Abstract
Background
Ketamine is receiving increasing attention as a rapid-onset antidepressant in patients suffering from major depressive disorder (MDD) with treatment resistance or severe suicidal ideation. Ketamine modulates several neurotransmitter systems, including norepinephrine via the norepinephrine transporter (NET), both peripherally and centrally. The locus coeruleus (LC), which has high NET concentration, has been attributed to brain networks involved in depression. Thus we investigated the effects of single-dose of racemic ketamine on the LC using resting state functional MRI.
Methods
Fifty-nine healthy participants (mean age 25.57 ± 4.72) were examined in a double-blind, randomized, placebo-controlled study with 7 Tesla MRI. We investigated the resting state functional connectivity (rs-fc) of the LC before and one hour after subanesthetic ketamine injection (0.5 mg/kg), as well as associations between its rs-fc and a common polymorphism in the NET gene (rs28386840).
Results
A significant interaction of drug and time was revealed, and post hoc testing showed decreased rs-fc between LC and the thalamus after ketamine administration compared with baseline levels, including the mediodorsal, ventral anterior, ventral lateral, ventral posterolateral and centromedian nuclei. The rs-fc reduction was more pronounced in NET rs28386840 [AA] homozygous subjects than in [T] carriers.
Conclusions
We demonstrated acute rs-fc changes after ketamine administration in the central node of the norepinephrine pathway. These findings may contribute to understanding the antidepressant effect of ketamine at the system level, supporting modes of action on networks subserving aberrant arousal regulation in depression.
Keywords: Ketamine, Locus coeruleus (LC), Norepinephrine transporter (NET), Attention networks, Pharmacogenetics, Major depressive disorder (MDD)
Highlights
-
•
Ketamine decreased connectivity between locus coeruleus and bilateral thalamus in resting state fMRI.
-
•
This reduction of rs-fc between LC and thalamus was dependent on norepinephrine transporter genotype.
-
•
The central effects of ketamine involve norepinephrine and attention networks.
-
•
Antidepressive effects of ketamine may involve LC attention system.
1. Introduction
Ketamine has been identified as a rapidly acting antidepressant (Berman et al., 2000; Zarate et al., 2006; Murrough et al., 2013). The different properties of ketamine, including the cardiovascular side-effects (Short et al., 2018), are likely to arise due to its influence on multiple receptor types. Inhibition of the NMDA receptor as the primary mechanism of antidepressant action (Boyer et al., 1998) has recently been challenged (Zanos et al., 2016). Instead, changes in synaptic plasticity, relevant for the development of major depressive disorder (MDD), may be influenced by ketamine via factors such as AMPA receptor mediated elevation of brain-derived neurotrophic factor (BDNF) levels (Duman and Aghajanian, 2012; Zhou et al., 2014). While these mechanisms may explain long-term effects of ketamine, more research is necessary to reveal the pathways mediating acute ketamine effects, including other neurotransmitter systems (Williams and Schatzberg, 2016). Understanding the mechanisms underpinning the acute response to ketamine is particularly important, because the acute side-effects of ketamine have been proposed to be predictive of its long-term antidepressant effects (Luckenbaugh et al., 2014).
We focus on the known influence of ketamine on the norepinephrine system as a potential mechanism by which ketamine has an acute effect on brain function. Firstly, the acute effects of ketamine on blood pressure and heart rate are considered to be driven by the norepinephrine system (Baraka et al., 1973). Specifically, ketamine is known to inhibit the norepinephrine transporter (NET), as shown by in vitro experiments and in vivo studies in animals and humans (Baraka et al., 1973; Miletich et al., 1973; Doenicke et al., 1992; Hara et al., 1998; Nishimura et al., 1998; Hara et al., 2002; Zhao and Sun, 2008). Importantly, those effects are observed at plasma concentrations achievable with administration of subanaesthetic doses (Miletich et al., 1973; Niesters et al., 2012). We have described this relationship in more detail previously (Liebe et al., 2017). The NET is responsible for the recirculation of norepinephrine (NE) from the synaptic cleft into the presynaptic terminal (Schroeder and Jordan, 2012). The study of different NET genotypes provides an approach to exploring the role of NET in the antidepressant effects of ketamine. Indeed, one common NET polymorphism (rs28386840) has been associated with the severity of the ketamine-related initial blood pressure increase, indicating an acute influence of ketamine on the peripheral norepinephrine system (Liebe et al., 2017).
In the brain, the highest concentrations of NET have been found in the locus coeruleus (LC) (Schou et al., 2005; Arakawa et al., 2008) as well as in the medial thalamus (Takano et al., 2008). The LC is a small bilateral nucleus located at the bottom of the fourth ventricle in the brainstem. It is the main source of norepinephrine in the brain (Mouton et al., 1994) and projects to nearly the whole brain, including the limbic system and prefrontal cortex. LC activity plays an important role in controlling autonomic functions (Samuels and Szabadi, 2008) as well as in arousal and attention.
Human attention has been linked to the activity of three networks, each of which has been implicated in different attentional phases: the alerting network, the orienting network, and the executive control network (Corbetta and Shulman, 2002; Fan et al., 2005; Corbetta et al., 2008; Fan et al., 2009; Petersen and Posner, 2012). Within these concepts, the LC has been assigned to all three networks, but its relevance has been mostly emphasized in the alerting network, with a pronounced role in the “alerting effect” (Petersen and Posner, 2012).
Acute ketamine administration has a rapid impact on attention (Oranje et al., 2000). For example, ketamine resulted in an elevated false alarm rate and a reduced hit rate in a visual oddball task (Musso et al., 2011). Analogously, it also acutely impaired performance in the AX-CPT arousal task, in which a lower hit rate and an elevated context-dependent false alarm rate were reported following ketamine administration (Umbricht et al., 2000). In rats, ketamine evoked a response-depressing effect in the five-choice serial-reaction time task, shown by an increased number of omissions and a reduced response speed, without affecting motivation (Nikiforuk and Popik, 2014).
Animal studies indicate that ketamine influences LC function (Dawson et al., 2013; El Iskandrani et al., 2015), and human studies show that the event-related potential P300 wave – which has been linked to phasic LC activity (Corbetta et al., 2008) – has a reduced amplitude during an attentional response following ketamine (Oranje et al., 2000; Musso et al., 2011).
The LC is thus involved in both blood pressure homeostasis and in attention regulation and vigilance, potentially drawing together two of the acute effects of ketamine and suggesting that effects on the norepinephrine system may underlie both.
We therefore propose that ketamine has an influence on the LC via inhibition of the NET, leading to elevation of NE concentration in the synaptic clefts. This elevation leads to change in the resting state functional connectivity (rs-fc) of the LC within the alerting network, reflecting a more distractible state of arousal. Consequently, we also predict an influence of the NET genotype on functional connectivity change after ketamine.
Despite the indications presented here, most studies have assessed acute ketamine effects based on the hypothesis of a mechanism involving NMDA-receptors, and to date, no study has investigated the influence of ketamine on brain structures that are linked to the norepinephrine network, including the NET.
2. Methods
2.1. Subjects
The study was conducted as a double-blind, placebo-controlled, randomized parallel-design trial. 80 participants (33 women, 25.89 ± 5.29) were recruited by public advertisement and received financial award for their participation. The participants were in a state of good general health as determined by medical history, physical examination, blood laboratory tests, electrocardiography and toxicology findings. Participants with magnetic resonance imaging (MRI)-incompatible devices, regular medication or excessive caffeine intake and current or former drug abuse were excluded. All subjects completed the Mini International Neuropsychiatric Interview (M.I.N.I., German Version 5.0.0) (Ackenheil et al., 1999) and underwent additional interview by the study physician to rule out any DSM-IV psychiatric disorders. The study was approved by the institutional ethical review board of the University of Magdeburg, and all subjects gave written informed consent in accordance with the Declaration of Helsinki.
3. Study design
After a baseline MRI session, study participants were randomized to a 50 ml intravenous infusion of either 0.5 mg/kg racemic ketamine (Ketamine-ratiopharm 500 mg/10 ml) or saline (Berlin-Chemie Isotone NaCl 0.9%) outside the MRI scanner. The infusion was administered over 40 min via an infusion pump (Injectomat 2000, Fresenius Kabi GmbH) in a resting, supine position. Twenty minutes after the infusion was completed (duration of infusion 40 min), a post-infusion MRI session was conducted. Each MRI session lasted 60 min with collection of 13 min rs data after a 40 min spectroscopy run.
The whole study included other time points (i.e. 24 h MR session). Since our hypothesis was specific for acute effects on the NE system, we focused on the baseline and 1 h functional MRI (fMRI) data.
