Abstract
The sequence of events that lead to inflammation and fibrosing nonalcoholic steatohepatitis (NASH) is incompletely understood. Hence, we investigated the chronology of whole body, tissue, and cellular events that occur during the evolution of diet-induced NASH. Male C57Bl/6 mice were assigned to a fast-food (FF; high calorie, high cholesterol, high fructose) or standard-chow (SC) diet over a period of 36 wk. Liver histology, body composition, mitochondrial respiration, metabolic rate, gene expression, and hepatic lipid content were analyzed. Insulin resistance [homeostasis model assessment-insulin resistance (HOMA-IR)] increased 10-fold after 4 wk. Fibrosing NASH was fully established by 16 wk. Total hepatic lipids increased by 4 wk and remained two- to threefold increased throughout. Hepatic triglycerides declined from sixfold increase at 8 wk to threefold increase by 36 wk. In contrast, hepatic cholesterol levels steadily increased from baseline at 8 wk to twofold by 36 wk. The hepatic immune cell population altered over time with macrophages persisting beyond 16 wk. Mitochondrial oxygen flux rates of FF mice diet were uniformly lower with all the tested substrates (13–276 pmol·s−1·ml−1 per unit citrate synthase) than SC mice (17–394 pmol·s−1·ml−1 per unit citrate synthase) and was accompanied by decreased mitochondrial:nuclear gene copy number ratios after 4 wk. Metabolic rate was lower in FF mice. Mitochondrial glutathione was significantly decreased at 24 wk in FF mice. Expression of dismutases and catalase was also decreased in FF mice. The evolution of NASH in the FF diet-induced model is multiphasic, particularly in terms of hepatic lipid composition. Insulin resistance precedes hepatic inflammation and fibrosis. Mitochondrial dysfunction and depletion occur after the histological features of NASH are apparent. Collectively, these observations provide a unique overview of the sequence of changes that coevolve with the histological evolution of NASH.
NEW & NOTEWORTHY This study demonstrates in a first of kind longitudinal analysis, the evolution of nonalcoholic steatohepatitis (NASH) on a fast-food diet-induced model. Key findings include 1) hepatic lipid composition changes in a multiphasic fashion as NASH evolves; 2) insulin resistance precedes hepatic inflammation and fibrosis, answering a longstanding chicken-and-egg question regarding the relationship of insulin resistance to liver histology in NASH; and 3) mitochondrial dysfunction and depletion occur after the histological features of NASH are apparent.
Keywords: fatty liver, insulin resistance, hepatic fibrosis, mitochondrial respiration, inflammation metabolic function
complications of liver disease secondary to nonalcoholic steatohepatitis (NASH) is currently the third most common indication for liver transplantation in the United States and is on a trajectory to become the most common indication (11). In the vast majority of patients, NASH is the consequence of chronic overnutrition, with the histological course determined by a complex interaction among dietary composition, genetic (e.g., PNPLA3 genotype), and nongenetic (e.g., microbiome) factors. The net effect of these factors can produce a histological spectrum of disease, ranging from isolated steatosis to steatohepatitis with fibrosis and, in some cases, cirrhosis (8). Clinically, NASH is closely associated with obesity, insulin resistance (IR), dyslipidemia, lipotoxicity, oxidative stress, mitochondrial dysfunction, and endocrine dysfunction (10). However, an understanding of the hierarchical sequence of these events and their temporal relationship to the histological progression of NASH remains obscure.
Several diet-induced obesity models have been evaluated for their ability to induce features of NASH in animals. To date, excess of saturated fats, trans fats, and cholesterol has been shown to promote steatosis and steatohepatitis, either individually or in combination with other components such as sugars like fructose, sucrose, and glucose (12, 15, 25, 41, 55, 58). These experimental models have highlighted various mechanisms that contribute to NASH, including lipotoxicity (20, 34), glutathione depletion (23, 56), mitochondrial cholesterol loading (35), mitochondrial dysfunction (44, 52), and oxidative stress (9, 21, 33). Additionally, these and other genetic models have implicated several cellular pathways that drive NASH. Although we have learned much about the whole body, tissue, and cellular events that occur in nonalcoholic fatty liver disease (NAFLD) and NASH, human and animal studies of the pathophysiology of NAFLD and NASH have been limited by the cross-sectional nature of these studies and by difficulties in distinguishing primary cause(s) from secondary effects and epiphenomena related to obesity and liver disease. Identification of the specific metabolic/nutritional trigger(s) for NASH and a detailed, longitudinal analysis of the sequence of events that culminates in steatohepatitis and fibrosis are important gaps in our knowledge regarding the pathophysiology of NASH.
We have previously shown that genetically unaltered mice reared for a period of 6 mo on a diet enriched with saturated fats, cholesterol, and fructose, referred as the fast-food diet (FF), recapitulate the clinical physiology of NASH seen in humans, including obesity, hepatomegaly, steatofibrosis, IR, dyslipidemia, and the endocrine signature associated with NASH, viz., decreased serum growth hormone and adiponectin (12). Herein, we provide a dynamic analysis of the progression of fatty liver disease using the same mouse model, including detailed longitudinal studies of whole body and cellular physiology, histology, and mitochondrial function that coevolve with progression of the disease.
MATERIALS AND METHODS
Animals and experimental design.
All animal experiments were carried out humanely and approved by the Institutional Animal Care and Use Committee of the Mayo Clinic (Rochester, MN). Briefly, 16-wk-old male C57Bl/6 mice (n = 8–10 per group; Cat. No. 000664; Jackson Laboratories) were housed two mice to a cage for varying time periods (Fig. 1A) and were randomly assigned to one of two diets: standard chow (SC; Diet Pico Laboratory Rodent Diet) or fast food (AIN-76A Western Diet; 1810060; Test Diet). All animals were additionally provided fructose (23.1 g/l; Sigma F2543) and glucose (18.9 g/l, Sigma 49158) in their drinking water. The age at start of experiment was based on prior observations using wild-type mice as well as other transgenic mice. At the predetermined time points (Fig. 1A), food was removed 6 h before death. Mice were weighed and euthanized by CO2 inhalation. Blood was drawn by cardiac puncture, and serum was separated by centrifugation and frozen for analysis of triglycerides, cholesterol, insulin, growth hormone, and adiponectin. The liver was perfused in situ with ice-cold heparinized (100 U/ml) phosphate-buffered saline. Known weights of liver were transferred to buffer containing (in mM) 50 Tris, 100 KCl, 5 MgCl2, 1.8 ATP, and 1 EDTA (32) for mitochondrial isolation, and the rest of the tissue were flash frozen or preserved in 10% buffered formalin for further analysis. In mice that were reared for 36 wk, additional 1-mm cubes of tissue were preserved in Trumps fixative and used for ultra-thin microscopy as described earlier (30).
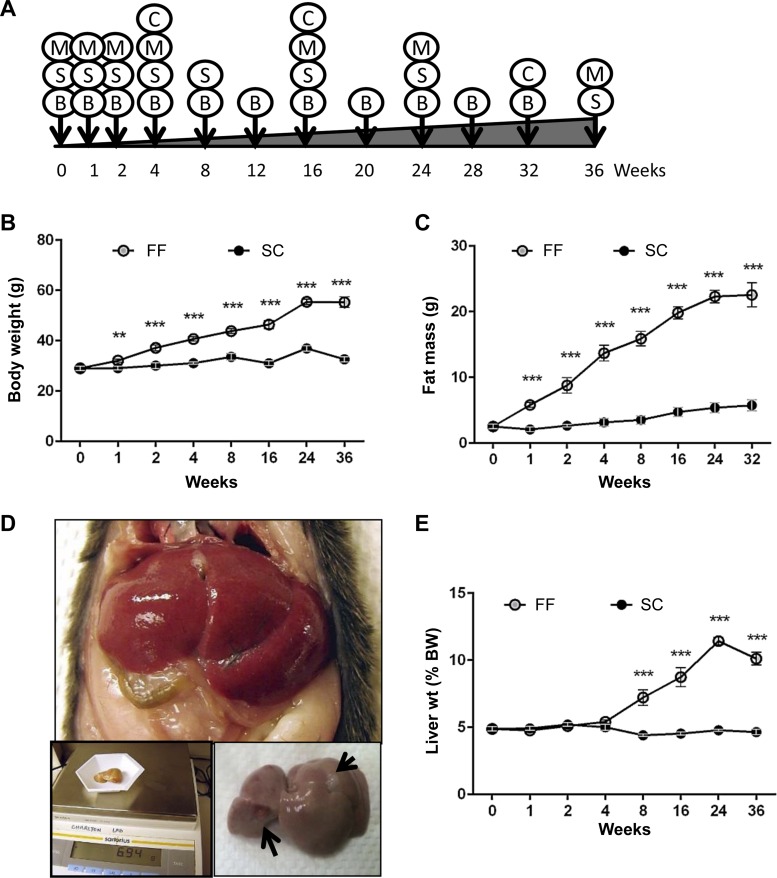
Fig. 1.
C57Bl/6 mice (n = 8–10), reared on fast-food (FF) diet, exhibit increased body weight, increased total fat mass, and increased steatosis. A: experimental design showing time (in wk) when data were collected. B, body, fat, and lean mass; C, Comprehensive Laboratory Animal Monitoring System (CLAMS); M, mitochondrial respiration; S, histological, physiological, and molecular profiling. C57Bl/6 mice on FF exhibited increased total body weight (B) and increased fat mass (C) as compared with standard-chow (SC) mice that were significantly higher in FF mice at all the time points beginning from 1 wk. D: representative in situ image of an enlarged liver of FF mouse at 36 wk approximating 11% of body wt (BW). Insets: dissected liver on scale bar, nodules on surface of the liver (arrows). E: liver weight was proportionate to body weight up to 4 wk and increased as percentage of BW thereafter. No such increase was observed in mice reared on SC. **P < 0.01; ***P < 0.001.
Body fat (nuclear magnetic resonance).
Lean mass and fat mass of experimental mice were quantified using nuclear magnetic resonance (EchoMRI, Houston, TX) as previously described (42).
Basal Metabolic rate and respiratory exchange ratio.
The Comprehensive Laboratory Animal Monitoring System (CLAMS; Oxymax Open Circuit Calorimeter System; Columbus Instruments) designed for the continuous monitoring of 16 mice at a time was used to measure oxygen consumption (V̇o2) and CO2 production (V̇co2) over a 48-h period (24-h fed and 24-h fasted). The V̇o2 and V̇co2 values were used to calculate the respiratory exchange ratio (RER), and V̇o2 and RER values were used to determine the basal metabolic rate (in kcal·kg−1·h−1).
Serum and hepatic biochemical assays.
Serum aspartate aminotransferase (AST) levels were measured using standardized and automated procedures of the diagnostic laboratory of the Mayo Clinic. Commercial ELISA kits were used to measure levels of insulin, growth hormone, and adiponectin (EZRMI-13K, EZRMGH-45K, and EZMADP-60K; Millipore). Total hepatic lipids were extracted from weighed tissue samples in a mixture of chloroform:isopropanol:NP40 (7:11:0.1). Cellular debris was removed by centrifugation. Solvents were evaporated overnight at 50°C and total lipid content determined gravimetrically. Hepatic and serum triglycerides and cholesterol were determined using commercial kits (E2CH-100, E2HL-100, and ETGA-100; Bio-Assay Systems). Total serum lipid is the sum of serum triglycerides and cholesterol.
