Abstract
The present study aimed to explore the efficiency of N-acetyl cysteine (NACC) or thymoquinone (TMQ) alone or in combination in the downregulation of inflammatory molecule expression and decreasing hepatic injury in response to sodium fluoride (SF). Sodium fluoride upregulated serum alanine and aspartate transferases activities, tumor necrosis factor α and hepatic malondialdehyde and nitric oxide levels, and the expression of cyclooxygenase 2, nuclear factor κB cell, and signal transducer and activator of transcription 3. In contrast, hepatic glutathione level, superoxide dismutase activity, and nuclear factor erythroid 2-related factor 2 expression were decreased. However, the concurrent treatment with antioxidants, alone or in combination, modulated the levels of these parameters. Histopathological examination revealed that SF treatment resulted in focal areas of massive hepatic degeneration and many degenerated hepatocytes, whereas the treatment with TMQ or NACC exhibited moderate improvement in cellular degeneration of the liver with many abnormal cells. Rats receiving a combination of TMQ and NACC showed marked improvement in cellular degeneration of liver with apparently normal hepatic architecture with very few degenerated hepatocytes. The results also revealed that the combination of TMQ and NACC is the most effective regimen in ameliorating SF toxicity, suggesting their efficacy against the toxicity of fluoride compounds. Their activities might be mediated via multiple molecular pathways.
Keywords: COX-2, NF-κB, sodium fluoride, STAT-3, Western blot, protein expression, protein expression, thymoquinone
Introduction
Sodium fluoride (SF), a pollutant, is used as an insecticide and antihelminthic agent.1,2 Fluoride can be present in the soil, water, and vegetation. It has been reported that chronic fluoride toxicity causes joint stiffness.3–6 Its deleterious effects on the reproductive system and kidney tubules are well-documented.7–9 It is known as a double-edged sword, and hence, it is an essential trace element in small doses to prevent dental caries and osteoporosis. Conversely, high doses cause harmful effects. It interferes with the metabolism of macromolecules,10,11 inhibits the activity of enzymes involved in glycolysis and Krebs cycle and fatty acid oxidation, and suppresses the formation of polypeptides that block DNA synthesis.12 The mechanism of fluoride-induced pathogenesis is accompanied by reactive oxygen species (ROS) and peroxyl and hydroxyl radical formation, resulting in oxidative stress (OS), apoptosis, and DNA damage.13,14
N-acetylcysteine (NACC) is a small molecule containing a thiol group and has an antioxidant effect. It can access intracellular compartments.15 Its therapeutic efficacy is contributed to the existent of the cysteinyl thiol group; the ability of thiol group to scavenge oxygen-free radicals is well established.15 N-acetylcysteine raises the intracellular concentrations of cysteine and in sequence decreases reduced glutathione (GSH) level.
Numerous active constituents from herbal and medical plants are widely documented for their favorable effects. Thymoquinone (TMQ) is known as an antioxidant and free radical scavenger.16 Thymoquinone exhibits various pharmacological and immunomodulatory actions17 and also has anti-inflammation, antioxidant,18 and antitumorigenic activities against different cancer diseases.19,20 Its protective role against oxidative damage of many organs induced by free radical-producing agents such as doxorubicin-induced cardiotoxicity and carbon tetrachloride-induced hepatotoxicity was well studied.21–23
The aim of this study is to explore the efficiency of NACC and/or TMQ alone or in combination in the downregulation of inflammatory molecule expression and hepatic injury in response to SF toxicity. Another aim is to study the influence of cyclooxygenase 2 (Cox2), nuclear factor κB (NFκB), signal transducer activator of transcription 3 (STAT-3), and nuclear factor erythroid 2-related factor 2 (Nrf2) protein expressions in SF toxicity and treatments.
Materials and Methods
Chemicals
All chemicals (high analytical grade) were purchased from Sigma and Merck (St. Louis, Missouri, United States); SF, NACC, and TMQ were purchased from Sigma Chemical Co (St Louis, Missouri). Commercial kit for the assay of alanine aminotransferase (ALT) was purchased from Randox (United Kingdom). Primary and secondary antibodies for COX-2, NF-κB, STAT-3, and Nrf2 were purchased from Santa Cruz Biotechnology (California, USA).
Experimental Animals
Fifty Wistar adult male albino rats weighing 170 to 210 g were kept at a temperature of 20°C to 22°C. The rats were procured from the Experimental Animal House, Faculty of Pharmacy, King Saud University, Saudi Arabia. They were fed with standard rat pellet chow with free access to tap water ad libitum. The experimental protocol was approved by the Experimental Animal Ethics Committee at the same University. After 1 week of acclimation, rats were divided into 5 groups, each of 10 rats.
