Abstract
Osteocytes, the most abundant bone cell in the adult skeleton, can function as mechanosensors directing osteoblast and osteoclast function in order to maintain optimal load bearing bone in addition to functioning as endocrine cells regulating phosphate metabolism. A controversial function, previously overlooked or denied, has been osteocytes as regulators of calcium metabolism. Early histologists upon observing enlarged osteocyte lacunae in bone sections proposed that mature osteocytes could remove their perilacunar matrix, a term called “osteocytic osteolysis”. New insights into this process have occurred during the last decade using novel technology thereby providing a means to identify molecular mechanisms responsible for osteocytic osteolysis. As release of calcium from a mineralized matrix requires a more acidic pH and specialized enzymes, it was proposed that osteocytes may utilize similar molecular mechanisms as osteoclasts to remove mineral. The idea that a cell descended from mesenchymal progenitors (the osteocyte) could function similarly to a cell descended from hematopoietic progenitors (the osteoclast) was challenged as being improbable. Here we review the molecular mechanisms behind this osteocyte function, the role of osteocytic osteolysis in health and disease, and the capacity of the osteocyte to reverse the osteolytic process by replacing the removed matrix, a revived osteoblast function.
Keywords: Osteocytic Osteolysis, Osteocyte, Osteoclast, Lactation, Parathyroid Hormone
Introduction
One remarkable characteristic of bone microarchitecture is the magnitude of the osteocyte network that lies hidden inside the mineralized matrix. Dendritic osteocytes connect to one another, to blood vessels and to bone surface cells enabling a global communication within bone tissue. Residing in a fluid-filled interstitium of lacunae and canaliculi, osteocytes sense the mechanical load that is being placed upon the skeleton during locomotion[1]. The transmission of the loading information from osteocytes is coupled with the secretion of factors i.e. sclerostin and RANKL, to directly regulate bone matrix turnover by osteoblasts and osteoclasts[2,3]. In the recently discovered multifunctionality of osteocytes, ranging from phosphate homeostasis to interaction with distant organs, this is just one of many ways through which osteocyte network connectivity contributes to bone health[4].
With an estimated 215 m2 lacuno-canalicular surface area in the human skeleton[5], the osteocyte network surface is several magnitudes larger than the bone surface consisting of osteoclasts, osteoblasts, and bone lining cells. One could assume that osteocytes utilize this characteristic feature and interact with their local perilacunar and pericanalicular matrix. Admittedly, the osteocyte that originates from a population of bone-forming osteoblasts might not be considered a bone-resorptive cell at a first glance. A closer look, however, confirms the inducible predominance of lytic, acidic lysosomes as organelles of mature osteocytes, and a rough appearance of the lacunar wall in calcium-demanding conditions[6].
An ample number of scientists have reported enlarged osteocyte lacunae in the past century and beyond, with Rigal and Vignal indicating active bone matrix dissolution by osteocytes as early as 1881[7]. Around the 1970s, bone resorption by osteocytes even seemed as important for the provision of calcium as osteoclast-dependent resorption[8]. Technical issues with specimen preparation and the lack of mechanistic data may have contributed to the countermovement against osteocytic osteolysis[9]. In fact, especially the description of osteolysis only based on large lacunae, as seen with woven vs. lamellar bones, or with mineralization defects should be avoided. We now understand that both: i) the demineralization and ii) the proteolysis of the perilacunar and pericanalicular matrix define osteocytic osteolysis but may contribute to a varying extent in different conditions.
The process of osteocytic osteolysis may have significant effects on bone physiology. The reversible remodelling of lacunar shapes and network connectivity could not only free calcium from the bone matrix, but could also affect the mechanosensation detected by osteocytes and alter bone turnover. Furthermore, lacuno-canalicular adaptations may contribute to local bone quality characteristics and fracture resistance. Within this review we summarize the current knowledge on osteocytic osteolysis starting off with its role in the physiological processes of lactation, hibernation, and mechanical loading, and continuing with its implications in several pathological scenarios including immobilization, hyperparathyroidism, glucocorticoid treatment, ovariectomy, and peri-implant bone loss. We have endeavoured to appraise existing evidence, highlight open remaining questions, and give an outlook on future directions with regards to the clinical importance of this process.
Methods
We searched electronic databases (PubMed/MEDLINE) using MeSH terms “Osteocyte” and “Osteolysis” or “Osteocyte” and “Perilacunar Remodeling” or “Osteocyte” and “Demineralization” up to November 30th 2017. The results of available in vitro and in vivo studies demonstrating osteocytic osteolysis in the most common physiological and pathophysiological settings, i.e. during lactation, immobilization and hyperparathyroidism are reviewed. In addition, other settings where osteocytic osteolysis could play a role are mentioned. We summarize possible mechanisms underscoring the osteocyte regulation of bone mineral/turnover and address controversies and open questions. Although perilacunar osteolysis has also been described in many other non-mammalian vertebrates this is beyond the scope of this review.
Results and discussion
Evidence of osteocytic osteolysis during lactation
Lactation in mammals is associated with a marked skeletal resorption to support milk production. In rodents, bone mineral content (BMC) or density (BMD) are reduced up to 25-30% as measured by dual-energy X-ray absorptiometry (DXA)[10], whereas bone materials properties (i.e. strength and toughness) are also severely compromised[11]. These effects are further enhanced through a calcium-restricted diet[12], and a larger number of suckling pups[13] (Figure 1). In lactating women, lumbar spine BMD decreases by 5% to 10% during 3 to 6 months of lactation[14], and lactation is positively correlated with vertebral fractures[15]. The sophisticated hormonal interplay regulating the maternal skeletal and mineral physiology during pregnancy, lactation, and weaning period has been elucidated in a recent extensive review[16].
Figure 1.
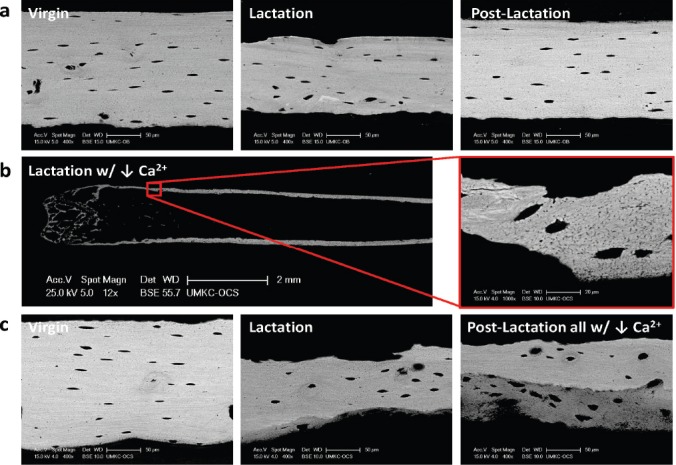
Effects of low calcium diet on osteocyte lacunar size. Backscattered electron microscopy was used to visualize osteocyte lacunae in the midshaft femoral cortex of 14 week old C57Bl/6N virgin, lactating (12 days) and post-lactating (7 days) mice (unpublished data from Jähn et al., JBMR 2017). (a) No significant differences in osteocyte lacunar size with lactation due to the small litters with C57Bl/6N mice (3-6 pups/litter). This is in contrast to the 10-12 pups/litter of CD1 mice inducing significant changes in osteocyte lacunar size (Qing et al., JBMR 2012). (b) Low calcium diet during lactation of C57Bl/6N mice leads to significant bone loss and significantly enlarged lacunae. (c) In this low calcium model with lactation, endosteal bone appears removed by osteoclastic resorption in addition to an increase in osteocyte lacunar size. This bone is replaced endosteally 7 days post-lactation. The 7 days post lactation was not sufficient time for complete reversal of bone loss particularly at the endocortical bone surface (bottom surface in the images). The poorly mineralized bone matrix (dark grey) with the presence of large lacunae suggests a less mature, less mineralized bone matrix. These observations imply that, in addition to the trabecular bone loss, bone loss during lactation is mainly occurring at the endosteal surface of cortical bone.
The enhanced resorption of the maternal skeleton during lactation is further supported by measurements of bone turnover markers, bone structure by high-resolution imaging, bone material properties and histomorphometric analyses. The classical mechanism of enhanced bone resorption is osteoclast-driven and affects primarily trabecular bone and endocortical surfaces[17,18]. However, if osteoclast-mediated bone resorption was the sole mechanism at play, then the inactivation of osteoclasts through a bisphosphonate would be expected to fully attenuate the decline in BMD during lactation. Since experimental data showed only a partial blocking of bone loss in lactating mice treated with pamidronate[19,20], it was hypothesized that an alternative osteolytic mechanism exists.
