Abstract
Advancements in in silico techniques of lead molecule selection have resulted in the failure of around 70% of new chemical entities (NCEs). Some of these molecules are getting rejected at final developmental stage resulting in wastage of money and resources. Unfavourable physicochemical properties affect ADME profile of any efficacious and potent molecule, which may ultimately lead to killing of NCE at final stage. Numerous techniques are being explored including nanocrystals for solubility enhancement purposes. Nanocrystals are the most successful and the ones which had a shorter gap between invention and subsequent commercialization of the first marketed product. Several nanocrystal-based products are commercially available and there is a paradigm shift in using approach from simply being solubility enhancement technique to more novel and specific applications. Some other aspects in relation to parenteral nanosuspensions are concentrations of surfactant to be used, scalability and in vivo fate. At present, there exists a wide gap due to poor understanding of these critical factors, which we have tried to address in this review. This review will focus on parenteral nanosuspensions, covering varied aspects especially stabilizers used, GRAS (Generally Recognized as Safe) status of stabilizers, scalability challenges, issues of physical and chemical stability, solidification techniques to combat stability problems and in vivo fate.
Abbreviations: ADME, absorption distribution metabolism elimination; ASEs, aerosols solvent extractions; AUC, area under curve; BBB, blood–brain barrier; BCS, Biopharmaceutical Classification System; BDP, beclomethasone dipropionate; CFC, critical flocculation concentration; CMC, critical micelle concentration; CLSM, confocal laser scanning microscopy; DMSO, dimethyl sulfoxide; EDI, estimated daily intake; EHDA, electrohydrodynamic atomization; EPAS, evaporative precipitation in aqueous solution; EPR, enhanced permeability and retention; FITC, fluorescein isothiocyanate; GRAS, Generally Recognized as Safe; HEC, hydroxyethylcellulose; HFBII, class II hydrophobin; HPC, hydroxypropyl cellulose; HPH, high-pressure homogenization; HPMC, hydroxypropyl methylcellulose; HP-PTX/NC, hyaluronic acid-paclitaxel/nanocrystal; IP, intraperitoneal; IM, intramuscular; IV, intravenous; IVIVC, in vivo–in vitro correlation; LD50, median lethal dose (50%); MDR, multidrug resistance effect; NCE, new chemical entities; PTX, paclitaxel; PEG, polyethylene glycol; P-gp, permeation glycoprotein; PVA, polyvinyl alcohol; QbD, quality by design; SC, subcutaneous; SEDS, solution enhanced dispersion by supercritical fluids; SEM, scanning electron microscopy; SFL, spray freezing into liquids; TBA, tert-butanol; TEM, transmission electron microscopy; US FDA, United States Food and Drug Administration; Vitamin E TPGS, d-α-tocopheryl polyethylene glycol 1000 succinate
KEY WORDS: Parenteral, Nanosuspension, Stabilizer, In vivo fate, Solidification, Scalability
Graphical abstract
In last decade nanocrystals have emerged as competent solubility enhancement techniques and nowadays there is a paradigm shift in using nanocrystallization approach from simply being solubility enhancement technique to more novel and specific applications. The present review provides a bird׳s eye view of parenteral nanosuspensions and advancements in technology/drug delivery fields.
1. Introduction
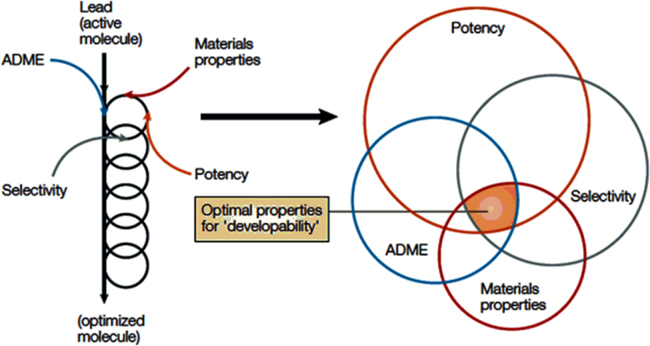
Lead molecule selection through in silico methods, including high throughput screening; have led to increase in the propensity of poorly water-soluble candidates being selected, and at the same time rejection of around 70% of these drug candidates due to their poor water solubility. Many of these new chemical entities (NCEs, around 40%)1 get processed and are killed at the final development stage resulting in wastage of money and resources. Because of their unacceptable physicochemical profile, such compounds are often considered as time and resources consuming only and are discarded either at initial or final stages, without any significant contribution in the synthesis pipeline2. Solubility and permeability are the two important factors governing oral bioavailability of compounds after oral absorption3. Deviation in solubility and permeability directs to unpredictable absorption profile and low oral bioavailability4. In the past few years, lead molecule optimization processes have governed the selection of molecules having potency and selectivity but these compounds lack suitable physicochemical properties because of their bulky structure and higher molecular mass. There is a dire need to incorporate appropriate physicochemical properties along with selectivity and potency5. Gardener et al.6 have nicely reported incorporation of physicochemical parameters in drug development process. Fig. 16 provides a glimpse of parameters to be taken care while designing a drug development strategy. To obtain an adequate amount of bioavailability, researchers have explored numerous techniques viz. use of co-solvents, micelle formation, cyclodextrin complexation, lipid-based nanoformulations, polymeric nanoparticulate systems, micronization, and nanonization. All aforementioned techniques have their own advantages and limitations7. Among these techniques, nanonization is one of the widely used technique for improvement in solubility and bioavailability, as evidenced by huge literature and approved marketed products such as Rapamune®, Emend®, Triglide®, Tricor®, Megace ES® already available in market8. Recent reports on the current market scenario of nanocrystals claim that it will occupy around 60% of a $136 billion nanotechnology-enabled drug delivery market by 20219. There are statistical predictions that the total market in 2021 will be $136 billion, with nanocrystals and other nanocarriers, of which $81 billion will be due to nanocrystal formulations alone. This itself is an indicative of vast market potential of nanocrystals10.
Figure 1.
Parameters to be critically monitored during initial screening of lead optimization. Reprinted (adapted) with permission from Ref. 6. Copyright © 2004 Nature Publishing Group.
These advancements are attributed to benefits offered by this technology such as ease of formulation, scale-up compatibilities, fewer controversies towards nanotoxicology and regulatory aspects11. Besides aforementioned advantages, nanocrystals offer easy conversion to solid dosage forms (tablets/capsules/powder for redispersion and many more). Nowadays, researchers are expanding horizons in nutraceuticals by using nanocrystals, as they offer an increase in saturation solubility, dissolution velocity and at the same time provide all the advantages of nanoformulation, with a slight addition of the surfactant12. The advent of nanotechnology has created a new boom in all fields, including chemical, physical and life sciences, which has given a new path for drug delivery in pharmaceuticals13, 14. Various routes for administration of nanocrystals7 are proposed as well, including oral15, parenteral16, dermal17, 18, systemic19, pulmonary20 and ocular21, 22, 23.
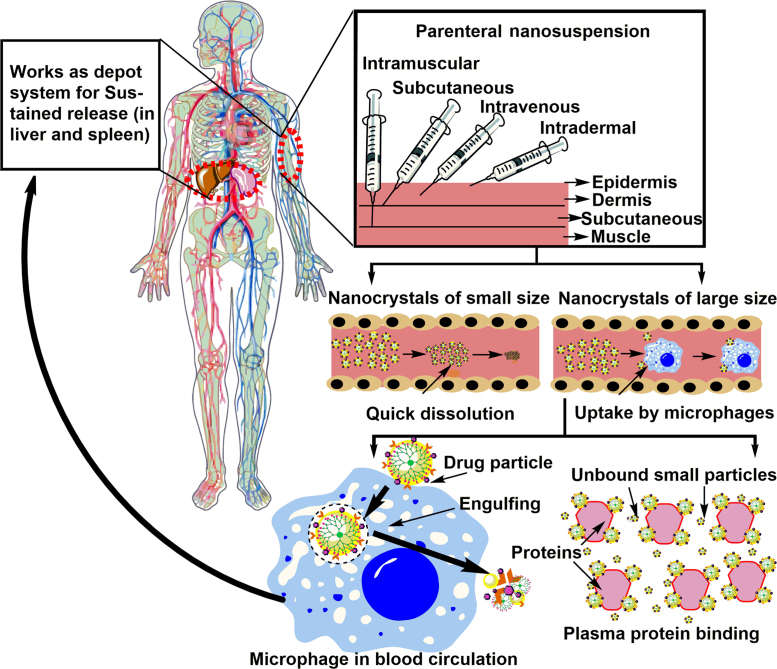
By definition, nanocrystals are crystals of the drug in nano-size range stabilized with only minute amounts of surfactants, therefore possessing higher theoretical drug loading as compared to any other nanoparticulate system. This high loading capacity makes these systems as better drug carriers for transporting the higher load of a drug to the targeted site for therapeutic activity24. Marketed formulations based on nanocrystals technologies are mostly for oral administration. The first nanocrystal-based formulation was approved in 1990s (Ambisome®) and since then almost forty commercial nanocrystal-based formulations are available in the market. Recently in vivo studies done on animal models have suggested the adaptability of nanocrystals formulation as suitable enough to be tuned to change improve targeting, pharmacodynamic and pharmacokinetics of less soluble drug candidates25. Parenteral administration, by all means, is critical and requires strict control over many parameters, like for parenteral nanosuspension, size becomes the main constraint as it decides the in vivo fate of administered nanocrystals. Researchers have reported that nanocrystals of size 200 nm and more dissolve slowly and are taken up by the macrophages in liver whereas nanocrystals of 100 nm size and below, dissolve rapidly26. All the basic information of solubility enhancement mechanism and preparation methods is already reviewed by many authors7, 27. The present review will focus on parenteral nanosuspension covering all aspects including surfactants used till date, preparation, stability issues and strategies to combat stability problems, in vivo fate after parenteral administration, marketed formulations and strategies to play with pharmacokinetics. In-general methods of preparation along with some advances and specific features of nanocrystals are discussed in brief.
2. Specific features and production
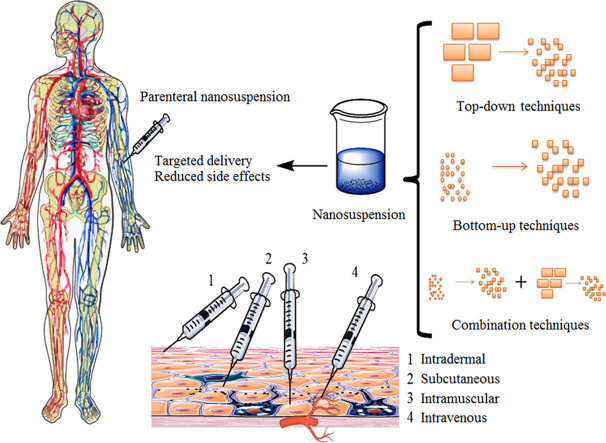
Parenteral aqueous nanosuspensions are prepared to deal with two major issues viz. to reduce the side effects of existing parenteral product or to target drug of interest to specific part of the body. Production techniques are classified either as bottom-up or top-down techniques7. In general top-down techniques start from larger size particles and produces crystals of a drug in nano-size range by using mechanical forces (attrition, cavitation, and others). In contrast to this, bottom-up technique starts from molecular level of drug and precipitation of drug crystals in nano range takes place in conditions, which influence nucleation process28. Advancements in technology include the use of supercritical fluid and solvent evaporation processes. Scalability issues at industrial level have made these advancements less preferable over already established ones2.
The present classification system of nanocrystals technologies broadly categorizes them into two generations, from which the first generation includes basic bottom-up and top-down technologies (nanoprecipitation, media milling, high-pressure homogenization (HPH) and others). Second generation production techniques have improved properties and are based on combinatory approaches (smartCrystal technology). Second generation techniques provide formulation scientists with benefits of faster production, better size reduction, improved physical stability and controlled pharmacokinetic profile of prepared nanocrystals. SamrtCrystal is “tool box” for various combination processes e.g. H42 (combination of spray drying and HPH) and H96 (combination of lyophilization and HPH). Nanocrystals of approximately 50 nm can be easily achieved with smartCrystal technologies26. Proceeding section will give a brief introduction about aforementioned production techniques.
2.1. Bottom-up method
The bottom-up nanoprecipitation technique has been utilized since decades for preparation of micronized particles. Use of this technique for nanocrystal production started in the late 1980s28. Bottom-up technique initiates from molecular level of drug (drug dissolved in organic solvent) and subsequently, nucleation is governed by various means, including solvent evaporation, ultrasonic waves, supercritical fluid and others. Solvents used in nanoprecipitation technique are the ones which have some amount of miscibility with antisolvent used29. Commonly used solvents are ethanol, methanol, acetone, dimethylsulfoxide, dichloromethane and many more. Most common choice of antisolvent is water; buffers are rarely used but their use has been reported as well. This production process is very tedious and has critical process parameters to be taken care of such as rate of precipitation and prevention of crystal growth. Sometimes there is a risk of residual organic solvents and complexity in process control which creates a barrier for scale-up7.
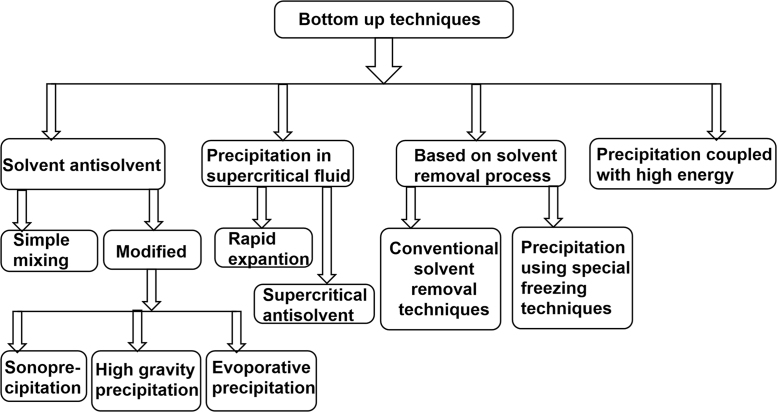
The BASF company have patented industrially useful nanosuspension precipitation method developed by Auweter, Horn and co-workers24 basically for colorants in colloidal dispersion form. This technology has been used by various industries to generate nanocrystals with the desired size range. Other bottom-up technologies such as high gravity controlled precipitation technology, liquid jet precipitation, sonocrystallization and multi-inlet vortex mixing are being explored in recent years30. Fig. 2 provides a glimpse of conventional and novel bottom-up techniques, utilized for effective production of nanocrystals. De Waard et al.31 developed a novel bottom-up process for fenofibrate to produce drug nanocrystals by controlled crystallization during freeze-drying. Numerous novel bottom-up methods are well-reported in existing literature. However; the acceptability of this technique is still limited to lab-scale production only, owing to poor understanding of critical process parameters, especially those affecting industrial scale production. This is one of the major reasons why most of the commercially available products are based on top-down technique only32.
Figure 2.
Advances in bottom-up techniques for preparation of nanocrystals.
2.2. Top-down method
Two basic size reduction techniques classified under top-down methods are wet milling and HPH7. The top-down techniques start with larger crystals of a drug, (µm in range) and size reduction takes place by virtue of applied mechanical forces as described in preceding sections. Wet milling technique is preferred mostly over dry (jet) milling because it gives particle size in the nm range. Most of the marketed products are reported to be based on pearl mill (bead mill) technology24. Table 1 provides brief information about various marketed products based on nanocrystal technology. Construction wise pearl mills consist of milling chamber, milling motor, and the recirculation chamber. Micronization by media milling is conducted by loading drug suspension in surfactant solution to the milling chamber. Milling media is added in optimized amount which is responsible for generation of forces utilized in size reduction. Process parameters and material attribute like the amount of the drug, amount of milling pearls used, milling speed, milling time and temperature must be controlled to obtain particles in the desired size range28. Another alternative top-down method was developed by Muller and co-workers, called piston-gap homogenizer, wherein the suspension is passed at high speed through a small orifice and during passage through the orifice collision of crystals results in the particle size reduction24, 33, 34, 35. Erosion from the milling pearl, during the milling process, is the major limitation of the method, but in recent years, yttrium stabilization of milling media has ameliorated this problem. Another technique is microfluidizer technology, which can generate smaller size crystallites with a collision of two fluid streams at very high pressure2. Microfluidizer technology is one of the top-down processes which is based on the principle of jet-stream homogenization. Either Z-type or Y-type of collision chamber is used. The cavitation forces generated during the process, particle collision and shear forces are responsible for particle size reduction up to desired range36. Apart from aforementioned limitations, top-down techniques make the drug substance more prone towards phase transition, which can ultimately affect the in vivo performance of the drug product7, 37.
