Abstract
Objectives:
To assess the usefulness of periapical dental radiograph as a screening tool aimed at early signs of osteoporosis in postmenopausal periodontal patients and root surface evaluation using spectrochemical analysis.
Methods:
This study was conducted at the Department of Periodontics, Riyadh Elm University, Riyadh, Saudi Arabia, for 12 months between December 2016 and November 2017. Two groups consisted healthy postmenopausal women having chronic periodontitis and postmenopausal women having chronic periodontitis with osteoporosis. Osteoporosis were evaluated for plaque index (PI); gingival index (GI); clinical attachment level (CAL); probing pocket depth (PPD), and bone mineral density (BMD). A standardized digital dental periapical radiographs were taken for every patient. The spectrochemical analysis was carried out using the self-assembled Laser-Induced Breakdown Spectroscopy (LIBS) system used for qualitative and quantitative analysis of Calcium (Ca), Potassium (K), Phosphorus (P), Fluoride (F), and Magnesium (Mg)
Results:
There was no statistically significant difference between both groups for GI and PI. Similarly PPD and CAL were showing the difference but statistically, significant difference was only for CAL. Value of distance starting from cement enamel junction to the alveolar crest (CEJ-AC) and BMD were having a statistically significant variance between both groups. The differences between osteoporotic and control group were statistically significant regarding Ca with the mean higher in the control group. Furthermore, the variances between the groups in both K and Mg were statistically significant with higher mean in the osteoporotic group (p<0.05).
Conclusion:
The clinical, radiographic, and experimental findings of this study indicated that osteoporosis has a direct effect on the progression rate of periodontal tissue destruction and dental radiographic can be suggested as a screening tool for an early sign of osteoporosis.
Osteoporosis is characterized by low bone mass, worsening of the skeletal microarchitecture, compromising trabecular and cortical bone material. Osteoporosis is a multifactorial chronic systemic disease otherwise physiological process associated with aging.1 Periodontitis and osteoporosis are very commonly seen in the old aged people.2 A number of research studies, systematic reviews and meta-analyses studies proposed possible connotations of periodontal disease to osteoporosis.3-7 Thus, it is very essential to appraise the actual impact of bone density on periodontal illness to explore the relationship of bone mineral density to the progression of periodontal disease.8 The diagnosis of osteoporosis and the criteria are established by World Health Organization (WHO) at the Consensus Development Conference. Osteoporosis is defined as a bone mineral density T score less than -2.5 SD. Individuals are considered normal with T score at least -1 SD or more.9 Patients are considered having periodontitis with periodontal bone resorption in the least 4 teeth with one or more having probing depth 4 mm together with clinical attachment loss of 3 mm apical to the cemento-enamel junction at the same site and bleeding is seen on probing which can be further confirmed by radiographic analysis.10,11 In disputed circumstances, when clinical description is same to criteria but inadequate to make any conclusion, radiographs are good to confirm the presence of periodontal disease.10,11 This prospective cross-sectional study was conducted to assess the usefulness of periapical dental radiographs as a screening tool for early signs of osteoporosis in female adult periodontic patients; and root surface was evaluated using spectrochemical analysis.
Methods
The purpose of this cross-sectional study was explained to all patients and consent form were obtained. This prospective study was conducted for 12 months period, between December 2016 and December 2017 and this study was approved by the Research Ethics Committee, Riyadh Elm University, Riyadh, Saudi Arabia. The study population includes a total of 60 women aged 50-70 years were classified into 2 groups depending on bone mineral density (BMD) measured using dual-energy x-ray absorptiometry (DXA) at femur neck and lumbar spine. Group I consisted 30 healthy postmenopausal women with generalized chronic periodontitis as a control. Group II included 30 postmenopausal women with generalized chronic periodontitis and osteoporosis matched for age, geographic area, and socioeconomic status with a control group who visited the Department of Periodontics for consultation.