Mitochondrial isolation and measurement of oxidative capacity.
Mitochondria were isolated from fresh liver tissue by differential centrifugation using previously described methods (32). Postisolation, mitochondria were resuspended in buffer containing (in mM) 500 EGTA, 3 MgCl2, 60 lactobionate, 20 taurine, 10 potassium phosphate, 20 HEPES, 110 sucrose, 20 histidine, 0.02 vitamin E succinate, 3 glutathione, 0.01 leupeptine, 2 glutamate, 2 malate, and 2 ATP-Mg. All chemicals and reagents for buffer preparations for mitochondrial isolation and for related assays described below were purchased from Sigma-Aldrich. Mitochondrial oxidative capacity was measured polarographically with a Clark-type electrode (Oroboros Instruments) as previously described (32). Briefly, mitochondria isolated from equivalent quantities of tissue were incubated in respiration buffer, containing 110 mM sucrose, 60 mM potassium lactobionate, 0.5 mM EGTA, 1 g/l essential fat-free BSA, 3 mM MgCl2, 20 mM taurine, 10 mM KH2PO4, and 20 mM HEPES (pH 7.1) and allowed to equilibrate. Oxygen flux rates were measured after addition of the following substrates into the respiration chamber in succession: 10 mM glutamate in combination with 2 mM malate, 2.5 mM ADP, 10 mM succinate, 0.5 µM rotenone, and 0.02% oligomycin. As citrate synthase is a marker for intact mitochondria, oxygen flux rates were normalized to citrate synthase activity rates.
Mitochondrial number.
Total genomic DNA was extracted from liver tissue (Dneasy Blood and tissue kit; 69504; Qiagen). Mitochondrial-to-nuclear DNA ratios were calculated using quantitative real-time PCR data for two mitochondrial genes cytochrome-b and 12S and one nuclear gene β-actin. The ratio of mitochondrial DNA to nuclear DNA provides an index of cellular mitochondrial number. The primer sequences were as follows: 1) cytochrome-b: forward, 5′-TCGCGGCCCTAGCAATCGTT-3′; reverse, 5′-TGGGGTGGGGTGTTTAGTGGAT-3′; 2) 12S: forward, 5′-CCAGCCACCGCGGTCATACG-3′; reverse, 5′-CCCAGTTTGGGTCTTAGCTGTCGT-3′; and 3) β-actin: forward, 5′-CGCTCAGGAGGAGCAATGAT-3′, reverse, 5′-TCCTTAGCTTGGTGAGGGTG-3′. Data on ratios of mitochondrial DNA to nuclear DNA were then normalized to that of age-matched mice reared on SC.
Citrate synthase activity.
Citrate synthase activity was measured in mitochondrial isolates using the methods previously described (1, 3). Citrate synthase activity is expressed as micromoles per milliliter per minute.
Mitochondrial and total glutathione.
Total glutathione was measured in isolated mitochondria and in whole liver homogenates using a commercial kit (Cat. No. ADI-900-160; Enzo Life Sciences).
Histology, NASH scoring, and immunohistochemistry.
Histology and immunostaining of tissue sections were carried out by the core facility of the Mayo Clinic. Briefly, deparaffinized, hydrated serial sections of liver were stained with hematoxylin and eosin, Masson’s trichrome, and Picrosirius red using standardized protocols. Additionally, serial sections were stained for α-smooth muscle actin (Asma; NB110-55432, Novus Biologicals) F4/80 (MCA497EL; Serotec), neutrophils (CL8993A; Cedarlane), and cluster of differentiation 3 (Cd3; A0452; Dako). Staining for all antibodies was optimized to ensure specificity with no background staining. Bound antibodies were detected using diaminobenzidine tetrahydrochloride or romulin AEC (RAEC810 L; Biocare Medical). Sections were counterstained with hematoxylin. Quantitative analysis of stained sections was performed as previously described using the NIS Elements Basic Research software (Nikon) (24). Data are presented as percentages of stained tissue area or as number of cells per field of view visualizing 10–35 fields of view per section. Histological scoring of stained sections for steatosis grade, inflammation, and hepatic fibrosis was carried out by a blinded pathologist (T. Mounajjed) as per the Brunt classification (8).
Quantitative real-time PCR.
Total RNA was isolated from frozen liver tissue using the RNeasy Plus kit (74104, Qiagen). Equal quantities of total RNA was reverse transcribed into cDNA using random hexamers (1708891; iScript cDNA synthesis kit; Bio-Rad). Real-time PCR was performed on an iCycler (Bio-Rad) using primers previously described (12, 27). Gene expression results were normalized to the mean of two housekeeping genes: 18S and GAPDH. Data are presented as fold changes over that of age-matched SC mice.
Statistics.
Data are presented as means ± SE representing replications within an experiment. All analyses were done in duplicates and occasionally in quadruplicates. The Student’s t-test was used to calculate statistical significance between treatments in age-matched mice. P = 0.05 was considered as significant.
RESULTS
Whole body, liver weight, and fat mass.
Animals reared on the FF diet gained weight rapidly, becoming significantly different from mice reared on the SC diet as early as 1 wk following feeding, attaining maximum weight gain at 24 wk, after which growth remained stable (Table 1 and Fig. 1B). Overall, total body weight gain in animals reared on the FF diet was 26.3 g as compared with 3.5 g for mice reared on the SC diet. Paralleling weight gains, quantitative magnetic resonance determinations of fat mass indicated a significantly higher accumulation of fat in animals reared on the FF diet averaging 19.8 g of body fat, amounting to 41% body weight over 36 wk as compared with 3.2 g (9.5% of body wt) for mice on the SC diet (Fig. 1C). Obese mice on FF also gained lean mass that was significantly higher than SC mice from 16 wk onward. Although gross liver weight was significantly increased in FF mice as early as 2 wk, as percentage of body weight, liver mass increased from 8 wk onward maximizing at 24 wk (11.4%) and marginally decreasing to 10.1% at 36 wk (Table 1 and Fig. 1, D and E). Spontaneous hepatic tumors (adenomas) were observed in 2 of 10 mice maintained on the FF diet for 36 wk (Fig. 1D).
Table 1.
Phenotype and serum biochemistry of animals reared on standard chow diet vs. a fast food diet
| Weeks (No./Group) |
||||||||
|---|---|---|---|---|---|---|---|---|
| 0 (6) | 1 (8) | 2 (8) | 4 (8) | 8 (8) | 16 (8) | 24 (8) | 36 (10) | |
| Initial body wt, g | ||||||||
| SC | 29.0 ± 0.8 | 29.5 ± 0.6 | 30.4 ± 0.8 | 30.2 ± 0.6 | 29.8 ± 2.1 | 29.2 ± 2.3 | 31.1 ± 1.1 | 29.5 ± 3.0 |
| FF | — | 29.0 ± 1.0 | 31.8 ± 0.4 | 31.1 ± 0.7 | 29.1 ± 1.1 | 29.0 ± 1.6 | 28.7 ± 2.9 | 30.4 ± 2.4 |
| Final body wt, g | ||||||||
| SC | — | 29.1 ± 0.7 | 30.1 ± 1.0 | 31.1 ± 0.4 | 33.6 ± 1.2 | 31.1 ± 2.7 | 36.9 ± 3.2 | 32.6 ± 3.5 |
| FF | — | 32.1 ± 0.6** | 37.2 ± 1.1*** | 40.7 ± 0.8*** | 43.8 ± 0.9*** | 46.4 ± 4.4*** | 55.5 ± 3.0*** | 55.3 ± 6.6*** |
| Liver wt, g | ||||||||
| SC | 1.4 ± 0.0 | 1.4 ± 0.1 | 1.6 ± 0.1 | 1.6 ± 0.1 | 1.5 ± 0.1 | 1.4 ± 0.2 | 1.8 ± 0.2 | 1.6 ± 0.2 |
| FF | — | 1.5 ± 0.1 | 1.9 ± 0.1* | 2.2 ± 0.1*** | 3.2 ± 0.3*** | 4.1 ± 1.3*** | 6.4 ± 0.7*** | 5.7 ± 1.5*** |
| Serum AST, IU/ml | ||||||||
| SC | 84 ± 29 | 152 ± 19 | 154 ± 27 | 198 ± 41 | 360 ± 79 | 382 ± 95 | 606 ± 266 | 458 ± 252 |
| FF | — | 311 ± 67* | 131 ± 22 | 282 ± 10 | 753 ± 114 | 853 ± 217*** | 1022 ± 272 | 698 ± 117 |
| Glucose, mg/dl | ||||||||
| SC | 179 ± 10 | 174 ± 7 | 167 ± 4 | 173 ± 7 | 184 ± 6 | 165 ± 22 | 191 ± 32 | 161 ± 40 |
| FF | — | 196 ± 10 | 210 ± 12** | 218 ± 24 | 198 ± 11 | 207 ± 41* | 216 ± 41 | 196 ± 33* |
| Insulin, ng/ml | ||||||||
| SC | 0.56 ± 0.15 | 0.84 ± 0.17 | 0.57 ± 0.18 | 0.42 ± 0.06 | 2.0 ± 0.11 | 1.78 ± 0.57 | 2.1 ± 0.75 | 1.89 ± 1.29 |
| FF | — | 1.51 ± 0.35*** | 0.37 ± 0.04 | 0.64 ± 0.05 | 6.55 ± 2.47*** | 6.10 ± 2.46*** | 5.64 ± 1.53*** | 5.09 ± 2.0*** |
| QUIKI | ||||||||
| SC | 0.3 ± 0.01 | 0.28 ± 0.01 | 0.30 ± 0.01 | 0.30 ± 0.01 | 0.25.0 ± 0.00 | 0.26 ± 0.01 | 0.25 ± 0.01 | 0.27 ± 0.04 |
| FF | — | 0.26 ± 0.01 | 0.30 ± 0.01 | 0.28 ± 0.00 | 0.22 ± 0.00*** | 0.22 ± 0.01*** | 0.22 ± 0.00*** | 0.23 ± 0.01*** |
| Triglycerides, mmol/l | ||||||||
| SC | 1.57 ± 0.17 | 1.59 ± 0.17 | 1.51 ± 0.15 | 1.40 ± 0.16 | 2.04 ± 0.17 | 0.97 ± 0.16 | 1.75 ± 0.16 | 0.90 ± 0.25 |
| FF | — | 1.57 ± 0.23 | 1.63 ± 0.14 | 1.45 ± 0.15 | 1.60 ± 0.15 | 0.86 ± 0.15 | 1.66 ± 0.24 | 0.72 ± 0.17 |
| HDL cholesterol, mg/dl | ||||||||
| SC | 66.2 ± 2.3 | 83.8 ± 18.3 | 97.5 ± 7.7 | 104.6 ± 5.7 | 66.6 ± 13.1 | 105.0 ± 8.5 | 67.3 ± 8.9 | 115.7 ± 25.5 |
| FF | — | 138.2 ± 16.7*** | 140.4 ± 5.2*** | 131.6 ± 1.8** | 73.0 ± 12.5 | 141.4 ± 36.7** | 101.1 ± 6.6*** | 156.1 ± 32.4** |
| LDL cholesterol, mg/dl | ||||||||
| SC | 32.4 ± 1.6 | 38.0 ± 13.4 | 47.2 ± 6.7 | 40.2 ± 2.2 | 74.4 ± 14.7 | 72.6 ± 12.8 | 52.5 ± 5.0 | 88.0 ± 23.9 |
| FF | — | 74.8 ± 17.5*** | 93.7 ± 11.9** | 98.2 ± 3.9*** | 115.3 ± 17.7*** | 136.7 ± 23.7*** | 108.8 ± 6.3*** | 135.1 ± 30.8*** |
| Total serum Lipids, mg/dl | ||||||||
| SC | 213.3 ± 24.7 | 244.7 ± 25.4 | 278.4 ± 21.2 | 268.4 ± 17.2 | 322.0 ± 23.1 | 263.4 ± 6.52 | 274.6 ± 20.8 | 283.7 ± 13.6 |
| FF | — | 352.3 ± 20.8* | 378.6 ± 18.8** | 358.2 ± 14.0** | 332.3 ± 21.6 | 354.2 ± 17.3*** | 356.8 ± 21.0** | 355.0 ± 16.9** |
| Growth Hormone, ng/ml | ||||||||
| SC | 6.8 ± 1.4 | 5.8 ± 1.8 | 8.5 ± 3.0 | 4.6 ± 1.9 | 5.97 ± 1.38 | 7.7 ± 4.9 | 9.63 ± 2.65 | 5.4 ± 4.8 |
| FF | — | 5.0 ± 1.8 | 2.2 ± 0.4** | 2.8 ± 1.0** | 6.16 ± 119 | 2.6 ± 1.1 | 8.33 ± 3.59 | 5.1 ± 4.4 |
| Adiponectin, ng/ml | ||||||||
| SC | 8,696 ± 423 | 7,810 ± 670 | 7,598 ± 1370 | 5,163 ± 750 | 8,578 ± 266 | 10,519 ± 1,504 | 7,426 ± 475 | 11,884 ± 3,144 |
| FF | — | 7,920 ± 1019 | 5,956 ± 583 | 4,321 ± 160 | 8,680 ± 220 | 9,303 ± 2,558 | 6,752 ± 328 | 8,491 ± 1,205** |
Values are means ± SE.
