Abstract
Hypertension is the major risk factor for morbidity and mortality from myocardial infarction, stroke, heart failure, and chronic kidney disease. Despite its importance, the pathogenesis of essential hypertension is poorly understood. During the past several years, it has become evident that T cells contribute to hypertension. Activated T cells accumulate in the perivascular space and the kidney and release cytokines that promote vascular dysfunction and end-organ damage. Although dendritic cells play a pivotal role in initiating adaptive immune responses, T cells have taken center stage in studies implicating the immune system in the genesis of hypertension. The mechanisms by which T cells are activated and the antigens involved are poorly understood. We recently showed that hypertension is associated with increased dendritic cell production of the TH17 polarizing cytokines, IL-6, IL-1β, and IL-23. This occurs in part by increased superoxide production via NADPH oxidase and protein modification by highly reactive isolevuglandins (IsoLGs). IsoLGs are produced via the isoprostane pathway of free radical-mediated lipid peroxidation and, when adducted to proteins, have the potential to act as neoantigens. In this review, we discuss recent advances in our understanding of the role of antigen-presenting dendritic cells in the pathophysiology of hypertension and highlight potential neoantigens that may contribute to this disease.
Keywords: dendritic cells, neoantigens, isolevuglandins, hypertension
hypertension is a major risk factor for cardiovascular disease, including stroke, heart failure, myocardial infarction, and renal failure. One-third of all adults in the United States has hypertension and another third has “prehypertension” which often progresses to overt hypertension in 2 years (29, 35, 52). About 70% of adults over 70 years of age are hypertensive (83). Despite its importance and extensive research, the etiology of most forms of hypertension is not known, and blood pressure remains poorly controlled in a substantial portion of hypertensive individuals despite treatment (9).
Several years of research have shown that inflammation is a fundamental process that underlies the development of hypertension. Emerging evidence from many laboratories including our own suggests that adaptive immunity plays a major role in the development of hypertension and that T cells are critical to this process. Various hypertensive stimuli, including angiotensin II, catecholamines, aldosterone, and excess salt cause inflammatory T cells to infiltrate the kidney and vasculature and release cytokines which promote sodium retention and vasoconstriction, blood pressure elevation, and end-organ damage. Immunosuppression ameliorates the end-organ damage resulting from hypertension (48, 50), and the renin-angiotensin system regulates immune responses (53). Previous studies in our laboratory have reported that cells of the adaptive immune system are involved in the pathogenesis of hypertension (22). Mice lacking lymphocytes (RAG-1−/− mice) develop blunted hypertension, vascular dysfunction, and vascular oxidative stress in response to various stimuli, including angiotensin II, norepinephrine, and deoxycorticosterone acetate (DOCA)-salt. Adoptive transfer of T cells restores hypertension in these animals. In addition, mice with severe combined immunodeficiency are partially protected from experimental hypertension (12), as are mice lacking the proinflammatory T-cell cytokines interleukin 17A (IL-17A), interferon-γ (IFN-γ), and tumor necrosis factor-α (TNF-α) (39, 44, 56, 85). These data show that T cells and their cytokines contribute to the development of hypertension. However, the mechanisms by which T cells are activated in hypertension are poorly understood, and the neoantigens involved are not known. Dendritic cells (DCs) are the major professional antigen-presenting cells and play a central role in the immune system. The present review discusses the role of DCs in the pathophysiological mechanisms that underlie the development and progression of hypertension.
General Overview of Dendritic Cells
Antigen-presenting cells (APCs) are the initiators of immune responses and include B cells, macrophages, and DCs. Of these, DCs are the most potent and therefore termed professional APCs. DCs were first discovered in 1973 by Dr. Ralph Steinman who received the 2011 Nobel Prize in Physiology for this discovery. Dr. Steinmann showed that DCs, not macrophages, are the most potent stimulators of T-cell activation (3). DCs develop in the bone marrow, migrate as immature cells to sites of potential pathogen invasion, and survey the peripheral microenvironment for evidence of tissue damage and antigens. Once they encounter an antigen, they become activated and mature into immune stimulatory effector cells. They capture, process, and present the antigens in the groove of their major histocompatibility complexes (MHCs) class I and II to T-cell receptors (TCRs) (40). Classical antigen presentation studies showed that intracellular self and non-self-antigens are processed in the proteasome into peptides which are recognized by CD8+ cells in the context of class 1 MHCs. By contrast, extracellular proteins captured from the environment are phagocytized by APCs, processed in the lysosome, and presented by MHC class II molecules to CD4+ T cells (40). DCs can also process extracellular antigens and present them in MHC class I to CD8+ T cells through a process called cross-presentation. This unique property provides the immune system with an important mechanism for generating tolerance to self-proteins as well as viral immunity (24).
For full T-cell activation, antigen presentation must be accompanied by costimulatory signals. The best described costimulatory signal is that involving interaction of the B7 ligands CD80 or CD86 on DCs with CD28 on T cells. Another costimulatory molecule, CD70, interacts with T-cell CD27, and is particularly important in activation of memory CD8+ T cells. In the absence of costimulation, T cells undergo apoptosis (44). There is also an upregulation of CCR7 by DCs which mediates their migration into draining lymphoid tissues where they present antigens to T-cell receptors and activate immune responses (41). In addition, activated DCs produce cytokines that guide T-cell polarization to specific effector phenotypes. For example, transforming growth factor (TGF)-β, interleukins IL-1β, IL-6, IL-23, and IL-21 are known to polarize T cells to an IL-17-producing phenotype (44).
An important function of DCs is to enable the immune system to differentiate self from non-self and to determine the magnitude and quality of T-cell responses. They present self-antigens to T cells thereby maintaining tolerance, which once broken, can result in autoimmune disease. Persistent autoimmune memory is the biggest challenge in the management of all immune-mediated diseases. It is currently impossible to suppress autoreactive T cells while maintaining immunity against potentially harmful invading pathogens. Delivery of appropriate doses of disease-specific antigens has recently been shown to effectively expand regulatory T cells in a number of autoimmune disease models. Clemente-Casares et al. (11), through a series of elegant studies, showed that systemic delivery of nanoparticles coated with various autoimmune, disease-relevant antigens resulted in specific expansion of regulatory T cells and subsequent suppression of disease.
