Structured Abstract
Background
Endothelial dysfunction is the first stage of the atherosclerotic cascade, and independently associated with cardiovascular events. We evaluated the associations of longitudinal changes in weight, waist circumference, body fat percentage and lean mass index with changes in endothelial function.
Methods
521 community-based subjects who belonged to hypertensive sibships and had no history of myocardial infarction or stroke had their anthropometric measures and endothelial function assessed a mean of 8.5 years apart. Endothelial function was assessed with brachial artery ultrasound, yielding measures of flow-mediated dilation and reactive hyperemia. We used multivariable linear regression with generalized estimating equations to assess the associations of longitudinal changes (Δ) in anthropometric measures with Δ flow-mediated dilation and reactive hyperemia, adjusting for potential confounders.
Results
Mean ± standard deviation age was 57.6±8.7 years, 58% were women, and 72% were hypertensive. Most (84%) were overweight or obese at baseline. At end of follow-up, flow-mediated dilation and reactive hyperemia increased by 1.9±7.6% and 51.2±605.8% on average, respectively. In multivariable linear regression, changes in anthropometric measures were not associated with changes in flow-mediated dilation. However, Δ weight (β±SE: −9.00±2.35), Δ waist circumference (−6.78±2.21) and Δ body fat percentage (−19.72±5.62, P<0.0001 for each) were inversely associated with Δ reactive hyperemia. Δ lean mass index was not associated with Δ reactive hyperemia.
Conclusions
Long-term increases in weight, waist circumference and body fat percentage are associated with progressive worsening of microvascular endothelial function, but not conduit vessel endothelial function, in subjects without a history of cardiovascular events, independently of risk factors.
Keywords: endothelium, obesity, weight gain, body fat, flow-mediated dilation, reactive hyperemia
1. Introduction
The social, economic, and human costs of cardiovascular (CV) disease continue to escalate. However, despite scientific advances in the past decades, the number of effective CV therapies and viable therapeutic targets remains limited. Importantly, once the atherosclerotic process commences, containing its progression is difficult, and reversing established structural abnormalities is improbable. For these reasons, to contain the health and economic burden of CV diseases, there is a critical need to enhance early detection of individuals at risk in order to enhance primary prevention strategies, arrest the pathogenic process, and improve health and outcomes.
With the goals of understanding CV pathogenesis and identifying targets for early detection and prevention of CV disease, scientists have often turned their attention to the vascular endothelium. The endothelium is a highly active structure capable of producing several substances that modulate vascular tone and reactivity, and inhibit cell proliferation and thrombogenesis.1 Thus, it is essential for maintaining vascular health;1 and it is now well established that adverse alterations in endothelial function represent the first abnormalities in the atherogenic cascade.2 The role of the endothelium on CV diseases has been further corroborated by longitudinal clinical studies, which have highlighted the association endothelial dysfunction with future development of myocardial infarction and stroke independently of aging and conventional CV risk factors.3 Thus, measures of endothelial dysfunction represent a viable target for early detection of individuals at higher CV risk, and for identifying environmental and heritable factors that may contribute to the initiation of the atherogenic cascade.
Obesity is a known risk factor for CV disease,4, 5 and cross-sectionally associated with endothelial dysfunction.6 Acutely, purposeful weight gain leads to impairment of endothelial function.7 However, whether non-purposeful weight gain detrimentally affects endothelial function in free-living individuals remains unknown. This is the knowledge gap that we sought to address in the present study. We hypothesized that longitudinal increases in measures of total and central obesity would be associated with worsening of endothelial function. To this end, we studied a community-based cohort to evaluate whether changes in weight, waist circumference (WC), body fat percentage (BF%) and lean mass were associated with worsening in conduit artery and microvascular endothelial function over time.
2. Subjects, Materials and Methods
2.1. Study Participants and Baseline Characteristics
The study was approved by the Mayo Clinic’s Institutional Review Board and participants gave informed consent. The cohort consisted of 557 community-based non-Hispanic white participants from the Genetic Epidemiology Network of Arteriopathy (GENOA) study who had their endothelial function assessed on 2 separate occasions (baseline: between January 2003 and December 2008; follow up: between October 2009 and December 2011). The GENOA study is a cohort study of hypertensive sibships from Olmsted County, MN, USA. We excluded 36 participants with a history of myocardial infarction or stroke, leaving 521 participants for the present analyses.
On the day of each study visit, participants met with the study coordinator and completed a comprehensive questionnaire that included demographic and medical information. Methods for assessment of vital signs and baseline laboratory tests are described in the Supplemental Methods.