Table 1.
Nanosuspension based marketed products.
| Product name | Drug used | Indication | Marketed by (company) | Production technique | Administration route | Solved problem | Refs. |
|---|---|---|---|---|---|---|---|
| Tricor® | Fenofibrate | Hypercholesterolemia | Abbot pharma | Elan nanocrystals | Oral | Solubility enhancement | 39, 40 |
| Sporanox® | Itraconazole | Antifungal | Janssen pharma | Sporanox | Oral | Oral bioavailability | 41 |
| Invega sustenna® | Paliperidone palmitate | Antipsychotic | Janssen pharma | Elan nanocrystals | Intravenous | Modified treatment | 42 |
| Rapamune® | Sirolimus | Immunosuppressant | Wyeth | Elan nanocrystals | Oral | Dissolution and erratic absorption | 43, 44 |
| Triglide® | Fenofibrate | Hypercholesterolemia | First horizon pharma | Skye pharma | Oral | Oral bioavailability | 45 |
| Emend® | Aprepitant | Antiemetic | Merck | Elan nanocrystals | Oral | Absorption | 8, 46, 47 |
2.3. Combination technologies
Combination techniques simply combine bottom-up and top-down technologies. It is a two-step process which involves nanoprecipitation in the first step followed by HPH2, 7.
In the context of parenteral nanosuspension, it is very difficult to control various process parameters and Baxter is one of the industries focusing on intravenous suspensions of drug molecule presently. The smartCrystal technology is one amongst the best-suited techniques, combining benefits of fast production and at the same time production of very small nanocrystals, as already mentioned in preceding sections24. Recent advances in the field of combination techniques are triple combination i.e. precipitation-lyophilization-homogenization technique, supercritical fluid methods, spray freezing into liquids (SFL), solution-enhanced dispersion by the supercritical fluids (SEDS), aerosol solvent extraction (ASES) and evaporative precipitation into an aqueous solution (EPAS)30.
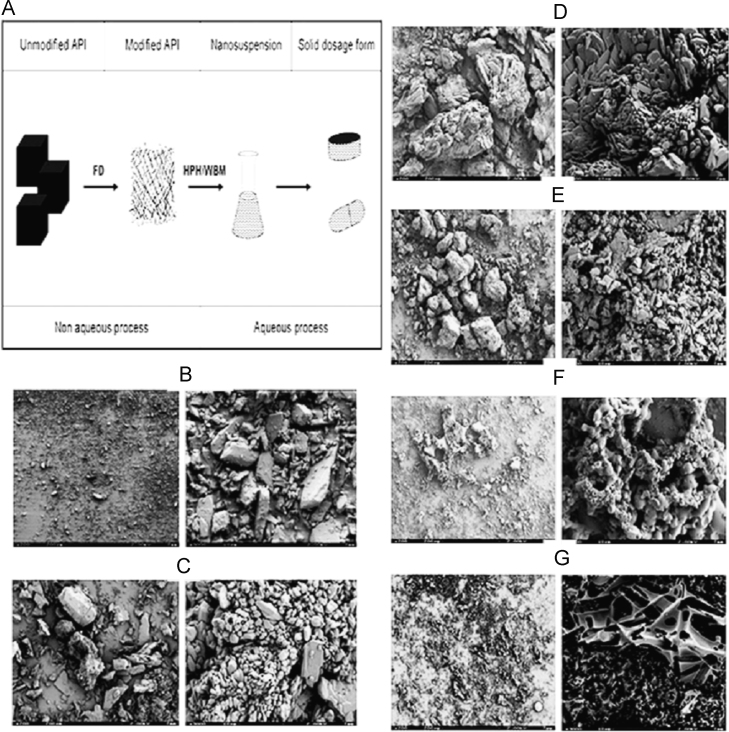
Salazar et al.38 have reported a comparative evaluation of a combination approach (freeze drying followed by HPH/wet ball milling) with conventional top-down technique and found combination approach to be better regarding size control. Glibenclamide was taken as a model drug and two subsequent steps were reported to be performed including freeze drying followed by wet ball milling. Authors have shown the effect of solvents (dimethyl sulfoxide: tert-butanol) on the porosity of powder obtained after the first step of freeze drying. Scanning electron microscopic (SEM) images obtained for different ratios of dimethyl sulfoxide and tert-butanol showed that the material obtained was porous and fragile with different morphologies. Fig. 3 gives a glimpse of the process as well as SEM images obtained for the different combination of solvents38. At the industrial scale, the major challenge is to obtain less than 100 nm particle size using conventional approach only, and in such situation, combinatory approaches are very useful36.
Figure 3.
SEM pictures of the freeze-dried powders: (B) unmodified API; (C) dimethyl sulfoxide:tert-butanol (DMSO:TBA), 90:10 (low TBA); (D) 75:25, (E) 50:50 (medium); (F) 25:75; and (G) 10:90 (high TBA). Left: 200 µm scale bar/right: 10 µm scale bar (3000× magnification). Reprinted (adapted) with permission from Ref. 38. Copyright © 2012 Elsevier.
3. Parenteral nanosuspension
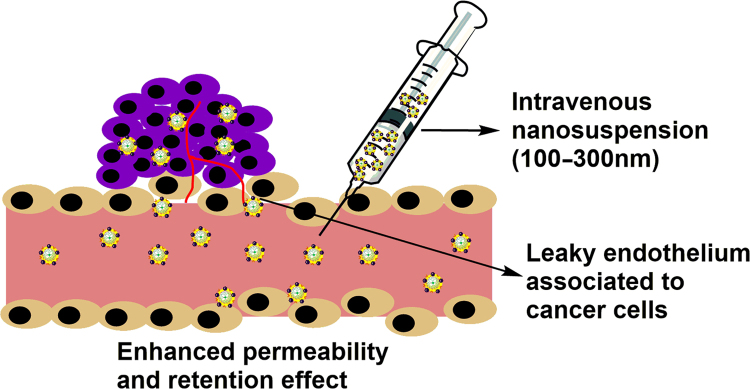
Currently, nanosuspension technology has been broadly applied to resolve the problem of poor water solubility for drug candidates administered by either route, oral or parenteral. Nanocrystals are delivered parenterally to address two main issues viz. to reduce toxicity issues of non-aqueous existing formulation and to provide targeted effect48. A Parenterally administered nanosuspension formulation offers reduced toxicity as compared to conventional drug deliveries16. Size range between 100 and 300 nm is ideal for the enhanced permeability and retention effect (EPR effect) to achieve drug concentration in solid tumors which are reported to have very dense vasculature7. Proceeding section will deal with stabilizers used till date, recent advances, and applications of parenteral nanosuspension.
3.1. Stabilizers used in parental nanocrystals
Being very versatile technique, nanocrystals are preferred over numerous other techniques of solubility enhancement and have emerged as leading technique in last decade. Nanocrystals are solid drug particles surrounded by the layer of a stabilizing agent. Preparation of nanocrystals is quite easy, but the stability and selection of stabilizer in optimum concentration are critical in formulation development. Not all the stabilizers are suitable for parenteral use, due to associated toxicity and microbial load issues. Polymers and surfactants are used as stabilizers for pharmaceutical nanocrystals49 and the amount used for stabilization is very critical. Sinha et al.32 have nicely mentioned the effect of concentration of surfactants as well as polymers regarding critical micelle concentration (CMC) and critical flocculation concentration (CFC) respectively. In general, it is assumed that higher the concentration, better the chances of adsorption and stabilization of nanocrystals, but beyond a concentration level (CMC/CFC) micellar solubilization phenomenon is prominent leading to Ostwald ripening and system destabilization32. By this means, selection of surfactant type and concentration to be used is important. Reports have shown lecithin to be the stabilizer of choice for parenteral and autoclavable nanosuspension50. Proceeding section deals with various stabilizers reported in the production of parenteral nanosuspension.
3.1.1. Poloxamers
Poloxamers are synthetic polymers consisting of a chain made up of closely packed block copolymers of ethylene oxide and propylene oxide. Commonly used concentrations for nanosuspension preparation is 0.2% to 0.6% by intramuscular and intravenous route51, 52. They are widely used in the preparation of nanoparticles53, micelle carriers54, thermo-sensitive hydrogels55 and as tissue scaffold56 in biomedicine applications following their physical and chemical properties. They are generally considered as safe for topical, oral and parenteral applications and are approved by FDA as a pharmaceutical ingredient and food additives. Nowadays, their use as a stabilizing agent is very common for nanocrystal formulations. Fabrication of poloxamers involves the addition of ethylene oxide to polyoxypropylene glycol, which again is a product of the reaction between polyethylene glycol (PEG) and propylene oxide. These types of block polymers are reported to be more advantageous as stabilizers compare to traditional homopolymers57. Commonly used poloxamer in nanocrystals formulations is poloxamer 188 and (Pluronic® F68) poloxamer 407 (Pluronic® F127). Both of these polymeric stabilizers are the non-ionic linear triblock copolymer consisting of the hydrophobic central segment of polypropylene oxide and two hydrophilic side segments of polyethylene oxide. The adsorption on crystal surface is governed by hydrophobic polypropylene oxide chains. However, the reverse structured poloxamers are more advantageous, due to inter-particle bridging and aggregation after nanocrystals formation as compared to Pluronics® 58. During selection of stabilizers several factors affect stability and particle size of the nanosuspension formulation viz. morphology of polymers, functional groups, hydrophilic or hydrophobic ratio in stabilizer molecule and molecular weight59, 60. Polymer composition has the direct effect on the stability of nanosuspension. Polymers and other stabilizers used should have optimum hydrophilic to lipophilic balance in controlling the crystal growth and enhancing the wettability of poorly soluble drug61. Stabilizers work by two mechanisms of stabilization viz. steric stabilization as well as electrostatic stabilization62. Adsorption plays a key role in providing steric stabilization by means of surface adsorbed bulky groups, while the formation of electrical double layer results in electrostatic stabilization. The surfactants having small molecular weights are generally reported to facilitate the dissolution of hydrophobic drugs61. Functional group present on stabilizer and drug surface also plays an important role in particle size and stability63.
Several authors have reported nanocrystals of drug (nimodipine, paclitaxel, indomethacin, budesonide) stabilized with the help of single or the combinations of poloxamers64. Hydrophilic polymers are considered less competent stabilizers for nanocrystals formulation as compared to amphiphilic polymers and surfactants, due to the absence of thermodynamic driving forces required to adsorb on hydrophobic surfaces of nanocrystals drug particles62. Additionally, amphiphilic polymers are known to increase wettability and decrease the interfacial tension of nanocrystals thus providing better stabilization. The selection of stabilizer totally depends on the drug compound of interest in the formulation, but due to versatile and advantageous nature of the poloxamer, they are effective for most of the compounds from biopharmaceutical classification system-II (BCS class-II)49. Viitala et al. have reported better stabilization of itraconazole nanosuspension prepared with the aid of poloxamers whereas other surfactants screened, failed to do so58, 65, 66. A wide range of Pluronics® with different grades is reported for the preparation of parenteral nanosuspensions. Danhier et al.67 have reported parenteral (intravenous) nanosuspension of a novel poorly water-soluble anti-cancer multi-targeted kinase inhibitor, MTKi-327 with the aid of Pluronic® F108. Authors have investigated the role of four different formulation approaches to tackle the problem of poor water solubility viz. polymeric nanoparticles, parenteral nanosuspension, polymeric micelles and solution prepared with the aid of Captisol®. Results of comparative analysis of aforementioned approaches suggested nanosuspension to be effective formulation approach due to higher tolerated dose and 2.4-fold increase in aqueous solubility as compared to all other investigational approaches67. Table 2 provides inactive ingredient guide (IIG) and GRAS status of commonly used stabilizers in parenteral nanosuspension.
Table 2.
Commonly used surfactants in parenteral nanosuspensions with their daily uptake limits.
| Nos. | Ingredient | Chemical structure (Monomeric units)n | GRAS status and daily uptake limits with a particular route of administration | IIG limit (maximum potency/unit dose) | Refs. |
|---|---|---|---|---|---|
| 1 | Hydroxypropyl cellulose (HPC) | 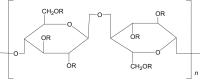 |
|
* | 52, 68 |
| 2 | Hypromellose (HPMC) | 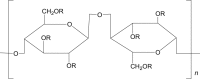 |
|
* | 52, 68 |
| 3 | Leucine | 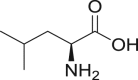 |
|
IV-52.6% | 52, 69, 70 |
| 4 | Poloxamer F68, F127 | 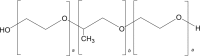 |
|
Poloxamer F68: | 52, 68 |
| IM-0.2%, | |||||
| IV-0.6%, | |||||
| 5 | Polyethyleneglycol (PEG) 400 | 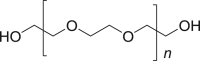 |
|
IM-20%, | 52, 68 |
| IV-75.58% | |||||
| 6 | Polysorbate 80 | 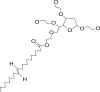 |
|
IM-12%, | 52, 68 |
| IV infusion-58.5% | |||||
| 7 | Polyvinylalcohol (PVA) | 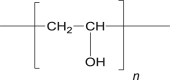 |
|
Intravitreal-0.12 mg | 52, 68 |
| 8 | Povidon | 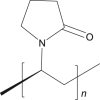 |
|
IV-0.2%, IM-0.9% | 52, 68 |
| 9 | 2-Pyrrolidone | 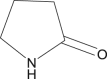 |
|
SC-25.85% | 52, 68 |
| 10 | Sodium lauryl sulfate (SLS) |
|
* | 52, 68 | |
| 11 | Cross povidone | 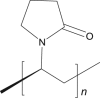 |
|
IM-0.02% | 52, 68 |
| 12 | Chitosan | 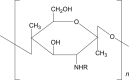 |
|
* | 52 |
| 13 | Cyclodextrin | 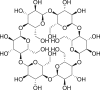 |
|
IV-5% | 52, 68 |
| 14 | d-α-Tocopheryl polyethylene glycol 1000 succinate (vitamin E TPGS) |
|
IV-225 mg/L | 52, 68 | |
| 15 | Arginine | 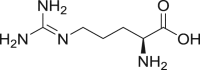 |
|
IM-78%, | 52, 68, 71, 72 |
| IV-39.5% | |||||
| 16 | Proline | 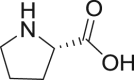 |
|
IV infusion-35.6% | 73, 51, 68 |
| 17 | Serum albumin |  |
|
IV-2% | 52, 68 |
| 18 | Soluplus® | 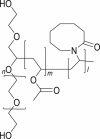 |
* | * | 74, 75 |
Parameter not found in the literature. SC, Subcutaneous; IM, Intramuscular; IV, Intravenous; IP, Intraperitoneal
3.1.2. Amino acid derived co-polymers
The amino acid derivative polypeptides are commonly used in the parenteral nanocrystal formulation as a stabilizer. Albumin (intravenous concentration allowed is 2%), lysine, leucine (intravenous concentration allowed is 52.6%) and transferrin are amongst the most common among copolymers which are used to achieve dual application of both surface stabilization and targeting. Albumin is one of the anciently used moieties having single polypeptide chain of 585 amino acids, containing protein and enzyme. In aqueous nanocrystal, formulation leucine acts as a lubricant as well as anti-adherent due to its hydrophobic nature. These co-polymers are reported to show enhanced stabilization effect when used in combination with each other. On similar lines, Frank and Boeck66 have introduced two amino acid derivatives for parenteral nanocrystals formulation viz. proline (intravenous concentration allowed is 35.6%) and arginine (intravenous and intramuscular concentration allowed is 78% and 39.5%, respectively)52, 68. Authors proposed the use of these stabilizers to formulate BI XX drug in the form of nanocrystals. Use of aforementioned amino acid-based stabilizers has been reported to achieve desired particle size and physical stability of the formulation.