In a study using backscatter scanning electron microscopy (BSEM), we showed that lactating mice are characterized by significantly larger osteocyte lacunae in the tibia, femur, and vertebra, as compared with virgin controls[21]. This difference ceased to exist after 7 days of forced weaning, indicating that lactation provoques a transient osteocytic perilacunar remodeling, in contrast to previous data in rats[22]. Furthermore, we identified osteoclast-specific marker expression by osteocytes during lactation[21]. A targeted deletion of parathyroid hormone (PTH) receptor 1 (PTHR1) prevented osteocyte-specific remodeling and upregulation of osteoclast markers, whereas treatment with PTH related peptide (PTHrP) led to the opposite effects, demonstrating the importance of PTHrP signaling through the osteocyte PTHR1[21]. This is in accordance with the known upregulation of PTHrP into the systemic circulation and into milk during lactation[19,23-25]. Since PTHR1 signaling also induces the production of Receptor Activator of NF-κB Ligand (RANKL)[26,27], and osteocytes are major contributors of RANKL production[28], it seems possible that during lactation osteocytes participate in both, a direct remodeling of their environment and a stimulation of osteoclast-mediated resorption through upregulation of RANKL[29]. Conversely, after conditional knock-out of the PTHR1 in osteocytes, a low-calcium diet translated into an impaired homeostatic calcemic response, i.e. hypocalcemia in mice, indicating that osteocytes are involved in mineral mobilization[30]. Providing further insight into the mechanism through which osteocytes achieve perilacunar remodeling and calcium mobilization, we showed that osteocytes generate a mild acidic environment through expression of the proton pump in an analogous manner to osteoclasts, and that this cell-induced acidification is modulated through PTHrP[31]. The study further emphasized the important role of osteocyte viability. Osteocytes appear to withstand mild acidic conditions longer than other cell types investigated[31]. This aspect is of functional importance, as osteocytes do not form a sealed resorption pit like osteoclasts do, so they must be protected from the acidic milieu which ensues during osteocytic osteolysis. The mechanisms responsible for this protection are unknown.
Lactation in rodents and humans is physiologically characterized by a surge in prolactin production and a decline in estradiol and progesterone[19,32,33]. In spite of the fact that low estrogen concentrations probably contribute to the enhanced bone resorption noted during lactation[16], the extent of this contribution is not adequately clarified. Although the simulation of an estrogen-deficient state through ovariectomy in rodents results in bone loss, lactation is associated comparatively with a much more prominent and rapid decline in bone mass and mineral content[34]. The effects of estrogen withdrawal in osteocyte viability are consistent with studies showing enhanced osteocyte apoptosis in humans and rodents[35-37]. In a study investigating the impact of estrogen loss on the osteocyte lacunar-canalicular network, Ciani et al. reported an increased solute transport around osteocytes of the proximal tibia of ovariectomized rats probably because of an enhanced lacunar-canalicular porosity[38]. Prolactin levels surge during early lactation initiating milk production and prolactin is a known stimulator of PTHrP production[39]. During recent years evidence has emerged for a direct action of prolactin in bone cells. Using a model of anterior pituitary transplantation to induce hyperprolactinemia, Seriwatanachai et al. showed that prolactin enhances bone turnover by downregulating osteoprotegerin, while concurrently upregulating RANKL production[40]. A possible direct regulatory role for prolactin in bone modeling was investigated by inhibiting prolactin in bromocriptine-treated pregnant and lactating rats[41]. Further studies are warranted to elucidate whether prolactin is directly implicated in osteocyte-driven perilacunar remodeling.
Another hormone which has been implicated in the pathophysiology of enhanced bone resorption during lactation is calcitonin. Although serum measurements of calcitonin concentrations in rodents and humans have not yielded thoroughly consistent data, most studies reported high calcitonin concentrations during lactation[42-45]. Since calcitonin inhibits lactation by suppressing prolactin release by the pituitary[46,47], a protective role for the maternal skeleton has been envisaged through a negative feedback-loop to reduce the production of PTHrP in the mammary gland during lactation[16,44]. Intriguingly, lactating calcitonin receptor global knockout mice did not show a difference in osteoclastic bone resorption, but depicted a larger osteocyte lacunar area in cortical bone as compared to littermate controls, providing evidence for a protective role of the calcitonin receptor through inhibition of osteocytic osteolysis[48]. This is in accordance with previous data describing a protective role for calcitonin inhibition of periosteocytic demineralization in disuse osteoporosis[49], or familial bone dysplasia[50].
A recent study identified matrix metalloproteinase 13 (MMP-13) as an essential factor for lactation-induced osteocyte perilacunar remodeling[51]. MMPs are multifunctional proteins, which mainly act as coupling factors of bone remodeling under physiological conditions, whereas their overexpression usually results in enhanced bone resorption and osteolysis[52]. Using MMP-13 deficient virgin and lactating mice and their wild-type littermates, Tang et al. showed that MMP-13 is indispensable for osteocyte perilacunar remodeling and its loss results in compromised bone quality and strength[51]. An upregulation of osteocyte mRNA and protein expression of MMP-13 was further confirmed in a mouse model of X-linked hypophosphatemia (XLH), which is also characterized by larger osteocyte lacunae in both the tibia and calvaria[53]. Finally, MMP-13 expression was reduced in mice with conditional osteocyte deletion of the TGF-β receptor II, which also depicted impaired perilacunar remodeling and impaired bone quality, indicating that TGF-β signaling is involved in these processes[54].
While osteoclast-mediated bone resorption is primarily localized in trabecular bone and endocortical surfaces, osteocytic osteolysis occurs both in trabecular and cortical bone[16,29,55,56]. Using BSEM, Raman microspectroscopy, and microindentation in lactating mice, Kaya et al. showed that the increased volume of lacunar and canalicular space also translates into a decrease in elastic modulus, although the overall matrix mineral content remains unaltered[57].
The reversibility of bone mass and structure changes after cessation of lactation is of particular clinical importance. In mice, weaning is associated with a decline in osteoclast numbers and up-regulation of osteoblasts and their precursors[21,58], whereas osteocyte lacunar size returns to normal and becomes similar to virgin littermates[59]. These changes markedly improve bone mass and microarchitecture[16]. In a study by Brembeck at al.,[82] women were followed up to 18 months postpartum. In women who had stopped breastfeeding by 9 months and experienced at least 9 months of recovery no significant decreases in BMD in trabecular or cortical bone were noted as compared to non-lactating controls. Since this study did not include pre-lactation/pregnancy data of the lactating women investigated, it is not possible to determine to what degree these women had recovered to their pre-pregnancy bone mineral status[60]. Despite the fact that existing epidemiological studies report no permanent BMD reduction and no increased fracture risk, differences in study design and study populations need to be taken into considearation[61-63]. Although existing data tend to support the notion that lactation in rodents and humans is not associated with long-term negative effects for the maternal skeleton[64], further studies, especially examining the effects of repetitive lactation, are warranted in order to better characterize the effect of pregnancy and lactation on female skeletal health.
Osteocytic osteolysis in immobilization and mechanical loading
The impact of immobilization on bone mass and bone mineral has been well documented with studies reporting a decline in BMD and bone strength[65,66]. Nevertheless, the direct effect of immobilization on osteocyte lacunar properties is a subject of controversy. After initial reports of osteocytic osteolysis in rats immobilized for 10 days by spinal cord severing[67], in arctic squirrels during hibernation[68], and in monkeys subjected to microgravity through spaceflight[69], subsequent studies yielded discrepant results. In our tail-suspension unloading mouse model, no osteocytic remodeling was observed despite documented bone loss[21], and comparable results were noted in a more recent study using a model of botox-induced immobilization[70]. Conversely, the canalicular diameter and expression of matrix metalloproteinases (MMP) 1, 3, and 10 are increased in female mice subjected to microgravity on a space shuttle mission, indicating active osteocytic osteolysis in this model[71]. Using a model of hindlimb suspension, Lloyd et al. demonstrated that mechanical unloading in rodents leads to osteocytic osteolysis in cortical bone and could be reversed through deletion of connexin 43, a pivotal gap junction protein in bone[72]. Furthermore, in a model of sciatic neurectomy as a means to induce severe osteoporosis in rats, a significant enlargement of osteocyte lacunae was depicted by using two-photon excitation microscopy, and local acidification was noted using a fluorescent pH sensor[73]. A possible explanation for these discrepant results could lie in the fact that in most studies two-dimensional techniques were used for the depiction and analysis of osteocytes, which entails a method-eminent risk of errors, since osteocytes are three-dimensional objects[74]. Alternatively, different mouse strains and the age of mice used and/or the different unloading models applied could have contributed to different results.
