Abstract
Objectives:
To determine the average health-related quality of life (HRQOL) score levels and their determinants in patients with predialysis chronic kidney disease (CKD).
Methods:
A systematic literature search was conducted for relevant observational studies published between April 2007 and April 2017 in MEDLINE, EBSCOhost, and CINAHL databases.
Results:
Thirteen observational studies with a total sample of 8635 subjects comprising 53.3% male with an aggregate mean age of 59.5 (SD 14.9) years were included in this review. Of the 8 generic HRQOL domains of the Short-Form Health Surveys, Social Functioning had the highest mean score whereas General Health had the lowest mean score in patients with predialysis CKD. Physical component summary (PCS) was more impaired than mental component summary (MCS). The determinants of poor HRQOL in predialysis CKD patients included both modifiable risk factors such as comorbidities (namely anxiety and depression), low serum hemoglobin level, sedentary lifestyle, unemployment and non-modifiable risk factors such as poor glomerular filtration rate, female gender, and older age. The risk factors impeded PCS more than MCS.
Conclusion:
Several risk factors influence HRQOL impairment in patients with predialysis CKD, with PCS being more impacted than MCS. The risk factors for poor HRQOL are important for future research and for improving renal care in patients with predialysis CKD.
PROSPERO registration number: CRD42018093385
Chronic kidney disease (CKD) afflicts approximately 500 million adults worldwide,1 majority of whom are in the asymptomatic predialysis stages.2 Impaired health-related quality of life (HRQOL) is one of the important outcomes among patients with CKD.1,3 Health-related quality of life is essentially the measure self-perceived functioning and wellbeing and is usually assessed with standardized patient-reported outcome measures (PROMs) such as the Short Form-36 (SF-36)4 and EuroQol (EQ-5D).5
The SF-36 is the most widely used generic PROM.4 It contains 36 multidimensional questions and profiles HRQOL under 8 broad domains, namely physical functioning (PF), role physical (RP), bodily-pain (BP), role-emotional (RE), vitality (VT), general health (GH), social functioning (SF), and mental health (MH), which are amenable into 2 summary components, physical component summary (PCS) and mental component summary (MCS).6
Health-related quality of life has emerged as an important outcome measure in patients with CKD,5 where its impairment showed increased risks of adverse clinical outcomes such as cardiovascular (CV) events and death.7 Several clinical and nonclinical factors affect HRQOL in CKD patients,5,8 and the control of these factors improves HRQOL, which in turn increases overall health outcomes and lessens disease burden in patients with CKD.9 However, the majority of the studies on HRQOL and its determinants in patients with CKD have been conducted on patients with end-stage renal disease (ESRD) undergoing renal replacement therapy (RRT)10,11 and only a few on patients with predialysis CKD.5,7,12 The lack of extensive studies on HRQOL among the latter group necessitated the need for this review to synthesize findings, highlight study gaps, and make recommendations for future studies.13
Thus, this review was carried out to estimate the average HRQOL scores in patients with predialysis CKD, as measured by the variants of the SF-36 health survey,4 including the Kidney Disease Quality of Life (KDQOL), and synthesize risk factors for poor HRQOL in this population.
Methods
Search strategy
A systematic search in Medline/PubMed, EBSCOhost, and CINAHL databases using Medical Subject Headings (MeSH) terms were carried out to identify articles on HRQOL and its risk factors in patients with predialysis CKD. The search terms were “determinants”, “risk factors”, “health-related quality of life”, “predialysis”, “renal disease”, and “chronic kidney disease.” Alternative terms such as “QOL”, “HRQOL”, and “CKD” were included, and all searches were limited to original research articles published between April 2007 and April 2017. Hand search for relevant grey literature was also carried out.
Study eligibility criteria
Studies were included if they met all the following inclusion criteria: original observational study in design (cross-sectional and/or cohort); subjects consisted of adults with non-dialysis, predialysis CKD (>18 years old), regardless of comorbidity present; HRQOL (measured by any variant of SF-36, including the KDQOL) and risk factors were measured; published in English Language and between April 2007 and April 2017. Studies were excluded if HRQOL tools other than the variants of SF-36 were used or not specified at all, or the study examined predialysis CKD and ESRD patients on RRT together (to prevent the influence of RRT on HRQOL scores), or the patients were aged less than 18 years.
Studies selection and data extraction
One author (MMA) searched for the articles, while another author (LN) vetted the articles from their titles and abstracts for relevance to set criteria and extracted the ones that met the inclusion criteria for further scrutiny. Two authors (MMA & NAAT) independently matched the retrieved articles with the set inclusion criteria. Another (MRWAH) moderated any discrepancy between 2 assessors. Data from the selected studies were obtained using a modified JBI Data Extraction Form for Observational Studies.14 The adjustment was to allow the extraction of data under additional terms. Overall, the retrieved information were: study method and statistical analyses, setting (country), sample size, CKD classification method, mean age, HRQOL tool used and results on HRQOL and risk factors.
