Abstract
Background
Elevation in central venous pressure (CVP) plays a fundamental pathophysiologic role in Fontan circulation. Because there is no sub-pulmonary ventricle in this system, CVP also provides the driving force for pulmonary blood flow. We hypothesized that this would make Fontan patients more susceptible to even low-level elevation in pulmonary vascular resistance index (PVRI), resulting in greater systemic venous congestion and adverse outcomes.
Methods
Adult Fontan patients and controls without congenital heart disease undergoing clinical evaluation that included cardiac catheterization and echocardiography were examined retrospectively. Outcomes including all-cause mortality and the development of Fontan associated diseases (FAD, defined as protein losing enteropathy, cirrhosis, heart failure hospitalization, arrhythmia, or thromboembolism) were assessed from longitudinal assessment.
Results
As compared to controls (n=82), Fontan patients (n=164) were younger (36 vs 45 years, p<0.001), more likely to be on anticoagulation or antiplatelet therapy, and more likely to have atrial arrhythmia or cirrhosis. There was a strong correlation between CVP and PVRI in the Fontan group (r=0.79, p<0.001), but there was no such relationship in controls. Elevated PVRI identified patients at increased risk for FAD (HR 1.92, 95% CI 1.39–2.41, p=0.01), and composite endpoint of FAD and/or death (HR 1.89, 95% CI 1.32–2.53, p=0.01) per 1 WU*m2 increment.
Conclusions
Systemic venous congestion, which is the primary factor in the pathogenesis of FAD and death, is related to even low-level abnormalities in pulmonary vascular function. Multicenter studies are needed to determine whether interventions targeting pulmonary vascular structure and function can improve outcomes in the Fontan population.
Keywords: Central venous pressure, pulmonary vascular resistance, Fontan associated diseases, Fontan failure
INTRODUCTION
Adult congenital heart disease is an important and understudied cause of heart failure.1 One challenging and poorly-understood cohort within the spectrum of congenital heart disease includes patients with Fontan circulation.1 The Fontan physiology is unique because it relies on passive (non-pulsatile) pulmonary blood flow to perfuse the lungs allowing for gas exchange, and provide preload to the systemic ventricle.2 In the absence of an effective subpulmonary ventricle, an obligatory increase in central venous pressure (CVP) is required to maintain the transpulmonary gradient necessary for pulmonary blood flow.2 Chronic increase in CVP causes systemic venous congestion that promotes Fontan associated diseases (FAD) such as arrhythmia, cirrhosis, protein losing enteropathy and heart failure.2–6
Previous studies have shown that non-pulsatile pulmonary blood flow as with the Fontan circulation results in pulmonary vascular disease due to dysregulation of the nitric oxide pathway and endothelial dysfunction.7, 8 In the normal biventricular circulation, slight increases in pulmonary vascular resistance (PVR) are well-tolerated, since the right ventricle can accelerate venous blood against this increased impedance. However in the venous circulation resistance is very low, and thus in the Fontan circuit, any increase in PVR could markedly influence CVP. We hypothesized that in the absence of a subpulmonary ventricle in the Fontan physiology, PVR becomes the primary determinant of CVP, and that this would be related to adverse outcomes including death and the development of FAD.
METHODS
Patient Selection
We identified all adult patients with the Fontan circulation (>18 years) that underwent cardiac catheterization at Mayo Clinic Rochester, Minnesota between January 1, 1990 and December 31, 2015. Fontan patients were compared to patients without congenital heart disease referred for invasive hemodynamic exercise testing because of exertional dyspnea during the same study period but who were found to display no identifiable cardiac cause of symptoms. The Mayo Clinic Institutional Review Board approved this study and waived informed consent for the patients that provided research authorization.
Invasive and Clinical Data Collection
Medical records were reviewed in detail including clinical notes, echocardiograms, cardiac catheterization reports, and surgical notes. All cardiac catheterization procedures were reviewed and invasive hemodynamic data were analyzed. Central venous pressure (CVP), pulmonary artery (PA) pressures, and PA wedge pressures (PAWP), were recorded at end expiration taken as the average of ≥3 beats.
