Abstract
Objective
This study examined the phenotypic effects of adipocyte-specific oncostatin M receptor (OSMR) loss in chow-fed mice.
Methods
Chow-fed adipocyte-specific OSMR knockout (FKO) mice and littermate OSMRfl/fl controls were studied. Tissue weights, insulin sensitivity, adipokine production, and stromal cell immunophenotypes were assessed in epididymal fat (eWAT); serum adipokine production was also assessed. In vitro, adipocytes were treated with oncostatin M (OSM) and adipokine gene expression assessed.
Results
Body weights, fasting blood glucose levels, and eWAT weights did not differ between genotypes. However, the eWAT of OSMRFKO mice was modestly less responsive to insulin stimulation than that of OSMRfl/fl mice. Notably, significant increases in adipokines including C-reactive protein, lipocalin 2, intercellular adhesion molecule-1 (ICAM-1), and insulin-like growth factor binding protein 6, were observed in the eWAT of OSMRFKO mice. In addition, significant increases in fetuin A and ICAM-1 were detected in OSMRFKO serum. Flow cytometry revealed a significant increase in leukocyte number and modest, but not statistically significant, increases in B and T cells in the eWAT of OSMRFKO mice.
Conclusions
The chow-fed OSMRFKO mouse exhibits adipose tissue dysfunction and increased pro-inflammatory adipokine production. These results suggest that intact adipocyte OSM-OSMR signaling is necessary for adipose tissue immune cell homeostasis.
Keywords: adipose tissue, inflammation, oncostatin M, adipokines
INTRODUCTION
A coordination among healthy adipocytes and stromal vascular cells (SVCs, including immune cells, preadipocytes, and endothelial cells) in the local adipose tissue environment is critical to maintain homeostasis of both adipose tissue and systemic metabolism. Obesity is intimately coupled to a chronic, low-grade inflammatory state characterized by leukocyte infiltration of adipose tissue and subsequent modulation of the tissue adipokine profile. Activation of infiltrating leukocytes and promotion of pro-inflammatory adipokine production can enable adipose tissue dysfunction and lead to temporal effects on metabolic homeostasis (1, 2, 3, 4).
Oncostatin M (OSM), an adipokine belonging to the interleukin-6/gp130 family of cytokines, regulates a variety of physiological and pathological processes (5, 6). First recognized in 1986 for its anti-tumorigenic effects (7), it is now evident that OSM can regulate many other biological processes in a cell-type dependent manner (8). OSM is a unique gp130 cytokine in that it has its own specific receptor subunit (OSM receptor β or OSMR) that heterodimerizes with gp130 to create a functional OSM receptor complex, which produces the majority of OSM effects (9). Adipocytes in particular exhibit extensive responses to OSM (10, 11), and OSMR is highly expressed in adipose tissue (12). Importantly, though adipocytes express OSMR, they do not produce OSM itself. Rather, OSM is produced in various adipose tissue SVCs including macrophages, T cells, and B cells (10, 12). Increased adipose tissue expression of OSM and OSMR are correlates of obesity in both mouse and man (12). We have previously shown that adipocyte-specific deletion of OSMR in mice (referred to here as OSMRFKO mice) is associated with augmented adipose tissue inflammation and systemic insulin resistance and obesity (10). Notably, adipose tissue OSM expression itself is elevated in obese OSMRFKO mice (10). While we have demonstrated its role in perpetuating inflammation and systemic insulin resistance in the obese condition (10), it remains unclear what potential effects disrupted adipose tissue OSM signaling has in the absence of obesity.
With the present study, we examined the function of adipose tissue OSM signaling in the absence of an obesogenic stimulus such as a high-fat diet. Our novel observations demonstrate that, in the absence of obesity, OSMRFKO mice exhibit leukocyte accumulation, increased pro-inflammatory adipokine production, and a modest reduction of adipose tissue insulin responsiveness. These effects occur in the absence of differences in body weight or adiposity. These results corroborate our previous observations that intact adipocyte OSM signaling is necessary for maintenance of adipose tissue homeostasis, and demonstrate that adipose tissue dysfunction precedes systemic metabolic dysfunction in this model.
METHODS
Animals and Husbandry
Mixed background OSMRFKO mice and homozygous floxed littermates (OSMRfl/fl) were obtained from our in-house colony, which was generated using adiponectin–Cre and OSMR floxed mice as previously described (10). All mice used in these studies were male, were 6–9 months of age, and were maintained on LabDiet #5015 (LabDiet, St. Louis, MO) from weaning (3 weeks of age) until study termination. Mice were housed in a temperature (22° ± 2° Celsius) - and humidity-controlled (45–55%) room under a 12-hour light/dark cycle, and allowed ad libitum access to food and water. Tissue OSMR and gp130 gene and protein expression, 4-hour blood glucose measurement, and eWAT insulin sensitivity studies (acute intraperitoneal insulin injections) were performed on one cohort of mice. Acute OSM injection studies were conducted in a second cohort of mice, tissue adipokine studies were conducted in mice from the protein expression cohort and in a third cohort. Serum adipokine studies were also conducted in third cohort. Flow cytometry studies were performed in an additional cohort of 6-month old mice. The Pennington Biomedical Research Center Institutional Animal Care and Use Committee approved all studies (protocol #961P).
Acute OSM injection experiments
Fed mice (n=4 OSMRfl/fl and n=4 OSMRFKO) were given acute injections of 200 ng recombinant murine OSM (mOSM; R&D Systems; Minneapolis, MN; catalog #495-MO-025) or vehicle (sterile 0.1% BSA in PBS) and sacrificed 15 minutes later. Epididymal fat pads were excised, snap frozen, and stored at −80°C for immunoblotting analyses as described below.
Cell culture and treatments
Murine 3T3-L1 preadipocytes were grown to 2 days post-confluence in DMEM with 10% fetal bovine serum (FBS) and differentiated as described previously (10). One day prior to beginning OSM treatment of adipocytes, medium was replaced with DMEM containing 5% FBS. Cells were pretreated with 50 μM of the ERK inhibitor U0126 for 1 hour, and then 1nM recombinant mOSM or vehicle (0.1% BSA in PBS) was added as indicated. Six hours later, medium was removed, and cells were washed with PBS and then harvested for RNA extraction. Experiments were performed in duplicate.