While aberrant costimulation and activation of T cells promotes development of autoimmunity, a number of coinhibitory pathways have been shown to limit autoimmune disease. One of the coinhibitory pathways involves binding of the programmed death-ligand 1 (PD-L1) to PD-1 or B7.1, which inhibits proliferation and activation of CD8+ T cells. A protective role of the PD-1/PD-L1 inhibitory pathway has been demonstrated in autoimmune diseases including rheumatoid arthritis and systemic erythematosus (86). During pregnancy, the PD-1/PD-L1 pathway functions to maintain balance between regulatory T cells and T-helper 17 cells, and this is altered in pre-eclampsia (69). However, the role of PD-L1 expression in hypertension has not yet been investigated.
Dendritic Cells as a Bridge between Innate and Adaptive Immunity
DCs not only influence T-cell function, but also activate other cells of the adaptive immune system including B-lymphocytes and natural killer cells. The innate immune response occurs when DCs recognize pathogen-associated molecular patterns (PAMPs) including viral nucleic acids, bacterial molecules such as lipopolysaccharide (LPS), lipoproteins, flagellin, peptidoglycan, and CpG DNA (45). In addition to PAMPs, nonmicrobial danger signals from apoptotic and necrotic host cells known as danger-associated molecular patterns (DAMPs) also trigger innate immune responses (2). These PAMPs and DAMPs are recognized by host cells via pattern recognition receptors (PRRs) including Toll-like receptors (TLRs), Nod-like receptors, and RIGI-like receptors (1, 20, 67). PRRs have been shown to play a pivotal role in inducing immune activation in DCs, thus bridging the innate to the adaptive immune system. Most of the studies implicating PRRs in hypertension and vascular dysfunction have focused on TLRs (4, 21, 42, 43, 68). The specific role of PRR expression on DCs in hypertension still remains to be elucidated.
Dendritic Cell Subtypes and Cardiovascular Disease
In humans, DCs are broadly categorized into CD11C-/CD123+ plasmacytoid (p)DCs and CD11c+/CD123− conventional (c)DCs or myeloid (m)DCs, which arise from CD34+ hematopoietic precursors in the bone marrow (8, 18, 38, 57, 60). A third type of DCs, the monocyte-derived DCs (MoDCs), differentiates from monocytes in response to inflammation (57). Although the classification of DCs is still evolving and there are considerable differences in markers that define human and mouse DCs, the subsets in both steady state and inflammation have been recently reviewed in mice and humans (47, 57). Both mDCs and pDCs have been shown to play a role in cardiovascular disease, including coronary heart disease, peripheral arterial disease, and atherosclerosis (10, 87). In 2006, Niessner et al. (54) showed that pDCs accumulate in the atherosclerotic plaque and activate cytotoxic T cells via increased production of IFN-α. Niessner et al. (55) subsequently showed that IFN-α amplifies inflammation and leads to plaque destabilization, sensitizing APCs toward pathogen-derived TLR4 ligands. In contrast, Christ et al. (14) showed that pDCs are reduced in both mouse and human atherosclerotic lesions and that selective deletion of pDCs is protective against atherosclerosis. These contradictory findings are likely due to dynamic changes in this cell type during different stages of disease progression.
mDCs have also been implicated in cardiovascular disease. Yilmaz et al. (80) showed that mDCs are activated and co-localize with T cells in coronary artery lesions in patients with Kawasaki disease. DC numbers are decreased in the circulation after stroke, coronary heart disease, and chronic kidney disease, likely due to increased recruitment to the sites of inflammation (59, 79, 81, 82). In fact, Yilmaz et al. and others (33, 78) found that DCs accumulate in the myocardium after acute myocardial infarction. While substantial progress has been made in studying the role of DCs in cardiovascular disease, we are only beginning to understand the role of this cell type in the genesis of hypertension, which is the major risk factor for cardiovascular disease. Below, we discuss recent advances in our knowledge about the role of DCs in hypertension and outline potential therapeutic approaches targeting this cell type.
Dendritic Cells and Hypertension
Although T cells have taken a center stage in studies implicating the immune system in the genesis of hypertension, a few studies from several laboratories including our own suggest that DCs play a major role. In 2010 Vinh et al. (71) showed that T-cell activation in hypertension requires the classical T-cell receptor ligation and costimulation via an interaction with B7 ligands on APCs. The authors found that during experimental hypertension, there is increased expression of the B7 ligand CD86 in DCs. They also showed that blockade of the B7/CD28 T-cell costimulation axis by treatment with CTLA4-Ig or genetic deletion of B7 ligands prevents both angiotensin II and DOCA-salt-induced hypertension. Importantly, the authors demonstrated that this blockade of the costimulatory signal could reverse already established hypertension.
In addition to costimulatory engagement of the DC B7 ligands by T-cell CD28, which activates naïve T cells, an important interaction involving CD70 on APCs and CD27 on T cells is required for formation of memory T cells (16, 19) and has been implicated in the pathogenesis of hypertension. Recently, we found that in a mouse model of repeated hypertensive challenges, there is increased surface expression of CD70 on DCs in the kidney and activation of memory T cells to produce cytokines, including IL-17A and IFN-ϒ (27). In this study, we induced the first hypertensive challenge using L-NAME followed by a wash out period. This sensitized immune cells and rendered the mice susceptible to salt-induced hypertension. We previously showed that DCs from hypertensive mice drive memory T-cell proliferation and prime naïve mice to develop hypertension in response to a subpressor dose of angiotensin II (30). These studies suggest that hypertension may be antigen mediated, rather than a nonspecific inflammatory event. Furthermore, these studies provide a rationale for evaluation of the efficacy of drugs that target costimulation in treating hypertension that is refractory to available treatments.
Isolevuglandins as Potential Neoantigens in Hypertension
While it is well established that activation of the adaptive immune system contributes to malignant hypertension, the antigens involved are not known. In 1985, Salomon and Miller (64) discovered that prostaglandin H2, a labile endoperoxide intermediate in the cyclooxygenase pathway, undergoes nonenzymatic rearrangement to form γ-ketoaldehydes, which he named isolevuglandins because of their structural similarity to levulinic acid. Iyer et al. (28) later found that these compounds are highly reactive, forming covalent bonds with lysines on proteins, leading to formation of cross-linked proteins. In 1990, Morrow et al. (49) discovered that arachidonic acid undergoes free radical catalyzed lipid peroxidation leading to formation of a series of similar compounds in vivo, in rats and humans. Brame et al. (5) subsequently showed that these compounds are also formed in abundance as products of the isoprostane pathway by rearrangement of the prostaglandin H2-like endoperoxide intermediates. Most importantly, compared with the cyclooxygenase pathway, which yields only 2 γ-ketoaldehydes, the isoprostane pathway yields 64 different isolevuglandin isomers. It is not well understood how these IsoLGs affect cellular function. However, in 2002 Davies et al. (15) showed that the cross-linked proteins formed by reaction with IsoLGs produced by both the isoprostane and the cyclooxygenase pathway inhibit the ability of the proteasome to degrade normal proteins. Proteasome inhibition has been shown to induce expression of inflammatory genes via the NF-κB pathway (13).