4. FMRI data acquisition, processing and analysis
Image acquisition was performed using a 7 Tesla Magnetom scanner (Siemens) with the following parameters for T1 anatomical images: 3D-MPRAGE sequence, TE = 2.73 ms, TR = 2300 ms, T1 = 1050 ms, flip angle = 7°, bandwidth = 140 Hz/pixel, acquisition matrix = 320 × 320 × 224, isometric voxel size = 0.8 mm3. Resting state fMRI data were acquired eyes-closed and with the following parameters: 62 axial slices, acquisition matrix = 106 × 106, field of view = 212 × 212 mm, slice thickness = 2 mm, leading to a high resolution of 2 mm isotropic, repetition time (TR) = 2800 ms, echo time (TE) = 22 ms, flip angle = 80°, 280 volumes in total). Raw data quality was visually checked for each dataset and each time point. Subjects were excluded if artifacts were observed at any time point (Supplementary Fig. 1), which led to exclusion of 19 datasets, leaving 61 subjects for further analysis. Data preprocessing was conducted using scripts developed in the 1000 Functional Connectomes project (https://www.nitrc.org/projects/fcon_1000/) with several modifications. This pipeline implemented motion correction, spatial smoothing with a Gaussian kernel (FWHM = 4 mm), temporal filtering with a bandpass filter (0.005–0.1 Hz) and removal of linear temporal trends, and regressed out the mean time course of white matter, cerebrospinal fluid and six affine motion parameters. No slice time correction was performed because of its minimal impact in task-free functional connectivity analysis at the TR used in this study (Wu et al., 2011), as well as our long acquisition time of 13 min. We additionally regressed out motion outliers identified by a framewise displacement (FD) > 0.2 (Power et al., 2012), calculated with fsl_motion_outliers in FSL.
As a confirmatory analysis, we additionally preprocessed the data with physiological noise regressors (heart beats and respiration), created with the FSL PNM toolbox (Brooks et al., 2008). For this analysis, we excluded another eight subjects due to poor physiological data recordings.
The fMRI data of each subject were coregistered to the corresponding individual high-resolution anatomical images and then normalized to the Montreal Neurological Institute (MNI) template (ICBM152) with 2 × 2 × 2 mm3 resolution.
The seed was defined based on a previous probability template of LC (Keren et al., 2009). Two subjects were excluded because of a mismatch after registration, compared to the targeted probability template (Supplementary Fig. 2).
The calculation of rs-fc included extraction of the time series from the average LC seed, computation of voxel-wise correlations with the extracted time series, Z-transformation of the correlations and registration of the Z-transformed correlations to standard space using AFNI (https://afni.nimh.nih.gov/) and FSL (https://fsl.fmrib.ox.ac.uk/).
Calculation of second level statistics for the subjects that passed the preprocessing steps (ketamine group n = 29, 12 women; placebo group n = 30, 14 woman) was performed using SPM12 (http://www.fil.ion.ucl.ac.uk/spm). There was no age difference between the two treatment groups (t(57) = 0.810; p = .421).
Specific analysis steps: Statistic images were assessed for cluster-wise significance by using a cluster-defining threshold of p = .001. To investigate the baseline rs-fc from LC to all the brain, we conducted a one-sample t-test including both groups (ketamine, placebo) for the resting state scan before infusion. A mixed-design ANOVA was performed to assess the time (baseline, after ketamine or placebo infusion) by group (ketamine n = 29, placebo n = 30) interaction of the rs-fc of the LC to whole brain. As a post-hoc test, we conducted a paired-t-test within the ketamine and placebo groups with the factor time (baseline, one hour after ketamine administration) to investigate the change in rs-fc in each group on a whole brain level. To account for multiple testing (two groups), we defined a conservative p < .025 for rejection of the H0 in both of these analyses. We then aimed to corroborate the result of the paired-t-test with the physiological noise corrected data using a small volume correction corresponding to regions of interest revealed by the interaction.
Genotyping of blood samples with regard to the NET gene rs28386840 was performed following a protocol published previously (Liebe et al., 2017). To test the influence of genotype on rs-fc change, we extracted the change of the mean rs-fc beta-weights for the significant result in the ketamine group (baseline, directly after ketamine administration) and compared the groups ([AA] n = 15, [T]-carrier n = 13, no genetic data for one participant) using the non-parametric U Test for two independent samples. Regional activity was tested by the same analysis for the mediodorsal (MD) thalamic subregion based on WFU Pick-Atlas.
MRI figures were created with Mango (Research Imaging Institute, UTHSCSA) and the WFU Pick-Atlas (Maldjian et al., 2003).
5. Results
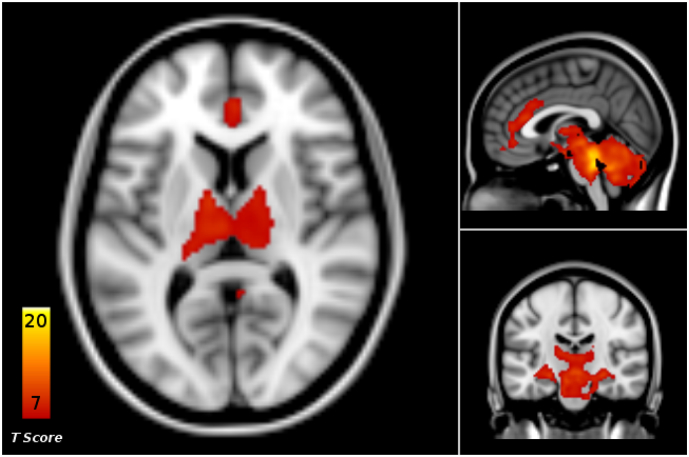
The LC rs-fc at baseline showed connections with the cingulate cortex, temporal superior gyrus, putamen, caudate nucleus, pallidum, hippocampus, precuneus, thalamus, cerebellum and brainstem regions (Fig. 1, Supplementary Table 1).
Fig. 1.
Baseline resting state functional connectivity (rs-fc) between locus coeruleus (LC) and the whole brain, across all patients in the placebo and ketamine groups (p < .05, FWE cluster level corrected).
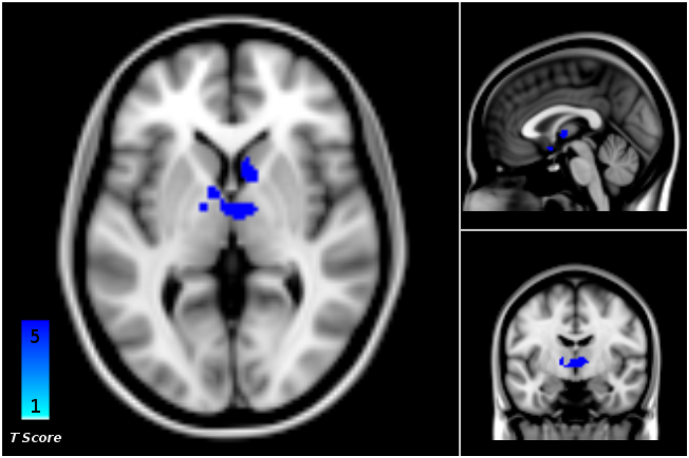
The whole brain ANOVA revealed a significant drug (ketamine, placebo) by time (baseline, 1 h after start of infusion) interaction in the bilateral thalamus (n = 59, p < .001, cluster size k = 614, FWE cluster level corrected, Fig. 2).
Fig. 2.
Whole-brain analysis of variance (ANOVA) revealed a significant time-by-group (baseline, 1 h; ketamine, placebo) interaction of resting state functional connectivity (rs-fc) between the locus coeruleus (LC) seed and the bilateral thalamus (p < .001, FWE cluster level corrected, cluster size = 614).
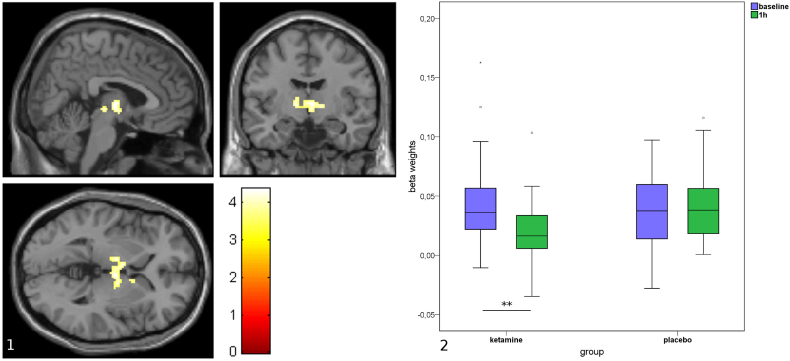
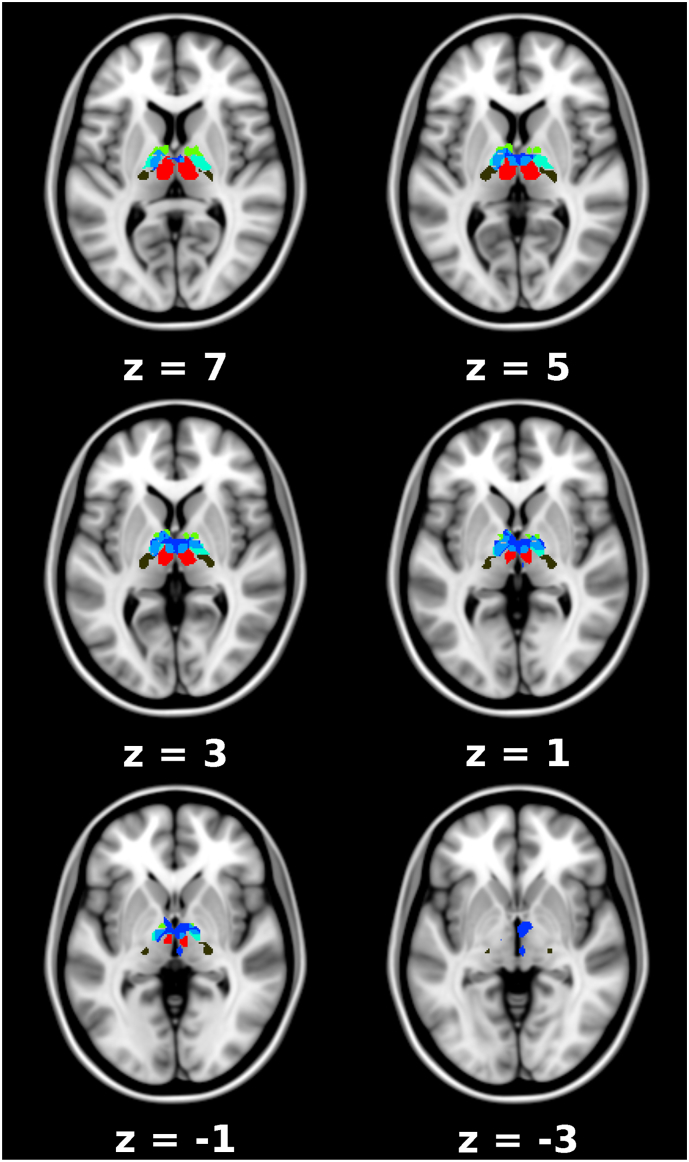
Follow-up analysis showed that this interaction was driven by a rs-fc-decrease between the LC and the bilateral thalamus in the ketamine group (n = 29, p = .008, cluster size k = 252, FWE cluster level corrected, Fig. 3, Fig. 4), which was not found in the placebo group. Further investigation of the thalamic nuclei revealed decreased rs-fc in the ventral anterior (VA), ventral lateral (VL) and mediodorsal nuclei bilaterally, and in the left ventral posterolateral (VPL) nucleus and the right centromedian nucleus (CM) (Fig. 4, Supplementary Table 2). There were no further significant findings.