Gene expression analyses
Total RNA was isolated from indicated mouse tissues or 3T3-L1 adipocytes using an RNeasy Mini Kit (Qiagen; Germantown, MD) and yield determined by spectrophotometry (NanoDrop Technologies; Wilmington, DE) as previously described (10). cDNA was synthesized using the High Capacity cDNA Reverse Transcription kit (Applied Biosystems; Foster City, CA) with the SYBR Green system (Clontech; Mountain View, CA). Relative quantification of mRNA expression was analyzed using an ABI Prism 7900 Sequence Detection System (Applied Biosystems; Foster City, CA). Sequences for mouse primers (Integrated DNA Technologies; San Diego, CA) appear in Table 1.
Table 1.
Sequences for qPCR primers used in this study.
| Gene | Forward Sequence | Reverse Sequence |
|---|---|---|
| Crp | CAG CAG CAT CCA TAG CCA T | TGC TTC CAG AGA CAC ATA GGA |
| FetA (Ahsg) | CTT CAG GGA TTC AAA CAG GTC T | CAA AGC ATG GCA AGT GGT C |
| Glut1 (Slc2a1) | AGT TCG GCT ATA ACA CTG GTG | GTG GTG AGT GTG GTG GAT G |
| Glut4 (Slc2a4) | TCT TAT TGC AGC GCC TGA G | GAG AAT ACA GCT AGG ACC AGT G |
| gp130 | AGG AGA AAT AGA AGC CAT AGT CG | TGG AAG GAT CAG GAA CAT TAG G |
| Icam1 | CTG TGC TTT GAG AAC TGT GG | GGT CCT TGC CTA CTT GCT G |
| Igfbp6 | TCT ATG TGC CAA ACT GTG ACC | CTG AGT GCT TCC TTG ACC ATC |
| Lcn2 | TGC AAG TGG CCA CCA CGG AC | GCA TTG GTC GGT GGG GAC AGA GA |
| Osmr | CGT TCC CCT GTG AGG CCG AG | TCC TCC AAG ACT TCG CTT CGG G |
| Ppia | CCA CTG TCG CTT TTC GCC GC | TGC AAA CAG CTC GAA GGA GAC GC |
| Ubb | CCA GTG GGC AGT GAT GG | GCT TAC CAT GCA ACA AAA CCT |
Immunoblotting
Proteins were separated on 7.5% (OSMR, gp130) or 10% (Akt, ERK, phosphoSTAT3/5) polyacrylamide gels containing sodium dodecyl sulfate and transferred to nitrocellulose membranes as previously described (10). After transfer, membranes were blocked and incubated with goat anti-OSMR (R&D Systems; Minneapolis, MN; catalog #AF662), rabbit anti-STAT5A (Santa Cruz Biotechnology; Dallas, TX; catalog #sc-1081), or mouse anti-phosphoSTAT5 (Millipore Sigma; Burlington MA; catalog #05-495) primary antibodies (1:1000 dilution) overnight at 4°C. Results were visualized with appropriate horseradish peroxidase-conjugated secondary antibodies (1:15,000 dilution) (Jackson ImmunoResearch Laboratories; West Grove, PA) and SuperSignal Chemiluminescent Substrate (ThermoFisher Scientific; Waltham, MA; catalog# 34580), using a Mini-Med film processor. All immunoblots were performed in triplicate.
Serum and tissue adipokine arrays
Adipokine protein expression levels in serum (n=3 per genotype) or eWAT (n=6–7 per genotype) were assessed using mouse Proteome Profiler Adipokine Array kits (R&D Systems; Minneapolis, MN; catalog# ARY013) according to manufacturer’s instructions. Briefly, eWAT was homogenized in PBS with 10 μg/mL each aprotinin, leupeptin, and pepstatin. After homogenization, Triton X-100 was added to each sample at 1% final concentration. Samples were frozen at −80°C, thawed, and centrifuged at 10,000 × g for 5 minutes at 4°C to remove cellular debris. Sample protein concentrations were quantified using a bicinchoninic acid assay with bovine serum albumin as the standard (Sigma-Aldrich, St. Louis, MO). Serum assays were performed using 100ul of mouse serum as specified in the kit instructions. Membranes were incubated with chemiluminescence reagents and then exposed to autoradiography film for times ranging from one to five minutes. Film from the five-minute exposure was used for densitometry analyses, which were performed with Image Studio Lite software (Version 3.1; Li-Cor; Lincoln, NE). Densitometry values for each adipokine were normalized to values for the reference spot on each membrane. Resulting values were then compared between genotypes.
Isolation of eWAT SVCs for immunophenotyping
Freshly excised eWAT was weighed, pooled (2–3 mice per pooled sample; 4 pooled samples per genotype), and fractionated using previously described methods (10, 13), with minor modifications (14) as briefly described here. Tissues were minced in ice-cold digestion buffer (low glucose DMEM without phenol red; 5% heat-inactivated FBS; 1 mg/ml Type II collagenase), transferred to 50 ml conical tubes, and placed into a shaking water bath at 37°C. Tissues were digested for 45 minutes at 100 rpm, with tubes being shaken vigorously by hand every 10 minutes. In the last 5 minutes of the digestion procedure, EDTA was added at a final concentration of 10mM. Each slurry was then passed through a pre-wet 100 μM cell strainer into a 50-ml conical tube and the strainer rinsed with 10 ml PBS. Slurries were centrifuged at 500xg for 10 minutes at 4°C. After centrifugation, floating adipocytes and supernatant were removed and RBC lysis buffer was added to the remaining SVF pellet and incubated for 5 minutes. Lysis buffer was neutralized with PBS, the SVF mixture passed through a 40μm cell strainer into a 50 ml tube, the strainer rinsed twice with 5 ml PBS, and the tubes centrifuged at 500xg for 10 minutes at 4°C. The resulting SVF pellet was resuspended in 3 ml of PBS and placed on ice for processing for flow cytometry analyses. Viable cells were counted on a hemacytometer using trypan blue exclusion.
Flow cytometry analysis of SVCs
SVCs (5 × 105 – 1 × 106 cells per tube) were isolated as described above and suspended in flow cytometry staining buffer (eBioscience/Thermo Fisher; Waltham, MA; catalog #00-4222) and incubated in Fc block for 10 minutes on ice. Cells were stained with appropriate antibodies for 45 minutes at 4°C in the dark. All flow cytometry antibodies are listed in Table 2. Stained SVCs were washed twice in PBS, fixed in 1% paraformaldehyde in PBS, and analyzed on FACS Aria or FACS Calibur flow cytometers using Flow Jo software (Version 10.0; TreeStar; Ashland, OR). Adipose tissue macrophages are identified as CD45+, CD64+ (14, 15). Adipose tissue T cells are identified as CD45+, CD3+ (15). Adipose tissue B cells are identified as CD45+, CD19+, and B220+. Preadipocytes were identified as CD45-, CD31-, Sca1+ (16) and endothelial cells were identified as CD45-, CD31+. Samples were analyzed on FACS Aria or FACS Calibur flow cytometers. Data were analyzed using FlowJo software (Version 10.0; TreeStar; Ashland, OR). Gating strategies for flow cytometry analyses are based on those previously described for adipose SVCs (3, 14, 15); auto fluorescence (no antibody) controls, single stain controls, and fluorescence minus one isotype controls were used in each immunophenotyping panel for compensation settings and to determine gating. Data were normalized per gram of eWAT or as a percentage of SVCs, as indicated.