3.1.3. d-α-Tocopheryl polyethylene glycol 1000 succinate (Vitamin E TPGS)
Vitamin E TPGS is a waxy material obtained from esterification of vitamin E carboxylic group with PEG 1000 which is water soluble in nature. It is a non-ionic multi-role surfactant used widely in the pharmaceutical, nutraceutical and cosmetic formulations76. Commonly used concentrations are 200–300 mg/L by intravenous route52, 77. It is used mainly in the formulation of self-emulsifying drug delivery system owing to its emulsifying properties. As a water-soluble compound vitamin E TPGS is also used as an effective source of natural vitamin E for both therapeutic and nutritional purposes. Physical properties of vitamin E TPGS makes it a good plasticizer for several newer applications. Since so many years, it was used in oral formulations, but nowadays it is also explored for its use in parenteral and topical delivery routes. Due to its antioxidant properties, vitamin E TPGS is attractive for industries dealing in cosmetics. Very high physical stability of vitamin E TPGS makes it less vulnerable towards effects of light, heat, oxygen and oxidizing agents. It is stable up to 4 years in closely packed container at room temperature and presents stability issues only when in contact with basic medium49. Vitamin E TPGS in combination with the PEG is reported to give extended release, especially of drugs having a short half-life in plasma and enhancing the bioavailability of drug78. It is having amphiphilic structure, owing to the presence of alkyl tail and hydrophilic polar head, with comparatively low CMC of 0.02% (w/w), and hydrophilic-lipophilic balance value of 13.2. Due to these aforementioned properties of vitamin E TPGS, it is used in various drug delivery systems such as nano formulations, liposomes, prodrug, nutraceuticals, cosmeceuticals and micellar formulations.
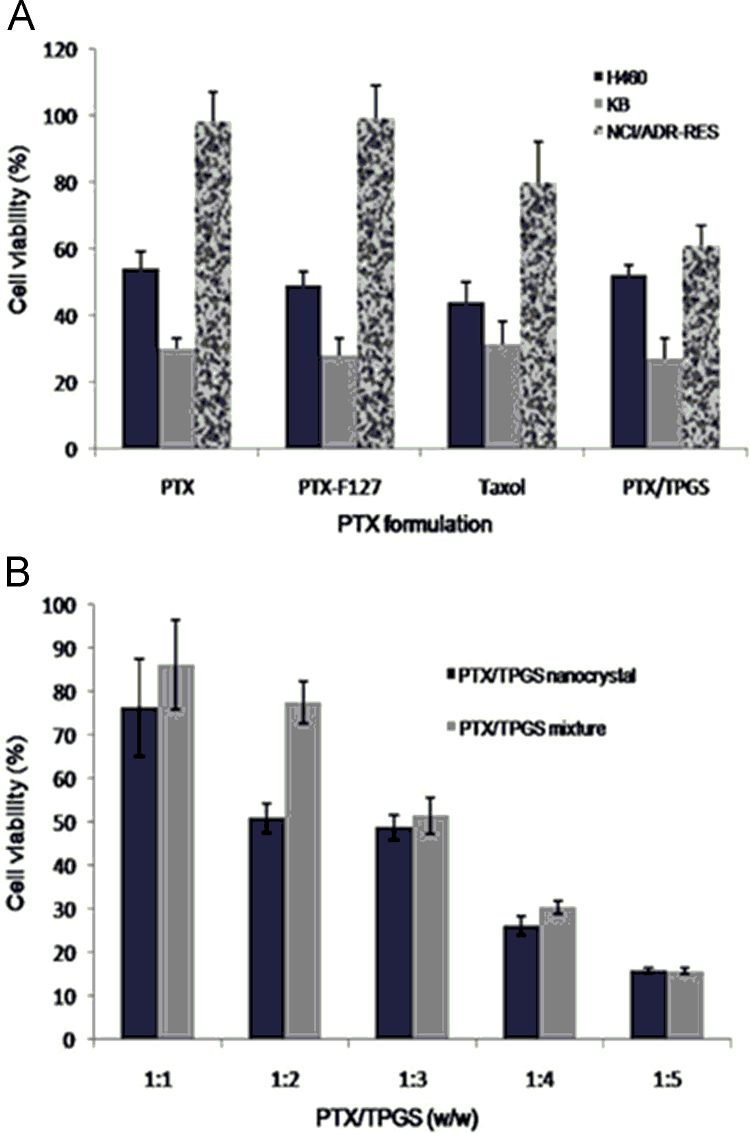
Apart from all other benefits, vitamin E TPGS has permeation glycoprotein (P-gp) inhibitory activities and is used to overcome the multidrug resistance (MDR) effect in case of anticancer drugs by ameliorating cellular uptake. Many researchers are working in this direction and paclitaxel/docetaxel nanocrystals are successfully developed with vitamin E TPGS showing improved anticancer profile79. Liu et al.80 have investigated the effect of TPGS stabilized paclitaxel nanocrystals on KB, H460, and NCI/ADR-RES cells and found the aforementioned formulation to be more effective as compared to nanocrystals stabilized with Pluronic® F127 and marketed formulation Taxol®. Fig. 480 provides an overview of % cell viability in various cell lines using vitamin E TPGS as a stabilizer.
Figure 4.
Cell viability after being treated with different formulations for 48 h. A, Effects of paclitaxel/TPGS nanocrystals, paclitaxel/F127 nanocrystals, Taxol and paclitaxel at the same 5 μmol/L paclitaxel concentration on NCI/ADR-RES, KB and H460 cells. B, Effects of paclitaxel/TPGS nanocrystals (10 μmol/L) with different amount of TPGS and paclitaxel/TPGS mixture (10 μmol/L) with different amount of TPGS. Reprinted (adapted) with permission from Ref. 80. Copyright © 2010 American chemical society.
Vitamin E TPGS is well reported for its stabilization as well as solubilization effects in various oral and parenteral formulations76. Baert et al.81 have reported rilpivirine nanosuspension stabilized with vitamin E TPGS, where it was used for long-acting parenteral formulations providing prophylactic treatment in HIV.
Ge et al.82 have reported preparation of ursolic acid nanocrystals utilizing vitamin E TPGS as a stabilizer and found many folds enhancement in bioavailability as compared to other techniques, due to P-gp inhibition effect on intestinal epithelium cells. Several such examples are reported in the literature showing P-gp83 inhibitory effect of this compound, however, covering them all is beyond the scope of this article84. All these properties of vitamin E TPGS coupled with low toxicity profile makes it a suitable candidate for parenteral applications.
3.1.4. Soluplus®
It is an innovative excipient that gives new levels of solubility and bioavailability of poorly water-soluble drugs. Chemically, it is a polyvinyl caprolactam-polyvinyl acetate-PEG graft copolymer. The allowed concentration of Soluplus® by intravenous and intramuscular routes is 75% and 20% respectively52, 72. Soluplus® is an effective excipient and has been used in formulation development of poorly water-soluble drugs utilizing spray drying, hot melt extrusion, electrospinning, solvent casting, high shear dispersions, solvent evaporation, thermal heating, co-milling and ball milling. Soluplus® was developed by BASF industries to be used in the preparation of the solid solution, but it is used for various other applications including, as a binder in wet granulation or dry binder in direct compression for tableting, owing to its versatile nature. Soluplus® exhibits high flowability and controllable extrudability features which makes it suitable for use as stabilizers in pharmaceuticals49.
Yang et al.85 have reported fenofibrate nanocrystals stabilized with the combination of hydroxypropylmethylcellulose (HPMC) and Soluplus® prepared with media milling technique. Authors have reported better physical stability of prepared nanocrystals due to weaker Ostwald׳s ripening effect of Soluplus®. Additionally, this excipient was found to improve bioavailability by altering drug permeability in intestine. The permeation studies performed in vitro in Caco-2 cell line and in vivo transport studies in Beagle dogs show safety and permeability enhancer property of the Soluplus® in nanocrystals formulation as compared to pure drug86. All these studies are performed for oral/intragastric administration and use of Soluplus® via parenteral route still needs to be explored concerning its toxicity.
3.1.5. Cellulose-based polymers
HPMC is a cellulose-based polymer, used in pharmaceutical development and is considered as a non-toxic and non-irritant ingredient. It is semi-synthetic non-ionic polymer widely used in nanocrystals formulations to provide physical stabilization using steric stabilization mechanisms. HPMC is suitable for various routes of administration including ocular, oral and parenteral routes. Characteristics of prolonged contact with mucosa, low toxicity, high swelling ability, and lubrication effect, makes it most appropriate excipient for ophthalmic nanosuspensions87. The concentration of 5.50 g/day is commonly used for HPMC via intravenous route52.
Tuomela et al.88 have recently reported the use of HPMC in ocular drug delivery as a stabilizer in intraocular pressure releasing nanosuspension formulation. The formulation was developed using HPMC as a stabilizer by wet milling method at different PH conditions. Optimization of nanocrystals formulation with various concentrations revealed 25% (w/w) HPMC to be giving better stabilization. Due to the presence of hydroxypropoxy and methoxy groups on the HPMC surface, it provides good attachment of brinzolamide with hydrogen bonding. High molecular weight also plays an important role for steric stabilization in the above-mentioned drug formulation88.
Apart from HPMC other cellulose-based non-ionic polymers are also used as stabilizers in preparation of nanocrystals viz. hydroxyethyl cellulose (HEC), hydroxypropyl cellulose (HPC) and others. The hydrogen bonding between polymer and drug surface inhibits the crystal growth by virtue of which all these acts as good stabilizer49.
Ghosh et al.89 have studied the effect of different polymeric stabilizers (HPMC, polyvinyl pyrrolidone, and HPC) on process parameter associated variabilities in size and polydispersity of nanosuspension. They used the quality by design (QbD) approach and found that the type of polymeric stabilizers plays an important role in the production of the nano range particles. Among all other polymeric stabilizers compared, authors found HPMC stabilized nanosuspension was exhibiting least variations in final size and dispersity upon exposure to the different processing parameters. Overall results revealed that the choice of stabilizer to be used is a key factor along with the concentration to be used in the successful development of stable nanosuspension. Authors reported steric stabilization and hydrogen bond making tendency of polymer, responsible for better stabilization effect89.
3.1.6. Hydrophobins
These are protein-based surfactants obtained from filaments fungi which stabilize nanocrystals by forming the self-assembling monolayer with hydrophilic-hydrophobic interfaces. Hydrophobin forms a protein shell around hydrophobic particles which gives dispersion in water and restricts the aggregation of particles. These are classified into two classes based on of their hydropathy and nature of amino acid sequence and have a significant change in their structural assembly90. Hydrophobins are nontoxic in nature and have the activity to prevent immune response by making a layer around nanoparticles. Adsorption of these between hydrophilic–hydrophobic interfaces without losing their folded structure is possible due to its amphiphilic nature. Nowadays, different classes of hydrophobins are used to improve solubility and bioavailability. Apart from it, these can be used in targeted and controlled drug delivery systems. Because of its good stability, versatile nature and good adsorption properties, it is used to produce various nanoparticles successfully. Valo et al.90 have reported nanocrystals of drug beclomethasone dipropionate stabilized with hydrophobins and found it to be physically stable system depicted by transmission electron microscopy (TEM) images of stabilized nanocrystals (Fig. 5) prepared with nanoprecipitation method. Till date hydrophobin׳s use as a stabilizing agent is reported only for the topical and oral nanosuspensions, that leaves a lot of room for expanding its horizons for parenteral administration, provided suitable toxicity are generated first90, 91.
Figure 5.
TEM images showing beclomethasone dipropionate (BDP) precipitation in deionized water (A) without class II hydrophobin (HFBII), (B) with 0.005% HFBII, (C) with 0.05% HFBII, and (D) with 0.1% HFBII (Scale bar: 0.5 µm). Reprinted (adapted) with permission from Ref. 90. Copyright © 2010 American chemical society.
3.1.7. Miscellaneous
Above mentioned stabilizers are commonly used for the production of nanosuspensions. Apart from these some non-conventional stabilizers are also used these days92. Polyvinyl alcohol (PVA) is water soluble synthetic polymer used as an effective stabilizer in nanocrystals formulations. Xia et al.93 have reported PVA stabilized nitrendipine nanocrystals to enhance the dissolution rate and oral bioavailability. Prepared nanosuspension (combination method of precipitation-ultrasonication) showed particle size and zeta potential of 209±9 nm and –13.9±1.9 mV respectively. The in vitro dissolution of nitrendipine was found to be increased by nanonization of drug particles93. Intravenously allowed level of PVA in general is 0.12 mg52.
Recently a research group have reported the use of chitosan as a cationic stabilizer for preparation of itraconazole nanocrystals using the conventional method of HPH method and investigated its stabilization effect. Physical stability of prepared nanocrystals was found to be significantly improved without any changes or new modification observed in crystal structure94. Another research group working on chitosan-based atorvastatin nanocrystals investigated the effect of the cationic charge of stabilizer on the nanocrystals particle size and stability. Nanocrystals of the drug were prepared with desired specifications and effect of chitosan as potential stabilizer has been evaluated94.
3.2. Recent advances
Application of nanocrystals in parenteral drug delivery is explored very rapidly nowadays due to its important role in solubility and bioavailability enhancement7. As already described in preceding sections aqueous parenteral nanosuspensions are prepared for two reasons viz. either to overcome side effects of existing parenteral formulation or to provide targeting effects. Passive targeting can be achieved by the interplay of particle size as nanocrystals in the range of 100–300 nm are reported to be better candidates for EPR effect as shown in Fig. 6. Most of the times anticancer drugs including paclitaxel, docetaxel, curcumin, camptothecin and others are formulated using nanocrystals technology for the intravenous administration80 keeping in view the passive targeting effect. For active targeting effect surface, modified nanocrystals are being explored in recent years. Recently, Liu et al.95 have reported Paclitaxel nanocrystal formulation coated with vitamin E TPGS, where surface coating served the dual purpose of stabilization as well as P-gp inhibition. As already described in preceding sections, vitamin E TPGS works as virtuous P-gp inhibitor as well as a stabilizer; authors have combined these properties to overcome multidrug resistance of various anticancer drugs. This changing trend is itself indicative of a paradigm shift from simply solubility enhancement to more novel and specific applications of nanocrystals. Table 3 enlist recently reported nanosuspensions used for parenteral applications.
Figure 6.
EPR effect shown by smaller size nanocrystals.
Table 3.