Experimental Design
Fifty rats were allocated into 5 groups, each of 10 rats; the first group was the normal control and was given distilled water, the second group was intoxicated with 10 mg/kg SF24 once daily, the third group was treated with TMQ at a dose of 10 mg/kg/d intraperitoneally,25 the fourth group was treated with 20 mg/kg/d of NACC,26 and the fifth group was treated with TMQ and NACC. All treatments were given daily along with SF for 1 month.
Rats were killed; serum was separated by blood centrifugation at 3000 rpm for 20 minutes. Some livers were homogenized in phosphate buffer to yield 20% homogenates. The homogenates were centrifuged for 20 minutes at 3000 rpm at 5°C and were kept at −80°C. Other parts of the livers were rapidly frozen under liquid nitrogen for Western blotting. Three livers from each group were kept in 4% formalin for histopathological examination.
Biochemical Serum Analysis
Determination of serum content of ALT and aspartate aminotransferase
The content of ALT and aspartate aminotransferase (AST) was determined using the kits obtained from Randox.
Determination of lipid peroxidation (malondialdehyde) in the hepatic tissues
The degree of lipid peroxidation in the liver tissue was determined according to the method of Uchiyama and Mihara.27
Determination of hepatic GSH content
The content of GSH was determined by the method of Ellman.28
Determination of hepatic total nitrite content (nitric oxide)
The total nitrite content was measured according to the method of Moshage et al.29
Determination of superoxide dismutase activity
The activity of superoxide dismutase (SOD) was measured according to the method of Marklund and Marklund.30
Determination of tumor necrosis factor level
The level of tumor necrosis factor α (TNF-α) in the serum was measured using a high-sensitive rat enzyme-linked immunosorbent assay kit (Immuno-Biological Laboratories Co, Ltd, Takasaki-Shi, Gunma, Japan).
Histological Analysis
The liver samples were stored in 4% paraformaldehyde embedded in paraffin wax. Thinly sliced sections were used for the histopathological examination using hematoxylin and eosin (H&E) stain.
Western Blot Analysis
Western blotting was performed to determine the expression of COX-2, NF-κB light-chain enhancer of activated B cells, STAT-3, and Nrf2. The proteins bands were visualized using the ECL-Plus detection system (Amersham Life Sciences, Little Chalfont, Buckinghamshire, United Kingdom), according to the manufacturer’s instruction. Positive immunoreactive bands were quantified densitometrically and compared with that of the control.31 Liver sections were homogenated in lysis buffer (20 mM) 4-(2-Hydroxyethyl)piperazine-1-ethanesulfonic acid, N-(2-Hydroxyethyl)piperazine-N′-(2-ethanesulfonic acid), (HEPES), 2 mM MgCl2, 1 mM EDTA, 1 mM dithiothreitol (DTT), 0.1% sodium dodecyl sulfate, 1 mM phenylmethane sulfonyl fluoride, pH 7.4 on ice. The supernatants were harvested by centrifugation at 12 000g at 4°C for 10 minutes. Protein concentrations were determined by a Bradford assay. Protein (20 mg) was separated on a 12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred to polyvinylidene fluoride membranes.
Statistical Analysis
The data are expressed as mean ± standard error of the mean. Comparisons between different groups were performed by the 1-way analysis of variance, followed by Tukey-Kramer multiple comparisons test. The level of significance was set at P < .05, P < .01, and P < .001. The statistical analyses were conducted using the software GraphPad Prism version 5 (GraphPad Prism, San Diego, California) and SPSS version 21 (IBM).
Results
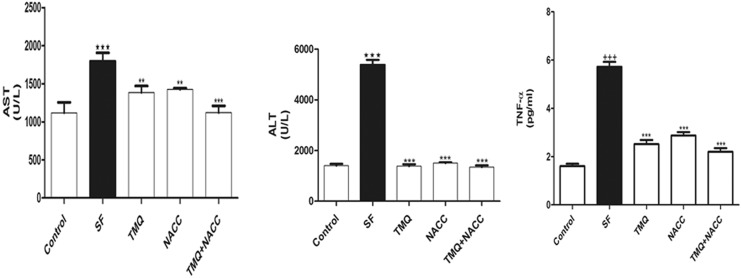
Effect of SF and TMQ or/and NACC on the levels of serum ALT and AST (Figure 1) revealed the effect of SF, TMQ, or NACC and their combination on serum liver function. Sodium fluoride exposure resulted in a significant increase in the activity of ALT and AST. Coadministration of TMQ or/and NACC resulted in a significant decrease in these activities compared to the control group (Figure 1). Also, serum TNF-α was markedly increased upon SF intoxication and ameliorated by the antioxidants in question.
Figure 1.