Table 2.
Flow cytometry antibodies used in this study.
| Antibody | Clone | Vendor |
|---|---|---|
| CD45.2 | 104 | eBioscience/ThermoFisher |
| CD64 | X54-5/7.1 | BD Biosciences |
| CD31 | 390 | eBioscience/ThermoFisher |
| CD19 | 1D3 | eBioscience/ThermoFisher |
| B220 | RA3-6B2 | eBioscience/ThermoFisher |
| CD3 | 145-2C11 | eBioscience/ThermoFisher |
| Sca1 | D7 | eBioscience/ThermoFisher |
| CD16/CCD32 (Fc block) | 93 | eBioscience/ThermoFisher |
| Mouse IgG2aκ | eBM2a | eBioscience/ThermoFisher |
| Mouse IgG1κ | P3.6.2.8.1 | eBioscience/ThermoFisher |
| Rat IgG2aκ | eBR2a | eBioscience/ThermoFisher |
| Armenian hamster IgG | eBio299Arm | eBioscience/ThermoFisher |
Statistical analyses
GraphPad Prism software was used for all statistical analyses (Version 7.0; GraphPad Software; La Jolla, CA), and results are expressed as mean ± SEM. Differences between genotypes were analyzed using the Student’s t-test, with results considered significant when p<0.05.
RESULTS
In this study, we examined the phenotypic effects of adipocyte-specific oncostatin M receptor loss (OSMRFKO) in chow-fed male mice and littermate control OSMRfl/fl mice. Specifically, we examined tissue weight, insulin responsiveness, adipokine production, and stromal cell immunophenotypes in epididymal fat pads (eWAT), as well as serum adipokine production.
Adipose and non-adipose expression of oncostatin receptor subunits
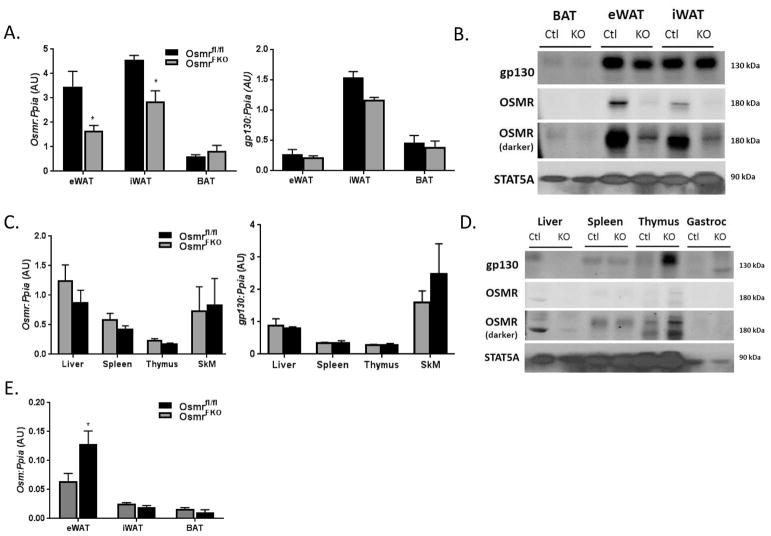
We observed significant decreases in OSMR gene and protein expression in two white fat depots from OSMRFKO mice when compared to OSMRfl/fl mice (Figures 1A and 1B). The levels of gp130 gene and protein expression did not differ significantly between the control and knockout mice. There were no significant differences in either OSMR or gp130 gene and protein expression between genotypes in brown adipose tissue (Figures 1A & 1B) or in non-adipose tissues examined (Figures 1C & 1D). Notably, expression of OSM itself was significantly increased in the eWAT of OSMRFKO mice (Figure 1E); this increase in OSM expression also occurs in obese high-fat fed OSMRFKO mice (10).
Figure 1. Expression levels of OSMR and gp130 are not altered in non-adipose tissues.
Tissue expression of OSMR and gp130 in various adipose depots at the A) gene and B) protein levels in OSMRfl/fl (CTL) and OSMRFKO (KO) chow-fed mice. Non-adipose tissue expression of OSMR and gp130 at the C) gene and D) protein levels was also measured. E) Gene expression of OSM itself was also measured in various adipose depots. Total RNA in various tissues was purified and analyzed by real-time PCR. Cyclophilin A (Ppia) was used as an endogenous control. Protein (100 ug for adipose tissues, 50 ug for all other tissues) was subjected to Western blot analysis. Data are shown as mean ± SEM. For PCR, n=8 per genotype (eWAT), n=3–4 per genotype (inguinal WAT (iWAT), brown adipose tissue (BAT), other tissues). ***p < 0.001, *p<0.05 vs. OSMRfl/fl. AU, arbitrary units.
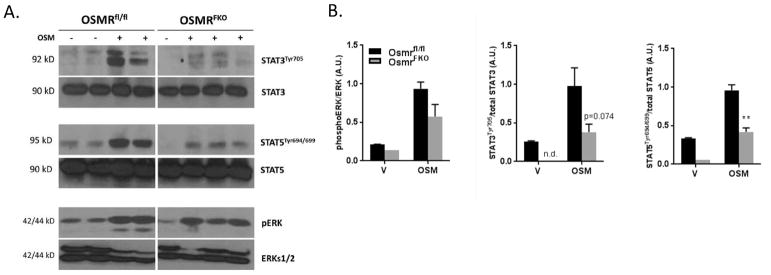
Adipose tissue response to an acute OSM challenge
Acute OSM injection in OSMRfl/fl mice resulted in robust phosphorylation of STAT3 and STAT5 tyrosine residues in eWAT. The reduced STAT phosphorylation in OSMRFKO mice (Figures 2A and 2B) indicated a substantial decrease in responsiveness to an OSM challenge. The observed effect on STAT5 phosphorylation was significant (p=0.0084; Figure 2B), while the effect on STAT3 followed the same trend but did not reach significance (p=0.074; Figure 2B). These data also suggest that adipocyte OSMR is responsible for the majority of OSM signaling in eWAT. Notably, basal levels of STAT3 and STAT5 tyrosine phosphorylation in vehicle-injected mice were lower in the OSMRFKO group (Figures 2A and 2B). OSM is known to induce ERKs 1&2 phosphorylation, but we did not observe a significant reduction in ERK activation following acute OSM injection in OSMRFKO mice compared to floxed littermate controls.