Methodological quality assessment
Two authors (LN & JT) assessed the retrieved articles for quality using a modified Joanna Briggs Institute Meta-Analysis of Statistics Assessment and Review Instrument (JBI-MAStARI) standardized critical appraisal tool.15 The kappa inter-rated reliability of 85% was achieved with the use of SPSS v16.16
Statistical analysis
In 3 studies,17-19 the mean and standard deviation (SD) was calculated from the given component HRQOL scores, SD and sample size using “Analysis of Variance with summary data” procedure in Statistical Package for Social Sciences Version 16.20 The HRQOL summary scales PCS (PF, RP, BP, and GH) and MCS (VT, SF, RE, and MH) were also computed from pooling the scores of the constituent domains in 2 studies21,22 using the same statistical procedure above. The pooled weighted means and SDs of the SF domains were also calculated using the statistical procedure above. However, the results on the association between HRQOL and risk factors are those available in each individual article, and no meta-regression was conducted. The reported statistical difference (p<0.05 as significant) was also reported as available in the studies.
Results
Study search and selection
Two thousand and 3 articles (n=2003) were identified from searches across the databases and 51 articles were retrieved after vetting. A further screening of the retrieved articles excluded 38 articles for ESRD/combined ESRD and CKD patients (n=18), irrelevance to set objectives (n=10), weak quality (n=6), used non-included HRQOL tools (n=3), and inaccessibility (n=1). Thirteen studies consisting of cross-sectional (n=10) and prospective cohort (n=3) studies were included in this review, following the preferred reporting items for systematic reviews and meta-analysis (PRISMA) guideline.23 Figure 1 summarized the characteristics of included studies.
Figure 1.
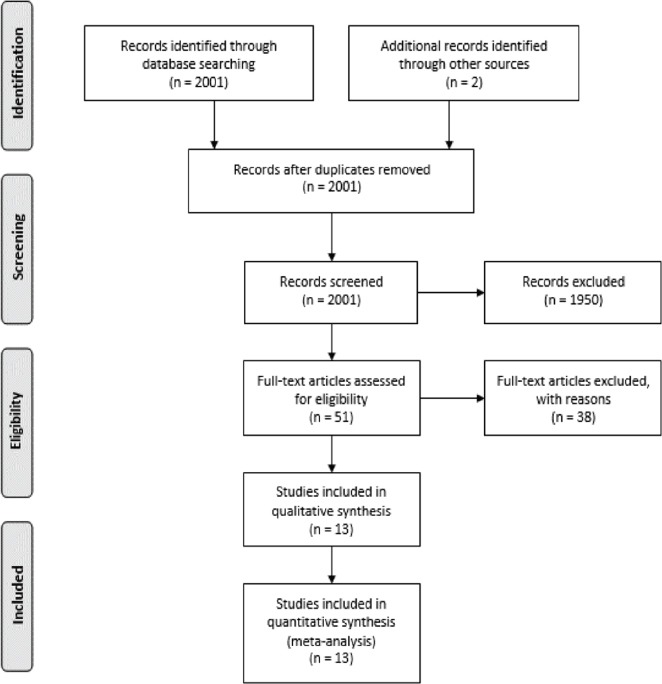
PRISMA flow diagram of study search and selection.23
By study design, 9 studies were cross-sectional, 2 prospective cohorts, one prospective observational, and one ‘transversal descriptive.’ Sample size varied from 57 to 3837.7,24 The combined sample size (n) of subjects in this review was 8635, of which 4033 (46.7%) were female. The aggregated mean age was 59.5 (SD=14.9) years. Ten studies estimated glomerular filtration rate (eGFR) using variations of Modification of Diet in Renal Disease Formula (MDRD), 3 studies17,22,25 used CKD-Epidemiology Collaboration Creatinine Equation (CKD-EPI), and 1 study used Cockroft Gault formula alongside MDRD.26 All studies classified CKD according to the US National Kidney Foundation Kidney Disease Outcomes Quality Initiative (NKF KDOQI)27 (Table 1).
Table 1.
Characteristics of the included studies (baseline).