Liver function enzymes (ALT and AST) and inflammatory marker (TNF-α) in hepatic tissues of rats in control, SF-intoxicated, and all treated groups. Data are presented as mean ± SEM (N = 6). +++ P ≤ .001 versus control and ***P ≤ .001 versus SF-intoxicated group. ALT indicates alanine aspartate; AST, aspartate transferase; TNF-α, tumor necrosis factor α; SEM, standard error of the mean.
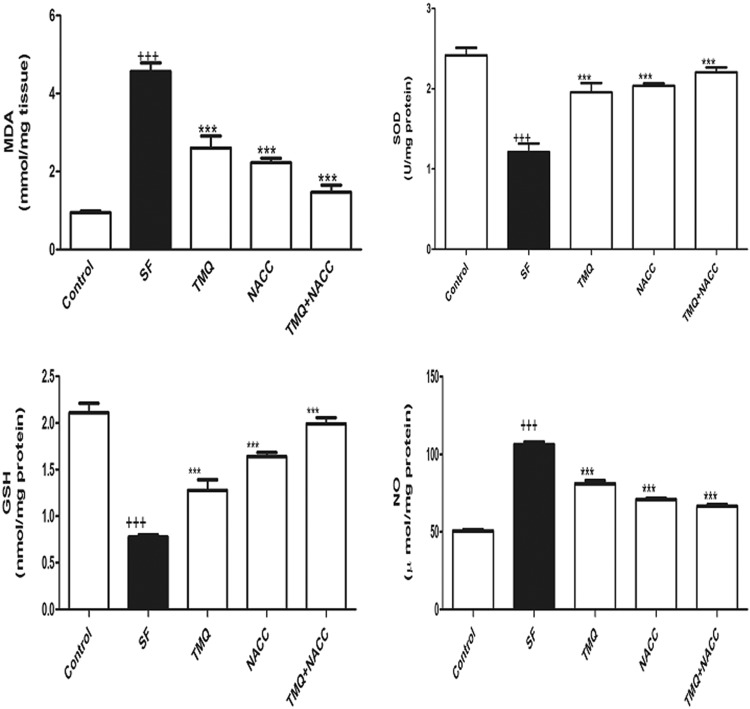
Effect of SF and TMQ or/and NACC treatments on hepatic tissue administration of SF evoked significant increment in hepatic malondialdehyde (MDA; P < 0.001) and nitric oxide (NO) levels compared to the control group. The TMQ and/or NACC administration to SF-intoxicated rats alleviated the enhanced MDA and NO levels in the liver tissue (Figure 2). The activity of SOD and the level of GSH in the liver homogenates were reduced significantly (P < .001) in response to SF treatment. Ingestion of TMQ and/or NACC to SF-intoxicated rats successfully restored their values matched to the control group (Figure 2).
Figure 2.
Oxidative stress and antioxidant biomarkers (MDA, SOD, GSH, and NO) in hepatic tissues of rats in control, SF-intoxicated, and all treated groups. Data are presented as mean ± SEM (N = 6). +++ P ≤ .001 versus control and ***P ≤ .001 versus SF-intoxicated group. GSH indicates glutathione; MDA, malondialdehyde; NO, nitric oxide; SEM, standard error of the mean; SOD, superoxide dismutase.
Western Blot
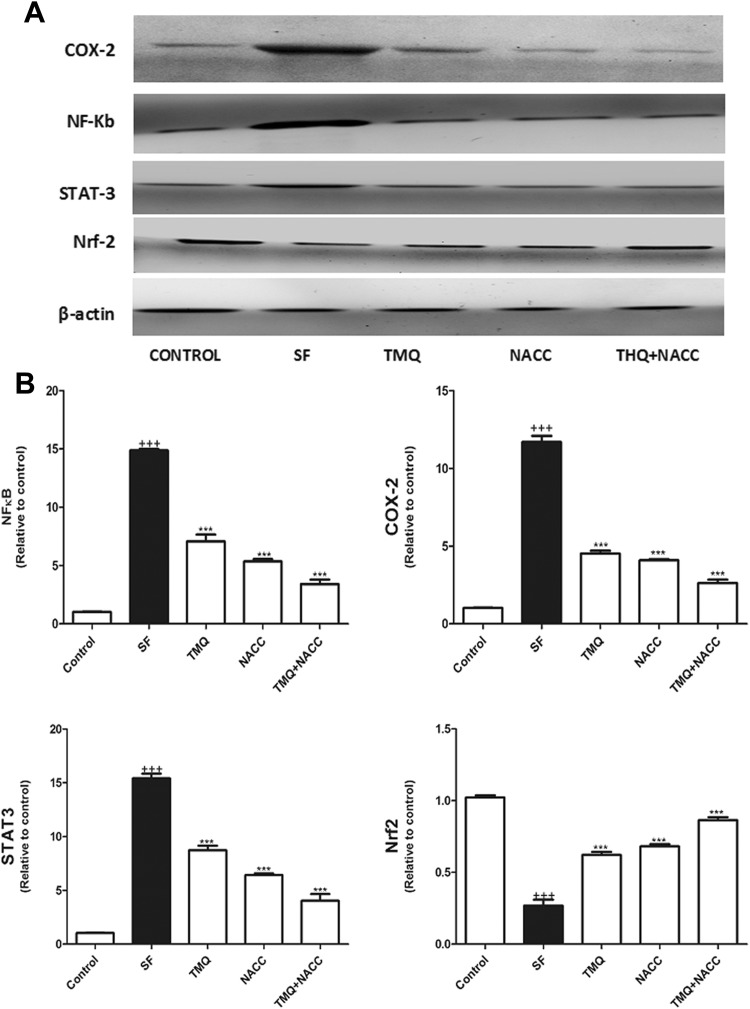
Western blot analysis results indicated that SF administration induced a significant elevation in COX-2, NF-κB, and STAT-3 with concomitant significant depletion in Nrf2 protein expressions in hepatic tissue compared with control (P < .001), while TMQ and/or NACC administration alleviated the activation