SC, standard chow; FF, fast food.
P < 0.05;
P < 0.01;
P < 0.001 for age-matched SC vs. FF.
Serum transaminases, IR, serum lipids, and serum hormone profile.
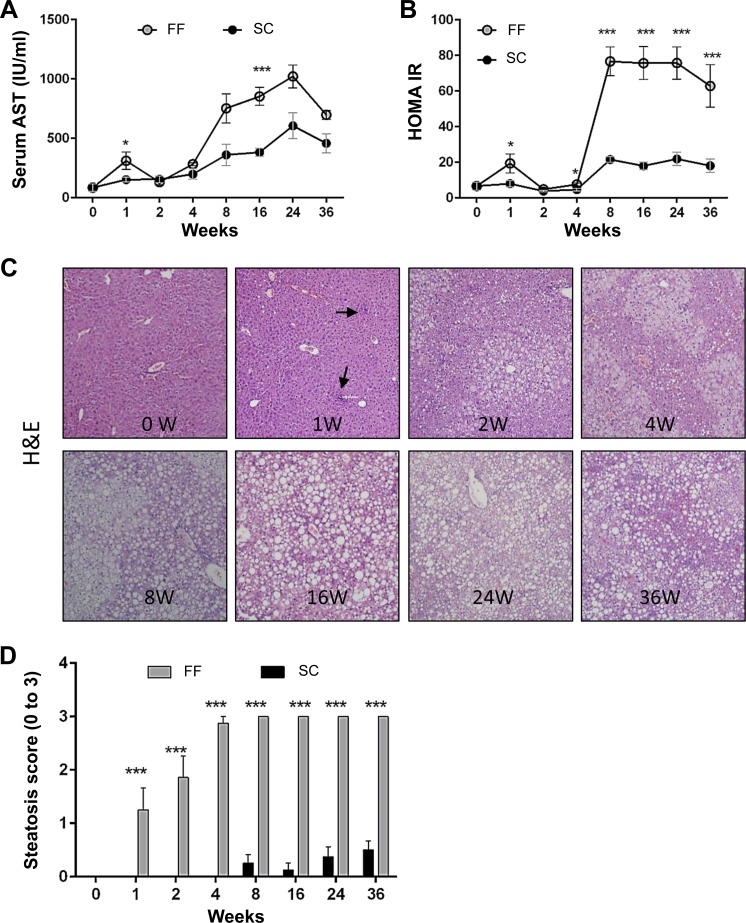
Serum AST levels were significantly higher in FF mice within a week of introducing the diet, normalized to baseline level by 4 wk, increased precipitously by 8 wk and thereafter remained 10- to 12-fold elevated (Fig. 2A). Serum glucose levels remained marginally higher in mice on the FF diet (Table1). Serum insulin levels followed the same trends as that of serum AST. Consequently, homeostasis model assessment-insulin resistance (HOMA-IR), a derivative of glucose and insulin levels, also followed the same trends as that of serum AST and insulin, being significantly greater in animals on the FF diet by 1 wk and 10- to 12-fold increased beyond 4 wk (Fig. 2B). IR was observed by 4 wk (Fig. 2B). There was no difference in serum triglyceride levels between mice on the SC or FF diets. Serum HDL and LDL cholesterol levels increased twofold within a week and thereafter remained two- to threefold increased over that of SC mice throughout the experimental period. The total sum of serum triglyceride and cholesterol levels in FF mice was stably elevated from 1 wk throughout the experimental period (Table 1). Growth hormone and adiponectin levels remained lower in FF mice than mice on the SC diet, becoming statistically significant only at later time points (Table 1).
Fig. 2.
Elevation of serum transaminases, insulin resistance, and onset of steatosis occurs rapidly in FF mice (n = 8–10 samples all groups and time points). A: serum AST levels increased by 1 wk, normalized to baseline by 4 wk, and increased sharply thereafter. AST levels in SC mice were also increased over baseline. B: insulin resistance [homeostasis model assessment-insulin resistance (HOMA-IR)] increased by 1 wk, normalized to baseline by 4 wk, and increased sharply thereafter. SC mice showed a marginal increase in HOMA-IR over baseline. C: hematoxylin and eosin sections of mice reared on FF demonstrate rapid onset of steatosis over time. Hepatic fat accumulation was largely microvesicular at earlier time points of 1 to 4 wk and macrovesicular thereafter. Inflammatory infiltrates are visible as early as 1 wk (black arrows). No ballooning was observed. D: histological scoring for steatosis indicate that maximum scores were attained by 4 wk, which persisted throughout the experimental period. By comparison, SC mice attained a minimal score of 0.45 ± 0.18 by 36 wk (magnification, ×200). *P < 0.05; ***P < 0.001.
Histology.
Hematoxylin and eosin-stained sections were scored for steatosis, inflammation, and fibrosis as per the classification of Brunt, with scores of 0 to 3 assigned for steatosis, 0 to 3 for inflammation, and 0 to 4 for fibrosis.
Steatosis.
The onset of steatosis was rapid in animals reared on FF (Fig. 2C). By 4 wk, six of eight mice in the group received maximum scores of 3, and all animals in the FF groups were ranked at three for all later time points (Fig. 2D). Steatosis was largely microvesicular at the early time points while macrovesicular steatosis predominated from 4 wk onward. In animals reared on SC, in contrast, low-grade steatosis occurred rarely beyond 8 wk (Fig. 2D).
Inflammation.
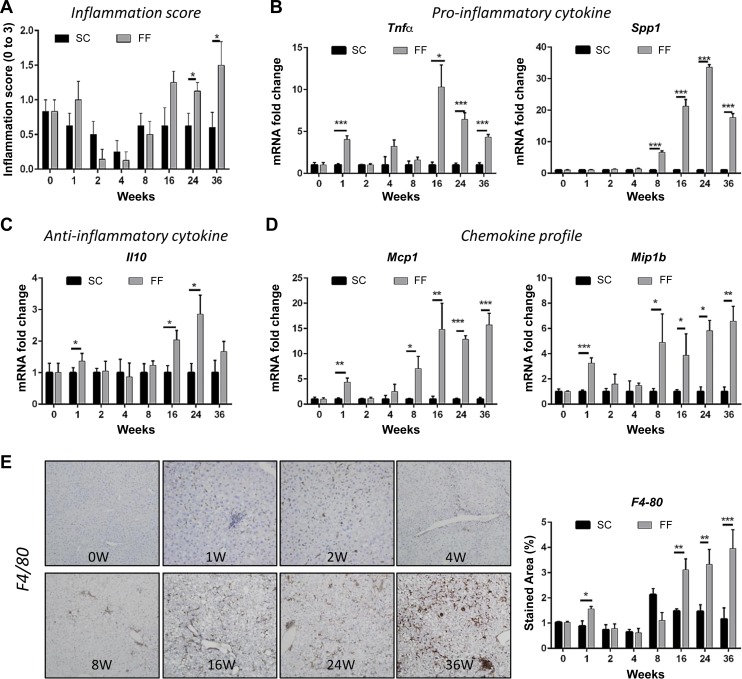
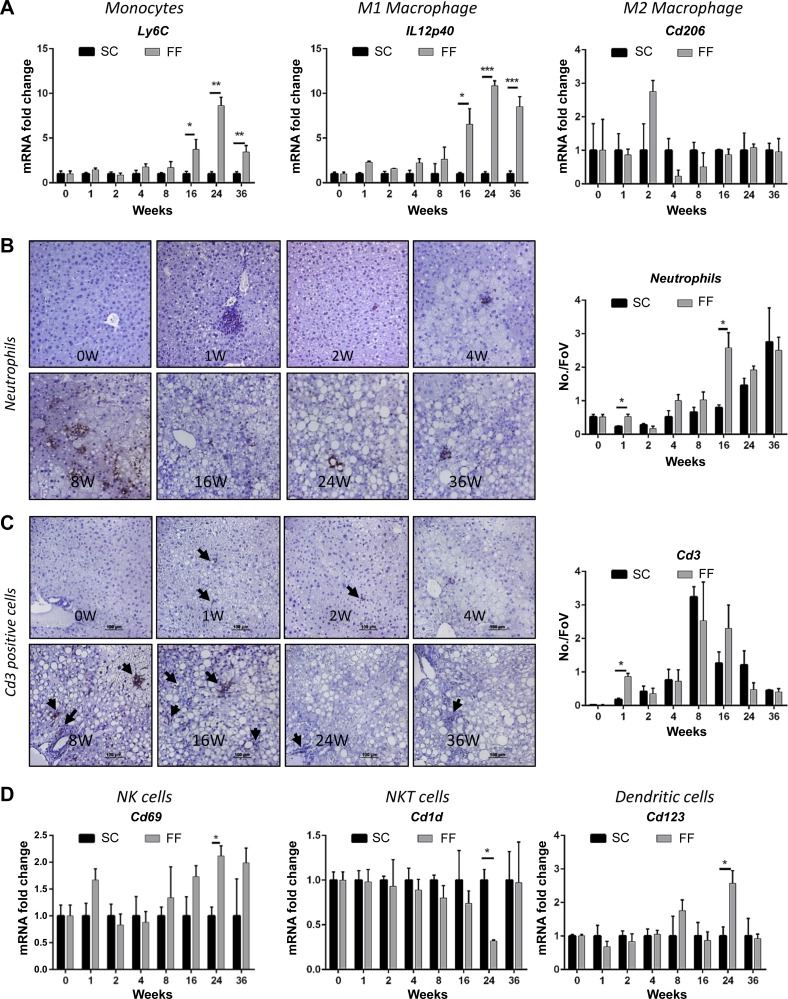
In correlation with increased serum AST levels, there was an initial increase in histological scoring for inflammation that normalized to baseline by 4 wk and increased progressively from 8 wk onward (Fig. 3A). This phenomenon was more evident in animals reared on FF than in the SC diet-fed mice. Histologically, intra-acinar inflammation, commonly associated with severe NASH, was evident from the 16 wk in hematoxylin and eosin-stained sections that was corroborated by staining for F4/80 (Figs. 2C and 3E). There was no evidence of ballooning. At the molecular level, gene expression of proinflammatory cytokine Tnfα was significantly elevated in FF mice at 1 wk and from 16 wk onward (Fig. 3B); there was no difference in Il1β and Il6 remained decreased from 4 wk onward (data not shown). Interestingly, Il10, an anti-inflammatory cytokine trended similar to Tnfa expression levels (Fig. 3, B and C). Spp1 (osteopontin) an extracellular matrix protein involved in proinflammatory signaling increased significantly from 8 wk onward becoming greater than 30-fold increased over SC mice by 24 wk (Fig. 3B). Mcp1 and Mip1β, the two chemokines most well recognized as chemoattractants to monocytes and macrophages, also showed a transient increase at 1 wk and remained elevated from 8 wk onward (Fig. 3D). In parallel, immunostaining (Fig. 3E) and gene expression for F4/80 (data not shown), a marker for macrophages, showed an increase at 1 wk, followed by a surge from 16 wk onward. Gene expression of Ly6C, a marker for monocytes recruited from peripheral circulation, and IL12p40, marker for proinflammatory macrophage, was significantly increased from 16 wk (Fig. 4A). By contrast expression of M2 macrophage marker, Cd206 remained close to baseline levels from 16 wk after an insignificant and transient increase at 2 wk (Fig. 4A). Transient increases at 1 wk and 16 wk were observed for neutrophils and for Cd3 positive T cells at 1 wk (Fig. 4, B and C) by immunostaining. Gene expression assays for Cd69 (NK cell marker), Cd123 (dendritic cell marker) and Cd1d (NKT cells) indicated significantly elevated levels of NK and dendritic cells at 24 wk but a decline in NKT cells (Fig. 4D).