Recently reported nanosuspension based parenteral formulations.
| Nos. | Active ingredient | Category | Method of nanosuspension production | Surfactant used | Concentration of surfactant used | Purpose of the study | Refs. |
|---|---|---|---|---|---|---|---|
| 1 | p-Terphynyl derivative | Anticancer | Precipitation-microfluidization method | Pluronic F-68 | 0.08%, w/v | Solubility enhancement | 16 |
| 2 | Etoposide | Anticancer | High pressure homogenization –solvent precipitation | Bovin serum albumin | 3%, w/v | Targeted delivery | 96 |
| 3 | Azithromycin | Antibiotic | Reactive precipitation method | Soybean Lecithin | 1% | Targeted delivery | 97 |
| 4 | Fenofibrate | Reduces cholesterol level | Stirred ball mill | HPMC | 5%, 7.5%, 10% | Controlled delivery | 98 |
| 5 | Silybin | Hepatoprotective | High pressure homogenization | Lecithin Poloxamer 188 | 0.2% and 0.1%, respectively | Solubility enhancement | 99 |
| 6 | Simvastatin | Lipid lowering agent | Nanoprecipitation technique | PVP K-30SLS | 4% and 0.02%, respectively | Solubility enhancement | 100 |
| 7 | Itraconazole | Anti-fungal | _ | Poloxamer 188 | – | Solubility enhancement | 19 |
| 8 | Clofazimine | Antimycobacterial | High pressure homogenizer | Pluronic F-68 | 0.5% | Targeted delivery, Stability | 101 |
| 9 | Paclitaxel | Anticancer | High-pressure homogenizer | Human serum albumin PEG | 4.5% and 10%. respectively | Targeted delivery | 102 |
| 10 | Hyaluronic acid and paclitaxel | Anticancer | Electrostatic attraction method | Pluronic F-127 Chitosan | – | Targeted delivery | 103 |
| 11 | Paclitaxel | Anticancer | Antisolvent precipitation method (probe sonication) | PVP K-30 | 0.01% | Targeted delivery | 68 |
| 12 | Bexarotene | Anticancer | High pressure homogenizer | Pluronic F-68 | 0.02% | Solubility enhancement | 104 |
| 13 | Paclitaxel | Anticancer | High pressure homogenizer | Sodium poly styrene sulphonate | 0.1%, 0.1%, 0.5% and 0.5%, respectively | Solubility enhancement | 105 |
| Glycon Chitosan | |||||||
| Tween 80 | |||||||
| 14 | Bexarotene | Anticancer | Precipitation + microfluidization | Pluronic F-68 | 0.5% | Targeted delivery | 106 |
| 15 | Paclitaxel 1-methyltryptophan | Anticancer | Nano-precipitation method | PVA | 2% of both | Targeted delivery | 107 |
| Pluronic F-68 | |||||||
| 16 | Paclitaxel | Anticancer | Wet media milling method | Cremophor EL | 0.1% | Targeted delivery | 108 |
| 17 | Paclitaxel | Anticancer | Nano-precipitation method | TPGS | 0.4% | Toxicity and PK study | 109 |
| 18 | Paclitaxel | Anticancer | Nano-precipitation method | TPGS | 0.4% | Drug resistance study | 110 |
| 19 | Paclitaxel | Anticancer | High pressure homogenization | Poloxamer 188 | 0.08% | PK evaluation study | 64 |
| PEG-400 | |||||||
| Tween-80 | |||||||
| CMC-Na | |||||||
| Tyloxapol | |||||||
| 20 | Paclitaxel | Anticancer | Emulsification+high speed homogenization | Poloxamer-188 | 0.5% | Solubility enhancement | 111 |
| 21 | Quercetin | Antioxidant | HPS | Tween-80 | 2% | Solubility enhancement | 37 |
| Bead milling | |||||||
| Cave precipitation | |||||||
| 22 | Baicalein | Anticancer | Antisolvent recrystalization + high pressure homogenization | Polysorbate -80 | 0.1%–0.5% | Solubility enhancement | 20 |
| SLS | |||||||
| Poloxamer-188 | |||||||
| 23 | Puerarin | Cardiovascular system | High pressure homogenization | Sodium dodecyl sulphate | 3.75% | Solubility enhancement | 112 |
| 24 | Docetaxel | Anticancer | High shear homogenization | Pluronic F-127 | 1%–5% | Targeted delivery | 113 |
| Pluronic F-68 | |||||||
| Tween-80 | |||||||
| 25 | Curcumin | Anticancer | Media milling | Tween-80 | 0.2% | Solubility enhancement | 114 |
| 26 | Benzimidazole | Anticancer | Nanoprecipitation, media milling | Pluronic F-108 | 10% | Bioavailability enhancement | 115 |
| Tween-80 | |||||||
| 27 | Itraconazole | Antimycotic agent | Pearl milling technique | Poloxamer-407 | 3% | Solubility enhancement | 51 |
| 28 | Nimodipine | Calcium-channel blocker | High pressure homogenization | Poloxamer-188 | 0.6% | Solubility and bioavailability enhancement | 116 |
| Tween-80 | 0.5% | ||||||
| 29 | Asulacrine | Anticancer | High pressure homogenization | Poloxamer | 1% | Bioavailability enhancement | 117 |
| 30 | Nevirapine | Antiretroviral | High pressure homogenization | Tween-80 | 1.0% | Targeted delivery | 118 |
| Poloxamer-188 | 0.5%0.3% | ||||||
| PVP | |||||||
| 31 | Oridonin | Anticancer | High pressure homogenization | Pluronic f-68 | 0.5% | PK and tissue distribution study | 119 |
| Lecithin | |||||||
| 32 | 1,3-Dicyclohexylurea | Antihypertensive | Wet media milling | PVP | 20% | PK evaluation | 120 |
| 33 | Ibuprofen | Anti-inflammatory | Nano-precipitation | Tween-80 | 0.02% | Bioavailability enhancement | 121 |
| 34 | Acyclovir | Antiviral | Nano-precipitation | Tween-80 | 0.02% | Bioavailability enhancement | 122 |
| 35 | Hydrocortisone, prednisolone | Glucocorticoid | High-pressure homogenization | Pluronic F-68 | 0.1% | Targeted and delayed drug delivery | 123 |
| 36 | Fluticasone | Anti-inflammatory | Wet media milling technique | Tween-80 | 0.5% | Targeted drug delivery | 124 |
| 37 | Sulfacetamide | Antibiotic | Nano-precipitation | Pluronic F-68 | 1% | Targeted drug delivery | 125 |
| 38 | Cloricromene | Antithrombotic and antiplatelet | Nano-precipitation | Tween-80 | 0.02% | Bioavailability and stability enhancement | 126 |
| 39 | Camptothecin | Antitumor | Anti-solvent precipitation with sonication | Pluronic F-127 | Not reported | Targeted delivery | 127 |
| PVP-K30 | |||||||
| HPMC | |||||||
| PEG 8000 | |||||||
| 40 | Paclitaxel | Antitumor | Wet media milling | Pluronic F-127 | 1% | Targeted delivery | 128 |
| 41 | Budesonide | Glucocorticoid | High pressure homogenization | Span 85 | 0.5% | Targeted delivery | 129 |
| Lecithin | 0.5% | ||||||
| 42 | Deacety mycoepoxydiene | Anticancer | High pressure homogenization | Poloxamer 188 | 0.5% | Solubility enhancement | 130 |
| Lecithin | 0.5% | ||||||
| HPMC | 0.1% | ||||||
| PVP | 0.1% | ||||||
| 43 | Atovaquone | Antimicrobial | High pressure homogenization | Tween 80 | 0.1% | Targeted delivery | 131 |
| Poloxamer 184 | 0.1% | ||||||
| 44 | Bupravaquonone | Antibiotic | High pressure homogenization | Poloxamer 188 | 1.0% | Targeted delivery | 132 |
| Lecithin | 0.5% | ||||||
| 45 | Omeprazol | Proton pump inhibitor | High pressure homogenization | Poloxamer 188 | 1% | Stability enhancement | 133 |
׳–׳ Data not available.
Apart from surface modifications for active targeting, effects of nanocrystal size on passive targeting is explored as well. Use of novel stabilizing agents to enhance stability and achieve desired size range is reported by authors. Strict control over size range indirectly exerts an effect on passive targeting and lymphatic clearance. Newer stabilizers which are more biocompatible are being explored these days to address toxicity issues as well8, 25.
Frank and co-authors66 developed nanosuspension formulation utilizing novel amino acid stabilizers (proline and arginine) utilizing media milling process and found it physically stable showing enhanced dissolution and bioavailability of drug BIXX.
On similar lines, Zakir et al.134 recently reported amphotericin B nanocrystal-based formulation for parenteral administration. They have used Tween 80, Pluronic® F68 and Soya lecithin as the stabilizers to achieve stability and desired particle size range. Nanocrystal formulation for the parenteral administration with smaller particle size was successfully produced with the NanoEdgeTM technology134. The technology utilizes basic precipitation process followed by annealing step applying high energy either in the form of shear or thermal energies33. The combinational step of imparting high shear or thermal energy provides an important role in nano-sizing.
Nanotechnology gives a lot of new opportunities in the field of drug delivery as well as challenges while focusing on targeting aspects. Targeting to the specific site is one of the major challenges and offers several avenues of research. Nanocrystals surface can be suitably modified with the specific targeting ligand to avoid cellular uptake of the nanocrystals, as well as targeting to specific site135. Nowadays researchers are focusing on means surface modifications which are more complex instead of the simple step of coating.
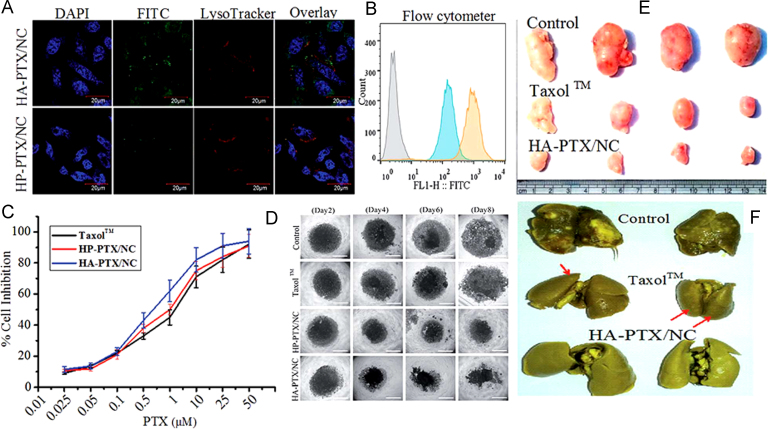
Recently Sharma et al.103 have reported hyaluronic acid anchored paclitaxel nanocrystals for the enhanced anticancer effect of the drug. Drug nanocrystals of <250 nm were prepared and cell based in vitro evaluation of HA-paclitaxel/NC on 2D monolayer and 3D spheroids demonstrated that hyaluronic acid coating was able to significantly improve the therapeutic efficacy (Fig. 7)103 as compared to free drug and marketed formulation. Authors reported a significant increase in circulation lifetime of these surface modified nanocrystals as evidenced by 8.4-fold increase in area under curve the (AUC). At the same time, the enhanced anticancer effect was contributed to the interaction of hyaluronic acid with CD44 receptor which significantly enhanced the cellular uptake of these nanocrystals. Reduced lung metastasis and reduced toxicity in LA-7 tumor-bearing rat model was observed in comparison to marketed formulation Taxol™103.
Figure 7.
Fluorescein isothiocyanate (FITC) tagged nanocrystals uptake by (A) Confocal laser scanning microscopy (CLSM), (B) Flow cytometry (blue: hyaluronic acid-paclitaxel/nanocrystal (HP-PTX/NC); orange: HP-PTX/NC); (C) Cytotoxicity of paclitaxel formulation on MDA-MB 231 cells after 48 h, M, mol/L; (D) Microscopic images of MDA-MB-231 spheroids on 2–8 days after incubation of different formulations at alternate days (paclitaxel equivalent dose 0.5 µmol/L). Scale bar 500 µm. (E) and (F), Antitumor efficacy of control, Taxol™ and HA-PTX/NCs against LA-7 mammary gland rat cancer model at a dose equivalent to 10 mg/kg paclitaxel. (E) Morphology of the harvested tumors at the end of the study; (F) Lungs isolated from animals of different groups at the end of tumor regression study to demonstrate metastasis of tumor cells to lungs. Reprinted (adapted) with permission from Ref. 103. Copyright © 2016 Royal Society of Chemistry.
Apart from targeting to cancer cells, use of apolipoprotein E, Tween 80 and poloxamer 188 are well reported for effective transport against the blood–brain barrier (BBB) and can be effectively used for decoration of drug nanocrystal surface, requiring higher transport from BBB. Table 4 reports some of the patented nanosuspension drug products.
Table 4.
Patents related to parenteral nanosuspension.
| No. | Title | Area of invention/final product description | Advantages/applications/claims | Refs. |
|---|---|---|---|---|
| 1 | An aqueous suspension of submicron 9-hydroxyrisperidone fatty acid esters | A pharmaceutical composition suitable as a depot formulation for administration via IM or SC route | Administration by IM or SC injection provides therapeutically effective formulation by improving bioavailability | 42 |
| 2 | Pharmaceutical nanosuspension for medicament administration as systems with increased saturation solubility and rate of solution | Preparation of nanosuspension formulation | Nanosuspension formulation provides good bioavailability by increasing the saturation solubility and dissolution rate compared with powders of active compounds | 136 |
| 3 | Nano-particulate formulation of fenofibrate | Nanosuspension prepared using vitamin E TPGS as a stabilizer | Stable fenofibrate nano-particulate (nanosuspension) with vitamin E-TPGS without the addition of any stabilizer | 137 |
| 4 | Parenteral and oral formulations of benzimidazole | Parenteral and oral nanosuspension | Benzimidazole nanosuspension formulation for oral and parenteral delivery, which provides a better effect on oral bioavailability and good therapeutic efficacy | 115 |
| 5 | Novel compositions | The final product as nanosuspension for parenteral administration | Spironolactone nanosuspension preparation for solubility enhancement and improving bioavailability by increasing saturation solubility | 137 |
In on similar lines, Zhan et al.60 have reported camptothecin nanocrystals decorated with silver nanoparticles. Authors have reported excellent dispersion properties along with enhanced dissolution rate and cellular uptake. Silver nanoparticles are reported to inhibit P-gp and authors have tried to combine advantages obtained from the broad-spectrum anticancer agent and P-gp inhibition in single nanocrystal formulation. Prepared nanocrystals were tested for in vitro cytotoxicity in both drug sensitive and resistant cell lines and found them to be showing extreme and indiscriminate cytotoxicity in both the cells.
Recently, authors are working in the field of chemically modified drug nanocrystals and exploring the field to incorporate properties like sustained release as well as enhanced circulation time. Zhang et al.139 have reported PEGylated paclitaxel nanocrystals with prolonged circulation time. For this purpose pre-treatment of drug paclitaxel was done with PEG 5000 and then nanocrystals were prepared by simple nanoprecipitation method augmented by probe sonication. An average particle size of paclitaxel nanocrystals and PEGylated paclitaxel nanocrystals was found to be 240 and 330 nm, respectively. PEG molecules were found to cover drug nanocrystals with 11.54 nm fixed aqueous layer thickness. Surface modified nanocrystals showed higher stability in both the storage conditions as well as physiological conditions. In breast cancer xenograft mice model and lung cancer model, the modified nanocrystals revealed higher anticancer effect as compared to simple drug nanocrystals. The results were quite promising and the prepared formulation was giving better activity when compared with the marketed formulation, Taxol®.
4. In-vivo fate of nanocrystals after parenteral delivery
As mentioned in preceding sections, nanocrystals have emerged as potential drug delivery platform for solubility enhancement of poorly water-soluble drug. Apart from the solubility advantages, nanocrystals can be surface-tailored for more advanced applications including targeting via the parenteral route. Parenteral administration has advantages of immediate onset of action, reduced dose, and efficacious targeting. Due to these advantages offered by this route of administration, drugs having problems of first-pass metabolism, faster degradation in the gastrointestinal tract and erratic absorption are suitable candidates for parenteral administration. When talking about parenteral administration toxicity-related issues of excipients are to be taken care. A well-known example of the fact is Cremophor EL® which is an integral component of marketed formulation Taxol®. The aforementioned surfactant was found to initiate histamine release eliciting hypersensitivity reactions140. Nanocrystals formulation contains very few excipients (stabilizer in minute quantities) and141, 142, toxicity issues related to it are very less. When nanocrystal formulation is prepared, there are many factors to be taken care as they affect the in vivo behavior of nanocrystals.
4.1. In vivo fate of nanocrystals administered intravenously
4.1.1. Nanocrystals with smaller size
Particle size is one of the important factors affecting the in vivo fate of drug nanocrystals. Nanocrystals with a smaller particle size (less than 100 nm) behave like solution due to very fast dissolution and in contrary, larger crystals show higher cellular uptake by the reticuloendothelial system upon intravenous administration. In practice, the in vivo and in vitro correlation (IVIVC) is difficult to establish after intravenous administration of nanosuspension owing to either short residence time or fast precipitation. Hence it is extremely important for a formulation scientist to establish meaningful correlation in IVIVC studies140.
