 High pressure state-of-the-art synchrotron XRD in black-phosphorus has solved apparent contradictions about the stability of the A7 layered structure in pnictogens, highlighting the importance of the s–p orbital mixing in the formation of the p-sc structure.
High pressure state-of-the-art synchrotron XRD in black-phosphorus has solved apparent contradictions about the stability of the A7 layered structure in pnictogens, highlighting the importance of the s–p orbital mixing in the formation of the p-sc structure.
Abstract
Black phosphorus was studied by state-of-the-art synchrotron X-ray diffraction in a Diamond Anvil Cell during room temperature compression in the presence of He, H2, N2 and Daphne Oil 7474. The data demonstrate that the existence of the pseudo simple-cubic (p-sc) structure above 10.5 GPa is an intrinsic feature of P independent from the pressure transmitting medium. In the case of He, the pressure evolution of the lattice parameters and unit cell volume of P across the A17, A7 and p-sc structures was obtained and the corresponding EOS derived, providing a deeper insight on the recently reported p-sc structure. The results presented in this letter highlight the key role of the s–p orbital mixing in the formation and stabilization of the p-sc structure up to ∼30 GPa, solving apparent contradictions emerging from previous literature and finally bringing order to the sequence of the high pressure A7 layered structure in group 15 elements.
The sequence of the high pressure structures of phosphorus at room T represents a striking anomaly in comparison to the other group 15 elements having higher atomic number. P is indeed the only element of the group exhibiting the A17 orthorhombic layered structure (Cmce, Z = 8),1,2 commonly known as black phosphorus (bP), which is actually the thermodynamically stable allotrope of the element at ambient conditions and currently represents the starting material for the synthesis of phosphorene3,4 (ESI1†). In contrast, As, Sb and Bi crystallize in rhombohedral A7 (R3[combining macron]m, Z = 2), another layered structure adopted by P only above ∼5 GPa, which can be described in this case by the stacking of blue phosphorene layers.5 Furthermore, whereas the high pressure limit of the A7 structure decreases in the group with increasing atomic number, according to current literature its pressure value in P (11 GPa) is located below that of As (25 GPa).6,7 In both A17 and A7 layered structures each P atom hosts an electron lone pair and is three-coordinated to atoms belonging to the same layer, with three longer interatomic distances with respect to atoms in the adjacent layers.
Above 10.5 GPa the layered A7 structure is reported to transform to a metallic non-layered simple-cubic structure (sc, Pm3[combining macron]m, Z = 1) stable up to 103 GPa, in which P is hexa-coordinated with six equivalent interatomic distances8 (ESI2†).
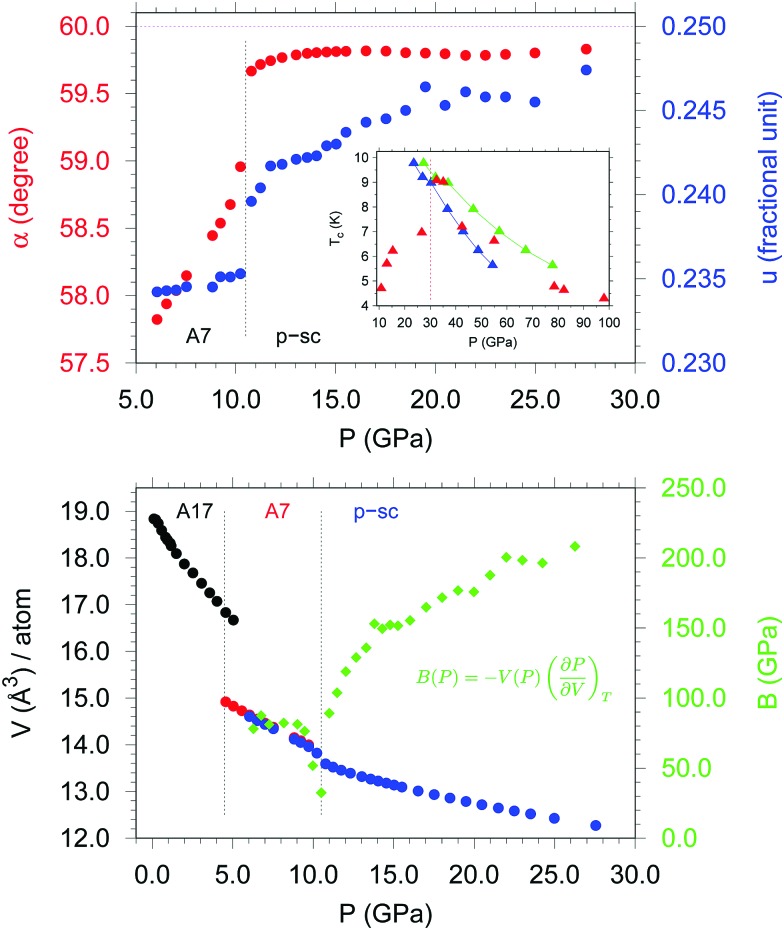