Fig. 3.
Decrease in resting state functional connectivity (rs-fc) from locus coeruleus (LC) to the thalamus following ketamine (p = .008, FWE cluster level corrected, cluster size = 252, Panel 1). Change in rs-fc between LC seed and thalamus in ketamine and placebo groups at baseline and 1 h after administration. Decrease of rs-fc is only significant in the ketamine group (p = .008, FWE cluster level corrected, Panel 2).
Fig. 4.
Decrease in locus coeruleus (LC) resting state functional connectivity (rs-fc) to thalamic subnuclei in the ketamine group, axial view. Significant thalamus cluster (blue, MNI coordinates 4, −10, 0) including VA (yellow-green), VL (turquoise), MD (red), VPL (brown) nuclei and the right CM (smaller blue cluster). The overlap between the significant cluster and the thalamic subnuclei is marked in light blue. Numbers indicate the z-coordinate within the brain in MNI space.
In the dataset analyzed following the second preprocessing (controlling for physiological noise), a rs-fc reduction from LC to the thalamus was also identified (p = .018, FWE small volume peak level corrected).
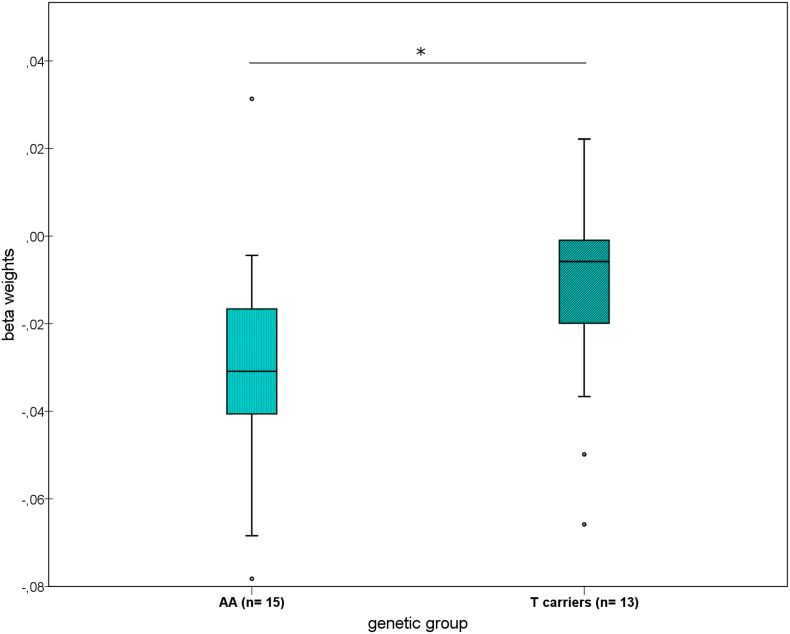
The reduction of rs-fc between LC and thalamus was dependent on NET rs28386840, with a greater decrease in the [AA] homozygotes than in the [T] carriers (U = 55, p = .050, Fig. 5). In the MD nuclei of the thalamus, on which we focused due to previous detection of NET in the medial thalamus as well as the LC (Takano et al., 2008), the difference was even more pronounced (U = 54, p = .045).
Fig. 5.
Differing changes in resting state functional connectivity (rs-fc) from locus coeruleus (LC) to thalamus in the ketamine group according to NET genotype (*p = .05).
6. Discussion
Following subanaesthetic ketamine administration, we found a decrease in rs-fc between the LC and the thalamus peaking at the mediodorsal, ventral anterior, ventral lateral bilateral, left ventral posterolateral and right centromedian nucleus. Genetic evidence further suggested an underlying noradrenergic mechanism, as this decrease was modulated by the NET rs28386840 gene with a greater decrease in [AA] genotype than in [T] carriers.
These results are compatible with the proposed effects of ketamine on attention regulation, since the rs-fc change observed here concerns the first path of the associated attention network.
The thalamus has been classified as a bottom-up regulatory entity for alertness, with its activation interpreted as facilitating increased response readiness after an external warning stimulus (Périn et al., 2010). This effect is thought to support target detection and to improve awareness in tasks. Accordingly, the thalamus has been shown to be activated in the alerting effect (Fan et al., 2005), together with the LC (Xuan et al., 2016).
Recent studies also describe the role of the thalamus in executive attentional control. Elevated alertness was associated with high activity in the thalamus, which was interpreted as increasing the signal-to-noise ratio and facilitating a reduction of distraction in attentional performance (Sadaghiani and D'Esposito, 2015; Coste and Kleinschmidt, 2016).
Furthermore, the attentional networks revealed by task-based studies show a strong overlap with networks identified in the resting condition (Fox et al., 2006; Vincent et al., 2008), and an involvement of the thalamus has been shown in the resting-state “salience network” (Seeley et al., 2007). The salience network has been suggested to constitute a core regulatory entity of human consciousness due to its responsibility for “higher-order” stimulus selection (Seeley et al., 2007), and within this network, the MD has been shown to regulate behavioral flexibility (Parnaudeau et al., 2013; Peters et al., 2016).
The observed connectivity decrease between LC and thalamus could indicate a change in the regulation of arousal in terms of a disruption of the alerting function, a promotion of non-selective sensory signal detection and an enhancement of behavioral flexibility. We suggest that this disconnection within the attention network may constitute a neurophysiological correlate of the known ketamine-related impairment in attentional tasks (Krystal et al., 1994; Umbricht et al., 2000; Oranje et al., 2000; Musso et al., 2011). We postulate that this decoupling of LC-thalamic control with thalamic nuclei involved in attentional processing leads to more nonspecific sensory signal detection and a higher susceptibility to non-focused sensory signals in the thalamus. Conceptual work has explained the delusion-like symptoms after ketamine, which include disembodiment and impaired control of cognition (Vollenweider and Kometer, 2010), as stemming from reduced top-down signaling and increased bottom-up signaling (Corlett et al., 2009; Powers et al., 2015). We suggest that the increased bottom-up signaling may be facilitated by the decrease of functional LC-thalamic connection found in our study.
Accordingly, the different roles of the thalamic nuclei that we found to be disconnected from LC are related to the well-recognized actions of ketamine regarding attentional performance and motor response (Musso et al., 2011), memory (Salvadore et al., 2010) and sensory signal perception (Vollenweider and Kometer, 2010). The right CM nucleus of the thalamus is directly related to attentional processing (Kinomura et al., 1996; Metzger et al., 2013). The MD nucleus connects subcortical structures to the cortex and is anatomically connected with the LC (Montagnese et al., 2003). Evidence indicates that the MD is also involved in attention (Van der Werf et al., 2003) and forms a part of the salience network (Seeley et al., 2007). It is also involved in sexual arousal (Walter et al., 2008) and in memory processing (Aggleton and Brown, 1999; Sweeney-Reed et al., 2016). The VPL, as part of the ventral posterior nucleus, conducts sensory information from the ascending medial lemniscus to the primary and secondary somatosensory cortex areas (Behrens et al., 2003). VA and VL nuclei are involved in motor circuits, including the preparation of movement (McFarland and Haber, 2002; Johansen-Berg et al., 2005).