of COX-2, NF-κB, and STAT-3 expressions and increased the expression of Nrf2 compared with an SF-treated group (P ≤ .001; Figure 3). Of the aforementioned measured parameters, the treatment with the combination of TMQ and NACC was the most effective regimen in ameliorating SF toxicity.
Figure 3.
A, Western blot analysis of the expression of NF-κB, COX-2, STAT3, and NrF2 proteins in control, SF-intoxicated, and all treated groups. B, The densitometry analysis of the expression of NF-κB, COX-2, STAT3, and NrF2 proteins in control, SF-intoxicated, and all treated groups. (Data corrected by β-actin and expressed as protein/β-actin). Data are presented as mean ± SEM (N = 6). +++ P ≤ .001 versus control and ***P ≤ .001 versus SF-intoxicated group. COX-2 indicates cyclooxygenase-2; NAC, N-acetylcysteine; NF-κB, nuclear factor-κB; NrF2, nuclear factor erythroid 2-related factor 2; SEM, standard error of the mean; SF, sodium fluoride; STAT-3, signal transducer and activator of transcription 3; TMQ, thymoquinone.
Histological Examination
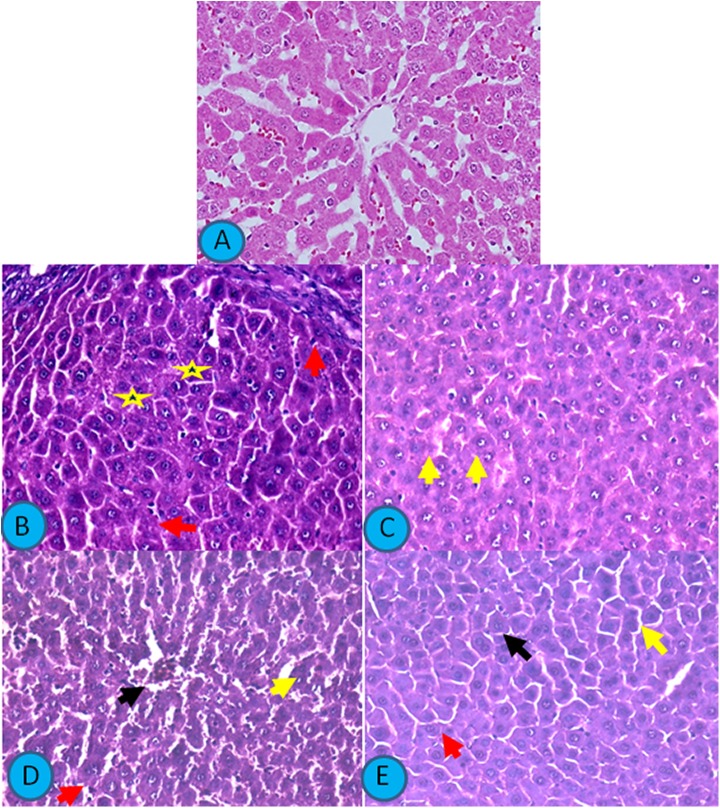
Figure 4 presents light photomicrographs of H&E-stained sections of the liver. Liver of control rat revealed normal hepatic architecture, normal hepatocytes, and blood sinusoids. Sodium fluoride treatment caused focal areas of massive hepatic degeneration and many degenerated hepatocytes, while TMQ administration caused mild improvement in the hepatic changes with many degenerated cells. Liver from rat receiving NACC also showed moderate improvement in hepatic cellular degeneration with many abnormal cells, whereas liver from rat receiving TMQ and NACC showed the marked improvement in the hepatic cellular degeneration with apparently normal hepatic architecture with very few degenerated hepatocytes.