Fig. 3.
Hepatic inflammation is increased in FF mice (n = 8–10, all groups). A: histological scores for hepatic inflammation decreased from baseline over 2–8 wk and increased over baseline from 16 wk onward. In SC mice (n = 8–10 all groups), scores for hepatic inflammation remained at or below baseline throughout. B: the cytokine Tnfα was increased transiently at 1 wk and persistently beyond 16 wk. The cytokine Spp1 was persistently increased beyond 8 wk. C: the anti-inflammatory cytokine was also significantly increased at 1, 16, and 24 wk. D: gene expression of the chemokines Mcp1 and Mip1β were significantly elevated at 1 wk and for all time points beyond 8 wk. E: representative images of liver tissue sections immunostained for macrophage marker F4/80 indicated increased presence of infiltrates at 1, at 2, and from 8 wk onward. Quantified data from image analysis of sections stained for F4-80 indicated significant upregulated expression at 1 wk, and further at 16, 24, and 36 wk (n = 8–10 per time point). *P < 0.05; **P < 0.01; ***P < 0.001.
Fig. 4.
Markers of hepatic inflammatory infiltrate cell types are shown (n = 8–10 samples all groups and time points). Gene expression of Ly6C, a marker for monocytes recruited from peripheral circulation, and IL12p40, marker for proinflammatory macrophages, were significantly increased from 16 wk (A). Expression of M2 macrophage marker, Cd206 remained close to baseline levels from 16 wk after an insignificant and transient increase at 2 wk (A). Transient increases at 1 and 16 wk were observed for neutrophils and for Cd3 positive T cells at 1 wk (B and C) by immunostaining. Gene expression assays for Cd 69 (NK cell marker), Cd123 (dendritic cell marker), and Cd1d (NKT cells) indicated significantly elevated levels of natural killer (NK) and dendritic cells at 24 wk but a decline in NKT cells (D). *P < 0.05; **P < 0.01; ***P < 0.001.
Fibrosis.
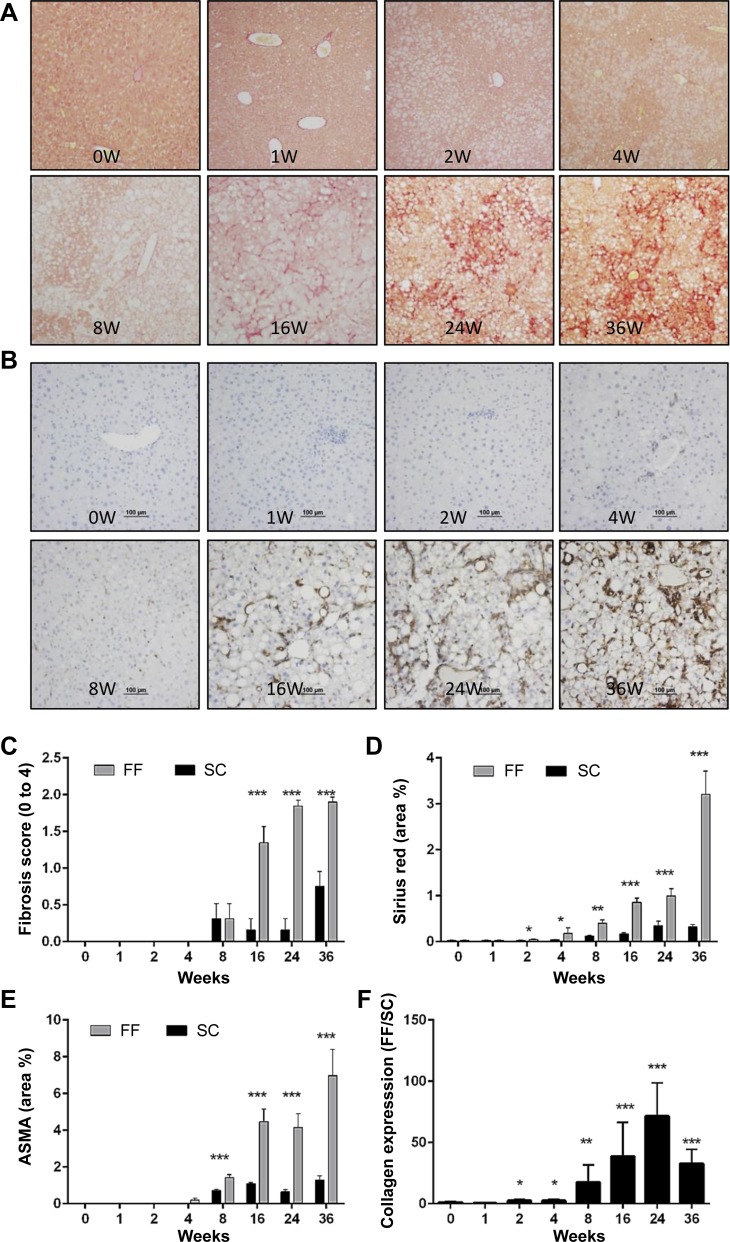
Histological evidence of fibrosis was evident by sirius red staining in animals reared on FF by 8 wk (Fig. 5, A and B). By 16 wk, seven of eight animals in the group showed strong evidence of fibrosis. By 24 wk, 75% of the animals had scored 2 for fibrosis (Fig. 5, A–C). These observations were corroborated by quantitative analysis of sirius red-stained liver sections (Fig. 5D). The presence of activated fibroblasts was evidenced by immunostaining for Asma from 4 wk onward (Fig. 5, B and E). Significantly increased gene expression of collagen1α1 occurred as early as 2 wk (Fig. 5F) and was persistently elevated in FF mice.
Fig. 5.
Hepatic fibrosis is enhanced in FF mice (n = 8–10 samples all groups and timepoints). A: representative images of liver tissue sections stained for Picrosirius red indicate progressive increase in collagen fibril from 8 wk onward. Fibrosis is “chicken mesh” in pattern, characteristic of nonalcoholic steatohepatitis (NASH). B: representative images of liver tissue sections stained for α-smooth muscle actin (Asma) show the presence of activated fibroblasts from 4 wk onward. C: histological scoring for fibrosis indicate progressive increase in hepatic fibrosis from 8 wk onward. Extent of hepatic fibrosis was determined by image analysis of sirius red (D) and ASMA stained area (E). F: hepatic gene expression of collagen indicated a progressive increase from 2 wk onward. *P < 0.05; **P < 0.01; ***P < 0.001.
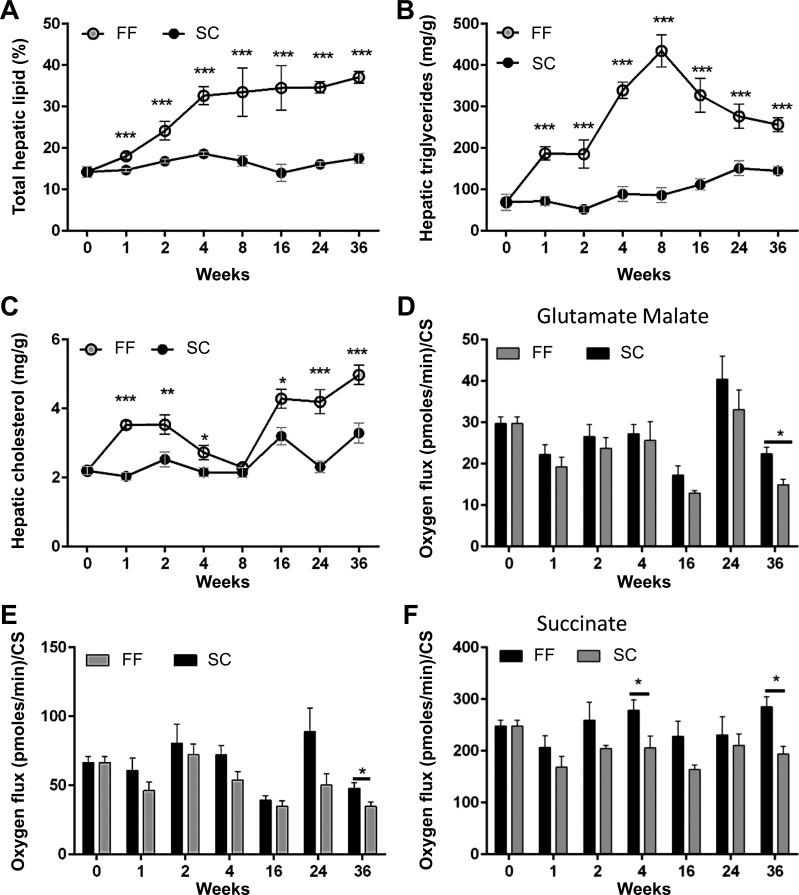
Hepatic triglycerides and cholesterol.
Total hepatic lipids started accumulating as early as one week in the liver of mice on the FF diet peaking at 8 wk and plateauing thereafter (Fig. 6A). Hepatic triglyceride maximized at sixfold increase over baseline by 8 wk (Fig. 6B) but declined gradually to a threefold increase by 36 wk. Hepatic cholesterol on the other hand followed the same trajectory as that of IR (Fig. 6C).