Cardiac output was determined by the Fick technique using assumed O2 consumption in Fontan patients and directly measured O2 consumption in controls along with directly measured O2 contents in the pulmonary arterial and systemic blood samples.9 Cardiac index was calculated by the quotient of cardiac output and body surface area. Pulmonary vascular resistance index (PVRI) was calculated by (mean pulmonary artery pressure – PAWP)/cardiac index. Systemic vascular resistance index was calculated by (mean systemic arterial pressure – Fontan pressure)/cardiac index. Plasma volume at the time of catheterization was estimated by: (1-hematocrit) (a + [b weight in kg]), where a = 1,530 in men and 864 in women, and b= 41 in men and 47.9 in women.10
Study Endpoints and Definitions
The presence of FAD was defined as in previous studies,11, 12 including 1 or more of the following: protein losing enteropathy (elevated stool α-1 antitrypsin concentration >54 mg/dl with decreased serum albumin <3.5 g/dl and accompanying symptoms); cirrhosis (liver stiffness >5.0 kPa by magnetic resonance elastography or stage 4 fibrosis on histology); heart failure hospitalization (admission for worsening heart failure signs and symptoms requiring intravenous diuretics); arrhythmia (atrial or ventricular); and thromboembolism (thrombus within the Fontan pathway, intracardiac thrombus, stroke or other systemic arterial embolus). Death was defined as all-cause mortality. Only one event was counted per patient in the censor for the composite endpoint of FAD and/or death.
Statistical Analysis
Statistical analyses were performed with JMP software (version 10.0; SAS Institute Inc). Categorical variables were reported as percentages, and continuous variables were reported as mean ± standard deviation or median (interquartile range) for skewed data. Categorical variables were compared using the χ2 test or Fisher exact test, and continuous variables were compared with a 2-sided, unpaired t test or Wilcoxon rank sum test, as appropriate. Linear regression was performed to examine relationships between continuous variables.
In order to evaluate the impact of elevated PVRI on FAD and/or death, we divided the Fontan patients into high PVRI group (PVRI>2 WU*m2) and low PVRI group (PVRI ≤ 2 WU*m2) based on the partition value used in prior publications.13, 14 A Cox proportional hazards model was used to determine the association between Fontan hemodynamic variables and the occurrence of FAD and/or death. The variables included in the univariate model were chosen a priori based on their previously demonstrated association with outcome in Fontan patients.11, 12, 15, 16 Variables that reached statistical significance in univariate analysis were included in the multivariate analysis. The risk for each variable was expressed as hazard ratio (HR) and 95% confidence interval (CI). A p value less than 0.05 was considered statistically significant.
RESULTS
There were 164 patients in the Fontan group and 82 controls. As compared to controls, the Fontan patients were younger, more likely to be on anticoagulation or antiplatelet therapy, and more likely to have atrial arrhythmia or cirrhosis (Table 1). Among the Fontan patients, the mean age at the time of Fontan operation was 8±5 years, 103 (63%) had systemic left ventricle, and 105 (64%) had atriopulmonary Fontan connection. The most common congenital heart disease diagnoses were tricuspid atresia (n=65, 40%) and double inlet left ventricle (n=52, 32%).