The in vivo stability of nanocrystals is a big issue especially when it comes to their fate inside the body. Dissolution rate and half-life in vivo are dependent on the size of nanocrystals, the hydrophobicity of drug and presence of a stabilizer. After intravenous administration biodistribution can be mostly affected by the state of agglomeration and surface characteristics of particles.
4.1.2. Larger nanocrystals
Nanocrystals greater than 500 nm are not taken up by cells because of their big size and slowly undergo dissolution or bind to the plasma proteins or are taken up by macrophages. Surface deposition of plasma proteins on nanocrystal surface can lead to aggregation or opsonization by macrophages. Nanocrystals of this size are phagocytosed by macrophages (considered as foreign particles) and may cause toxicity143. This mechanism of phagocytosis and clearance is very fast and takes around 5 min to phagocytose almost 90% of administered dose. Macrophages associated with Kupffer cells play an important role and are responsible for the accumulation of most of the dose in the liver. After liver, spleen is the main component responsible for the same phenomenon. This internalization changes the whole pharmacokinetics of nanosuspension and inversely affects higher dissolution rates. After being taken up by liver macrophages, these cells work as a depot of drug and slowly release drug of interest, which is in absolute contrast to the mechanism of drug release exhibited by nanocrystals under normal conditions144. Fig. 8 provides an overview of the in vivo fate of drug nanocrystals after parenteral administration.
Figure 8.
The in vivo fate of drug nanocrystals following intravenous administration.
Route of administration decides which path of nanoparticle will follow after administration and how body cells will consider that nanoparticles. In case of nanocrystals, cellular uptake mechanisms can be of different type after entering to the bloodstream. The paracellular transport mechanism is proposed by some authors, which can be enhanced by the use of surfactants like sodium deoxycholate and others. Particles of 100 nm can be easily transported through this mechanism144. However, there is little data available to validate this proof of concept. Another mechanism of cellular uptake is clathrin-mediated endocytosis, non-clathrin mediated endocytosis, phagocytosis, and pinocytosis. Following the intravenous route of administration, nanocrystals remain as such or are present in the form of a molecular dispersion. As mentioned in earlier sections their fate will be eventually decided by the size and surface properties of nanocrystals depending upon which they will be either solubilized or phagocytized25.
Shegokar et al.2 have reported the impact of nanocrystals size on body distribution. Nevirapine nanosuspension of particle size 480 nm was administered intravenously to observe its in vivo fate. Findings of the study were in accordance with theoretical perception. Around 40% of the administered dose was found in liver and 37% reached to the spleen. The reasons behind this were postulated as nanocrystals size, which was big enough to be taken up by macrophages.
4.2. In vivo fate of nanocrystals administered by an intramuscular/subcutaneous route
Contrary to intravenous administration, drug nanosuspensions administered intra-muscularly or subcutaneously (with smaller size) are reported to show sustained drug release irrespective of nanocrystal size. Wei et al.145 have reported intramuscular administration of curcumin didecanoate nanosuspension and found drug release to be sustained. Similarly, rilpivirine nanosuspension of size 200 nm was reported to show sustained plasma release profile up to 3 months, when administered as single intramuscular or subcutaneous injection135. The in vivo fate of nanocrystals can be suitably modified upon surface modification. Opsonisation is the most prominent phenomenon that can occur after intravenous administration and can be avoided by surface modification with PEG, which also enhances circulation time of these nanoparticles. Similarly, surface modification using vitamin E TPGS can lead to P-gp efflux inhibition. However, as mentioned earlier; IVIVC correlation is highly needed, to evaluate drug efficacy and avoid probable toxicity.
5. Challenges associated with the development of parenteral nanocrystals
Currently, there are very few marketed formulations based on parenteral nanosuspension technology. United States Food and Drug Administration (US FDA) has approved Invega Sustenna®, the first monthly antipsychotic injection, for use with Elan Drug Technologies (NanoCrystal technology) developed by Janssen Pharmaceuticals in 2012. Invega Sustenna® is the fifth licensed product approved by the US FDA using Elan׳s NanoCrystal technology for various formulations42. Paliperidone palmitate is an antipsychotic agent belonging to the category of benzisoxazole derivatives. Nanocrystal formulation of a mentioned drug for parenteral use is prepared with the aid of polysorbate 20, PEG 4000, citric acid monohydrate, disodium hydrogen phosphate anhydrous, sodium dihydrogen phosphate monohydrate, sodium hydroxide, and water for injection as inactive ingredients.
Sporanox IV® is another nanocrystal-based parenteral formulation of antifungal drug itraconazole. Contrary to oral route (Emend®, Rapamune®, Tricor®, Triglide® and others) as mentioned above, there are very less parenteral products based on nanocrystal technologies2. The reason behind this could be various challenges associated with development and effectiveness of parenteral nanosuspensions. Physical stability, stability during application and shedding of targeting ligand are some of the bottlenecks hindering the development of parenteral nanocrystal-based formulations. Proceeding sections will deal with challenges associated with parenteral nanocrystal in details.
5.1. Challenges of physical stability
Nanosuspension system is colloidal dispersion which is stabilized by stabilizers (surfactants, polymers or permutation of both). It is a thermodynamically unstable system having a tendency to agglomerate or enhances crystal growth. Despite the fact that the nanosuspension technology has been broadly investigated, stability is still a major concern and creates trouble for industrial scale up. Moreover, the knowledge on the practical correlation between the stability and stabilizer efficacy of nanosuspension formulation is quite insufficient. Stabilizer plays a vital role to put off aggregation of nanosuspension formulation. However, different drug requires different stabilizers in varying concentration and it is unlikely to arrive at a rule of thumb, applicable to all. Unfortunately, there is no experiential or theoretical guideline for the stabilizer selection and its optimization. There is still very little practical knowledge about the stability and stabilizer effectiveness of the formulation. Therefore, to select an appropriate stabilizer for a given nanosuspension formulation is often an important task for formulation scientist. Moreover, the amount and type of stabilizers affect the physical stability and in vivo performance of formulation. Some common stability problems are frequently observed in nanosuspension formulation146.
5.1.1. Aggregation
Selection of stabilizer type plays an important role in the stabilization and often improper selection can induce aggregation/other stability issues during prolonged storage. Ostwald ripening is a most common phenomenon occurring in nanosuspensions of heterogeneous nature which is most likely the case with most of the developed nanosuspensions146. In fact, aggregation is an intrinsic property of nanosuspension formulation, which is due to the propensity of a nanonized system to decrease the Gibbs free energy and initiation of Ostwald ripening. Proper stabilizer selection offering an excellent interaction potential between stabilizer and drug is necessary for the proper stabilization of preparation. When nanosuspension is produced by the top-down approach the surface area largely increases and due to that aggregation of nanosized particles happens. Therefore, the use of stabilizers is extremely important to cover up the surface of nanoparticles during preparation process147.
5.1.2. Crystalline transformation
To improve physical stability, solidification is considered a good method and various techniques like spray drying, freeze drying, electrospraying and pelletization are used. Formulation of the amorphous form requires very less energy than the crystal form formation and exhibits higher saturation solubility and higher dissolution velocity than the crystalline state. As a result, the crystalline drug is more stable and mostly best choice for the nanosuspension preparation. Once the amorphous material is produced, there is a risk of crystalline transformation during release or storage and thus crystalline transformation is an unavoidable thing in storage stage of the nanosuspension. Due to crystalline transformation, solubility may vary and may result in the alteration in plasma drug concentrations148.
5.1.3. Sedimentation/creaming
Nanosuspension formulations are mostly colloidal dispersion and are neither like true solutions nor a coarse dispersion rather an intermediate between them. Sedimentation is an incident of appearance for unstable nanosuspension formulation147. The primary step of instability is aggregation and Ostwald ripening. Nevertheless, when the gravity of drug nanoparticles becomes larger than their buoyancy force provided by dispersion system, the sedimentation takes place. Flocculation is also one of the types of sedimentation and it may occur by neutralization charge, particle- polymer complex, depletion flocculation or due to polymer bridging mechanism. The flocculation depends upon the two things polymer and its property of interaction with nanocrystals surface149.
5.1.4. Crystal growth
Crystal growth is the common phenomenon in the colloidal suspension and it is known as Ostwald ripening which is responsible for the change in size distribution and particle size150. According to Ostwald–Freundlich equation, smaller particles have more saturation solubility than bigger particles and this creates concentration gradient between small and large particles. The molecules in formulation disseminate the small particles (higher concentration surrounding areas) from larger particles (surrounded with lower drug concentration) and create an unsaturated solution in the vicinity of large particles which leads to a process of the Ostwald ripening and crystal growth. In other terms, the process of the diffusion leaves an unsaturated solution surrounding the small particles, giving rise to drug crystallization on large particles. The stabilizers play an essential role here i.e. they reduce the interfacial tension between the solid particles and a liquid medium and thereby avoid the Ostwald ripening. Verma et al.151 have reported the preparation of indomethacin nanosuspension stabilized using various stabilizers, the effect of the stabilizer concentration and its type on Ostwald ripening.
5.2. Challenges of chemical instability
Commonly, the nanosuspension formulations are prepared using water as a dispersion medium. Though non-aqueous solvents are also used to the same extent, the major proportion is still aqueous. Consequently, the chemical stability, like oxidation and hydrolysis, are the major concerns in the nanosuspension formulation. Appropriate packaging is a potential approach ensuring the chemical stability of formulation by protecting the drug from chemical hazards, light sensitivity, oxidation and hydrolysis152. Conversion of final product into the solid and concentrated state protects the drug from chemical hazards, but hydrolyzable compounds are still very difficult to protect146. It was reported that a significant enhancement of chemical stability of quercetin nanocrystal formulation occurred by using evaporative precipitation method and HPH considerably improved chemical stability and dissolution rate153.
5.3. Challenges due to the shedding of targeting ligand
Targeting ligands, in general, are not covalently attached to the surface of nanocrystals and physical adsorption is the prime most concepts utilized for surface modification. Physical adsorption provides weak bonding which could be unstable in biological environments and is a major challenge when the foremost objective is to provide targeting to a specific site. In some cases, this ligand shedding is desirable, as in case of P-gp substrate stabilized with vitamin E TPGS. Shedding of this ligand is involved in P-gp inhibition and provides better efficacy59.
6. Strategies to combat stability issues
Various solidification techniques are used to overcome issues related to physical as well as chemical stability. Solidification results in physical stability of nanosuspensions, as particle motion is hindered and chances of collision become less. Removal of aqueous part reduces/ends most of the chemical reactions resulting in chemical stability. Researchers have explored various solidification techniques viz. freeze drying, spray drying, the formation of hydrogels, pelletization, printing and electrospraying which are suitable for removal of aqueous content and enhancing physical as well as chemical stability. Among them freeze drying154, spray freeze drying155, printing155, coating155 are the techniques already reported for solidification of parenteral nanosuspension. Other possible solidification techniques, which can be explored in the near future based on the properties of drug incorporated and excipients used, are also discussed briefly. Proceeding section will deal with some of the solidifications techniques in brief.
6.1. Freeze drying
Freeze drying technique (well-known as lyophilization) is predominantly used for solidification of pharmaceuticals. Freeze drying technique is considered a safe technique for solidification of heat sensitive products like vaccines and various protein-containing formulations including nanosuspension. Freezing, primary drying, and secondary drying are three main steps in the process of lyophilization. Freezing step converts most of the aqueous component to the ice while primary drying involves sublimation of ice crystals. Secondary drying step removes an unfrozen aqueous component from the system59. Cryoprotectants are added to avoid the particle aggregation in formulation during the lyophilization process. Finally, the product is obtained as highly porous powder cake with very less water content. Selection of cryoprotectants and freezing rate both are critical parameters in this technique.
6.2. Spray drying
Spray drying technique is very simple and convenient technique for the conversion of liquid formulation into the solid powder156. This technique is working on the principle of spraying liquid in the form of small droplets to a preheated chamber by using nozzle and dried at a predefined temperature. Inlet temperature and spraying rate are the critical process parameters for this technique157. Nowadays, an electro-spraying technique is widely used for the solidification of liquid formulation which utilizes electrical current for atomization in place of pressurized air59.
6.3. Spray-freeze drying
Spray-freeze drying technique is a fusion of both the aforementioned techniques. This technique is particularly used for the thermo-sensitive products (vaccines, proteins, etc.). In this technique, nanosuspension is passed through a nozzle to obtain droplets and then these are immediately frozen in liquid nitrogen. The remaining process is similar to freeze drying. Spray freeze-drying produces injectable as well as inhalable delivery products156. The technique provides a good alternative especially for thermo-sensitive drug compounds and provides less particle size in comparison to the simple spray dried product.
6.4. Coating
Deposition of nanosuspension on the exterior of pellets, granules, sugar beads with the help of coater is another technique for the solidification of nanosuspension. This method requires an adequate quantity of excipients to stabilize the system and also acts as coating agents156. He et al.158 have reported preparation of indomethacin nanosuspension by precipitation– ultrasonication method using various proteins obtained from food as stabilizers. In this study, solidification was carried out using coating onto the pellets and results obtained suggested faster dissolution profile than the unprocessed crystals158.
6.5. Incorporation in granules and pellets
Incorporation of nanosuspension in granules and pellets is another approach used for solidification of the nanosuspension formulation156, In this technique, nanosuspension is incorporated in the granules or pellets utilizing various instruments such as fluidized-bed granulator, spheronizer etc. Mitri et al.159 have reported preparation of the lutein nanosuspension by HPH method and further solidification here was done with the formation of pellets by incorporation of nanosuspension using the spheronization technique. Finally, these prepared pellets were filled into capsules. This prepared formulation has shown higher saturation solubility and increased release rate159. However, this technique is reportedly not used for parenteral nanosuspension so far.
6.6. Printing
Printing technique is based on the principle of deposition of liquid or dispersed drug for manufacturing solid oral or pulmonary dosage forms. These techniques play an important role for narrow therapeutic index drugs and administering an amalgamation of drugs by printing more than one layer via barrier coating. A significant application of printing technology is a preparation of personalized medicines156. Nowadays, Inkjet printing, flexographic printing, and 3D printing are three mainly applied printing technologies used, which appears to be promising for use in parenteral nanocrystal-based formulations.
6.7. Using aerosol flow reactor
The aerosol flow reactor is one of patented technology for solidification of nanosuspension formulation for better stability. It is a single step continuous method with atomization of liquid flow via nitrogen (carrier gas) for solidification156. The liquid feed is atomized by nebulizer and the droplets produced are suspended in a carrier gas (N2) which are then fused in the heated tubular laminar flow reactor with constant temperature, where solidification occurs. Laaksonen et al.160 prepared the nanoparticulate indomethacin by aerosol flow reactor and wet milling technique. Particles obtained from this technique were showing good release and dissolution profile. Detailed investigation of nanocrystals produced using this technique is however still lacking for it to be used safely for parenteral administration.
6.8. Electrospraying
Electrohydrodynamic atomization (EHDA) is a technique based on electromechanical and hydrodynamic forces working in combination with each other. Thakkar et al.161 have recently reported preparation of erlotinib nanosuspension utilizing nanoprecipitation technique. A comparative evaluation of electrospraying and lyophilization techniques was done utilizing electrospraying as novel solidification technique. Flow rate, applied voltage, and tip to collector distance are critical parameters to be taken care with the use of this technique. While this technique was explored for obtaining a nano sprayed powder for orally dispersible tablets, it would be equally interesting to investigate the feasibility of using this technique for parenteral application. Electrospraying offers the advantage of using a different polymer which could also be used to obtain customized release rate of parenteral formulation.