Fig. 6.
Hepatic lipid accumulation in FF mice (n = 8–10 samples all groups and timepoints). A: total hepatic lipid content in FF mice increased gradually and plateaued at around 40% of liver weight while hepatic lipid stores remained close to baseline in mice on SC. B: total hepatic triglycerides maximized at 8 wk and thereafter declined gradually. C: hepatic cholesterol content increased gradually beyond 8 wk in FF mice. Mitochondrial respiration rates normalized to citrate synthase activity were lower at all times and for all substrates in FF mice (D–F). Decreases in oxygen consumption were statistically significant for all substrates at 36 wk in FF mice. *P < 0.05; **P < 0.01; ***P < 0.001.
Whole body metabolism.
Overall, O2 consumption, CO2 production, and metabolic rate were lower in mice fed FF over all time points and under both fed and fasted conditions (Table 2). Physical activity scores for mice fed the FF were significantly lower than those of mice on SC for all time points under fasted conditions and additionally under fed conditions at 32 wk (Table 2). Total energy expenditure under both fed and fasting conditions were also lower in FF mice (data not shown). By 32 wk, there was significant difference in endurance as measured by distance run and in grip strength between the SC and FF mice (data not shown).
Table 2.
Oxygen consumption, carbon-dioxide production, metabolic rate, and activity associated with rearing mice on a SC diet vs. a FF diet
| Fed |
Fasted |
|||
|---|---|---|---|---|
| Day | Night | Day | Night | |
| Oxygen consumption, ml/h | ||||
| 4 wk | ||||
| SC | 2,610 ± 117 | 3,197 ± 116 | 2,300 ± 85 | 2,605 ± 85 |
| FF | 2,255 ± 39** | 2,766 ± 72** | 2,145 ± 48 | 2,451 ± 68 |
| 16 wk | ||||
| SC | 2,446 ± 116 | 2,931 ± 112 | 2,259 ± 101 | 2,567 ± 85 |
| FF | 2,375 ± 69 | 2,854 ± 87 | 2,182 ± 68 | 2,454 ± 101 |
| 32 wk | ||||
| SC | 2,165 ± 92 | 2,765 ± 161 | 1,976 ± 104 | 2,282 ± 68 |
| FF | 2,027 ± 53 | 2,536 ± 65 | 1,868 ± 54 | 2,137 ± 61 |
| CO2 production, ml/h | ||||
| 4 wk | ||||
| SC | 2,139 ± 143 | 2,763 ± 133 | 1,651 ± 71 | 1,851 ± 63 |
| FF | 1,653 ± 37** | 2,113 ± 61** | 1,502 ± 35 | 1,705 ± 48 |
| 16 wk | ||||
| SC | 2,078 ± 130 | 2559 ± 118 | 1,685 ± 89 | 1,846 ± 65 |
| FF | 1,899 ± 40 | 2256 ± 74* | 1,606 ± 54 | 1,728 ± 71 |
| 32 wk | ||||
| SC | 1,788 ± 105 | 2,339 ± 181 | 1,497 ± 91 | 1,634 ± 51 |
| FF | 1,626 ± 61 | 2,030 ± 74 | 1,374 ± 40 | 1,506 ± 55 |
| Metabolic rate, kj·kg−1·day−1 | ||||
| 4 wk | ||||
| SC | 12.6 ± 0.6 | 15.6 ± 0.6 | 10.8 ± 0.4 | 12.2 ± 0.4 |
| FF | 10.6 ± 0.2** | 13.2 ± 0.3** | 10.0 ± 0.2 | 11.5 ± 0.3 |
| 16 wk | ||||
| SC | 11.9 ± 0.6 | 14.3 ± 0.6 | 10.7 ± 0.5 | 12.1 ± 0.4 |
| FF | 11.4 ± 0.3 | 13.7 ± 0.4 | 10.3 ± 0.3 | 11.5 ± 0.5 |
| 32 wk | ||||
| SC | 10.5 ± 0.5 | 13.4 ± 0.8 | 9.4 ± 0.5 | 10.7 ± 0.3 |
| FF | 9.7 ± 0.3 | 12.2 ± 0.3 | 8.8 ± 0.3 | 10.0 ± 0.3 |
| Activity, events/12 h | ||||
| 4 wk | ||||
| SC | 9,896 ± 1,187 | 28,560 ± 2,086 | 11,477 ± 1,486 | 51,438 ± 4,172 |
| FF | 6,563 ± 563* | 25,873 ± 2,432 | 7,399 ± 704* | 35,270 ± 4,103* |
| 16 wk | ||||
| SC | 13,419 ± 2,475 | 38,629 ± 4,168 | 17,852 ± 1,937 | 61,364 ± 6,153 |
| FF | 7,770 ± 1,673* | 28,319 ± 3,914 | 9,237 ± 1,606* | 37,513 ± 5,719* |
| 32 wk | ||||
| SC | 7,681 ± 706 | 34,051 ± 3,978 | 12,315 ± 1,360 | 45,977 ± 4,229 |
| FF | 5,890 ± 602* | 22,323 ± 2,094* | 7,286 ± 1,421* | 26,733 ± 1,959* |
Values are means ± SE.
SC, standard chow; FF, fast food.
P < 0.05;
P < 0.01 for age-matched SC vs. FF.
Mitochondrial function, glutathione, and 4-hydroxynonenal status.
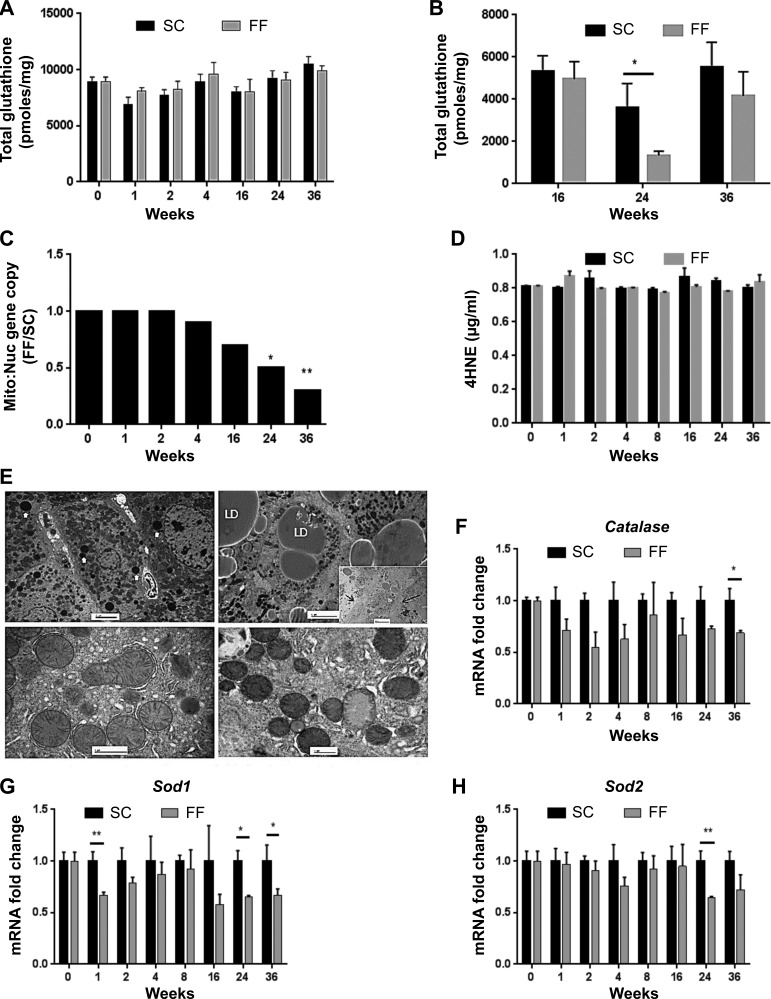
Mitochondrial respiration rates normalized to citrate synthase activity were lower at all times and for all substrates in FF mice (Fig. 6, D–F). Significant decrease in oxygen consumption was observed with all substrates at 36 wk in FF mice. Citrate synthase activity was similar between both SC and FF mice at all times (data not shown). Total glutathione levels remained equivalent in liver homogenates of mice reared on SC and FF diets for all time points (Fig. 7A). Mitochondrial glutathione concentration, however, decreased significantly in mice reared on the FF diet at 24 wk, recovering by 36 wk (Fig. 7B). There was no difference in hepatic concentrations of 4-hydroxynonenal (4-HNE) adducts, a marker of oxidative stress, between SC-and FF-fed mice at any time point (Fig. 7D). Mitochondria:-to-nuclear gene expression ratios (in age-matched experimental mice), which provide an index of mitochondrial number, decreased steadily after 4 wk in FF mice, becoming significantly different from that of SC mice at 24 and 36 wk (Fig. 7C). Decreased mitochondrial number within hepatocytes of FF mice is also discernible in ultra-thin micrographs taken at 36 wk (Fig. 7E). Gene expression of cytosolic catalase, Cat, was reduced over all time points with significant decrease at 36 wk. Expression of cytosolic dismutase, Sod1, showed a significant decrease at 1, 24, and 36 wk. Mitochondrial dismutase Sod2 was significantly decreased at 24 wk.
Fig. 7.
Indexes of mitochondrial function are shown. Total glutathione levels remained equivalent in liver homogenates of mice reared on SC and FF diets for all time points (A) (n = 8–10 samples all groups and time points). Mitochondrial glutathione concentration, however, decreased significantly in mice reared on the FF diet at the 24 wk, recovering by 36 wk (B). There was no difference in hepatic concentrations of 4-hydroxynonenal (4-HNE) adducts, a marker of oxidative stress, between SC and FF fed mice at any time point (C). Mitochondria-to-nuclear gene expression ratios (in age-matched experimental mice), which provide an index of mitochondrial number, decreased steadily after 4 wk in FF mice, becoming significantly different (C). Decreased mitochondrial number within hepatocytes of FF mice is also discernible in ultra-thin micrographs taken at 36 wk (E). F: gene expression of Cat (catalase) is decreased over all time points with significant decrease at 36 wk. G: gene expression of cytosolic dismutase Sod1 is significantly decreased at 1 wk and beyond 24 wk. H: gene expression of mitochondrial dismutase Sod2 is significantly decreased at 24 wk (n = 8 per time point). *P < 0.05; **P < 0.01.
DISCUSSION
This article provides an overview of the dynamic changes that occur during the evolution of NASH in genetically unaltered C57Bl/6 mice reared on a FF diet, a model that closely recapitulates the human condition (12). The timeframe of this longitudinal analysis (36 wk) was sufficient to reproduce all elements of nonalcoholic fatty liver disease from initiation of steatosis to hepatitis and, eventually, to fibrosis. The most important outcome of this study is the ability to link the histological evolution of NASH with the morphological, physiological, and metabolic changes that characterize disease progression.