Inclusion criteria were postmenopausal Saudi women who experienced natural menopause (aged 50-70 years) and presence of at least 14 natural teeth to provide a reasonable number of teeth together with the presence of at least one hopeless tooth. Exclusion criteria were postmenopausal women with no precise medical history in hospital records; with a past of surgically induced menopause, smoking, alcohol abuse, bone destructive lesions of the jaw, diabetes mellitus, thyroid diseases, chronic renal problems, hormone replacement therapy, corticosteroids, chemotherapy and/or radiotherapy and connective tissue disorders.
Clinical assessment
Comprehensive periodontal examination was evaluated for every patient included gingival index,10,11 plaque index,10 probing pocket depth (PPD),11 clinical attachment level (CAL).11 Probing pocket depth and CAL were recorded using a standard UNC periodontal probe at 6 sites per tooth (mesiobuccal, buccal, distobuccal, distolingual, lingual and mesiolingual) from cemento-enamel junction (CEJ) that is a fixed point to the deepest probing depth. Clinical attachment level and PPD measurements helps to evaluate periodontal destruction. It used a straight probe (in millimetres) to measure the distance of the base of the gingival pocket to that cemento-enamel junction (CAL) or the gingival margin (PPD).
The 6 selected teeth (16, 21, 24, 36, 41 and 44) (Ramfjord teeth) were used as described by Ramfjord for the plaque index (PI), gingival index (GI), and CAL and if one of them is missing the adjacent tooth was considered as a replacement, as the selected teeth have been used to represent the entire dentition. Ramfjord periodontal disease indexed is a modification of Russells PI, which is primarily concerned with accurate measurement and emphasis on recording of attachment level of periodontal tissue relative to CE junction.
Radiographic evaluation
A standardized digital dental periapical radiographs were taken for every patient in osteoporotic and non-osteoporotic groups for the interproximal alveolar bone between mandibular second premolar and first molar using Rinn XCP device for extension cone parallel technique at the selected sites.12 All exposures were standardized at 70 kV and 10 mA. The periapical radiographs were viewed on a monitor; where contrast and brightness were adjusted to enhance the image quality. The images were viewed on a computer screen using Adobe Photoshop CS according to the procedure described earlier.12 The area of interest was marked using the magnetic lasso tool for evaluation of 1) BMD of the interproximal alveolar bone between mandibular second premolar and first molar using gray level scales. 2) Linear measurement of alveolar bone level as the distance from CEJ to alveolar crest (CEJ-AC).
Root surface analysis
Extraction of hopeless teeth was carried out in all the patients, and each sample of extracted teeth was scaled by ultrasonic scalar for calculus removal then washed out in distilled water and air-dried then kept in a solution of 10% formalin with water in small bottles. The spectrochemical analysis was carried out for root cementum using the self-assembled Laser-Induced Breakdown Spectroscopy (LIBS) system. Laser-Induced Breakdown Spectroscopy spectra of different teeth samples were recorded over a 240-670nm wavelength range for qualitative and quantitative analysis of Calcium (Ca), Phosphorus (P), Fluoride (F), Potassium (K), and Magnesium (Mg).
Statistical analysis
Data were collected and analyzed using the Statistical Package for Social Science, Version 22.0 (Armonk, NY: IBM Corp.). Descriptive statistics (mean and standard deviation) was used to present the overview of the findings. Based on the Shapiro-Wilk test of normality (p>0.05), independent samples t-test (parametric test) was used to compare means of (PI, GI, PPD, and CAL) and (CEJ-AC and alveolar bone BMD). Probability (p value) ≤ 0.05 was considered to be statistically significant.
Results
Sixty female patients with a mean age of 51.23 years suffering from chronic periodontitis were selected from Outpatient Clinics, Department of Periodontics, Riyadh Elm University, Riyadh, Saudi Arabia, for 12 months between December 2016 and November 2017, and who visited for consultation. The mean ± SD age group was 50.27 ± 4.72 in the non-osteoporotic and 52.2 ± 4.54 in the osteoporotic group.