2.2. Anthropometric assessment
Height, weight and WC were obtained at baseline and during the follow-up visit. Height was measured with a stadiometer, weight was measured with an electronic scale, and WC was measured with a measuring tape at the level of the umbilicus. Participants were classified as being centrally obese if their WC was > 102 cm in men and > 88cm in women.8 Body mass index (BMI) was calculated as weight (in Kg)/height (in m)2. Patients were classified as having underweight, normal, overweight or obese BMI according to World Health Organization (WHO) criteria.8 BF% was calculated using the formula: (1.20 × BMI) + (0.23 × Age) − (10.8 × sex) − 5.4; where sex=1 for men and 0 for women. This validated formula provides accurate measures of body composition, with prediction errors that are compatible to those obtained from direct measures of body fat such as skinfold thickness of bioelectrical impedance.9 Lean mass index (LMI) was calculated as BMI x (1-BF%) as previously described.10
2.3. Assessment of Endothelial Function
Endothelial function was assessed non-invasively during the baseline and follow-up visits using brachial artery ultrasound, a technique that yields measures of conduit artery endothelial (flow-mediated dilation, FMD) and microvascular (reactive hyperemia, RH) endothelial function. Participants were asked to fast and refrain from smoking, ingesting caffeine, or taking anti-hypertensive medications for 12 hours prior to the study visit. Brachial artery ultrasound was performed by trained technicians while participants were laying supine in a dark, quiet room with controlled temperature, according to published guidelines11 and described in detail in the Supplemental Methods. FMD was calculated as [(mBAD – rBAD)/rBAD] x 100, where mBAD is the maximum brachial artery diameter during hyperemia following cuff deflation, and rBAD is the resting brachial artery diameter. RH was calculated as a [(dFlow – rFlow)/rFlow] x 100, where dFlow is hyperemic post-deflation brachial artery flow and rFlow is resting brachial artery flow.
2.4. Biomarkers
To further elucidate mechanisms underlying the link between weight and fat gain on endothelial function, we also measured circulating levels of adipocytokines (leptin, resistin and adiponectin) and inflammatory markers [C-reactive protein (CRP), homocysteine, Intercellular Adhesion Molecule 1 (ICAM-1), vascular cell adhesion molecule 1 (VCAM-1), E-selectin, P-selectin, homocysteine, interleukin-6 (IL-6) and interleukin-18 (IL-18)] at baseline. Details about biomarker measurement, performance and quality control are summarized in the Supplemental Methods.
2.5. Statistical Methods
Continuous variables were reported as mean ± standard deviation (SD) for normally distributed variables, and as median (interquartile range, IQR) for skewed variables. Nominal variables were reported as n and %. Longitudinal changes (Δ) in weight, BMI, WC, BF, LMI, FMD and RH were calculated by subtracting values obtained at the baseline visit from the values obtained at the follow-up visit.
We used multivariable linear regression models adjusted for sex, baseline age, blood pressure, renal function, lipids, baseline FMD or RH, follow-up time, history of hypertension, diabetes and smoking, and use of statins and anti-hypertensives to assess the associations of Δ weight, Δ BMI, Δ WC, Δ BF% and Δ LMI (independent variables, separate models for each anthropometric measure) with Δ FMD and Δ RH (dependent variables). Only covariates significantly associated with the dependent variable were included in the final models, but baseline age, sex and follow-up time were forced into all models. In all models, we tested interactions terms for changes in all anthropometric measures with age and sex; and for Δ BMI*Δ WC. To aid in interpretation, we also performed multivariable logistic regression models to predict FMD increase (ΔFMD<0) and RH increase (ΔRH<0).
To determine whether adipocytokines and inflammatory markers mediated associations of Δ anthropometric measures with worsening of endothelial function, we followed the principles outlined by Baron and Kenny 12 (see Supplemental Methods).
All regression analyses were performed using generalized estimating equations to account for the familial relatedness of the participants. All analyses were performed using SPSS vs. 22 (IBM Corp., Armonk, New York, U.S.A.), and a 2-tailed P-value ≤0.05 was considered statistically significant.
3. Results
Participant characteristics are summarized in Table 1. After a mean follow-up of 8.5±1.0 years, 54% of participants gained weight, which was predominantly due to a gain in BF% despite a decline in LMI (Table 1). At the end of follow-up, 41% of participants experienced a decline in FMD (magnitude of decline: −4.8±4.7%), and 46% experienced a decline in RH (magnitude of decline: −441.5±382.7%); although the mean values for these endothelial function measures increased over time in the whole group (Table 1), as those who experienced increased FMD and RH over time had a greater magnitude of increase (+6.6±5.4% and +462.9±419.4%, respectively). Baseline characteristics according to weight loss or gain groups are shown in Supplemental Table S1. Those who gained the greatest amounts of weight (>10Kg) were more likely to be younger men than those who maintained, lost or gained less weight. They were also more likely to be smokers, have higher glomerular filtration rate and lower leptin levels than those who lost weight. Conversely, those who lost the most weight (>10Kg) were more likely to be heavier at the first evaluation, and had higher baseline BMI, WC and BF% than those who lost less or gained weight. Baseline FMD and RH were similar across all groups. A summary of the brachial artery ultrasound parameters at baseline and at follow-up can be found in Supplemental Table S2.
Table 1.
