Abstract
Mammalian carboxylesterases (CEs) are key enzymes from the serine hydrolase superfamily. In the human body, two predominant carboxylesterases (CES1 and CES2) have been identified and extensively studied over the past decade. These two enzymes play crucial roles in the metabolism of a wide variety of endogenous esters, ester-containing drugs and environmental toxicants. The key roles of CES in both human health and xenobiotic metabolism arouse great interest in the discovery of potent CES modulators to regulate endobiotic metabolism or to improve the efficacy of ester drugs. This review covers the structural and catalytic features of CES, tissue distributions, biological functions, genetic polymorphisms, substrate specificities and inhibitor properties of CES1 and CES2, as well as the significance and recent progress on the discovery of CES modulators. The information presented here will help pharmacologists explore the relevance of CES to human diseases or to assign the contribution of certain CES in xenobiotic metabolism. It will also facilitate medicinal chemistry efforts to design prodrugs activated by a given CES isoform, or to develop potent and selective modulators of CES for potential biomedical applications.
Keywords: Human carboxylesterases, CES1, CES2, Substrate preference, Inhibitor spectra, Inducer
Graphical abstract
Human carboxylesterases (CES) play crucial roles in both endo- and xenobiotic metabolism, which arouse great interest in the discovery of potent CES modulators to regulate endobiotic metabolism or to improve the outcomes of ester drugs. This review covers the structural and catalytic features of human CES, tissue distributions, biological functions and substrate specificities of two predominant human CES, as well as the significance and recent progress on the discovery of human CES modulators.
1. Introduction
Mammalian carboxylesterases (CES, E.C. 3.1.1.1) are essential members of the serine hydrolase superfamily, which are localized within the lumen of the endoplasmic reticulum in many tissues1, 2, 3. As their name implies, CES catalyze the ester cleavage of a large number of structurally diverse ester- or amide-containing substrates into the corresponding alcohol and carboxylic acid1, 2, 3. Actually, CES can hydrolyze ester, thioester, amide, and carbamate linkages in a wide variety of endo- and xenobiotic compounds, thus playing key roles in both endobiotic metabolism, and in activation and/or detoxification of xenobiotics3, 4, 5. In the human body, three CES have been identified, although human carboxylesterase 1 (CES1) and human carboxylesterase 2 (CES2) are the two extensively studied isoenzymes involved in xenobiotic metabolism3, 4, 5, 6, 7, 8. Both CES1 and CES2 play crucial roles in the metabolism of various ester xenobiotics including many ester drugs (such as oseltamivir, clopidogrel, irinotecan and capecitabine) and environmental toxicants (such as pyrethroids)9, 10, 11, 12, 13. These two enzymes are also known to metabolize endogenous esters including cholesteryl esters, triacylglycerols and other endogenous lipids, thus playing vital physiological functions in lipid homeostasis14, 15, 16, 17, 18.
Over the past twenty years, many studies have provided powerful insight into the roles of CES in metabolic diseases and xenobiotic metabolism14. The key roles of CES in both endogenous and xenobiotic metabolism have attracted great interest in the discovery of CES modulators to regulate lipid metabolism or to enhance the activity of ester drugs19, 20, 21, 22, 23, 24. This review covers the structural and catalytic features of CES, tissue distribution, biological functions, substrate specificities and inhibitor profiles of two predominant CES, as well as the significance and recent progress on the discovery of CES modulators. It will be very helpful for pharmacologists to explore the relevance of CES to human diseases or to confirm the contribution of CES in xenobiotic metabolism. In addition, it will assist medicinal chemists in designing ideal prodrugs which can be activated by a given CES isoform in the human body, or to develop more potent inhibitors/inducers of CES.
2. Structural features and catalytic properties of CES
2.1. Structural features of CES
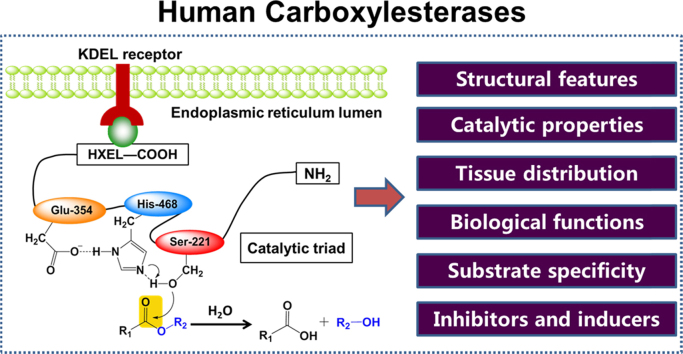
CES belong to the α/β-hydrolase fold superfamily of proteins. The majority of mammalian carboxylesterases are intracellular proteins found predominantly in the microsomal fraction that encompass the endoplasmic reticulum (ER)3, 25, 26. Microsomal CES from human, rabbit, and mouse carry the HXEL motifs of the KDEL consensus ER retrieval sequence at their C-terminal (such as HIEL and HTEL for CES1 and CES2, respectively), which is essential for the localization of these enzymes to the ER lumen in mammalian cells26. Following cleavage of the C-terminal signal peptide, microsomal carboxylesterases can be released from their membrane-associated state, suggesting that these enzymes are not transmembrane proteins but soluble proteins that reside in the ER lumen9.
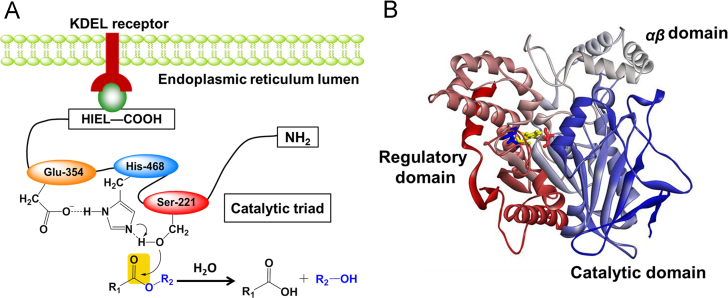
The three-dimensional (3D) structures of several mammalian CEs including human carboxylesterase 1 (CES1) have been solved by X-ray crystallography with several ligands9, 25, 26, 27, 28, 29, 30, 31. As depicted in Fig. 1, CES1 is composed by a central catalytic domain, an αβ domain, and an adjacent regulatory domain which containing the low-affinity surface ligand-binding Z-site28, 30, 31. The X-ray crystal structure of CES1 demonstrated its existence as monomer, trimer, or hexamer, with substrate-dependent equilibrium of homooligomer formation. In contrast, CES2 and CES3 exist as monomers. Both sequence aligments and secondary sequence predictions have suggested that these three CES are members of α/β hydrolase family32, 33, 34. Although the 3D structures of CES2 and CES3 have not been reported, the 3D structure modelling of both CES2 and CES3 can be downloaded from the SWISS-MODEL repository (a database of annotated 3D protein structure models generated by the SWISS-MODEL homology-modelling pipeline).
Figure 1.
The structural features of CES1. (A) The scheme for catalyzing (hydrolysis) ester groups; (B) The 3D structure of CES1. The catalytic triad including Ser221, Glu354 and His468 are colored in red, yellow and blue, respectively.
The molecular properties of CES1 and CES2 are listed in Table 16, 9, 19, 25, 27, 29. Similar to all reported serine hydrolases, the catalytic domain of human CEs contain a catalytic triad (such as Ser221, Glu354, and His468 in CES1) at the interface of the three domains, which is highly conserved among all mammalian carboxylesterases and is crucial for carboxylesterases-mediated catalysis (Fig. 1B)9, 27. Mutation of any residue of the catalytic triad will lead to the loss of carboxylesterase activity18. Furthermore, the oxyanion hole formed by Gly142 and Gly143 in the HGGG motif is also highly conserved among all mammalian carboxylesterases and is essential for carboxylesterase activity. Notably, the active cavity of human CES1 is quite large (about 1,300 Å3) and is lined mainly by hydrophobic amino acids, except the residues (such as Ser221) of the catalytic triad. The residue Ser221 divides the whole ligand-binding pocket of CES1 into two pockets, one is a rigid pocket which makes CES1 selective for those substrates with small acyl group, and another is a flexible pocket which makes CES1 promiscuous towards a wide range of esters with various acyl groups28. These features make CES1 capable of interacting with a wide variety of chemically diverse ligands28.
Table 1.
| Property | CES1 | CES2 |
|---|---|---|
| Molecular weight | 60 kD (monomer), 180 kD (trimer) | 60 kD (monomer) |
| Isoelectric point | 5.6—5.8 | 4.8—5.0 |
| Optimal pH | 6.5 | 7.5—8.0 |
| C-terminal signal peptide | HIEL | HTEL |
| Catalytic triad | Ser221, Glu354 and His468 | Ser228, Glu345 and His457 |
| Glycosylation site | Asn-X-Thr and Asn79 | Asn-X-Ser/Thr, Asn103and Asn267 |
2.2. The catalytic properties of CES
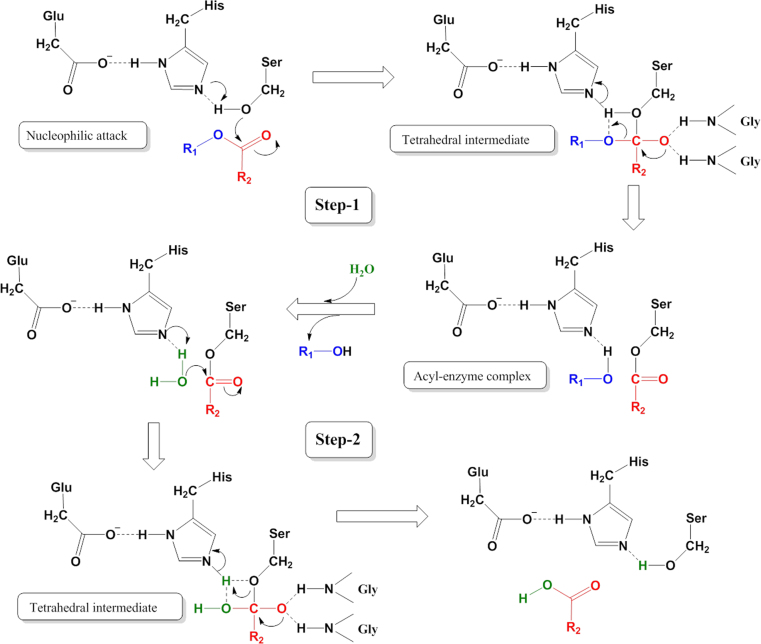
The CES hydrolyze substrates using a classic base-catalysed mechanism via a two-step reaction which is conserved in all serine hydrolases, including proteases, peptidases and lipases (Fig. 2)3, 5, 6. This process is dependent on an essential catalytic triad which is generally composed by three residues (Ser, His, and Glu) within the active cavity of mammalian carboxylesterases3, 5, 6. First, a nucleophilic attack by the base-activated serine oxygen atom (such as Ser221 in human CES1) on the carbonyl carbon of the substrate lead to the formation of an acyl-enzyme intermediate and the release of an alcohol, thiol, or amine product (Fig. 2). Second, the acyl-enzyme intermediate is attacked in an identical fashion with water acting as the nucleophile, leading to release of the carboxylic acid metabolite, which regenerates the carboxylesterase to its original state with the free serine residue5, 6.
Figure 2.
The two-step catalytic mechanism of mammalian carboxylesterases.