In 2014 we published a novel pathway in which DCs are activated in hypertension by proteins modified by IsoLGs. We found that during hypertension, there is increased DC production of superoxide, which is NADPH oxidase dependent. This was associated with formation of IsoLG-modified proteins which accumulate in DCs and act as neoantigens, promoting an autoimmune-like state and hypertension (30). DCs that accumulate IsoLGs have increased expression of the B7 ligands CD80 and CD86 and produce large amounts of IL-6, IL-1β, and IL-23, which are known to promote differentiation of naïve T cells into IL-17A-producing cells. Indeed, we found that when cocultured with primed T cells, these DCs drive T-cell proliferation and cytokine production, including IL-17A, TNF-α, and IFN-γ which contribute to hypertension. Adoptive transfer of these activated DCs primed naïve recipient mice to develop hypertension in response to a subpressor dose of angiotensin II. These studies suggest that IsoLGs act as neoantigens presented by DCs to T cells in hypertension.
Isolevuglandin Cross-Presentation in Hypertension
As mentioned above, cross-presentation is a process where extracellular antigens are phagocytosed by DCs and presented in MHC class 1 to CD8+ T cells, and this is important in maintaining tolerance and inducing viral immunity (24, 25). In a recent study, by examining T-cell receptor usage, we found that an oligo-clonal population of particularly CD8+ T cells contributes to hypertension (70). We also found that similar to intracellularly-formed IsoLGs, exposing DCs to IsoLG-modified renal homogenates preferentially enhances their ability to drive CD8+ proliferation (30). Moreover, DCs primed with aortic homogenates from mice with vascular specific overexpression of the NADPH oxidase subunit p22phox had increased production of cytokines including IL-1β, IL-6, TGF-β1, and GM-CSF. These cells markedly activated T cells from mice of the same genotype but not T cells from wild-type mice to proliferate and produce cytokines including IL-17A, IL-17F, TNF-α, and IFN-γ (76). These observations support the concept that increased oxidative stress leads to formation of IsoLG protein adducts that are presented on MHC class 1 molecules to CD8+ T cells.
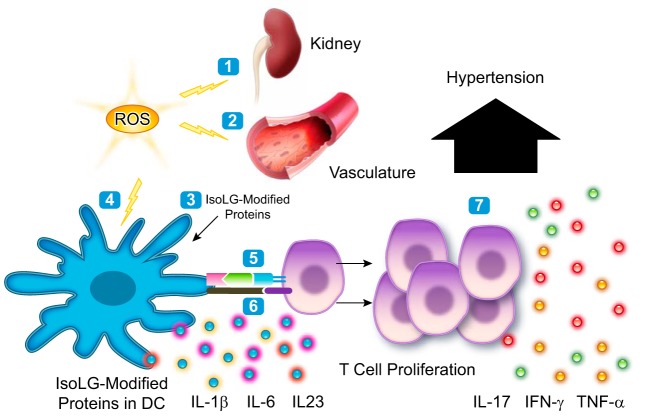
The IsoLG protein adducts that activate DCs can be formed intracellularly when the APC is exposed to increase oxidative stress or in other organs, including the kidney (30) or the vasculature (76) (Fig. 1). Of note, a number of studies have demonstrated a pivotal role of NADPH oxidase-derived superoxide in altering antigen cross-presentation in the context of MHC class 1 (34). Reactive oxygen species have also been shown to regulate class I peptide selection during antigen processing (58). Thus cross-presentation is a fundamental process that may link ROS formation in tissues to DC activation, inflammation, and hypertension. The mechanisms by which both endogenous and exogenous IsoLG-modified peptides are processed and presented in MHC class 1 to T cell in hypertension need to be elucidated.
Fig. 1.
Paradigm illustrating how isolevuglandins (IsoLGs) activate dendritic cells (DCs) leading to hypertension. During hypertension, there is increased oxidative stress in the kidney (1) and the vasculature (2) leading to formation of isolevuglandin-protein adducts. Dendritic cells take up the altered proteins (3). Increased oxidative stress can also lead to formation of isolevuglandin-protein adducts directly inside dendritic cells (4). The altered proteins are processed and presented in MHC to T cells by dendritic cells (5). The dendritic cells increase expression of costimulatory molecules and produce cytokines which activate T cells (6). The activated T cells proliferate and produce cytokines leading to hypertension (7).
What are the specific hypertensive stimuli that lead to DC activation and accumulation of IsoLGs? We previously reported that while in vivo exposure of mice to angiotensin II results in a fivefold increase in DC superoxide production, in vitro exposure only had a minimum effect (30). This suggests that stimuli other than angiotensin II are contributing to DC activation in vivo. Angiotensin II is known to stimulate the central nervous system and promote T-cell activation in hypertension (7). In 2013 Deo et al. (17) found that norepinephrine increases NADPH oxidase-dependent superoxide production in human peripheral blood mononuclear cells. In a recent study, we found that norepinephrine dose-dependently increases IsoLG formation in DCs in vitro (77). In keeping with this finding, renal sympathetic outflow promoted DC activation and accumulation of IsoLG adducts during angiotensin II infusion, and this was reversed by renal denervation (77). Interestingly, norepinephrine stimulation increases superoxide production in T cells but has an inhibitory effect on T lymphocyte activation (6). These studies suggest that sympathetic nerves contribute to DC activation via increased superoxide production and IsoLG adduct formation leading to T-cell activation and hypertension.
Recently, it has become recognized that interstitial sodium can accumulate in the interstitial spaces of humans with hypertension (32). In 2013 two studies showed that these higher concentrations of sodium can activate T cells via signaling molecules such as NFAT5 and the salt-sensing kinase serum and glucocorticoid regulated kinase 1 (SGK1) (31, 75). It is not known whether elevated sodium concentrations can activate DCs via formation of immunogenic IsoLGs.