7. Conclusion and future prospects
In comparison to other nanocarriers, nanocrystals are most successful as evidenced by the lesser time between inventions to market stage. A total number of products in the market (Tricor® a blockbuster product with sales of > 1 billion US dollar per year) and ongoing clinical trials further support this fact. Toxicity issues associated with nanocrystals are negligible as these are made majorly of the drug itself, thus get easily dissolved without leaving any residual carrier and can be of size >100 nm. Again uptake of drug nanocrystals by macrophages is majorly governed by the interplay of size/surface properties and can affect drug release (sustained release by working as depot system). Higher drug concentrations are associated with toxicity issues, especially in case of anticancer molecules which are very potent. However, very few articles talks about the in vivo fate of nanocrystals administered through various routes. The prime bottleneck is IVIVC of nanocrystal-based formulation which is needed to have a better understanding of its in vivo fate. A surface modification which provides stable interactions even at physiological conditions is one more thrust area of research; however, undesirable targeting ligand shedding still pose a major challenge. Some researchers have focused on pre-treatment of drug (PEGylation of drug followed by nanocrystals preparation) with the ligand to provide stable binding, which requires the involvement of chemical processes and needs further systematic investigation. Most of the nanocrystal-based studies lack stability under physiological conditions, a critical parameter which can affect most desirable properties i.e. solubility enhancement of nanocrystals. An investigation in directions above is needed for better understanding of nanocrystal technology and for providing new insights in this field before it could be safely extrapolated to formulations delivered parenterally.
Acknowledgment
Authors are thankful to Department of Pharmaceuticals, the Ministry of Chemicals and Fertilizers, Government of India, for providing financial assistance in the form of Fellowships. Authors would like to thank Department of Science and Technology, India for providing funding to conduct research work (DST INSPIRE LSBM-13 and SERB EMR/2016/007966).
Footnotes
Peer review under responsibility of Institute of Materia Medica, Chinese Academy of Medical Sciences and Chinese Pharmaceutical Association.
References
- 1.Lu Y., Li Y., Wu W. Injected nanocrystals for targeted drug delivery. Acta Pharm Sin B. 2016;6:106–113. doi: 10.1016/j.apsb.2015.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shegokar R., Müller R.H. Nanocrystals: industrially feasible multifunctional formulation technology for poorly soluble actives. Int J Pharm. 2010;399:129–139. doi: 10.1016/j.ijpharm.2010.07.044. [DOI] [PubMed] [Google Scholar]
- 3.Braddock M. Royal Society of Chemistry; Cambridge: 2016. Nanomedicines: design, delivery and detection. [Google Scholar]
- 4.Verma S., Burgess D. Solid nanosuspensions: the emerging technology and pharmaceutical applications as nanomedicine. In: Kulshreshtha A., Singh O., Wall G., editors. Pharmaceutical suspensions. Springer; New York: 2009. pp. 285–318. [Google Scholar]
- 5.Müller R.H., Benita S., Böhm B.H. CRC Press; 1998. Emulsions and nanosuspensions for the formulation of poorly soluble drugs. [Google Scholar]
- 6.Gardner C.R., Walsh C.T., Almarsson Ö. Drugs as materials: valuing physical form in drug discovery. Nat Rev Drug Discov. 2004;3:926. doi: 10.1038/nrd1550. [DOI] [PubMed] [Google Scholar]
- 7.Sun B., Yeo Y. Nanocrystals for the parenteral delivery of poorly water-soluble drugs. Curr Opin Solid State Mater Sci. 2012;16:295–301. doi: 10.1016/j.cossms.2012.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chin W.W.L., Parmentier J., Widzinski M., Tan E.H., Gokhale R. A brief literature and patent review of nanosuspensions to a final drug product. J Pharm Sci. 2014;103:2980–2999. doi: 10.1002/jps.24098. [DOI] [PubMed] [Google Scholar]
- 9.Nanotechnology for drug delivery: global market for nanocarriers. Available from: 〈http://www.cientifica.com/research/market-reports/nanotechnology-for-drug-delivery-global-market-for-nanocarriers/〉.
- 10.Kanwar S. Nano medicine breakthrough a giant leap for India. Times India News. 24 August 2015. Available from: 〈https://timesofindia.indiatimes.com/home/science/Nano-medicine-breakthrough-a-giant-leap-for-India/articleshow/48646117.cms〉
- 11.Müller R., Jacobs C., Kayser O. Nanosuspensions as particulate drug formulations in therapy: rationale for development and what we can expect for the future. Adv Drug Deliv Rev. 2001;47:3–19. doi: 10.1016/s0169-409x(00)00118-6. [DOI] [PubMed] [Google Scholar]
- 12.Kumar S., Burgess D.J. Nanosuspensions. In: Wright J., Burgess D., editors. Long acting injections and implants. Springer; Boston: 2012. pp. 239–261. [Google Scholar]
- 13.Rabinow B.E. Nanosuspensions in drug delivery. Nat Rev Drug Discov. 2004;3:785. doi: 10.1038/nrd1494. [DOI] [PubMed] [Google Scholar]
- 14.Wang G.D., Mallet F.P., Ricard F., Heng J.Y. Pharmaceutical nanocrystals. Curr Opin Chem Eng. 2012;1:102–107. [Google Scholar]
- 15.Ghosh I., Schenck D., Bose S., Ruegger C. Optimization of formulation and process parameters for the production of nanosuspension by wet media milling technique: effect of vitamin E TPGS and nanocrystal particle size on oral absorption. Eur J Pharm Sci. 2012;47:718–728. doi: 10.1016/j.ejps.2012.08.011. [DOI] [PubMed] [Google Scholar]
- 16.Tian X., Li H., Zhang D., Liu G., Jia L., Zheng D. Nanosuspension for parenteral delivery of a p-terphenyl derivative: preparation, characteristics and pharmacokinetic studies. Colloid Surf B. 2013;108:29–33. doi: 10.1016/j.colsurfb.2013.02.038. [DOI] [PubMed] [Google Scholar]
- 17.Zhai X., Lademann J., Keck C.M., Müller R.H. Nanocrystals of medium soluble actives—Novel concept for improved dermal delivery and production strategy. Int J Pharm. 2014;470:141–150. doi: 10.1016/j.ijpharm.2014.04.060. [DOI] [PubMed] [Google Scholar]
- 18.Mishra P.R., Al Shaal L., Müller R.H., Keck C.M. Production and characterization of Hesperetin nanosuspensions for dermal delivery. Int J Pharm. 2009;371:182–189. doi: 10.1016/j.ijpharm.2008.12.030. [DOI] [PubMed] [Google Scholar]
- 19.Rabinow B., Kipp J., Papadopoulos P., Wong J., Glosson J., Gass J. Itraconazole IV nanosuspension enhances efficacy through altered pharmacokinetics in the rat. Int J Pharm. 2007;339:251–260. doi: 10.1016/j.ijpharm.2007.02.030. [DOI] [PubMed] [Google Scholar]
- 20.Zhang J., Lv H., Jiang K., Gao Y. Enhanced bioavailability after oral and pulmonary administration of baicalein nanocrystal. Int J Pharm. 2011;420:180–188. doi: 10.1016/j.ijpharm.2011.08.023. [DOI] [PubMed] [Google Scholar]
- 21.Khan M.S., Vishakante G.D., Bathool A. Development and characterization of pilocarpine loaded Eudragit nanosuspensions for ocular drug delivery. J Biomed Nanotechnol. 2013;9:124–131. doi: 10.1166/jbn.2013.1475. [DOI] [PubMed] [Google Scholar]
- 22.Thassu D., Chader G.J. CRC Press; Boca Raton: 2012. Ocular drug delivery systems: barriers and application of nanoparticulate systems. [Google Scholar]
- 23.Das S., Suresh P.K. Nanosuspension: a new vehicle for the improvement of the delivery of drugs to the ocular surface. Appl Amphotericin B Nanomed. 2011;7:242–247. doi: 10.1016/j.nano.2010.07.003. [DOI] [PubMed] [Google Scholar]
- 24.Müller R.H., Gohla S., Keck C.M. State of the art of nanocrystals–special features, production, nanotoxicology aspects and intracellular delivery. Eur J Pharm Biopharm. 2011;78:1–9. doi: 10.1016/j.ejpb.2011.01.007. [DOI] [PubMed] [Google Scholar]
- 25.Gao L., Liu G., Ma J., Wang X., Zhou L., Li X. Drug nanocrystals: in vivo performances. J Control Release. 2012;160:418–430. doi: 10.1016/j.jconrel.2012.03.013. [DOI] [PubMed] [Google Scholar]
- 26.Keck C., Kobierski S., Mauludin R., Müller R.H. Second generation of drug nanocrystals for delivery of poorly soluble drugs: smartcrystals technology. Dosis. 2008;24:124–128. [Google Scholar]
- 27.Ghosh I., Bose S., Vippagunta R., Harmon F. Nanosuspension for improving the bioavailability of a poorly soluble drug and screening of stabilizing agents to inhibit crystal growth. Int J Pharm. 2011;409:260–268. doi: 10.1016/j.ijpharm.2011.02.051. [DOI] [PubMed] [Google Scholar]
- 28.Gao L., Zhang D., Chen M. Drug nanocrystals for the formulation of poorly soluble drugs and its application as a potential drug delivery system. J Nanopart Res. 2008;10:845–862. [Google Scholar]
- 29.Chan H.-K., Kwok P.C.L. Production methods for nanodrug particles using the bottom-up approach. Adv Drug Deliv Rev. 2011;63:406–416. doi: 10.1016/j.addr.2011.03.011. [DOI] [PubMed] [Google Scholar]
- 30.Junyaprasert V.B., Morakul B. Nanocrystals for enhancement of oral bioavailability of poorly water-soluble drugs. Asian J Pharm. 2015;10:13–23. [Google Scholar]
- 31.De Waard H., Hinrichs W., Frijlink H. A novel bottom–up process to produce drug nanocrystals: controlled crystallization during freeze-drying. J Control Release. 2008;128:179–183. doi: 10.1016/j.jconrel.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 32.Sinha B., Müller R.H., Möschwitzer J.P. Bottom-up approaches for preparing drug nanocrystals: formulations and factors affecting particle size. Int J Pharm. 2013;453:126–141. doi: 10.1016/j.ijpharm.2013.01.019. [DOI] [PubMed] [Google Scholar]
- 33.Junghanns J.-U.A., Müller R.H. Nanocrystal technology, drug delivery and clinical applications. Int J Nanomed. 2008;3:295. doi: 10.2147/ijn.s595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Van Eerdenbrugh B., Van den Mooter G., Augustijns P. Top-down production of drug nanocrystals: nanosuspension stabilization, miniaturization and transformation into solid products. Int J Pharm. 2008;364:64–75. doi: 10.1016/j.ijpharm.2008.07.023. [DOI] [PubMed] [Google Scholar]
- 35.Thakkar S., Shah V., Misra M., Kalia K. Nanocrystal based drug delivery system: conventional and current scenario. Recent Pat Nanotechnol. 2017;11:130–145. doi: 10.2174/1872210510666161014122439. [DOI] [PubMed] [Google Scholar]
- 36.Möschwitzer J.P. Drug nanocrystals in the commercial pharmaceutical development process. Int J Pharm. 2013;453:142–156. doi: 10.1016/j.ijpharm.2012.09.034. [DOI] [PubMed] [Google Scholar]
- 37.Kakran M., Shegokar R., Sahoo N.G., Al Shaal L., Li L., Müller R.H. Fabrication of quercetin nanocrystals: comparison of different methods. Eur J Pharm Biopharm. 2012;80:113–121. doi: 10.1016/j.ejpb.2011.08.006. [DOI] [PubMed] [Google Scholar]
- 38.Salazar J., Ghanem A., Müller R.H., Möschwitzer J.P. Nanocrystals: comparison of the size reduction effectiveness of a novel combinative method with conventional top-down approaches. Eur J Pharm Biopharm. 2012;81:82–90. doi: 10.1016/j.ejpb.2011.12.015. [DOI] [PubMed] [Google Scholar]
- 39.Ryde NP, Ruddy SB, inventors. Solid dose nanoparticulate compositions comprising a synergistic combination of a polymeric surface stabilizer and dioctyl sodium sulfosuccinate. United States patent US6375986B1; 2002.
- 40.Ryde T, Gustow EE, Ruddy SB, Jain R, Patel R, Wilkins MJ, inventors. Nanoparticulate fibrate formulations. United States patent US7276249B2; 2007.
- 41.Namburi RR, Kerr JE, inventors. Oral itraconazole formulations and methods of making the same. United States patent US20020150620A1; 2003.
- 42.François MKJ, Dries WMAC, Basstanie EDG, inventors. Aqueous suspensions of submicron 9-hydroxyrisperidone fatty acid esters. United States patent US6555544B2; 2003.
- 43.Nagi AS, inventors. Rapamycin formulations for oral administration. United States patent US5989591A. 1999.
- 44.Hu X., Lin C., Chen D., Zhang J., Liu Z., Wu W. Sirolimus solid self-microemulsifying pellets: formulation development, characterization and bioavailability evaluation. Int J Pharm. 2012;438:123–133. doi: 10.1016/j.ijpharm.2012.07.055. [DOI] [PubMed] [Google Scholar]
- 45.Pace GW, Mishra AK, Snow RA, Parikh I, Guivarc׳h P-HW, inventors. Spray drying process and compositions of fenofibrate. United States patent US6696084B2; 2004.
- 46.Bosch HW, Liversidge E, Shelukar SD, Thompson KC, inventors. Pharmaceutical composition of a tachykinin receptor antagonist. United States patent US20040214746A1; 2012.