Notably, several mammalian CES (especially CES1) can perform transesterification reactions. When ethyl alcohol is present in the CES1 reaction system, alcohol replaces water to attack the acyl-enzyme intermediate to generate an ethyl ester product. One of the well-studied examples of this reaction is the formation of cocaethylene in individuals abusing both cocaine and alcohol32, 33, 34, 35, 36. Under these conditions, the ethyl group from ethanol replaces the methyl group of cocaine to produce a more toxic metabolite cocaethylene34, 35. Furthermore, CES1 can catalyze the creation of cholesteryl esters from cholesterol and fatty acids, as well as to generate fatty acid ethyl esters (FAEEs) from fatty acyl-Coenzyme A (CoA) and ethanol, using a transesterification reaction36, 37. Because CES have cholesteryl esters hydrolysis and FAEE hydrolysis activities, the formation of these endogenous esters is the result of the balance between typical hydrolysis reactions and transesterification reactions.
3. Tissue distribution and substrate specificity of CES
Although CES1 and CES2 share 47% amino acid sequence identity, these two enzymes exhibit extremely different substrate distribution and specificity5, 6. Typically, CES1 and CES2 are highly expressed in the epithelia of most metabolic organs including liver, intestine and kidney, indicating that these two enzymes play a protective role against xenobiotics. CES1 is abundantly expressed in the liver and adipocyte, with lesser amounts in the kidney, monocytes, lung, intestine, testis, heart, and macrophages1, 8, 38, 39, 40, 41, 42, 43, 44, 45. In contrast, CES2 is expressed mainly in the small intestine and colon, but also observed in kidney, liver, heart, brain and testis1, 8, 42. Quantitative data on CES abundance in the human liver microsomes (HLMs) and liver cytosol (HLC) have been reported. Protein levels of CES1 and CES2 in 16 individual HLMs were 402 and 29.8 pmol/mg, respectively, while in HLC were 54.5 and 2.76 pmol/mg, respectively39. Furthermore, the secreted forms of CES and very high activity levels of CES were detected in rodent blood, yet CES1 and CES2 activities were barely detected in human blood43, 44. Notably, the expression profiles of CES1 and CES2 in tumour tissues or cancer cell lines differ markedly from those of healthy cells. For example, human Caco-2 cells mainly express the CES1 isoenzyme, yet protein levels of CES1 in the human intestine are extremely low42. In addition, CES2 is overexpressed in several types of cancer or cancer cell lines, including multiple myeloma, thyroid papillary carcinoma, esophageal squamous carcinoma, and kidney adenocarcinoma46. CES2 expression in cancer cell lines and tumour tissue significantly correlate with bioactivation of several cancer prodrugs (such as irinotecan and LY2334737, the prodrug of gemcitabine), in agreement with the anticancer effects of these drugs46, 47, 48. These results suggest that the rational design of CES2-bioactivated prodrugs will be very useful for cancer therapy.
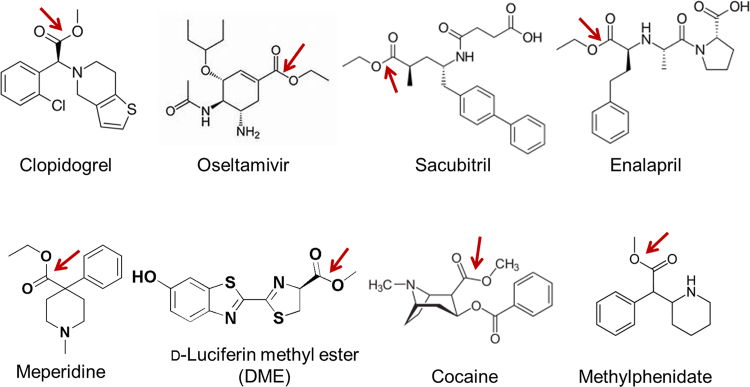
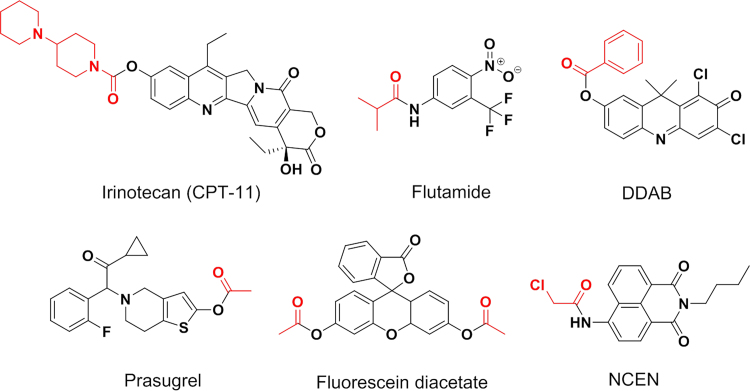
Human carboxylesterases have a broad substrate specificity, which can hydrolyze a vast number of endo- and xenobiotic substrates with ester, thioester, carbamate, and amide bonds (Fig. 3, Fig. 4, and Supplementary Information Table S1)4, 5, 6, 9, 10, 11, 24, 49, 50, 51, 52, 53, 54, 55, 56, 57. Over the past decade, many studies have reported that CES1 and CES2 exhibit distinct substrate specificities. The representative substrates for CES1 and CES2 have been depicted in Fig. 3, and Fig. 4, respectively4, 5, 6, 9, 10, 11, 49, 50, 51, 52, 53, 54, 55, 56, 57. In general, CES1 prefers to metabolize the ester substrates that contain a small alcohol group and a bulky acyl group, such as enalapril, oseltamivir, imidapril, clopidogrel, meperidine, D-luciferin methyl ester, and the illegal drugs heroin and cocaine5, 6, 49. In contrast, CES2 prefers to hydrolyse esters with a relatively large alcohol group and a small acyl group, such as irinotecan, prasugrel, capecitabine, flutamide, and fluorescein diacetate9, 50, 51, 52, 57. However, several substrates with a small acyl group, such as R-propionyl propranolol, can also be hydrolyzed by CES1. As mentioned above, CES1 has two ligand-binding pockets, one is a rigid pocket and another is a flexible pocket, which makes CES1 promiscuous towards a vast number of substrates32. The substrate specificity of CES3 has not been extensively studied, but CES3 has been found with irinotecan hydrolysis activity but exhibits much lower hydrolysis activity, compared with CES257. Based on the substrate specificities of both CES1 and CES2, some optical probe substrates have been recently developed for assessing the real activities of CES1 or CES2 in complex biological systems (Supplementary Information Table S2)49, 51, 58, 59, 60, 61, 62, 63, 64. These optical probes provide practical and efficient tools for high-throughput screening (HTS) of CES modulators in cell/tissue preparations or even in living cells, due to the inherent advantages including non-destructive, highly sensitive, easily managed, and applicable to HTS assay49, 58, 59, 60, 61, 62, 63, 64, 65, 66, 67, 68, 69.
Figure 3.
Substrate specificity of CES1. Generally, CES1 prefers to hydrolyze the substrates containing a small alcohol group and a bulky acyl group.
Figure 4.
Substrate specificity of CES2. CES2 prefers to hydrolyze the esters with a relatively large alcohol group and a small acyl group.
4. Biological functions of CES
The primary physiological function of CES appears to be xenobiotic metabolism. Over the past twenty years, CES have been regarded as the classic xenobiotic-metabolizing enzymes which are responsible for in the metabolism of a variety of ester-containing drugs, prodrugs, and environmental toxins. Many clinical drugs with ester moieties can be readily hydrolyzed by CES. Such compounds include the anticancer prodrugs (such as irinotecan, capecitabine), opioids and stimulants (cocaine, heroin, and meperidine), angiotensin-converting enzyme inhibitors (enalapril, temocapril, imidapril and quinapril), and other drugs with ester moieties (oseltamivir, clopidogrel, flumazenil, procaine, oxybutynin, delapril, flutamide, and prasugrel)3, 4, 5, 6, 70. In addition to drug metabolism, CES also participate in the detoxification and metabolic clearance of many ester-containing toxicants, such as the pyrethroid insecticides (deltamethrin and permethrin)12, 71. Notably, strong inhibition of CES may slow down the hydrolysis of CES substrates, which may affect their pharmacokinetic properties and thus modulate their activities in vivo13, 22. For example, irinotecan (CES2 substrate) can trigger severe delayed diarrhea due to the overproduction of SN-38 (the hydrolytic product of irinotecan) in the small intestine, but co-administration with potent CES2 inhibitors may ameliorate CPT-11 caused severe diarrhea in patients and thus improve the therapeutic effect72, 73. Besides the well-known roles of CES in xenobiotic metabolism, these enzymes have recently been studied for their participation in endogenous metabolism. As one of the most abundant serine hydrolases found in human hepatocytes and adipocytes, CES1 is responsible for the hydrolysis of a vast number of endogenous esters (such as cholesteryl esters and triacylglycerols) thereby participating in physiological and pathological processes, such as lipid metabolism, cholesterol homeostasis, and fatty liver disease14, 74, 75, 76. Notably, it has been shown that the protein expression and enzymatic activities of CES1 in adipose tissues from obese and type 2 diabetic patients are markedly elevated compared to lean subjects, yet treatment with CE1 inhibitors has multiple beneficial effects on both glucose and lipid homeostasis in mice20. More recently, CES2 was shown to exhibit triacylglycerol and diacylglycerol hydrolase activity, and its expression and function in liver are strongly related to several metabolic diseases, such as obesity and non-alcoholic steatohepatitis16, 20, 77.
Besides key roles in both endo- and xenobiotic metabolism, CES also have other biological roles, such as trafficking and retention of proteins in the endoplasmic reticulum (ER). In the ER, CES1 and CES2 appear to regulate protein trafficking, including release of proteins. For instance, CES1 can directly bind to the C-reactive protein (CRP) and retain this small protein before its release into the plasma78. Both CES use a region of amino acid sequence adjacent to the ‘side door’, which is comprised of the loop between α15 and β18, to contact CRP. These CEs could hold a small reservoir of CRP within the ER, and then release it during the stage of tissue injury. It has also been reported that CES can directly interact with β-glucuronidases, the enzymes responsible for the removal of glucuronic acid moieties which are typically conjugated to drugs and endobiotics by the UDP-glucuronosyltransferase (UGT) enzymes, in the ER79. Although the interactions between β-glucuronidases with CEs have not been extensively investigated, several studies have demonstrated that some compounds (such as organophosphate) are capable of inducing the release of β-glucuronidases from the ER by disrupting the β-glucuronidase-CES1 complex79.
5. Genetic polymorphisms of CES
The genomic structures of CES1 and CES2 has been ascertained, and both are located on 16q13-q22.180. Over the past decade, a vast number of single-nucleotide polymorphisms (SNPs) have been reported in the NCBI SNP database. It is worth note that the allele and haplotype frequencies of known SNPs showed significant differences among ethnic groups. For instance, the G143E and the D260fs variants were two important functional SNPs in Caucasian populations, while these two CES1 polymorphisms were not found in a Korean population80. To date, a number of functional genetic variants of CES1 and CES2 have been reported, which may be associated with substantial individual variations in the responses to pharmacologic therapies (Table 210, 38, 70, 81, 82). Clopidogrel pharmacotherapy has been associated with substantial inter-individual variability in clinical responses. Zhu et al.10 found the CES1 variants G143E and D260fs showed diminished enzymatic activity, which impaired the hydrolysis of clopidogrel and 2-oxo-clopidogrel. Tang et al.38 reported the antiplatelet agent aspirin could be hydrolyzed by CES2 and the CES2 variant A139T showed decreased CES activity accounting for the decreased aspirin hydrolysis. The association between SNPs in the human CES2 gene and CPT-11 hydrolysis has also been investigated81, 83. Among Japanese subjects, the CES2 variants rs72547531 and rs72547532 were associated with decreased CES2 activity and reduced CPT-11 hydrolysis activity in vivo81. Taking into account that both CES1 and CES2 are the major enzymes responsible for the hydrolysis of many clinically-used ester drugs, the SNPs of CES are therefore recognized as important pharmacogenetic regulators of xenobiotic ester metabolism and therefore treatment outcomes with these drugs. Functional analysis of the novel genetic polymorphisms of CES that can lead to clinically significant alterations in pharmacokinetics and drug responses of their substrates has become essential.