Participant characteristics
| Variable | Baseline (mean±SD) or n (%) | Change during follow-up, % or median (IQR) |
|---|---|---|
| Age, years | 57.6±8.7 | --- |
| Women, n (%) | 302 (58%) | --- |
| Systolic blood pressure, mmHg | 130±16 | --- |
| Diastolic blood pressure, mmHg | 75±8 | -- |
| Total cholesterol, mmol/L | 5.13±0.85 | --- |
| HDL cholesterol, mmol/L | 1.35±0.39 | --- |
| LDL cholesterol, mmol/L | 3.12±0.80 | --- |
| Triglycerides, mmol/L | 1.73±0.97 | --- |
| Hypertension, n (%) | 375 (72%) | --- |
| Diabetes, n (%) | 58 (11%) | --- |
| Smoking, n (%) | 233 (45%) | --- |
| eGFR, ml/s/1.73 m2 | 1.09±0.21 | --- |
| Statin use, n (%) | 154 (30%) | --- |
| Anti-hypertensive use, n (%) | 351 (68%) | --- |
| Height, cm | 168.8±8.9 | --- |
| Weight, Kg | 87.6±19.4 | + 0.6 (−3.8, +4.6) |
| BMI, Kg/m2 | 30.7±5.8 | +0.07 (−1.4, +1.6) |
| Underweight (BMI <18.5Kg/m2), n (%) | 0 | 0 |
| Normal BMI (18.5–24.9 Kg/m2), n (%) | 81 (16%) | −2% |
| Overweight BMI (25–29.9 Kg/m2), n (%) | 177 (34%) | +3% |
| Obese BMI (≥30 Kg/m2), n (%) | 262 (50%) | −1% |
| Waist circumference, cm | 100.2±15.7 | + 0.4 (−4.4, +5.7) |
| Centrally obese, n (%) | 336 (65%) | −1% |
| BF%, % | 50.9±8.6 | + 2.2 (+0.3, +3.8) |
| LMI, Kg/m2 | 14.7±2.0 | −0.6 (−0.4, −0.8) |
| FMD, % | 5.9±4.9 | + 1.6 (−2.6, +6.0) |
| Reactive hyperemia, % | 805±452 | +60.3 (−277.3, +369.0) |
BF%: body fat percentage. BMI: body mass index. eGFR: estimated glomerular filtration rate. FMD: brachial artery flow mediated dilation. HDL: high-density lipoprotein. LDL: low-density lipoprotein. LMI: lean mass index
Independent predictors of Δ FMD were baseline age (β±SE: −0.15±0.05, P=0.002), time between studies (0.69±0.36, P=0.05), baseline FMD (−0.78±0.07, P<0.0001) and use of statins (1.48±0.74, P=0.05). Independent predictors of ΔFMD<0 were older baseline age (odds ratio (OR) and 95% confidence interval (CI) for 1 year increase in age: 1.06 (1.03, 1.09), P<0.0001), higher baseline FMD (each 1 SD increase in baseline FMD: 3.23 (2.49, 4.25), P<0.0001), shorter time between studies (each 1 year increase in time: 0.77 (0.60, 0.98), P=0.04) and the absence of diabetes (0.41 (0.20, 0.81), P=0.009). Independent predictors of Δ RH were: baseline systolic (−6.12±1.67, P<0.0001) and diastolic (9.49±2.663, P<0.0001) blood pressure, serum triglycerides (−0.85±0.23, P<0.0001) and baseline RH (−0.86±0.05, P<0.0001). Independent predictors of ΔRH<0 were higher baseline systolic blood pressure (OR and 95% CI for each 5 mmHg increase: 1.09 (1.01, 1.18), P=0.04) and higher baseline RH (each 1 SD increase in baseline RH: 5.04 (3.66, 7.16), P<0.0001).
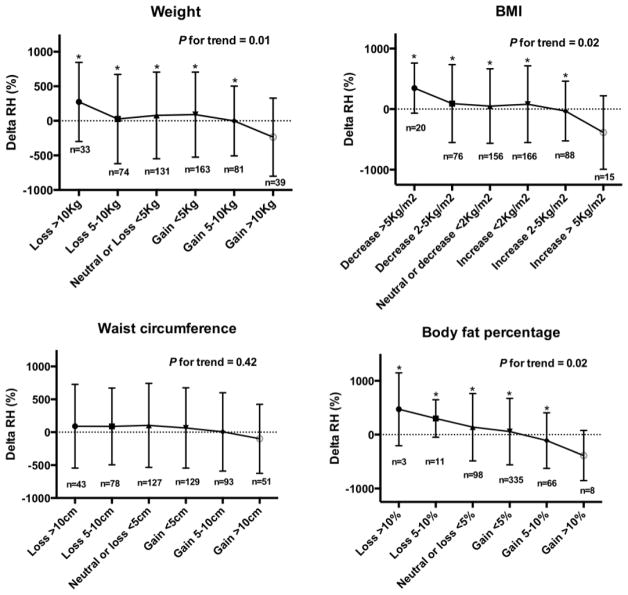
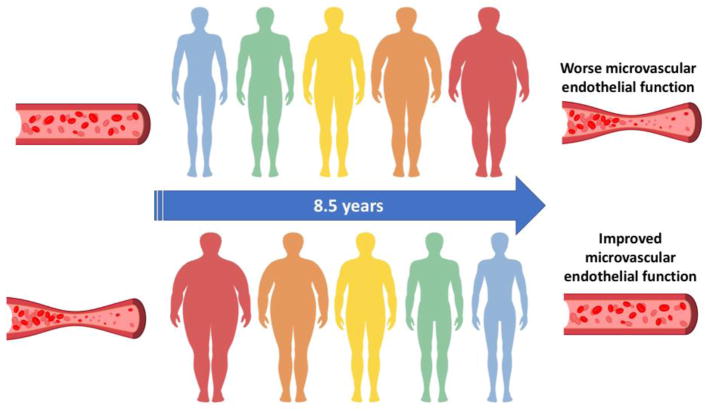
Results of the linear regression models are summarized in Table 2. Longitudinal increases in weight, BMI, WC and BF% were associated with declines in RH over time, but not with changes in FMD. Inferences remained unchanged when we added baseline anthropometric measures as additional covariates in the models (analyses not shown). Figure 1 depicts the effects of changes in weight, WC, BMI and BF% on changes in RH over time. Only those individuals who experienced the greatest increases in weight, BMI and BF% experienced deterioration in microvascular endothelial function over time. Conversely, those who lost the most weight/BMI/BF% had the greatest improvements in microvascular endothelial function over time. Figure 2 summarizes the main findings of the study. Neither age nor sex was a significant effect modifier of these associations. The interaction term ΔBMI*ΔWC was not statistically significant in the prediction of ΔFMD or ΔRH.