The defining feature of fatty liver disease is the accumulation of hepatic lipids, and this was evident within a week in mice reared on FF. By 8 wk and for all further time points, all animals attained the maximum steatosis score of 3 as per Brunt classification. A similar trend was also observed for total hepatic lipid content (maximizing at ~40% of tissue weight by 8 wk), suggesting that a threshold for hepatic fat accumulation had been attained. Maximum gains in body weight and total body fat occurred during this early phase. Hepatomegaly was initiated by 4 wk, with liver mass expanding from 5.4% of body wt at 4 wk to 11% by 24 wk. By contrast, liver mass remained stable at <5% of body wt in SC mice. One of the most striking findings of this study was the dynamic nature of hepatic lipid composition during the evolution of NASH. Hepatic triglyceride content initially increased sixfold by 8 wk but thereafter declined, despite stable total hepatic lipid content. Although the mechanism of the multiphasic pattern of hepatic triglyceride content cannot be determined from these experiments, the eventual decline in hepatic triglyceride content may have contributed to hepatic sensitivity to lipotoxicity. In contrast to triglycerides, hepatic cholesterol steadily increased after 8 wk. In the serum, LDL cholesterol levels remained persistently high, HDL cholesterol levels paralleled hepatic cholesterol, and triglycerides were similar to that of SC mice throughout the experimental period. Total serum lipid, however, was stably elevated from 1 wk throughout the study period reinforcing the notion that while lipids, particularly cholesterol, are in constant flux between the liver and peripheral organs, excess circulating cholesterol is preferentially transferred to the liver over time. Histologically, increasing hepatic cholesterol content and decreasing triglyceride content coincided with the onset of fibrosis. A protective effect of hepatic triglycerides against inflammation and fibrosis in NASH has been previously reported in animal models of NASH (14, 59). While the mechanism of the hepatoprotective effect of triglycerides is not definitively known, it has been suggested that hepatic triglycerides may buffer the lipotoxic effects of free fatty acids (59). In contrast, hepatic cholesterol accumulation is implicated in many pathological mechanisms: disruption of membrane integrity of mitochondrial and endoplasmic reticulum, triggering mitochondrial oxidative injury and endoplasmic reticulum stress and promoting generation of toxic oxysterols (39). It is worth noting that in this study, hepatic cholesterol accumulation coincided with decline in mitochondrial: nuclear gene copy number signifying a loss of mitochondria. Cholesterol has also been directly implicated in promoting fatty liver disease both clinically and in rodent models of NASH (18, 20, 28, 37, 55).
This study also demonstrated that the physiological impact on other consequences of the FF diet was also nonlinear. By 1 wk, introduction of the FF diet induced rapid and transient changes characterized by increased insulin secretion, serum AST, proinflammatory cytokines, chemokines, and the increased recruitment of immune cells. Of interest, the anti-inflammatory cytokine Il10 was also similarly increased. All parameters returned to basal levels by 4 wk. Beyond 8 wk, however, a steep and sustained surge in HOMA-IR was complemented by parallel surges in serum AST, inflammatory cytokine Spp1, and chemokines Mcp1 and Mip1b. A significantly increased pool of macrophages and neutrophils with recruitment of Ly6C-positive monocytes from peripheral circulation was evident at 16 wk along with increased gene expression of Tnfα. These observations suggest that the 4- to 8-wk period marks a critical phase in disease evolution characterized by precipitous changes in IR, onset of hepatomegaly, with initiation of proinflammatory and chemokine signaling that presage the recruitment of multiple immune cell types to the liver resulting in steatohepatitis. Whether IR and inflammation are causally related cannot be determined from these studies. However, we have previously shown that mice fed a high-fat diet (60% of energy) alone attained the same weight gain as that of mice on a FF diet, exhibited similar levels of IR and steatosis, but had normal levels of serum AST and normal liver weights with no evidence of NASH (12), suggesting that IR and steatohepatitis may not be causally linked. Nevertheless, our current data confirm that IR is an early event in NASH (2, 40).
The contribution of immune cells to initiation and perpetuation of injury in fatty liver disease remains incompletely understood. The liver is enriched in cells of both myeloid and lymphoid lineage (macrophages, dendritic, T, NK, and NKT cells) but under nonpathological conditions tamps down overactivation of resident and transitioning immune cells (45, 54). In the present study, introduction of the FF diet induced a significantly increased and transient accrual of hepatic macrophages, neutrophils, and Cd3-positive T cells with a marginal increase in NK cells. Abatement of this influx occurred in tandem with steady-state levels of hepatic lipids over the following week and decreasing cholesterol levels over the subsequent weeks. A second influx of immune cells occurred later at 16 wk and was again characterized by the presence of multiple cell types: macrophages, neutrophils, recruited monocytes, and additionally at 24 wk by NK and dendritic cells. By contrast, depletion of NKT cells was observed at 24 wk. Furthermore, among the macrophage population, the proinflammatory M1 phenotype predominated while the M2 phenotype remained at basal levels. Overall, this would suggest that in fatty liver disease progression, macrophages are the most persistent immune cell type; DC and NK cells are latecomers, and NKT cells are depleted, while neutrophils and T cells remain at basal levels.
A vast body of literature exists that demonstrates the contribution of individual immune cells to the pathogenicity of NASH (4, 26, 46, 53) often assigning dichotomous roles in amplifying and in resolving inflammation (16). A major role has been attributed to macrophages (29, 38). Our observations indicate that while macrophages indeed represent the most persistent cell type beyond 16 wk, the overall immune profile alters with disease progression.
Fibrosis, as determined by pathological evaluation, was initiated in the FF mice by 8 wk and was well established by 16 wk. However, image analysis of tissue sections stained for Asma and for collagen, as well as gene expression for Coll1a1, indicated an early low activation of the profibrotic pathway by 2 wk, ahead of either the development of IR or proinflammatory signaling. An early and low induction of collagen gene transcription has been proposed to be on the basis of lipotoxicity mediated by increased local FFA (5, 34). Indeed, HuH7 cells exposed to low concentrations of palmitate (400 µM) display modest time-dependent increase in Tgfß1 and Coll1a1 (2-fold by 24 h) gene expression (data not shown). This would indicate activation of the profibrotic signaling pathway independent of inflammation. This notion is further supported by low histological scores for inflammation and baseline values for serum AST and the proinflammatory signaling genes Tnfα and Spp1 within the 2- to 4-wk period. Activation of fibroblasts was observed by 4 and 16 wk, and heavy deposits of ECM collagen fibrils were accompanied by inflammatory infiltrates and elevated levels of Tnfα and Spp1 reproducing the most common observations associated with fibrosis, the coexistence of fibrosis with inflammation. Little is known about the interaction between hepatocyte lipid accumulation and fibrotic signaling in vivo, although hepatocytes exposed to an admixture of oleic acid and palmitate were shown to induce proinflammatory signaling (13) and induce activation of stellate cells when in close contact (22).
Two factors implicated as key mediators of metabolic function in NASH are mitochondrial dysfunction (6) and oxidative stress (48). To more clearly understand their role in the evolution of NASH, we assessed metabolic function using a closed monitoring system that measured O2 consumption versus CO2 production, determined mitochondrial respiration using standard polarographic techniques, and assessed oxidative stress via determinations of 4-HNE, cellular and mitochondrial glutathione, and gene expression of antioxidant enzymes. Together, these data sets provide insights into metabolic function at the whole body, cellular, and organelle level in NASH. Data from the closed monitoring system indicated decreased V̇o2 consumption and a corresponding decrease in V̇co2 levels in mice reared on FF for all time points monitored. Consequently, the metabolic rate was decreased in FF mice for all time points. The decrease in metabolic rate was multifactorial, associated with decreased physical activities, decreased grip strength, decreased work output, and decreased total energy expenditure across all time points. Concurrently, respiration rates of liver mitochondria were decreased in FF mice with all substrates and for all time points despite equivalent citrate synthase between FF and SC mice. Significant impairment of mitochondrial respiration rate occurred only at 24 and 36 wk. Furthermore, this impairment occurred in the context of significantly reduced mitochondrial numbers as evidenced by a decreased mitochondrial-to- nuclear gene ratio. While the basis of decreasing mitochondrial numbers cannot be determined from these studies, the data suggest a certain degree of mitochondrial flexibility indicating that 1) mitochondrial respiration can adapt to reduced numbers, 2) overall mitochondrial health is a function of both number and respiratory capacity, and 3) mitochondrial respiratory dysfunction in NASH accrues gradually over a prolonged period of time. Some of these phenomena have been previously observed either in clinical NASH or in other animal models of NASH. For example, as seen in the current study, decreased activity of all the mitochondrial respiratory complexes was observed in NASH patients even though citrate synthase activity was similar to that of healthy patients (43). Reduced mitochondrial numbers have also been reported in clinical NASH (57), and mitophagy has been reported in steatotic hepatocytes associated with ethanol feeding (17). In a rodent model of NASH, mitochondrial dysfunction was attributed to impaired function of respiratory complexes I and II that led to reduced ATP content despite normal enzyme activity (50), whereas no differences in mitochondrial dysfunction (based on oxygen consumption, ATP synthesis, membrane potential) were observed between obese, steatotic, hyperinsulinemic, and lean Zucker rats (19). It must be noted that mitochondrial dysfunction is an all-encompassing term that collectively refers to several phenomena: morphological abnormalities, impairment in enzyme function, respiratory complex dysfunction, ATP synthesis, a decrease in mitochondrial numbers, and/or loss of membrane potential. In isolated mitochondria, mitochondrial respiration rate following addition of ADP is considered a true measure of mitochondrial health (7). In this context, our data would suggest that mitochondrial health, as evidenced by respiration rates, remains preserved through the early histological changes of fatty liver disease and is compromised in concert with a decline in mitochondrial numbers.
Oxidative stress, a term that refers to injury induced by reactive oxygen species (ROS), occurs when the production of free radicals, a byproduct of mitochondrial respiration, overwhelms the innate antioxidant defenses (7). Measurement of the levels of dismutases, glutathione, and peroxidation products offer some insights into the status of oxidative stress at the cellular level. Glutathione depletion, particularly in the mitochondria, has been invoked as an early and possibly precipitating event in NASH (35, 36). However, in the FF mice, despite a significant decrease in cytosolic dismutase Sod1 at 1 wk and reduced levels at 24 and 36 wk, there was no difference in cellular glutathione levels or in 4HNE between SC or FF mice at any time point. A transient drop in mitochondrial glutathione at 24 wk reflected the drop in mitochondrial dismutase Sod2 but was reversed by 36 wk, suggesting a possible adaptive mechanism. Significantly decreased mitochondrial respiration occurred in parallel to diminished catalase gene expression over all time points. While these data corroborate our previous observations with DNA oxidation product 8 OH-dG in mice fed for 6 mo on the FF diet (12), they run counter to the notion that oxidative stress and mitochondrial dysfunction are key early events that mediate disease progression to NASH (6, 35, 36, 47, 48). One possible explanation for this disparity may be that most publications that report oxidative stress measure ROS, a transient product, that the innate antioxidant systems may well be capable of scavenging in vivo. Interestingly, our own group has previously demonstrated decreased hepatic gene expression of dismutases and catalase in clinical NASH (51). Multiple mechanisms exist in vivo to dispose of ROS (glutathione reductase/peroxidase, dismutases, and catalase). Shortfalls in any one arm may be compensated by others with no apparent long-term effects. However, it is possible that transient declines may have other physiological consequences, such as the induction of hepatic collagen (49) or diminished lipophagy (31). However, taken collectively, our data would suggest that metabolic dysfunction in NASH is characterized by decreased metabolic rate and a decelerated mitochondrial respiration rate that is mediated at least in part by reduced mitochondrial numbers.