Figure 2. OSMRFKO mice are less responsive to an acute OSM challenge.
A) Effects of acute OSM or vehicle injection (15 min) on STAT and ERK phosphorylation in eWAT of chow-fed OSMRfl/fl (n=4) or OSMRFKO mice (n=4). 75 ug protein was subjected to Western blot analysis. B) Densitometry analyses were conducted using Image Studio software. Data are shown as mean ± SEM and are representative of two independent experiments. AU, arbitrary units.
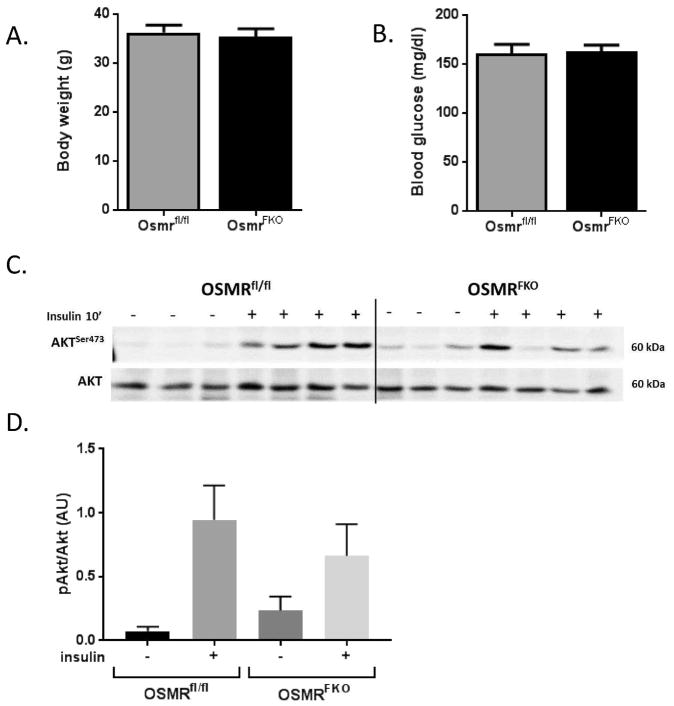
Body weight and blood glucose
Body weights did not significantly differ between OSMRfl/fl and OSMRFKO mice in any of the experimental cohorts at the time of sacrifice (Figure 3A). Fasting blood glucose levels also were not different between the two genotypes at the time of sacrifice (Figure 3B).
Figure 3. OSMRFKO mice exhibit a modest blunting of insulin-induced Akt phosphorylation.
A) Body weights, B) 4-hour fasting blood glucose, and C) protein expression levels of Akt, phosphoAkt, and phosphoERK in eWAT from chow-fed OSMRfl/fl and OSMRFKO (KO) mice. Body weights and blood glucose levels were not significantly different between genotypes. 100 ug protein was subjected to Western blot analysis. KO mice exhibited a slight diminution in Akt phosphorylation (S473) in eWAT. Densitometry analysis of D) pAkt/Akt ratio was conducted using Image Studio software. For vehicle injections, n=3 per genotype and for insulin injections, n=4 per genotype. Data are shown as mean ± SEM. AU, arbitrary units.
Insulin sensitivity and adipokine levels
As a measure of insulin sensitivity, we examined insulin-stimulated Akt phosphorylation in eWAT. Seven mice from each genotype were given intraperitoneal vehicle or insulin injections and sacrificed 10 minutes later. Most of the insulin-injected OSMFKO mice had reduced Akt phosphorylation compared to floxed controls. However, when the results from all the mice were quantitated, the effects did not reach statistical significance (Figure 3C). There were no differences in total Akt levels (Figure 3C and 3D). Further, we did observe any significant changes in eWAT Glut1 or Glut4 gene expression levels [Glut1 relative expression: 1.082 ± 0.125 AU (OSMRfl/fl) vs 1.028 ±0.061 AU (OSMRFKO); Glut4 relative expression: 1.222 ± 0.237 AU (OSMRfl/fl) vs 1.238 ± 0.139 AU (OSMRFKO)] in eWAT.
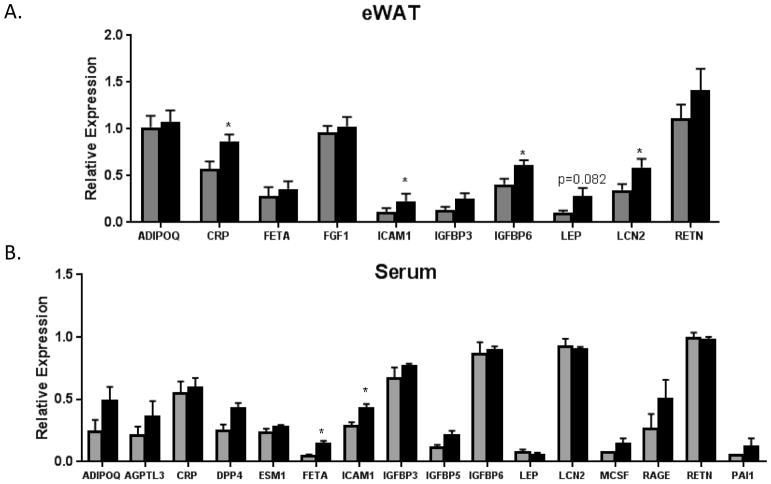
Significant increases in CRP, ICAM-1, IGFBP6, and LCN2 were observed in OSMRFKO eWAT (Figure 4A), while ICAM-1 and FETA were elevated in OSMRFKO serum (Figure 4B). The elevated ICAM-1 in both serum and eWAT in OSMRFKO mice suggests that the eWAT may be the source of increasing circulating ICAM-1, although additional studies are required to confirm this hypothesis.
Figure 4. Increased pro-inflammatory adipokine expression in eWAT and serum of OSMRFKO mice.
Protein expression levels of various adipokines in A) eWAT (n=6–7 per genotype) and B) serum (n=3 per genotype) of chow-fed OSMRfl/fl (grey bars) and OSMRFKO (black bars) mice. CRP, ICAM-1, IGFBP6, and LCN2 expression levels were significantly increased in eWAT of the OSMRFKO mice when compared to OSMRfl/fl mice, and we observed significant increases in FETA and ICAM-1 in the serum of OSMRFKO mice when compared to OSMRfl/fl mice. Data are presented as mean ± SEM. *p<0.05 vs. OSMRfl/fl.