Table 2.
Multivariable linear regression models results showing associations of changes in anthropometric measures with changes in endothelial function over time
| Δ FMD models | Δ RH models | |||
|---|---|---|---|---|
| β±SE | P-value | β±SE | P-value | |
| Δ weight, 1 Kg | 0.001±0.05 | 0.99 | −9.00±2.35 | <0.0001 |
| Δ BMI, 1 Kg/m2 | −0.06±0.10 | 0.56 | −23.68±6.64 | <0.0001 |
| Δ WC, 1cm | −0.005±0.04 | 0.89 | −6.78±2.21 | 0.002 |
| Δ BF%, 1% | −0.05±0.08 | 0.56 | −19.72±5.62 | <0.0001 |
| Δ LMI, 1 Kg/m2 | −0.73±0.59 | 0.28 | 45.45±48.57 | 0.91 |
WC: waist circumference. Other abbreviations as in Table 1.
Figure 1. Effects of increases in anthropometric measures with changes in reactive hyperemia over time.
BF: body fat percentage. BMI: body mass index. WC: waist circumference. RH: Reactive hyperemia.
* indicates P≤0.05 for comparison with the group with highest weight/BMI/WC/BF gain.
Figure 2. Summary of effects of weight gain and loss on microvascular endothelial function.
After an average follow-up of 8.5 years, longitudinal gains in weight, waist circumference, body mass index and body fat percentage were associated with worsening of microvascular endothelial function. Conversely, weight loss over time was associated with improvement of microvascular endothelial function.
Biomarker levels were log-transformed to reduce skewness. We found no significant associations, adiponectin, resistin, CRP, homocysteine, ICAM-1, VCAM-1, IL-18, E-selectin or P-selectin with Δ FMD or Δ RH (analyses not shown). However, baseline log leptin (β±SE: −89.75±34.27, P= 0.009) and log IL-6 (β±SE: −91.93±34.90, P=0.009) were independently associated with Δ RH. We then regressed log leptin and log IL-6 on Δ weight, Δ BMI, Δ WC and Δ BF% and found that Δ weight (−0.02±0.004, P<0.0001), Δ BMI (−0.04±0.01, P=0.002) and Δ BF% (−0.03±0.01, P=0.002) were significantly associated with baseline leptin. Conversely, Δ weight (−0.005±0.003, P=0.161), Δ WC (−0.006±0.003, P=0.069), ΔBMI (−0.002±0.010, P=0.106) and ΔBF% (−0.014±0.008, P=0.100) were not significantly associated with baseline IL-6. As the final step, we repeated the multivariable linear regression models, this time including log leptin or log IL-6 as an additional covariates, and found that the associations of Δ weight, Δ BMI, Δ WC and Δ BF% with Δ RH were strengthened rather than weakened by the addition of leptin or IL-6 to the models (Table 3). Leptin and IL-6 remained inversely and significantly associated with Δ RH in these models (P≤0.05 in each of the models). Thus, the associations of leptin and changes in anthropometric measures with microvascular endothelial dysfunction are independent of each other, and leptin is not a mediator of the association of Δ weight, Δ BMI and Δ BF% with ΔRH.
Table 3.
Associations of Δ weight, Δ BMI and Δ BF% with Δ RH with and without leptin in the model
| Baseline model | Baseline model + log leptin | Baseline model + log IL-6 | ||||
|---|---|---|---|---|---|---|
| β±SE | P-value | β±SE | P-value | β±SE | P-value | |
| Δ weight | −9.00±2.35 | <0.0001 | −10.21±2.36 | <0.0001 | −9.79±2.71 | 0.0003 |
| Δ WC | −6.94±2.55 | 0.007 | −8.02±2.55 | 0.002 | −7.43±2.532 | 0.004 |
| Δ BF% | −19.72±5.62 | <0.0001 | −22.64±5.56 | <0.0001 | −21.82±6.32 | 0.0007 |
| Δ BMI | −23.68±6.64 | <0.0001 | −27.08±6.60 | <0.0001 | −25.82±7.59 | 0.0007 |
Lastly, since higher BMI has been associated with greater resting forearm blood flow,13 presumably to support greater forearm mass, we repeated the ΔRH models after additional adjustment for resting forearm flow. We found that inferences remained unchanged, and Δ weight (P=0.002), Δ WC, (P=0.02) Δ BMI (P=0.02) and Δ BF% (P=0.002) remained independently associated with ΔRH.