A protein which regulates re-shaping of osteocyte lacunae under unloading conditions is sclerostin, well-described to stimulate osteocyte-driven osteoclast activity through RANKL[75]. Initial reports implicated sclerostin in the decrease of pH in human and murine osteocyte-like cells[76], and the expression of sclerostin was found to be upregulated in osteocytes after sciatic neurectomy[77]. Lately the application of recombinant sclerostin in a bone tibial culture system of osteocytes devoid of other cell types also led to an increase of osteocyte lacunar size and upregulation of bone resorption marker genes[78]. Taken together these observations indicate that sclerostin acts not only as a potent antagonist of bone formation, but also as a promotor of bone resorption. The differential mechanical loading information perceived by an osteocyte does appear to induce the structural rearrangement of its lacunae. As seen with immobilization and the classical absence of high impact loads, a dose response curve might exist as it does for osteoclast and osteoblast-dependent bone turnover. Whether catabolic loading regimes, general health status, or age affect such responses has to be determined.
In contrast to unloading and immobilization, the application of mechanical force has mostly been linked to enlargement of osteocyte lacunae to date[79-82]. Possible explanations include, on the one hand, an enhancement of bone resorption via mechanical loading, whereby osteocytes are either stimulated by the mechanical deformation of the perilacunar bone matrix or by fluid flow which is created by shear stresses acting on the osteocyte cell processes[79-81]. On the other hand, mechanical loading also induces new bone formation, and previous studies have described larger osteocyte lacunae in sites recently formed due to loading[82].
Evidence of osteocytic osteolysis in hyperparathyroidism
The notion that PTH as a major calciotropic hormone instigates osteocytes to mobilize calcium through removal of perilacunar mineral was developed in the 1970s when evidence of metabolic osteocyte activation combined with acute calcium plasma concentrations were reported when PTH was administered to thyroparathyroidectomized rats[67,83]. With the development of more sophisticated techniques in later years these initial findings were reproduced and peri-canalicular demineralization was demonstrated in detail using contact microradiography and synchroton X-ray tomography in rodents which were continuously treated with PTH[84,85]. Similar morphological changes occur in patients suffering from hyperparathyroidism based on histological evaluation of iliac crest biopsies[86,87], as well as in patients with renal osteodystrophy[88] or hemodialysis patients through the use of light and electron microscopy[89].
Osteocytic osteolysis and vitamin D
Vitamin D is another pivotal calciotropic hormone closely intertwined with osteocytes. Active vitamin D [1,25(OH)2D3] is primarily produced by the kidney and induces osteocyte production of fibroblast growth factor 23 (FGF23), a potent phosphaturic hormone[90]. However, some interactions between vitamin D and osteocytes are less well defined. Evidence exists that osteocytes can also produce 1,25(OH)2D3[90], but its action is poorly understood. Another subject of active investigation is whether 1,25(OH)2D3 influences perilacunar remodeling. Initial reports on thyroparathyroidectomized rats fed a normal diet and administered 1,25(OH)2D3 in various dosages failed to demonstrate an effect of active vitamin D on the contour of osteocyte lacunae[91]. Conversely, vitamin D deficient humans showed signs of enlarged osteocyte lacunae in histological images[92]. Using advanced 3D high-resolution techniques, we reported that individuals afflicted with vitamin D deficiency and surface osteoidosis had a 14% higher osteocyte lacunar volume than healthy controls[74]. Mice lacking the vitamin D receptor presented with fewer osteocytes, which were characterized by enlarged lacunar area[93]. Individuals with low vitamin D serum concentrations showed also a higher number of empty osteocyte lacunae in iliac crest bone cores[93]. In addition, the surviving osteocytes presented with increased expression of Cathepsin K indicating active osteolysis. These findings highlight a role of vitamin D signaling in osteocyte number, viability and morphology[74,93], but the molecular mechanisms remain unclear.
Osteocytic osteolysis in other settings
Osteocytic osteolysis in glucocorticoid-induced osteoporosis
Glucocorticoid-induced osteoporosis constitutes the most common form of secondary osteoporosis and is characterized by a rapid loss of bone mass, especially in the trabecular compartment[94]. Although traditionally attributed to glucocorticoid actions to inhibit bone formation and transiently increase bone resorption[94], existing data also provides evidence for morphological changes of osteocytes and the presence of hypomineralized bone in the osteocytic microenvironment under glucocorticoid treatment[95]. Using synchrotron microCT und BSEM in a mouse model of induced hypercorticosteronaemia and osteoporosis, Karunaratne et al. showed a reduction of bone quality due to disruption of intracortical architecture and osteocytic osteolysis[96]. Furthermore, highlighting the role of MMPs in the process of osteocytic osteolysis, Sun et al. demonstrated intense immunoreaction of MMP-2 and MMP-13 in the enlarged osteocytic lacunae of prednisolone-treated mice[97]. Conversely, a recent study in osteonecrosis demonstrated a glucocorticoid-induced suppression of osteocyte perilacunar remodeling in rodents and humans, underling the potential differential modulation of osteocytic osteolysis by glucocorticoids in the setting of established bone disease[98].
Periprosthetic osteocytic osteolysis
Revision surgery of joint prostheses is often necessary because of loosening caused by peri-implant bone loss, and studies in animals and humans have highlighted the role of prosthetic wear particles in this procedure[99]. Polyethylene wear particles, which are commonly used for joint replacement, up-regulate the expression of osteoclastic genes in a 3D-culture system of the osteocyte-like MLO-Y4 cells[100], a finding which was later confirmed in vivo in a mouse model of calvarial osteolysis, and in patients undergoing total hip replacement therapy for osteoarthritis[101]. These studies established that osteocytic osteolysis also contributes to peri-implant bone loss, in addition to osteocytic apoptosis induced by prosthetic wear particles[102-104].
Tumor-associated osteocytic osteolysis
A paucity of data exists on tumor-associated osteocytic osteolysis, with Cramer et al. reporting enlarged osteocytic lacunae in osteolytic metastases of patients with lung cancer[105], and Bonucci describing coastal crystals along the border of the osteocytic lacunae adjacent to bone metastases, a finding which could suggest perilacunar demineralization[106]. More research is warranted to address this issue and to elucidate the pathogenetic mechanisms underlining osteocytic osteolysis in the setting of malignant bone disease.
Osteocytic osteolysis due to calcium deficiency vs. enlarged osteocyte lacunae due to new bone formation
There are several bone turnover changes in human cohorts where the analysis of osteocyte lacunar morphology may reveal enlarged osteocyte lacunae in comparison to controls. Paget’s disease of bone is the second most common bone disease, where a local high bone turnover situation may result in substantially enlarged osteocyte lacunae[107]. Here, rapid formation of new bone tissue may lead to the formation of immature bone with a high osteocyte lacunar area, which is quite distinct from the process of active perilacunar matrix removal by the osteocytes (Figure 2). In addition, suture fusion in human cranial sutures is a process associated with changes in bone turnover[108]. During suture fusion, osteocyte lacunar volume is higher than in cases where the cranial sutures are already fused[108]. Therefore, large lacunae might reflect a relative calcium deficiency due to a high demand for calcium during rapid formation of bone[109,110], in contrast to smaller lacunae that can be found in more mature bone packets.
Figure 2.