Conclusions and Perspectives
Much of the groundwork in understanding the role of the immune system in hypertension has been performed on experimental animals treated with angiotensin II, catecholamines, aldosterone, and excess salt. Although these are often elevated in human hypertension, the most commonly used approaches to study hypertension in animal models are eliminating different subtypes of cells or their cytokines and genetic deletion of signaling molecules. Humans are undoubtedly more difficult to study because of inherent genetic and environmental variability, and these approaches are neither practical nor directly translatable. Despite these barriers, the recent genome-wide association studies have helped to identify a number of human key driver genes for hypertension. One of the top key driver genes was the lymphocyte adaptor protein LNK/SH2B3 (26), and we recently found that mice lacking this gene are predisposed to inflammation and hypertension (63). Moreover, emerging new tools like the CRISPR/Cas9 and targeted genome editing hold great promise to generate knock-in models that more faithfully mimic human hypertension.
With regard to DCs, there are differences in some surface markers and cytokine production patterns between humans and mice. One example is that unlike mouse DCs, human DCs do not express CD8 on their surface (65). However, there are some similarities between mouse and human DCs. For example, some of the human thymic DCs are CD11c+CD11b−CD45ROlo and lack myeloid markers, as do mouse thymic CD8+ DCs. Similarly, the human thymic DCs that are CD11chiCD11b+CD45ROhi and express myeloid markers are equivalent to mouse CD8- myeloid DCs (65). In a recent study, we found that monocyte-derived CD11chiCD11b+ DCs were the most activated and had the most accumulation of IsoLG adducts (30). Similarly monocytes from hypertensive patients had increased accumulation of IsoLG-adducted peptides and expression of activation marker CD83 (30). These studies suggest that there is considerable overlap and similarities between murine and human DCs. Thus murine studies can be extremely informative in understanding human hypertension.
IsoLG-adduct formation is a new posttranslational protein modification that might have a role in numerous conditions where lipid oxidation occurs. Emerging evidence has implicated other posttranslational protein modifications, such as citrullination and carbamoylation, in autoimmune disease, including rheumatoid arthritis (46, 51). In humans, monocyte-derived DCs have been widely used as a tool for cancer immunotherapy. These recent studies provide a rationale for these approaches in hypertension.
While we know that inflammation and oxidative stress are implicated in the genesis of hypertension, we are only beginning to unravel the mechanisms by which these two interact in the etiology of this important disease. IsoLG protein modifications provide a mechanism by which chronic oxidative stress can lead to systemic immune activation and hypertension. Despite substantial evidence implicating oxidative stress in the genesis of hypertension, to date, clinical trials with antioxidants have failed to show efficacy (23, 36, 37, 61, 66, 72, 73, 84). There may be major methodological flaws with some of these trials, including using doses of antioxidants below that required to significantly alter lipid peroxidation (62). Nevertheless, when very high doses of antioxidants are used, they are paradoxically associated with increased cardiovascular mortality (74). Because reactive oxygen species have important physiological roles, it is quite possible that their complete elimination is neither possible nor desirable. Thus rather than focusing on antioxidants, it is time to examine the possibility of scavenging IsoLGs. Because these are downstream products of oxidative stress, reducing their levels may have significantly fewer adverse effects and provide greater protection. Clearly further research is needed to understand the mechanisms by which IsoLG adducts are formed and to determine specifically what proteins are involved. Identifying the altered peptides may lead to important immunomodulatory therapeutic approaches for not only hypertension but also other autoimmune conditions where lipid oxidation occurs.
GRANTS
This work was supported by American Heart Association Strategically Focused Research Network Grant 14SFRN20420046 and National Heart, Lung, and Blood Institute Grant K01-HL-130497.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
K.B.D. and A.K. prepared figures; K.B.D. and A.K. drafted manuscript; K.B.D., S.S.D., and A.K. edited and revised manuscript; S.SD and A.K. approved final version of manuscript.
REFERENCES
- 1.Akira S, Takeda K. Toll-like receptor signalling. Nat Rev Immunol : 499–511, 2004. doi: 10.1038/nri1391. [DOI] [PubMed] [Google Scholar]
- 2.Benko S, Philpott DJ, Girardin SE. The microbial and danger signals that activate Nod-like receptors. Cytokine : 368–373, 2008. doi: 10.1016/j.cyto.2008.07.013. [DOI] [PubMed] [Google Scholar]
- 3.Birmingham K. Ralph Steinman. Nat Med : 1078, 2001. doi: 10.1038/nm1001-1078. [DOI] [PubMed] [Google Scholar]
- 4.Bomfim GF, Dos Santos RA, Oliveira MA, Giachini FR, Akamine EH, Tostes RC, Fortes ZB, Webb RC, Carvalho MH. Toll-like receptor 4 contributes to blood pressure regulation and vascular contraction in spontaneously hypertensive rats. Clin Sci (Lond) : 535–543, 2012. doi: 10.1042/CS20110523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brame CJ, Salomon RG, Morrow JD, Roberts LJ II. Identification of extremely reactive γ-ketoaldehydes (isolevuglandins) as products of the isoprostane pathway and characterization of their lysyl protein adducts. J Biol Chem : 13139–13146, 1999. doi: 10.1074/jbc.274.19.13139. [DOI] [PubMed] [Google Scholar]