4. Discussion
In a large community-based cohort without baseline clinical history of coronary heart disease or stroke, we have demonstrated that weight and body fat gain, and worsening of central obesity over an 8.5 year period are associated with deterioration of microvascular endothelial function, but not conduit artery endothelial function. This was specific to individuals who had the most extreme gains in weight, BMI and body fat, and not observed among those who had modest or moderate gains. Further, individuals who lost the most weight/BMI/body fat over the same 8.5 year time period experience the greatest improvements in microvascular endothelial function. In addition, we found that the effects of weight gain on endothelial function are not mediated by adipocytokines or inflammatory markers. To the best of our knowledge, this is the first report of the effects of long-term, non-purposeful weight gain and loss on endothelial function in free-living individuals. Our findings are important for elucidating the mechanisms through which obesity may contribute to CV disease, and for highlighting the importance of minimizing weight gain associated with aging as means to prevent the deleterious effects of excess adiposity on arterial health.
Both total and central obesity have well established associations with future risk of CV diseases 14, 15 and death. 16–18 Mechanisms linking obesity to CV disease are multiple. Indirectly, obesity is associated with CV disease through its link with conventional risk factors. However, potential direct effects of obesity on atherogenesis have also been described.19 Visceral adipose tissue is a source of pro- and anti-inflammatory adipocytokines, and an imbalance favoring inflammation may be implicated in atherosclerosis in obese subjects.19 In addition, fat accumulation in obese humans correlates closely with greater oxidative stress; 20 and free fatty acids activate he innate immune system, triggering inflammasome activation.21
Another proposed mechanism through which obesity contributes to CV diseases is endothelial dysfunction.22 On a molecular level, increased adiposity is associated with lower nitric oxide production and availability,23 increased prostanoid-dependent vasoconstriction and vascular thromboxane receptor gene expression.24 An imbalance in these homeostatic mechanisms at the endothelial level is likely to contribute to hypertension, atherogenesis and thrombosis associated with obesity. Confirming these biological observations, clinical studies have also demonstrated a cross-sectional association of obesity with endothelial dysfunction. 25,26 Only two previous studies have prospectively assessed the effects of short-tem weight gain on endothelial function in human adults, allowing causal inferences. Romero-Corral et al studied 43 healthy young adults who were assigned to either gain (+4Kg) or maintain weight over 8 weeks,7 and found that although FMD did not change among weight maintainers, it decreased by 1.3% in weight gainers. Gupta et al. studied 14 healthy adults who received an over-feeding prescription for 8 weeks. As a result, the relative hyperemia index (measured by peripheral arterial tonometry) decreased by 21% on average; although this study did not have a control group. Despite this mechanistic evidence of a direct effect of short term weight gain on endothelial function, the long-lasting effects of non-purposeful weight gain on endothelial dysfunction, better reflecting pathophysiological mechanisms occurring in ‘real life’, have been largely unknown to date. This is the knowledge gap that our study has filled. Based on our findings, we can propose that long term weight gain (specifically more extreme weight gain, greater than 10 kg over 8.5 years) may detrimentally affects microvascular endothelial function. Results were robust for all anthropometric measures, further corroborating our findings.
Adipose tissue is now well established as an active endocrine and paracrine organ, participating in several metabolic processes and secreting several cytokines that contribute to inflammation and insulin resistance.19 Leptin’s main function pertains to control food intake and energy expenditure.27 However, leptin is also considered a pro-inflammatory cytokine 28 that is structurally similar to IL-6 and IL-12.29 In our study, higher baseline leptin and IL-6 levels were independently associated with worsening in reactive hyperemia over time. Through mediation analyses we discovered that the effects of leptin, IL-6 and weight gain on worsening of microvascular endothelial function were independent of each other. Therefore, although leptin and IL-6 have known inflammatory and vascular effects that may be implicated in atherogenesis, results from our study suggest that the detrimental effects of weight and fat gain on endothelial function occur through mechanisms other than those mediated by leptin and IL-6.
Other interesting findings from our study were the fact that (1) 46% of participants lost weight over time, (2) the overall improvement in reactive hyperemia with time (except in extreme weight gainers, as outlined above), and (3) the fact that weight and fat gain were associated with worsening of microvascular endothelial function, but not with conduit artery endothelial function. The temporal trends in weight observed in our study are comparable to population-based cohorts of similar age and BMI.30 Our findings of greater reactive hyperemia over time mirror the cross-sectional observations from the Framingham study, where increasing age was also positively correlated with peripheral arterial tonometry ratio.31 Neither Framingham nor our study evaluated specific biological mechanisms leading to this increase over time, but based on the results from our study, we can speculate that weight loss over time could be contributing to the improvement in reactive hyperemia – a hypothesis that is amenable to testing in mechanistic studies. It is also possible that the results from our study could have been mediated by exercise patterns among the participants. In a recent meta-analysis, exercise training significantly improved endothelial function.32 Since exercise training is also expected to contribute to weight maintenance or loss, greater levels of exercise over time could potentially underlie the inverse association between weight gain and microvascular endothelial function observed in our study. Regarding the differential associations of changes in anthropometric measures with FMD and reactive hyperemia, this should be interpreted in light of the different physiological roles of conduit and resistance arteries. Whereas decreased nitric oxide release in response to stimuli is central do conduit artery endothelial dysfunction, in the microvasculature nitric oxide may primarily modulate tissue metabolism, 33 highlighting potentially different regulatory mechanisms in conduit and resistance arteries. Furthermore, FMD has been shown to be negatively correlated with hypertension,34 while reactive hyperemia better correlates to metabolic risk factors such as diabetes, dyslipidemia and body mass index,13, 31, 34 confirming that different biological mechanisms influence these parameters. For these reasons, it has been proposed that micro and macrovascular endothelial dysfunction might reflect different pathophysiologic stages, with conduit artery endothelial dysfunction being more relevant in subjects with existing atherosclerosis, and microvascular endothelial dysfunction providing an earlier indication of risk.35 This may explain the detrimental effects of extreme weight gain on microvascular, but not conduit artery endothelial function observed in our study, since participants had no history of clinically manifest atherosclerotic disease at recruitment.