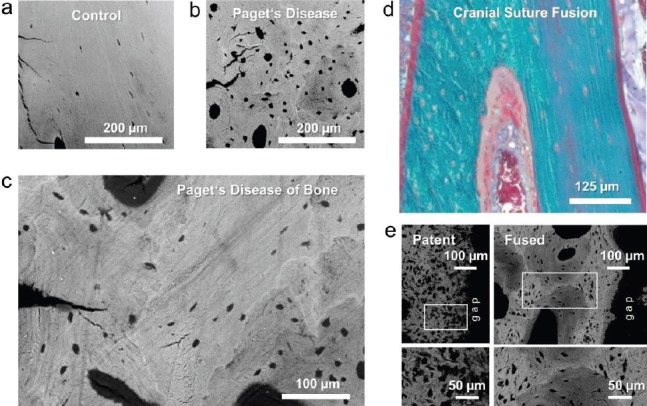
Pagetic bone is representative of larger osteocyte lacunae not dependent upon osteocytic osteolysis. Backscattered electron microscopy images of human iliac crest biopsies from a bone-healthy-age matched control (a) and a patient with Paget’s disease of bone (b, c). (b) Enlarged lacunae in Pagetic bone (c) Areas of less mineralized woven bone most likely caused by high pathological bone turnover typical of Paget’s Disease. (Images a-c adapted from Zimmermann EA, … Busse B. Modifications to Nano- and Microstructural Quality and the Effects on Mechanical Integrity in Paget’s Disease of Bone. JBMR 2015;30;264–73). (d) Histological image of a human cranial suture during fusion (Mason Goldner trichrome staining) showing the presence of both woven and lamellar bone. (e) Backscattered electron microscopy images of a specimen from an infant with a patent cranial suture demonstrating large osteocyte lacunae due to the woven bone for comparison to the specimen from an infant with premature fusion due to cranial synostosis showing lamellar bone with relatively smaller osteocytes. (Images d-e adapted from Regelsberger J, … Busse B. Changes to the cell, tissue and architecture levels in cranial suture synostosis reveal a problem of timing in bone development. ECM Journal 2012;24;441-58).
Evidence that osteocytes have matrix deposition and mineralization ability
The significant role of osteocytes in the mineralization process is well-contested and accompanies the transition of osteoblasts to osteocytes and the expression of genes promoting mineralization, such as dentin matrix protein 1 (DMP1) and the phosphate-regulating gene with homologies to endopeptidases on the X-chromosome (PHEX)[111]. More recently, Phospho1, a soluble cytosolic phosphatase, has been identified as a pivotal regulator of skeletal mineralization and mice lacking PHOSPHO1 are characterized by a hypomineralized matrix and an increased osteocytic lacunar and vascular porosity, suggesting that osteoblast-to-osteocyte transition may be accelerated in the absence of PHOSPHO1[112]. Another recent study has highlighted the role of ecto-nucleotidase pyrophosphatase/phosphodiesterase 1 (NPP1) to regulate tissue mineralization[113]. Although osteoclast formation and resorptive activity are unaffected by NPP1 deletion, mice lacking NPP1 displayed fewer and small osteocyte lacunae, probably because of a localized increase in the amount of matrix mineralisation, which could have a negative impact on osteocyte function and survival[113]. Thus, an intrinsic feedback mechanism to reverse osteocytic osteolysis seems to be at play here, whereby osteocytes actively deposit matrix through upregulation of DMP1, PHEX, Phospho1, and other proteins essential for mineralization. A similar process could characterize the post-lactation period.
Summary and outlook
The removal of perilacunar matrix by osteocytes is now a well-accepted process (Figure 3). Osteocytes are able to acidify their lacunar-canalicular space through the production of protons via the carbonic anhydrase 2 and the release of protons via the proton pumping vacuolar ATPases. While this serves to demineralize the bone matrix and free calcium, MMP-13, tartrate resistance acid phosphatase and cathepsin K remove the organic components of the perilacunar matrix. Several scenarios induce osteocytic osteolysis including activation of PTHR1 by both its ligands PTH and PTHrP, TGFβ signaling, as well as the absence of physiological load potentially via increased expression of sclerostin (Figure 3). On the other hand, calcitonin seems to counteract the removal of perilacunar bone matrix by osteocytes mediated by its action through the calcitonin receptor.
Figure 3.
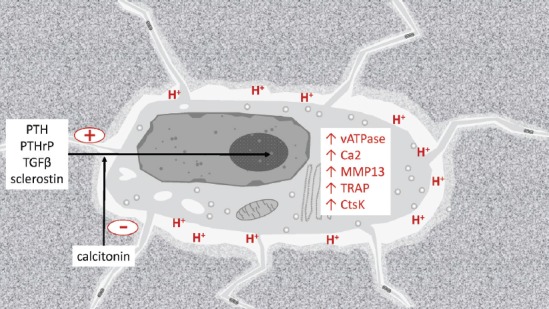
Schematic image of an osteolytic osteocyte in its lacuna. The activation of the PTHR1 via PTH or PTHrP, also the action of TGFβ on its receptor TGFβR2, as well as the absence of strain and therefore the expression of sclerostin can induce the osteolytic state, while calcitonin acts inhibitory. Both the demineralization via acidification involving the Ca2 and the vATPase, and the proteolytic degradation via MMP13, TRAP, and CtsK are implicated in the osteolytic activity of osteocytes.
Up-coming therapeutic modalities demonstrate that the osteocyte is a promising target for the treatment of bone diseases. The mechanistic details and the regulatory circuits involved in osteocytic osteolysis are still to be determined, but an approach to prevent osteocytic osteolysis seems possible and could prevent local bone loss and deterioration of bone quality. So far, the process appears to be mainly utilized in pathological conditions, whereas lactation-induced osteocytic osteolysis suggests it is originally a normal physiological process. A baseline level of activation may exist that could serve osteocytes in maintaining their perilacunar space, or adapt their lacunar volumes to different loading scenarios. The role of demineralization and proteolysis and remineralization by osteocytes will need to be investigated further, but one could imagine that on this small scale, bone is structurally adapted to fit metabolic needs. This would also imply a key role for osteocyte-directed production of osteoid and mineralization, which needs to be address in the future.
Osteocyte lacunae are essentially ellipsoid three-dimensional objects. Using quantitative 2D data may lead to incomplete results due to non-consideration of osteocyte lacunar orientation, i.e. osteocytes orientation in long bones is dependent on both anatomical and biomechanical characteristics; osteocyte lacunae short axis and long axis are not distributed uniformly in regions of interest. When quantifying osteocyte lacunar density and osteocyte lacunar volume, utilization of 3D techniques as X-Ray nanotomography, high-resolution micro-computed tomography, or other methods collecting image stacks for 3D reconstruction will provide ideal datasets for quantification. Slight changes in the dimensions of osteocyte lacunae (i.e. short axis and long axis) will result in large changes in osteocyte lacunar volume.
In summary, recent research in the field of osteocytic osteolysis has indicated that this process has significant physiological and pathophysiological importance, but further studies are warranted to precisely define the mechanisms involved in osteocytic perilacunar remodeling and the relative contribution of osteocytic osteolysis versus osteoclast-driven bone resorption. The thorough elucidation of these mechanisms will lead to a better understanding of bone quality in health and disease.
Grant support
This work was supported by the MedDrive Starting Grant and the Frauenhabilitatiosstipendium of TU Dresden to ET, the DFG SPP 1629 ThyroidTransAct to MR, the DFG BU 2562/3-1 to BB, the DFG JA 2654/1-1 to KJ and the NIH NIA PO1AC39355 to LFB.
Authors’ roles
Drafting manuscript: ET, KJ. Revising manuscript content: ET, KJ, MR, BB, and LFB. Approving final version of manuscript: ET, KJ, MR, BB, and LFB.
Footnotes
E. Tsourdi reports grants from the University Clinic Dresden, Medical Faculty. K. Jaehn, M. Rauner and B. Busse report grants from DFG. L. Bonewald reports grants from NIH.