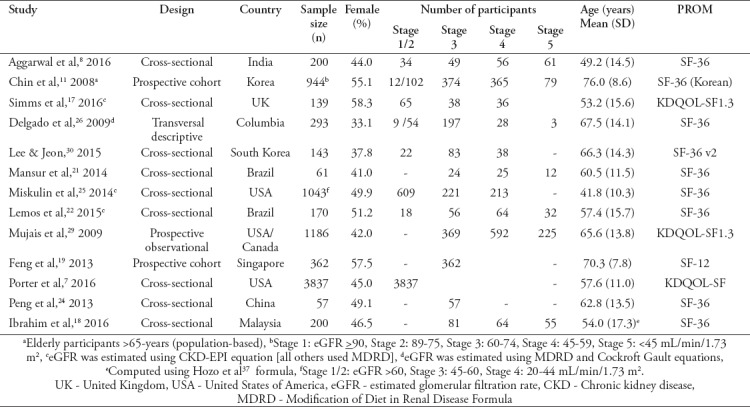
Two studies were population-based; one was among subjects aged above 65 years,28 and the other was among subjects aged 55 and above.19 Two other studies17,25 were carried out on patients diagnosed with autosomal dominant polycystic kidney disease (ADPKD) and impaired renal function. The SF tools were self-administered or administered with help.
Majority of the studies recruited patients with comorbid conditions, mainly hypertension, diabetes mellitus (DM), cardiovascular disease (CVD), history of cardiovascular accident (CVA), myocardial infarction (MI), congestive heart failure (CHF), peripheral vascular disease (PVD), coronary heart disease (CHD), anxiety and depression. In one study,28 71.1% of the sample (n=944) had hypertension, 20.9% DM, 16.5% depression, 10.1% history of CVA, and 7.6% CHD. In two studies, patients with major comorbidities such as active cancer, serious neurological disorders, history of CVA, infectious and inflammatory disease,22 strokes and dementia18 were excluded. Patients with serious incapacitating physical or mental conditions were excluded in all the studies. The studies measured HRQOL with SF-36v1 (n=9/13), KDQOL-SF (n=2), SF-36v2 (n=1), and SF-12 (n=1). For prospective studies, only baseline values were included in this review.
HRQOL scores in patients with predialysis CKD
On average, SF domain had the highest mean score of 76.2 (SD=26.5) over 100. Role-emotional with 75.8 (SD=34.4) was higher than MH with 71.8 (SD=20.6) and BP with 67.1 (SD=26.9). The mean scores for PF was 65.2 (SD=29.8) and RP was 65.2 (SD=37.4), while VT domain score was 54.7 (SD=23.0). The domain with the least score was GH with 51.9 (SD=22.7). For summary scores, MCS with a mean score of 52.3 (SD=14.5) was higher than PCS with a mean score of 46.0 (SD=15.6) (Table 2).
Table 2.
Mean scores of HRQOL in predialysis CKD patients (measured with SF-36, SF-36 v2, SF-12 & KDQOL-SF) at baseline.
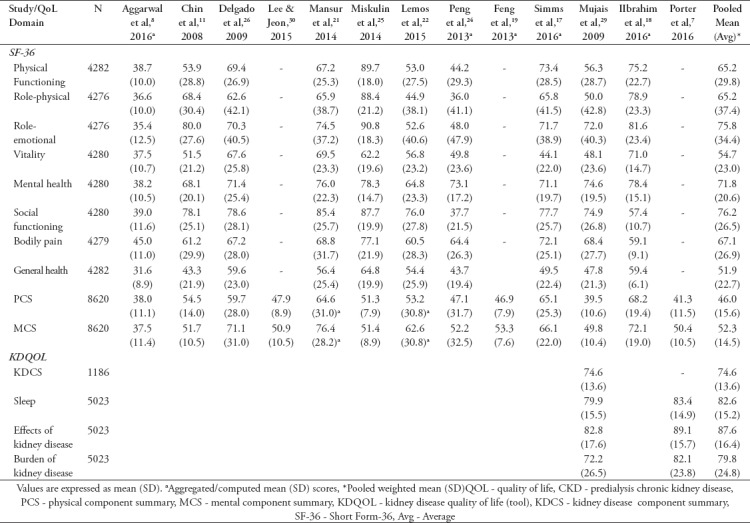
The average mean score of kidney disease component summary (KDCS) in one study7 was 74.6 (SD= 13.6), while the average mean scores of sleep domain in 2 studies7,29 were 82.6 (SD=14.7), effects of kidney disease was 87.6 (SD=16.4) and burden of kidney disease domains was 79.8 (SD=24.8) (Table 2).