4.1. Limitations
The main strengths of our study are the large community-based nature of our cohort and the long prospective follow-up, making our study the largest to report longitudinal effects of weight gain and loss on endothelial function, and the first to demonstrate a detrimental effect of weight and fat gain on microvascular endothelial function. Our study has several limitations. We only had anthropometric and endothelial function data from 2 visits, preventing us from evaluating the roles of short-term vs. longstanding weight gain or loss on endothelial function. Although our study is unable to identify the precise mechanisms linking weight gain to worsening microvascular endothelial function, it sends a strong message of potential hazardous effects of weight gain on vascular health in the long term, which is an important message with widespread clinical applicability. Our study also serves as a solid foundation for future mechanistic studies to identify mechanisms linking weight gain to worse vascular health. Further, since our cohort only included non-Hispanic white subjects, results may not be directly applicable to other ethnicities. In addition, our study is observational, thus no direct causal inferences can be made. Further, we did not have data on exercise habits or levels of fitness, thus we cannot determine whether decreased fitness over time mediated the findings from our study. Also, we did not have a biochemical assessment of insulin levels and insulin resistance, which is a potential mechanism mediating the link between weight/fat gain and microvascular endothelial dysfunction. Lastly, we did not have direct measurements of body composition. However, the formulas used for estimation of body composition are validated and provide accurate measures, with prediction errors compatible to those obtained from skinfold thickness of bioelectrical impedance.9
4.2. Conclusions
In a longitudinal study, we have demonstrated that weight gain over time (specifically extremes of weight gain, greater than 10Kg and greater than 10% gain in body fat) are associated with significant worsening of microvascular endothelial function, while weight maintenance and modest to moderate weight gain over time did not detrimentally affect microvascular endothelial function. Comparatively, those who lost the most weight (>10Kg and greater than 10% loss in body fat) experienced the greatest improvements in microvascular endothelial function over time. Our findings support a potential role for weight gain in the initiation of an adverse cascade that culminates in atherogenesis, and highlight the importance of avoiding the weight gain that commonly accompanies aging as means of primary prevention of vascular diseases.
Supplementary Material
Highlights.
After a mean follow-up of 8.5 years, increases in weight body mass index, waist circumference and body fat percentage over time were associated with worsening of microvascular endothelial function (assessed by forearm reactive hyperemia).
These associations were only observed among individuals who gained the most weight (> 10 Kg in weight, >5 Kg/m2 in BMI, >10 cm in waist circumference and >10% in body fat percentage) over time.
Conversely, those who lost weight over time experienced improvement in microvascular endothelial function.
Our findings support a potential role for weight gain in the initiation of an adverse cascade that culminates in atherogenesis, and highlight the importance of avoiding the weight gain that commonly accompanies aging as means of primary prevention of vascular diseases.
Acknowledgments
Sources of Funding
This work was supported by Grant HL89354 from the National Institutes of Health. Dr. Thais Coutinho is a Clinician Scientist supported by a Heart and Stroke Foundation of Ontario Clinician Scientist Phase I Award, and holds the Chair for Women’s Heart Health at the University of Ottawa Heart Institute.
Footnotes
Disclosures
None of the authors have any conflicts of interest to disclose.