Adipokine expression in 3T3-L1 adipocytes
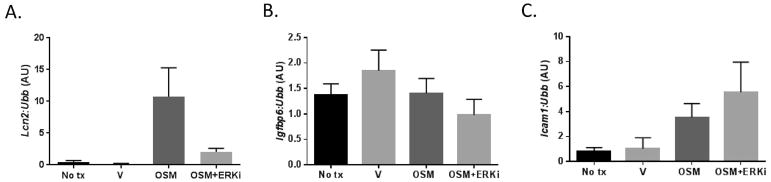
The lack of adipocyte OSMR signaling is associated with increased OSM expression in eWAT of OSMRFKO mice, both in the current study (Figure 1E) and with high-fat diet feeding (10). As shown in Figure 4A, OSMRFKO increased LCN2 levels in eWAT. To determine if OSM could induce Lcn2 gene expression in a cell-autonomous manner, we studied the effects of OSM in cultured adipocytes. We observed that a 6-hour treatment with OSM strongly induced Lcn2 expression in 3T3-L1 adipocytes. Use of the MEK inhibitor, U0126, demonstrated that this effect was primarily ERK-dependent (Figure 5A). The efficacy of ERK inhibition was confirmed by western blotting that demonstrated a significant reduction in ERK phosphorylation following inhibitor treatment (data not shown). OSM, however, did not induce Igfbp6 expression in adipocytes (Figure 5B). Interestingly, OSM induced Icam1 expression and ERK inhibition further increased this effect (Figure 5C). Previous studies have reported Crp and FetA expression and secretion by adipocytes (17, 18); however, we were not able to detect either gene in our experiments in 3T3-L1 adipocytes.
Figure 5. Adipokine gene expression in 3T3-L1 adipocytes in response to OSM treatment and/or ERK inhibition.
Fully differentiated 3T3-L1 adipocytes were treated with 1 nM or OSM or vehicle for 6 hours in the presence/absence of the ERK inhibitor U0126. Gene expression levels of A) Lcn2, B) Igfbp6, C) Icam1, Crp, and FetA were assessed, but Crp and FetA were not detected. Total RNA was purified from cells and analyzed by real-time PCR. Ubiquitin b (Ubb) was used as an endogenous control.
Immunophenotyping of SVC populations
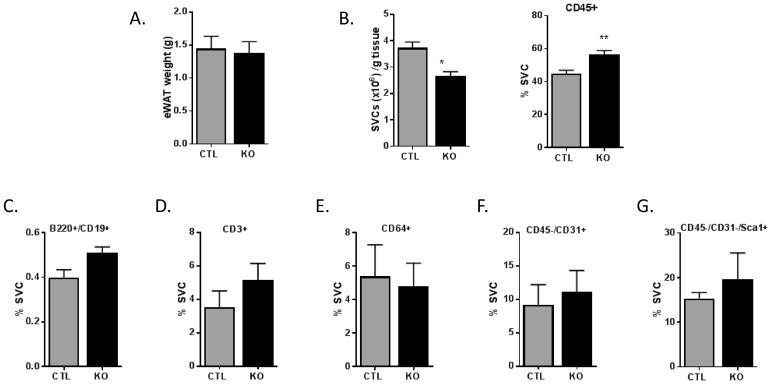
Though eWAT weights did not differ (Figure 6A), OSMRfl/fl mice had a significantly higher number of SVCs per gram of eWAT than OSMRFKO mice (Figure 6B). However, immunophenotyping analyses indicated significantly higher CD45+ leukocytes in OSMRFKO eWAT when compared to OSMRfl/fl eWAT (Figure 6B). While not significant, there were trends towards increased CD19+/B220+ B cells (p=0.07) and increased CD3+ T cells in OSMRFKO eWAT when compared to OSMRfl/fl control mice (Figures 6C and 6D, respectively). No obvious trends were observed in CD64+ adipose tissue macrophages or in endothelial cells between groups (Figures 6E and 6F). Although not statistically significant, a modest increase in Sca1+ preadipocytes was observed in the OSMRFKO mice (Figure 6G).
Figure 6. Leukocyte accumulation and altered immune cell populations in eWAT of OSMRFKO mice.
A) Epididymal fat pad (eWAT) weight, B) number of SVF cells per gram eWAT and CD45+ leukocytes as determined by flow cytometry in chow-fed OSMRfl/fl (CTL; grey bars) and OSMRFKO (KO; black bars) mice (n=4 groups of pooled tissue per genotype; 2–3 mice per pool). Quantification of eWAT stromal vascular cells was performed using flow cytometry and data are presented as % SVCs for C) B cells (CD19+/B220+), D) T cells (CD3+), E) macrophages (CD64+), F) endothelial cells (CD31+), and G) preadipocytes (CD31-/Sca1+). Data presented are mean ± SEM. *p<0.05 and ** p<0.01 vs. OSMRfl/fl.
DISCUSSION
Our previous work indicates that OSMRFKO mice exhibit systemic insulin resistance and adipose tissue inflammation when challenged with a high-fat diet (10). Here, we studied the phenotype of OSMRFKO mice in the absence of a high-fat diet challenge and more closely examined the adipose tissue inflammatory profile in the OSMRFKO mouse. There are several significant observations from this study. Notably, adipocyte-specific OSMR deletion does not alter body mass or fasting blood glucose, but does modestly blunt adipose tissue insulin sensitivity in chow-fed mice. In addition, adipose tissue and serum pro-inflammatory adipokine expression levels are increased in chow-fed OSMRFKO mice. These results are consistent with the significant increase in the number of adipose tissue leukocytes (Figure 6B) and the trend towards increased T cell and B cell numbers, despite no changes in adipose tissue weight. The observed phenotypic effects in chow-fed OSMRFKO mice are not due to alterations in gp130 expression in adipose tissue or in gp130 and/or OSMR expression in non-adipose tissues. Taken together, these results and our previous data (10) demonstrate that the adipose tissue dysfunction in OSMRFKO mouse is characteristic of the genotype itself and occurs in the absence of obesity.