Collectively, our observations provide a unique overview of the series of changes that coevolve with the histological evolution of NASH. Although the study is cross sectional, the multiplicity of observations provides insights into the nonlinear nature of disease progression. A number of questions such as the molecular signaling pathways that are induced at the early, critical, and late stages of disease progression remain to be resolved. However, we believe that this delineation of events will facilitate a more nuanced analysis on specific features of the disease in the future.
In summary, this study demonstrates that in the FF diet-induced fatty liver disease model, NASH develops in a multiphasic manner marked by fluctuations in IR and inflammation that occur in concert with variations in hepatic content of two primary lipid components, triglyceride and cholesterol. IR, proinflammatory, and profibrotic signaling precede mitochondrial respiratory dysfunction, while the role of oxidative stress appears to be relatively minor.
GRANTS
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grant R01-DK-41978 and Clinical and Translational Science Award Grant UL1TR000135.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
A.K., T.S.A., T.M., S.P.H., A.M., T.A.W., N.K.L., and I.R.L. performed experiments; A.K., T.S.A., T.M., S.P.H., A.M., T.A.W., N.K.L., I.R.L., S.N., G.J.G., and M.C. analyzed data; A.K., T.S.A., T.M., S.P.H., T.A.W., N.K.L., I.R.L., S.N., G.J.G., and M.C. interpreted results of experiments; A.K. and M.C. prepared figures; A.K., A.M., T.A.W., N.K.L., I.R.L., S.N., G.J.G., and M.C. drafted manuscript; A.K., T.S.A., S.P.H., and M.C. edited and revised manuscript; A.K., T.S.A., T.M., S.P.H., A.M., T.A.W., N.K.L., I.R.L., S.N., G.J.G., and M.C. approved final version of manuscript; M.C. conceived and designed research.
REFERENCES
- 1.Abdelmegeed MA, Yoo SH, Henderson LE, Gonzalez FJ, Woodcroft KJ, Song BJ. PPARalpha expression protects male mice from high fat-induced nonalcoholic fatty liver. J Nutr : 603–610, 2011. doi: 10.3945/jn.110.135210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Adkins Y, Schie IW, Fedor D, Reddy A, Nguyen S, Zhou P, Kelley DS, Wu J. A novel mouse model of nonalcoholic steatohepatitis with significant insulin resistance. Lab Invest : 1313–1322, 2013. doi: 10.1038/labinvest.2013.123. [DOI] [PubMed] [Google Scholar]
- 3.Alp PR, Newsholme EA, Zammit VA. Activities of citrate synthase and NAD+-linked and NADP+-linked isocitrate dehydrogenase in muscle from vertebrates and invertebrates. Biochem J : 689–700, 1976. doi: 10.1042/bj1540689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baeck C, Wehr A, Karlmark KR, Heymann F, Vucur M, Gassler N, Huss S, Klussmann S, Eulberg D, Luedde T, Trautwein C, Tacke F. Pharmacological inhibition of the chemokine CCL2 (MCP-1) diminishes liver macrophage infiltration and steatohepatitis in chronic hepatic injury. Gut : 416–426, 2012. doi: 10.1136/gutjnl-2011-300304. [DOI] [PubMed] [Google Scholar]
- 5.Bechmann LP, Kocabayoglu P, Sowa JP, Sydor S, Best J, Schlattjan M, Beilfuss A, Schmitt J, Hannivoort RA, Kilicarslan A, Rust C, Berr F, Tschopp O, Gerken G, Friedman SL, Geier A, Canbay A. Free fatty acids repress small heterodimer partner (SHP) activation and adiponectin counteracts bile acid-induced liver injury in superobese patients with nonalcoholic steatohepatitis. Hepatology : 1394–1406, 2013. doi: 10.1002/hep.26225. [DOI] [PubMed] [Google Scholar]
- 6.Begriche K, Massart J, Robin MA, Bonnet F, Fromenty B. Mitochondrial adaptations and dysfunctions in nonalcoholic fatty liver disease. Hepatology : 1497–1507, 2013. doi: 10.1002/hep.26226. [DOI] [PubMed] [Google Scholar]
- 7.Brand MD, Nicholls DG. Assessing mitochondrial dysfunction in cells. Biochem J : 297–312, 2011. doi: 10.1042/BJ20110162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brunt EM. Non-alcoholic fatty liver disease: what’s new under the microscope? Gut : 1152–1158, 2011. doi: 10.1136/gut.2010.218214. [DOI] [PubMed] [Google Scholar]
- 9.Caballero F, Fernández A, Matías N, Martínez L, Fucho R, Elena M, Caballeria J, Morales A, Fernández-Checa JC, García-Ruiz C. Specific contribution of methionine and choline in nutritional nonalcoholic steatohepatitis: impact on mitochondrial S-adenosyl-L-methionine and glutathione. J Biol Chem : 18528–18536, 2010. doi: 10.1074/jbc.M109.099333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chalasani N, Younossi Z, Lavine JE, Diehl AM, Brunt EM, Cusi K, Charlton M, Sanyal AJ; American Gastroenterological Association; American Association for the Study of Liver Diseases; American College of Gastroenterology . The diagnosis and management of non-alcoholic fatty liver disease: practice guideline by the American Gastroenterological Association, American Association for the Study of Liver Diseases, and American College of Gastroenterology. Gastroenterology : 1592–1609, 2012. doi: 10.1053/j.gastro.2012.04.001. [DOI] [PubMed] [Google Scholar]
- 11.Charlton M. Evolving aspects of liver transplantation for nonalcoholic steatohepatitis. Curr Opin Organ Transplant : 251–258, 2013. doi: 10.1097/MOT.0b013e3283615d30. [DOI] [PubMed] [Google Scholar]
- 12.Charlton M, Krishnan A, Viker K, Sanderson S, Cazanave S, McConico A, Masuoko H, Gores G. Fast food diet mouse: novel small animal model of NASH with ballooning, progressive fibrosis, and high physiological fidelity to the human condition. Am J Physiol Gastrointest Liver Physiol : G825–G834, 2011. doi: 10.1152/ajpgi.00145.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chavez-Tapia NC, Rosso N, Tiribelli C. Effect of intracellular lipid accumulation in a new model of non-alcoholic fatty liver disease. BMC Gastroenterol : 20, 2012. doi: 10.1186/1471-230X-12-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Choi CS, Savage DB, Kulkarni A, Yu XX, Liu ZX, Morino K, Kim S, Distefano A, Samuel VT, Neschen S, Zhang D, Wang A, Zhang XM, Kahn M, Cline GW, Pandey SK, Geisler JG, Bhanot S, Monia BP, Shulman GI. Suppression of diacylglycerol acyltransferase-2 (DGAT2), but not DGAT1, with antisense oligonucleotides reverses diet-induced hepatic steatosis and insulin resistance. J Biol Chem : 22678–22688, 2007. doi: 10.1074/jbc.M704213200. [DOI] [PubMed] [Google Scholar]
- 15.Deng QG, She H, Cheng JH, French SW, Koop DR, Xiong S, Tsukamoto H. Steatohepatitis induced by intragastric overfeeding in mice. Hepatology : 905–914, 2005. doi: 10.1002/hep.20877. [DOI] [PubMed] [Google Scholar]
- 16.Duffield JS, Forbes SJ, Constandinou CM, Clay S, Partolina M, Vuthoori S, Wu S, Lang R, Iredale JP. Selective depletion of macrophages reveals distinct, opposing roles during liver injury and repair. J Clin Invest : 56–65, 2005. doi: 10.1172/JCI200522675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eid N, Ito Y, Otsuki Y. Mitophagy in steatotic hepatocytes of ethanol-treated wild-type and Parkin knockout mice. Am J Physiol Gastrointest Liver Physiol : G513–G514, 2015. doi: 10.1152/ajpgi.00254.2015. [DOI] [PubMed] [Google Scholar]
- 18.Farrell GC, van Rooyen D. Liver cholesterol: is it playing possum in NASH? Am J Physiol Gastrointest Liver Physiol : G9–G11, 2012. doi: 10.1152/ajpgi.00008.2012. [DOI] [PubMed] [Google Scholar]
- 19.Flamment M, Foufelle F. [Importance of phospholipids in ER stress and fatty liver disease]. Med Sci (Paris) : 13–15, 2012. doi: 10.1051/medsci/2012281004. [DOI] [PubMed] [Google Scholar]
- 20.Gan LT, Van Rooyen DM, Koina ME, McCuskey RS, Teoh NC, Farrell GC. Hepatocyte free cholesterol lipotoxicity results from JNK1-mediated mitochondrial injury and is HMGB1 and TLR4-dependent. J Hepatol : 1376–1384, 2014. doi: 10.1016/j.jhep.2014.07.024. [DOI] [PubMed] [Google Scholar]
- 21.García-Ruiz I, Solís-Muñoz P, Fernández-Moreira D, Grau M, Colina F, Muñoz-Yagüe T, Solís-Herruzo JA. High-fat diet decreases activity of the oxidative phosphorylation complexes and causes nonalcoholic steatohepatitis in mice. Dis Model Mech : 1287–1296, 2014. doi: 10.1242/dmm.016766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Giraudi PJ, Becerra VJ, Marin V, Chavez-Tapia NC, Tiribelli C, Rosso N. The importance of the interaction between hepatocyte and hepatic stellate cells in fibrogenesis induced by fatty accumulation. Exp Mol Pathol : 85–92, 2015. doi: 10.1016/j.yexmp.2014.12.006. [DOI] [PubMed] [Google Scholar]
- 23.Haque JA, McMahan RS, Campbell JS, Shimizu-Albergine M, Wilson AM, Botta D, Bammler TK, Beyer RP, Montine TJ, Yeh MM, Kavanagh TJ, Fausto N. Attenuated progression of diet-induced steatohepatitis in glutathione-deficient mice. Lab Invest : 1704–1717, 2010. doi: 10.1038/labinvest.2010.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hartono SP, Knudsen BE, Lerman LO, Textor SC, Grande JP. Combined effect of hyperfiltration and renin angiotensin system activation on development of chronic kidney disease in diabetic db/db mice. BMC Nephrol : 58, 2014. doi: 10.1186/1471-2369-15-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hebbard L, George J. Animal models of nonalcoholic fatty liver disease. Nat Rev Gastroenterol Hepatol : 35–44, 2011. doi: 10.1038/nrgastro.2010.191. [DOI] [PubMed] [Google Scholar]