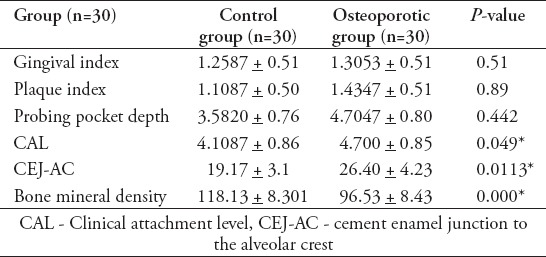
Table 1 summarized the mean±SD of clinical parameters of the control group and osteoporotic group.
Table 1.
Mean±standard deviation of clinical parameters of the control group and osteoporotic group.
Figures 1 & 2 show the radiographic results for the control group and osteoporotic group.
Figure 1.
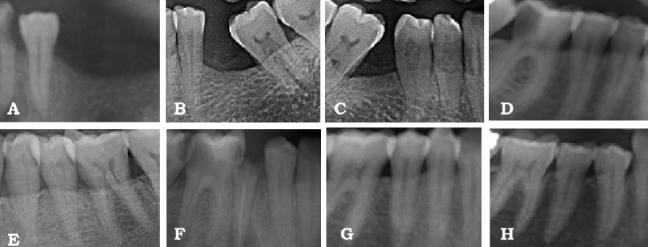
Radiographic results of group 1 showing: A) generalized horizontal bone loss (A-H), missing adjacent teeth (A-C), mesial migration of molars B, C), proximal caries (A, D, F), periapical infection with endodontically treated root stump (F).
Figure 2.
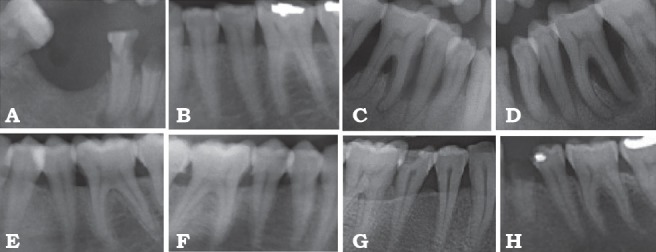
Radiographic results of group 2 showing: A) generalized horizontal and A-H) vertical bone loss, missing adjacent teeth (A), endo perio lesions (A, C, D), dental filling (B, H), calculus (C, D, F, G), root stumps (A, H).
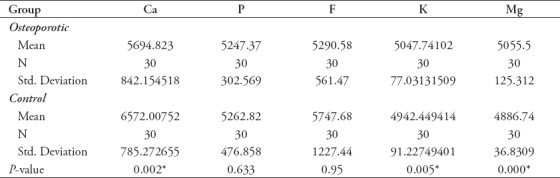
The root surface for each extracted tooth was analyzed by laser-induced breakdown spectroscopy (LIBS) to detect the intensities for Ca, P, F, K, and Mg. Statistical analysis Mann-Whitney U test (non-parametric) revealed that the mean ± SD intensity in arbitrary unit (a.u) of Ca, P, and F was higher in the osteoporotic group, while K and Mg were higher in the control group. The differences between osteoporotic and control groups were statistically significant (p<0.05) regarding Ca with the mean higher in the control group. Furthermore, the differences between the groups in both K and Mg were statistically significant with higher mean in the osteoporotic group (p<0.05). On the other hand, the differences between both groups regarding P and F were statistically not significant with higher mean in control group (Table 2).
Table 2.
Mean±standard deviation of Calcium (Ca), Phosphorus (P), Fluoride (F), Potassium (K), and Magnesium (Mg) between osteoporotic and control groups.