Recently, a synchrotron X-ray diffraction (XRD) study has shed new light on the phase diagram of P, demonstrating the presence of a two-step mechanism for the A7 to sc transition and unveiling the existence of an intermediate pseudo simple-cubic (p-sc) structure in the pressure range extending from 10.5 GPa up to at least ∼30 GPa9 (ESI3†). Indeed, adopting a rhombohedral cell description (R3[combining macron]m) for both A7 and sc structures, whereas above 10.5 GPa the angle α rapidly approaches the ∼60° limit value characteristic of the sc structure, at ∼30 GPa the atomic position u is still far from the 0.250 value expected in the sc structure, thus causing a metric pseudo-symmetry9 (Fig. 3, upper panel and Fig. 4 in ref. 9). The existence of the p-sc structure, originating from a pressure dependent competition between the s–p mixing and the electrostatic contribution, has two remarkable implications. First, from a chemical point of view, the presence of three shorter and three longer interlayer distances, in contrast with the six equivalent ones expected in the sc structure, structurally relates p-sc to A7, thus significantly raising the pressure limit where the layered phases of P exist. Second, the observation of the p-sc structure has provided new experimental evidence to explain the long-debated anomalous pressure evolution of the superconduting critical temperature Tc in P below 30 GPa.10–12 Experimental data indicate a maximum of Tc at ∼30 GPa. Nevertheless, whereas calculations assuming a sc structure satisfactorily reproduce the pressure evolution of Tc above this limit, they catastrophically fail at lower pressure. The existence of the p-sc structure in this pressure range, responsible for a different electron–phonon coupling with respect to the sc structure, provides new evidence to solve this issue (inset in Fig. 3, upper panel).
Fig. 3. Upper panel: Room T pressure evolution of the angle α (red) and atomic position u (blue) of the rhombohedral cell (R3[combining macron]m) in the A7 and p-sc structures of P, with the magenta dotted line indicating their limit values in the sc structure (respectively 60 and 0.250) and the black dotted line marking the formation of p-sc at 10.5 GPa. The inset, adapted from ref. 22, shows the anomalous pressure evolution of experimental Tc (red triangles),12 exhibiting a maximum at ∼30 GPa, in contrast to calculations assuming a sc structure (blue and green triangles),22 with the dotted line marking the room T pressure limit up to which p-sc has been instead demonstrated to exist.9 Lower panel: Room T pressure evolution of the atomic volume (left y axis) of P across the A17 (black), A7 (red and blue) and p-sc (blue) structures and of the bulk modulus (right y axis) across the A7 and p-sc structures (green). The blue solid circles refer to the data obtained by Rietveld refinement in the A7 and p-sc structures. The minimum in the pressure evolution of the bulk modulus at 10.5 GPa, corresponding to the onset of the A7 to p-sc transformation,9 highlights the characteristic anomalous behavior reported for first order structural phase transitions.20,21 .
The results presented in this communication unveil a third fundamental implication, which reconciles the chemical and structural high pressure behavior of P with those of heavier elements in group 15 of the periodic table. The results allow us to correlate the p-sc structure to the expected sequence of the high pressure limit for the A7 structure in group 15 elements as a function of the atomic number, solving a long-debated inconsistency within the group, which has fundamental relevance in structural chemistry6,7 and providing a deeper insight on the recently reported p-sc structure.