Table 1.
Baseline Clinical Characteristics of Fontan and Control Groups
| Demographic variables | Fontan (N=164) | Control (N=82) | p |
|---|---|---|---|
| Age, years | 36 (25–43) | 45 (35 – 52) | <0.001 |
| Male | 88 (54%) | 26 (32%) | 0.01 |
| Body mass index, kg/m2 | 27 ± 3 | 28 ± 6 | 0.12 |
| Body surface area, m2 | 1.9 ± 03 | 1.9 ± 0.2 | 0.31 |
| Laboratory data | |||
| Hemoglobin, g/dl | 15.1 ± 0.4 | 13.3 ± 0.6 | 0.01 |
| Albumin, g/dl | 4.2 ± 0.4 | 4.4 ± 0.6 | 0.23 |
| Creatinine, mg/dl | 1.1 ± 0.4 | 0.9 ± 0.2 | 0.18 |
| NT-pro BNP, pg/ml | 191 (114 – 746) | 47 (25 – 82) | <0.001 |
| Estimated plasma volume, ml | 2971± 423 | 2703 ± 311 | 0.06 |
| Medications | |||
| Diuretics | 45 (27%) | 14 (18%) | 0.12 |
| RAAS antagonist | 46 (28%) | 15 (19%) | 0.13 |
| Beta blockers | 31 (20%) | 20 (24%) | 0.56 |
| Comorbidities | |||
| Hypertension | 1 (1%) | 32 (39%) | <0.001 |
| Hyperlipidemia | 42 (26%) | 26 (32%) | 0.21 |
| Diabetes | 14 (9%) | 5 (6%) | 0.41 |
NT-pro BNP: N-terminal pro-B-type natriuretic peptide; RAAS: Renin angiotensin aldosterone system antagonist
Hemodynamic Differences between Groups
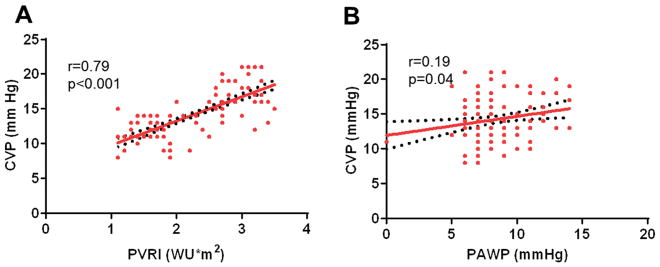
As compared to controls, Fontan patients had lower systemic arterial and mixed venous saturations and oxygen contents, lower cardiac index, and lower systemic vascular resistance (Supplementary Table 1). CVP was significantly higher in Fontan patients than controls, but PVRI values were similar. There was a strong correlation between CVP and PVRI (r=0.79, p<0.001) but CVP was only marginally related to PAWP (r=0.19, p=0.04) in the Fontan group (Figure 1). In contrast, there was no relationship between CVP and PVRI in the control group.
Figure 1.
Figure 1A: Linear correlation of central venous pressure (CVP) vs pulmonary vascular resistance index (PVRI) in Fontan patients
Figure 1B: Linear correlation of central venous pressure (CVP) vs pulmonary artery wedge pressure (PAWP) in Fontan patients
Impact of Elevated PVRI in Fontan Patients
In order to assess the impact of PVRI on the other hemodynamic indices, we divided the Fontan cohort into 2 groups based on predetermined cut point of high PVRI Fontan (>2.0 WU*m2) and low PVRI Fontan (≤2.0 WU*m2). Among the 164 Fontan patients in the study, 91 (55%) had high PVRI while 73 (45%) had low PVRI. By design, the mean PVRI was greater in the high PVRI group compared to the low PVRI group (2.7 ± 0.5 vs 1.6 ± 0.4 WU*m2, p<0.001). Compared to the low PVRI Fontan group, the high PVRI Fontan group had lower peak oxygen consumption, more likely to have atriopulmonary Fontan connection, and tended to be older at the time of cardiac catheterization and (Supplementary Table 2).
PVRI and Fontan Related Clinical Outcomes
The following FAD events occurred either at the time of cardiac catheterization or during follow-up: cirrhosis (n=35, 21%), protein-losing enteropathy (n=13, 8%), thromboembolism (n=25, 15%), heart failure hospitalization (n=46, 28%), and atrial arrhythmia (n=76, 46%). The endpoint of any FAD occurred in 112 patients (68%). Elevated PVRI was found to be a significant predictor of the development of FAD, independent of other known predictors (HR 1.92, 95% CI 1.39 – 2.41, p=0.01) per 1 WU*m2 increment, Table 2.
Table 2.