Discussion
Periodontal disease is considered as localized tissue damage while osteoporosis represents a systemic condition, both show bone resorption as their main feature and share many other risk factors such as hormonal influence and the presence of cytokines. The present study showed that there is no statistically significant difference between osteoporotic group and control group in PI, GI, and PPD which indicated that the inflammatory process was not higher in the osteoporotic group and demonstrated a direct relationship between bleeding on probing and the presence/amount of subgingival deposits.13 Clinical attachment level found to be significantly more in the osteoporotic group in many studies.14-19 The present study concluded that the CAL was statistically significantly more in the osteoporotic group than the control group. Changes in systemic bone density simultaneously entail changes in the height and density of the alveolar bone. In an evaluation of the root surface minerals using LIBS, we found that the Ca intensity was statistically significant lower in osteoporotic group than that in the control group (p≤0.05) which raises a question about the effect of osteoporosis on mineralized tissues other than bone. The P intensity in osteoporotic group found to be closed to that found in control group. The mean F intensity was higher in control group but the difference was not statistically significant. Magnesium and K intensities found to be statistically significant higher in osteoporotic group than in control group. Many studies about elemental composition of cementum, GCF and saliva between periodontitis patients and gingivitis or normal patients were implemented and showed different results. Selvig and Zander,20 found that the chemical analysis showed high content of Ca, P, and Mg in the cervical cementum of periodontally involved teeth than those of healthy teeth. Nakata et al21 found no change in Ca concentration in any comparison, but P and Mg concentrations tended to be higher in periodontally diseased cementum than in normal cementum. Bang et al22 found that K values in gingival crevicular fluid (GCF) tended to be significantly higher in patients with severe periodontitis. Calcium, P, and F found to be higher in exposed cementum while no differences found in Mg.23 Koregol et al24 found that there was significantly higher level of K while Ca levels were slightly higher in GCF of periodontitis group in comparison to gingivitis group. Calcium and P level was seen to be significantly high in the saliva of periodontitis patients and no statistical significant difference was observed in Mg concentration in comparison to control group.25 The high level of Mg inhibits the formation of hydroxyapatite crystals in bone by contending with calcium and forms pyrophosphate insoluble salt, which is not degraded by the enzymes.27 Thereby, high Mg inhibit osteoblast differentiation and mineralizing activity which is seen in an in vitro study.26 The root surface mineral content revealed that the cementum of osteoporotic patients showed higher magnesium and potassium levels and lower calcium levels compared to non-osteoporotic patients.28,29 A study of Hayhoe et al,30 showed positively association between dietary magnesium and potassium with reduced fracture risk in postmenopausal women. Thus, we signify a good-quality diet containing adequate magnesium and potassium intake would be helping in reducing the risk of osteoporosis. Normal serum magnesium level, compared with suboptimal concentration, has also been shown to be clinically associated with a reduced risk of incident fracture.30 There is a need to define how generalizable the results of these analyses can help to understand the relation between intake of these micronutrients, bone health, and osteoporosis.
Severe gum ailment, bone damage around teeth, loss of teeth, loose dentures, or ill-fitting dentures are early cautionary signs of osteoporosis.29 The utmost useful bony landmarks were observed due to dental radiography, by which one can evaluate the trabecular pattern and cortical structures to determine bone destruction. An additional observation in radiograph give in depth changes of osteoporosis which included reduction of overall bone density, thinner and less dense cortical bone and shift of trabecular pattern.31 In general clinical practitioners use a vast expanse of dental radiography in their clinical work, but in most cases, a valuable information of patients’ osteoporosis is not collected. If such information through radiograph is available a dentist would be able to refer women under 65 for a bone densitometry test.32 Study of Erdogan et al33 2009 showed a close association of radiodensitometric scores with the number of remaining teeth, CAL, and bone density. Low skeletal bone mineral density was similarly indicated by tooth loss, increased CAL, and matched with reduced density on digital periapical radiographs. Lindh et al34 suggested that sparse trabecular pattern in the radiographic image is indicative of osteoporosis, and can be a potential method to identify it. The clinical, radiographic and experimental findings of this study indicated that osteoporosis has a direct effect on the progression rate of periodontal tissue destruction that might be related to osteoporotic alveolar bone and/or to the change of subgingival environment due to changes in the root surface mineral contents or subgingival bacteria. The different methodology, type of intraoral radiograph used the mean age of the participants affect the results of the studies examining alveolar bone BMD. Standard periapical dental radiographs of the mandible using special visual software program can be used as screening tool for finding early signs of low bone density.
