Abstract
Nebulization delivery of adeno-associated virus serotype 1 encoding sarcoplasmic reticulum Ca2+-ATPase2a (AAV1.SERCA2a) gene was examined in a Yukatan miniature swine model of chronic pulmonary hypertension (n = 13). Nebulization of AAV1.SERCA2a resulted in homogenous distribution of vectors, lower pulmonary vascular resistance, and a trend towards better long-term survival compared to control animals.
Keywords: gene therapy, airway delivery, post-capillary pulmonary hypertension, nebulization
Pulmonary vascular remodeling attributable to proliferation of pulmonary vascular smooth muscle cells (PVSMC) underlies the pathophysiology of pulmonary hypertension (PH).1 Emerging evidence suggests that dysregulation of calcium cycling in PVSMCs promotes a phenotype switch from a contractile to a proliferative phenotype owing, in part, to concomitant downregulation of sarcoplasmic reticulum Ca2+-ATPase 2 (SERCA2) isoforms.2,3 Gene transfer of SERCA2a has demonstrated potential in prevention of this aberrant phenotype switch in vitro and in vivo and shown efficacy as a therapeutic in pre-clinical PH models.4–6 Recently, we reported that aerosolized intra-airway delivery of adeno-associated virus type 1 (AAV1) carrying the SERCA2a gene (AAV1.SERCA2a) inhibited progression of PH by ameliorating pulmonary vascular remodeling in a Yorkshire pig model of post-capillary PH.6 In this study, we used a metal sprayer device inserted directly into the trachea via the endotracheal tube to deliver AAV1.SERCA2a, which is not feasible for clinical use in severely ill PH patients who would benefit from a minimally invasive delivery system. Therefore, the objective of the current study was to investigate the safety and efficacy of AAV1.SERCA2a gene therapy using nebulization delivery as a less invasive and clinically applicable means of vector delivery. We also extended follow-up to four months after the gene delivery in order to determine if the efficacy of AAV1.SERCA2a gene therapy was maintained in the long term.
Methods
The study was performed in accordance with the Guidelines for the Care and Use of Laboratory Animals, with approval granted by the Icahn School of Medicine at Mount Sinai Institutional Animal Care and Use Committee. The general study protocol, post-capillary PH model creation, and methods to evaluate PH have been published previously,6 except for using different pig strain, different gene delivery method, and a longer follow-up. Due to the expected large weight gain during the long-term observation in the Yorkshire swine, studies were performed in Yukatan miniature swine (Sinclair Bioresources, ME, USA). Briefly, female Yukatan miniature swine underwent pulmonary vein banding as described previously.7 Two months after surgery, when PH is established, a total of 13 animals were randomized to receive either 2.0 × 1013 of AAV-1.SERCA2a (3 mL) (n = 6) or 0.9% saline (3 mL) (n = 7) using nebulization delivery (Aeroneb Solo, Aerogen) in circuit with the endotracheal tube. The Aeroneb Solo produces a particle size of 1–5 µm, which is smaller than that of a Microsprayer (Penn-Century, Inc.) used in the previous study (16–22 µm).6 The animals were ventilated with ∼3 mmHg of positive end-expiratory pressure for 15 min after the end of nebulization delivery. Hemodynamic and echocardiographic evaluations were conducted at two and four months after gene delivery in surviving animals (Fig. 1a). The animals were euthanized if they developed refractory symptoms of right ventricular (RV) failure or at completion of the study. Upon euthanasia, the lung tissues were collected for histology and molecular analysis. More detailed methods are available in the supplemental materials.
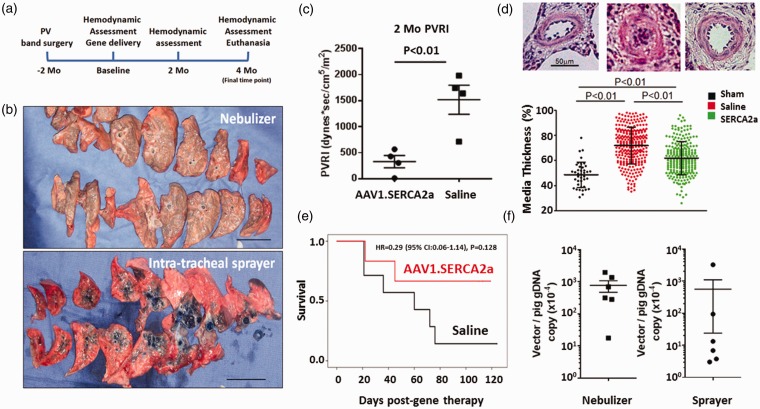
Fig. 1.