Hemodynamic and Clinical Risk Factors for Fontan Associated Diseases
| Univariate | Multivariate | |||
|---|---|---|---|---|
| HR (95% CI) | p | HR (95% CI) | p | |
| Hemodynamic variables | ||||
| PVRI, per 1 WU*m2 increment | 2.22 (1.71 – 2.88) | <0.001 | 1.92 (1.39 – 2.41) | 0.01 |
| Cardiac index, per 1 l/min/m2 decrease | 1.04 (0.82 – 1.15) | 0.13 | --- | --- |
| PAWP, per 1 mm Hg increment | 1.69 (1.11 – 2.19) | 0.04 | 1.09 (0.59–2.75) | 0.24 |
| Ejection fraction <30% | 1.39 (0.96 – 1.71) | 0.08 | --- | --- |
| ≥ Moderate AVV regurgitation | 0.96 (0.33 – 1.84) | 0.21 | --- | --- |
| SVRI, per dynes*s/cm5/m2 decrease | 1.49 (0.89 – 2.65) | 0.06 | ||
| Clinical variables | ||||
| Age at cath, per 5 y increment | 2.31 (1.44 – 3.28) | <0.001 | 1.51 (0.93 – 2.71) | 0.06 |
| Age at Fontan op, per 2 y increment | 1.61 (0.97 – 1.94) | 0.07 | --- | --- |
| Male gender | 0.96 (0.53 – 1.42) | 0.49 | --- | --- |
| Peak VO2 <50% of predicted | 1.94 (0.86–3.11) | 0.12 | --- | --- |
| Left ventricle morphology | 1.11 (0.61–2.43) | 0.28 | --- | --- |
| Atriopulmonary Fontan | 2.14 (1.53–2.59) | < 0.001 | 1.55 (1.11–2.02) | 0.04 |
| Heterotaxy | 1.14 (0.21 – 1.95) | 0.21 | --- | --- |
| Paced rhythm | 1.08 (0.31 – 2.37) | 0.41 | --- | --- |
| Warfarin | 1.42 (0.61 – 2.61) | 0.36 | --- | --- |
| Diuretics | 1.33 (0.87 – 2.88) | 0.18 | --- | --- |
HR: hazard ratio; CI: confidence interval; y: year; PVRI: pulmonary vascular resistance index; CI: cardiac index; PAWP: pulmonary artery wedge pressure; AVV: atrioventricular valve; cath: catheterization; op: Operation; SVRI: Systemic vascular resistance index, VO2 oxygen consumption
Over a mean follow-up of 7.1 ± 2.2 years there were 31 deaths (19%). Cause of death was perioperative death after cardiac surgery in 9, sudden death in 6, heart failure/thromboembolism in 3, sepsis in 2, and unknown/multifactorial in 12. A composite endpoint of FAD and all-cause mortality occurred in 116 (72%). Again elevated PVRI was an independent predictor of the composite endpoint of development of FAD and all-cause mortality (HR 1.89, 95% CI 1.32 – 2.53, p=0.01) per 1 WU*m2 increment.
DISCUSSION
Although the long-term survival after the Fontan operation has improved, the life expectancy in the Fontan population is still significantly lower than that of patients with biventricular circulation.17, 18 The excess mortality in this population is largely due to FAD.16, 19 The pathogenesis of most FAD is driven by systemic venous congestion caused by chronic elevation in CVP.2 In this study we demonstrated a direct relationship between PVRI and CVP, and an association between high PVRI and Fontan related clinical outcomes.
Impact of CVP on Fontan Related Clinical Outcomes
The current study demonstrates a strong correlation between PVRI and CVP in Fontan physiology (r=0.79, p<0.001), in contrast to biventricular physiology where CVP was uncoupled from PVRI. This is expected because the functional right ventricle can easily cope with low grade elevations in PVRI without causing frank right heart failure. However, in the absence of an effective sub-pulmonary ventricle, even minor increases in resistance in the circuit can lead to dramatic upstream congestion, elevation in CVP, and stagnation of venous flow. This can promote thromboembolism as well as deleterious effects upon a number of organ systems including the liver, gut, and kidneys mediated by tissue edema from venous congestion.
The impact of CVP elevation in the Fontan circuit has been described. A retrospective study reported occurrence of protein losing enteropathy in 26 of 354 (7.3%) pediatric and adult Fontan patients within the first two decades after Fontan operation.20 High CVP was identified as a risk factor for protein losing enteropathy in that cohort. A different study assessing outcomes in 44 Fontan patients with protein losing enteropathy showed that elevated CVP (>15 mmHg) was a risk factor for death.4 Elevated CVP has also been reported as a risk factor for cirrhosis and portal hypertension in a cohort of 64 Fontan patents.21 The detrimental impact of elevated CVP is not restrictive to the liver and gut but has been reported as a risk factor for several Fontan related adverse events.2–6
Determinants of CVP
CVP provides the driving force for pulmonary blood flow in the Fontan circulation.2 As impedance to pulmonary blood flow (ventricular filling pressures and PVR) increase, CVP must rise in order to maintain the transpulmonary gradient required for passive pulmonary blood flow, gas exchange, and systemic ventricular filling. In the current study we demonstrated that CVP was directly related to PVRI but was independent of PAWP (ventricular filling pressure). CVP is also related to plasma volume and venous tone. Plasma volume tended to be greater in Fontan patients, but not to the extent that would explain the marked disparity in CVP between groups.
Pulmonary Vascular Disease as Potential Target for Intervention
In the current study, elevated PVRI was an independent predictor for FAD, and also independent predictor for the composite endpoint of FAD and all-cause mortality. These findings suggest that minor elevations in PVRI, even within the range that is considered normal in the biventricular circulation, may increase risk of adverse outcomes in patients with Fontan palliation.