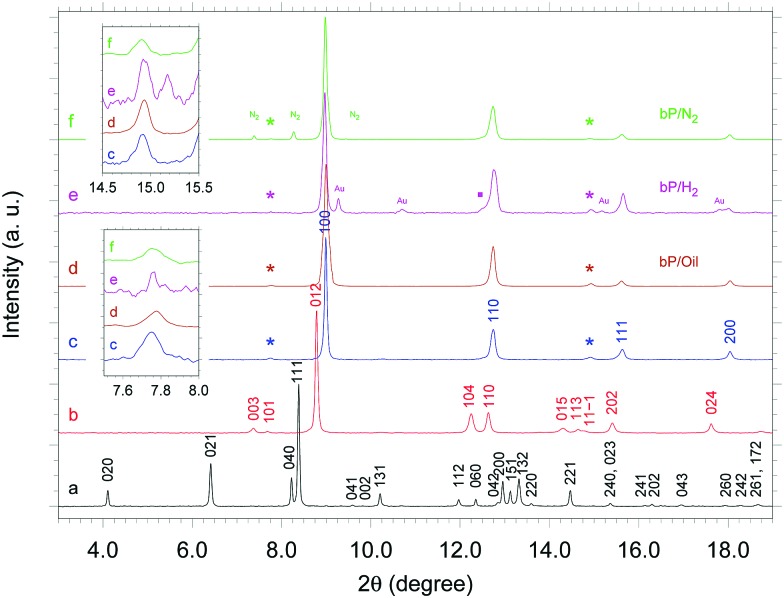
Synchrotron XRD patterns were acquired during room T compression and decompression of bP in the presence of He, H2, N2 and Daphne Oil 7474 (ESI4†). For each sample compression was started at pressure lower than 1 GPa, where P is in the A17 phase and all the investigated pressure transmitting media (PTMs) are fluid. Pressure was then slowly increased in steps of a few tenths of GPa up to 27.6 GPa, waiting for stabilization before further change. XRD patterns were acquired at every pressure value and information on the pressure evolution of the unit cell volume and on the lattice parameters across the A17 to A7 and the A7 to sc phase transitions was obtained. Representative XRD diffraction patterns at ∼11 GPa, in the presence of the different PTMs, are shown in Fig. 1. According to literature the A7 to sc phase transition, reported to occur sharply, should be already completed at this pressure. Nevertheless, all the patterns clearly show the presence of the two extra peaks characteristic of the recently discovered p-sc structure,9 indicating that its formation is related neither to the application of hydrostatic/non-hydrostatic conditions nor to the solidification of the PTM (He = 11.5 GPa,13 H2 = 5.4 GPa,14 N2 = 2.4 GPa,15 Daphne Oil 7474 = 3.7 GPa16). The presented data unambiguously demonstrate that the p-sc structure, recently observed using He as PTM,9 is an intrinsic feature of the element.
Fig. 1. Room T XRD patterns of bP in the presence of He at 0.2 GPa (A17, black a trace), at 6.5 GPa (A7, red b trace) and at 11.2 GPa (p-sc, blue c trace) and in the presence of Daphne Oil 7474 (p-sc, dark-orange d trace), H2 (p-sc, magenta e trace) and N2 (p-sc, green f trace). The asterisks mark the two characteristic extra reflections which identify the p-sc structure9 and are not expected in the sc one. The two insets show a zoom of the XRD patterns in the 2θ ranges corresponding to the two extra reflections. Peak indexing of the A17, A7 and p-sc structure is also shown.9 The peaks labeled by Au and N2, respectively in the bP/H2 and bP/N2 samples, are due to the gold ring and to crystalline N2. The weak peak marked by a solid square and the slight asymmetry on the peak at 2θ ∼ 14.9 degree in the diffraction pattern of the bP/H2 sample are due to persisting A7 domains disappearing on further compression.
The use of He as hydrostatic PTM and the high resolution of our pressure sampling allowed us to acquire data of significantly improved quality and precision compared to existing literature.8,17,18 Rietveld refinement of the XRD data (ESI5†) allowed to obtain the pressure evolutions of the lattice parameters (Fig. S1, ESI†) and of the unit cell volume. In agreement with previous studies, our data indicate a larger decrease with pressure of the interlayer distances both in the A17 and A7 structure, compared to the other directions. Interestingly, this is the direction along which the electron lone pairs, originating from the s–p orbital mixing, point towards each other. Their perturbation is responsible for the A17 to A7 phase transition, where interlayer bond reconstruction occurs with electron lone pair redistribution, leading to layers of different conformation for a more efficient packing, as described in the mechanism proposed by Boulfelfel et al.19 Also in the A7 structure the electron lone pairs point to the interlayer spacing and are thus preferentially perturbed during compression. With increasing pressure, the reduction of the interlayer spacing further perturbs the electron lone pairs up to induce interlayer bond formation. The key role of the s–p orbital mixing in stabilizing the presence of electron lone pairs at tetrahedral P sites versus the sc octahedral coordination is further highlighted by the pressure delay of the P atoms in assuming the positions expected in the sc structure,9 which is responsible for the lattice distortion observed in the p-sc structure.