Based on our observations of connectivity changes, it is intriguing to interpret our findings in terms of the underlying neuroanatomy and physiology of the LC norepinephrine system. The implicit prerequisite of directed connections is supported by anatomical and animal studies reporting effective connectivity between the LC and the thalamus (Samuels and Szabadi, 2008). The firing mode of the thalamus is influenced by norepinephrine, which subsequently increases thalamic neuronal excitability and responsiveness, and is associated with particular vigilance states (McCormick et al., 1991). More precisely, the different LC firing patterns directly influence the thalamic reaction to sensory stimuli (Berridge and Waterhouse, 2003). In line with the Adaptive Gain Theory (Aston-Jones and Cohen, 2005a), the different LC firing patterns have the capability either to promote non-selectively all sensory inputs to the thalamus or to facilitate selective responses observable in connectivity changes between LC, thalamus and sensory cortex (Devilbiss et al., 2012). Aston-Jones expected an increase in false alarm errors, problems with discrimination between targets and a lower threshold for unspecific sensory stimuli with LC tonic activity (Aston-Jones and Cohen, 2005a; Corbetta et al., 2008), which is exactly what was found as an effect of ketamine administration (Oranje et al., 2000; Umbricht et al., 2000; Musso et al., 2011; Nikiforuk and Popik, 2014).
We speculate that ketamine might influence the LC firing mode via inhibition of the NET, which shows its highest expression level in the LC. This inhibition would cause a local elevation of NE levels that could directly affect the LC self-regulating system (Samuels and Szabadi, 2008; Okamoto et al., 2012) and influence LC firing modes.
The modulation of ketamine-related LC–thalamic rs-fc changes by a NET genetic variant with a greater response in [AA] homozygotes further supports the idea that the mechanism of action of ketamine involves these norepinephrine pathways. According to our hypothesis, [T] allele carriers would exhibit lower NET activity and thus decreased clearance of norepinephrine from the synaptic cleft (Kim et al., 2006; Schroeder and Jordan, 2012). This suggests that differential NE levels are likely to constitute the distinctive element between the two groups. Neuronal network models of LC function relate high local NE levels in the LC to high tonic LC activity (Usher et al., 1999; Aston-Jones and Cohen, 2005b).
Accordingly, one would primarily expect an increased ketamine effect on rs-fc in the [T] allele carriers. However the overall mechanism needs to incorporate other, systemic effects, which occur at the same time. The [T] genotype is thought to have decreased promoter function (Kim et al., 2006), and accordingly, we found a stronger initial blood pressure rise in [T] carriers during ketamine administration (Liebe et al., 2017). One hour after the start of the infusion, however, [T] carriers showed a lesser response in terms of LC-thalamic connectivity when compared to [AA] carriers. Blood pressure increase after administration of NET inhibitors likely results from two mechanisms: peripheral NE rise, which elevates blood pressure and – to a lesser degree – a centrally mediated counter regulation that lowers blood pressure (Okamoto et al., 2012). The LC tightly controls the sympathetic tone and influences short-term blood pressure homeostasis (Samuels and Szabadi, 2008). A diminished change of LC tone in [T] carriers could conceptually be linked to a reduced central counter regulation in response to the increased initial blood pressure rise.
Contrary to these findings, another recent study indicated higher NET availability in the thalamus (Sigurdardottir et al., 2016) in healthy [T] carriers, supporting potential alternative explanations. More research considering the distinct underlying network dynamics is necessary to reveal the complex interactions between NET genetics, NET availability and function, and connectivity differences.
A connectivity decrease between the LC and thalamus, similar to the one found in our fMRI study, has also been shown measuring local cerebral glucose utilization in mice receiving ketamine (Dawson et al., 2013). Ketamine was associated with an elevated mean firing rate in LC norepinephrine neurons in mice (El Iskandrani et al., 2015) – which we would interpret as a shift to the non-specific arousal condition.
The current findings indicate that the mechanism underlying the antidepressant effect of ketamine may also involve the norepinephrine system. Previous research has shown that norepinephrine and the LC are substantially involved in broad depression-like behaviors and modulated by a wide range of antidepressant drugs (Moret and Briley, 2011). In animal studies, neuronal loss in the LC has resulted in depressive symptoms (Szot et al., 2016), and the regulation of LC firing modes is essential in stress vulnerability (Curtis et al., 2012) and social defeat (Isingrini et al., 2016).
Consistent with our understanding, in a positron emission tomography (PET) study, higher NET availability in MDD patients compared to controls has been found in both the LC and in the thalamus, particularly in a thalamic subregion projecting to the frontal cortex (Moriguchi et al., 2017). Moreover, visual attention was maintained in the MDD patients conducting the Trail Making Test and was positively correlated with NET availability (Moriguchi et al., 2017).
Reducing alertness would counteract a hypothesized hyperalertness in MDD which has been found in electroencephalography (EEG) vigilance studies (Hegerl et al., 2012; Olbrich et al., 2012). MDD patients were not impaired in a visual attention task and performed even better than healthy controls in a study using the flanker task (Dillon et al., 2015). Accordingly, reducing an increased attentional focus may be in line with a therapeutic mode of action based on a mechanism involving reduced LC-thalamic connectivity.
Taken together, it seems plausible that patients with MDD may have an altered alarm system with high NE levels in brain structures that are a functional part of this network and effectively innervated by the LC – especially the medial thalamus. Long-term NE elevation could induce upregulation of the NET in the thalamus (Lee et al., 1983; Weinshenker et al., 2002; Macey et al., 2003) and explain the observed elevated thalamic NET availability in MDD patients. Ketamine may acutely disrupt a long-term activated alarm mode of the LC. This hypothesis would be in line with recent ideas suggesting a reduction of arousal to treat depressive symptomatology, at least in a subset of MDD patients (Spirito et al., 2011). Such a mechanism has been partly discussed with respect to other antidepressant mechanisms such as sleep deprivation, which convergingly also leads to very rapid antidepressant effects, similar to ketamine (Boland et al., 2017). Finally, beyond the antidepressant effect, a shift in attentional focus could relate to further acute effects of ketamine such as pain relief (May 2007; Niesters et al., 2012).
Since ketamine possesses a high affinity for NMDA receptors (Sleigh et al., 2014), and the thalamus was highlighted in the ketamine model of schizophrenia (Frohlich and Van Horn, 2014; Vukadinovic, 2014), NMDA receptor inhibition mediated thalamic-LC rs-fc change (Höflich et al., 2015) could provide an alternative explanation for our results. To our knowledge, however, there is no evidence in the literature that the thalamus is a structure with high NMDA receptor density or enhanced ketamine NMDA receptor binding, which would support the hypothesis of a preferred NMDA receptor action on that structure in healthy volunteers, while our hypothesis is based on structural evidence for a particularly high NET density in the LC and thalamus (Schou et al., 2005; Arakawa et al., 2008; Takano et al., 2008). Interestingly, strong efferent projections from LC to thalamus have been found (Samuels and Szabadi, 2008) to influence sensory signal perception in the thalamus (Devilbiss and Waterhouse, 2011), whereas no known “top-down” control from thalamus to LC exists. Moreover, the known sympathomimetic cardiovascular side-effects of ketamine reflect the relevance of its NET inhibitory component. In contrast, such effects are not manifest in other NMDA receptor inhibitors lacking this specific component, such as memantine (Parsons et al., 1999) or 2-amino-5-phosphono-valerate (AP5) (Lin et al., 1995). Furthermore, we found genetic differences in NET reflected in both the cardiovascular side-effects (Liebe et al., 2017) and in brain connectivity, further highlighting the relevance of this transporter in the mechanism of action of ketamine. Nevertheless, NMDA receptor inhibition during ketamine infusion has been shown to induce rs-fc changes in other brain areas (Kraguljac et al., 2017), and we cannot exclude the possiblity that these brain regions may influence the change in LC-thalamic rs-fc.
We did not find a change of rs-fc to all brain areas that are known to be anatomically connected to the LC. However, our strong findings of altered LC-thalamic rs-fc are not surprising, considering the known functional importance of LC-thalamic connectivity (Devilbiss and Waterhouse, 2011) and the previously identified strong LC-thalamic rs-fc at baseline (Zhang et al., 2016). The preferred modulation of LC-thalamic rs-fc may also be based on the high NET density found in both of these areas (Schou et al., 2005; Arakawa et al., 2008; Takano et al., 2008), and some neocortical brain areas receiving efferent projections from the LC may be modulated by other mechanisms of action of ketamine (Kraguljac et al., 2017).
7. Limitations
There are some limitations to this study. Because ketamine has transient side-effects, optimization of blinding through an active placebo control, for instance with midazolam, has been suggested (Murrough et al., 2013). However, due to ethical considerations in a study with healthy participants, saline infusion was the most feasible option. It is not possible to measure the LC activity modes directly in human subjects. However, further research could include more indirect measurements such as pupil fluctuations or other autonomous nervous system markers that are related both to LC activity and to the dorsal attention network (Alnæs et al., 2014). We used a probabilistic template (Keren et al., 2009) to determine the location of the LC. Future work could include measurements of the LC location at a subject level using neuromelanine sensitive sequences (Sasaki et al., 2006; Betts et al., 2017). Compared to global estimation methods, including a range of frequency-domain analyses applied to multiple channels simultaneously (Zang et al., 2007) and independent component analysis, in which all information channels are incorporated (McKeown et al., 1998), the seed-based rs-fc analysis applied here has the disadvantage that possible relationships and influences resulting from the interplay of multiple brain areas may be missed. However, the current approach provides the benefit of a more straightforward interpretability constrained by a priori hypotheses (Cole et al., 2010). While genetic studies often involve small group sample sizes, a known nearly 1:1 distribution of risk/non-risk carriers in the population (Kohli et al., 2011) led to comparable groups for our analysis ([AA] n = 15, [T]-carriers n = 13). We did not assess ketamine plasma levels, because there are no strong indications that differences in ketamine metabolism affect psychomimetic, antidepressant or sympathomimetic effects in subjects without premedication (Peltoniemi et al., 2016).