Table 2.
Significant genetic variants of CES and the associated effects on drug metabolism10, 38, 70, 81, 82.
| SNP | Drug | Function |
|
|---|---|---|---|
| In vitro | In vivo | ||
| CES1 rs2244613 | Dabigatran etexilate | - | Decrease in trough concentrations of dabigatran etexilate |
| CES1 rs71647871 | Methylphenidate | Decrease the catalytic function of CES1 | Required lower doses of methylphenidate for symptom reduction |
| CES1 rs71647871 | Oseltamivir | Decrease the catalytic function of CES1 | - |
| CES1 rs71647871 | Clopidogrel | Decrease the catalytic function of CES1 | Significantly higher levels of active clopidogrel metabolite (P = 0.001) and better clopidogrel response |
| CES1 rs121912777 | Oseltamivir | - | Increased oseltamivir AUC and 23% smaller carboxylatetooseltamivir AUC |
| CES1 rs3785161 | Imidapril | - | The responder rate was significantly higher |
| CES2 A139T | Aspirin | 40% Maximal decrease in CES2 functioning and, thus, decreased aspirin hydrolysis | - |
| CES2 rs72547531 | Irinotecan | Associated with low in vitro expression and function of CES2 | Reduced in vivo CES2 activity in irinotecantreated patients |
| CES2 rs72547532 | Irinotecan | Associated with low in vitro expression and function of CES2 | Reduced in vivo CES2 activity in irinotecantreated patients |
-Not assessed.
6. CES inhibitors
The key roles of CES in both human health and xenobiotic metabolism arouse great interest in the discovery of potent modulators to regulate enzyme expression in order to modulate endogenous metabolism or to improve patient responses to ester drugs. With this goal in mind, many small molecule inhibitors or inducers of CES have been identified with the specific intention of altering enzyme activity for therapeutic purposes.
6.1. Clinical drugs and pharmaceutical excipients
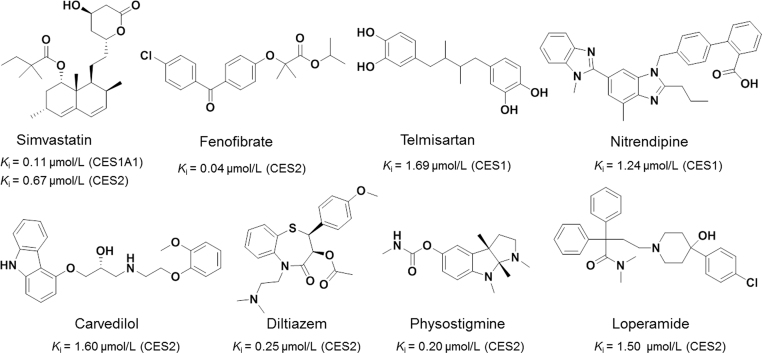
The crucial roles of CES in the metabolism of many ester-containing drugs suggest that some drugs might serve as CES inhibitors with the potential to cause significant drug—drug interactions38, 84, 85. Some antihyperlipidemic drugs, such as simvastatin and fenofibrate, could significantly inhibit the catalytic activities of CES86. It was reported that simvastatin was a potent inhibitor against imidapril hydrolysis in recombinant CES1 with the Ki value of 0.11 µmol/L, while CES2-mediated irinotecan (CPT-11) hydrolysis could be strongly inhibited by both fenofibrate and simvastatin (Fig. 5)72, 87, 88, 89, 90, 91. The antihypertensive drugs telmisartan and nitrendipine, displayed strong inhibitory effects on CES1 with the Ki values of 1.69 and 1.24 µmol/L, respectively87. Carvedilol and diltiazem showed excellent inhibitory effects against CES2 with the Ki values of 1.60 and 0.25 µmol/L, respectively72. Physostigmine, an anticholinesterase drug, was reported to be a strong CES2 inhibitor with the Ki value of 0.20 µmol/L88. Loperamide was often used to treat CPT-11 associated diarrhea, and it was a potent and selective CES2 inhibitor (IC50=1.5 µmol/L)72. Pharmaceutical excipients are applied to obtained appropriate biopharmaceutical and physicochemical properties89, 90. But this has been neglected as evidenced by the lack of mechanisms to evaluate excipient safety outside the new drug application process. Zhang et al.91 found that sodium lauryl sulfate (SLS) and polyoxyl 40 hydrogenated castor oil (RH40) could significantly inhibit CES1-mediated imidapril hydrolysis, and Tween 20 could dramatically inhibit CES2-mediated CPT-11 hydrolysis. These results indicate that some pharmaceutical excipients, such as SLS, RH40 and Tween 20, may attenuate carboxylesterases activity, therefore such inhibitions should be regarded with some care during drug administration.
Figure 5.
Clinical drugs as inhibitors of CES.
6.2. Natural products
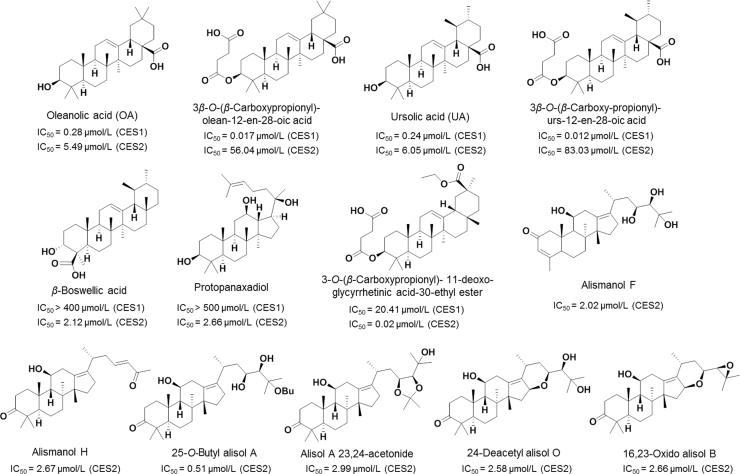
Natural products have been an important source of potential drug leads and inspiration for medicinal chemists to develop more potent modulators for a given enzyme via efficient chemical modifications92, 93, 94. However, their use against molecular targets has diminished over the past two decades, due to the technical barriers to screening natural products95. Zou et al. 96, 97 have collected a series of natural triterpenoids and characterized their inhibitory effects against CES using DME (D-luciferin methyl ester, a probe for CES1) and DDAB (6,8-dichloro-9,9-dimethyl-7-oxo-7,9-dihydroacridin-2-yl benzoate, a probe for CES2) as the specific substrates for high-throughput screening of inhibitors against CES1 and CES2, respectively. Two pentacyclic triterpenoids, ursolic acid (UA) and oleanolic acid (OA), exhibited strong inhibitory effects on CES1 (Fig. 6)97, 98, 99, 100. By structural modifications on OA and UA, two derivatives including 3β-O-(β-carboxy-propionyl)-urs-12-en-28-oic acid and 3β-O-(β-carboxypropionyl)-olean-12-en-28-oic acid were obtained, which displayed very strong inhibitory effects against CES1 (Ki value as 0.012 µmol/L and 0.017 µmol/L, respectively) and high selectivity over CES2 (6919-fold and 3296-fold against CES2, respectively). Guided by the structure-CES2 inhibition relationships of a series of glycyrrhetinic acid (GA) derivatives, Zou et al.98 designed and developed a novel compound 3-O-(β-carboxypropionyl)- 11-deoxo-glycyrrhetinic acid-30-ethyl ester as the most potent inhibitor against CES2 (IC50 = 20 nmol/L). This compound showed high selectivity over CES1 (>1000-fold), which is 3463-fold more potent than the parent compound GA. Recently, 22 protostane triterpenoids have been isolated from the rhizome of Alismaorientale99. Among them, five could potently inhibit CES2, with IC50 values less than 10 µmol/L. The inhibition kinetics demonstrated that alismanol F could inhibit the CES2-catalyzed 4-benzoyl-N-butyl-1,8-naphthalimide hydrolysis with the Ki value of 1.76 µmol/L via mixed inhibition. Zhang et al.100 investigated the inhibitory effects of 22 protostane triterpenoids including 10 new protostane-type triterpenoids from the phytochemical investigation of A. orientalis, on CES2. Among them, five compounds, including alismanol H, 25-O-butyl alisol A, alisol A 23,24-acetonide, 24-deacetyl alisol O, 16,23-oxido alisol B displayed strong inhibitofry effects on CES2, with the IC50 values less than 3.0 µmol/L (Fig. 6)97, 98, 99, 100, 101.
Figure 6.
Triterpenoids as inhibitors of CES.
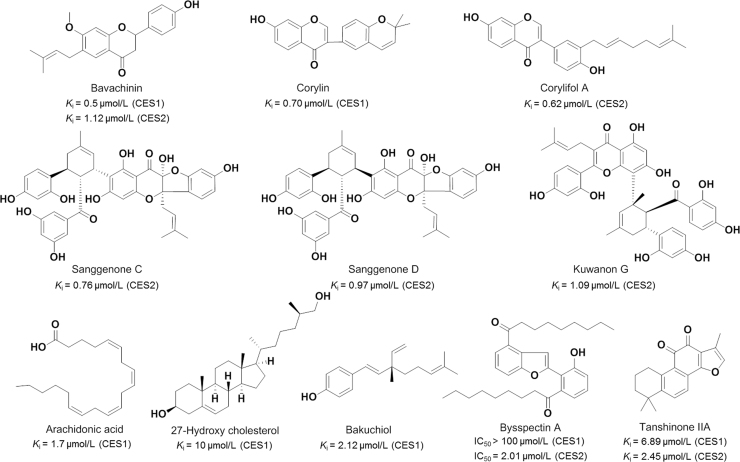
Flavonoids are a large group of polyphenolic products widely distributed in vegetables, fruits, and beverages such as wine and tea102, 103. Recent research has revealed that some natural flavonoids are strong inhibitor against both CES1 and CES2 (Fig. 7)98, 99, 100. Bavachinin and corylin significantly inhibited the CES1-mediated BMBT hydrolysis with low Ki values as 0.5 µmol/L and 0.7 µmol/L, respectively, while corylifol A found in Fructus Psoraleae (also named Bu-gui-shi), is a potent inhibitor against CES2 with the Ki value of 0.62 μmol/L100, 101, 104, 105, 106. More recently, three major constituent from the root-bark of white mulberry (also named Sang-bai-pi) including sanggenone D, kuwanon G, and sanggenone C, could strongly inhibit CES2-mediated FD hydrolysis in HLM via non-competitive manner107. Furthermore, some naturally occurring fatty acids displayed potential inhibitory effects on the hydrolytic activities of CES117. In contrast to saturated fatty acids, unsaturated fatty acids displayed more potent inhibitory effects on CES1, and arachidonic acid demonstrated strong inhibitory effects on CES1 with the Ki value of 1.7 µmol/L. 27-Hydroxycholesterol (27-HC), an oxidized form of cholesterol, also showed promising inhibitory activity against CES1 and high selectivity over CES217. Further investigation on the inhibitory behavior of 27-HC demonstrated that 27-HC functioned as a noncompetitive inhibitor against CES1, with the very low Ki value (10 nmol/L).