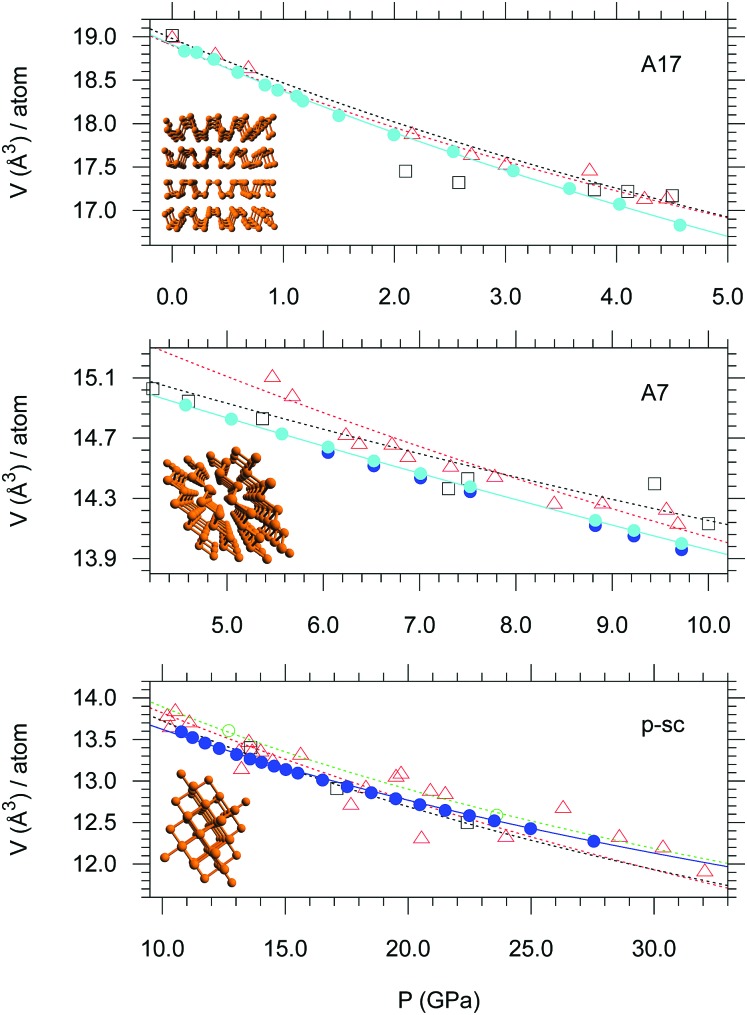
The pressure evolution of the atomic volume in the different structures was then fitted using a Vinet EOS to obtain the atomic volume at ambient pressure V0, the bulk modulus B0 and its first derivative B′ for the A17, A7 and p-sc structures. These fits are shown in Fig. 2 and the obtained fit parameters are listed in Table S2, ESI† where previous literature data referring to the sc structure are also shown for comparison (ESI6†). Our V0, B0 and B′ values are in very good agreement with literature ones8,17,18 for the A17 and A7 structures. Nevertheless, the fit of our data for the p-sc structure provides significantly different values of B0 (31.5 ± 6.4) and B′ (9.7 ± 0.7) compared to those reported in literature for the sc structure, which are respectively higher and lower. Furthermore, our V0 for the p-sc structure is slightly higher than literature values for the sc one and almost identical to those for the A7 one within the experimental error. This apparently contrasting result for the p-sc structure is however not surprising, because our values show the highest precision and refer exactly to the p-sc structure in the pressure range of its existence (ESI6†).
Fig. 2. EOS of bP in the A17, A7 and p-sc pressure ranges. The solid circles (cyan and blue) represent the data acquired in this study during the compression of bP using He as PTM. The blue points were obtained by Rietveld refinement of the XRD data. Empty symbols refer to the data reported by Kikegawa et al.17 (red triangles), Clark et al.18 (black squares) and Akahama et al.8 (green circles). The corresponding EOS type and fit parameters are indicated in Table S1 (ESI†). A drawing of each structure, obtained by a cif file in the corresponding pressure range, is also shown.