Figure 4.
Light photomicrographs of H&E-stained sections of the liver. Scale bar 400 µm. A, Liver from control rat showing normal hepatic architecture with normal hepatocytes and blood sinusoids. B, Liver from rat receiving SF showing focal areas of massive hepatic degeneration (yellow star) and many degenerated hepatocytes (red arrows). C, Liver from rat receiving thymoquinone showing mild improvement in the hepatic changes with many degenerated cells (yellow arrow). D, Liver from rat receiving N-acetylcysteine showing moderate improvement in hepatic cellular degeneration with many abnormal cells (arrows). E, Liver from rat receiving thymoquinone and N-acetylcysteine showing marked improvement in the hepatic cellular degeneration with apparently normal hepatic architecture with very few degenerated hepatocytes (arrows). H&E indicates hematoxylin and eosin; SF, sodium fluoride.
Discussion
Fluoride, one of the most dangerous environmental pollutants, disrupts metabolic pathways at toxic doses.32 Its effect on many organs, such as liver, pancreas, lungs, heart, skeletal muscles, and kidney, is well established.33–36 In the present study, SF increased the activity of serum ALT and AST and the level of TNF-α, hepatic MDA, and NO with contaminant decrease in GSH level and SOD activity. Liver dysfunction after SF administration is due to increased OS, leading to liver injury. Abnormal levels of some serum biomarkers, such as ALT and AST, are attributed to the destruction of the membrane structure of the hepatic cells, leading to a noticeable increase in ALT activity.37 Lipid peroxidation is the essential index of OS, and MDA is the end product of lipid peroxidation and reflects the degree of lipid peroxidation.11
Glutathione is an antioxidant toward ROS and is considered as a primary indicator of the OS.35 Chen et al38 and Liu et al39 reported that in broiler chickens, high dietary fluoride increases the MDA content in their serum. The present data showed that the activity of SOD and GSH level were significantly decreased in the liver after SF treatment. This finding designated that SF can induce the high generation of ROS and reactive nitrogen species. Herein, NO level was significantly increased in the liver after treatment with SF compared with the control group. The analogous observation was gained by Zhou et al40 and Liu et al39 who reported that fluoride-induced OS is involved in the morphological damage and dysfunction of the liver in female mice and elevated serum NO levels in chicks, respectively.
The elevated NO level induced by fluoride could disrupt protein functions, affect energy metabolism, reduce adenosine triphosphate and NADPH, and cause DNA damage and react with superoxide anions (O2 −), to produce peroxynitrite (ONOO–), which is responsible for oxidation and destruction and hence cytotoxicity.41,42 This anion is capable to diffuse across plasma cell. Based on the presence of iron, thiols, and SOD, peroxynitrite undergoes 3 types of reactions, leading to depletion of thiols radical, chain peroxidation, and nitrosylation of proteins.43
The bioactivities of TNF-α are mediated through the activation of the NF-κB pathway.44 The crucial role of TNF-α in many diseases is that the mediation of organs injury is well-documented.45,46
Herein, SF induced a significant rise in hepatic NF-κB, COX-2, and STAT-3, while Nrf2expression was decreased; this is in agreement with the findings of Afolabi et al,47 Gutowska et al,48 Jain et al,49 and Mukhopadhyay et al,50 who declared that fluoride affected the previous parameters in the same manner.
Cyclooxygenase expression is linked with inflammation51 and is released by a variety of pro-inflammatory stimulus.52,53 It was documented that Nrf2 is the key manager of all cellular oxidation–antioxidation system at the transcriptional level.54
From the current investigation, it can be concluded that the consumption of TMQ or/and NACC to SF-intoxicated rats successfully restored ALT and AST activities; SOD, MDA, GSH, and NO levels; and COX-2, NF-κB, and STAT3 protein expressions back to control values. This positive effect of either TMQ or/and NACC was formally recognized in different models of hepatotoxicity.16,55
The STAT3 protein expression is one of the major members of the unit of signal transducers and activators of transcription that functions by adaptable cell growth, differentiation, and angiogenesis and participates in the pathogenesis of diabetic retinopathy.56 It is the first time to evaluate the effect of SF on STAT3.
Histological examination reported that SF treatment caused focal areas of massive hepatic degeneration and many degenerated hepatocytes, while TMQ administration caused mild improvement in the hepatic changes with many degenerated cells. Liver from rat receiving NACC also showed moderate improvement in hepatic cellular degeneration with many abnormal cells. Liver from rat receiving the combination of TMQ and NACC showed marked improvement in the hepatic cellular degeneration with apparently normal hepatic architecture with very few degenerated hepatocytes.