- 6.Case AJ, Zimmerman MC. Redox-regulated suppression of splenic T-lymphocyte activation in a model of sympathoexcitation. Hypertension : 916–923, 2015. doi: 10.1161/HYPERTENSIONAHA.114.05075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Case AJ, Zimmerman MC. Sympathetic-mediated activation versus suppression of the immune system: consequences for hypertension. J Physiol : 527–536, 2016. doi: 10.1113/JP271516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chang CC, Wright A, Punnonen J. Monocyte-derived CD1a+ and CD1a− dendritic cell subsets differ in their cytokine production profiles, susceptibilities to transfection, and capacities to direct Th cell differentiation. J Immunol : 3584–3591, 2000. doi: 10.4049/jimmunol.165.7.3584. [DOI] [PubMed] [Google Scholar]
- 9.Chobanian AV. Shattuck Lecture. The hypertension paradox—more uncontrolled disease despite improved therapy. N Engl J Med : 878–887, 2009. doi: 10.1056/NEJMsa0903829. [DOI] [PubMed] [Google Scholar]
- 10.Christ A, Temmerman L, Legein B, Daemen MJ, Biessen EA. Dendritic cells in cardiovascular diseases: epiphenomenon, contributor, or therapeutic opportunity. Circulation : 2603–2613, 2013. doi: 10.1161/CIRCULATIONAHA.113.003364. [DOI] [PubMed] [Google Scholar]
- 11.Clemente-Casares X, Blanco J, Ambalavanan P, Yamanouchi J, Singha S, Fandos C, Tsai S, Wang J, Garabatos N, Izquierdo C, Agrawal S, Keough MB, Yong VW, James E, Moore A, Yang Y, Stratmann T, Serra P, Santamaria P. Expanding antigen-specific regulatory networks to treat autoimmunity. Nature : 434–440, 2016. doi: 10.1038/nature16962. [DOI] [PubMed] [Google Scholar]
- 12.Crowley SD, Song YS, Lin EE, Griffiths R, Kim HS, Ruiz P. Lymphocyte responses exacerbate angiotensin II-dependent hypertension. Am J Physiol Regul Integr Comp Physiol : R1089–R1097, 2010. doi: 10.1152/ajpregu.00373.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cullen SJ, Ponnappan S, Ponnappan U. Proteasome inhibition up-regulates inflammatory gene transcription induced by an atypical pathway of NF-κB activation. Biochem Pharmacol : 706–714, 2010. doi: 10.1016/j.bcp.2009.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Daissormont IT, Christ A, Temmerman L, Sampedro Millares S, Seijkens T, Manca M, Rousch M, Poggi M, Boon L, van der Loos C, Daemen M, Lutgens E, Halvorsen B, Aukrust P, Janssen E, Biessen EA. Plasmacytoid dendritic cells protect against atherosclerosis by tuning T-cell proliferation and activity. Circ Res : 1387–1395, 2011. doi: 10.1161/CIRCRESAHA.111.256529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Davies SS, Amarnath V, Montine KS, Bernoud-Hubac N, Boutaud O, Montine TJ, Roberts LJ II. Effects of reactive γ-ketoaldehydes formed by the isoprostane pathway (isoketals) and cyclooxygenase pathway (levuglandins) on proteasome function. FASEB J : 715–717, 2002. doi: 10.1096/fj.01-0696fje. [DOI] [PubMed] [Google Scholar]
- 16.Denoeud J, Moser M. Role of CD27/CD70 pathway of activation in immunity and tolerance. J Leukoc Biol : 195–203, 2011. doi: 10.1189/jlb.0610351. [DOI] [PubMed] [Google Scholar]
- 17.Deo SH, Jenkins NT, Padilla J, Parrish AR, Fadel PJ. Norepinephrine increases NADPH oxidase-derived superoxide in human peripheral blood mononuclear cells via α-adrenergic receptors. Am J Physiol Regul Integr Comp Physiol : R1124–R1132, 2013. doi: 10.1152/ajpregu.00347.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dzionek A, Fuchs A, Schmidt P, Cremer S, Zysk M, Miltenyi S, Buck DW, Schmitz J. BDCA-2, BDCA-3, and BDCA-4: three markers for distinct subsets of dendritic cells in human peripheral blood. J Immunol : 6037–6046, 2000. doi: 10.4049/jimmunol.165.11.6037. [DOI] [PubMed] [Google Scholar]
- 19.Feau S, Garcia Z, Arens R, Yagita H, Borst J, Schoenberger SP. The CD4+ T-cell help signal is transmitted from APC to CD8+ T-cells via CD27-CD70 interactions. Nat Commun : 948, 2012. doi: 10.1038/ncomms1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fritz JH, Ferrero RL, Philpott DJ, Girardin SE. Nod-like proteins in immunity, inflammation and disease. Nat Immunol : 1250–1257, 2006. doi: 10.1038/ni1412. [DOI] [PubMed] [Google Scholar]
- 21.Goulopoulou S, McCarthy CG, Webb RC. Toll-like receptors in the vascular system: sensing the dangers within. Pharmacol Rev : 142–167, 2016. doi: 10.1124/pr.114.010090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Guzik TJ, Hoch NE, Brown KA, McCann LA, Rahman A, Dikalov S, Goronzy J, Weyand C, Harrison DG. Role of the T cell in the genesis of angiotensin II induced hypertension and vascular dysfunction. J Exp Med : 2449–2460, 2007. doi: 10.1084/jem.20070657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Heart Protection Study Collaborative Group MRC/BHF Heart Protection Study of antioxidant vitamin supplementation in 20,536 high-risk individuals: a randomised placebo-controlled trial. Lancet : 23–33, 2002. doi: 10.1016/S0140-6736(02)09328-5. [DOI] [PubMed] [Google Scholar]
- 24.Heath WR, Carbone FR. Cross-presentation in viral immunity and self-tolerance. Nat Rev Immunol : 126–134, 2001. doi: 10.1038/35100512. [DOI] [PubMed] [Google Scholar]
- 25.Heath WR, Carbone FR. Cross-presentation, dendritic cells, tolerance and immunity. Annu Rev Immunol : 47–64, 2001. doi: 10.1146/annurev.immunol.19.1.47. [DOI] [PubMed] [Google Scholar]
- 26.Huan T, Meng Q, Saleh MA, Norlander AE, Joehanes R, Zhu J, Chen BH, Zhang B, Johnson AD, Ying S, Courchesne P, Raghavachari N, Wang R, Liu P, O’Donnell CJ, Vasan R, Munson PJ, Madhur MS, Harrison DG, Yang X, Levy D. Integrative network analysis reveals molecular mechanisms of blood pressure regulation. Mol Syst Biol : 799, 2015. doi: 10.15252/msb.20145399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Itani HA, Xiao L, Saleh MA, Wu J, Pilkinton MA, Dale BL, Barbaro NR, Foss JD, Kirabo A, Montaniel KR, Norlander AE, Chen W, Sato R, Navar LG, Mallal SA, Madhur MS, Bernstein KE, Harrison DG. CD70 exacerbates blood pressure elevation and renal damage in response to repeated hypertensive stimuli. Circ Res : 1233–1243, 2016. doi: 10.1161/CIRCRESAHA.115.308111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Iyer RS, Ghosh S, Salomon RG. Levuglandin E2 crosslinks proteins. Prostaglandins : 471–480, 1989. doi: 10.1016/0090-6980(89)90096-8. [DOI] [PubMed] [Google Scholar]