To obtain further insight on the formation of the p-sc structure, the pressure evolution of the atomic volume was used to calculate the pressure evolution of the bulk modulus B. This evolution is known to exhibit an overall increase of the bulk modulus with pressure, with a characteristic anomalous softening of the bulk modulus in the low symmetry phase for first order structural phase transitions20,21 (Fig. 3, lower panel). Even if both A7 and p-sc structures are described by a rhombohedral cell and no high or low symmetry phase can be strictly identified, the p-sc one is certainly more similar to the high symmetry sc structure. This observation provides conclusive evidence of the first order character of the A7 to p-sc structural transformation (ESI in ref. 9).
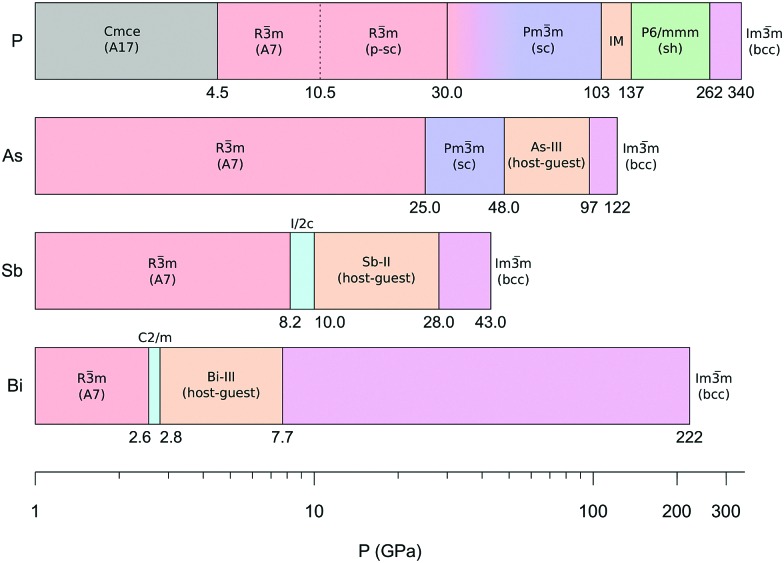
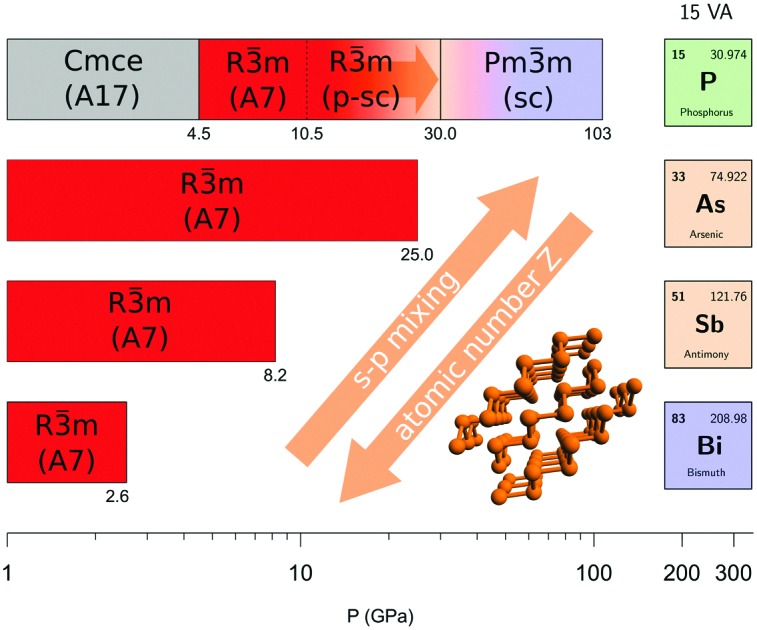
The high pressure structures in group 15 elements have been thoroughly studied due to the appearance of characteristic occurrences, like the rarely observed sc structure in P and As and the presence of incommensurate host–guest structures in the heavier elements.7 However, among the group peculiarities, two of them, both involving P, evidently stand out. The first one is related to the existence of the A17 structure. Indeed, with the exception of N, all the heavier pnictogens (P, As, Sb and Bi) exhibit crystalline layered structures at ambient conditions. Nevertheless, whereas the thermodynamically stable structure of P at ambient conditions is orthorhombic A17, As, Sb and Bi crystallize at ambient conditions adopting the rhombohedral A7 structure,6,7 which appears in P only above 4.8 GPa (Fig. 4). Structurally, A17 in P represents an isolated exception in group 15 elements. The second one concerns the high pressure limit for the A7 structure (R3[combining macron]m, Z = 2), which in P, As, Sb and Bi is the last high pressure structure showing evidence of layers. Whereas in As (25 GPa), Sb (8.2 GPa) and Bi (2.55 GPa) this pressure limit decreases with increasing atomic number Z, according to literature the A7 structure in P, appearing at ∼5 GPa, only extends up to 10.5 GPa, apparently contrasting the trend of the other elements (see dotted line in Fig. 4).