Edited by: P. Makras
References
- 1.Klein-Nulend J, van der Plas A, Semeins CM, Ajubi NE, Frangos JA, Nijweide PJ, Burger EH. Sensitivity of osteocytes to biomechanical stress in vitro. FASEB J. 1995;9(5):441–5. doi: 10.1096/fasebj.9.5.7896017. [DOI] [PubMed] [Google Scholar]
- 2.van Bezooijen RL, Roelen BA, Visser A, van der Wee-Pals L, de Wilt E, Karperien M, et al. Sclerostin is an osteocyte-expressed negative regulator of bone formation, but not a classical BMP antagonist. J Exp Med. 2004;199(6):805–14. doi: 10.1084/jem.20031454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nakashima T, Hayashi M, Fukunaga T, Kurata K, Oh-Hora M, Feng JQ, et al. Evidence for osteocyte regulation of bone homeostasis through RANKL expression. Nat Med. 2011;17(10):1231–4. doi: 10.1038/nm.2452. [DOI] [PubMed] [Google Scholar]
- 4.Dallas SL, Prideaux M, Bonewald LF. The osteocyte: an endocrine cell and more. Endocr Rev. 2013;34(5):658–90. doi: 10.1210/er.2012-1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Buenzli PR, Sims NA. Quantifying the osteocyte network in the human skeleton. Bone. 2015;75:144–50. doi: 10.1016/j.bone.2015.02.016. [DOI] [PubMed] [Google Scholar]
- 6.Semba T, Tolnai S, Bélanger LF. Observations on the fine structure of chick embryo osteocytes;effect of parathyroid extract. 6th Int Congr Electron Microsc. 1966:569–70. [Google Scholar]
- 7.Rigal A, Vignal W. Recherches experimentales sur la formation du cal et sur les modifications des tissus dans les pseudoarthroses. Arch Physiol. 1881;8:419–58. [Google Scholar]
- 8.Bélanger LF. Osteocytic osteolysis. Calcif Tissue Res. 1969;4(1):1–12. doi: 10.1007/BF02279101. [DOI] [PubMed] [Google Scholar]
- 9.Parfitt AM. The cellular basis of bone turnover and bone loss: a rebuttal of the osteocytic resorption - bone flow theory. Clin Orthop Relat Res. 1977;127:236–47. [PubMed] [Google Scholar]
- 10.Gonen E, Sahin I, Ozbek M, Kovalak E, Yologlu S, Ates Y. Effects of pregnancy and lactation on bone mineral density, and their relation to the serum calcium, phosphorus, calcitonin, and parathyroid hormone levels in rats. J Endocrinol Invest. 2005;28(4):322–26. doi: 10.1007/BF03347197. [DOI] [PubMed] [Google Scholar]
- 11.Peng TC, Kusy RP, Garner SC, Hirsch PF, De Blanco MC. Influence of lactation and pregnancy +lactation on mechanical properties and mineral content of the rat femur. J Bone Miner Res. 1987;2(3):249–57. doi: 10.1002/jbmr.5650020312. [DOI] [PubMed] [Google Scholar]
- 12.Gruber HE, Stover SJ. Maternal and weanling bone: the influence of lowered calcium intake and maternal dietary history. Bone. 1994;15(2):167–76. doi: 10.1016/8756-3282(94)90704-8. [DOI] [PubMed] [Google Scholar]
- 13.Peng TC, Garner SC, Kusy RP, Hirsch PF. Effect of number of suckling pups and dietary calcium on bone mineral content and mechanical properties of femurs of lactating rats. Bone Miner. 1998;3(4):293–304. [PubMed] [Google Scholar]
- 14.Cross NA, Hillman LS, Allen SH, Krause GF. Changes in bone mineral density and markers of bone remodeling during lactation and postweaning in women consuming high amounts of calcium. J Bone Miner Res. 1995;10(09):1312–20. doi: 10.1002/jbmr.5650100907. [DOI] [PubMed] [Google Scholar]
- 15.Kovacs CS, Ralston SH. Presentation and management of osteoporosis presenting in association with pregnancy or lactation. Osteoporos Int. 2015;26(9):2223–41. doi: 10.1007/s00198-015-3149-3. [DOI] [PubMed] [Google Scholar]
- 16.Kovacs CS. Maternal mineral and bone metabolism during pregnancy, lactation, and post-weaning. Physiol Rev. 2016;96(2):449–547. doi: 10.1152/physrev.00027.2015. [DOI] [PubMed] [Google Scholar]
- 17.Honda A, Kurabayashi T, Yahata T, Tomita M, Matsushita H, Takakuwa K, et al. Effects of pregnancy and lactation on trabecular bone and marrow adipocytes in rats. Calcif Tissue Int. 2000;67(5):367–72. doi: 10.1007/s002230001145. [DOI] [PubMed] [Google Scholar]
- 18.Kent GN, Price RI, Gutteridge DH, Smith M, Allen JR, Bhagat CI, et al. Human lactation: forearm trabecular bone loss, increased bone turnover, and renal conservation of calcium and inorganic phosphate with recovery of bone mass following weaning. J Bone Miner Res. 1990;5(4):361–69. doi: 10.1002/jbmr.5650050409. [DOI] [PubMed] [Google Scholar]
- 19.VanHouten JN, Wysolmerski JJ. Low estrogen and high parathyroid hormone-related peptide levels contribute to accelerated bone resorption and bone loss in lactating mice. Endocrinology. 2003;144(12):5521–9. doi: 10.1210/en.2003-0892. [DOI] [PubMed] [Google Scholar]
- 20.Ardeshirpour L, Dann P, Adams DJ, Nelson T, VanHouten J, Horowitz MC, et al. Weaning triggers a decrease in receptor activator of nuclear factor-kappaB ligand expression, widespread osteoclast apoptosis, and rapid recovery of bone mass after lactation in mice. Endocrinology. 2007;148(8):3875–86. doi: 10.1210/en.2006-1467. [DOI] [PubMed] [Google Scholar]
- 21.Qing H, Ardeshirpour L, Pajevic PD, Dusevich V, Jahn K, Kato S, et al. Demonstration of osteocytic osteolysis perilacunar/canalicular remodeling in mice during lactation. J Bone Miner Res. 2012;27(5):1018–29. doi: 10.1002/jbmr.1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rasmussen P. Calcium deficiency, pregnancy, and lactation in rats. Microscopic and microradiographic observations on bones. Calcif Tissue Res. 1977;23(1):95–102. doi: 10.1007/BF02012772. [DOI] [PubMed] [Google Scholar]
- 23.Kovacs CS, Kronenberg HM. Maternal-fetal calcium and bone metabolism during pregnancy, puerperium, and lactation. Endocr Rev. 1997;18(6):832–72. doi: 10.1210/edrv.18.6.0319. [DOI] [PubMed] [Google Scholar]
- 24.VanHouten JN. Maternal calcium and bone metabolism during lactation. Curr Opin Endocrinol Diabetes. 2005;12(6):477–82. [Google Scholar]
- 25.VanHouten JN, Dann P, Stewart AF, Watson CJ, Pollak M, Karaplis AC, et al. Mammary-specific deletion of parathyroid hormone-related protein preserves bone mass during lactation. J Clin Invest. 2003;112(9):1429–36. doi: 10.1172/JCI19504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xiong J, O'Brien CA. Osteocyte RANKL: new insights into the control of bone remodeling. J Bone Miner Res. 2012;27(3):499–505. doi: 10.1002/jbmr.1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.O'Brien CA, Plotkin LI, Galli C, Goellner JJ, Gortazar AR, Allen MR, et al. Control of bone mass and remodeling by PTH receptor signaling in osteocytes. PLoS One. 2008;3(8):e2942. doi: 10.1371/journal.pone.0002942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nakashima T, Hayashi M, Fukunaga T, Kurata K, Oh-Hora M, Feng JQ, et al. Evidence for osteocyte regulation of bone homeostasis through RANKL expression. Nat Med. 2011;17(10):1231–4. doi: 10.1038/nm.2452. [DOI] [PubMed] [Google Scholar]