All authors have approved the final article.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Widlansky ME, Gokce N, Keaney JF, Jr, Vita JA. The clinical implications of endothelial dysfunction. J Am Coll Cardiol. 2003;42:1149–60. doi: 10.1016/s0735-1097(03)00994-x. [DOI] [PubMed] [Google Scholar]
- 2.Esper RJ, Nordaby RA, Vilarino JO, Paragano A, Cacharron JL, Machado RA. Endothelial dysfunction: a comprehensive appraisal. Cardiovasc Diabetol. 2006;5:4. doi: 10.1186/1475-2840-5-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Halcox JP, Schenke WH, Zalos G, Mincemoyer R, Prasad A, Waclawiw MA, Nour KR, Quyyumi AA. Prognostic value of coronary vascular endothelial dysfunction. Circulation. 2002;106:653–8. doi: 10.1161/01.cir.0000025404.78001.d8. [DOI] [PubMed] [Google Scholar]
- 4.Hubert HB, Feinleib M, McNamara PM, Castelli WP. Obesity as an independent risk factor for cardiovascular disease: a 26-year follow-up of participants in the Framingham Heart Study. Circulation. 1983;67:968–77. doi: 10.1161/01.cir.67.5.968. [DOI] [PubMed] [Google Scholar]
- 5.Lavie CJ, De Schutter A, Parto P, Jahangir E, Kokkinos P, Ortega FB, Arena R, Milani RV. Obesity and Prevalence of Cardiovascular Diseases and Prognosis-The Obesity Paradox Updated. Prog Cardiovasc Dis. 2016;58:537–47. doi: 10.1016/j.pcad.2016.01.008. [DOI] [PubMed] [Google Scholar]
- 6.Steinberg HO, Chaker H, Leaming R, Johnson A, Brechtel G, Baron AD. Obesity/insulin resistance is associated with endothelial dysfunction. Implications for the syndrome of insulin resistance. J Clin Invest. 1996;97:2601–10. doi: 10.1172/JCI118709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Romero-Corral A, Sert-Kuniyoshi FH, Sierra-Johnson J, Orban M, Gami A, Davison D, Singh P, Pusalavidyasagar S, Huyber C, Votruba S, Lopez-Jimenez F, Jensen MD, Somers VK. Modest visceral fat gain causes endothelial dysfunction in healthy humans. J Am Coll Cardiol. 2010;56:662–6. doi: 10.1016/j.jacc.2010.03.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Obesity: preventing and managing the global epidemic. Report of a WHO consultation. World Health Organ Tech Rep Ser. 2000;894:i–xii. 1–253. [PubMed] [Google Scholar]
- 9.Deurenberg P, Weststrate JA, Seidell JC. Body mass index as a measure of body fatness: age- and sex-specific prediction formulas. Br J Nutr. 1991;65:105–14. doi: 10.1079/bjn19910073. [DOI] [PubMed] [Google Scholar]
- 10.Schutz Y, Kyle UU, Pichard C. Fat-free mass index and fat mass index percentiles in Caucasians aged 18–98 y. Int J Obesity Rel Metabol Dis. 2002;26:953–60. doi: 10.1038/sj.ijo.0802037. [DOI] [PubMed] [Google Scholar]
- 11.Corretti MC, Anderson TJ, Benjamin EJ, Celermajer D, Charbonneau F, Creager MA, Deanfield J, Drexler H, Gerhard-Herman M, Herrington D, Vallance P, Vita J, Vogel R. Guidelines for the ultrasound assessment of endothelial-dependent flow-mediated vasodilation of the brachial artery: a report of the International Brachial Artery Reactivity Task Force. J Am Coll Cardiol. 2002;39:257–65. doi: 10.1016/s0735-1097(01)01746-6. [DOI] [PubMed] [Google Scholar]
- 12.Baron RM, Kenny DA. The moderator-mediator variable distinction in social psychological research: conceptual, strategic, and statistical considerations. J Pers Soc Psychol. 1986;51:1173–82. doi: 10.1037//0022-3514.51.6.1173. [DOI] [PubMed] [Google Scholar]
- 13.Kullo IJ, Malik AR, Santos S, Ehrsam JE, Turner ST. Association of cardiovascular risk factors with microvascular and conduit artery function in hypertensive subjects. Am J Hypertens. 2007;20:735–42. doi: 10.1016/j.amjhyper.2007.01.015. [DOI] [PubMed] [Google Scholar]
- 14.de Koning L, Merchant AT, Pogue J, Anand SS. Waist circumference and waist-to-hip ratio as predictors of cardiovascular events: meta-regression analysis of prospective studies. Eur Heart J. 2007;28:850–6. doi: 10.1093/eurheartj/ehm026. [DOI] [PubMed] [Google Scholar]
- 15.Yusuf S, Hawken S, Ounpuu S, Bautista L, Franzosi MG, Commerford P, Lang CC, Rumboldt Z, Onen CL, Lisheng L, Tanomsup S, Wangai P, Jr, Razak F, Sharma AM, Anand SS. Obesity and the risk of myocardial infarction in 27,000 participants from 52 countries: a case-control study. Lancet. 2005;366:1640–9. doi: 10.1016/S0140-6736(05)67663-5. [DOI] [PubMed] [Google Scholar]
- 16.Whitlock G, Lewington S, Sherliker P, Clarke R, Emberson J, Halsey J, Qizilbash N, Collins R, Peto R. Body-mass index and cause-specific mortality in 900 000 adults: collaborative analyses of 57 prospective studies. Lancet. 2009;373:1083–96. doi: 10.1016/S0140-6736(09)60318-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Coutinho T, Goel K, Correa de Sa D, Carter RE, Hodge DO, Kragelund C, Kanaya AM, Zeller M, Park JS, Kober L, Torp-Pedersen C, Cottin Y, Lorgis L, Lee SH, Kim YJ, Thomas R, Roger VL, Somers VK, Lopez-Jimenez F. Combining body mass index with measures of central obesity in the assessment of mortality in subjects with coronary disease: role of “normal weight central obesity”. J Am Coll Cardiol. 2013;61:553–60. doi: 10.1016/j.jacc.2012.10.035. [DOI] [PubMed] [Google Scholar]