Finally, our claims on antidepressant effects need substantiation in a patient group, particularly because chronic conditions such as hyperarousal may lead to a more differentiated response than the one observed in our healthy sample.
8. Conclusions
We provide evidence that ketamine has an acute impact on brain structures known to be involved in attentional processing. We interpret our findings in the context of attentional impairment symptoms after ketamine administration and consider how they may explain aspects of its antidepressant effect. We expand our finding of NET rs28386840 polymorphism dependent differences in the physiological side-effects by revealing NET-dependent LC connectivity changes in the brain, further underlining the potential importance of norepinephrine in ketamine antidepressant treatment and side-effects. We suggest further research on the functional modification of brain activity by ketamine with respect to attentional processing and norepinephrine networks.
Funding
T. Liebe was supported by a thesis scholarship from the University of Magdeburg, Medical Faculty.
Prof. M. Walter, Dr. B. Schott received support from the German Research Foundation (SFB 779/A06 to Prof. Walter, SFB 779/A08 to Dr. Schott, and DFG Wa 2673/4–1 to Prof. Walter), the Centre for Behavioral Brain Sciences (CBBS NN05 for Prof. Walter), and Leibniz Association (Park für Forschung und Innovation to Prof. Walter). L. Colic received a scholarship from German Research Foundation (SFB 779, 2013–2016). M. Woelfer was supported through a research stipend from the Otto-von-Guericke-University Magdeburg and German Academic Exchange Service.
Statement of interest
Prof. Walter has received research support from Janssen Pharmaceutical Research for an IIT on ketamine in patients with major depression. Further research support to Prof. Walter was from HEEL Gmbh, not related to this study or the substances used. Others declare no potential conflict of interest.
Acknowledgments
We would like to thank Jörg Stadler, Renate Blobel-Lüer, Claus Tempelmann and Andreas Fügner for their help and technical advice during data acquisition. We acknowledge support by Deutsche Forschungsgemeinschaft and Open Access Publishing Fund of University of Tübingen.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.nicl.2018.09.001.
Appendix A. Supplementary data
Supplementary material
References
- Ackenheil M., Stotz-Ingenlath G., Dietz-Bauer R., Vossen A. 1999. Mini International Neuropsychiatric Interview. (German Version 5.0.0, DSM-IV) [Google Scholar]
- Aggleton J.P., Brown M.W. Episodic memory, amnesia, and the hippocampal-anterior thalamic axis. Behav. Brain Sci. 1999;22:425–444. (discussion 444-489) [PubMed] [Google Scholar]
- Alnæs D., Sneve M.H., Espeseth T., Endestad T., van de Pavert S.H.P., Laeng B. Pupil size signals mental effort deployed during multiple object tracking and predicts brain activity in the dorsal attention network and the locus coeruleus. J. Vis. 2014;14 doi: 10.1167/14.4.1. [DOI] [PubMed] [Google Scholar]
- Arakawa R., Okumura M., Ito H., Seki C., Takahashi H., Takano H., Nakao R., Suzuki K., Okubo Y., Halldin C., Suhara T. Quantitative analysis of norepinephrine transporter in the human brain using PET with (S,S)-18F-FMeNER-D2. J. Nucl. Med. Off. Publ. Soc. Nucl. Med. 2008;49:1270–1276. doi: 10.2967/jnumed.108.051292. [DOI] [PubMed] [Google Scholar]
- Aston-Jones G., Cohen J.D. Adaptive gain and the role of the locus coeruleus-norepinephrine system in optimal performance. J. Comp. Neurol. 2005;493:99–110. doi: 10.1002/cne.20723. [DOI] [PubMed] [Google Scholar]
- Aston-Jones G., Cohen J.D. An integrative theory of locus coeruleus-norepinephrine function: adaptive gain and optimal performance. Annu. Rev. Neurosci. 2005;28:403–450. doi: 10.1146/annurev.neuro.28.061604.135709. [DOI] [PubMed] [Google Scholar]
- Baraka A., Harrison T., Kachachi T. Catecholamine levels after ketamine anesthesia in man. Anesth. Analg. 1973;52 [PubMed] [Google Scholar]
- Behrens T.E.J., Johansen-Berg H., Woolrich M.W., Smith S.M., Wheeler-Kingshott C.a.M., Boulby P.A., Barker G.J., Sillery E.L., Sheehan K., Ciccarelli O., Thompson A.J., Brady J.M., Matthews P.M. Non-invasive mapping of connections between human thalamus and cortex using diffusion imaging. Nat. Neurosci. 2003;6:750–757. doi: 10.1038/nn1075. [DOI] [PubMed] [Google Scholar]
- Berman R.M., Cappiello A., Anand A., Oren D.A., Heninger G.R., Charney D.S., Krystal J.H. Antidepressant effects of ketamine in depressed patients. Biol. Psychiatry. 2000;47:351–354. doi: 10.1016/s0006-3223(99)00230-9. [DOI] [PubMed] [Google Scholar]
- Berridge C.W., Waterhouse B.D. The locus coeruleus–noradrenergic system: modulation of behavioral state and state-dependent cognitive processes. Brain Res. Rev. 2003;42:33–84. doi: 10.1016/s0165-0173(03)00143-7. [DOI] [PubMed] [Google Scholar]
- Betts M.J., Cardenas-Blanco A., Kanowski M., Jessen F., Düzel E. In vivo MRI assessment of the human locus coeruleus along its rostrocaudal extent in young and older adults. NeuroImage. 2017;163:150–159. doi: 10.1016/j.neuroimage.2017.09.042. [DOI] [PubMed] [Google Scholar]
- Boland E.M., Rao H., Dinges D.F., Smith R.V., Goel N., Detre J.A., Basner M., Sheline Y.I., Thase M.E., Gehrman P.R. Meta-Analysis of the Antidepressant Effects of Acute sleep Deprivation. J. Clin. Psychiatry. 2017;78:e1020–e1034. doi: 10.4088/JCP.16r11332. [DOI] [PubMed] [Google Scholar]
- Boyer P.A., Skolnick P., Fossom L.H. Chronic administration of imipramine and citalopram alters the expression of NMDA receptor subunit mRNAs in mouse brain. A quantitative in situ hybridization study. J. Mol. Neurosci. MN. 1998;10:219–233. doi: 10.1007/BF02761776. [DOI] [PubMed] [Google Scholar]
- Brooks J.C.W., Beckmann C.F., Miller K.L., Wise R.G., Porro C.A., Tracey I., Jenkinson M. Physiological noise modelling for spinal functional magnetic resonance imaging studies. NeuroImage. 2008;39:680–692. doi: 10.1016/j.neuroimage.2007.09.018. [DOI] [PubMed] [Google Scholar]
- Cole D.M., Smith S.M., Beckmann C.F. Advances and pitfalls in the Analysis and Interpretation of Resting-State FMRI Data. Front. Syst. Neurosci. 2010;4 doi: 10.3389/fnsys.2010.00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbetta M., Shulman G.L. Control of goal-directed and stimulus-driven attention in the brain. Nat. Rev. Neurosci. 2002;3:201–215. doi: 10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- Corbetta M., Patel G., Shulman G.L. The reorienting system of the human brain: from environment to theory of mind. Neuron. 2008;58:306–324. doi: 10.1016/j.neuron.2008.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corlett P.R., Frith C.D., Fletcher P.C. From drugs to deprivation: a Bayesian framework for understanding models of psychosis. Psychopharmacology. 2009;206:515–530. doi: 10.1007/s00213-009-1561-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coste C.P., Kleinschmidt A. Cingulo-opercular network activity maintains alertness. NeuroImage. 2016;128:264–272. doi: 10.1016/j.neuroimage.2016.01.026. [DOI] [PubMed] [Google Scholar]
- Curtis A.L., Leiser S.C., Snyder K., Valentino R.J. Predator stress Engages Corticotropin-releasing factor and Opioid Systems to Alter the Operating Mode of Locus Coeruleus Norepinephrine Neurons. Neuropharmacology. 2012;62:1737–1745. doi: 10.1016/j.neuropharm.2011.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson N., Morris B.J., Pratt J.A. Subanaesthetic ketamine treatment alters prefrontal cortex connectivity with thalamus and ascending subcortical systems. Schizophr. Bull. 2013;39:366–377. doi: 10.1093/schbul/sbr144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devilbiss D.M., Waterhouse B.D. Phasic and tonic patterns of locus coeruleus output differentially modulate sensory network function in the awake rat. J. Neurophysiol. 2011;105:69–87. doi: 10.1152/jn.00445.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devilbiss D.M., Waterhouse B.D., Berridge C.W., Valentino R. Corticotropin-releasing factor acting at the locus coeruleus disrupts thalamic and cortical sensory-evoked responses. Neuropsychopharmacol. Off. Publ. Am. Coll. Neuropsychopharmacol. 2012;37:2020–2030. doi: 10.1038/npp.2012.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dillon D.G., Wiecki T., Pechtel P., Webb C., Goer F., Murray L., Trivedi M., Fava M., McGrath P.J., Weissman M., Parsey R., Kurian B., Adams P., Carmody T., Weyandt S., Shores-Wilson K., Toups M., McInnis M., Oquendo M.A., Cusin C., Deldin P., Bruder G., Pizzagalli D.A. A computational analysis of flanker interference in depression. Psychol. Med. 2015;45:2333–2344. doi: 10.1017/S0033291715000276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doenicke A., Angster R., Mayer M., Adams H.A., Grillenberger G., Nebauer A.E. The action of S-(+)-ketamine on serum catecholamine and cortisol. A comparison with ketamine racemate. Anaesthesist. 1992;41:597–603. [PubMed] [Google Scholar]