Risk factors for HRQOL in predialysis CKD. Poor renal function (eGFR)
The lower the renal function, the worse the HRQOL scores and vice versa. This trend was consistent across all the studies. In one study, patients with eGFR >30 had higher PCS (44.0, SD=7.2) and MCS (44.8, SD=11.0) mean scores than the PCS (32.1, SD=7.4) and MCS (33.0, SD=7.6) mean scores of patients with eGFR <30 (p<0.01 in both PCS and MCS comparison).8 In another study, the PCS score was 75.3 (SD=21.5) and MCS score was 69.6 (SD=22.1) for patients with eGFR >60 versus PCS score of 49.2 (SD=24.2) and MCS score of 60.1 (SD=24.0) in patients with eGFR <30, respectively (p<0.005 in both comparisons).17 Patients with moderate CKD (eGFR: 30-59) had much higher HRQOL mean scores of: PCS: 40.4 (SD=10.8); MCS: 51.3 (SD=9.0), compared with advanced CKD (eGFR: <15) patients with PCS mean score of 37.9 (SD=10.5) and MCS mean score of 47.4 (SD=11.5) (p<0.005 in both).29 Similar differences were reported in other studies.7,28
Estimated glomerular filtration rate (eGFR), as a continuous variable, showed high correlation with PCS (r=0.70) and moderate correlation with MCS scores (r=0.67) (p<0.01 in both)8 but showed no significant correlation with either PCS (r=0.16) or MCS (r=-0.12) in one study.30 There was a significant linear relationship between eGFR with PCS scores (p<0.005),26 with MCS (p<0.01),29 and with both PCS and MCS, as a dichotomized variable (eGFR <45) (p<0.005).28 A multilinear regression in one study showed eGFR as one of the 6 continuous variables, others being age, serum hemoglobin (HB), C-reactive protein (CRP), blood urea, and serum sodium, that predicted PCS scores, accounting for 48.1% of the variance (p<0.005; adjusted R2=48.1%); and one of the 4 variables (others being CRP, mean arterial pressure, and blood urea) for MCS scores, explaining 44.6% of the variance (p<0.005; adjusted R2=44.6%).8 Another study reported patients with 20 points more eGFR had 5.6 times of odds of having higher PCS scores (p<0.01) and 0.67 odds for MCS (p>0.05).7 Estimated glomerular filtration rate as a dichotomized variable (eGFR<45) predicted both PCS and MCS in a multilinear regression (PCS: β=-5.22; MCS: β=-0.06; p<0.005 in both) in another study.28
Biochemical variables
Serum hemoglobin level showed low-to-moderate correlation with PCS and MCS (p<0.01).8,26,28 Hemoglobin level was however not correlated with either PCS or MCS in one study30 or MCS in another study.26 Serum albumin showed a strong positive association with PCS (b=7.4) and MCS (b=5.0) scores (p<0.005) only at univariate level but not at multivariate level (p>0.05).28 Blood glucose showed no association with either PCS or MCS.8,28 Serum creatinine and CRP were moderately correlated with poorer PCS and MCS (p<0.01)8 but showed no relationship with HRQOL in 2 studies.28,30 Other variables that showed moderate but significant (negative) relationship with PCS and MCS were blood urea, serum uric acid, serum phosphorus, and erythrocyte sedimentation rate. Mean arterial pressure (MAP) and serum calcium and serum potassium showed negligible but important correlations with HRQOL (p<0.05 in PCS).8 Patient with “no” urine albumin-to-creatinine ratio had better PCS and MCS scores than those “with” microalbuminuria and those with an ACR ratio of >300 (p<0.005).7 Urine protein, urine RBC, triglyceride, blood pressure (systolic and diastolic) showed no statistical association with either PCS or MCS.28
Comorbidities
There exists a strong negative linear relationship between comorbidities and lower HRQOL. Similar to renal function and gender (female) and eGFR, comorbidities impeded PCS more than MCS in predialysis CKD patients. Patients with CHF had lower PCS mean scores (35.4, SD=9.7) than patients without CHF (40.3, SD=10.6) (p<0.005), but comparable MCS mean scores (49.1, SD=11.6 versus 50.2, SD=10.1; p>0.05).29 Similar patterns of a significant difference in PCS and comparable MCS scores were seen in CKD patients with hypertension (PCS 47.0, SD=9.1 versus 50.7, SD=7.9, p<0.05; MCS 51.4, SD=10.8 versus 49.6, SD=9.5, p>0.05);30 MI (PCS 36.1, SD=10.0 versus 40.2, SD=10.6, p<0.005; MCS 50.7, SD=10.6 versus 49.9, SD=10.2, p>0.05); and DM (PCS 37.3, SD=10.6 versus 41.6, SD=10.2, p<0.005; MCS 49.7, SD=10.7 versus 50.3, SD=9.9, p>0.05).29 In another study, the difference in HRQOL in CKD patients with and without hypertension was only seen in PCS (40.6, SD=11.4 versus 45.1, SD=11.6, p<0.005) and not in MCS (50.7, SD=10.4 versus 50.3, SD=10.5, p>0.05),7 similar to findings in another study.30
Another finding reported the odds of having impaired PCS and MCS between hypertensive and non-hypertensive patients with predialysis CKD were not different,29 while another study reported that hypertension was not associated with either PCS or MCS.28 Patients with CVA were however at 6.3 and 3.3 times of odds of having poorer PCS and MCS scores than those without CVA (p<0.005).28 Cardiovascular accident and depression were the 2 out of 5 comorbidities (others being hypertension, DM, and CHF), and 2 of 7 variables out of the total 24 variables, that significantly contributed to the MLR model predicting HRQOL (PCS and MCS).28 Patients with a history of either MI (84%), CHF (77%), PVD (45%) or DM (55%) were more likely to have impaired PCS than patients without the corresponding comorbidities (p<0.005); however, the odds were not different in MCS, except for DM (where patients were 33% more likely to present with impaired MCS scores than those without it, p<0.005).29 In another study, no difference in PCS and MCS scores were seen in patients with CVD or DM and those without the comorbidities (p>0.05).30
However, in other studies, patients with CVD,8 anxiety, and depression17,19,24 showed much lower PCS and MCS scores compared with those without the respective conditions (p<0.005). Depression showed high but negative linear relationship with both PCS (b=-9.9) and MCS (b=-8.9), (p<0.005).28 Other studies reported moderate but significant negative correlation between depression and HRQOL summary scores.17,19,24 Patients with both anxiety and depression have twice as less HRQOL scores (PCS 32.4, SD=13.2; MCS 37.3, SD=12.9) than patients with neither of the 2 comorbidities (PCS 62.5, SD=19.4; MCS 65.5, SD=16.6), p<0.005 in both comparisons.24 Other conditions such as frailty,21 ADKPD diagnosis,17 use of beta blockers,29 and symptoms clusters30 were reasonably associated with poorer PCS and MCS scores.