Figure 7.
Natural products as inhibitors of CES.
Bakuchiol, a natural phenolic compound isolated from Fructus Psoraleae, displayed strong inhibitory effects against CES2101. The Ki value of bakuchiol against CES2-mediated FD hydrolysis is 2.12 μmol/L and the inhibition type was non-competitive inhibition. Bysspectin A, a polyketide-derived octaketide dimer, displayed selective inhibitory effect on CES2-mediated FD hydrolysis108. Further investigation suggested that bysspectin A functioned as a competitive inhibitor against CES2, and the O-atom of the C-3′ phenolic or the O-atom at the funan ring could strongly interact with the Ser-288 (the key amino acid of the catalytic triad) of CES2 via hydrogen bonding. Hatfield et al.109, 110 found that Salvia miltiorrhiza root extracts demonstrated strong inhibitory effects on CES, due to the presence of tanshinones. These bioactive compounds have been found to be potent inhibitors of both CES1 and CES2, while most of these tanshinone-type compounds could inhibit neither human acetylcholinesterase (AchE) nor human butyrylcholinesterase (BchE). Furthermore, both tanshinones and S. miltiorrhiza root extracts could inhibit the hydrolysis of CPT-11 by the cell-based assays109, 110.
6.3. Other compounds
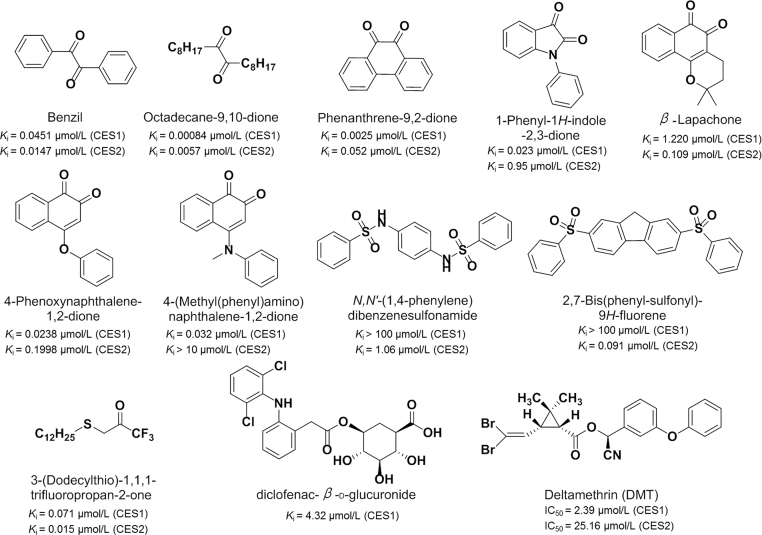
1,2-Diones including benzils, alkyl-1,2-diones, isatins, and 1,2-quinones (Fig. 8)109, 110, 111 have been identified as the most important chemical compounds for CEs inhibition with Ki values in the nanomolar range, which demonstrate potent and selective inhibitory effects toward CES1 or CES2; these agents do not exhibit inhibitory effects on human AchE and BchE. Benzene sulfonamides usually displayed potent inhibitory effects against CES2 and relative high selectivity over CES1, and had no inhibitory effects on either human AchE and BchE24, 112. The SAR analysis of benzene sulfonamides revealed that the relative hydrophobicity of these sulfonamides is an important factor affecting the inhibitory potency to CES2. Trifluoroketones were generally inhibitors to CES with Ki values at the nanomolar range, and mostly demonstrated poor specificity toward CES1 or CES2, but these compounds displayed weak inhibition towards human AchE and BchE113, 114. Clopidogrel acyl-β-D-glucuronide, the phase-II metabolites of clopidogrel carboxylic acid by uridine diphosphate glucuronosyltransferases (UGTs), could inhibit CES1-mediated 4-nitrophenyl acetate hydrolysis with the Ki values of 4.32 µmol/L, but did not significantly inhibit CES2115, 116. Pyrethroids are popular household insecticides for their relatively low toxicity to mammals in contrast to organophosphorus insecticides117, 118. Recently, Lei et al.119 found that six commonly used pyrethroids showed moderate inhibitory effects on CES. Among them, deltamethrin demonstrated strong inhibitory effects toward CES1 with the IC50 value of 2.39 μmol/L. Further investigation demonstrated that deltamethrin was a competitive inhibitor toward CES1-mediated BMBT hydrolysis, but acted as a noncompetitive inhibitor against CES1-mediated DME or DMCB hydrolysis in HLM.
Figure 8.
Inhibitors of CES.
7. CES inactivators
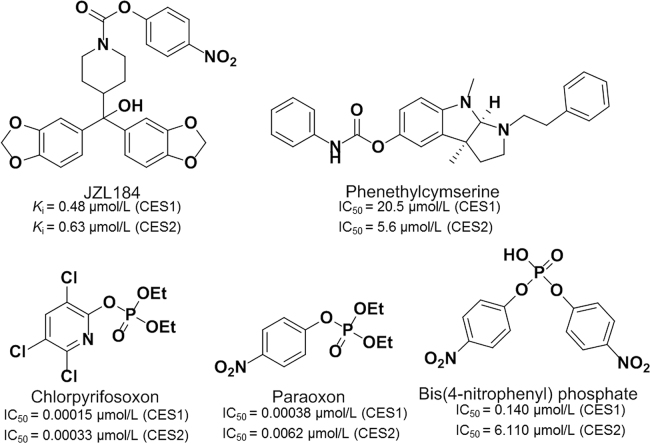
Carbamate compounds were developed as pharmaceutical agents specifically targeting members of the serine hydrolase superfamily via covalent binding and modification of serine at the active site120 These compounds, as potent inhibitors of AchE, have been widely used for the pest control in domestic animals and agriculture. However, several cholinesterase inhibitors containing the carbamate moiety, such as JZL184 and phenethylcymserine (Fig. 9)120, 121, 122, 123, 124, 125, 126, 127, 128, 129, were found to be CES inhibitors120, 121, 122. But all these compounds displayed poor isoform selectivity towards various CES. Organophosphate (OP) insecticides are inhibitors to AChE, which exert their toxicity through the termination of nerve impulses by metabolism of the neurotransmitter acetylcholine123, 124. A number of serine hydrolases including cholinesterases and carboxylesterases (CES1 and CES2) could be significantly inhibited following exposure to OPs125, 126. OPs could react with CES and generate a stable phosphate ester that is covalently linked to the catalytic residue (such as Ser-221 of CES1) of CES. Several OPs, including chlorpyrifos oxon, paraoxon, and bis(4-nitrophenyl)phosphate (BNPP), are potent irreversible inhibitors of CES with IC50 values at the nanomolar level127, 128, 129.
Figure 9.
Inactivators of CES.
8. CES inducers
Over the past two decades, most researchers have focused on the discovery of CES inhibitors. In contrast, only a few studies were conducted to explore the regulation of CES expression. Recent studies have reported the relevance of CES to some metabolic disorders (such as diabetes and obesity), indicating these enzymes might be potential targets for treatment of these metabolic diseases. Especially, the key roles of CES1 and CES2 in lipid metabolism in which the protein expression in liver and adipose tissues are strongly related with several human diseases such as non-alcoholic steatohepatitis, and obesity14. Thus, it is necessary to discover more potent CES modulators to regulate the expression or function of CES, and then to modulate endogenous metabolism or to improve the therapeutic effect of patients administrated with ester drugs.
Generally, microsomal enzyme inducers (MEIs) exert their effects on target genes through constitutive androstane receptor (CAR), pregnane X receptor (PXR), nuclear factor erythroid 2-related factor 2 (Nrf2), and peroxisome proliferator-activated receptors (PPARs)130, 131, 132, 133, 134, 135. Recent studies have demonstrated that the expression of mammalian CES could be regulated via activation of CAR, AhR, PPARs, LXR, PXR, hepatocyte nuclear factor-4α (HNF-4α), and/or Nrf2 transcriptional pathways130, 131, 132. It is worth noting that most of these investigations focus on the transcriptional regulation of rodent Ces genes. A few studies have indicated Nrf2 can be activated by MEIs and induced CES1 in human tumour cells133. Moreover, a PXR-activating agent, rifampicin, caused moderate induction of both CES1 and CES2 gene expression in human hepatocytes134. Future investigations should be conducted to explore whether these signalling pathways associated with CES expression in rodent animals are conserved in humans.
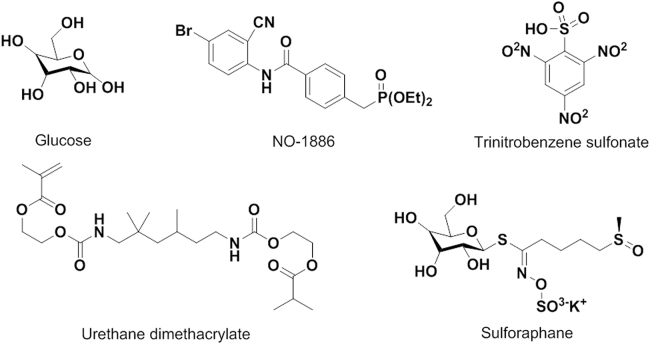
As depicted in Fig. 10132, 133, 134, 135, 136, 137, 138, 139 and (Supplementary Information Table S3)133, 136, some endogenous and exogenous substances have been confirmed with regulatory effects on the expression of mammalian CES. Treatment of mice with glucose could induce hepatic CE1 expression in vivo, due to glucose significantly activated the promoter activity of CES1 and increased acetylation of histone 3 and 4 in the CES1 chromatin137. Xu et al.130 suggested that cholic acid or FXR agonist induced the expression of hepatic CES1, then reduced the levels of plasma cholesterol, hepatic TG, and plasma TG. CES1 could also be highly induced by sensitizers and antioxidants in several cell lines. Recently, Chen et al.133 reported that sensitizer trinitrobenzene sulfonate (TNBS) and antioxidant sulforaphane induce CES1 through a novel element, nuclear factor-E2 related factor-2, in primary hepatocytes and cell lines (human fibrosarcoma cell line HT1080 and Huh7). Another report showed that NO1886 (Ibrolipim), a lipopreotein lipase-promoting agent, could slightly induce CES1 and CES2 in primary cultures of cryopreserved human hepatocytes136. In addition, urethane dimethacrylate (UDMA) was proved to induce the mRNA expression of CES2 in human dental pulp cells, without regulating the CES1 or CES3 mRNA expression138. In addition, gambogic acid decreased the protein expression of CES1 and CES2 via a dose-dependent manner, and the hydrolytic activities of CES1 and CES2 were also significantly decreased upon addition of gambogic acid139.
Figure 10.
Inducers of CES.