- Duman R.S., Aghajanian G.K. Synaptic dysfunction in depression: potential therapeutic targets. Science. 2012;338:68–72. doi: 10.1126/science.1222939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Iskandrani K.S., Oosterhof C.A., El Mansari M., Blier P. Impact of subanesthetic doses of ketamine on AMPA-mediated responses in rats: an in vivo electrophysiological study on monoaminergic and glutamatergic neurons. J. Psychopharmacol. (Oxf.) 2015;29:792–801. doi: 10.1177/0269881115573809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan J., McCandliss B.D., Fossella J., Flombaum J.I., Posner M.I. The activation of attentional networks. NeuroImage. 2005;26:471–479. doi: 10.1016/j.neuroimage.2005.02.004. [DOI] [PubMed] [Google Scholar]
- Fan J., Gu X., Guise K.G., Liu X., Fossella J., Wang H., Posner M.I. Testing the behavioral interaction and integration of attentional networks. Brain Cogn. 2009;70:209–220. doi: 10.1016/j.bandc.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox M.D., Corbetta M., Snyder A.Z., Vincent J.L., Raichle M.E. Spontaneous neuronal activity distinguishes human dorsal and ventral attention systems. Proc. Natl. Acad. Sci. U. S. A. 2006;103:10046–10051. doi: 10.1073/pnas.0604187103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frohlich J., Van Horn J.D. Reviewing the ketamine model for schizophrenia. J. Psychopharmacol. Oxf. Engl. 2014;28:287–302. doi: 10.1177/0269881113512909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara K., Yanagihara N., Minami K., Ueno S., Toyohira Y., Sata T., Kawamura M., Brüss M., Bönisch H., Shigematsu A., Izumi F. Ketamine interacts with the noradrenaline transporter at a site partly overlapping the desipramine binding site. Naunyn Schmiedeberg's Arch. Pharmacol. 1998;358:328–333. doi: 10.1007/pl00005261. [DOI] [PubMed] [Google Scholar]
- Hara K., Minami K., Ueno S., Toyohira Y., Tsutsui M., Shigematsu A., Yanagihara N. Up-regulation of noradrenaline transporter in response to prolonged exposure to ketamine. Naunyn Schmiedeberg's Arch. Pharmacol. 2002;365:406–412. doi: 10.1007/s00210-002-0534-1. [DOI] [PubMed] [Google Scholar]
- Hegerl U., Wilk K., Olbrich S., Schoenknecht P., Sander C. Hyperstable regulation of vigilance in patients with major depressive disorder. World J. Biol. Psychiatry Off. J. World Fed. Soc. Biol. Psychiatry. 2012;13:436–446. doi: 10.3109/15622975.2011.579164. [DOI] [PubMed] [Google Scholar]
- Höflich A., Hahn A., Küblböck M., Kranz G.S., Vanicek T., Windischberger C., Saria A., Kasper S., Winkler D., Lanzenberger R. Ketamine-Induced Modulation of the Thalamo-Cortical Network in healthy Volunteers as a Model for Schizophrenia. Int. J. Neuropsychopharmacol. 2015;18 doi: 10.1093/ijnp/pyv040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isingrini E., Perret L., Rainer Q., Amilhon B., Guma E., Tanti A., Martin G., Robinson J., Moquin L., Marti F., Mechawar N., Williams S., Gratton A., Giros B. Resilience to chronic stress is mediated by noradrenergic regulation of dopamine neurons. Nat. Neurosci. 2016;19:560–563. doi: 10.1038/nn.4245. [DOI] [PubMed] [Google Scholar]
- Johansen-Berg H., Behrens T.E.J., Sillery E., Ciccarelli O., Thompson A.J., Smith S.M., Matthews P.M. Functional-anatomical validation and individual variation of diffusion tractography-based segmentation of the human thalamus. Cereb. Cortex N. Y. N. 2005;1991(15):31–39. doi: 10.1093/cercor/bhh105. [DOI] [PubMed] [Google Scholar]
- Keren N.I., Lozar C.T., Harris K.C., Morgan P.S., Eckert M.A. In vivo mapping of the human locus coeruleus. NeuroImage. 2009;47:1261–1267. doi: 10.1016/j.neuroimage.2009.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim C.-H., Hahn M.K., Joung Y., Anderson S.L., Steele A.H., Mazei-Robinson M.S., Gizer I., Teicher M.H., Cohen B.M., Robertson D., Waldman I.D., Blakely R.D., Kim K.-S. A polymorphism in the norepinephrine transporter gene alters promoter activity and is associated with attention-deficit hyperactivity disorder. Proc. Natl. Acad. Sci. U. S. A. 2006;103:19164–19169. doi: 10.1073/pnas.0510836103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinomura S., Larsson J., Gulyás B., Roland P.E. Activation by attention of the human reticular formation and thalamic intralaminar nuclei. Science. 1996;271:512–515. doi: 10.1126/science.271.5248.512. [DOI] [PubMed] [Google Scholar]
- Kohli U., Hahn M.K., English B.A., Sofowora G.G., Muszkat M., Li C., Blakely R.D., Stein C.M., Kurnik D. Genetic variation in the presynaptic norepinephrine transporter is associated with blood pressure responses to exercise in healthy humans. Pharmacogenet. Genomics. 2011;21:171–178. doi: 10.1097/FPC.0b013e328344f63e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraguljac N.V., Frölich M.A., Tran S., White D.M., Nichols N., Barton-McArdle A., Reid M.A., Bolding M.S., Lahti A.C. Ketamine modulates hippocampal neurochemistry and functional connectivity: a combined magnetic resonance spectroscopy and resting-state fMRI study in healthy volunteers. Mol. Psychiatry. 2017;22:562–569. doi: 10.1038/mp.2016.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krystal J.H., Karper L.P., Seibyl J.P., Freeman G.K., Delaney R., Bremner J.D., Heninger G.R., Bowers M.B., Charney D.S. Subanesthetic effects of the noncompetitive NMDA antagonist, ketamine, in humans. Psychotomimetic, perceptual, cognitive, and neuroendocrine responses. Arch. Gen. Psychiatry. 1994;51:199–214. doi: 10.1001/archpsyc.1994.03950030035004. [DOI] [PubMed] [Google Scholar]
- Lee C.M., Javitch J.A., Snyder S.H. Recognition sites for norepinephrine uptake: regulation by neurotransmitter. Science. 1983;220:626–629. doi: 10.1126/science.6301013. [DOI] [PubMed] [Google Scholar]
- Liebe T., Li S., Lord A., Colic L., Krause A.L., Batra A., Kretzschmar M.A., Sweeney-Reed C.M., Behnisch G., Schott B.H., Walter M. Factors Influencing the Cardiovascular Response to Subanesthetic Ketamine: a Randomized, Placebo-Controlled Trial. Int. J. Neuropsychopharmacol. 2017;20:909–918. doi: 10.1093/ijnp/pyx055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin J.C., Tsao W.L., Wang Y. Cardiovascular effects of NMDA in the RVLM of spontaneously hypertensive rats. Brain Res. Bull. 1995;37:289–294. doi: 10.1016/0361-9230(95)00014-6. [DOI] [PubMed] [Google Scholar]
- Luckenbaugh D.A., Niciu M.J., Ionescu D.F., Nolan N.M., Richards E.M., Brutsche N.E., Guevara S., Zarate C.A. Do the dissociative side effects of ketamine mediate its antidepressant effects? J. Affect. Disord. 2014;159:56–61. doi: 10.1016/j.jad.2014.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macey D.J., Smith H.R., Nader M.A., Porrino L.J. Chronic cocaine self-administration upregulates the norepinephrine transporter and alters functional activity in the bed nucleus of the stria terminalis of the rhesus monkey. J. Neurosci. 2003;23:12–16. doi: 10.1523/JNEUROSCI.23-01-00012.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maldjian J.A., Laurienti P.J., Kraft R.A., Burdette J.H. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. NeuroImage. 2003;19:1233–1239. doi: 10.1016/s1053-8119(03)00169-1. [DOI] [PubMed] [Google Scholar]
- May A. Neuroimaging: visualising the brain in pain. Neurol. Sci. Off. J. Ital. Neurol. Soc. Ital. Soc. Clin. Neurophysiol. 2007;28(Suppl. 2):S101–S107. doi: 10.1007/s10072-007-0760-x. [DOI] [PubMed] [Google Scholar]