The results of the present study revealed that the combination of TMQ and NACC is the most effective regimen in the amelioration of SF toxicity. The hepatoprotective effects of NACC and TMQ against the toxicity of fluoride compounds might be mediated via multiple molecular mechanisms and can be considered a promising candidate against hepatic damage induced by SF toxicity.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by a grant from the Research Center of the Female Scientific and Medical Colleges, Deanship of Scientific Research, King Saud University, Saudi Arabia.
ORCID iD: Hanaa M. Ali  http://orcid.org/0000-0002-6870-7585
http://orcid.org/0000-0002-6870-7585
References
- 1. Machalinski B, Zejmo M, Stecewicz I, Machalinska A, Machoy Z, Ratajczak MZ. The influence of sodium fluoride on the clonogenicity of human hematopoietic progenitor cells: preliminary report. Fluoride. 2000;33(4):168–173. [Google Scholar]
- 2. Pain GN. Fluoride is a developmental nephrotoxin—coming to a kidney near you. 2017. doi:10.13140/RG.2.2.10999.62884. [Google Scholar]
- 3. Kant V, Verma PK, Pankaj NK, Raina R. Experimental osteo-fluorosis in goats of Jammu and Kashmir. Feedback. 2009. b;4(2):45. [Google Scholar]
- 4. Kant V, Verma PK, Pankaj NK, et al. Haematological profile of subacute oral toxicity of fluoride and ameliorative efficacy of aluminium sulphate in goats. Toxicol Int. 2009. a;16(1):31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Meena C, Toteja GS, Bala K, Mohanty SS. Assessment of non-skeletal fluorosis in children of Jaipur district of Rajasthan. India Int J Sci Res. 2017;5(12):3–5. [Google Scholar]
- 6. Pereira AG, Chiba FY, de Lima Coutinho Mattera MS, et al. Effects of fluoride on insulin signaling and bone metabolism in ovariectomized rats. J Trace Elem Med Biol. 2017;39:140–146. [DOI] [PubMed] [Google Scholar]
- 7. McCune D, Weinstein L. Effects of fluorides, air pollution and plant life. 2002;163–250. [Google Scholar]
- 8. Sharma J, Solanki M, Solanki D. Sodium fluoride toxicity on reproductive organs of female albino rats. Asian J Exp Sci. 2007;21(2):359–364. [Google Scholar]
- 9. Moemeni H, Moallem M, Parsian H, et al. A comparison between serum lead status in elderly osteopenic and osteoporotic patients versus healthy control. West Indian Med J. 2015. doi:10.7727/wimj.2015;103. [Google Scholar]
- 10. Blaszczyk I, Birkner E, Kasperczyk S. Influence of methionine on the toxicity of fluoride in the liver of rats. Biol Trace Elem Res. 2011;139(3):325–331. [DOI] [PubMed] [Google Scholar]
- 11. Nabavi SM, Nabavi SF, Eslami S, Moghaddam AH. In vivo protective effects of quercetin against sodium fluoride-induced oxidative stress in the hepatic tissue. Food Chem. 2012;132:931–935. [Google Scholar]
- 12. Hordyjewska A, Pasternak K. Influence of fluoride on the organism of human. J Elementol. 2004;9(4):883–887. [Google Scholar]
- 13. Wang AG, Xia T, Chu QL, et al. Effect of fluoride on lipid peroxidation, DNA damage and apoptosis in human embryo hepatocytes. Biomed Environ Sci. 2004;17(2):217–222. [PubMed] [Google Scholar]
- 14. Thangapandiyan S, Miltonprabu S. Molecular mechanism of fluoride induced oxidative stress and its possible reversal by chelation therapy. Res Rev J Toxicol. 2013;3(2):1–11. [Google Scholar]
- 15. Reichenberger F, Tamm M. N-acetylcysteine in the therapy of chronic bronchitis. Pneumologie. 2002;56(12):793–797. [DOI] [PubMed] [Google Scholar]
- 16. Abdel-Wahab WM. Protective effect of thymoquinone on sodium fluoride-induced hepatotoxicity and oxidative stress in rats. J Basic Appl Zool. 2013;66(5):263–270. [Google Scholar]
- 17. Ali BH, Blunden G. Pharmacological and toxicological properties of Nigella sativa. Phytother Res. 2003;17(4):299–295. [DOI] [PubMed] [Google Scholar]
- 18. Houghton PJ, Zarka R, Delasheras B, Hoult JR. Fixed oil of Nigella sativa and derived thymoquinone inhibit eicosanoid generation in leukocytes and membrane lipid peroxidation. Planta Med. 1995;61(1):33–36. [DOI] [PubMed] [Google Scholar]