9. Summary
Over the past twenty years, the molecular properties, substrate specificities and the biological roles of human carboxylesterases (including CES1 and CES2) in both endo- and xenobiotic metabolism have been extensively studied. Recent studies have suggested that CES participate in the hydrolysis of a vast number of endogenous esters and thus serve as therapeutic targets for the treatment of a variety of human metabolic disorders. The importance of CES in both human health and xenobiotic metabolism arouse great interest in the discovery of potent CES modulators. The development of new optical substrates for CES1 and CES2 has made the screening of CES modulators more convenient and efficient. Although many CES inhibitors with various scaffolds have been reported, their ability to target intracellular CES combined with their in vivo efficacy and safety profiles have not been well-studied. In contrast to the structurally diverse CES inhibitors, the inducers of CES are rarely reported and most studies focus on the transcriptional regulation of rodent Ces genes. Thus, it is necessary to find and develop more potent CES inducers by using cell-based assays. In addition, more in-depth investigations on the physiological functions of CES, the relevance of CES to human diseases, the species differences between human CES and other mammalian CES, as well as the interactions between CES and ligands, should be conducted in near future. These studies will be very helpful for revealing the crucial roles of CES in human health and diseases, as well as for the discovery and development of CES modulators with potential biomedical applications.
Acknowledgments
This work was supported by the National Key Research and Development Program of China (2016YFC1303900, 2017YFC1700200, 2017YFC1702000), the National Scientific and Technological Major Projects of China (2017ZX09101004), the National Natural Science Foundation of China (81703604, 81773687, 21602219, 81573501 and 81473181), Program of Shanghai Academic/Technology Research Leader (18XD1403600), and the Innovative Entrepreneurship Program of High-level Talents in Dalian (2016RQ025).
Footnotes
Peer review under responsibility of Institute of Materia Medica, Chinese Academy of Medical Sciences and Chinese Pharmaceutical Association.
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.apsb.2018.05.005.
Appendix A. Supplementary material
Supplementary material
.
References
- 1.Sanghani S.P., Sanghani P.C., Schiel M.A., Bosron W.F. Human carboxylesterases: an update on CES1, CES2 and CES3. Protein Pept Lett. 2009;16:1207–1214. doi: 10.2174/092986609789071324. [DOI] [PubMed] [Google Scholar]
- 2.Satoh T., Hosokawa M. Structure, function and regulation of carboxylesterases. Chem Biol Interact. 2006;162:195–211. doi: 10.1016/j.cbi.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 3.Satoh T., Hosokawa M. The mammalian carboxylesterases: from molecules to functions. Annu Rev Pharmacol Toxicol. 1998;38:257–288. doi: 10.1146/annurev.pharmtox.38.1.257. [DOI] [PubMed] [Google Scholar]
- 4.Ross M.K., Crow J.A. Human carboxylesterases and their role in xenobiotic and endobiotic metabolism. J Biochem Mol Toxicol. 2007;21:187–196. doi: 10.1002/jbt.20178. [DOI] [PubMed] [Google Scholar]
- 5.Hosokawa M. Structure and catalytic properties of carboxylesterase isozymes involved in metabolic activation of prodrugs. Molecules. 2008;13:412–431. doi: 10.3390/molecules13020412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Imai T. Human carboxylesterase isozymes: catalytic properties and rational drug design. Drug Metab Pharmacokinet. 2006;21:173–185. doi: 10.2133/dmpk.21.173. [DOI] [PubMed] [Google Scholar]
- 7.Redinbo M.R., Potter P.M. Mammalian carboxylesterases: from drug targets to protein therapeutics. Drug Discov Today. 2005;10:313–325. doi: 10.1016/S1359-6446(05)03383-0. [DOI] [PubMed] [Google Scholar]
- 8.Satoh T., Taylor P., Bosron W.F., Sanghani S.P., Hosokawa M., La Du. B.N. Current progress on esterases: from molecular structure to function. Drug Metab Dispos. 2002;30:488–493. doi: 10.1124/dmd.30.5.488. [DOI] [PubMed] [Google Scholar]
- 9.Potter P.M., Wolverton J.S., Morton C.L., Wierdl M., Danks M.K. Cellular localization domains of a rabbit and a human carboxylesterase: influence on irinotecan (CPT-11) metabolism by the rabbit enzyme. Cancer Res. 1998;58:3627–3632. [PubMed] [Google Scholar]
- 10.Zhu H.J., Wang X., Gawronski B.E., Brinda B.J., Angiolillo D.J., Markowitz J.S. Carboxylesterase 1 as a determinant of clopidogrel metabolism and activation. J Pharmacol Exp Ther. 2013;344:665–672. doi: 10.1124/jpet.112.201640. [DOI] [PubMed] [Google Scholar]
- 11.Zhu H.J., Markowitz J.S. Activation of the antiviral prodrug oseltamivir is impaired by two newly identified carboxylesterase 1 variants. Drug Metab Dispos. 2009;37:264–267. doi: 10.1124/dmd.108.024943. [DOI] [PubMed] [Google Scholar]
- 12.Nishi K., Huang H., Kamita S.G., Kim I.H., Morisseau C., Hammock B.D. Characterization of pyrethroid hydrolysis by the human liver carboxylesterases CES-1 and CES-2. Arch Biochem Biophys. 2006;445:115–123. doi: 10.1016/j.abb.2005.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Imai T., Ohura K. The role of intestinal carboxylesterase in the oral absorption of prodrugs. Curr Drug Metab. 2010;11:793–805. doi: 10.2174/138920010794328904. [DOI] [PubMed] [Google Scholar]
- 14.Lian J., Nelson R., Lehner R. Carboxylesterases in lipid metabolism: from mouse to human. Protein Cell. 2018;9:178–195. doi: 10.1007/s13238-017-0437-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Alam M., Ho S., Vance D.E., Lehner R. Heterologous expression, purification, and characterization of human triacylglycerol hydrolase. Protein Expr Purif. 2002;24:33–42. doi: 10.1006/prep.2001.1553. [DOI] [PubMed] [Google Scholar]
- 16.Ruby M.A., Massart J., Hunerdosse D.M., Schönke M., Correia J.C., Louie S.M. Human carboxylesterase 2 reverses obesity-induced diacylglycerol accumulation and glucose intolerance. Cell Rep. 2017;18:636–646. doi: 10.1016/j.celrep.2016.12.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Crow J.A., Herring K.L., Xie S., Borazjani A., Potter P.M., Ross M.K. Inhibition of carboxylesterase activity of THP1 monocytes/macrophages and recombinant human carboxylesterase 1 by oxysterols and fatty acids. Biochim Biophys Acta. 2010;1801:31–41. doi: 10.1016/j.bbalip.2009.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Alam M., Vance D.E., Lehner R. Structure-function analysis of human triacylglycerol hydrolase by site-directed mutagenesis: identification of the catalytic triad and a glycosylation site. Biochemistry. 2002;41:6679–6687. doi: 10.1021/bi0255625. [DOI] [PubMed] [Google Scholar]
- 19.Wang D.D., Zou L.W., Jin Q., Hou J., Ge G.B., Yang L. Recent progress in the discovery of natural inhibitors against human carboxylesterases. Fitoterapia. 2017;117:84–95. doi: 10.1016/j.fitote.2017.01.010. [DOI] [PubMed] [Google Scholar]
- 20.Dominguez E., Galmozzi A., Chang J.W., Hsu K.L., Pawlak J., Li W. integrated phenotypic and activity-based profiling links Ces3 to obesity and diabetes. Nat Chem Biol. 2014;10:113–121. doi: 10.1038/nchembio.1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Crow J.A., Middleton B.L., Borazjani A., Hatfield M.J., Potter P.M., Ross M.K. Inhibition of carboxylesterase 1 is associated with cholesteryl ester retention in human THP-1 monocyte/macrophages. Biochim Biophys Acta. 2008;1781:643–654. doi: 10.1016/j.bbalip.2008.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yoon K.J.P., Hyatt J.L., Morton C.L., Lee R.E., Potter P.M., Danks M.K. Characterization of inhibitors of specific carboxylesterases: development of carboxylesterase inhibitors for translational application. Mol Cancer Ther. 2004;3:903–909. [PubMed] [Google Scholar]
- 23.Xu Y., Zhang C., He W., Liu D. Regulations of xenobiotics and endobiotics on carboxylesterases: a comprehensive review. Eur J Drug Metab Pharmacokinet. 2016;41:321–330. doi: 10.1007/s13318-016-0326-5. [DOI] [PubMed] [Google Scholar]
- 24.Hicks L.D., Hyatt J.L., Stoddard S., Tsurkan L., Edwards C.C., Wadkins R.M. Improved, selective, human intestinal carboxylesterase inhibitors designed to modulate 7-ethyl-10-[4-(1-piperidino)-1-piperidino]carbonyloxycamptothecin (irinotecan; CPT-11) toxicity. J Med Chem. 2009;52:3742–3752. doi: 10.1021/jm9001296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Furihata T., Hosokawa M., Koyano N., Nakamura T., Satoh T., Chiba K. Identification of di-(2-ethylhexyl) phthalate-induced carboxylesterase 1 in C57BL/6 mouse liver microsomes: purification, cDNA cloning, and baculovirus-mediated expression. Drug Metab Dispos. 2004;32:1170–1177. doi: 10.1124/dmd.104.000620. [DOI] [PubMed] [Google Scholar]
- 26.Robbi M., Beaufay H. The COOH terminus of several liver carboxylesterases targets these enzymes to the lumen of the endoplasmic reticulum. J Biol Chem. 1991;266:20498–20503. [PubMed] [Google Scholar]
- 27.Bencharit S., Morton C.L., Howard-Williams E.L., Danks M.K., Potter P.M., Redinbo M.R. Structural insights into CPT-11 activation by mammalian carboxylesterases. Nat Struct Biol. 2002;9:337–342. doi: 10.1038/nsb790. [DOI] [PubMed] [Google Scholar]
- 28.Bencharit S., Morton C.L., Xue Y., Potter P.M., Redinbo M.R. Structural basis of heroin and cocaine metabolism by a promiscuous human drug-processing enzyme. Nat Struct Biol. 2003;10:349–356. doi: 10.1038/nsb919. [DOI] [PubMed] [Google Scholar]
- 29.Bencharit S., Morton C.L., Hyatt J.L., Kuhn P., Danks M.K., Potter P.M. Crystal structure of human carboxylesterase 1 complexed with the Alzheimer's drug tacrine: from binding promiscuity to selective inhibition. Chem Biol. 2003;10:341–349. doi: 10.1016/s1074-5521(03)00071-1. [DOI] [PubMed] [Google Scholar]