Gender (female)
Female patients with predialysis CKD showed poorer HRQOL than their male counterparts. The difference was more pronounced in PCS than in MCS.7,8,30 In one study, men had PCS mean scores of 41.0 (SD=10.2) versus 37.7 (SD=10.8) in women (p<0.005), and MCS scores of 51.2 (SD=9.6) versus 48.4 (SD=11.0) in females (p<0.005), respectively.29 The trend of women showing poorer HRQOL in both summary scores (PCS and MCS) persisted in other studies,7,8,25,28,30 and was even more pronounced in one study with PCS mean score of 52.2 (SD=42.2) versus 70.4 (SD=45.7) in men, and MCS mean scores of 66.4 (SD=33.7) versus of 87.7 (SD=55.6) in men (p<0.01 in both).26
A multiple linear regression (MLR) model showed a significant negative relationship between female gender and HRQOL; PCS (B=-6.19, p<0.0001) and MCS (B=-2.09, p=0.015).28 A simple linear regression test in another study showed similar negative linear relationship between female gender with PCS (b=-2.64; p<0.005) and MCS (b=-2.79; p<0.01).29 Female gender, alongside age, explained 39% variance of PCS (female, B=-16.0; age, B=-0.9 age, p<0.005 in both variables) and 25% variance of MCS (female, B=-18.3; age, B=-4.2; p<0.005 in both) in a, MLR model in one study;17 12.5% of variance for PCS (female, B=-13.7; age, B=-0.7, p<0.005 in both variables) in analysis of covariance (ANCOVA) in another study.26 Another study reported that women were 2.19 and 1.65 times of odds of having lower PCS and MCS scores (p<0.001 in both variables) than their male counterparts.7
Age
Older age was associated with poorer HRQOL scores, especially the PCS. However, older patients scored much higher MCS than younger patients in 3 studies.7,29,30 Older age, like female gender, was more associated with impairment of the PCS than MCS. One study reported that patients aged 65 years and above scored poorer PCS, 58.4 (SD=44.9), compared with patients aged <65 years, 72.2 (SD=39.9), (p<0.005). However, the mean MCS scores were comparable, 82.9 (SD=45.1) in the former group versus 79.4 (SD=52.1 significantly) in the latter group (p>0.05).26 Age showed low correlation (r=-0.35 in PCS, r=-0.33 in PCS, r=0.21 in MCS, p<0.01 in both comparisons),8,26 low linear relationship (b=-0.45 in PCS, -0.20 in MCS, p<0.005 in both)28 with HRQOL. Similar findings were reported in other studies.26,29 Age also showed a significant but modest linear relationship with HRQOL scores in multivariate analyses.7,17,26,28 Generally, the association between older age and HRQOL was negative with the PCS and fairly positive with MCS.
Other sociodemographic variables
Patients who engaged in regular exercise (defined as “exercise regimen for a minimum of 30 minutes three times or greater, per week”) were 5.11 and 2.65 times of odds of having better PCS and MCS scores, respectively than sedentary patients (p<0.005 in both).28 Higher and longer duration of education was associated with higher HRQOL scores. Patients with >16 years of education had better PCS mean score of 49.4 (SD=7.3) than those with ≤9 years with mean PCS score of 44.4 (SD=10.5), p<0.05, but comparable MCS scores (51.9, SD=9.2 versus 51.8, SD=11.4, p>0.05).30 Patients who graduated college scored higher in both PCS and MCS than patients with some college education, high school, and less than high school graduates (p<0.005).7 Employed patients had higher PCS score (p<0.01) but comparable MCS score (p>0.05).30
Patients with higher household incomes (versus lower-income), patients who never smoked (versus past or ‘currently smoking’ status), patients who were non-Hispanic white (versus non-Hispanic black and Hispanic), and patients with BMI <30 kg/m2 (versus >30) had better PCS and MCS scores (p<0.005).7 Higher family income positively influenced both PCS and MCS.22 Having a spouse was also associated with higher PCS and MCS scores in one study28 but no association was found in another study.30 Using illicit drugs (undefined) was associated with lower MCS but not PCS.7 Body mass index (>25 versus <25 kg/m2) showed neither association8 nor a elationship28 with PCS or MCS, even as a continuous variable (p>0.05).