Nebulization of AAV1.SERCA2a results in homogenous vector distribution and improves survival in a pig model of chronic PH. (a) Study protocol. Baseline functional evaluation followed by gene delivery was conducted 2 months after the PH induction surgery. Animals were followed up to 4 months after gene delivery. (b) Intra-airway delivery of Evans Blue dye using a nebulizer (top) or an intra-tracheal sprayer (bottom) method. Nebulizer delivery resulted in more homogeneous distribution with weak dye staining, whereas sprayer delivery exhibited more focal and concentrated distribution of dye. (c) Pigs treated with nebulized AAV1.SERCA2a had lower PVR index compared to the saline-treated pigs 2 months after treatment. (d) Representative hematoxylin and eosin staining of the lung tissues from sham, saline-treated, and AAV1.SERCA2a-treated animals. AAV1.SERCA2a-treated animals exhibited decreased pulmonary vascular medial thicknesS. (e) PH pigs treated with AAV1.SERCA2a nebulization therapy had better survival compared to those treated with saline. (f) Vector genome copy number relative to the pig genome in the lungs. An average of the lower left and right lung lobes is shown following nebulized delivery (n = 6) or intra-tracheal sprayer administration (n = 6). Error bars indicate ± SEM.
Results
To demonstrate that nebulization was at least comparable to the intra-tracheal aerosol method, we delivered Evans Blue dye via nebulization (n = 1) or the intra-tracheal sprayer (n = 1)6 (Fig. 1b). Nebulization delivery resulted in a more homogeneous distribution of dye evidenced by lighter blue staining across a greater area of the lungs compared to the intra-tracheal sprayer delivery method, which concentrated the dye in lung central segments (Fig. 1b). After nebulization delivery, the endotracheal tube also retained Evans Blue dye due to precipitation on the tube. To account for potential vector loss within the endotracheal tube and nebulizer, we therefore used a dose of 2.0 × 1013 vg (twice the dose used in our prior study [1013 vg]).6
Similar to Yorkshire pigs, Yukatan miniature swine developed PH two months after the pulmonary vein banding surgery; however, Yukatan miniature swine were less tolerant of the elevated pulmonary pressures and resistance, and many animals in the saline-treated group developed refractory RV failure before reaching the study endpoint. Therefore, we evaluated the therapeutic efficacy of nebulized AAV1.SERCA2a at two months after delivery. Consistent with our prior results, SERCA2a-treated animals had lower pulmonary vascular resistance (PVR) (Fig. 1c), consistent with the beneficial effect of SERCA2a gene therapy on pulmonary vascular remodeling (Fig. 1d). Although the number of animals that survived to the study endpoint was numerically higher in the AAV1.SERCA2a-treated group than the saline-treated group, the study was underpowered to detect differences between the treatment groups (Fig. 1e).
Vector genome distribution was measured by reverse transcription polymerase chain reaction (RT-PCR) as an indicator of virus distribution by nebulization in both the left and right lung lobes from all AAV1.SERCA2a-treated animals. After nebulization, vector genome copy numbers were similar in the left and right lung lobes consistent with homogenous virus distribution. There were also numerically higher vector genome copy numbers detected in the collected lung tissues from animals that had AAV1.SERCA2a delivered by nebulization as compared to the intra-tracheal sprayer method (Fig. 1f). There were no complications associated with nebulization delivery in the AAV1.SERCA2a- or saline-treated animals.
Discussion
In this study, we found that AAV1.SERCA2a delivery via nebulization is safe and results in homogenous distribution of vectors in both the left and right lung lobes with good retention of viral genome copy numbers. Similar to our prior study,6 nebulized AAV1.SERCA2a gene therapy was associated with improvement in PH; however, the large number of deaths in the saline-treated control group before the study endpoint (four months after gene therapy) precluded systematic evaluation of long-term efficacy. The animals treated with AAV1.SERCA2a had a trend towards better survival despite the small study sample size. The deaths in the AAV1.SERCA2a group early after gene therapy may potentially be related to the delayed SERCA2a protein expression using AAV system, which requires about 3–4 weeks to achieve stable expression of transferred SERCA2a. We believe that the numerically higher survival rate in the SERCA2a-treated group with lower PVR at two months after gene delivery strongly support the therapeutic efficacy of nebulization SERCA2a gene therapy in PH.