- 29.Julius S, Nesbitt SD, Egan BM, Weber MA, Michelson EL, Kaciroti N, Black HR, Grimm RH Jr, Messerli FH, Oparil S, Schork MA; Trial of Preventing Hypertension (TROPHY) Study Investigators . Feasibility of treating prehypertension with an angiotensin-receptor blocker. N Engl J Med : 1685–1697, 2006. doi: 10.1056/NEJMoa060838. [DOI] [PubMed] [Google Scholar]
- 30.Kirabo A, Fontana V, de Faria AP, Loperena R, Galindo CL, Wu J, Bikineyeva AT, Dikalov S, Xiao L, Chen W, Saleh MA, Trott DW, Itani HA, Vinh A, Amarnath V, Amarnath K, Guzik TJ, Bernstein KE, Shen XZ, Shyr Y, Chen SC, Mernaugh RL, Laffer CL, Elijovich F, Davies SS, Moreno H, Madhur MS, Roberts J II, Harrison DG. DC isoketal-modified proteins activate T cells and promote hypertension. J Clin Invest : 4642–4656, 2014. doi: 10.1172/JCI74084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kleinewietfeld M, Manzel A, Titze J, Kvakan H, Yosef N, Linker RA, Muller DN, Hafler DA. Sodium chloride drives autoimmune disease by the induction of pathogenic TH17 cells. Nature : 518–522, 2013. doi: 10.1038/nature11868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kopp C, Linz P, Dahlmann A, Hammon M, Jantsch J, Müller DN, Schmieder RE, Cavallaro A, Eckardt KU, Uder M, Luft FC, Titze J. 23Na magnetic resonance imaging-determined tissue sodium in healthy subjects and hypertensive patients. Hypertension : 635–640, 2013. doi: 10.1161/HYPERTENSIONAHA.111.00566. [DOI] [PubMed] [Google Scholar]
- 33.Kretzschmar D, Betge S, Windisch A, Pistulli R, Rohm I, Fritzenwanger M, Jung C, Schubert K, Theis B, Petersen I, Drobnik S, Mall G, Figulla HR, Yilmaz A. Recruitment of circulating dendritic cell precursors into the infarcted myocardium and pro-inflammatory response in acute myocardial infarction. Clin Sci (Lond) : 387–398, 2012. doi: 10.1042/CS20110561. [DOI] [PubMed] [Google Scholar]
- 34.Lam GY, Huang J, Brumell JH. The many roles of NOX2 NADPH oxidase-derived ROS in immunity. Semin Immunopathol : 415–430, 2010. doi: 10.1007/s00281-010-0221-0. [DOI] [PubMed] [Google Scholar]
- 35.Lenfant C, Chobanian AV, Jones DW, Roccella EJ; Joint National Committee on the Prevention, Detection, Evaluation, and Treatment of High Blood Pressure . Seventh report of the Joint National Committee on the Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC 7): resetting the hypertension sails. Hypertension : 1178–1179, 2003. doi: 10.1161/01.HYP.0000075790.33892.AE. [DOI] [PubMed] [Google Scholar]
- 36.Lonn E, Bosch J, Yusuf S, Sheridan P, Pogue J, Arnold JM, Ross C, Arnold A, Sleight P, Probstfield J, Dagenais GR; HOPE and HOPE-TOO Trial Investigators . Effects of long-term vitamin E supplementation on cardiovascular events and cancer: a randomized controlled trial. JAMA : 1338–1347, 2005. doi: 10.1001/jama.293.11.1338. [DOI] [PubMed] [Google Scholar]
- 37.Lonn E, Yusuf S, Dzavik V, Doris C, Yi Q, Smith S, Moore-Cox A, Bosch J, Riley W, Teo K; SECURE Investigators . Effects of ramipril and vitamin E on atherosclerosis: the study to evaluate carotid ultrasound changes in patients treated with ramipril and vitamin E (SECURE). Circulation : 919–925, 2001. doi: 10.1161/01.CIR.103.7.919. [DOI] [PubMed] [Google Scholar]
- 38.MacDonald KP, Munster DJ, Clark GJ, Dzionek A, Schmitz J, Hart DN. Characterization of human blood dendritic cell subsets. Blood : 4512–4520, 2002. doi: 10.1182/blood-2001-11-0097. [DOI] [PubMed] [Google Scholar]
- 39.Madhur MS, Lob HE, McCann LA, Iwakura Y, Blinder Y, Guzik TJ, Harrison DG. Interleukin 17 promotes angiotensin II-induced hypertension and vascular dysfunction. Hypertension : 500–507, 2010. doi: 10.1161/HYPERTENSIONAHA.109.145094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mantegazza AR, Magalhaes JG, Amigorena S, Marks MS. Presentation of phagocytosed antigens by MHC class I and II. Traffic : 135–152, 2013. doi: 10.1111/tra.12026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Martín-Fontecha A, Sebastiani S, Höpken UE, Uguccioni M, Lipp M, Lanzavecchia A, Sallusto F. Regulation of dendritic cell migration to the draining lymph node. J Exp Med : 615–621, 2003. doi: 10.1084/jem.20030448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.McCarthy CG, Goulopoulou S, Wenceslau CF, Spitler K, Matsumoto T, Webb RC. Toll-like receptors and damage-associated molecular patterns: novel links between inflammation and hypertension. Am J Physiol Heart Circ Physiol : H184–H196, 2014. doi: 10.1152/ajpheart.00328.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.McCarthy CG, Webb RC. The toll of the gridiron: damage-associated molecular patterns and hypertension in American football. FASEB J : 34–40, 2016. doi: 10.1096/fj.15-279588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.McMaster WG, Kirabo A, Madhur MS, Harrison DG. Inflammation, immunity, and hypertensive end-organ damage. Circ Res : 1022–1033, 2015. doi: 10.1161/CIRCRESAHA.116.303697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Medzhitov R. Recognition of microorganisms and activation of the immune response. Nature : 819–826, 2007. doi: 10.1038/nature06246. [DOI] [PubMed] [Google Scholar]