Our current results in OSMRFKO mice contrast with those of the global OSMR knockout mice fed a chow diet (19). Global OSMR knockout mice of similar age to the OSMRFKO mice in our study have increases in body mass, eWAT and iWAT mass, liver mass, and food intake when compared to OSMRfl/fl mice (19). We did not observe changes in any of these parameters in chow-fed OSMRFKO mice. Further, global OSMR knockout mice also exhibit significant increases in blood glucose and serum insulin and are generally glucose intolerant and insulin resistant (19), while our OSMRFKO mice are not. However, much like the OSMRFKO mice, global OSMR knockout mice exhibit increased adipose tissue inflammation. The vast differences in these phenotypes underscore the importance of OSMR in hematopoiesis, liver development, and other developmental functions, and suggest that many of the metabolic effects observed in the global OSMR knockout mice are due to non-adipocyte effects (19, 20, 21). Some of the molecular mechanisms by which specific disruption of adipocyte OSM-OSMR signaling axis promotes adipose tissue dysfunction still remain unclear, although our current data provide new information.
As a first step toward examining drivers of the adipose tissue dysfunction in chow-fed OSMRFKO mice, we generated adipokine profiles using antibody arrays. We analyzed 38 adipokines, and found four of them were differentially expressed at significant levels: C-reactive protein (CRP), intercellular adhesion molecule 1 (ICAM-1), insulin-like growth factor binding protein 6 (IGFBP6), and lipocalin 2 (LCN2). Various cell types produce these proteins, all of which have established effects in inflammation. Immune cells can produce all four of these proteins; however, adipocytes are reported to produce at least two of them (CRP and LCN2). CRP is an acute phase protein synthesized primarily by the liver in response to cytokines or other inflammatory stimuli. Previous reports suggest that adipocytes can produce CRP in response to inflammatory stimuli (17, 18), although we were unable to detect Crp gene expression in our in vitro 3T3-L1 adipocyte experiments. Adipocytes produce LCN2 in response to pro-inflammatory cytokine exposure (22, 23, 24) and several immune cell types also produce LCN2 (25). However, substantial evidence indicates that circulating LCN2 is liver–derived and produced in response to infection or injury (26, 27), so it is not surprising that the changes we observed in LCN2 levels in this study were only at the level of the eWAT itself and not in serum (Figure 4B). The role of LCN2 in adipose tissue is generally thought to be pro-inflammatory, since increasing its expression promotes neutrophil recruitment and induces pro-inflammatory cytokine production (28, 29), although anti-inflammatory effects have been reported in other tissues (30, 31). Our in vitro studies clearly indicate that OSM potently induces adipocyte LCN2 expression and that this induction is ERK-dependent (Figure 5A). TNFα and IFNγ are also potent inducers of LCN2 in cultured murine adipocytes (23).
ICAM-1 is a cell surface glycoprotein found on endothelial cells and leukocytes that is induced by pro-inflammatory cytokine production and interacts with β-integrins to facilitate leukocyte migration (32). Several studies report that adipocytes can express ICAM-1 (33, 34, 35). Our in vitro experiments indicated that, while Icam1 gene expression was very low in basal conditions, but OSM treatment substantially induced Icam1 mRNA levels in adipocytes (Figure 5). The significant elevations of ICAM-1 in both serum and eWAT in OSMRFKO mice when compared to floxed controls (Figure 4) is consistent with the elevated OSM levels we observe in in OSMRFKO mice. Our flow cytometry data also demonstrated increased leukocyte numbers in OSMRFKO adipose tissue. Given its known functions in leukocyte migration and recruitment, we hypothesize that ICAM-1 is either induced in adipocytes by immune cell-derived factors, produced by immune cells alone, or both, and may act to effectively trap leukocytes in the eWAT of the OSMRFKO mouse. Further studies are required to evaluate this hypothesis.
Increased levels of fetuin A (FETA), a negative acute phase protein primarily secreted from liver (36), are associated with metabolic syndrome and insulin resistance in humans and animals (37, 38, 39). In adipose tissue, FETA serves as an endogenous toll-like receptor 4 (TLR4) ligand, where it binds fatty acids for presentation to TLR4 and subsequently triggers pro-inflammatory cytokine production via the NF-kB pathway (38). FETA can also promote macrophage migration into adipose tissue (37) and directly interfere with insulin receptor action to mediate insulin resistance (39). Data from these previous studies coincide with results obtained in OSMRFKO mice where we observed significant increases in serum FETA (Figure 4B) and a modest blunting of eWAT insulin signaling in comparison to OSMRfl/fl mice.
A family of six IGFBPs controls the activity of insulin-like growth factors; the IGFBPs all serve to inhibit IGF actions. Of these IGFBPs, IGFBP6 has garnered interest for its high specificity for IGF-II and for its IGF-independent roles, such as the promotion of apoptosis and inhibition of angiogenesis (40, 41). Literature addressing the possible role of IGFBP6 in adipose tissue is scant; however, recent evidence suggests that in some inflammatory conditions IGFBP6 may be a possible chemotactic factor (40). In this study, we observed significant increases in IGFBP6 levels in OSMRFKO eWAT when compared to OSMRfl/fl eWAT. It is possible that, if IGFBP6 acts with chemotactic ability in adipose tissue, the increased IGFBP6 in OSMRFKO eWAT could be partially responsible for the significantly higher number of leukocytes measured in the tissue.
Collectively, our prominent adipokine array results suggest a local pro-inflammatory environment in the adipose tissue of the OSMRFKO mouse that occurs in the absence of obesity. In further support of these observations, flow cytometry analyses revealed elevations of leukocytes, B cells, and T cells in OSMRFKO eWAT (Figure 6). While it remains unclear as to which cells in the adipose tissue are responsible for the increased adipokine production, it is clear that the loss of adipocyte OSM-OSMR signaling disrupts adipose tissue homeostasis in the absence of obesity. Likely, more than one cell type is contributing to this effect, as there is known crosstalk among cell types in adipose tissue. Future studies will more closely examine each cell type in the adipose tissue of the OSMRFKO mouse to better determine their roles in contributing to disrupted adipose tissue homeostasis. Despite the evident adipose tissue dysfunction in chow-fed OSMRFKO mice, a systemic metabolic challenge, such as an obesogenic diet, is required to produce whole-body insulin resistance while perpetuating adipose tissue inflammation in these animals (10). Taken together, these results and our previous results strongly suggest the need for intact adipocyte OSM-OSMR signaling in maintaining adipose tissue homeostasis.
What is already known about this subject?
Adipose tissue oncostatin M (OSM) protein and gene expression are significantly induced in obese rodents and in people with obesity.
Obese mice with adipocyte-specific deletion of the oncostatin M receptor (OSMR) exhibit systemic insulin resistance and increased adipose tissue immune cell infiltration.
What does our study add?
Altered pro-inflammatory adipokine production observed in the adipocyte-specific OSMR knockout mouse occurs in the absence of high-fat diet-induced obesity.
Adipose tissue immune cell infiltration and adipose tissue dysfunction are present in non-obese adipocyte-specific OSMR knockout mice.