- 19. Norwood AA, Tan M, May M, Tucci M, Benghuzzi H. Comparison of potential chemotherapeutic agents, 5-fluoruracil, green tea, and thymoquinone on colon cancer cells. Biomed Sci Instrum. 2006;42:350–356. [PubMed] [Google Scholar]
- 20. Wilson-Simpson F, Vance S, Benghuzzi H. Physiological responses of ES-2 ovarian cell line following administration of epigallocatechin-3-gallate (EGCG), thymoquinone (TQ), and selenium (SE). Biomed Sci Instrum. 2007;43:378–383. [PubMed] [Google Scholar]
- 21. Nagi MN, Alam K, Badary OA, Al-Sawaf HA, Al-Bekairy AM. Thymoquinone protects against carbon tetrachloride hepatotoxicity in mice via antioxidant mechanism. Biochem Mol Biol Int. 1999;47(1):15–19. [DOI] [PubMed] [Google Scholar]
- 22. Nagi MN, Mansour MA. Protective effect of thymoquinone against doxorubicin-induced cardiotoxicity in rats: a possible mechanism of protection. Pharmacol Res. 2000;41(3):283–289. [DOI] [PubMed] [Google Scholar]
- 23. Badary OA, Taha RA, Gamal el-Din AM, Abdel-Wahab MH. Thymoquinone is a potent superoxide anion scavenger. Drug Chem Toxicol. 2003;26(2):87–98. [DOI] [PubMed] [Google Scholar]
- 24. Abbas M, Siddiqi MH, Khan K, Zahra K, Naqvi AU. Hematological evaluation of sodium fluoride toxicity in Oryctolagus cuniculus. Toxicol Rep. 2017;4:450–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hosseini SM, Taghiabadi E, Abnous K, Hariri AT, Pourbakhsh H, Hosseinzadeh H. Protective effect of thymoquinone, the active constituent of Nigella sativa fixed oil, against ethanol toxicity in rats. Iran J Basic Med Sci. 2017;20(8):927–939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Akgun E, Caliskan C, Celik HA, Ozutemiz AO, Tuncyurek M, Aydin HH. Effects of N-acetylcysteine treatment on oxidative stress in acetic acid-induced experimental colitis in rats. J Int Med Res. 2005;33(2):196–206. [DOI] [PubMed] [Google Scholar]
- 27. Uchiyama M, Mihara M. Determination of malondialdehyde precursor in tissues by thiobarbituric acid test. Anal Biochem. 1978;86(1):271–278. [DOI] [PubMed] [Google Scholar]
- 28. Ellman GL. Tissue sulfhydryl group. Arch Biochem Biophys. 1959;82(1):70–77. [DOI] [PubMed] [Google Scholar]
- 29. Moshage H, Kok B, Huizenga JR, Jansen PL. Nitrite and nitrate determinations in plasma: a critical evaluation. Clin Chem. 1995;41(6):892–896. [PubMed] [Google Scholar]
- 30. Marklund S, Marklund G. Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur J Biochem. 1974;47:469–474. [DOI] [PubMed] [Google Scholar]
- 31. Jackson L, Wu K, Mahida Y, Jenkins D, Hawkey C. Cyclooxygenase (COX) 1 and 2 in normal, inflamed, and ulcerated human gastric mucosa. Gut. 2000;47(6):762–770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Azmat R, Talat R, Ahmed K. The length–weight relationship: condition factor and impact of fluoride concentration in Johnius belangerii of Arabian Sea. Res J Environ Toxicol. 2007;1(3):138–143. [Google Scholar]
- 33. Kołodziejczyk L, Put A, Grzela P. Liver morphology and histochemistry in rats resulting from ingestion of sodium selenite and sodium fluoride. Fluoride. 2000;33(1):6–16. [Google Scholar]
- 34. Chinoy NJ. Fluoride stress on antioxidant defense systems. Fluoride. 2003;36(3):138–141. [Google Scholar]
- 35. Sinha M, Manna P, Sil PC. Terminalia arjuna protects mouse hearts against sodium fluoride-induced oxidative stress. J Med Food. 2008;11(4):733–740. [DOI] [PubMed] [Google Scholar]
- 36. Stawiarska-Pieta B, Paszczela A, Grucka-Mamczar E, Szaflarska-Stojko E, Birkner E. The effect of antioxidative vitamins A and E and coenzyme Q on the morphological picture of the lungs and pancreata of rats intoxicated with sodium fluoride. Food Chem Toxicol. 2009;47(10):2544–2550. [DOI] [PubMed] [Google Scholar]
- 37. Borlak J, Chougule A, Singh PK. How useful are clinical liver function tests in vitro human hepatotoxicity assays? Toxicol In Vitro. 2014;28(5):784–795. [DOI] [PubMed] [Google Scholar]