- 46.Ménard HA, Lapointe E, Rochdi MD, Zhou ZJ. Insights into rheumatoid arthritis derived from the Sa immune system. Arthritis Res : 429–432, 2000. doi: 10.1186/ar122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Merad M, Sathe P, Helft J, Miller J, Mortha A. The dendritic cell lineage: ontogeny and function of dendritic cells and their subsets in the steady state and the inflamed setting. Annu Rev Immunol : 563–604, 2013. doi: 10.1146/annurev-immunol-020711-074950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mervaala E, Müller DN, Park JK, Dechend R, Schmidt F, Fiebeler A, Bieringer M, Breu V, Ganten D, Haller H, Luft FC. Cyclosporin A protects against angiotensin II-induced end-organ damage in double transgenic rats harboring human renin and angiotensinogen genes. Hypertension : 360–366, 2000. doi: 10.1161/01.HYP.35.1.360. [DOI] [PubMed] [Google Scholar]
- 49.Morrow JD, Hill KE, Burk RF, Nammour TM, Badr KF, Roberts LJ II. A series of prostaglandin F2-like compounds are produced in vivo in humans by a non-cyclooxygenase, free radical-catalyzed mechanism. Proc Natl Acad Sci USA : 9383–9387, 1990. doi: 10.1073/pnas.87.23.9383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Muller DN, Shagdarsuren E, Park JK, Dechend R, Mervaala E, Hampich F, Fiebeler A, Ju X, Finckenberg P, Theuer J, Viedt C, Kreuzer J, Heidecke H, Haller H, Zenke M, Luft FC. Immunosuppressive treatment protects against angiotensin II-induced renal damage. Am J Pathol : 1679–1693, 2002. doi: 10.1016/S0002-9440(10)64445-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mydel P, Wang Z, Brisslert M, Hellvard A, Dahlberg LE, Hazen SL, Bokarewa M. Carbamylation-dependent activation of T cells: a novel mechanism in the pathogenesis of autoimmune arthritis. J Immunol : 6882–6890, 2010. doi: 10.4049/jimmunol.1000075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Narayan KM, Ali MK, Koplan JP. Global noncommunicable diseases—where worlds meet. N Engl J Med : 1196–1198, 2010. doi: 10.1056/NEJMp1002024. [DOI] [PubMed] [Google Scholar]
- 53.Nataraj C, Oliverio MI, Mannon RB, Mannon PJ, Audoly LP, Amuchastegui CS, Ruiz P, Smithies O, Coffman TM. Angiotensin II regulates cellular immune responses through a calcineurin-dependent pathway. J Clin Invest : 1693–1701, 1999. doi: 10.1172/JCI7451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Niessner A, Sato K, Chaikof EL, Colmegna I, Goronzy JJ, Weyand CM. Pathogen-sensing plasmacytoid dendritic cells stimulate cytotoxic T-cell function in the atherosclerotic plaque through interferon-α. Circulation : 2482–2489, 2006. doi: 10.1161/CIRCULATIONAHA.106.642801. [DOI] [PubMed] [Google Scholar]
- 55.Niessner A, Shin MS, Pryshchep O, Goronzy JJ, Chaikof EL, Weyand CM. Synergistic proinflammatory effects of the antiviral cytokine interferon-α and Toll-like receptor 4 ligands in the atherosclerotic plaque. Circulation : 2043–2052, 2007. doi: 10.1161/CIRCULATIONAHA.107.697789. [DOI] [PubMed] [Google Scholar]
- 56.Norlander AE, Saleh MA, Kamat NV, Ko B, Gnecco J, Zhu L, Dale BL, Iwakura Y, Hoover RS, McDonough AA, Madhur MS. Interleukin-17A regulates renal sodium transporters and renal injury in angiotensin II-induced hypertension. Hypertension : 167–174, 2016. doi: 10.1161/HYPERTENSIONAHA.116.07493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.O’Keeffe M, Mok WH, Radford KJ. Human dendritic cell subsets and function in health and disease. Cell Mol Life Sci : 4309–4325, 2015. doi: 10.1007/s00018-015-2005-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Park B, Lee S, Kim E, Cho K, Riddell SR, Cho S, Ahn K. Redox regulation facilitates optimal peptide selection by MHC class I during antigen processing. Cell : 369–382, 2006. doi: 10.1016/j.cell.2006.08.041. [DOI] [PubMed] [Google Scholar]
- 59.Paul K, Kretzschmar D, Yilmaz A, Bärthlein B, Titze S, Wolf G, Busch M; GCKD-Study Investigators . Circulating dendritic cell precursors in chronic kidney disease: a cross-sectional study. BMC Nephrol : 274, 2013. doi: 10.1186/1471-2369-14-274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Piccioli D, Tavarini S, Borgogni E, Steri V, Nuti S, Sammicheli C, Bardelli M, Montagna D, Locatelli F, Wack A. Functional specialization of human circulating CD16 and CD1c myeloid dendritic-cell subsets. Blood : 5371–5379, 2007. doi: 10.1182/blood-2006-08-038422. [DOI] [PubMed] [Google Scholar]
- 61.Rapola JM, Virtamo J, Ripatti S, Huttunen JK, Albanes D, Taylor PR, Heinonen OP. Randomised trial of α-tocopherol and β-carotene supplements on incidence of major coronary events in men with previous myocardial infarction. Lancet : 1715–1720, 1997. doi: 10.1016/S0140-6736(97)01234-8. [DOI] [PubMed] [Google Scholar]
- 62.Roberts LJ II, Oates JA, Linton MF, Fazio S, Meador BP, Gross MD, Shyr Y, Morrow JD. The relationship between dose of vitamin E and suppression of oxidative stress in humans. Free Radic Biol Med : 1388–1393, 2007. doi: 10.1016/j.freeradbiomed.2007.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Saleh MA, McMaster WG, Wu J, Norlander AE, Funt SA, Thabet SR, Kirabo A, Xiao L, Chen W, Itani HA, Michell D, Huan T, Zhang Y, Takaki S, Titze J, Levy D, Harrison DG, Madhur MS. Lymphocyte adaptor protein LNK deficiency exacerbates hypertension and end-organ inflammation. J Clin Invest : 1189–1202, 2015. doi: 10.1172/JCI76327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Salomon RG, Miller DB. Levuglandins: isolation, characterization, and total synthesis of new secoprostanoid products from prostaglandin endoperoxides. Adv Prostaglandin Thromboxane Leukot Res : 323–326, 1985. [PubMed] [Google Scholar]
- 65.Shortman K, Liu YJ. Mouse and human dendritic cell subtypes. Nat Rev Immunol : 151–161, 2002. doi: 10.1038/nri746. [DOI] [PubMed] [Google Scholar]
- 66.Stephens NG, Parsons A, Brown MJ, Schofield PM, Kelly F, Cheeseman K, Mitchinson MJ. Randomised controlled trial of vitamin E in patients with coronary disease: Cambridge Heart Antioxidant Study (CHAOS). Lancet : 781–786, 1996. doi: 10.1016/S0140-6736(96)90866-1. [DOI] [PubMed] [Google Scholar]