- 30.Kim K.K., Song H.K., Shin D.H., Hwang K.Y., Choe S., Yoo O.J. Crystal structure of carboxylesterase from Pseudomonas fluorescens, an alpha/beta hydrolase with broad substrate specificity. Structure. 1997;5:1571–1584. doi: 10.1016/s0969-2126(97)00306-7. [DOI] [PubMed] [Google Scholar]
- 31.Fleming C.D., Edwards C.C., Kirby S.D., Maxwell D.M., Potter P.M., Cerasoli D.M. Crystal structures of human carboxylesterase 1 in covalent complexes with the chemical warfare agents soman and tabun. Biochemistry. 2007;46:5063–5071. doi: 10.1021/bi700246n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Holmes R.S., Glenn J.P., VandeBerg J.L., Cox L.A. Baboon carboxylesterases 1 and 2: sequences, structures and phylogenetic relationships with human and other primate carboxylesterases. J Med Primatol. 2009;38:27–38. doi: 10.1111/j.1600-0684.2008.00315.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Parker R.B., Laizure S.C. The effect of ethanol on oral cocaine pharmacokinetics reveals an unrecognized class of ethanol-mediated drug interactions. Drug Metab Dispos. 2010;38:317–322. doi: 10.1124/dmd.109.030056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dean R.A., Christian C.D., Sample R.H., Bosron W.F. Human liver cocaine esterases: ethanol-mediated formation of ethylcocaine. FASEB J. 1991;5:2735–2739. doi: 10.1096/fasebj.5.12.1916095. [DOI] [PubMed] [Google Scholar]
- 35.Brzezinski M.R., Abraham T.L., Stone C.L., Dean R.A., Bosron W.F. Purification and characterization of a human liver cocaine carboxylesterase that catalyzes the production of benzoylecgonine and the formation of cocaethylene from alcohol and cocaine. Biochem Pharmacol. 1994;48:1747–1755. doi: 10.1016/0006-2952(94)90461-8. [DOI] [PubMed] [Google Scholar]
- 36.Beckemeier M.E., Bora P.S. Fatty acid ethyl esters: potentially toxic products of myocardial ethanol metabolism. J Mol Cell Cardiol. 1998;30:2487–2494. doi: 10.1006/jmcc.1998.0812. [DOI] [PubMed] [Google Scholar]
- 37.Bora P.S., Guruge B.L., Miller D.D., Chaitman B.R., Ruyle M.S. Purification and characterization of human heart fatty acid ethyl ester synthase/carboxylesterase. J Mol Cell Cardiol. 1996;28:2027–2032. doi: 10.1006/jmcc.1996.0195. [DOI] [PubMed] [Google Scholar]
- 38.Tang M., Mukundan M., Yang J., Charpentier N., LeCluyse E.L., Black C. Antiplatelet agents aspirin and clopidogrel are hydrolyzed by distinct carboxylesterases, and clopidogrel is transesterificated in the presence of ethyl alcohol. J Pharmacol Exp Ther. 2006;319:1467–1476. doi: 10.1124/jpet.106.110577. [DOI] [PubMed] [Google Scholar]
- 39.Sato Y., Miyashita A., Iwatsubo T., Usui T. Simultaneous absolute protein quantification of carboxylesterases 1 and 2 in human liver tissue fractions using liquid chromatography-tandem mass spectrometry. Drug Metab Dispos. 2012;40:1389–1396. doi: 10.1124/dmd.112.045054. [DOI] [PubMed] [Google Scholar]
- 40.Diczfalusy M.A., Bjorkhem I., Einarsson C., Hillebrant C.G., Alexson S.E. Characterization of enzymes involved in formation of ethyl esters of long-chain fatty acids in humans. J Lipid Res. 2001;42:1025–1032. [PubMed] [Google Scholar]
- 41.Williams E.T., Wang H., Wrighton S.A., Qian Y.W., Perkins E.J. Genomic analysis of the carboxylesterases: identification and classification of novel forms. Mol Phylogenet Evol. 2010;57:23–34. doi: 10.1016/j.ympev.2010.05.018. [DOI] [PubMed] [Google Scholar]
- 42.Taketani M., Shii M., Ohura K., Ninomiya S., Imai T. Carboxylesterase in the liver and small intestine of experimental animals and human. Life Sci. 2007;81:924–932. doi: 10.1016/j.lfs.2007.07.026. [DOI] [PubMed] [Google Scholar]
- 43.Li B., Sedlacek M., Manoharan I., Boopathy R., Duysen E.G., Masson P. Butyrylcholinesterase, paraoxonase, and albumin esterase, but not carboxylesterase, are present in human plasma. Biochem Pharmacol. 2005;70:1673–1684. doi: 10.1016/j.bcp.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 44.Lv X., Wang D.D., Feng L., Wang P., Zou L.W., Hao D.C. A highly selective marker reaction for measuring the activity of human carboxylesterase 1 in complex biological samples. RSC Adv. 2016;6:4302–4309. [Google Scholar]
- 45.Imai T., Imoto M., Sakamoto H., Hashimoto M. Identification of esterases expressed in Caco–2 cells and effects of their hydrolyzing activity in predicting human intestinal absorption. Drug Metab Dispos. 2005;33:1185–1190. doi: 10.1124/dmd.105.004226. [DOI] [PubMed] [Google Scholar]
- 46.Yano H., Kayukawa S., Iida S., Nakagawa C., Oguri T., Sanda T. Overexpression of carboxylesterase–2 results in enhanced efficacy of topoisomerase I inhibitor, irinotecan (CPT–11), for multiple myeloma. Cancer Sci. 2006;99:2309–2314. doi: 10.1111/j.1349-7006.2008.00936.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Xu G., Zhang W., Ma M.K., McLeod H. Human carboxylesterase 2 is commonly expressed in tumor tissue and is correlated with activation of irinotecan. Clin Cancer Res. 2002;8:2605–2611. [PubMed] [Google Scholar]
- 48.Pratt S.E., Durland-Busbice S., Shepard R.L., Heinz-Taheny K., Iversen P.W., Dantzig A.H. Human carboxylesterase-2 hydrolyzes the prodrug of gemcitabine (LY2334737) and confers prodrug sensitivity to cancer cells. Clin Cancer Res. 2013;19:1159–1168. doi: 10.1158/1078-0432.CCR-12-1184. [DOI] [PubMed] [Google Scholar]
- 49.Wang D.D., Jin Q., Zou L.W., Hou J., Lv X., Lei W. A bioluminescent sensor for highly selective and sensitive detection of human carboxylesterase 1 in complex biological samples. Chem Commun. 2016;52:3183–3186. doi: 10.1039/c5cc09874b. [DOI] [PubMed] [Google Scholar]
- 50.Imai T., Taketani M., Shii M., Hosokawa M., Chiba K. Substrate specificity of carboxylesterase isozymes and their contribution to hydrolase activity in human liver and small intestine. Drug Metab Dispos. 2006;34:1734–1741. doi: 10.1124/dmd.106.009381. [DOI] [PubMed] [Google Scholar]
- 51.Wang J., Williams E.T., Bourgea J., Wong Y.N., Patten C.J. Characterization of recombinant human carboxylesterases: fluorescein diacetate as a probe substrate for human carboxylesterase 2. Drug Metab Dispos. 2011;39:1329–1333. doi: 10.1124/dmd.111.039628. [DOI] [PubMed] [Google Scholar]
- 52.Yoshida T., Fukami T., Kurokawa T., Gotoh S., Oda A., Nakajima M. Difference in substrate specificity of carboxylesterase and arylacetamide deacetylase between dogs and humans. Eur J Pharm Sci. 2018;111:167–176. doi: 10.1016/j.ejps.2017.09.040. [DOI] [PubMed] [Google Scholar]
- 53.Thomsen R., Rasmussen H.B., Linnet K., The INDICES Consortium In vitro drug metabolism by human carboxylesterase 1: focus on angiotensin-converting enzyme inhibitors. Drug Metab Dispos. 2014;42:126–133. doi: 10.1124/dmd.113.053512. [DOI] [PubMed] [Google Scholar]
- 54.Williams E.T., Jones K.O., Ponsler G.D., Lowery S.M., Perkins E.J., Wrighton S.A. The biotransformation of prasugrel, a new thienopyridine prodrug, by the human carboxylesterases 1 and 2. Drug Metab Dispos. 2008;36:1227–1232. doi: 10.1124/dmd.107.020248. [DOI] [PubMed] [Google Scholar]
- 55.Shi J., Wang X.W., Nguyen J., Wu A., Bleske B., Zhu H.J. Sacubitril is selectively activated by carboxylesterase 1 (CES1) in the liver and the activation is affected by CES1 genetic variation. Drug Metab Dispos. 2016;44:554–559. doi: 10.1124/dmd.115.068536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Takahashi S., Katoh M., Saitoh T., Nakajima M., Yokoi T. Allosteric kinetics of human carboxylesterase 1: species differences and interindividual variability. J Pharm Sci. 2008;97:5434–5445. doi: 10.1002/jps.21376. [DOI] [PubMed] [Google Scholar]
- 57.Sanghani S.P., Quinney S.K., Fredenburg T.B., Davis W.I., Murry D.J., Bosron W.F. Hydrolysis of irinotecan and its oxidative metabolites, 7-ethyl-10-[4-N-(5-aminopentanoic acid)-1-piperidino] carbonyloxycamptothecin and 7-ethyl-10-[4-(1-piperidino)-1-amino]-carbonyloxycamptothecin, by human carboxylesterases CES1A1, CES2, and a newly expressed carboxylesterase isoenzyme, CES3. Drug Metab Dispos. 2004;32:505–511. doi: 10.1124/dmd.32.5.505. [DOI] [PubMed] [Google Scholar]
- 58.Feng L., Liu Z.M., Xu L., Lv X., Ning J., Hou J. A highly selective long-wavelength fluorescent probe for the detection of human carboxylesterase 2 and its biomedical applications. Chem Commun (Camb) 2014;50:14519–14522. doi: 10.1039/c4cc06642a. [DOI] [PubMed] [Google Scholar]
- 59.Feng L., Liu Z.M., Hou J., Lv X., Ning J., Ge G.B. A highly selective fluorescent ESIPT probe for the detection of Human carboxylesterase 2 and its biological applications. Biosens Bioelectron. 2015;65:9–15. doi: 10.1016/j.bios.2014.10.002. [DOI] [PubMed] [Google Scholar]
- 60.Jin Q., Feng L., Wang D.D., Dai Z.R., Wang P., Zou L.W. A two-photon ratiometric fluorescent probe for imaging carboxylesterase 2 in living cells and tissues. ACS Appl Mater Interfaces. 2015;7:28474–28481. doi: 10.1021/acsami.5b09573. [DOI] [PubMed] [Google Scholar]
- 61.Liu Z.M., Feng L., Ge G.B., Lv X., Hou J., Cao Y.F. A highly selective ratiometric fluorescent probe for in vitro monitoring and cellular imaging of human carboxylesterase 1. Biosens Bioelectron. 2014;57:30–35. doi: 10.1016/j.bios.2014.01.049. [DOI] [PubMed] [Google Scholar]
- 62.Liu Z.M., Feng L., Hou J., Lv X., Ning J., Ge G.B. A ratiometric fluorescent sensor for highly selective detection of human carboxylesterase 2 and its application in living cells. Sens Actuat B Chem. 2014;205:151–157. [Google Scholar]
- 63.Jin Q., Feng L., Wang D.D., Wu J.J., Hou J., Dai Z.R. A highly selective near-infrared fluorescent probe for carboxylesterase 2 and its bioimaging applications in living cells and animals. Biosens Bioelectron. 2016;83:193–199. doi: 10.1016/j.bios.2016.04.075. [DOI] [PubMed] [Google Scholar]
- 64.Ding L.L., Tian Z.H., Hou J., Weng Z.M., Cui J.N., Yang L. Design and development of fluorescent probe substrates for carboxylesterase 1 using BODIPY as the basic fluorophore. Acta Pharm Sin. 2017;52:58–65. [PubMed] [Google Scholar]