A number of small, single center studies have begun to explore the role of pulmonary vasodilators in patients with the Fontan palliation.13, 22–25 In a prospective, non-controlled study of 24 pediatric and adult Fontan patients with pulmonary vascular disease defined as PVRI > 2 WU*m2, the use of endothelin receptor antagonists resulted in a significant drop in PVRI after 6 months.13 There was a concomitant increase in cardiac index, and improvement in New York Heart Association (NYHA) functional class and peak oxygen consumption in most of the patients.13 In a different study, 24 Fontan patients with baseline PVRI >2.5 WU*m2 received sildenafil for 3 months. This resulted in a drop in PVRI from 3.9 to 1.7 WU*m2 with concordant improvement in cardiac index, NYHA functional class and 6 minute walk distance.22 Several other studies have demonstrated the beneficial effect of pulmonary vasodilators in Fontan patients both in terms of reduction in PVRI, cardiac output augmentation and improvement in exercise capacity.23–25
All these studies have been focused on relatively short term effects of pulmonary vasodilators, and there is no data available on long-term effects. Furthermore, it remains unclear what the mechanism of PVR elevation is in the Fontan circulation, and to what extent these low grade elevations in PVRI are due to vasoconstriction, vascular remodeling or both. The results of the current study suggest that interventions to reduce PVR over longer durations may have favorable effects on systemic venous congestion, and this merits testing in multicenter placebo-controlled trials.
Limitations
The cross sectional nature of hemodynamic assessments does not permit assessment of causality. This is a single center retrospective study reporting outcomes in an older Fontan cohort, which may limit generalizability to other populations. The results of the study may have been influenced, to some extent, by selection bias and other potential confounders because of the retrospective study design. There are several other determinants of Fontan hemodynamics such as power loss, wall shear stress, ventriculoarterial coupling etc. that were not tested for in our study. Controls were older than Fontan patients, but this would only be expected to bias any group differences toward the null, since pulmonary vascular disease worsens with normal aging.26
Conclusions
In the current study we demonstrated a direct correlation between CVP and PVRI in patients with Fontan physiology, in contrast to the normal biventricular circulation where CVP was independent of PVRI. Further study is indicated to evaluate the effect of pulmonary vaso-modulators on CVP as potential strategy for reducing morbidity and mortality in adults with the Fontan circulation.
Supplementary Material
Highlights.
Pulmonary vascular resistance is the primary determinant of systemic venous congestion
Systemic venous congestion is the primary factor in the pathogenesis of Fontan associated diseases (FAD) and death
Therapy targeted a pulmonary vascular resistance may modify risk of FAD and death
Acknowledgments
Dr. Egbe is supported by National Heart, Lung, and Blood Institute (NHLBI) grant K23 HL141448-01. Dr. Borlaug is supported by RO1 HL128526 and U10 HL110262. Dr. Reddy is supported by National Heart, Lung, and Blood Institute (NHLBI) grant number T32 HL007111.