Fig. 4. High pressure phases of group 15 elements. Data were taken from literature ref. 7 and references therein, except for the A17, A7 and p-sc structures of P.9 The dotted line in P indicates the A7 to sc transition pressure previously reported in literature. The observation of the p-sc structure up to at least 30 GPa extends the existence of a rhombohedral unit cell (R3[combining macron]m) above the pressure limit where the same cell is reported in As. For all the elements the high pressure limit of the bcc structures (Im3[combining macron]m) represents the highest experimental pressure at which this structure has been observed.
The observation of the p-sc structure and the identification of the effects responsible for its formation provide the key to interpret and reconsider these two anomalies. As emphasized by adopting the same rhombohedral cell description (R3[combining macron]m), the p-sc structure is indeed structurally related to A7, featuring three shorter and three longer interatomic distances, thus extending the high pressure limit for the layered structures of P up to at least 30 GPa. This experimental observation places this limit for an A7-like structure in P above the corresponding A7 limit for As, bringing order to the sequence of the A7 high pressure limits in group 15 elements, which now decreases down in the group with increasing atomic number (Fig. 4). From a chemical point of view, this occurrence consistently relates to the interpretation provided for the existence of the p-sc structure in P as a consequence of the stronger s–p orbital mixing compared to higher Z elements. The extent of the s–p mixing in P is indeed such as to stabilize the A17 structure at ambient conditions, as explained by Seo and Hoffmann,23 and decreases in the group with increasing atomic number, mirroring the A7 high pressure limit.
In summary, high quality XRD data about the A17, A7 and p-sc structures of bP, acquired up to ∼30 GPa using different PTMs, are presented in this study, demonstrating that the p-sc structure is an intrinsic feature of the element and that a first order transition characterizes its formation. The results correlate the p-sc structure in P to the A7 structure in As, Sb and Bi, bringing order to the sequence of the high pressure limit for the A7 structure in group 15 elements as a function of the atomic number, thus solving a long-debated inconsistency within the group, which has fundamental relevance in the structural chemistry of the elements6,7 and potential applicative implications for the synthesis, functionalization and stabilization of phosphorene based materials and heterostructures. Once more, like in the case of C for group 1424 and O for group 16,25,26 pressure has been shown here to be an extremely powerful tool for tuning the electronic properties of matter, thus enhancing the similarities between the structural and chemical properties of the lighter and heavier elements belonging to the same group and reconciling apparent discrepancies in their ambient pressure properties.
Thanks are expressed to EC through the European Research Council (ERC) for funding the project PHOSFUN “Phosphorene functionalization: a new platform for advanced multifunctional materials” (Grant Agreement No. 670173) through an ERC Advanced Grant. This study was also supported by the Alfred P. Sloan Foundation under the initiative The Extreme Physics and Chemistry Community of the Deep Carbon Observatory (DCO), by the Italian Ministero dell'Istruzione, dell'Università e della Ricerca (MIUR) and by Ente Cassa di Risparmio di Firenze under the project Firenze Hydrolab2.0. The authors acknowledge the European Synchrotron Radiation Facility for provision of synchrotron radiation facilities and thank M. Mezouar, A. B. Cairns, G. Garbarino and J. Jacobs for assistance in using beamline ID27.
Conflicts of interest
There are no conflicts to declare.