- 29.Wysolmerski JJ. Osteocytes remove and replace perilacunar mineral during reproductive cycles. Bone. 2013;54(2):230–36. doi: 10.1016/j.bone.2013.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Powell WF, Jr, Barry KJ, Tulum I, Kobayashi T, Harris SE, Bringhurst FR, et al. Targeted ablation of the PTH/PTHrP receptor in osteocytes impairs bone structure and homeostatic calcemic responses. J Endocrinol. 2011;209(1):21–32. doi: 10.1530/JOE-10-0308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jähn K, Kelkar S, Zhao H, Xie Y, Tiede-Lewis LM, Dusevich V, et al. Osteocytes acidify their microenvironment in response to PTHrP in vitro and in lactating mice in vivo. J Bone Miner Res. 2017;32(8):1761–72. doi: 10.1002/jbmr.3167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ardeshirpour L, Brian S, Dann P, VanHouten J, Wysolmerski J. Increased PTHrP and decreased estrogens alter bone turnover but do not reproduce the full effects of lactation on the skeleton. Endocrinology. 2010;151(12):5591–601. doi: 10.1210/en.2010-0566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Paoletti AM, Orru M, Floris L, Guerriero S, Ajossa S, Romagnino S, et al. Pattern of bone markers during pregnancy and their changes after delivery. Horm Res. 2003;59(1):21–9. doi: 10.1159/000067935. [DOI] [PubMed] [Google Scholar]
- 34.Anderson JJB, Garner SC, Mar MH, Boass A, Toverud SU, Parikh I. The ovariectomized, lactating rat as an experimental model for osteopenia: calcium metabolism and bone changes. Bone and Mineral. 1990;11(1):43–53. doi: 10.1016/0169-6009(90)90014-7. [DOI] [PubMed] [Google Scholar]
- 35.Tomkinson A, Reeve J, Shaw RW, Noble BS. The death of osteocytes via apoptosis accompanies estrogen withdrawal in human bone. J Clin Endocrinol Metab. 1997;82(9):3128–35. doi: 10.1210/jcem.82.9.4200. [DOI] [PubMed] [Google Scholar]
- 36.Tomkinson A, Gevers EF, Wit JM, Reeve J, Noble BS. The role of estrogen in the control of rat osteocyte apoptosis. J Bone Miner Res. 1998;13(8):1243–50. doi: 10.1359/jbmr.1998.13.8.1243. [DOI] [PubMed] [Google Scholar]
- 37.Emerton KB, Hu B, Woo AA, Sinofsky A, Hernandez C, Majeska RJ, et al. Osteocyte apoptosis and control of bone resorption following ovariectomy in mice. Bone. 2010;46(3):577–83. doi: 10.1016/j.bone.2009.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ciani C, Sharma D, Doty SB, Fritton SP. Ovariectomy enhances mechanical load-induced solute transport around osteocytes in rat cancellous bone. Bone. 2014;59:229–34. doi: 10.1016/j.bone.2013.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Thiede MA. The mRNA encoding a parathyroid hormone-like peptide is produced in mammary tissue in response to elevations in serum prolactin. Mol Endocrinol. 1989;3(9):1443–47. doi: 10.1210/mend-3-9-1443. [DOI] [PubMed] [Google Scholar]
- 40.Seriwatanachai D, Thongchote K, Charoenphandhu N, Pandaranandaka J, Tudpor K, Teerapornpuntakit J, et al. Prolactin directly enhances bone turnover by raising osteoblast-expressed receptor activator of nuclear factor kappaB ligand/osteoprotegerin ratio. Bone. 2008;42(3):535–46. doi: 10.1016/j.bone.2007.11.008. [DOI] [PubMed] [Google Scholar]
- 41.Suntornsaratoon P, Wongdee K, Krishnamra N, Charoenphandhu N. Femoral bone mineral density and bone mineral content in bromocriptine-treated pregnant and lactating rats. Am J Physiol Endocrinol Metab. 2010;299(3):E426–36. doi: 10.1152/ajpendo.00134.2010. [DOI] [PubMed] [Google Scholar]
- 42.Blahosova A, Neradilova M, Velicky J, Titlbach M, Marsikova L, Reisenauer R. Dynamics of changes of calcium and phosphorus metabolism in relation to the morphology of parafollicular thyroid cells in rats during lactation and forced weaning. Endokrinologie. 1974;63(1):122–36. [PubMed] [Google Scholar]
- 43.Toverud SU, Cooper CW, Munson PL. Calcium metabolism during lactation: elevated blood levels of calcitonin. Endocrinology. 1978;103(2):472–9. doi: 10.1210/endo-103-2-472. [DOI] [PubMed] [Google Scholar]
- 44.Stevenson JC, Hillyard CJ, MacIntyre Cooper H, Whitehead MI. A physiological role for calcitonin: protection of the maternal skeleton. Lancet. 1979;2(8146):769–70. doi: 10.1016/s0140-6736(79)92117-2. [DOI] [PubMed] [Google Scholar]
- 45.Greer FR, Lane J, Ho M. Elevated serum parathyroid hormone, calcitonin, and 1,25-dihydroxyvitamin D in lactating women nursing twins. Am J Clin Nutr. 1984;40(3):562–8. doi: 10.1093/ajcn/40.3.562. [DOI] [PubMed] [Google Scholar]
- 46.Ren Y, Sun YP, Shah GV. Calcitonin inhibits prolactin promoter activity in rat pituitary GGH3 cells: evidence for involvement of p42/44 mitogen-activated protein kinase in calcitonin action. Endocrine. 2003;20(1-2):13–22. doi: 10.1385/ENDO:20:1-2:13. [DOI] [PubMed] [Google Scholar]
- 47.Tohei A, VandeGarde B, Arbogast LA, Voogt JL. Calcitonin inhibition of prolactin secretion in lactating rats: mechanism of action. Neuroendocrinology. 2000;71(5):327–32. doi: 10.1159/000054553. [DOI] [PubMed] [Google Scholar]
- 48.Clarke MV, Russell PK, Findlay DM, Sastra S, Anderson PH, Skinner JP, et al. A role for the calcitonin receptor to limit bone loss during lactation in female mice by inhibiting osteocytic osteolysis. Endocrinology. 2015;156(9):3203–14. doi: 10.1210/en.2015-1345. [DOI] [PubMed] [Google Scholar]
- 49.Duriez R, Duriez J. Periosteocyte demineralization in disuse osteoporosis. The effect of calcitonin. Int Orthop. 1981;5(4):299–304. doi: 10.1007/BF00271086. [DOI] [PubMed] [Google Scholar]
- 50.Nunez EA, Horwith M, Krook L, Whalen JP. An electron microscopic investigation of human familial bone dysplasia. Inhibition of osteocyticosteolysis and induction of osteocytic formation of elastic fibers following calcitonin treatment. Am J Pathol. 1979;94(1):1–18. [PMC free article] [PubMed] [Google Scholar]
- 51.Tang SY, Herber RP, Ho SP, Alliston T. Matrix metalloproteinase-13 is required for osteocytic perilacunar remodeling and maintains bone fracture resistance. J Bone Miner Res. 2012;27(9):1936–50. doi: 10.1002/jbmr.1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Paiva KBS, Granjeiro JM. Matrix metalloproteinases in bone resorption, remodeling and repair. Prog Mol Biol Transl Sci. 2017;148:203–303. doi: 10.1016/bs.pmbts.2017.05.001. [DOI] [PubMed] [Google Scholar]
- 53.Tokarz D, Martins JS, Petit ET, Lin CP, Demay MB, Liu ES. Hormonal regulation of osteocyte perilacunar and canalicular remodeling in the Hyp mouse model of XLH. J Bone Miner Res. 2017;33(3):499–509. doi: 10.1002/jbmr.3327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dole NS, Mazur CM, Acevedo C, Lopez JP, Monteiro DA, Fowler TW, et al. Osteocyte-intrinsic TGF-βsignaling regulates bone quality through perilacunar/canalicular remodeling. Cell Rep. 2017;21(9):2585–96. doi: 10.1016/j.celrep.2017.10.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Teti A, Zallone A. Do osteocytes contribute to bone mineral homeostasis? Osteocytic osteolysis revisited. Bone. 2009;44(1):11–6. doi: 10.1016/j.bone.2008.09.017. [DOI] [PubMed] [Google Scholar]
- 56.Liu X, Ardeshirpour L, VanHouten JN, Shane E, Wysolmerski JJ. Site-specific changes in bone microarchitecture, mineralization, and stiffness during lactation and after weaning in mice. J Bone Miner Res. 2012;27(4):865–75. doi: 10.1002/jbmr.1503. [DOI] [PubMed] [Google Scholar]