Acknowledgment
The authors are grateful to the deanship of Scientific Research, King Saud University for funding through Vice Deanship of Scientific Research Chairs.
Footnotes
References
- 1.Guiglia R, Di-Fede O, Lo-Russo L, Sprini D, Rini GB, Campisi G. Osteoporosis, Jawbones and Periodontal Disease. Med Oral Patol Oral Cir Bucal. 2013;18:e93–e99. doi: 10.4317/medoral.18298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chang WP, Chang WC, Wu MS, Pai JT, Guo YC, Chen KC, Liu ME, Chiu WT, Hung KS. Population-Based 5-Year Follow-Up Study in Taiwan of Osteoporosis and Risk of Periodontitis. J Periodontol. 2014;85:E24–E30. doi: 10.1902/jop.2013.130256. [DOI] [PubMed] [Google Scholar]
- 3.Takahashi O, Yoshihara A, Nakamura K, Miyazaki H. Association between periodontitis and systemic bone mineral density in Japanese community-dwelling postmenopausal women. J Dent. 2012;40:304–311. doi: 10.1016/j.jdent.2012.01.005. [DOI] [PubMed] [Google Scholar]
- 4.Payne JB, Reinhardt RA, Nummikoski PV, Patil KD. Longitudinal alveolar bone loss in postmenopausal osteoporotic/osteopenic women. Osteoporos Int. 1999;10:34–40. doi: 10.1007/s001980050191. [DOI] [PubMed] [Google Scholar]
- 5.Tezal M, Wactawski-Wende J, Grossi SG, Ho AW, Dunford R, Genco RJ. The relationship between bone mineral density and periodontitis in postmenopausal women. J Periodontol. 2000;71:1492–1498. doi: 10.1902/jop.2000.71.9.1492. [DOI] [PubMed] [Google Scholar]
- 6.Nicopoulou-Karayianni K, Tzoutzoukos P, Mitsea A, Karayiannis A, Tsiklakis K, Jacobs R, et al. Tooth Loss and Osteoporosis: The Osteodent Study. J Clin Periodontol. 2009;36:190–197. doi: 10.1111/j.1600-051X.2008.01365.x. [DOI] [PubMed] [Google Scholar]
- 7.Sultan N, Rao J. Association between Periodontal Disease and Bone Mineral Density in Postmenopausal Women: A Cross Sectional Study. Med Oral Patol Oral Cir Bucal. 2011;16:E440–E447. doi: 10.4317/medoral.16.e440. [DOI] [PubMed] [Google Scholar]
- 8.Lockhart PB, Bolger AF, Papapanou PN, Osinbowale O, Trevisan M, Levison ME, et al. Periodontal disease and atherosclerotic vascular disease: Does the evidence support an independent association?: A scientific statement from the American Heart Association. Circulation. 2012;125:2520–2544. doi: 10.1161/CIR.0b013e31825719f3. [DOI] [PubMed] [Google Scholar]
- 9.Kanis JA, on behalf of the WHO Scientific Group . Assessment of osteoporosis at the primary health care level. Technical Report. WHO Collaborating Centre for Metabolic Bone Diseases, University of Sheffield, UK. Geneva: WHO; 2007. [Google Scholar]
- 10.Meseli SE, Kuru B, Kuru L. Relationships between initial probing depth and changes in the clinical parameters following non-surgical periodontal treatment in chronic periodontitis. Journal of Istanbul University Faculty of Dentistry. 2017;51:11–17. doi: 10.17096/jiufd.40993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goodson JM, Haffajee AD, Socransky SS, Kent R, Teles R, Hasturk H, Bogren A, Dyke T, Wennstrom J, Lindhe J. Control of periodontal infections: a randomized controlled trial I. The primary outcome attachment gain and pocket depth reduction at treated sites. J Clin Periodontol. 2012;39:526–536. doi: 10.1111/j.1600-051X.2012.01870.x. [DOI] [PubMed] [Google Scholar]