- 47.Kalepu S., Nekkanti V. Insoluble drug delivery strategies: review of recent advances and business prospects. Acta Pharm Sin B. 2015;5:442–453. doi: 10.1016/j.apsb.2015.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Barret E.R. Vol. 166. CRC Press; Boca Raton: 2007. Nanosuspension for parenteral delivery. (Nanoparticulate drug delivery system). [Google Scholar]
- 49.Tuomela A., Hirvonen J., Peltonen L. Stabilizing agents for drug nanocrystals: effect on bioavailability. Pharmaceutics. 2016;8:16. doi: 10.3390/pharmaceutics8020016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shah C.N. Nanosuspension: a novel approach toenhance solubility of poorly watersoluble drugs. Pharma Sci Monit. 2016:7. [Google Scholar]
- 51.Nakarani M., Misra A., Patel J., Vaghani S. Itraconazole nanosuspension for oral delivery: formulation, characterization and in vitro comparison with marketed formulation. Daru. 2010;18:84. [PMC free article] [PubMed] [Google Scholar]
- 52.Rowe R.C., Sheskey P.J., Quinn M. 7th edition. Vol. 18. Pharmaceutical Press; Landon: 2013. Handbook of pharmaceutical excipients; p. 544. [Google Scholar]
- 53.Tang X., Liang Y., Feng X., Zhang R., Jin X., Sun L. Co-delivery of docetaxel and Poloxamer 235 by PLGA–TPGS nanoparticles for breast cancer treatment. Mater Sci Eng C. 2015;49:348–355. doi: 10.1016/j.msec.2015.01.033. [DOI] [PubMed] [Google Scholar]
- 54.Jindal N., Mehta S. Nevirapine loaded Poloxamer 407/Pluronic P123 mixed micelles: optimization of formulation and in vitro evaluation. Colloid Surf B. 2015;129:100–106. doi: 10.1016/j.colsurfb.2015.03.030. [DOI] [PubMed] [Google Scholar]
- 55.Chen X., Zhi F., Jia X., Zhang X., Ambardekar R., Meng Z. Enhanced brain targeting of curcumin by intranasal administration of a thermosensitive poloxamer hydrogel. J Pharm Pharmacol. 2013;65:807–816. doi: 10.1111/jphp.12043. [DOI] [PubMed] [Google Scholar]
- 56.Yap L.-S., Yang M.-C. Evaluation of hydrogel composing of Pluronic F127 and carboxymethyl hexanoyl chitosan as injectable scaffold for tissue engineering applications. Colloid Surf B. 2016;146:204–211. doi: 10.1016/j.colsurfb.2016.05.094. [DOI] [PubMed] [Google Scholar]
- 57.Zhang W., Haman K.J., Metzger J.M., Hackel B.J., Bates F.S., Lodge T.P. Quantifying binding of ethylene oxide–propylene oxide block copolymers with lipid bilayers. Langmuir. 2017;33:12624–12634. doi: 10.1021/acs.langmuir.7b02279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Liu P., Viitala T., Kartal-Hodzic A., Liang H., Laaksonen T., Hirvonen J. Interaction studies between indomethacin nanocrystals and PEO/PPO copolymer stabilizers. Pharm Res. 2015;32:628–639. doi: 10.1007/s11095-014-1491-3. [DOI] [PubMed] [Google Scholar]
- 59.Wang Y., Zheng Y., Zhang L., Wang Q., Zhang D. Stability of nanosuspensions in drug delivery. J Control Release. 2013;172:1126–1141. doi: 10.1016/j.jconrel.2013.08.006. [DOI] [PubMed] [Google Scholar]
- 60.Zhan H., Zhou X., Cao Y., Jagtiani T., Chang T.-L., Liang J.F. Anti-cancer activity of camptothecin nanocrystals decorated by silver nanoparticles. J Mater Chem B. 2017;5:2692–2701. doi: 10.1039/c7tb00134g. [DOI] [PubMed] [Google Scholar]
- 61.Li H.-Y., Seville P., Williamson I., Birchall J.C. Dispersibility of spray-dried formulations for pulmonary drug delivery. J Pharm Pharmacol. 2004;56:S10. [Google Scholar]
- 62.Lee J., Lee S.-J., Choi J.-Y., Yoo J.Y., Ahn C.-H. Amphiphilic amino acid copolymers as stabilizers for the preparation of nanocrystal dispersion. Eur J Pharm Sci. 2005;24:441–449. doi: 10.1016/j.ejps.2004.12.010. [DOI] [PubMed] [Google Scholar]
- 63.Choi J.-Y., Yoo J.Y., Kwak H.-S., Nam B.U., Lee J. Role of polymeric stabilizers for drug nanocrystal dispersions. Curr Appl Phys. 2005;5:472–474. [Google Scholar]
- 64.Wang Y., Li X., Wang L., Xu Y., Cheng X., Wei P. Formulation and pharmacokinetic evaluation of a paclitaxel nanosuspension for intravenous delivery. Int J Nanomed. 2011;6:1497. doi: 10.2147/IJN.S21097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bitterlich A., Laabs C., Krautstrunk I., Dengler M., Juhnke M., Grandeury A. Process parameter dependent growth phenomena of naproxen nanosuspension manufactured by wet media milling. Eur J Pharm Biopharm. 2015;92:171–179. doi: 10.1016/j.ejpb.2015.02.031. [DOI] [PubMed] [Google Scholar]
- 66.Frank K.J., Boeck G. Development of a nanosuspension for iv administration: from miniscale screening to a freeze dried formulation. Eur J Pharm Sci. 2016;87:112–117. doi: 10.1016/j.ejps.2016.03.003. [DOI] [PubMed] [Google Scholar]
- 67.Danhier F., Ucakar B., Vanderhaegen M.-L., Brewster M.E., Arien T., Préat V. Nanosuspension for the delivery of a poorly soluble anti-cancer kinase inhibitor. Eur J Pharm Biopharm. 2014;88:252–260. doi: 10.1016/j.ejpb.2014.05.014. [DOI] [PubMed] [Google Scholar]
- 68.Mahapatra L., Swathi A., Biswal B., Jena A. Formulation and evaluation of paclitaxel nanocrystals for parenteral administration by using PVP. Int J Pharma Res Health Sci. 2017;5:1424–1430. [Google Scholar]
- 69.Electronic code of Federal Regulations: title 21 Food and Drugs Part 101 Food Labeling, 2017. Available from: 〈https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?CFRPart=101〉.
- 70.Shanmugam S., Im H.T., Sohn Y.T., Kim Y.-I., Park J.-H., Park E.-S. Enhanced oral bioavailability of paclitaxel by solid dispersion granulation. Drug Dev Ind Pharm. 2015;41:1864–1876. doi: 10.3109/03639045.2015.1018275. [DOI] [PubMed] [Google Scholar]
- 71.Shao A., Hathcock J.N. Risk assessment for the amino acids taurine, l-glutamine and l-arginine. Regul Toxicol Pharm. 2008;50:376–399. doi: 10.1016/j.yrtph.2008.01.004. [DOI] [PubMed] [Google Scholar]
- 72.Arginine. Available from: 〈http://pubchem.ncbi.nlm.nih.gov/compound/6322#Section=2D-structure〉. 27 December 2017.
- 73.Winter R. Crown Archetype; New York: 2009. A consumer׳s dictionary of food additives: descriptions in plain English of more than 12,000 ingredients both harmful and desirable found in foods. [Google Scholar]
- 74.Paaver U., Tamm I., Laidmäe I., Lust A., Kirsimäe K., Veski P. Soluplus graft copolymer: potential novel carrier polymer in electrospinning of nanofibrous drug delivery systems for wound therapy. Biomed Res Int. 2014;2014 doi: 10.1155/2014/789765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Shamma R.N., Basha M. Soluplus®: a novel polymeric solubilizer for optimization of Carvedilol solid dispersions: formulation design and effect of method of preparation. Powder Technol. 2013;237:406–414. [Google Scholar]
- 76.Guo Y., Luo J., Tan S., Otieno B.O., Zhang Z. The applications of vitamin E TPGS in drug delivery. Eur J Pharm Sci. 2013;49:175–186. doi: 10.1016/j.ejps.2013.02.006. [DOI] [PubMed] [Google Scholar]
- 77.Available from: 〈https://www.accessdata.fda.gov/scripts/cder/iig/index.cfm?Event=BasicSearch.page〉. (27-12-2017).
- 78.Zhang Z., Tan S., Feng S.-S. Vitamin E TPGS as a molecular biomaterial for drug delivery. Biomaterials. 2012;33:4889–4906. doi: 10.1016/j.biomaterials.2012.03.046. [DOI] [PubMed] [Google Scholar]
- 79.Feng S.-S., Mei L., Anitha P., Gan C.W., Zhou W. Poly(lactide) vitamin E derivative/montmorillonite nanoparticle formulations for the oral delivery of Docetaxel. Biomaterials. 2009;30:3297–3306. doi: 10.1016/j.biomaterials.2009.02.045. [DOI] [PubMed] [Google Scholar]
- 80.Liu Y., Huang L., Liu F. Paclitaxel nanocrystals for overcoming multidrug resistance in cancer. Mol Pharm. 2010;7:863–869. doi: 10.1021/mp100012s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Baert L., van‘t Klooster G., Dries W., François M., Wouters A., Basstanie E. Development of a long-acting injectable formulation with nanoparticles of rilpivirine (TMC278) for HIV treatment. Eur J Pharm Biopharm. 2009;72:502–508. doi: 10.1016/j.ejpb.2009.03.006. [DOI] [PubMed] [Google Scholar]
- 82.Ge Z.-Q., Du X.-Y., Huang X.-N., Qiao B. Enhanced oral bioavailability of ursolic acid nanoparticles via antisolvent precipitation with TPGS1000 as a stabilizer. J Drug Deliv Sci Technol. 2015;29:210–217. [Google Scholar]
- 83.Collnot E.-M., Baldes C., Wempe M.F., Kappl R., Hüttermann J., Hyatt J.A. Mechanism of inhibition of P-glycoprotein mediated efflux by vitamin E TPGS: influence on ATPase activity and membrane fluidity. Mol Pharm. 2007;4:465–474. doi: 10.1021/mp060121r. [DOI] [PubMed] [Google Scholar]
- 84.Collnot E.-M., Baldes C., Schaefer U.F., Edgar K.J., Wempe M.F., Lehr C.-M. Vitamin E TPGS P-glycoprotein inhibition mechanism: influence on conformational flexibility, intracellular ATP levels, and role of time and site of access. Mol Pharm. 2010;7:642–651. doi: 10.1021/mp900191s. [DOI] [PubMed] [Google Scholar]
- 85.Yang H., Teng F., Wang P., Tian B., Lin X., Hu X. Investigation of a nanosuspension stabilized by Soluplus® to improve bioavailability. Int J Pharm. 2014;477:88–95. doi: 10.1016/j.ijpharm.2014.10.025. [DOI] [PubMed] [Google Scholar]
- 86.Linn M., Collnot E.-M., Djuric D., Hempel K., Fabian E., Kolter K. Soluplus® as an effective absorption enhancer of poorly soluble drugs in vitro and in vivo. Eur J Pharm Sci. 2012;45:336–343. doi: 10.1016/j.ejps.2011.11.025. [DOI] [PubMed] [Google Scholar]
- 87.El‐Sousi S., Nácher A., Mura C., Catalán‐Latorre A., Merino V., Merino‐Sanjuán M. Hydroxypropylmethylcellulose films for the ophthalmic delivery of diclofenac sodium. J Pharm Pharmacol. 2013;65:193–200. doi: 10.1111/j.2042-7158.2012.01587.x. [DOI] [PubMed] [Google Scholar]
- 88.Tuomela A., Liu P., Puranen J., Rönkkö S., Laaksonen T., Kalesnykas G. Brinzolamide nanocrystal formulations for ophthalmic delivery: reduction of elevated intraocular pressure in vivo. Int J Pharm. 2014;467:34–41. doi: 10.1016/j.ijpharm.2014.03.048. [DOI] [PubMed] [Google Scholar]
- 89.Ghosh I., Schenck D., Bose S., Liu F., Motto M. Identification of critical process parameters and its interplay with nanosuspension formulation prepared by top down media milling technology–a QbD perspective. Pharm Dev Technol. 2013;18:719–729. doi: 10.3109/10837450.2012.723720. [DOI] [PubMed] [Google Scholar]
- 90.Valo H.K., Laaksonen Pi.H., Peltonen L.J., Linder M.B., Hirvonen J.T., Laaksonen T.J. Multifunctional hydrophobin: toward functional coatings for drug nanoparticles. ACS Nano. 2010;4:1750–1758. doi: 10.1021/nn9017558. [DOI] [PubMed] [Google Scholar]
- 91.Paukkonen H., Ukkonen A., Szilvay G., Yliperttula M., Laaksonen T. Hydrophobin-nanofibrillated cellulose stabilized emulsions for encapsulation and release of BCS class II drugs. Eur J Pharm Sci. 2017;100:238–248. doi: 10.1016/j.ejps.2017.01.029. [DOI] [PubMed] [Google Scholar]
- 92.Ntoutoume G.M.N., Granet R., Mbakidi J.P., Brégier F., Léger D.Y., Fidanzi-Dugas C. Development of curcumin–cyclodextrin/cellulose nanocrystals complexes: new anticancer drug delivery systems. Bioorg Med Chem Lett. 2016;26:941–945. doi: 10.1016/j.bmcl.2015.12.060. [DOI] [PubMed] [Google Scholar]
- 93.Xia D., Quan P., Piao H., Piao H., Sun S., Yin Y. Preparation of stable nitrendipine nanosuspensions using the precipitation–ultrasonication method for enhancement of dissolution and oral bioavailability. Eur J Pharm Sci. 2010;40:325–334. doi: 10.1016/j.ejps.2010.04.006. [DOI] [PubMed] [Google Scholar]
- 94.Kurakula M., El-Helw A., Sobahi T.R., Abdelaal M.Y. chitosan based atorvastatin nanocrystals: effect of cationic charge on particle size, formulation stability, and in-vivo efficacy. Int J Nanomed. 2015;10:321. doi: 10.2147/IJN.S77731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Liu H., Ma Y., Liu D., Fallon J.K., Liu F. The effect of surfactant on paclitaxel nanocrystals: an in vitro and in vivo study. J Biomed Nanotechnol. 2016;12:147–153. doi: 10.1166/jbn.2016.2127. [DOI] [PubMed] [Google Scholar]
- 96.Wang Z., Li Z., Zhang D., Miao L., Huang G. Development of etoposide-loaded bovine serum albumin nanosuspensions for parenteral delivery. Drug Deliv. 2015;22:79–85. doi: 10.3109/10717544.2013.871600. [DOI] [PubMed] [Google Scholar]
- 97.Hou C.-D., Wang J.-X., Le Y., Zou H.-K., Zhao H. Preparation of azithromycin nanosuspensions by reactive precipitation method. Drug Dev Ind Pharm. 2012;38:848–854. doi: 10.3109/03639045.2011.630394. [DOI] [PubMed] [Google Scholar]
- 98.Hill A., Geißler S., Weigandt M., Mäder K. Controlled delivery of nanosuspensions from osmotic pumps: zero order and non-zero order kinetics. J Control Release. 2012;158:403–412. doi: 10.1016/j.jconrel.2011.12.005. [DOI] [PubMed] [Google Scholar]
- 99.Wang Y., Zhang D., Liu Z., Liu G., Duan C., Jia L. In vitro and in vivo evaluation of silybin nanosuspensions for oral and intravenous delivery. Nanotechnology. 2010;21:155104. doi: 10.1088/0957-4484/21/15/155104. [DOI] [PubMed] [Google Scholar]
- 100.Pandya V.M., Patel J.K., Patel D.J. Formulation, optimization and characterization of simvastatin nanosuspension prepared by nanoprecipitation technique. Pharm Lett. 2011;3:129–140. [Google Scholar]
- 101.Peters K., Leitzke S., Diederichs J., Borner K., Hahn H., Müller R. Preparation of a clofazimine nanosuspension for intravenous use and evaluation of its therapeutic efficacy in murine Mycobacterium avium infection. J Antimicrob Chemotherapy. 2000;45:77–83. doi: 10.1093/jac/45.1.77. [DOI] [PubMed] [Google Scholar]
- 102.Yin T., Dong L., Cui B., Wang L., Yin L., Zhou J. A toxic organic solvent-free technology for the preparation of PEGylated paclitaxel nanosuspension based on human serum albumin for effective cancer therapy. Int J Nanomed. 2015;10:7397. doi: 10.2147/IJN.S92697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Sharma S., Singh J., Verma A., Teja B.V., Shukla R.P., Singh S.K. Hyaluronic acid anchored paclitaxel nanocrystals improves chemotherapeutic efficacy and inhibits lung metastasis in tumor-bearing rat model. RSC Adv. 2016;6:73083–73095. [Google Scholar]
- 104.Li L., Liu Y., Wang J., Chen L., Zhang W., Yan X. Preparation, in vitro and in vivo evaluation of bexarotene nanocrystals with surface modification by folate-chitosan conjugates. Drug Deliv. 2016;23:79–87. doi: 10.3109/10717544.2014.904455. [DOI] [PubMed] [Google Scholar]
- 105.Sharma S., Verma A., Teja B.V., Shukla P., Mishra P.R. Development of stabilized paclitaxel nanocrystals: in-vitro and in-vivo efficacy studies. Eur J Pharm Sci. 2015;69:51–60. doi: 10.1016/j.ejps.2014.11.012. [DOI] [PubMed] [Google Scholar]
- 106.Wang Y., Rong J., Zhang J., Liu Y., Meng X., Guo H. Morphology, in vivo distribution and antitumor activity of bexarotene nanocrystals in lung cancer. Drug Dev Ind Pharm. 2017;43:132–141. doi: 10.1080/03639045.2016.1225752. [DOI] [PubMed] [Google Scholar]
- 107.Calleja P., Irache J., Zandueta C., Martínez-Oharriz C., Espuelas S. A combination of nanosystems for the delivery of cancer chemoimmunotherapeutic combinations: 1-methyltryptophan nanocrystals and paclitaxel nanoparticles. Pharmacol Res. 2017 doi: 10.1016/j.phrs.2017.09.004. [DOI] [PubMed] [Google Scholar]
- 108.Chiang P.-C., Gould S., Nannini M., Qin A., Deng Y., Arrazate A. Nanosuspension delivery of paclitaxel to xenograft mice can alter drug disposition and anti-tumor activity. Nanoscale Res Lett. 2014;9:156. doi: 10.1186/1556-276X-9-156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Gao L., Liu G., Kang J., Niu M., Wang Z., Wang H. Paclitaxel nanosuspensions coated with P-gp inhibitory surfactants: i. Acute toxicity and pharmacokinetics studies. Colloid Surf B. 2013;111:277–281. doi: 10.1016/j.colsurfb.2013.06.004. [DOI] [PubMed] [Google Scholar]
- 110.Gao L., Liu G., Ma J., Wang X., Wang F., Wang H. Paclitaxel nanosuspension coated with P-gp inhibitory surfactants: ii. Ability to reverse the drug-resistance of H460 human lung cancer cells. Colloid Surf B. 2014;117:122–127. doi: 10.1016/j.colsurfb.2014.02.016. [DOI] [PubMed] [Google Scholar]
- 111.Li Y., Zhao X., Zu Y., Zhang Y. Preparation and characterization of paclitaxel nanosuspension using novel emulsification method by combining high speed homogenizer and high pressure homogenization. Int J Pharm. 2015;490:324–333. doi: 10.1016/j.ijpharm.2015.05.070. [DOI] [PubMed] [Google Scholar]
- 112.Yi Y., Tu L., Hu K., Wu W., Feng J. The construction of puerarin nanocrystals and its pharmacokinetic and in vivo–in vitro correlation (IVIVC) studies on beagle dog. Colloid Surf B. 2015;133:164–170. doi: 10.1016/j.colsurfb.2015.04.054. [DOI] [PubMed] [Google Scholar]
- 113.Pawar V.K., Gupta S., Singh Y., Meher J.G., Sharma K., Singh P. Pluronic F-127 stabilised docetaxel nanocrystals improve apoptosis by mitochondrial depolarization in breast cancer cells: pharmacokinetics and toxicity assessment. J Biomed Nanotechnol. 2015;11:1747–1763. doi: 10.1166/jbn.2015.2158. [DOI] [PubMed] [Google Scholar]
- 114.Onoue S., Takahashi H., Kawabata Y., Seto Y., Hatanaka J., Timmermann B. Formulation design and photochemical studies on nanocrystal solid dispersion of curcumin with improved oral bioavailability. J Pharm Sci. 2010;99:1871–1881. doi: 10.1002/jps.21964. [DOI] [PubMed] [Google Scholar]
- 115.Chow DS-L, Gupta P, Qi Y, Shah J, Wisniecki P, inventors. Parenteral and oral formulations of benzimidazoles. United States patent US20090048322A1; 2008.