- 26.Henning JR, Graffeo CS, Rehman A, Fallon NC, Zambirinis CP, Ochi A, Barilla R, Jamal M, Deutsch M, Greco S, Ego-Osuala M, Bin-Saeed U, Rao RS, Badar S, Quesada JP, Acehan D, Miller G. Dendritic cells limit fibroinflammatory injury in nonalcoholic steatohepatitis in mice. Hepatology : 589–602, 2013. doi: 10.1002/hep.26267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ibrahim SH, Hirsova P, Tomita K, Bronk SF, Werneburg NW, Harrison SA, Goodfellow VS, Malhi H, Gores GJ. Mixed lineage kinase 3 mediates release of C-X-C motif ligand 10-bearing chemotactic extracellular vesicles from lipotoxic hepatocytes. Hepatology : 731–744, 2016. doi: 10.1002/hep.28252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ioannou GN, Van Rooyen DM, Savard C, Haigh WG, Yeh MM, Teoh NC, Farrell GC. Cholesterol-lowering drugs cause dissolution of cholesterol crystals and disperse Kupffer cell crown-like structures during resolution of NASH. J Lipid Res : 277–285, 2015. doi: 10.1194/jlr.M053785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jindal A, Bruzzì S, Sutti S, Locatelli I, Bozzola C, Paternostro C, Parola M, Albano E. Fat-laden macrophages modulate lobular inflammation in nonalcoholic steatohepatitis (NASH). Exp Mol Pathol : 155–162, 2015. doi: 10.1016/j.yexmp.2015.06.015. [DOI] [PubMed] [Google Scholar]
- 30.Krishnan A, Li X, Kao WY, Viker K, Butters K, Masuoka H, Knudsen B, Gores G, Charlton M. Lumican, an extracellular matrix proteoglycan, is a novel requisite for hepatic fibrosis. Lab Invest : 1712–1725, 2012. doi: 10.1038/labinvest.2012.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kurahashi T, Hamashima S, Shirato T, Lee J, Homma T, Kang ES, Fujii J. An SOD1 deficiency enhances lipid droplet accumulation in the fasted mouse liver by aborting lipophagy. Biochem Biophys Res Commun : 866–871, 2015. doi: 10.1016/j.bbrc.2015.10.052. [DOI] [PubMed] [Google Scholar]
- 32.Lanza IR, Nair KS. Functional assessment of isolated mitochondria in vitro. Methods Enzymol : 349–372, 2009. doi: 10.1016/S0076-6879(09)05020-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Laurent A, Nicco C, Tran Van Nhieu J, Borderie D, Chéreau C, Conti F, Jaffray P, Soubrane O, Calmus Y, Weill B, Batteux F. Pivotal role of superoxide anion and beneficial effect of antioxidant molecules in murine steatohepatitis. Hepatology : 1277–1285, 2004. doi: 10.1002/hep.20177. [DOI] [PubMed] [Google Scholar]
- 34.Malhi H, Barreyro FJ, Isomoto H, Bronk SF, Gores GJ. Free fatty acids sensitise hepatocytes to TRAIL mediated cytotoxicity. Gut : 1124–1131, 2007. doi: 10.1136/gut.2006.118059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Marí M, Caballero F, Colell A, Morales A, Caballeria J, Fernandez A, Enrich C, Fernandez-Checa JC, García-Ruiz C. Mitochondrial free cholesterol loading sensitizes to TNF- and Fas-mediated steatohepatitis. Cell Metab : 185–198, 2006. doi: 10.1016/j.cmet.2006.07.006. [DOI] [PubMed] [Google Scholar]
- 36.Marí M, Colell A, Morales A, Caballero F, Moles A, Fernández A, Terrones O, Basañez G, Antonsson B, García-Ruiz C, Fernández-Checa JC. Mechanism of mitochondrial glutathione-dependent hepatocellular susceptibility to TNF despite NF-kappaB activation. Gastroenterology : 1507–1520, 2008. doi: 10.1053/j.gastro.2008.01.073. [DOI] [PubMed] [Google Scholar]
- 37.Min HK, Kapoor A, Fuchs M, Mirshahi F, Zhou H, Maher J, Kellum J, Warnick R, Contos MJ, Sanyal AJ. Increased hepatic synthesis and dysregulation of cholesterol metabolism is associated with the severity of nonalcoholic fatty liver disease. Cell Metab : 665–674, 2012. doi: 10.1016/j.cmet.2012.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Miura K, Yang L, van Rooijen N, Ohnishi H, Seki E. Hepatic recruitment of macrophages promotes nonalcoholic steatohepatitis through CCR2. Am J Physiol Gastrointest Liver Physiol : G1310–G1321, 2012. doi: 10.1152/ajpgi.00365.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Musso G, Gambino R, Cassader M. Cholesterol metabolism and the pathogenesis of non-alcoholic steatohepatitis. Prog Lipid Res : 175–191, 2013. doi: 10.1016/j.plipres.2012.11.002. [DOI] [PubMed] [Google Scholar]
- 40.Nakamura A, Tajima K, Zolzaya K, Sato K, Inoue R, Yoneda M, Fujita K, Nozaki Y, Kubota KC, Haga H, Kubota N, Nagashima Y, Nakajima A, Maeda S, Kadowaki T, Terauchi Y. Protection from non-alcoholic steatohepatitis and liver tumourigenesis in high fat-fed insulin receptor substrate-1-knockout mice despite insulin resistance. Diabetologia : 3382–3391, 2012. doi: 10.1007/s00125-012-2703-1. [DOI] [PubMed] [Google Scholar]
- 41.Neuschwander-Tetri BA, Clark JM, Bass NM, Van Natta ML, Unalp-Arida A, Tonascia J, Zein CO, Brunt EM, Kleiner DE, McCullough AJ, Sanyal AJ, Diehl AM, Lavine JE, Chalasani N, Kowdley KV; NASH Clinical Research Network . Clinical, laboratory and histological associations in adults with nonalcoholic fatty liver disease. Hepatology : 913–924, 2010. doi: 10.1002/hep.23784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nixon JP, Zhang M, Wang C, Kuskowski MA, Novak CM, Levine JA, Billington CJ, Kotz CM. Evaluation of a quantitative magnetic resonance imaging system for whole body composition analysis in rodents. Obesity (Silver Spring) : 1652–1659, 2010. doi: 10.1038/oby.2009.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pérez-Carreras M, Del Hoyo P, Martín MA, Rubio JC, Martín A, Castellano G, Colina F, Arenas J, Solis-Herruzo JA. Defective hepatic mitochondrial respiratory chain in patients with nonalcoholic steatohepatitis. Hepatology : 999–1007, 2003. doi: 10.1002/hep.1840380426. [DOI] [PubMed] [Google Scholar]
- 44.Pirola CJ, Gianotti TF, Burgueño AL, Rey-Funes M, Loidl CF, Mallardi P, Martino JS, Castaño GO, Sookoian S. Epigenetic modification of liver mitochondrial DNA is associated with histological severity of nonalcoholic fatty liver disease. Gut : 1356–1363, 2013. doi: 10.1136/gutjnl-2012-302962. [DOI] [PubMed] [Google Scholar]
- 45.Racanelli V, Rehermann B. The liver as an immunological organ. Hepatology , Suppl 1: S54–S62, 2006. doi: 10.1002/hep.21060. [DOI] [PubMed] [Google Scholar]
- 46.Ramachandran P, Pellicoro A, Vernon MA, Boulter L, Aucott RL, Ali A, Hartland SN, Snowdon VK, Cappon A, Gordon-Walker TT, Williams MJ, Dunbar DR, Manning JR, van Rooijen N, Fallowfield JA, Forbes SJ, Iredale JP. Differential Ly-6C expression identifies the recruited macrophage phenotype, which orchestrates the regression of murine liver fibrosis. Proc Natl Acad Sci USA : E3186–E3195, 2012. doi: 10.1073/pnas.1119964109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rector RS, Thyfault JP, Uptergrove GM, Morris EM, Naples SP, Borengasser SJ, Mikus CR, Laye MJ, Laughlin MH, Booth FW, Ibdah JA. Mitochondrial dysfunction precedes insulin resistance and hepatic steatosis and contributes to the natural history of non-alcoholic fatty liver disease in an obese rodent model. J Hepatol : 727–736, 2010. doi: 10.1016/j.jhep.2009.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rolo AP, Teodoro JS, Palmeira CM. Role of oxidative stress in the pathogenesis of nonalcoholic steatohepatitis. Free Radic Biol Med : 59–69, 2012. doi: 10.1016/j.freeradbiomed.2011.10.003. [DOI] [PubMed] [Google Scholar]
- 49.Sakiyama H, Fujiwara N, Yoneoka Y, Yoshihara D, Eguchi H, Suzuki K. Cu, Zn-SOD deficiency induces the accumulation of hepatic collagen. Free Radic Res : 666–677, 2016. doi: 10.3109/10715762.2016.1164856. [DOI] [PubMed] [Google Scholar]
- 50.Serviddio G, Bellanti F, Vendemiale G, Altomare E. Mitochondrial dysfunction in nonalcoholic steatohepatitis. Expert Rev Gastroenterol Hepatol : 233–244, 2011. doi: 10.1586/egh.11.11. [DOI] [PubMed] [Google Scholar]
- 51.Sreekumar R, Rosado B, Rasmussen D, Charlton M. Hepatic gene expression in histologically progressive nonalcoholic steatohepatitis. Hepatology : 244–251, 2003. doi: 10.1053/jhep.2003.50290. [DOI] [PubMed] [Google Scholar]
- 52.Sunny NE, Parks EJ, Browning JD, Burgess SC. Excessive hepatic mitochondrial TCA cycle and gluconeogenesis in humans with nonalcoholic fatty liver disease. Cell Metab : 804–810, 2011. doi: 10.1016/j.cmet.2011.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Talukdar S, Oh DY, Bandyopadhyay G, Li D, Xu J, McNelis J, Lu M, Li P, Yan Q, Zhu Y, Ofrecio J, Lin M, Brenner MB, Olefsky JM. Neutrophils mediate insulin resistance in mice fed a high-fat diet through secreted elastase. Nat Med : 1407–1412, 2012. doi: 10.1038/nm.2885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Thomson AW, Knolle PA. Antigen-presenting cell function in the tolerogenic liver environment. Nat Rev Immunol : 753–766, 2010. doi: 10.1038/nri2858. [DOI] [PubMed] [Google Scholar]
- 55.Van Rooyen DM, Larter CZ, Haigh WG, Yeh MM, Ioannou G, Kuver R, Lee SP, Teoh NC, Farrell GC. Hepatic free cholesterol accumulates in obese, diabetic mice and causes nonalcoholic steatohepatitis. Gastroenterology : 1393–1403, 1403.e1–1403.e5, 2011. doi: 10.1053/j.gastro.2011.06.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.von Montfort C, Matias N, Fernandez A, Fucho R, Conde de la Rosa L, Martinez-Chantar ML, Mato JM, Machida K, Tsukamoto H, Murphy MP, Mansouri A, Kaplowitz N, Garcia-Ruiz C, Fernandez-Checa JC. Mitochondrial GSH determines the toxic or therapeutic potential of superoxide scavenging in steatohepatitis. J Hepatol : 852–859, 2012. doi: 10.1016/j.jhep.2012.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wei Y, Rector RS, Thyfault JP, Ibdah JA. Nonalcoholic fatty liver disease and mitochondrial dysfunction. World J Gastroenterol : 193–199, 2008. doi: 10.3748/wjg.14.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wouters K, van Gorp PJ, Bieghs V, Gijbels MJ, Duimel H, Lütjohann D, Kerksiek A, van Kruchten R, Maeda N, Staels B, van Bilsen M, Shiri-Sverdlov R, Hofker MH. Dietary cholesterol, rather than liver steatosis, leads to hepatic inflammation in hyperlipidemic mouse models of nonalcoholic steatohepatitis. Hepatology : 474–486, 2008. doi: 10.1002/hep.22363. [DOI] [PubMed] [Google Scholar]
- 59.Yamaguchi K, Yang L, McCall S, Huang J, Yu XX, Pandey SK, Bhanot S, Monia BP, Li YX, Diehl AM. Inhibiting triglyceride synthesis improves hepatic steatosis but exacerbates liver damage and fibrosis in obese mice with nonalcoholic steatohepatitis. Hepatology : 1366–1374, 2007. doi: 10.1002/hep.21655. [DOI] [PubMed] [Google Scholar]