Acknowledgments
Funding:
CME is supported by K01 DK106307 and JMS is supported by R01 DK052968 from the National Institutes of Health. This work used core facilities that are supported in part by COBRE (NIH P30 GM118430) and NORC (NIH P30 DK072476) center grants from the National Institutes of Health.
Footnotes
Disclosure: The authors declare no conflict of interest
LITERATURE CITED
- 1.Greenberg AS, Obin MS. Obesity and the role of adipose tissue in inflammation and metabolism. The American Journal of Clinical Nutrition. 2006;83:461S–465S. doi: 10.1093/ajcn/83.2.461S. [DOI] [PubMed] [Google Scholar]
- 2.Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW., Jr Obesity is associated with macrophage accumulation in adipose tissue. The Journal of Clinical Investigation. 2003;112:1796–1808. doi: 10.1172/JCI19246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cho KW, Zamarron BF, Muir LA, Singer K, Porsche CE, DelProposto JB, et al. Adipose Tissue Dendritic Cells Are Independent Contributors to Obesity-Induced Inflammation and Insulin Resistance. The Journal of Immunology. 2016;197:3650–3661. doi: 10.4049/jimmunol.1600820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lumeng CN, Saltiel AR. Inflammatory links between obesity and metabolic disease. The Journal of Clinical Investigation. 2011;121:2111–2117. doi: 10.1172/JCI57132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fasnacht N, Müller W. Conditional gp130 deficient mouse mutants. Seminars in Cell & Developmental Biology. 2008;19:379–384. doi: 10.1016/j.semcdb.2008.07.001. [DOI] [PubMed] [Google Scholar]
- 6.Heinrich PC, Behrmann I, Haan S, Hermanns HM, Muller-Newen G, Schaper F. Principles of interleukin (IL)-6-type cytokine signalling and its regulation. Biochemical Journal. 2003;374:1–20. doi: 10.1042/BJ20030407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zarling JM, Shoyab M, Marquardt H, Hanson MB, Lioubin MN, Todaro GJ. Oncostatin M: a growth regulator produced by differentiated histiocytic lymphoma cells. Proceedings of the National Academy of Sciences. 1986;83:9739–9743. doi: 10.1073/pnas.83.24.9739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wallace PM, MacMaster JF, Rouleau KA, Brown TJ, Loy JK, Donaldson KL, et al. Regulation of Inflammatory Responses by Oncostatin M. The Journal of Immunology. 1999;162:5547–5555. [PubMed] [Google Scholar]
- 9.Mosley B, De Imus C, Friend D, Boiani N, Thoma B, Park LS, et al. Dual Oncostatin M (OSM) Receptors: cloning and characterization of an alternative signaling subunit conferring OSM-specific receptor activation. Journal of Biological Chemistry. 1996;271:32635–32643. doi: 10.1074/jbc.271.51.32635. [DOI] [PubMed] [Google Scholar]
- 10.Elks CM, Zhao P, Grant RW, Hang H, Bailey JL, Burk DH, et al. Loss of Oncostatin M Signaling in Adipocytes Induces Insulin Resistance and Adipose Tissue Inflammation in Vivo. Journal of Biological Chemistry. 2016;291:17066–17076. doi: 10.1074/jbc.M116.739110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.White UA, Stewart WC, Stephens JM. Gp130 Cytokines Exert Differential Patterns of Crosstalk in Adipocytes Both In Vitro and In Vivo. Obesity. 2011;19:903–910. doi: 10.1038/oby.2010.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sanchez-Infantes D, White UA, Elks CM, Morrison RF, Gimble JM, Considine RV, et al. Oncostatin M Is Produced in Adipose Tissue and Is Regulated in Conditions of Obesity and Type 2 Diabetes. The Journal of Clinical Endocrinology & Metabolism. 2014;99:E217–E225. doi: 10.1210/jc.2013-3555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grant R, Youm Y-H, Ravussin A, Dixit VD. Quantification of Adipose Tissue Leukocytosis in Obesity. Methods in Molecular Biology. 2013;1040:195–209. doi: 10.1007/978-1-62703-523-1_15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cho KW, Morris DL, Lumeng CN. Flow Cytometry Analyses of Adipose Tissue Macrophages. Methods in Enzymology. 2014;537:297–314. doi: 10.1016/B978-0-12-411619-1.00016-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Morris DL, Cho KW, DelProposto JL, Oatmen KE, Geletka LM, Martinez-Santibanez G, et al. Adipose Tissue Macrophages Function As Antigen-Presenting Cells and Regulate Adipose Tissue CD4+ T Cells in Mice. Diabetes. 2013;62:2762–2772. doi: 10.2337/db12-1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Church C, Berry R, Rodeheffer MS. Isolation and Study of Adipocyte Precursors. Methods in Enzymology. 2014;537:31–46. doi: 10.1016/B978-0-12-411619-1.00003-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Calabro P, Chang DW, Willerson JT, Yeh ETH. Release of C-Reactive Protein in Response to Inflammatory Cytokines by Human Adipocytes: Linking Obesity to Vascular Inflammation. Journal of the American College of Cardiology. 2005;46:1112–1113. doi: 10.1016/j.jacc.2005.06.017. [DOI] [PubMed] [Google Scholar]
- 18.Memoli B, Procino A, Calabrò P, Esposito P, Grandaliano G, Pertosa G, et al. Inflammation may modulate IL-6 and C-reactive protein gene expression in the adipose tissue: the role of IL-6 cell membrane receptor. American Journal of Physiology-Endocrinology and Metabolism. 2007;293:E1030–E1035. doi: 10.1152/ajpendo.00697.2006. [DOI] [PubMed] [Google Scholar]
- 19.Komori T, Tanaka M, Senba E, Miyajima A, Morikawa Y. Lack of Oncostatin M Receptor β Leads to Adipose Tissue Inflammation and Insulin Resistance by Switching Macrophage Phenotype. Journal of Biological Chemistry. 2013;288:21861–21875. doi: 10.1074/jbc.M113.461905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tamura S, Morikawa Y, Tanaka M, Miyajima A, Senba E. Developmental expression pattern of oncostatin M receptor β in mice. Mechanisms of Development. 2002;115:127–131. doi: 10.1016/s0925-4773(02)00081-3. [DOI] [PubMed] [Google Scholar]