A key feature of this study was to evaluate nebulization as a method of minimizing the invasiveness of vector delivery while retaining efficacy. More homogenous distribution using the nebulizer is likely attributed to the smaller particle size generated by the nebulizer compared to the sprayer. Because of expected loss of vector outside the body using the nebulizer, we used twice the dose relative to the previous study which employed the direct intra-tracheal spraying method. In the clinical situation, part of the vector would be absorbed in the oral and main trachea instead of the tracheal tube, thus its effect must be carefully evaluated in the future. Although differences in pig strain, vector dose, and timing of analysis after vector delivery preclude a full direct comparison between our nebulization and intra-tracheal sprayer studies, beneficial effects of airway AAV1.SERCA2a gene therapy on PH are consistently demonstrated in these studies. It remains to be determined, however, which of the following has better efficacy to achieve global effects on vascular remodeling and, ultimately, RV remodeling and function: high local gene overexpression or low but homogeneous gene expression.
In summary, we demonstrated the safety and longer-term efficacy of AAV1.SERCA2a nebulization therapy in a clinically relevant animal model of advanced PH. While nebulization delivery showed excellent safety with a less invasive delivery method, whether efficacy is superior to intra-tracheal sprayer delivery requires further study. Our finding of potential improvement in survival with SERCA2a gene therapy also warrants further examination of this approach and translation to clinical studies.
Supplemental Material
Supplemental material for Safety and long-term efficacy of AAV1.SERCA2a using nebulizer delivery in a pig model of pulmonary hypertension by Shin Watanabe, Kiyotake Ishikawa, Maria Plataki, Olympia Bikou, Erik Kohlbrenner, Jaume Aguero, Lahouaria Hadri, Iratxe Zarragoikoetxea, Kenneth Fish, Jane A. Leopold and Roger J. Hajjar in Pulmonary Circulation
Acknowledgments
The authors acknowledge the Gene Therapy Resource Program (GTRP) of the National Heart, Lung, and Blood Institute, National Institutes of Health for providing the gene vectors used in this study. JA was supported by the Fundacion Alfonso Martin-Escudero. OB was supported by the Deutsche Herzstiftung. The authors also thank Lauren Leonardson, LVT, for her excellent technical support and expertise on the study.
Conflict of interest
The author(s) declare that there is no conflict of interest.
Funding
This work was supported by NIH R01 HL117505, HL 119046, HL129814, HL128072, HL131404, HL135093, a P50 HL112324 (to RJH), HL139963 (to KI), AHA-SDG 17SDG33410873 (to KI), and two Transatlantic Fondation Leducq grants.
References
- 1.Tuder RM, Archer SL, Dorfmuller P, et al. Relevant issues in the pathology and pathobiology of pulmonary hypertension. J Am Coll Cardiol 2013; 62(25 Suppl): D4–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lipskaia L, Bobe R, Chen J, et al. Synergistic role of protein phosphatase inhibitor 1 and sarco/endoplasmic reticulum Ca2+ -ATPase in the acquisition of the contractile phenotype of arterial smooth muscle cells. Circulation 2014; 129(7): 773–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kuhr FK, Smith KA, Song MY, et al. New mechanisms of pulmonary arterial hypertension: role of Ca(2)(+) signaling. Am J Physiol Heart Circ Physiol 2012; 302(8): H1546–1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lipskaia L, Hadri L, Lopez JJ, et al. Benefit of SERCA2a gene transfer to vascular endothelial and smooth muscle cells: a new aspect in therapy of cardiovascular diseases. Curr Vasc Pharmacol 2013; 11(4): 465–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hadri L, Kratlian RG, Benard L, et al. Therapeutic efficacy of AAV1.SERCA2a in monocrotaline-induced pulmonary arterial hypertension. Circulation 2013; 128(5): 512–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aguero J, Ishikawa K, Hadri L, et al. Intratracheal gene delivery of SERCA2a ameliorates chronic post-capillary pulmonary hypertension: a large animal model. J Am Coll Cardiol 2016; 67(17): 2032–2046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aguero J, Hadri L, Hammoudi N, et al. Inhaled gene transfer for pulmonary circulation. Methods Mol Biol 2017; 1521: 339–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material for Safety and long-term efficacy of AAV1.SERCA2a using nebulizer delivery in a pig model of pulmonary hypertension by Shin Watanabe, Kiyotake Ishikawa, Maria Plataki, Olympia Bikou, Erik Kohlbrenner, Jaume Aguero, Lahouaria Hadri, Iratxe Zarragoikoetxea, Kenneth Fish, Jane A. Leopold and Roger J. Hajjar in Pulmonary Circulation