- 12.Yelamali T, Saikrishna D. Role of Platelet Rich Fibrin and Platelet Rich Plasma in Wound Healing of Extracted Third Molar Sockets: A Comparative Study. J Maxillofac Oral Surg. 2015;14:410–416. doi: 10.1007/s12663-014-0638-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Checchi L, Montevecchi M, Checchi V, Zappulla F. The Relationship between Bleeding On Probing and Subgingival Deposits. An Endoscopical Evaluation. Open Dent J. 2009;3:154–160. doi: 10.2174/1874210600903010154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chhina K. Osteoporosis: A risk factor in periodontal disease. Indian Journal of Dental Sciences. 2010;2:4. [Google Scholar]
- 15.Mohammad AR, Hooper DA, Vermilyea SG, Mariotti A, Preshaw PM. An Investigation of the Relationship between systemic bone density and clinical periodontal status in Post-Menopausal Asian-American Women. Int Dent J. 2003;53:121–125. doi: 10.1111/j.1875-595x.2003.tb00735.x. [DOI] [PubMed] [Google Scholar]
- 16.Shen EC, Gau CH, Hsieh YD, Chang CY, Fu E. Periodontal Status in Post-Menopausal Osteoporosis: A Preliminary Clinical Study in Taiwanese Women. J Chin Med Assoc. 2004;67:389–393. [PubMed] [Google Scholar]
- 17.Iwasaki M, Taylor GW, Nakamura K, Yoshihara A, Miyazaki H. Association between Low Bone Mineral Density and Clinical Attachment Loss in Japanese Postmenopausal Females. J Periodontol. 2013;84:1708–1716. doi: 10.1902/jop.2013.120613. [DOI] [PubMed] [Google Scholar]
- 18.Aspalli SS, Shetty VS, Parab PG, Nagappa G, Devnoorkar A, Devarathnamma MV. Osteoporosis And Periodontitis: Is There A Possible Link? Indian J Dent Res. 2014;25:316–320. doi: 10.4103/0970-9290.138327. [DOI] [PubMed] [Google Scholar]
- 19.Juluri R, Prashanth E, Gopalakrishnan D, Kathariya R, Devanoorkar A, Viswanathan V, Romanos GE. Association of postmenopausal osteoporosis and periodontal disease: A double-blind case-control study. J Int Oral Health. 2015;7:119–123. [PMC free article] [PubMed] [Google Scholar]
- 20.Selvig KA, Zander HA. Chemical analysis and microradiography of cementum and dentin from periodontally diseased human teeth. Journal of Periodontology. 1962;33:303–310. [Google Scholar]
- 21.Nakata TM, Stepnick RJ, Zipkin I. Chemistry of Human Dental Cementum: The effect of age and fluoride exposure on the concentration of ash, Fluoride, Calcium, Phosphorus, and Magnesium. J Periodontol. 1972;43:115–124. doi: 10.1902/jop.1972.43.2.115. [DOI] [PubMed] [Google Scholar]
- 22.Inzitari R, Cabras T, Pisano E, Fanali C, Manconi B, Scarano E, et al. HPLC ESI MS analysis of oral human fluids reveals that gingival crevicular fluid is the main source of oral thymosins 4 and 10. J Sep Sci. 2009;32:57–63. doi: 10.1002/jssc.200800496. [DOI] [PubMed] [Google Scholar]
- 23.Selvig KA, Hals E. Periodontally diseased cementum studied by correlated microradiography, electron probe analysis and electron microscopy. J Periodontal Res. 1977;12:419–429. doi: 10.1111/j.1600-0765.1977.tb00137.x. [DOI] [PubMed] [Google Scholar]