- 116.Xiong R., Lu W., Yue P., Xu R., Li J., Chen T. Distribution of an intravenous injectable nimodipine nanosuspension in mice. J Pharm Pharmacol. 2008;60:1155–1159. doi: 10.1211/jpp.60.9.0006. [DOI] [PubMed] [Google Scholar]
- 117.Ganta S., Paxton J.W., Baguley B.C., Garg S. Formulation and pharmacokinetic evaluation of an asulacrine nanocrystalline suspension for intravenous delivery. Int J Pharm. 2009;367:179–186. doi: 10.1016/j.ijpharm.2008.09.022. [DOI] [PubMed] [Google Scholar]
- 118.Shegokar R., Jansch M., Singh K.K., Müller R.H. In vitro protein adsorption studies on nevirapine nanosuspensions for HIV/AIDS chemotherapy. Nanomedicine. 2011;7:333–340. doi: 10.1016/j.nano.2010.10.012. [DOI] [PubMed] [Google Scholar]
- 119.Gao L., Zhang D., Chen M., Duan C., Dai W., Jia L. Studies on pharmacokinetics and tissue distribution of oridonin nanosuspensions. Int J Pharm. 2008;355:321–327. doi: 10.1016/j.ijpharm.2007.12.016. [DOI] [PubMed] [Google Scholar]
- 120.Wahlstrom J.L., Chiang P.-C., Ghosh S., Warren C.J., Wene S.P., Albin L.A. Pharmacokinetic evaluation of a 1,3-dicyclohexylurea nanosuspension formulation to support early efficacy assessment. Nanoscale Res Lett. 2007;2:291. [Google Scholar]
- 121.Bucolo C., Maltese A., Puglisi G., Pignatello R. Enhanced ocular anti-inflammatory activity of ibuprofen carried by an eudragit RS100® nanoparticle suspension. Ophthalmic Res. 2002;34:319–323. doi: 10.1159/000065608. [DOI] [PubMed] [Google Scholar]
- 122.Dandagi P., Kerur S., Mastiholimath V., Gadad A., Kulkarni A. Polymeric ocular nanosuspension for controlled release of acyclovir: in vitro release and ocular distribution. Iran J Pharm Res. 2010:79–86. [Google Scholar]
- 123.Kassem M., Rahman A.A., Ghorab M., Ahmed M., Khalil R. Nanosuspension as an ophthalmic delivery system for certain glucocorticoid drugs. Int J Pharm. 2007;340:126–133. doi: 10.1016/j.ijpharm.2007.03.011. [DOI] [PubMed] [Google Scholar]
- 124.Chiang P.-C., Hu Y., Thurston A., Sommers C.D., Guzova J.A., Kahn L.E. Pharmacokinetic and pharmacodynamic evaluation of the suitability of using fluticasone and an acute rat lung inflammation model to differentiate lung versus systemic efficacy. J Pharm Sci. 2009;98:4354–4364. doi: 10.1002/jps.21714. [DOI] [PubMed] [Google Scholar]
- 125.Mandal B., Alexander K.S., Riga A.T. Sulfacetamide loaded Eudragit RL100 nanosuspension with potential for ocular delivery. J Pharm Pharm Sci. 2010;13:510–523. [PubMed] [Google Scholar]
- 126.Pignatello R., Ricupero N., Bucolo C., Maugeri F., Maltese A., Puglisi G. Preparation and characterization of eudragit retard nanosuspensions for the ocular delivery of cloricromene. AAPS PharmSciTech. 2006;7:E192–E198. doi: 10.1208/pt070127. [DOI] [PubMed] [Google Scholar]
- 127.Zhang H., Hollis C.P., Zhang Q., Li T. Preparation and antitumor study of camptothecin nanocrystals. Int J Pharm. 2011;415:293–300. doi: 10.1016/j.ijpharm.2011.05.075. [DOI] [PubMed] [Google Scholar]
- 128.De Smet L., Colin P., Ceelen W., Bracke M., Van Bocxlaer J., Remon J.P. Development of a nanocrystalline Paclitaxel formulation for HIPEC treatment. Pharm Res. 2012;29:2398–2406. doi: 10.1007/s11095-012-0765-x. [DOI] [PubMed] [Google Scholar]
- 129.Jacobs C., Müller R.H. Production and characterization of a budesonide nanosuspension for pulmonary administration. Pharm Res. 2002;19:189–194. doi: 10.1023/a:1014276917363. [DOI] [PubMed] [Google Scholar]
- 130.Wang Y., Liu Z., Zhang D., Gao X., Zhang X., Duan C. Development and in vitro evaluation of deacety mycoepoxydiene nanosuspension. Colloid Surf B. 2011;83:189–197. doi: 10.1016/j.colsurfb.2010.10.029. [DOI] [PubMed] [Google Scholar]
- 131.Shubar H.M., Dunay I.R., Lachenmaier S., Dathe M., Bushrab F.N., Mauludin R. The role of apolipoprotein E in uptake of atovaquone into the brain in murine acute and reactivated toxoplasmosis. J Drug Target. 2009;17:257–267. doi: 10.1080/10611860902718680. [DOI] [PubMed] [Google Scholar]
- 132.Jacobs C., Kayser O., Müller R. Production and characterisation of mucoadhesive nanosuspensions for the formulation of bupravaquone. Int J Pharm. 2001;214:3–7. doi: 10.1016/s0378-5173(00)00622-0. [DOI] [PubMed] [Google Scholar]
- 133.Möschwitzer J., Achleitner G., Pomper H., Müller R.H. Development of an intravenously injectable chemically stable aqueous omeprazole formulation using nanosuspension technology. Eur J Pharm Biopharm. 2004;58:615–619. doi: 10.1016/j.ejpb.2004.03.022. [DOI] [PubMed] [Google Scholar]
- 134.Zakir F., Sharma H., Kaur K., Malik B., Vaidya B., Goyal A.K. Nanocrystallization of poorly water soluble drugs for parenteral administration. J Biomed Nanotechnol. 2011;7:127–129. doi: 10.1166/jbn.2011.1234. [DOI] [PubMed] [Google Scholar]
- 135.Fuhrmann K., Gauthier M.A., Leroux J.-C. Targeting of injectable drug nanocrystals. Mol Pharm. 2014;11:1762–1771. doi: 10.1021/mp5001247. [DOI] [PubMed] [Google Scholar]
- 136.Muller RH, Becker R, Kruss B, Peters K, inventors. Pharmaceutical nanosuspensions for medicament administration as systems with increased saturation solubility and rate of solution. United States patent US5858410A; 1999.
- 137.Grenier P, Vergnault G, Nhamias A, inventors. Nanoparticulate formulations of fenofibrate. PCT filling patent WO2003013474A1; 2014.
- 138.Vergnault G, Grenier P, Nhamias A, inventors. Novel compositions. United States patent US20040208936A1; 2002.
- 139.Zhang H., Hu H., Zhang H., Dai W., Wang X., Wang X. Effects of PEGylated paclitaxel nanocrystals on breast cancer and its lung metastasis. Nanoscale. 2015;7:10790–10800. doi: 10.1039/c4nr07450e. [DOI] [PubMed] [Google Scholar]
- 140.Tuomela A., Saarinen J., Strachan C.J., Hirvonen J., Peltonen L. Production, applications and in vivo fate of drug nanocrystals. J Drug Deliv Sci Technol. 2016;34:21–31. [Google Scholar]
- 141.Gol D., Thakkar S., Misra M. Nanocrystal-based drug delivery system of risperidone: lyophilization and characterization. Drug Dev Ind Pharm. 2018;44:1–28. doi: 10.1080/03639045.2018.1460377. [DOI] [PubMed] [Google Scholar]
- 142.Thakkar S, Sharma K, Khurana S, BansalA K. Excipients and their functionality for enabling technologies in oral dosage forms. Pharmaceutical excipients: properties, functionality, and applications in research and industry. Wiley Online Library; 2016.p.97–143.
- 143.Leone F., Cavalli R. Drug nanosuspensions: a ZIP tool between traditional and innovative pharmaceutical formulations. Expert Opin Drug Deliv. 2015;12:1607–1625. doi: 10.1517/17425247.2015.1043886. [DOI] [PubMed] [Google Scholar]
- 144.Pawar V.K., Singh Y., Meher J.G., Gupta S., Chourasia M.K. Engineered nanocrystal technology: in-vivo fate, targeting and applications in drug delivery. J Control Release. 2014;183:51–66. doi: 10.1016/j.jconrel.2014.03.030. [DOI] [PubMed] [Google Scholar]
- 145.Wei X.-L., Han Y.-R., Quan L.-H., Liu C.-Y., Liao Y.-H. Oily nanosuspension for long-acting intramuscular delivery of curcumin didecanoate prodrug: preparation, characterization and in vivo evaluation. Eur J Pharm Sci. 2013;49:286–293. doi: 10.1016/j.ejps.2013.03.010. [DOI] [PubMed] [Google Scholar]
- 146.Wang Y., Zheng Y., Zhang L., Wang Q., Zhang D. Stability of nanosuspensions in drug delivery. J Control Release. 2013;172:1126–1141. doi: 10.1016/j.jconrel.2013.08.006. [DOI] [PubMed] [Google Scholar]
- 147.Detroja C., Chavhan S., Sawant K. Enhanced antihypertensive activity of candesartan cilexetil nanosuspension: formulation, characterization and pharmacodynamic study. Sci Pharm. 2011;79:635–652. doi: 10.3797/scipharm.1103-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Puri V., Dantuluri A.K., Kumar M., Karar N., Bansal A.K. Wettability and surface chemistry of crystalline and amorphous forms of a poorly water soluble drug. Eur J Pharm Sci. 2010;40:84–93. doi: 10.1016/j.ejps.2010.03.003. [DOI] [PubMed] [Google Scholar]
- 149.Van Eerdenbrugh B., Stuyven B., Froyen L., Van Humbeeck J., Martens J.A., Augustijns P. Downscaling drug nanosuspension production: processing aspects and physicochemical characterization. AAPS Pharm Sci Tech. 2009;10:44–53. doi: 10.1208/s12249-008-9170-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Wu L., Zhang J., Watanabe W. Physical and chemical stability of drug nanoparticles. Adv Drug Deliv Rev. 2011;63:456–469. doi: 10.1016/j.addr.2011.02.001. [DOI] [PubMed] [Google Scholar]
- 151.Verma S., Kumar S., Gokhale R., Burgess D.J. Physical stability of nanosuspensions: investigation of the role of stabilizers on Ostwald ripening. Int J Pharm. 2011;406:145–152. doi: 10.1016/j.ijpharm.2010.12.027. [DOI] [PubMed] [Google Scholar]
- 152.Müller R., Jacobs C. Buparvaquone mucoadhesive nanosuspension: preparation, optimisation and long-term stability. Int J Pharm. 2002;237:151–161. doi: 10.1016/s0378-5173(02)00040-6. [DOI] [PubMed] [Google Scholar]
- 153.Gao L., Liu G., Wang X., Liu F., Xu Y., Ma J. Preparation of a chemically stable quercetin formulation using nanosuspension technology. Int J Pharm. 2011;404:231–237. doi: 10.1016/j.ijpharm.2010.11.009. [DOI] [PubMed] [Google Scholar]
- 154.Beirowski J., Inghelbrecht S., Arien A., Gieseler H. Freeze-drying of nanosuspensions, part 3: investigation of factors compromising storage stability of highly concentrated drug nanosuspensions. J Pharm Sci. 2012;101:354–362. doi: 10.1002/jps.22745. [DOI] [PubMed] [Google Scholar]
- 155.Malamatari M., Somavarapu S., Taylor K.M., Buckton G. Solidification of nanosuspensions for the production of solid oral dosage forms and inhalable dry powders. Expert Opin Drug Deliv. 2016;13:435–450. doi: 10.1517/17425247.2016.1142524. [DOI] [PubMed] [Google Scholar]
- 156.Malamatari M., Somavarapu S., Taylor K.M., Buckton G. Solidification of nanosuspensions for the production of solid oral dosage forms and inhalable dry powders. Expert Opin Drug Deliv. 2016;13:435–450. doi: 10.1517/17425247.2016.1142524. [DOI] [PubMed] [Google Scholar]
- 157.Baba K., Nishida K. Steroid nanocrystals prepared using the Nano Spray Dryer B-90. Pharmaceutics. 2013;5:107–114. doi: 10.3390/pharmaceutics5010107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.He W., Lu Y., Qi J., Chen L., Yin L., Wu W. Formulating food protein-stabilized indomethacin nanosuspensions into pellets by fluid-bed coating technology: physical characterization, redispersibility, and dissolution. Int J Nanomed. 2013;8:3119. doi: 10.2147/IJN.S46207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Mitri K., Shegokar R., Gohla S., Anselmi C., Müller R.H. Lutein nanocrystals as antioxidant formulation for oral and dermal delivery. Int J Pharm. 2011;420:141–146. doi: 10.1016/j.ijpharm.2011.08.026. [DOI] [PubMed] [Google Scholar]
- 160.Laaksonen T., Liu P., Rahikkala A., Peltonen L., Kauppinen E.I., Hirvonen J. Intact nanoparticulate indomethacin in fast-dissolving carrier particles by combined wet milling and aerosol flow reactor methods. Pharm Res. 2011;28:2403. doi: 10.1007/s11095-011-0456-z. [DOI] [PubMed] [Google Scholar]
- 161.Thakkar S., Sharma D., Misra M. Comparative evaluation of electrospraying and lyophilization techniques on solid state properties of Erlotinib nanocrystals: assessment of In-vitro cytotoxicity. Eur J Pharm Sci. 2018;111:257–269. doi: 10.1016/j.ejps.2017.10.008. [DOI] [PubMed] [Google Scholar]