The variables associated with HRQOL impairment in predialysis or non-dialysis CKD patients are summarized in Table 3.
Table 3.
Factors associated with HRQOL impairment in predialysis CKD patients.

Discussion
Patients with predialysis CKD have impaired HRQOL which was associated with several risk factors, as shown in this review. The physical components were more impaired than the mental components. It is however not surprising that burden and effects of kidney disease, as measured by KDQOL in 2 studies7,29 were less impaired because the large majority of patients with predialysis CKD were asymptomatic.31 The HRQOL impairment and its risk factors in patients with predialysis CKD were confirmed with different patient-report outcome measures (PROMs) such as the EQ-5D.5 Likewise, studies on a combined CKD and ESRD samples reported similar HRQOL impairment and association with factors such as poor renal function, presence of comorbidities, race, older age (better MCS scores), female gender, sociodemographic variables (namely, low education and income), biochemical variables (namely, albumin and serum urea),12,32 as reported in this review.
Health-related quality of life impairment is associated with a higher risk of clinical outcomes in both predialysis CKD and ESRD patients.7,33 In predialysis CKD, low PCS score was associated with higher risks of CV events and all-cause death and low MCS score was associated with increased risk of all-cause death.7 Impaired HRQOL has shown significant correlations with increased risks of all-cause death and CKD progression, even after adjustment for other clinical and sociodemographic variables in patients with predialysis CKD,34 but not CKD progression in another study.5 While HRQOL scores were typically not predictors of CKD progression,5,7 other independent risk factors for HRQOL impairment such as anxiety and poor renal function35 among other factors highlighted in this text have been shown to predict CKD progression.
This review shows the various risk factors for HRQOL impairment (which is an important predictor of adverse clinical outcomes) in patients with predialysis CKD, suggesting the need for increased attention to patients with predialysis CKD by the renal care providers.11 For example, being female, having a poor renal function, having comorbid conditions among other factors shown in this text are an important cue to predict poor HRQOL. While many of the risk factors for poor HRQOL are largely unmodifiable, several others such as sedentary lifestyle, income, and unemployment are modifiable; therefore, more attention should be given to the modifiable ones to lessen the risk burden of adverse health outcome.11 Similarly, this review would help guide future studies on HRQOL and risk factors in patients with predialysis CKD, as only a few studies have been conducted using the SF-36 and its variants in this population.
This review is not without limitations. The studies which measured HRQOL using other widely used PROMs such as EuroQOL (EQ-5D) and Nottingham Health Profile (NHP) were not included in this study. Furthermore, the possibility of HRQOL scoring discrepancy exists as studies using different scoring methods and versions of the SF tool. In this review, a statistical method (ANOVA) was used to determine weighted average scores of HRQOL (by aggregating component scores) where overall scores were not reported. Further, only generic HRQOL scores (measured with SF-36 variants) reported in observational studies were considered; thus, caution must be applied in interpreting these results due to inherent flaws associated with observational studies and generic tools.36
In conclusion, the PCS score, compared with MCS scores, was most impaired in patients with non-dialysis or predialysis CKD. The risk factors for poor HRQOL in patients with predialysis CKD were more associated with PCS than with MCS. There is a need for more observational, longitudinal and interventional studies on improving HRQOL by controlling some of the highlighted risk factors in patients with predialysis CKD.
Acknowledgment
We would like to thank Science-Edit (http://www.sciencedit-dw.org/) for English language editing.