- 24.Koregol AC, More SP, Nainegali S, Kalburgi N, Verma S. Analysis of inorganic ions in the gingival crevicular fluid as indicators of periodontal disease activity: A clinico-biochemical study. Contemp Clin Dent. 2011;2:278–282. doi: 10.4103/0976-237X.91788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rajesh KS, Zareena SH, Kumar MA. Assessment of Salivary Calcium, Phosphate, Magnesium, Ph, and Flow Rate in Healthy Subjects, Periodontitis, and Dental Caries. Contemp Clin Dent. 2015;6:461–465. doi: 10.4103/0976-237X.169846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Leidi M, Dellera F, Mariotti M, Maier JA. High Magnesium Inhibits Human Osteoblast Differentiation In Vitro. Magnes Res. 2011;24:1–6. doi: 10.1684/mrh.2011.0271. [DOI] [PubMed] [Google Scholar]
- 27.Navarro-González JF, Mora-Fernández C, García-Pérez J. Clinical implications of disordered Magnesium homeostasis in chronic renal failure and dialysis. Semin Dial. 2009;22:37–44. doi: 10.1111/j.1525-139X.2008.00530.x. [DOI] [PubMed] [Google Scholar]
- 28.Haugeberg G, Uhlig T, Falch JA, Halse JI, Kvien TK. Bone mineral density and frequency of osteoporosis in female patients with rheumatoid arthritis: results from 394 patients in the Oslo County Rheumatoid Arthritis register. Arthritis & Rheumatology. 2000;43:522–530. doi: 10.1002/1529-0131(200003)43:3<522::AID-ANR7>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 29.Taguchi A, Ohtsuka M, Tsuda M, Nakamoto T, Kodama I, Inagaki K, et al. Risk of Vertebral Osteoporosis in Post-Menopausal Women with Alterations of the Mandible. Dentomaxillofac Radiol. 2007;36:143–148. doi: 10.1259/dmfr/50171930. [DOI] [PubMed] [Google Scholar]
- 30.Hayhoe RP, Lentjes MA, Luben RN, Khaw KT, Welch AA. Dietary magnesium and potassium intakes and circulating magnesium are associated with heel bone ultrasound attenuation and osteoporotic fracture risk in the EPIC-Norfolk cohort study, 2. Am J Clin Nutr. 2015;102:376–384. doi: 10.3945/ajcn.114.102723. [DOI] [PubMed] [Google Scholar]
- 31.Geraets WG, Verheij JG, van der Stelt PF, Horner K, Lindh C, Nicopoulou-Karayianni K, et al. Prediction of bone mineral density with dental radiographs. Bone. 2007;40:1217–1221. doi: 10.1016/j.bone.2007.01.009. [DOI] [PubMed] [Google Scholar]
- 32.Vlasiadis KZ, Damilakis J, Velegrakis GA, Skouteris CA, Fragouli I, Goumenou A, et al. Relationship between BMD, Dental panoramic radiographic findings and biochemical markers of bone turnover in diagnosis of osteoporosis. Maturitas. 2008;59:226–233. doi: 10.1016/j.maturitas.2008.01.006. [DOI] [PubMed] [Google Scholar]
- 33.Erdogan Ö, Incki KK, Benliday ıME, Şeydaoglu G, Kelekci S. Dental and radiographic findings as predictors of osteoporosis in postmenopausal women. Geriatr Gerontol Int. 2009;9:155–164. doi: 10.1111/j.1447-0594.2009.00518.x. [DOI] [PubMed] [Google Scholar]
- 34.Lindh C, Horner K, Jonasson G, Olsson P, Rohlin M, Jacobs R. The use of a visual assessment of dental radiographs for identifying women at risk of having osteoporosis: the Osteodent project. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2008;106:285–293. doi: 10.1016/j.tripleo.2007.09.008. [DOI] [PubMed] [Google Scholar]