- 57.Kaya S, Basta-Pljakic J, Seref-Ferlengez Z, Majeska RJ, Cardoso L, Bromage T, et al. Lactation-induced changes in the volume of osteocyte lacunar-canalicular space alter mechanical properties in cortical bone tissue. J Bone Miner Res. 2017;32(4):688–97. doi: 10.1002/jbmr.3044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Collins JN, Kirby BJ, Woodrow JP, Gagel RF, Rosen CJ, Sims NA, et al. Lactating Ctcgrp nulls lose twice the normal bone mineral content due to fewer osteoblasts and more osteoclasts, whereas bone mass is fully restored after weaning in association with up-regulation of Wnt signaling and other novel genes. Endocrinology. 2013;154(4):1400–13. doi: 10.1210/en.2012-1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Qing H, Bonewald LF. Osteocyte remodeling of the perilacunar and pericanalicular matrix. Int J Oral Sci. 2009;1(2):59–65. doi: 10.4248/ijos.09019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Brembeck P, Lorentzon M, Ohlsson C, Winkvist A, Augustin H. Changes in cortical volumetric bone mineral density and thickness, and trabecular thickness in lactating women postpartum. J Clin Endocrinol Metab. 2015;100(2):535–43. doi: 10.1210/jc.2014-2825. [DOI] [PubMed] [Google Scholar]
- 61.Alderman BW, Weiss NS, Daling JR, Ure CL, Ballard JH. Reproductive history and postmenopausal risk of hip and forearm fracture. Am J Epidemiol. 1986;124(2):262–7. doi: 10.1093/oxfordjournals.aje.a114384. [DOI] [PubMed] [Google Scholar]
- 62.Carranza-Lira S, Mera JP. Influence of number of pregnancies and total breast-feeding time on bone mineral density. Int J Fertil Womens Med. 2002;47(4):169–71. [PubMed] [Google Scholar]
- 63.Grainge MJ, Coupland CA, Cliffe S, Chilvers CE, Hosking DJ. Reproductive, menstrual and menopausal factors: which are associated with bone mineral density in early postmenopausal women? Osteoporos Int. 2001;12(9):777–87. doi: 10.1007/s001980170055. [DOI] [PubMed] [Google Scholar]
- 64.Kovacs CS. The skeleton is a storehouse of mineral that is plundered during lactation and (fully?) replenished afterwards. J Bone Miner Res. 2017;32(4):676–80. doi: 10.1002/jbmr.3090. [DOI] [PubMed] [Google Scholar]
- 65.Jiang SD, Dai LY, Jiang LS. Osteoporosis after spinal cord injury. Osteoporos Int. 2006;17(2):180–92. doi: 10.1007/s00198-005-2028-8. [DOI] [PubMed] [Google Scholar]
- 66.Thomsen JS, Morukov BV, Vico L, Alexandre C, Saparin PI, Gowin W. Cancellous bone structure of iliac crest biopsies following 370 days of head-down bed rest. Aviat Space Environ Med. 2005;76(10):915–22. [PubMed] [Google Scholar]
- 67.Krempien B, Friedrich E, Ritz E. Effect of PTH on osteocyte ultrastructure. Adv Exp Med Biol. 1978;103:437–50. doi: 10.1007/978-1-4684-7758-0_45. [DOI] [PubMed] [Google Scholar]
- 68.Wojda SJ, Gridley RA, McGee-Lawrence ME, Drummer TD, Hess A, Kohl F, et al. Arctic ground squirrels limit bone loss during the prolonged physical inactivity associated with hibernation. Physiol Biochem Zool. 2016;89(1):72–80. doi: 10.1086/684619. [DOI] [PubMed] [Google Scholar]
- 69.Rodionova NV, Oganov VS, Zolotova NV. Ultrastructural changes in osteocytes in microgravity conditions. Adv Space Res. 2002;30(4):765–70. doi: 10.1016/s0273-1177(02)00393-9. [DOI] [PubMed] [Google Scholar]
- 70.Bach-Gansmo FL, Wittig NK, Brüel A, Thomsen JS, Birkedal H. Immobilization and long-term recovery results in large changes in bone structure and strength but no corresponding alterations of osteocyte lacunar properties. Bone. 2016;91:139–47. doi: 10.1016/j.bone.2016.07.005. [DOI] [PubMed] [Google Scholar]
- 71.Blaber EA, Dvorochkin N, Lee C, Alwood JS, Yousuf R, Pianetta P, et al. Microgravity induces pelvic bone loss through osteoclastic activity, osteocytic osteolysis, and osteoblastic cell cycle inhibition by CDKN1a/p21. PLoS One. 2013;8(4):e61372. doi: 10.1371/journal.pone.0061372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lloyd SA, Loiselle AE, Zhang Y, Donahue HJ. Evidence for the role of connexin 43-mediated intercellular communication in the process of intracortical bone resorption via osteocytic osteolysis. BMC Musculoskelet Disord. 2014;15:122. doi: 10.1186/1471-2474-15-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sano H, Kikuta J, Furuya M, Kondo N, Endo N, Ishii M. Intravital bone imaging by two-photon excitation microscopy to identify osteocytic osteolysis in vivo. Bone. 2015;74:134–9. doi: 10.1016/j.bone.2015.01.013. [DOI] [PubMed] [Google Scholar]
- 74.Busse B, Bale HA, Zimmermann EA, Panganiban B, Barth HD, Carriero A, et al. Vitamin D deficiency induces early signs of aging in human bone, increasing the risk of fracture. Sci Transl Med. 2013;5(193):193ra88. doi: 10.1126/scitranslmed.3006286. [DOI] [PubMed] [Google Scholar]
- 75.Wijenayaka AR, Kogawa M, Lim HP, Bonewald LF, Findlay DM, Atkins GJ. Sclerostin stimulates osteocyte support of osteoclast activity by a RANKL-dependent pathway. PLoS One. 2011;6(10):e25900. doi: 10.1371/journal.pone.0025900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kogawa M, Wijenayaka AR, Ormsby RT, Thomas GP, Anderson PH, Bonewald LF, et al. Sclerostin regulates release of bone mineral by osteocytes by induction of carbonic anhydrase 2. J Bone Miner Res. 2013;28(12):2436–48. doi: 10.1002/jbmr.2003. [DOI] [PubMed] [Google Scholar]
- 77.Moustafa A, Sugiyama T, Prasad J, Zaman G, Gross TS, Lanyon LE, et al. Mechanical loading-related changes in osteocyte sclerostin expression in mice are more closely associated with the subsequent osteogenic response than the peak strains engendered. Osteoporos Int. 2012;23(4):1225–34. doi: 10.1007/s00198-011-1656-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kogawa M, Khalid KA, Wijenayaka AR, Ormsby RT, Evdokiou A, Anderson PH, et al. Recombinant sclerostin antagonizes effects of ex vivo mechanical loading in trabecular bone and increases osteocyte lacunar size. Am J Physiol Cell Physiol. 2018;314(1):C53–C61. doi: 10.1152/ajpcell.00175.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ferreyra RS, Ubios AM, Gendelman H, Cabrini RL. Enlargement of periosteocytic lacunae associated to mechanical forces. Acta Odontol Latinoam. 2000;13(1):31–8. [PubMed] [Google Scholar]
- 80.Lanyon LE. Osteocytes, strain detection, bone modeling and remodeling. Calcif Tissue Int. 1993;53(Suppl 1):S102–7. doi: 10.1007/BF01673415. [DOI] [PubMed] [Google Scholar]
- 81.Nicolella DP, Moravits DE, Gale AM, Bonewald LF, Lankford J. Osteocyte lacunae tissue strain in cortical bone. J Biomech. 2006;39(9):1735–43. doi: 10.1016/j.jbiomech.2005.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Meunier PJ, Coindre JM, Edouard CM, Arlot ME. Bone histomorphometry in Paget's disease. Quantitative and dynamic analysis of pagetic and nonpagetic bone tissue. Arthritis Rheum. 1980;23(10):1095–103. doi: 10.1002/art.1780231005. [DOI] [PubMed] [Google Scholar]
- 83.Talmage RV, Doppelt SH, Fondren FB. An interpretation of acute changes in plasma 45Ca following parathyroid hormone administration to thyroparathyroidectomized rats. Calcif Tissue Res. 1976;22(2):117–28. doi: 10.1007/BF02010351. [DOI] [PubMed] [Google Scholar]
- 84.Tazawa K, Hoshi K, Kawamoto S, Tanaka M, Ejiri S, Ozawa H. Osteocytic osteolysis observed in rats to which parathyroid hormone was continuously administered. J Bone Miner Metab. 2004;22(6):524–9. doi: 10.1007/s00774-004-0519-x. [DOI] [PubMed] [Google Scholar]
- 85.Nango N, Kubota S, Hasegawa T, Yashiro W, Momose A, Matsuo K. Osteocyte-directed bone demineralization along canaliculi. Bone. 2016;84:279–88. doi: 10.1016/j.bone.2015.12.006. [DOI] [PubMed] [Google Scholar]