- McCormick D.A., Pape H.C., Williamson A. Actions of norepinephrine in the cerebral cortex and thalamus: implications for function of the central noradrenergic system. Prog. Brain Res. 1991;88:293–305. doi: 10.1016/s0079-6123(08)63817-0. [DOI] [PubMed] [Google Scholar]
- McFarland N.R., Haber S.N. Thalamic relay nuclei of the basal ganglia form both reciprocal and nonreciprocal cortical connections, linking multiple frontal cortical areas. J. Neurosci. 2002;22:8117–8132. doi: 10.1523/JNEUROSCI.22-18-08117.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKeown M.J., Makeig S., Brown G.G., Jung T.P., Kindermann S.S., Bell A.J., Sejnowski T.J. Analysis of fMRI data by blind separation into independent spatial components. Hum. Brain Mapp. 1998;6:160–188. doi: 10.1002/(SICI)1097-0193(1998)6:3<160::AID-HBM5>3.0.CO;2-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metzger C.D., van der Werf Y.D., Walter M. Functional mapping of thalamic nuclei and their integration into cortico-striatal-thalamo-cortical loops via ultra-high resolution imaging—from animal anatomy to in vivo imaging in humans. Front. Neurosci. 2013;7 doi: 10.3389/fnins.2013.00024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miletich D.J., Ivankovic A.D., Albrecht R.F., Zahed B., Ilahi A.A. The effect of ketamine on catecholamine metabolism in the isolated perfused rat heart. Anesthesiology. 1973;39:271–277. doi: 10.1097/00000542-197309000-00003. [DOI] [PubMed] [Google Scholar]
- Montagnese C.M., Mezey S.E., Csillag A. Efferent connections of the dorsomedial thalamic nuclei of the domestic chick (Gallus domesticus) J. Comp. Neurol. 2003;459:301–326. doi: 10.1002/cne.10612. [DOI] [PubMed] [Google Scholar]
- Moret C., Briley M. The importance of norepinephrine in depression. Neuropsychiatr. Dis. Treat. 2011;7:9–13. doi: 10.2147/NDT.S19619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriguchi S., Yamada M., Takano H., Nagashima T., Takahata K., Yokokawa K., Ito T., Ishii T., Kimura Y., Zhang M.-R., Mimura M., Suhara T. Norepinephrine Transporter in Major Depressive Disorder: a PET Study. Am. J. Psychiatry. 2017;174:36–41. doi: 10.1176/appi.ajp.2016.15101334. [DOI] [PubMed] [Google Scholar]
- Mouton P.R., Pakkenberg B., Gundersen H.J., Price D.L. Absolute number and size of pigmented locus coeruleus neurons in young and aged individuals. J. Chem. Neuroanat. 1994;7:185–190. doi: 10.1016/0891-0618(94)90028-0. [DOI] [PubMed] [Google Scholar]
- Murrough J.W., Iosifescu D.V., Chang L.C., Al Jurdi R.K., Green C.E., Perez A.M., Iqbal S., Pillemer S., Foulkes A., Shah A., Charney D.S., Mathew S.J. Antidepressant efficacy of ketamine in treatment-resistant major depression: a two-site randomized controlled trial. Am. J. Psychiatry. 2013;170:1134–1142. doi: 10.1176/appi.ajp.2013.13030392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musso F., Brinkmeyer J., Ecker D., London M.K., Thieme G., Warbrick T., Wittsack H.-J., Saleh A., Greb W., de Boer P., Winterer G. Ketamine effects on brain function--simultaneous fMRI/EEG during a visual oddball task. NeuroImage. 2011;58:508–525. doi: 10.1016/j.neuroimage.2011.06.045. [DOI] [PubMed] [Google Scholar]
- Niesters M., Khalili-Mahani N., Martini C., Aarts L., van Gerven J., van Buchem M.A., Dahan A., Rombouts S. Effect of subanesthetic ketamine on intrinsic functional brain connectivity: a placebo-controlled functional magnetic resonance imaging study in healthy male volunteers. Anesthesiology. 2012;117:868–877. doi: 10.1097/ALN.0b013e31826a0db3. [DOI] [PubMed] [Google Scholar]
- Nikiforuk A., Popik P. The effects of acute and repeated administration of ketamine on attentional performance in the five-choice serial reaction time task in rats. Eur. Neuropsychopharmacol. J. Eur. Coll. Neuropsychopharmacol. 2014;24:1381–1393. doi: 10.1016/j.euroneuro.2014.04.007. [DOI] [PubMed] [Google Scholar]
- Nishimura M., Sato K., Okada T., Yoshiya I., Schloss P., Shimada S., Tohyama M. Ketamine inhibits monoamine transporters expressed in human embryonic kidney 293 cells. Anesthesiology. 1998;88:768–774. doi: 10.1097/00000542-199803000-00029. [DOI] [PubMed] [Google Scholar]
- Okamoto L.E., Shibao C., Gamboa A., Choi L., Diedrich A., Raj S.R., Black B.K., Robertson D., Biaggioni I. Synergistic effect of norepinephrine transporter blockade and ALPHA-2 antagonism on blood pressure in autonomic failure. Hypertension. 2012;59:650–656. doi: 10.1161/HYPERTENSIONAHA.111.184812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olbrich S., Sander C., Minkwitz J., Chittka T., Mergl R., Hegerl U., Himmerich H. EEG vigilance regulation patterns and their discriminative power to separate patients with major depression from healthy controls. Neuropsychobiology. 2012;65:188–194. doi: 10.1159/000337000. [DOI] [PubMed] [Google Scholar]
- Oranje B., van Berckel B.N., Kemner C., van Ree J.M., Kahn R.S., Verbaten M.N. The effects of a sub-anaesthetic dose of ketamine on human selective attention. Neuropsychopharmacol. Off. Publ. Am. Coll. Neuropsychopharmacol. 2000;22:293–302. doi: 10.1016/S0893-133X(99)00118-9. [DOI] [PubMed] [Google Scholar]
- Parnaudeau S., O'Neill P.-K., Bolkan S.S., Ward R.D., Abbas A.I., Roth B.L., Balsam P.D., Gordon J.A., Kellendonk C. Inhibition of mediodorsal thalamus disrupts thalamofrontal connectivity and cognition. Neuron. 2013;77:1151–1162. doi: 10.1016/j.neuron.2013.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons C.G., Danysz W., Quack G. Memantine is a clinically well tolerated N-methyl-D-aspartate (NMDA) receptor antagonist--a review of preclinical data. Neuropharmacology. 1999;38:735–767. doi: 10.1016/s0028-3908(99)00019-2. [DOI] [PubMed] [Google Scholar]
- Peltoniemi M.A., Hagelberg N.M., Olkkola K.T., Saari T.I. Ketamine: a Review of Clinical Pharmacokinetics and Pharmacodynamics in Anesthesia and Pain Therapy. Clin. Pharmacokinet. 2016;55:1059–1077. doi: 10.1007/s40262-016-0383-6. [DOI] [PubMed] [Google Scholar]
- Périn B., Godefroy O., Fall S., de Marco G. Alertness in young healthy subjects: an fMRI study of brain region interactivity enhanced by a warning signal. Brain Cogn. 2010;72:271–281. doi: 10.1016/j.bandc.2009.09.010. [DOI] [PubMed] [Google Scholar]
- Peters S.K., Dunlop K., Downar J. Cortico-Striatal-Thalamic Loop Circuits of the Salience Network: a Central Pathway in Psychiatric Disease and Treatment. Front. Syst. Neurosci. 2016;10 doi: 10.3389/fnsys.2016.00104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen S.E., Posner M.I. The attention system of the human brain: 20 years after. Annu. Rev. Neurosci. 2012;35:73–89. doi: 10.1146/annurev-neuro-062111-150525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power J.D., Barnes K.A., Snyder A.Z., Schlaggar B.L., Petersen S.E. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. NeuroImage. 2012;59:2142–2154. doi: 10.1016/j.neuroimage.2011.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powers A.R., Gancsos M.G., Finn E.S., Morgan P.T., Corlett P.R. Ketamine-Induced Hallucinations. Psychopathology. 2015;48:376–385. doi: 10.1159/000438675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadaghiani S., D'Esposito M. Functional Characterization of the Cingulo-Opercular Network in the Maintenance of Tonic Alertness. Cereb. Cortex N. Y. N. 2015;1991(25):2763–2773. doi: 10.1093/cercor/bhu072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvadore G., Cornwell B.R., Sambataro F., Latov D., Colon-Rosario V., Carver F., Holroyd T., Diazgranados N., Machado-Vieira R., Grillon C., Drevets W.C., Zarate C.A. Anterior cingulate desynchronization and functional connectivity with the amygdala during a working memory task predict rapid antidepressant response to ketamine. Neuropsychopharmacol. Off. Publ. Am. Coll. Neuropsychopharmacol. 2010;35:1415–1422. doi: 10.1038/npp.2010.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuels E., Szabadi E. Functional Neuroanatomy of the Noradrenergic Locus Coeruleus: its Roles in the Regulation of Arousal and Autonomic Function Part I: Principles of Functional Organisation. Curr. Neuropharmacol. 2008;6:235–253. doi: 10.2174/157015908785777229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki M., Shibata E., Tohyama K., Takahashi J., Otsuka K., Tsuchiya K., Takahashi S., Ehara S., Terayama Y., Sakai A. Neuromelanin magnetic resonance imaging of locus ceruleus and substantia nigra in Parkinson's disease. Neuroreport. 2006;17:1215–1218. doi: 10.1097/01.wnr.0000227984.84927.a7. [DOI] [PubMed] [Google Scholar]