References
- 1.Hill NR, Fatoba ST, Oke JL, Hirst JA, O'callaghan CA, Lasserson DS, et al. Global prevalence of chronic kidney disease. A systematic review and meta-analysis. PLoS One. 2016;11:1–18. doi: 10.1371/journal.pone.0158765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jha V, Garcia-Garcia G, Iseki K, Li Z, Naicker S, Plattner B, et al. Chronic kidney disease: Global dimension and perspectives. Lancet. 2013;382:260–272. doi: 10.1016/S0140-6736(13)60687-X. [DOI] [PubMed] [Google Scholar]
- 3.Levin A, Tonelli M, Bonventre J, Coresh J, Donner JA, Fogo AB, et al. Global kidney health 2017 and beyond: a roadmap for closing gaps in care, research, and policy. Lancet. 2017;6736:1–30. doi: 10.1016/S0140-6736(17)30788-2. [DOI] [PubMed] [Google Scholar]
- 4.Lins L, Carvalho FM. SF-36 total score as a single measure of health-related quality of life: scoping review. SAGE Open Med. 2016;4 doi: 10.1177/2050312116671725. 2050312116671725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jesky MD, Dutton M, Dasgupta I, Yadav P, Ng KP, Fenton A, et al. Health-related quality of life impacts mortality but not progression to end-stage renal disease in pre-dialysis chronic kidney disease: a prospective observational study. PLoS One. 2016;11:e0165675. doi: 10.1371/journal.pone.0165675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ware JE, Kosinski M. Interpreting SF-36 summary health measures: a response. Qual Life Res. 2001;10:405-13–20. doi: 10.1023/a:1012588218728. [DOI] [PubMed] [Google Scholar]
- 7.Porter AC, Lash JP, Xie D, Pan Q, DeLuca J, Kanthety R, et al. Predictors and outcomes of health-related quality of life in adults with CKD. Clin J Am Soc Nephrol. 2016;11:1154–1162. doi: 10.2215/CJN.09990915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aggarwal HK, Jain D, Pawar S, Yadav RK. Health-related quality of life in different stages of chronic kidney disease. QJM. 2016;109:711–716. doi: 10.1093/qjmed/hcw054. [DOI] [PubMed] [Google Scholar]
- 9.Chong K, Unruh M. Why does quality of life remain an under-investigated issue in chronic kidney disease and why is it rarely set as an outcome measure in trials in this population? Nephrol Dial Transplant. 2017;32(Suppl 2):ii47–ii52. doi: 10.1093/ndt/gfw399. [DOI] [PubMed] [Google Scholar]
- 10.Clarke AL, Yates T, Smith AC, Chilcot J. Patient's perceptions of chronic kidney disease and their association with psychosocial and clinical outcomes: A narrative review. Clin Kidney J. 2016;9:494–502. doi: 10.1093/ckj/sfw014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen SS, Al Mawed S, Unruh M. Health-related quality of life in end-stage renal disease patients: how often should we ask and what do we do with the answer? Blood Purif. 2016;41:218–224. doi: 10.1159/000441462. [DOI] [PubMed] [Google Scholar]
- 12.Manavalan M, Priyamvada PS, Majumdar A, Kumar KTH. Assessment of health-related quality of life and its determinants in patients with chronic kidney disease. Indian J Nephrol. 2017;27:37–43. doi: 10.4103/0971-4065.179205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Peters MDJ, Godfrey CM, Khalil H, McInerney P, Parker D, Soares CB. Guidance for conducting systematic scoping reviews. Int J Evid Based Health. 2015;13:141–146. doi: 10.1097/XEB.0000000000000050. [DOI] [PubMed] [Google Scholar]
- 14.Pearson A, Field J, Jordan Z. Appendix 3 data extraction tools. Evidence-Based Clin Pract Nurs Heal Care. 2009:183–186. [Google Scholar]
- 15.Joanna Briggs Institute. Critical Appraisal Tools. Available from: http://joannabriggs.org/research/critical-appraisal-tools.html .
- 16.Leech NL, Barrett KC, GA M. IBM SPSS for intermediate statistics: Use and interpretation. 5th ed. Abingdon (UK): Routledge; 2014. [Google Scholar]
- 17.Simms RJ, Thong KM, Dworschak GC, Ong ACM. Increased psychosocial risk, depression and reduced quality of life living with autosomal dominant polycystic kidney disease. Nephrol Dial Transplant. 2016;31:1130–1140. doi: 10.1093/ndt/gfv299. [DOI] [PubMed] [Google Scholar]
- 18.Ibrahim N, Aini Pakri Mohamed R, Teo S, Che Din N, Halim Abdul Gafor A, Ismail R, et al. Association between health-related quality of life and psychological distress at different stages of chronic kidney disease. Sains Malaysiana. 2016;45:753–759. [Google Scholar]
- 19.Feng L, Yap KB, Ng TP. Depressive symptoms in older adults with chronic kidney disease: Mortality, quality of life outcomes, and correlates. Am J Geriatr Psychiatry. 2013;21:570–579. doi: 10.1016/j.jagp.2012.12.020. [DOI] [PubMed] [Google Scholar]
- 20.IBM Support. ANOVA with summary data [Internet] 2016. [[cited 2017 June 4]]. Available from: http://www-01.ibm.com/support/docview.wss?uid=swg21476127 .