- 21.Tanaka M, Hirabayashi Y, Sekiguchi T, Inoue T, Katsuki M, Miyajima A. Targeted disruption of oncostatin M receptor results in altered hematopoiesis. Blood. 2003;102:3154–3162. doi: 10.1182/blood-2003-02-0367. [DOI] [PubMed] [Google Scholar]
- 22.Yan Q-W, Yang Q, Mody N, Graham TE, Hsu C-H, Xu Z, et al. The Adipokine Lipocalin 2 Is Regulated by Obesity and Promotes Insulin Resistance. Diabetes. 2007;56:2533–2540. doi: 10.2337/db07-0007. [DOI] [PubMed] [Google Scholar]
- 23.Zhao P, Elks CM, Stephens JM. The induction of lipocalin-2 expression in vivo and in vitro. Journal of Biological Chemistry. 2014;289:5960–5969. doi: 10.1074/jbc.M113.532234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhao P, Stephens JM. STAT1, NF-κB and ERKs play a role in the induction of lipocalin-2 expression in adipocytes. Molecular Metabolism. 2013;2:161–170. doi: 10.1016/j.molmet.2013.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Flo TH, Smith KD, Sato S, Rodriguez DJ, Holmes MA, Strong RK, et al. Lipocalin 2 mediates an innate immune response to bacterial infection by sequestrating iron. Nature. 2004;432:917. doi: 10.1038/nature03104. [DOI] [PubMed] [Google Scholar]
- 26.Meier EM, Pohl R, Rein-Fischboeck L, Schacherer D, Eisinger K, Wiest R, et al. Circulating lipocalin 2 is neither related to liver steatosis in patients with non-alcoholic fatty liver disease nor to residual liver function in cirrhosis. Cytokine. 2016;85:45–50. doi: 10.1016/j.cyto.2016.06.004. [DOI] [PubMed] [Google Scholar]
- 27.Sultan S, Pascucci M, Ahmad S, Malik I, Bianchi A, Ramadori P, et al. Lipocalin-2 is a major acute-phase protein in a rat and mouse model of sterile abscess. Shock. 2012;37:191–196. doi: 10.1097/SHK.0b013e31823918c2. [DOI] [PubMed] [Google Scholar]
- 28.Catalán V, Gómez-Ambrosi J, Rodríguez A, Ramírez B, Silva C, Rotellar F, et al. Increased adipose tissue expression of lipocalin-2 in obesity is related to inflammation and matrix metalloproteinase-2 and metalloproteinase-9 activities in humans. Journal of Molecular Medicine. 2009;87:803. doi: 10.1007/s00109-009-0486-8. [DOI] [PubMed] [Google Scholar]
- 29.Law IKM, Xu A, Lam KSL, Berger T, Mak TW, Vanhoutte PM, et al. Lipocalin-2 Deficiency Attenuates Insulin Resistance Associated With Aging and Obesity. Diabetes. 2010;59:872–882. doi: 10.2337/db09-1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moschen AR, Adolph TE, Gerner RR, Wieser V, Tilg H. Lipocalin-2: A Master Mediator of Intestinal and Metabolic Inflammation. Trends in Endocrinology & Metabolism. 2017;28:388–397. doi: 10.1016/j.tem.2017.01.003. [DOI] [PubMed] [Google Scholar]
- 31.Toyonaga T, Matsuura M, Mori K, Honzawa Y, Minami N, Yamada S, et al. Lipocalin 2 prevents intestinal inflammation by enhancing phagocytic bacterial clearance in macrophages. Scientific Reports. 2016;6:35014. doi: 10.1038/srep35014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Aljada A, Saadeh R, Assian E, Ghanim H, Dandona P. Insulin Inhibits the Expression of Intercellular Adhesion Molecule-1 by Human Aortic Endothelial Cells through Stimulation of Nitric Oxide1. The Journal of Clinical Endocrinology & Metabolism. 2000;85:2572–2575. doi: 10.1210/jcem.85.7.6677. [DOI] [PubMed] [Google Scholar]
- 33.Elgazar-Carmon V, Rudich A, Hadad N, Levy R. Neutrophils transiently infiltrate intra-abdominal fat early in the course of high-fat feeding. J Lipid Res. 2008;49:1894–1903. doi: 10.1194/jlr.M800132-JLR200. [DOI] [PubMed] [Google Scholar]
- 34.Permana PA, Menge C, Reaven PD. Macrophage-secreted factors induce adipocyte inflammation and insulin resistance. Biochemical and Biophysical Research Communications. 2006;341:507–514. doi: 10.1016/j.bbrc.2006.01.012. [DOI] [PubMed] [Google Scholar]
- 35.Soukas A, Socci ND, Saatkamp BD, Novelli S, Friedman JM. Distinct Transcriptional Profiles of Adipogenesis in Vivo and in Vitro. Journal of Biological Chemistry. 2001;276:34167–34174. doi: 10.1074/jbc.M104421200. [DOI] [PubMed] [Google Scholar]
- 36.Triffitt JT, Gebauer U, Ashton BA, Owen ME, Reynolds JJ. Origin of plasma α2HS-glycoprotein and its accumulation in bone. Nature. 1976;262:226. doi: 10.1038/262226a0. [DOI] [PubMed] [Google Scholar]
- 37.Chatterjee P, Seal S, Mukherjee S, Kundu R, Mukherjee S, Ray S, et al. Adipocyte Fetuin-A Contributes to Macrophage Migration into Adipose Tissue and Polarization of Macrophages. Journal of Biological Chemistry. 2013;288:28324–28330. doi: 10.1074/jbc.C113.495473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pal D, Dasgupta S, Kundu R, Maitra S, Das G, Mukhopadhyay S, et al. Fetuin-A acts as an endogenous ligand of TLR4 to promote lipid-induced insulin resistance. Nature Medicine. 2012;18:1279. doi: 10.1038/nm.2851. [DOI] [PubMed] [Google Scholar]
- 39.Srinivas PR, Wagner AS, Reddy LV, Deutsch DD, Leon MA, Goustin AS, et al. Serum alpha 2-HS-glycoprotein is an inhibitor of the human insulin receptor at the tyrosine kinase level. Molecular Endocrinology. 1993;7:1445–1455. doi: 10.1210/mend.7.11.7906861. [DOI] [PubMed] [Google Scholar]
- 40.Alunno A, Bistoni O, Manetti M, Cafaro G, Valentini V, Bartoloni E, et al. Insulin-Like Growth Factor Binding Protein 6 in Rheumatoid Arthritis: A Possible Novel Chemotactic Factor? Frontiers in Immunology. 2017;8:554. doi: 10.3389/fimmu.2017.00554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bach LA. Recent insights into the actions of IGFBP-6. Journal of Cell Communication and Signaling. 2015;9:189–200. doi: 10.1007/s12079-015-0288-4. [DOI] [PMC free article] [PubMed] [Google Scholar]