- Schou M., Halldin C., Pike V.W., Mozley P.D., Dobson D., Innis R.B., Farde L., Hall H. Post-mortem human brain autoradiography of the norepinephrine transporter using (S,S)-[18F]FMeNER-D2. Eur. Neuropsychopharmacol. J. Eur. Coll. Neuropsychopharmacol. 2005;15:517–520. doi: 10.1016/j.euroneuro.2005.01.007. [DOI] [PubMed] [Google Scholar]
- Schroeder C., Jordan J. Norepinephrine transporter function and human cardiovascular disease. AJP Heart Circ. Physiol. 2012;303:H1273–H1282. doi: 10.1152/ajpheart.00492.2012. [DOI] [PubMed] [Google Scholar]
- Seeley W.W., Menon V., Schatzberg A.F., Keller J., Glover G.H., Kenna H., Reiss A.L., Greicius M.D. Dissociable intrinsic connectivity networks for salience processing and executive control. J. Neurosci. 2007;27:2349–2356. doi: 10.1523/JNEUROSCI.5587-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Short B., Fong J., Galvez V., Shelker W., Loo C.K. Side-effects associated with ketamine use in depression: a systematic review. Lancet Psychiatry. 2018;5:65–78. doi: 10.1016/S2215-0366(17)30272-9. [DOI] [PubMed] [Google Scholar]
- Sigurdardottir H.L., Kranz G.S., Rami-Mark C., James G.M., Vanicek T., Gryglewski G., Kautzky A., Hienert M., Traub-Weidinger T., Mitterhauser M., Wadsak W., Hacker M., Rujescu D., Kasper S., Lanzenberger R. Effects of norepinephrine transporter gene variants on NET binding in ADHD and healthy controls investigated by PET. Hum. Brain Mapp. 2016;37:884–895. doi: 10.1002/hbm.23071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sleigh J., Harvey M., Voss L., Denny B. Ketamine – more mechanisms of action than just NMDA blockade. Trends Anaesth. Crit. Care. 2014;4:76–81. [Google Scholar]
- Spirito A., Esposito-Smythers C., Wolff J., Uhl K. Cognitive-behavioral therapy for adolescent depression and suicidality. Child Adolesc. Psychiatr. Clin. N. Am. 2011;20:191–204. doi: 10.1016/j.chc.2011.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweeney-Reed C.M., Zaehle T., Voges J., Schmitt F.C., Buentjen L., Kopitzki K., Richardson-Klavehn A., Hinrichs H., Heinze H.-J., Knight R.T., Rugg M.D. Pre-stimulus thalamic theta power predicts human memory formation. NeuroImage. 2016;138:100–108. doi: 10.1016/j.neuroimage.2016.05.042. [DOI] [PubMed] [Google Scholar]
- Szot P., Franklin A., Miguelez C., Wang Y., Vidaurrazaga I., Ugedo L., Sikkema C., Wilkinson C.W., Raskind M.A. Depressive-like behavior observed with a minimal loss of locus coeruleus (LC) neurons following administration of 6-hydroxydopamine is associated with electrophysiological changes and reversed with precursors of norepinephrine. Neuropharmacology. 2016;101:76–86. doi: 10.1016/j.neuropharm.2015.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takano A., Varrone A., Gulyás B., Karlsson P., Tauscher J., Halldin C. Mapping of the norepinephrine transporter in the human brain using PET with (S,S)-[18F]FMeNER-D2. NeuroImage. 2008;42:474–482. doi: 10.1016/j.neuroimage.2008.05.040. [DOI] [PubMed] [Google Scholar]
- Umbricht D., Schmid L., Koller R., Vollenweider F.X., Hell D., Javitt D.C. Ketamine-induced deficits in auditory and visual context-dependent processing in healthy volunteers: implications for models of cognitive deficits in schizophrenia. Arch. Gen. Psychiatry. 2000;57:1139–1147. doi: 10.1001/archpsyc.57.12.1139. [DOI] [PubMed] [Google Scholar]
- Usher M., Cohen J.D., Servan-Schreiber D., Rajkowski J., Aston-Jones G. The role of locus coeruleus in the regulation of cognitive performance. Science. 1999;283:549–554. doi: 10.1126/science.283.5401.549. [DOI] [PubMed] [Google Scholar]
- Van der Werf Y.D., Scheltens P., Lindeboom J., Witter M.P., Uylings H.B.M., Jolles J. Deficits of memory, executive functioning and attention following infarction in the thalamus; a study of 22 cases with localised lesions. Neuropsychologia. 2003;41:1330–1344. doi: 10.1016/s0028-3932(03)00059-9. [DOI] [PubMed] [Google Scholar]
- Vincent J.L., Kahn I., Snyder A.Z., Raichle M.E., Buckner R.L. Evidence for a frontoparietal control system revealed by intrinsic functional connectivity. J. Neurophysiol. 2008;100:3328–3342. doi: 10.1152/jn.90355.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vollenweider F.X., Kometer M. The neurobiology of psychedelic drugs: implications for the treatment of mood disorders. Nat. Rev. Neurosci. 2010;11:642–651. doi: 10.1038/nrn2884. [DOI] [PubMed] [Google Scholar]
- Vukadinovic Z. NMDA receptor hypofunction and the thalamus in schizophrenia. Physiol. Behav. 2014;131:156–159. doi: 10.1016/j.physbeh.2014.04.038. [DOI] [PubMed] [Google Scholar]
- Walter M., Bermpohl F., Mouras H., Schiltz K., Tempelmann C., Rotte M., Heinze H.J., Bogerts B., Northoff G. Distinguishing specific sexual and general emotional effects in fMRI-subcortical and cortical arousal during erotic picture viewing. NeuroImage. 2008;40:1482–1494. doi: 10.1016/j.neuroimage.2008.01.040. [DOI] [PubMed] [Google Scholar]
- Weinshenker D., White S.S., Javors M.A., Palmiter R.D., Szot P. Regulation of norepinephrine transporter abundance by catecholamines and desipramine in vivo. Brain Res. 2002;946:239–246. doi: 10.1016/s0006-8993(02)02889-5. [DOI] [PubMed] [Google Scholar]
- Williams N.R., Schatzberg A.F. NMDA antagonist treatment of depression. Curr. Opin. Neurobiol. 2016;36:112–117. doi: 10.1016/j.conb.2015.11.001. [DOI] [PubMed] [Google Scholar]
- Wu C.W., Chen C.-L., Liu P.-Y., Chao Y.-P., Biswal B.B., Lin C.-P. Empirical evaluations of slice-timing, smoothing, and normalization effects in seed-based, resting-state functional magnetic resonance imaging analyses. Brain Connect. 2011;1:401–410. doi: 10.1089/brain.2011.0018. [DOI] [PubMed] [Google Scholar]
- Xuan B., Mackie M.-A., Spagna A., Wu T., Tian Y., Hof P.R., Fan J. The activation of interactive attentional networks. NeuroImage. 2016 doi: 10.1016/j.neuroimage.2016.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zang Y.-F., He Y., Zhu C.-Z., Cao Q.-J., Sui M.-Q., Liang M., Tian L.-X., Jiang T.-Z., Wang Y.-F. Altered baseline brain activity in children with ADHD revealed by resting-state functional MRI. Brain Dev. 2007;29:83–91. doi: 10.1016/j.braindev.2006.07.002. [DOI] [PubMed] [Google Scholar]
- Zanos P., Moaddel R., Morris P.J., Georgiou P., Fischell J., Elmer G.I., Alkondon M., Yuan P., Pribut H.J., Singh N.S., Dossou K.S.S., Fang Y., Huang X.-P., Mayo C.L., Wainer I.W., Albuquerque E.X., Thompson S.M., Thomas C.J., Zarate C.A., Gould T.D. NMDAR inhibition-independent antidepressant actions of ketamine metabolites. Nature. 2016;533:481–486. doi: 10.1038/nature17998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarate C.A., Singh J.B., Carlson P.J., Brutsche N.E., Ameli R., Luckenbaugh D.A., Charney D.S., Manji H.K. A randomized trial of an N-methyl-D-aspartate antagonist in treatment-resistant major depression. Arch. Gen. Psychiatry. 2006;63:856–864. doi: 10.1001/archpsyc.63.8.856. [DOI] [PubMed] [Google Scholar]
- Zhang S., Hu S., Chao H.H., Li C.-S.R. Comparison with the Ventral Tegmental Area/Substantia Nigra Pars Compacta and the Effects of Age. Cereb. Cortex N. Y. N 1991. Vol. 26. 2016. Resting-State Functional Connectivity of the Locus Coeruleus in Humans; pp. 3413–3427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y., Sun L. Antidepressants modulate the in vitro inhibitory effects of propofol and ketamine on norepinephrine and serotonin transporter function. J. Clin. Neurosci. Off. J. Neurosurg. Soc. Australas. 2008;15:1264–1269. doi: 10.1016/j.jocn.2007.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou W., Wang N., Yang C., Li X.-M., Zhou Z.-Q., Yang J.-J. Ketamine-induced antidepressant effects are associated with AMPA receptors-mediated upregulation of mTOR and BDNF in rat hippocampus and prefrontal cortex. Eur. Psychiatry J. Assoc. Eur. Psychiatr. 2014;29:419–423. doi: 10.1016/j.eurpsy.2013.10.005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material