- 67.Takeuchi O, Akira S. Innate immunity to virus infection. Immunol Rev : 75–86, 2009. doi: 10.1111/j.1600-065X.2008.00737.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Thompson JA, Webb RC. Potential role of Toll-like receptors in programming of vascular dysfunction. Clin Sci (Lond) : 19–25, 2013. doi: 10.1042/CS20120673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tian M, Zhang Y, Liu Z, Sun G, Mor G, Liao A. The PD-1/PD-L1 inhibitory pathway is altered in pre-eclampsia and regulates T cell responses in pre-eclamptic rats. Sci Rep : 27683, 2016. doi: 10.1038/srep27683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Trott DW, Thabet SR, Kirabo A, Saleh MA, Itani H, Norlander AE, Wu J, Goldstein A, Arendshorst WJ, Madhur MS, Chen W, Li CI, Shyr Y, Harrison DG. Oligoclonal CD8+ T cells play a critical role in the development of hypertension. Hypertension : 1108–1115, 2014. doi: 10.1161/HYPERTENSIONAHA.114.04147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Vinh A, Chen W, Blinder Y, Weiss D, Taylor WR, Goronzy JJ, Weyand CM, Harrison DG, Guzik TJ. Inhibition and genetic ablation of the B7/CD28 T-cell costimulation axis prevents experimental hypertension. Circulation : 2529–2537, 2010. doi: 10.1161/CIRCULATIONAHA.109.930446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Virtamo J, Rapola JM, Ripatti S, Heinonen OP, Taylor PR, Albanes D, Huttunen JK. Effect of vitamin E and beta carotene on the incidence of primary nonfatal myocardial infarction and fatal coronary heart disease. Arch Intern Med : 668–675, 1998. doi: 10.1001/archinte.158.6.668. [DOI] [PubMed] [Google Scholar]
- 73.Vivekananthan DP, Penn MS, Sapp SK, Hsu A, Topol EJ. Use of antioxidant vitamins for the prevention of cardiovascular disease: meta-analysis of randomised trials. Lancet : 2017–2023, 2003. doi: 10.1016/S0140-6736(03)13637-9. [DOI] [PubMed] [Google Scholar]
- 74.Widder JD, Harrison DG. Can vitamin E prevent cardiovascular events and cancer? Nat Clin Pract Cardiovasc Med : 510–511, 2005. doi: 10.1038/ncpcardio0291. [DOI] [PubMed] [Google Scholar]
- 75.Wu C, Yosef N, Thalhamer T, Zhu C, Xiao S, Kishi Y, Regev A, Kuchroo VK. Induction of pathogenic TH17 cells by inducible salt-sensing kinase SGK1. Nature : 513–517, 2013. doi: 10.1038/nature11984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wu J, Saleh MA, Kirabo A, Itani HA, Montaniel KR, Xiao L, Chen W, Mernaugh RL, Cai H, Bernstein KE, Goronzy JJ, Weyand CM, Curci JA, Barbaro NR, Moreno H, Davies SS, Roberts LJ II, Madhur MS, Harrison DG. Immune activation caused by vascular oxidation promotes fibrosis and hypertension. J Clin Invest : 50–67, 2016. doi: 10.1172/JCI80761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Xiao L, Kirabo A, Wu J, Saleh MA, Zhu L, Wang F, Takahashi T, Loperena R, Foss JD, Mernaugh RL, Chen W, Roberts J II, Osborn JW, Itani HA, Harrison DG. Renal denervation prevents immune cell activation and renal inflammation in angiotensin II-induced hypertension. Circ Res : 547–557, 2015. doi: 10.1161/CIRCRESAHA.115.306010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Yilmaz A, Dietel B, Cicha I, Schubert K, Hausmann R, Daniel WG, Garlichs CD, Stumpf C. Emergence of dendritic cells in the myocardium after acute myocardial infarction - implications for inflammatory myocardial damage. Int J Biomed Sci : 27–36, 2010. [PMC free article] [PubMed] [Google Scholar]
- 79.Yilmaz A, Fuchs T, Dietel B, Altendorf R, Cicha I, Stumpf C, Schellinger PD, Blümcke I, Schwab S, Daniel WG, Garlichs CD, Kollmar R. Transient decrease in circulating dendritic cell precursors after acute stroke: potential recruitment into the brain. Clin Sci (Lond) : 147–157, 2009. doi: 10.1042/CS20090154. [DOI] [PubMed] [Google Scholar]
- 80.Yilmaz A, Rowley A, Schulte DJ, Doherty TM, Schröder NW, Fishbein MC, Kalelkar M, Cicha I, Schubert K, Daniel WG, Garlichs CD, Arditi M. Activated myeloid dendritic cells accumulate and co-localize with CD3+ T cells in coronary artery lesions in patients with Kawasaki disease. Exp Mol Pathol : 93–103, 2007. doi: 10.1016/j.yexmp.2007.01.007. [DOI] [PubMed] [Google Scholar]
- 81.Yilmaz A, Schaller T, Cicha I, Altendorf R, Stumpf C, Klinghammer L, Ludwig J, Daniel WG, Garlichs CD. Predictive value of the decrease in circulating dendritic cell precursors in stable coronary artery disease. Clin Sci (Lond) : 353–363, 2009. doi: 10.1042/CS20080392. [DOI] [PubMed] [Google Scholar]
- 82.Yilmaz A, Weber J, Cicha I, Stumpf C, Klein M, Raithel D, Daniel WG, Garlichs CD. Decrease in circulating myeloid dendritic cell precursors in coronary artery disease. J Am Coll Cardiol : 70–80, 2006. doi: 10.1016/j.jacc.2006.01.078. [DOI] [PubMed] [Google Scholar]
- 83.Yoon PW, Gillespie CD, George MG, Wall HK; Centers for Disease Control and Prevention (CDC) . Control of hypertension among adults—National Health and Nutrition Examination Survey, United States, 2005–2008. MMWR Suppl : 19–25, 2012. [PubMed] [Google Scholar]
- 84.Yusuf S, Dagenais G, Pogue J, Bosch J, Sleight P; The Heart Outcomes Prevention Evaluation Study Investigators . Vitamin E supplementation and cardiovascular events in high-risk patients. N Engl J Med : 154–160, 2000. doi: 10.1056/NEJM200001203420302. [DOI] [PubMed] [Google Scholar]
- 85.Zhang J, Rudemiller NP, Patel MB, Karlovich NS, Wu M, McDonough AA, Griffiths R, Sparks MA, Jeffs AD, Crowley SD. Interleukin-1 receptor activation potentiates salt reabsorption in angiotensin II-induced hypertension via the NKCC2 co-transporter in the nephron. Cell Metab : 360–368, 2016. doi: 10.1016/j.cmet.2015.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zhang Q, Vignali DA. Co-stimulatory and co-inhibitory pathways in autoimmunity. Immunity : 1034–1051, 2016. doi: 10.1016/j.immuni.2016.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zhang Y, Zhang C. Role of dendritic cells in cardiovascular diseases. World J Cardiol : 357–364, 2010. doi: 10.4330/wjc.v2.i11.357. [DOI] [PMC free article] [PubMed] [Google Scholar]