- 38. Chen T, Cui HM, Cui Y, Bai CM, Gong T. Decreased antioxidase activities and oxidative stress in the spleen of chickens fed on high-fluorine diets [Abstract]. Hum Exp Toxicol. 2011;30(9):1282–1286. [DOI] [PubMed] [Google Scholar]
- 39. Liu G, Chai C, Cui L. Fluoride causing abnormally elevated serum nitric oxide levels in chicks. Environ Toxicol Pharmacol. 2003;13(3):199–204. [DOI] [PubMed] [Google Scholar]
- 40. Zhou BH, Zhao J, Liu J, Zhang JL, Li J, Wang W. Fluoride-induced oxidative stress is involved in the morphological damage and dysfunction of liver in female mice. Chemosphere. 2015;139:504–511. [DOI] [PubMed] [Google Scholar]
- 41. Gross SS, Wolin MS. Nitric oxide: pathophysiological mechanisms. Annu Rev Physiol. 1995;57:737–769. [DOI] [PubMed] [Google Scholar]
- 42. Luperchio S, Tamir S, Tannenbaum SR. NO-induced oxidative stress and glutathione metabolism in rodent and human cells. Free Radic Biol Med. 1996;21(4):513–519. [DOI] [PubMed] [Google Scholar]
- 43. Fredstrom S. Nitric oxide, oxidative stress, and dietary antioxidants. Nutrition. 2002;18(6):537–539. [DOI] [PubMed] [Google Scholar]
- 44. Baker SJ, Reddy EP. Modulation of life and death by the TNF receptor superfamily. Oncogene. 1998;17(25):3261–3270. [DOI] [PubMed] [Google Scholar]
- 45. del Zoppo GJ. Microvascular changes during cerebral ischemia and reperfusion. Cerebrovasc Brain Metab Rev. 1994;6(1):47–96. [PubMed] [Google Scholar]
- 46. Feuerstein GZ, Liu T, Barone FC. Cytokines, inflammation, and brain injury: the role of tumor necrosis factor-alpha. Cerebrovasc Brain Metab Rev. 1994;6(4):341–360. [PubMed] [Google Scholar]
- 47. Afolabi OK, Oyewo EB, Adekunle AS, Adedosu OT, Adedeji AL. Oxidative indices correlate with dyslipidemia and pro-inflammatory cytokine levels in fluoride-exposed rats. Arh Hig Rada Toksikol. 2013;64(4):521–529. [DOI] [PubMed] [Google Scholar]
- 48. Gutowska I, Baranowska-Bosiacka I, Goschorska M, et al. Fluoride as a factor initiating and potentiating inflammation in THP1 differentiated monocytes/macrophages. Toxicol In Vitro. 2015;29(7):1661–1668. [DOI] [PubMed] [Google Scholar]
- 49. Jain A, Mehta VK, Chittora R, Mahdi AA, Bhatnagar M. Melatonin ameliorates fluoride induced neurotoxicity in young rats: an in vivo evidence. Asian J Pharm Clin Res. 2015;8(4):164–167. [Google Scholar]
- 50. Mukhopadhyay D, Priya P, Chattopadhyay A. Sodium fluoride affects zebrafish behaviour and alters mRNA expressions of biomarker genes in the brain: role of Nrf2/Keap1. Environ Toxicol Pharmacol. 2015;40(2):352–359. [DOI] [PubMed] [Google Scholar]
- 51. Park S, Ajtai K, Burghardt P. Inhibition of myosin ATPase by metal fluoride complexes. Biochem Biophys Acta. 1999;1430(1):127–140. [DOI] [PubMed] [Google Scholar]
- 52. Lessner SM, Prado HL, Waller EK, Galis ZS. Atherosclerotic lesions grow through recruitment and proliferation of circulating monocytes in a murine model. Am J Pathol. 2002;160(6):2145–2155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Loftin CD, Tiano HF, Langenbach R. Phenotypes of the COX-deficient mice indicate physiological and pathophysiological roles for COX-1 and COX-2. Prostaglandins Other Lipid Mediat. 2002;68-69:177–185. [DOI] [PubMed] [Google Scholar]
- 54. Lee JM, Johnson JA. An important role of Nrf2-ARE pathway in the cellular deference mechanism. J Biochem Mol Biol. 2004;37(2):139–143. [DOI] [PubMed] [Google Scholar]
- 55. Pawłowska-Góral K, Kurzeja E, Stec M. N-acetylcysteine protects against fluoride-induced oxidative damage in primary rat hepatocytes. Toxicol In Vitro. 2013;27(8):2279–2282. [DOI] [PubMed] [Google Scholar]
- 56. Zhong Z, Wen Z, Darnell JE., Jr Stat3: a STAT family member activated by tyrosine phosphorylation in response to epidermal growth factor and interleukin-6. Science. 1994;264(5155):95–98. [DOI] [PubMed] [Google Scholar]