- 65.Dai Z.R., Ge G.B., Feng L., Ning J., Hu L.H., Jin Q. A highly selective ratiometric two-photon fluorescent probe for human cytochrome P450 1A. J Am Chem Soc. 2015;137:14488–14495. doi: 10.1021/jacs.5b09854. [DOI] [PubMed] [Google Scholar]
- 66.Dai Z.R., Feng L., Jin Q., Cheng H., Li Y., Ning J. A practical strategy to design and develop an isoform-specific fluorescent probe for a target enzyme: cyp1a1 as a case study. Chem Sci. 2017;8:2795–2803. doi: 10.1039/c6sc03970g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Jin Q., Feng L., Zhang S.J., Wang D.D., Wang F.J., Zhang Y. Real-time tracking the synthesis and degradation of albumin in complex biological systems with a near-infrared fluorescent probe. Anal Chem. 2017;89:9884–9891. doi: 10.1021/acs.analchem.7b01975. [DOI] [PubMed] [Google Scholar]
- 68.Wang P., Xia Y.L., Zou L.W., Qian X.K., Dou T.Y., Jin Q. An optimized two-photon fluorescent probe for biological sensing and imaging of catechol-O-methyltransferase. Chem Eur J. 2017;23:10800–10807. doi: 10.1002/chem.201701384. [DOI] [PubMed] [Google Scholar]
- 69.Zou L.W., Wang P., Qian X.K., Feng L., Yu Y., Wang D.D. A highly specific ratiometric two-photon fluorescent probe to detect dipeptidyl peptidase IV in plasma and living systems. Biosens Bioelectron. 2017;90:283–289. doi: 10.1016/j.bios.2016.11.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Shi J., Wang X., Eyler R.F., Liang Y., Liu L., Mueller B.A. Association of oseltamivir activation with gender and carboxylesterase 1 genetic polymorphisms. Basic Clin Pharmacol Toxicol. 2016;199:555–561. doi: 10.1111/bcpt.12625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yang D., Pearce R.E., Wang X., Gaedigk R., Wan Y.J., Yan B. Human carboxylesterases CES1 and CES2: ontogenic expression, inter-individual variability and differential hydrolysis of oseltamivir, aspirin, deltamethrin and permethrin. Biochem Pharmacol. 2009;77:238–247. doi: 10.1016/j.bcp.2008.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Quinney S.K., Sanghani S.P., Davis W.I., Hurley T.D., Sun Z., Murry D.J. Hydrolysis of capecitabine to 5'-deoxy-5-fluorocytidine by human carboxylesterases and inhibition by loperamide. J Pharmacol Exp Ther. 2005;313:1011–1016. doi: 10.1124/jpet.104.081265. [DOI] [PubMed] [Google Scholar]
- 73.Alimonti A., Gelibter A., Pavese I., Satta F., Cognetti F., Ferretti G. New approaches to prevent intestinal toxicity of irinotecan-based regimens. Cancer Treat Rev. 2004;30:555–562. doi: 10.1016/j.ctrv.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 74.Faulds M.H., Dahlman-Wright K. Metabolic diseases and cancer risk. Curr Opin Oncol. 2012;24:58–61. doi: 10.1097/CCO.0b013e32834e0582. [DOI] [PubMed] [Google Scholar]
- 75.Alberti K.G., Eckel R.H., Grundy S.M., Zimmet P.Z., Cleeman J.I., Donato K.A. Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation. 2009;120:1640–1645. doi: 10.1161/CIRCULATIONAHA.109.192644. [DOI] [PubMed] [Google Scholar]
- 76.Bie J., Wang J., Marqueen K.E., Osborne R., Kakiyama G., Korzun W. Liver-specific cholesteryl ester hydrolase deficiency attenuates sterol elimination in the feces and increases atherosclerosis in ldlrv-/- mice. Arterioscler Thromb Vasc Biol. 2013;33:1795–1802. doi: 10.1161/ATVBAHA.113.301634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Li Y., Zalzala M., Jadhav K., Xu Y., Kasumov T., Yin L. Carboxylesterase 2 prevents liver steatosis by modulating lipolysis, endoplasmic reticulum stress, and lipogenesis and is regulated by hepatocyte nuclear factor 4 alpha in mice. Hepatology. 2016;63:1860–1874. doi: 10.1002/hep.28472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Yue C.C., Muller-Greven J., Dailey P., Lozanski G., Anderson V., Macintyre S. Identification of a C-reactive protein binding site in two hepatic carboxylesterases capable of retaining C-reactive protein within the endoplasmic reticulum. J Biol Chem. 1996;271:22245–22250. doi: 10.1074/jbc.271.36.22245. [DOI] [PubMed] [Google Scholar]
- 79.Zhen L.D., Baumann H., Novak E.K., Swank R.T. The signal for retention of the egasyn glucuronidase complex within the endoplasmic-reticulum. Arch Biochem Biophys. 1993;304:402–414. doi: 10.1006/abbi.1993.1368. [DOI] [PubMed] [Google Scholar]
- 80.Cha Y.J., Jeong H.E., Shin J.G., Kim E.Y., Yu K.S., Cho J.Y. Genetic polymorphisms of the Carboxylesterase 1 (CES1) gene in a Korean population. Transl Clin Pharm. 2014;22:30–34. [Google Scholar]
- 81.Kubo T., Kim S.R., Sai K., Saito Y., Nakajima T., Matsumoto K. Functional characterization of three naturally occurring single nucleotide polymorphisms in the CES2 gene encoding carboxylesterase 2 (HCE-2) Drug Metab Dispos. 2005;33:1482–1487. doi: 10.1124/dmd.105.005587. [DOI] [PubMed] [Google Scholar]
- 82.Nemoda Z., Angyal N., Tarnok Z., Gadoros J., Sasvari-Szekely M. Carboxylesterase 1 gene polymorphism and methylphenidate response in ADHD. Neuropharmacology. 2009;57:731–733. doi: 10.1016/j.neuropharm.2009.08.014. [DOI] [PubMed] [Google Scholar]
- 83.Sai K., Saito Y., Tatewaki N., Hosokawa M., Kaniwa N., Nishimaki-Mogami T. Association of carboxylesterase 1A genotypes with irinotecan pharmacokinetics in Japanese cancer patients. Br J Clin Pharmacol. 2010;70:222–233. doi: 10.1111/j.1365-2125.2010.03695.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Rhoades J.A., Peterson Y.K., Zhu H.J., Appel D.I., Peloquin C.A., Markowitz J.S. Prediction and in vitro evaluation of selected protease inhibitor antiviral drugs as inhibitors of carboxylesterase 1: a potential source of drug–drug interactions. Pharm Res. 2012;29:972–982. doi: 10.1007/s11095-011-0637-9. [DOI] [PubMed] [Google Scholar]
- 85.Laizure S.C., Herring V., Hu Z.Y., Witbrodt K., Parker R.B. The role of human carboxylesterases in drug metabolism: have we overlooked their importance? Pharmacotherapy. 2013;33:210–222. doi: 10.1002/phar.1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Fukami T., Takahashi S., Nakagawa N., Maruichi T., Nakajima M., Yokoi T. In vitro evaluation of inhibitory effects of antidiabetic and antihyperlipidemic drugs on human carboxylesterase activities. Drug Metab Dispos. 2010;38:2173–2178. doi: 10.1124/dmd.110.034454. [DOI] [PubMed] [Google Scholar]
- 87.Xu Y.J., Zhang C.L., Li X.P., Wu T., Ren X.H., Liu D. Evaluation of the inhibitory effects of antihypertensive drugs on human carboxylesterase in vitro. Drug Metab Pharmacokinet. 2013;28:468–474. doi: 10.2133/dmpk.dmpk-12-rg-143. [DOI] [PubMed] [Google Scholar]
- 88.Paré G., Eriksson N., Lehr T., Connolly S., Eikelboom J., Ezekowitz M.D. Genetic determinants of dabigatran plasma levels and their relation to bleeding. Circulation. 2013;127:1404–1412. doi: 10.1161/CIRCULATIONAHA.112.001233. [DOI] [PubMed] [Google Scholar]
- 89.Murthy S.N., Repka M.A. Excipient stability: a critical aspect in stability of pharmaceuticals. AAPS PharmSciTech. 2018;19:11. doi: 10.1208/s12249-017-0902-2. [DOI] [PubMed] [Google Scholar]
- 90.Patra M., Bhattacharya S., Patnaik M. Importance of propellants and excipients in pharmaceutical topical aerosol. Curr Drug Deliv. 2017;14:1106–1113. doi: 10.2174/1567201814666170309104245. [DOI] [PubMed] [Google Scholar]
- 91.Zhang C., Xu Y., Zhong Q., Li X., Gao P., Feng C. In vitro evaluation of the inhibitory potential of pharmaceutical excipients on human carboxylesterase 1A and 2. PLoS One. 2014;9:e93819. doi: 10.1371/journal.pone.0093819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Newman D.J., Cragg G.M. Natural products as sources of new drugs from 1981 to 2014. J Nat Prod. 2016;79:629–661. doi: 10.1021/acs.jnatprod.5b01055. [DOI] [PubMed] [Google Scholar]
- 93.Neves B.J., Andrade C.H., Cravo P.V.L. Natural products as leads in schistosome drug discovery. Molecules. 2015;20:1872–1903. doi: 10.3390/molecules20021872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Clement J.A. Recent progress in medicinal natural products drug discovery. Curr Top Med Chem. 2014;14:2758. doi: 10.2174/156802661424150105115653. [DOI] [PubMed] [Google Scholar]
- 95.Tabassum N., Tai H.M., Jung D.W., Williams D.R. Fishing for nature's hits: establishment of the zebrafish as a model for screening antidiabetic natural products. Evid Based Complement Altern Med. 2015:287847. doi: 10.1155/2015/287847. [2015] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Zou L.W., Dou T.Y., Wang P., Lei W., Weng Z.M., Hou J. Structure-activity relationships of pentacyclic triterpenoids as potent and selective inhibitors against human carboxylesterase 1. Front Pharmacol. 2017;8:435. doi: 10.3389/fphar.2017.00435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Zou L.W., Jin Q., Wang D.D., Qian Q.K., Hao D.C., Ge G.B. Carboxylesterase inhibitors: an update. Curr Med Chem. 2018;25 doi: 10.2174/0929867325666171204155558. 1627. [DOI] [PubMed] [Google Scholar]
- 98.Zou L.W., Li Y.G., Wang P., Zhou K., Hou J., Jin Q. Design, synthesis, and structure-activity relationship study of glycyrrhetinic acid derivatives as potent and selective inhibitors against human carboxylesterase 2. Eur J Med Chem. 2016;112:280–288. doi: 10.1016/j.ejmech.2016.02.020. [DOI] [PubMed] [Google Scholar]
- 99.Mai Z.P., Zhou K., Ge G.B., Wang C., Huo X.K., Dong P.P. Protostane triterpenoids from the rhizome of Alisma orientale exhibit inhibitory effects on human carboxylesterase 2. J Nat Prod. 2015;78:2372–2380. doi: 10.1021/acs.jnatprod.5b00321. [DOI] [PubMed] [Google Scholar]
- 100.Zhang Z.J., Huo X.K., Tian X.G., Feng L., Ning J., Zhao X.Y. Novel protostane-type triterpenoids with inhibitory human carboxylesterase 2 activities. RSC Adv. 2017;7:28702–28710. [Google Scholar]
- 101.Li Y.G., Hou J., Li S.Y., Lv X., Ning J., Wang P. Fructus Psoraleae contains natural compounds with potent inhibitory effects towards human carboxylesterase 2. Fitoterapia. 2015;101:99–106. doi: 10.1016/j.fitote.2015.01.004. [DOI] [PubMed] [Google Scholar]
- 102.Wen L., Jiang Y., Yang J., Zhao Y., Tian M., Yang B. Structure, bioactivity, and synthesis of methylated flavonoids. Ann N Y Acad Sci. 2017;1398:120–129. doi: 10.1111/nyas.13350. [DOI] [PubMed] [Google Scholar]