- 18.Coutinho T, Goel K, Correa de Sa D, Kragelund C, Kanaya AM, Zeller M, Park JS, Kober L, Torp-Pedersen C, Cottin Y, Lorgis L, Lee SH, Kim YJ, Thomas R, Roger VL, Somers VK, Lopez-Jimenez F. Central obesity and survival in subjects with coronary artery disease: a systematic review of the literature and collaborative analysis with individual subject data. J Am Coll Cardiol. 2011;57:1877–86. doi: 10.1016/j.jacc.2010.11.058. [DOI] [PubMed] [Google Scholar]
- 19.Lovren F, Teoh H, Verma S. Obesity and Atherosclerosis: Mechanistic Insights. Can J Cardiol. 2015;31:177–183. doi: 10.1016/j.cjca.2014.11.031. [DOI] [PubMed] [Google Scholar]
- 20.Furukawa S, Fujita T, Shimabukuro M, Iwaki M, Yamada Y, Nakajima Y, Nakayama O, Makishima M, Matsuda M, Shimomura I. Increased oxidative stress in obesity and its impact on metabolic syndrome. J Clin Invest. 2004;114:1752–61. doi: 10.1172/JCI21625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.De Nardo D, Latz E. NLRP3 inflammasomes link inflammation and metabolic disease. Trends Immunol. 2011;32:373–9. doi: 10.1016/j.it.2011.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Virdis A, Neves MF, Duranti E, Bernini G, Taddei S. Microvascular endothelial dysfunction in obesity and hypertension. Curr Pharm Des. 2013;19:2382–9. doi: 10.2174/1381612811319130006. [DOI] [PubMed] [Google Scholar]
- 23.Sansbury BE, Cummins TD, Tang Y, Hellmann J, Holden CR, Harbeson MA, Chen Y, Patel RP, Spite M, Bhatnagar A, Hill BG. Overexpression of endothelial nitric oxide synthase prevents diet-induced obesity and regulates adipocyte phenotype. Circulation Res. 2012;111:1176–89. doi: 10.1161/CIRCRESAHA.112.266395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Traupe T, Lang M, Goettsch W, Munter K, Morawietz H, Vetter W, Barton M. Obesity increases prostanoid-mediated vasoconstriction and vascular thromboxane receptor gene expression. Journal Hypertens. 2002;20:2239–45. doi: 10.1097/00004872-200211000-00024. [DOI] [PubMed] [Google Scholar]
- 25.Perticone F, Ceravolo R, Candigliota M, Ventura G, Iacopino S, Sinopoli F, Mattioli PL. Obesity and body fat distribution induce endothelial dysfunction by oxidative stress: protective effect of vitamin C. Diabetes. 2001;50:159–65. doi: 10.2337/diabetes.50.1.159. [DOI] [PubMed] [Google Scholar]
- 26.Martin BJ, Verma S, Charbonneau F, Title LM, Lonn EM, Anderson TJ. The relationship between anthropometric indexes of adiposity and vascular function in the FATE cohort. Obesity. 2013;21:266–73. doi: 10.1002/oby.20266. [DOI] [PubMed] [Google Scholar]
- 27.Friedman JM, Halaas JL. Leptin and the regulation of body weight in mammals. Nature. 1998;395:763–70. doi: 10.1038/27376. [DOI] [PubMed] [Google Scholar]
- 28.Lord GM, Matarese G, Howard JK, Baker RJ, Bloom SR, Lechler RI. Leptin modulates the T-cell immune response and reverses starvation-induced immunosuppression. Nature. 1998;394:897–901. doi: 10.1038/29795. [DOI] [PubMed] [Google Scholar]
- 29.La Cava A, Matarese G. The weight of leptin in immunity. Nat Rev Immunol. 2004;4:371–9. doi: 10.1038/nri1350. [DOI] [PubMed] [Google Scholar]
- 30.Sheehan TJ, DuBrava S, DeChello LM, Fang Z. Rates of weight change for black and white Americans over a twenty year period. Int J Obesity Rel Metabol Dis. 2003;27:498–504. doi: 10.1038/sj.ijo.0802263. [DOI] [PubMed] [Google Scholar]
- 31.Hamburg NM, Keyes MJ, Larson MG, Vasan RS, Schnabel R, Pryde MM, Mitchell GF, Sheffy J, Vita JA, Benjamin EJ. Cross-sectional relations of digital vascular function to cardiovascular risk factors in the Framingham Heart Study. Circulation. 2008;117:2467–74. doi: 10.1161/CIRCULATIONAHA.107.748574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Early KS, Stewart A, Johannsen N, Lavie CJ, Thomas JR, Welsch M. The Effects of Exercise Training on Brachial Artery Flow-Mediated Dilation: A Meta-analysis. J Cardiopulm Rehabil Prev. 2017;37:77–89. doi: 10.1097/HCR.0000000000000206. [DOI] [PubMed] [Google Scholar]
- 33.Trochu JN, Bouhour JB, Kaley G, Hintze TH. Role of endothelium-derived nitric oxide in the regulation of cardiac oxygen metabolism: implications in health and disease. Circulation research. 2000;87:1108–17. doi: 10.1161/01.res.87.12.1108. [DOI] [PubMed] [Google Scholar]
- 34.Hamburg NM, Palmisano J, Larson MG, Sullivan LM, Lehman BT, Vasan RS, Levy D, Mitchell GF, Vita JA, Benjamin EJ. Relation of brachial and digital measures of vascular function in the community: the Framingham heart study. Hypertension. 2011;57:390–6. doi: 10.1161/HYPERTENSIONAHA.110.160812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Flammer AJ, Anderson T, Celermajer DS, Creager MA, Deanfield J, Ganz P, Hamburg NM, Luscher TF, Shechter M, Taddei S, Vita JA, Lerman A. The assessment of endothelial function: from research into clinical practice. Circulation. 2012;126:753–67. doi: 10.1161/CIRCULATIONAHA.112.093245. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.