RWK, Chung RCK, Pang MYC. The effect of whole body vibration on balance, mobility and falls in older adults: a systematic review and meta-analysis. Maturitas. 2012;72(3):206–213. doi: 10.1016/j.maturitas.2012.04.009. [DOI] [PubMed] [Google Scholar]
- 38.Abercromby AFJ, Amonette WE, Layne CS, McFarlin BK, Hinman MR, Paloski WH. Vibration exposure and biodynamic responses during whole-body vibration training. Med Sci Sports Exerc. 2007;39(10):1794–1800. doi: 10.1249/mss.0b013e3181238a0f. [DOI] [PubMed] [Google Scholar]