Supplementary Material
Footnotes
†Electronic supplementary information (ESI) available. See DOI: 10.1039/c8cc03013h
References
- Hultgren R., Gingrich N. S., Warren B. E. J. Chem. Phys. 1935;3:351–355. [Google Scholar]
- Brown A., Rundqvist S. Acta Crystallogr. 1965;19:684–685. [Google Scholar]
- Liu H., Neal A. T., Zhu Z., Luo Z., Xu X., Tománek D., Ye P. D. ACS Nano. 2014;8:4033–4041. doi: 10.1021/nn501226z. [DOI] [PubMed] [Google Scholar]
- Li L., Yu Y., Ye G. J., Ge Q., Ou X., Wu H., Feng D., Chen X. H., Zhang Y. Nat. Nanotechnol. 2014;9:372–377. doi: 10.1038/nnano.2014.35. [DOI] [PubMed] [Google Scholar]
- Zhu Z., Tománek D. Phys. Rev. Lett. 2014;112:176802. doi: 10.1103/PhysRevLett.112.176802. [DOI] [PubMed] [Google Scholar]
- Müller U., Inorganic Structural Chemistry, Wiley, 2007. [Google Scholar]
- Katzke H., Tolédano P. Phys. Rev. B: Condens. Matter Mater. Phys. 2008;77:024109. [Google Scholar]
- Akahama Y., Kobayashi M., Kawamura H. Phys. Rev. B: Condens. Matter Mater. Phys. 1999;59:8520–8525. [Google Scholar]
- Scelta D., Baldassarre A., Serrano-Ruiz M., Dziubek K., Cairns A. B., Peruzzini M., Bini R., Ceppatelli M. Angew. Chem., Int. Ed. 2017;56:14135–14140. doi: 10.1002/anie.201708368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamura H., Shirotani I., Tachikawa K. Solid State Commun. 1984;49:879–881. [Google Scholar]
- Kawamura H., Shirotani I., Tachikawa K. Solid State Commun. 1985;54:775–778. [Google Scholar]
- Karuzawa M., Ishizuka M., Endo S. J. Phys.: Condens. Matter. 2002;14:10759. [Google Scholar]
- Pinceaux J.-P., Maury J.-P., Besson J.-M. J. Phys., Lett. 1979;40:307–308. [Google Scholar]
- Diatschenko V., Chu C. W., Liebenberg D. H., Young D. A., Ross M., Mills R. L. Phys. Rev. B: Condens. Matter Mater. Phys. 1985;32:381–389. doi: 10.1103/physrevb.32.381. [DOI] [PubMed] [Google Scholar]
- Vos W. L., Schouten J. A. J. Chem. Phys. 1989;91:6302–6305. [Google Scholar]
- Murata K., Yokogawa K., Yoshino H., Klotz S., Munsch P., Irizawa A., Nishiyama M., Iizuka K., Nanba T., Okada T., Shiraga Y., Aoyama S. Rev. Sci. Instrum. 2008;79:085101. doi: 10.1063/1.2964117. [DOI] [PubMed] [Google Scholar]
- Kikegawa T., Iwasaki H. Acta Crystallogr., Sect. B: Struct. Sci. 1983;39:158–164. [Google Scholar]
- Clark S. M., Zaug J. M. Phys. Rev. B: Condens. Matter Mater. Phys. 2010;82:134111. [Google Scholar]
- Boulfelfel S. E., Seifert G., Grin Y., Leoni S. Phys. Rev. B: Condens. Matter Mater. Phys. 2012;85:014110. [Google Scholar]
- Angel R. J. Rev. Mineral. Geochem. 2000;41:35–59. [Google Scholar]
- Angel R. J., High-Pressure Crystallography, Springer, Netherlands, 1st edn, 2004, pp. 21–36. [Google Scholar]
- Chan K. T., Malone B. D., Cohen M. L. Phys. Rev. B: Condens. Matter Mater. Phys. 2013;88:064517. [Google Scholar]
- Seo D.-K., Hoffmann R. J. Solid State Chem. 1999;147:26–37. [Google Scholar]
- Santoro M., Gorelli F. A., Bini R., Ruocco G., Scandolo S., Crichton W. A. Nature. 2006;441:857–860. doi: 10.1038/nature04879. [DOI] [PubMed] [Google Scholar]
- Gorelli F. A., Ulivi L., Santoro M., Bini R. Phys. Rev. Lett. 1999;83:4093–4096. [Google Scholar]
- Lundegaard L. F., Weck G., McMahon M. I., Desgreniers S., Loubeyre P. Nature. 2006;443:201–204. doi: 10.1038/nature05174. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.