- 103.Rice-Evans C.A., Miller N.J., Paganga G. Structure-antioxidant activity relationships of flavonoids and phenolic acids. Free Radic Bio Med. 1996;20:933–956. doi: 10.1016/0891-5849(95)02227-9. [DOI] [PubMed] [Google Scholar]
- 104.Santos-Buelga C., Feliciano A.S. Flavonoids: from structure to health issues. Molecules. 2017;22:477. doi: 10.3390/molecules22030477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Sun D.X., Ge G.B., Dong P.P., Cao Y.F., Fu Z.W., Ran R.X. Inhibition behavior of Fructus Psoraleae's ingredients towards human carboxylesterase 1 (hCES1) Xenobiotica. 2016;46:503–510. doi: 10.3109/00498254.2015.1091521. [DOI] [PubMed] [Google Scholar]
- 106.Weng Z.M., Ge G.B., Dou T.Y., Wang P., Liu P.K., Tian X.H. Characterization and structure-activity relationship studies of flavonoids as inhibitors against human carboxylesterase 2. Bioorg Chem. 2018;77:320–329. doi: 10.1016/j.bioorg.2018.01.011. [DOI] [PubMed] [Google Scholar]
- 107.Liu Y.J., Li S.Y., Hou J., Liu Y.F., Wang D.D., Jiang Y.S. Identification and characterization of naturally occurring inhibitors against human carboxylesterase 2 in White Mulberry Root-bark. Fitoterapia. 2016;115:57–63. doi: 10.1016/j.fitote.2016.09.022. [DOI] [PubMed] [Google Scholar]
- 108.Wu Y.Z., Zhang H.W., Sun Z.H., Dai J.G., Hu Y.C., Li R. Bysspectin A, an unusual octaketide dimer and the precursor derivatives from the endophytic fungus Byssochlamys spectabilis IMM0002 and their biological activities. Eur J Med Chem. 2018;145:717–725. doi: 10.1016/j.ejmech.2018.01.030. [DOI] [PubMed] [Google Scholar]
- 109.Hatfield M.J., Tsurkan L.G., Hyatt J.L., Edwards C.C., Lemoff A., Jeffries C. Modulation of esterified drug metabolism by tanshinones from Salvia miltiorrhiza ("Danshen") J Nat Prod. 2013;76:36–44. doi: 10.1021/np300628a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Hatfield M.J., Potter P.M. Carboxylesterase inhibitors. Expert Opin Ther Pat. 2011;21:1159–1171. doi: 10.1517/13543776.2011.586339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Hyatt J.L., Wadkins R.M., Tsurkan L., Hicks L.D., Hatfield M.J., Edwards C.C. Planarity and constraint of the carbonyl groups in 1,2-diones are determinants for selective inhibition of human carboxylesterase 1. J Med Chem. 2007;50:5727–5734. doi: 10.1021/jm0706867. [DOI] [PubMed] [Google Scholar]
- 112.Wadkins R.M., Hyatt J.L., Yoon K.J., Morton C.L., Lee R.E., Damodaran K. Discovery of novel selective inhibitors of human intestinal carboxylesterase for the amelioration of irinotecan-induced diarrhea: synthesis, quantitative structure-activity relationship analysis, and biological activity. Mol Pharmacol. 2004;65:1336–1343. doi: 10.1124/mol.65.6.1336. [DOI] [PubMed] [Google Scholar]
- 113.Wadkins R.M., Hyatt J.L., Edwards C.C., Tsurkan L., Redinbo M.R., Wheelock C.E. Analysis of mammalian carboxylesterase inhibition by trifluoromethylketone-containing compounds. Mol Pharmacol. 2007;71:713–723. doi: 10.1124/mol.105.021683. [DOI] [PubMed] [Google Scholar]
- 114.Gelb M.H., Svaren J.P., Abeles R.H. Fluoro ketone inhibitors of hydrolytic enzymes. Biochemistry. 1985;24:1813–1817. doi: 10.1021/bi00329a001. [DOI] [PubMed] [Google Scholar]
- 115.Di Meo F., Steel M., Nicolas P., Marquet P., Duroux J.L., Trouillas P. Acylglucuronide in alkaline conditions: migration vs. hydrolysis. J Mol Model. 2013;19:2423–2432. doi: 10.1007/s00894-013-1790-3. [DOI] [PubMed] [Google Scholar]
- 116.Buchheit D., Dragan C.A., Schmitt E.I., Bureik M. Production of ibuprofen acyl glucosides by human UGT2B7. Drug Metab Dispos. 2011;39:2174–2181. doi: 10.1124/dmd.111.041640. [DOI] [PubMed] [Google Scholar]
- 117.Kaneko H. Pyrethroids: mammalian metabolism and toxicity. J Agr Food Chem. 2011;59:2786–2791. doi: 10.1021/jf102567z. [DOI] [PubMed] [Google Scholar]
- 118.Moreno S.C., Silvério F.O., Picanço M.C., Alvarenga E.S., Pereira R.R., Santana Júnior P.A. New pyrethroids for use against Tuta absoluta (lepidoptera: gelechiidae): their toxicity and control speed. J Insect Sci. 2017;17:99. doi: 10.1093/jisesa/iex072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Lei W., Wang D.D., Dou T.Y., Hou J., Feng L., Yin H. Assessment of the inhibitory effects of pyrethroids against human carboxylesterases. Toxicol Appl Pharmacol. 2017;321:48–56. doi: 10.1016/j.taap.2017.02.018. [DOI] [PubMed] [Google Scholar]
- 120.Crow J.A., Bittles V., Borazjani A., Potter P.M., Ross M.K. Covalent inhibition of recombinant human carboxylesterase 1 and 2 and monoacylglycerol lipase by the carbamates JZL184 and URB597. Biochem Pharmacol. 2012;84:1215–1222. doi: 10.1016/j.bcp.2012.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Tsurkan L.G., Hatfield M.J., Edwards C.C., Hyatt J.L., Potter P.M. Inhibition of human carboxylesterases hCE1 and hiCE by cholinesterase inhibitors. Chem Biol Interact. 2013;203:226–230. doi: 10.1016/j.cbi.2012.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Scaloni A., Barra D., Jones W.M., Manning J.M. Human acylpeptide hydrolase. Studies on its thiol groups and mechanism of action. J Biol Chem. 1994;269:15076–15084. [PubMed] [Google Scholar]
- 123.Lee I., Eriksson P., Fredriksson A., Buratovic S., Viberg H. Developmental neurotoxic effects of two pesticides: behavior and biomolecular studies on chlorpyrifos and carbaryl. Toxicol Appl Pharmacol. 2015;288:429–438. doi: 10.1016/j.taap.2015.08.014. [DOI] [PubMed] [Google Scholar]
- 124.Scholz N.L., Truelove N.K., Labenia J.S., Baldwin D.H., Collier T.K. Dose-additive inhibition of chinook salmon acetylcholinesterase activity by mixtures of organophosphate and carbamate insecticides. Environ Toxicol Chem. 2006;25:1200–1207. doi: 10.1897/05-030r1.1. [DOI] [PubMed] [Google Scholar]
- 125.Kovach I.M. Structure and dynamics of serine hydrolase-organophosphate adducts. J Enzym Inhib. 1988;2:199–208. doi: 10.3109/14756368809040726. [DOI] [PubMed] [Google Scholar]
- 126.Nomura D.K., Durkin K.A., Chiang K.P., Quistad G.B., Cravatt B.F., Casida J.E. Serine hydrolase KIAA1363: toxicological and structural features with emphasis on organophosphate interactions. Chem Res Toxicol. 2006;19:1142–1150. doi: 10.1021/tx060117m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Heymann E., Mentlein R., Schmalz R., Schwabe C., Wagenmann F. A method for the estimation of esterase synthesis and degradation and its application to evaluate the influence of insulin and glucagon. Eur J Biochem. 1979;102:509–520. doi: 10.1111/j.1432-1033.1979.tb04267.x. [DOI] [PubMed] [Google Scholar]
- 128.Wei Y., Peng A.Y., Huang J. Inhibition of porcine liver carboxylesterase by phosphorylated flavonoids. Chem Biol Interact. 2013;204:75–79. doi: 10.1016/j.cbi.2013.04.010. [DOI] [PubMed] [Google Scholar]
- 129.Casida J.E., Quistad G.B. Serine hydrolase targets of organophosphorus toxicants. Chem Biol Interact. 2005;157-158:277–283. doi: 10.1016/j.cbi.2005.10.036. [DOI] [PubMed] [Google Scholar]
- 130.Xu J., Li Y., Chen W.D., Xu Y., Yin L., Ge X. Hepatic carboxylesterase 1 is essential for both normal and farnesoid X receptor-controlled lipid homeostasis. Hepatology. 2014;59:1761–1771. doi: 10.1002/hep.26714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Jones R.D., Taylor A.M., Tong E.Y., Repa J.J. Carboxylesterases are uniquely expressed among tissues and regulated by nuclear hormone receptors in the mouse. Drug Metab Dispos. 2013;41:40–49. doi: 10.1124/dmd.112.048397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Staudinger J.L., Xu C., Cui Y.J., Klaassen C.D. Nuclear receptor-mediated regulation of carboxylesterase expression and activity. Expert Opin Drug Metab Toxicol. 2010;6:261–271. doi: 10.1517/17425250903483215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Chen Y.T., Shi D., Yang D., Yan B. Antioxidant sulforaphane and sensitizer trinitrobenzene sulfonate induce carboxylesterase-1 through a novel element transactivated by nuclear factor-E2 related factor-2. Biochem Pharmacol. 2012;84:864–871. doi: 10.1016/j.bcp.2012.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Shi D., Yang J., Yang D., Yan B. Dexamethasone suppresses the expression of multiple rat carboxylesterases through transcriptional repression: evidence for an involvement of the glucocorticoid receptor. Toxicology. 2008;254:97–105. doi: 10.1016/j.tox.2008.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Rigano D., Sirignano C., Taglialatela-Scafati O. The potential of natural products for targeting PPARα. Acta Pharm Sin B. 2017;7:427–438. doi: 10.1016/j.apsb.2017.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Morioka Y., Nishimura M., Imai T., Suzuki S., Harada M., Satoh T. Assessment of induction of cytochrome p450 by NO-1886 (Ibrolipim), a lipoprotein lipase-promoting agent, in primary cultures of human Hepatocytes and in female rat liver. Drug Metab Pharmacokinet. 2006;21:19–28. doi: 10.2133/dmpk.21.19. [DOI] [PubMed] [Google Scholar]
- 137.Xu J., Yin L., Xu Y., Li Y., Zalzala M., Cheng G. Hepatic carboxylesterase 1 is induced by glucose and regulates postprandial glucose levels. PLoS One. 2014;9:e109663. doi: 10.1371/journal.pone.0109663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Chang H.H., Chang M.C., Wang H.H., Huang G.F., Lee Y.L., Wang Y.L. Urethane dimethacrylate induces cytotoxicity and regulates cyclooxygenase-2, hemeoxygenase and carboxylesterase expression in human dental pulp cells. Acta Biomater. 2014;10:722–731. doi: 10.1016/j.actbio.2013.10.006. [DOI] [PubMed] [Google Scholar]
- 139.Ning R., Wang X.P., Zhan Y.R., Qi Q., Huang X.F., Hu G. Gambogic acid potentiates clopidogrel-induced apoptosis and attenuates irinotecan-induced apoptosis through down-regulating human carboxylesterase 1 and 2. Xenobiotica. 2016;46:816–824. doi: 10.3109/00498254.2015.1125560. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material