- 21.Mansur H, Colugnati FA, Grincenkov FR, dos S, Bastos M. Frailty and quality of life: a cross-sectional study of Brazilian patients with pre-dialysis chronic kidney disease. Health Qual Life Outcomes. 2014;12:27. doi: 10.1186/1477-7525-12-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lemos CF, Rodrigues MP, Paulo Veiga JR, Veiga JRP. Family income is associated with quality of life in patients with chronic kidney disease in the pre-dialysis phase: a cross sectional study. Heal Qual Life Outcomes. 2015;13:1–9. doi: 10.1186/s12955-015-0390-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moher D, Liberati A, Tetzlaff J, Altman DG, Altman D, Antes G, et al. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009;6:e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Peng T, Hu Z, Guo L, Xia Q, Li D, Yang X. Relationship between psychiatric disorders and quality of life in nondialysis patients with chronic kidney disease. Am J Med Sci. 2013;345:218–221. doi: 10.1097/MAJ.0b013e318255a561. [DOI] [PubMed] [Google Scholar]
- 25.Miskulin DC, Abebe KZ, Chapman AB, Perrone RD, Steinman TI, Torres VE, et al. Health-related quality of life in patients with autosomal dominant polycystic kidney disease and CKD stages 1-4: a cross-sectional study. Am J Kidney Dis. 2014;63:214–226. doi: 10.1053/j.ajkd.2013.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Delgado CEY, Jaramillo MM, Orozco BEO, Santaella MHC, Nunez JJY, Munoz JPL, et al. Quality of life in patients with chronic kidney disease without dialysis or transplant: a random sample from two insurance companies. Medellin, Colombia, 2008. Nefrologia. 2009;29:548–556. doi: 10.3265/Nefrologia.2009.29.6.5490.en.full. [Spanish] [DOI] [PubMed] [Google Scholar]
- 27.National Kidney Foundation. Clinical practice guidelines for chronic kidney disease: evaluation, clasification and stratification. American Journal of Kidney Diseases. 2002;39:1–356. [PubMed] [Google Scholar]
- 28.Chin HJ, Song YR, Lee JJ, Lee SB, Kim KW, Na KY, et al. Moderately decreased renal function negatively affects the health-related quality of life among the elderly Korean population: A population-based study. Nephrol Dial Transplant. 2008;23:2810–2817. doi: 10.1093/ndt/gfn132. [DOI] [PubMed] [Google Scholar]
- 29.Mujais SK, Story K, Brouillette J, Takano T, Soroka S, Franek C, et al. Health-related quality of Life in CKD patients: Correlates and evolution over time. Clin J Am Soc Nephrol. 2009;4:1293–1301. doi: 10.2215/CJN.05541008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee SJ, Jeon JH. Relationship between symptom clusters and quality of life in patients at stages 2 to 4 chronic kidney disease in Korea. Appl Nurs Res. 2015;28:e13–e19. doi: 10.1016/j.apnr.2015.03.004. [DOI] [PubMed] [Google Scholar]
- 31.Qaseem A, Hopkins RH, Sweet DE, Starkey M, Shekelle P, EM B, et al. Screening, monitoring, and treatment of stage 1 to 3 chronic kidney disease: a clinical practice guideline from the Clinical Guidelines Committee of the American College of Physicians. Ann Intern Med. 2013;157:251–262. doi: 10.7326/0003-4819-159-12-201312170-00726. [DOI] [PubMed] [Google Scholar]
- 32.Zimbudzi E, Lo C, Ranasinha S, Gallagher M, Fulcher G, Kerr PG, et al. Predictors of health-related quality of life in patients with co-morbid diabetes and chronic kidney disease. PLoS One. 2016;11:12. doi: 10.1371/journal.pone.0168491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu WJ, Musa R, Chew TF, Lim CTS, Morad Z, Bujang A. Quality of life in dialysis: A Malaysian perspective. Hemodial Int. 2014;18:495–506. doi: 10.1111/hdi.12108. [DOI] [PubMed] [Google Scholar]
- 34.Tsai YC, Hung CC, Hwang SJ, Wang SL, Hsiao SM, Lin MY, et al. Quality of life predicts risks of end-stage renal disease and mortality in patients with chronic kidney disease. Nephrol Dial Transplant. 2010;25:1621–1626. doi: 10.1093/ndt/gfp671. [DOI] [PubMed] [Google Scholar]
- 35.McKercher C, Sanderson K, Jose MD. Psychosocial factors in people with chronic kidney disease prior to renal replacement therapy. Nephrology. 2013;18:585–591. doi: 10.1111/nep.12138. [DOI] [PubMed] [Google Scholar]
- 36.Wang MTM, Bolland MJ, Grey A. Reporting of limitations of observational research. JAMA Intern Med. 2015;175:1571–1157. doi: 10.1001/jamainternmed.2015.2147. [DOI] [PubMed] [Google Scholar]
- 37.Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol. 2005;5:13. doi: 10.1186/1471-2288-5-13. [DOI] [PMC free article] [PubMed] [Google Scholar]