- 86.Menieur P, Bernard J, Vignon G. The measurement of periosteocytic enlargement in primary and secondary hyperparathyroidism. Isr J Med Sci. 1971;7(3):482–5. [PubMed] [Google Scholar]
- 87.Mosekilde L, Melsen F. A tetracycline-based histomorphometric evaluation of bone resorption and bone turnover in hyperthyroidism and hyperparathyroidism. Acta Med Scand. 1978;204(1-2):97–102. doi: 10.1111/j.0954-6820.1978.tb08406.x. [DOI] [PubMed] [Google Scholar]
- 88.Bonucci E, Gherardi G. Osteocyte ultrastructure in renal osteodystrophy. Virchows Arch A Pathol Anat Histol. 1977;373(3):213–31. doi: 10.1007/BF00432238. [DOI] [PubMed] [Google Scholar]
- 89.Bonucci E, Gherardi G, Faraggiana T. Bone changes in hemodialyzed uremic subjects. Comparative light and electron microscope investigations. Virchows Arch A Pathol Anat Histol. 1976;371(3):183–98. doi: 10.1007/BF00433067. [DOI] [PubMed] [Google Scholar]
- 90.Lanske B, Densmore MJ, Erben RG. Vitamin D endocrine system and osteocytes. Bonekey Rep. 2014;3:494. doi: 10.1038/bonekey.2013.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Weisbrode SE, Capen CC, Norman AW. Light-and electron-microscopic evaluation of the effects of 1,25-dihydroxyvitamin D3 on bone of thyroparathyroidectomized rats. Am J Pathol. 1979;97(2):247–60. [PMC free article] [PubMed] [Google Scholar]
- 92.Brickley M, Mays S, Ives R. An investigation of skeletal indicators of vitamin D deficiency in adults: effective markers for interpreting past living conditions and pollution levels in 18th and 19th century Birmingham, England. Am J Phys Anthropol. 2007;132(1):67–79. doi: 10.1002/ajpa.20491. [DOI] [PubMed] [Google Scholar]
- 93.Rolvien T, Krause M, Jeschke A, Yorgan T, Püschel K, Schinke T, et al. Vitamin D regulates osteocyte survival and perilacunar remodeling in human and murine bone. Bone. 2017;103:78–87. doi: 10.1016/j.bone.2017.06.022. [DOI] [PubMed] [Google Scholar]
- 94.Hofbauer LC, Rauner M. Live and let die: effects of glucocorticoids on bone cells. Mol Endocrinol. 2009;23(10):1525–31. doi: 10.1210/me.2009-0069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Lane NE, Yao W, Balooch M, Nalla RK, Balooch G, Habelitz S, et al. Glucocorticoid-treated mice have localized changes in trabecular bone material properties and osteocyte lacunar size that are not observed in placebo-treated or estrogen-deficient mice. J Bone Miner Res. 2006;21(3):466–76. doi: 10.1359/JBMR.051103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Karunaratne A, Xi L, Bentley L, Sykes D, Boyde A, Esapa CT, et al. Multiscale alterations in bone matrix quality increased fragility in steroid induced osteoporosis. Bone. 2016;84:15–24. doi: 10.1016/j.bone.2015.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Sun B, Sun J, Han X, Liu H, Li J, Du J, et al. Immunolocalization of MMP 2, 9 and 13 in prednisolone induced osteoporosis in mice. Histol Histopathol. 2016;31(6):647–56. doi: 10.14670/HH-11-702. [DOI] [PubMed] [Google Scholar]
- 98.Fowler TW, Acevedo C, Mazur CM, Hall-Glenn F, Fields AJ, Bale HA, et al. Glucocorticoid suppression of osteocyte perilacunar remodeling is associated with subchondral bone degeneration in osteonecrosis. Sci Rep. 2017;7:44618. doi: 10.1038/srep44618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Harris WH. The first 50 years of total hip arthroplasty: lessons learned. Clin Orthop Relat Res. 2009;467(1):28–31. doi: 10.1007/s11999-008-0467-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Atkins GJ, Welldon KJ, Holding CA, Haynes DR, Howie DW, Findlay DM. The induction of a catabolic phenotype in human primary osteoblasts and osteocytes by polyethylene particles. Biomaterials. 2009;30(22):3672–81. doi: 10.1016/j.biomaterials.2009.03.035. [DOI] [PubMed] [Google Scholar]
- 101.Ormsby RT, Cantley M, Kogawa M, Solomon LB, Haynes DR, Findlay DM, et al. Evidence that osteocyte perilacunar remodelling contributes to polyethylene wear particle induced osteolysis. Acta Biomater. 2016;33:242–51. doi: 10.1016/j.actbio.2016.01.016. [DOI] [PubMed] [Google Scholar]
- 102.Zhang Y, Yan M, Yu A, Mao H, Zhang J. Inhibitory effects of β-tricalciumphosphate wear particles on osteocytes via apoptotic response and Akt inactivation. Toxicology. 2012;297(1-3):57–67. doi: 10.1016/j.tox.2012.04.002. [DOI] [PubMed] [Google Scholar]
- 103.Zawawi MS, Marino V, Perilli E, Cantley MD, Xu J, Purdue PE, et al. Parthenolide reduces empty lacunae and osteoclastic bone surface resorption induced by polyethylene particles in a murine calvarial model of peri-implant osteolysis. J Biomed Mater Res A. 2015;103(11):3572–9. doi: 10.1002/jbm.a.35484. [DOI] [PubMed] [Google Scholar]
- 104.Kanaji A, Caicedo MS, Virdi AS, Sumner DR, Hallab NJ, Sena K, et al. Co-Cr-Mo alloy particles induce tumor necrosis factor alpha production in MLO-Y4 osteocytes: a role for osteocytes in particle-induced inflammation. Bone. 2009;45(3):528–33. doi: 10.1016/j.bone.2009.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Cramer SF, Fried L, Carter KJ. The cellular basis of metastatic bone disease in patients with lung cancer. Cancer. 1981;48(12):2649–60. doi: 10.1002/1097-0142(19811215)48:12<2649::aid-cncr2820481217>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 106.Bonucci E. Pathophysiology of cancer metastases in bone and of the change they induce in bone remodeling. Rend Fis Accad Lincei. 2002;13:181–246. [Google Scholar]
- 107.Zimmermann EA, Köhne T, Bale HA, Panganiban B, Gludovatz B, Zustin J, et al. Modifications to nano- and microstructural quality and the effects on mechanical integrity in Paget's disease of bone. J Bone Miner Res. 2015;30(2):264–73. doi: 10.1002/jbmr.2340. [DOI] [PubMed] [Google Scholar]
- 108.Regelsberger J, Milovanovic P, Schmidt T, Hahn M, Zimmermann EA, Tsokos M, et al. Changes to the cell, tissue and architecture levels in cranial suture synostosis reveal a problem of timing in bone development. Eur Cell Mater. 2012;24:441–58. doi: 10.22203/ecm.v024a31. [DOI] [PubMed] [Google Scholar]
- 109.Kusuzaki K, Kageyama N, Shinjo H, Takeshita H, Murata H, Hashiguchi S, et al. Development of bone canaliculi during bone repair. Bone. 2000;27(5):655–9. doi: 10.1016/s8756-3282(00)00383-5. [DOI] [PubMed] [Google Scholar]
- 110.Hernandez CJ, Majeska RJ, Schaffler MB. Osteocyte density in woven bone. Bone. 2004;35(5):1095–9. doi: 10.1016/j.bone.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 111.Atkins GJ, Findlay DM. Osteocyte regulation of bone mineral: a little give and take. Osteoporos Int. 2012;23(8):2067–79. doi: 10.1007/s00198-012-1915-z. [DOI] [PubMed] [Google Scholar]
- 112.Javaheri B, Carriero A, Staines KA, Chang YM, Houston DA, Oldknow KJ, et al. Phospho1 deficiency transiently modifies bone architecture yet produces consistent modification in osteocyte differentiation and vascular porosity with ageing. Bone. 2015;81:277–91. doi: 10.1016/j.bone.2015.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Hajjawi MO, MacRae VE, Huesa C, Boyde A, Millán JL, Arnett TR, et al. Mineralisation of collagen rich soft tissues and osteocyte lacunae in Enpp1(-/-) mice. Bone. 2014;69:139–47. doi: 10.1016/j.bone.2014.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]