Abbreviations
- FAD
Fontan associated disease
- PVR
pulmonary vascular resistance
- PVRI
pulmonary vascular resistance index
- CVP
central venous pressure
- PAWP
pulmonary artery wedge pressure
- HR
hazard ratio
- CI
confidence interval
- NYHA
New York Heart Association
Footnotes
Conflict of Interest
None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Budts W, Roos-Hesselink J, Radle-Hurst T, Eicken A, McDonagh TA, Lambrinou E, Crespo-Leiro MG, Walker F, Frogoudaki AA. Treatment of heart failure in adult congenital heart disease: a position paper of the Working Group of Grown-Up Congenital Heart Disease and the Heart Failure Association of the European Society of Cardiology. Eur Heart J. 2016;37:1419–27. doi: 10.1093/eurheartj/ehv741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gewillig M, Brown SC. The Fontan circulation after 45 years: update in physiology. Heart. 2016;102:1081–6. doi: 10.1136/heartjnl-2015-307467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rychik J, Veldtman G, Rand E, Russo P, Rome JJ, Krok K, Goldberg DJ, Cahill AM, Wells RG. The precarious state of the liver after a Fontan operation: summary of a multidisciplinary symposium. Pediatr Cardiol. 2012;33:1001–12. doi: 10.1007/s00246-012-0315-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.John AS, Johnson JA, Khan M, Driscoll DJ, Warnes CA, Cetta F. Clinical outcomes and improved survival in patients with protein-losing enteropathy after the Fontan operation. J Am Coll Cardiol. 2014;64:54–62. doi: 10.1016/j.jacc.2014.04.025. [DOI] [PubMed] [Google Scholar]
- 5.Mori M, Hebson C, Shioda K, Elder RW, Kogon BE, Rodriguez FH, Jokhadar M, Book WM. Catheter-measured Hemodynamics of Adult Fontan Circulation: Associations with Adverse Event and End-organ Dysfunctions. Congenit Heart Dis. 2016;11:589–597. doi: 10.1111/chd.12345. [DOI] [PubMed] [Google Scholar]
- 6.Hebson CL, McCabe NM, Elder RW, Mahle WT, McConnell M, Kogon BE, Veledar E, Jokhadar M, Vincent RN, Sahu A, Book WM. Hemodynamic phenotype of the failing Fontan in an adult population. Am J Cardiol. 2013;112:1943–7. doi: 10.1016/j.amjcard.2013.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zongtao Y, Huishan W, Zengwei W, Hongyu Z, Minhua F, Xinmin L, Nanbin Z, Hongguang H. Experimental study of nonpulsatile flow perfusion and structural remodeling of pulmonary microcirculation vessels. Thorac Cardiovasc Surg. 2010;58:468–72. doi: 10.1055/s-0030-1250124. [DOI] [PubMed] [Google Scholar]
- 8.Binotto MA, Maeda NY, Lopes AA. Altered endothelial function following the Fontan procedure. Cardiol Young. 2008;18:70–4. doi: 10.1017/S1047951107001680. [DOI] [PubMed] [Google Scholar]
- 9.LaFarge CG, Miettinen OS. The estimation of oxygen consumption. Cardiovasc Res. 1970;4:23–30. doi: 10.1093/cvr/4.1.23. [DOI] [PubMed] [Google Scholar]
- 10.Ling HZ, Flint J, Damgaard M, Bonfils PK, Cheng AS, Aggarwal S, Velmurugan S, Mendonca M, Rashid M, Kang S, Papalia F, Weissert S, Coats CJ, Thomas M, Kuskowski M, Cohn JN, Woldman S, Anand IS, Okonko DO. Calculated plasma volume status and prognosis in chronic heart failure. Eur J Heart Fail. 2015;17:35–43. doi: 10.1002/ejhf.193. [DOI] [PubMed] [Google Scholar]
- 11.Egbe AC, Connolly HM, McLeod CJ, Ammash NM, Niaz T, Yogeswaran V, Poterucha JT, Qureshi MY, Driscoll DJ. Thrombotic and Embolic Complications Associated With Atrial Arrhythmia After Fontan Operation: Role of Prophylactic Therapy. J Am Coll Cardiol. 2016;68:1312–9. doi: 10.1016/j.jacc.2016.06.056. [DOI] [PubMed] [Google Scholar]
- 12.Egbe AC, Driscoll DJ, Khan AR, Said SS, Akintoye E, Berganza FM, Connolly HM. Cardiopulmonary exercise test in adults with prior Fontan operation: The prognostic value of serial testing. Int J Cardiol. 2017;235:6–10. doi: 10.1016/j.ijcard.2017.02.140. [DOI] [PubMed] [Google Scholar]
- 13.Agnoletti G, Gala S, Ferroni F, Bordese R, Appendini L, Pace Napoleone C, Bergamasco L. Endothelin inhibitors lower pulmonary vascular resistance and improve functional capacity in patients with Fontan circulation. J Thorac Cardiovasc Surg. 2017 doi: 10.1016/j.jtcvs.2017.01.051. [DOI] [PubMed] [Google Scholar]
- 14.Mitchell MB, Campbell DN, Boucek MM. Heart transplantation for the failing Fontan circulation. Semin Thorac Cardiovasc Surg Pediatr Card Surg Annu. 2004;7:56–64. doi: 10.1053/j.pcsu.2004.02.013. [DOI] [PubMed] [Google Scholar]
- 15.Egbe AC, Connolly HM, Dearani JA, Bonnichsen CR, Niaz T, Allison TG, Johnson JN, Poterucha JT, Said SM, Ammash NM. When is the right time for Fontan conversion? The role of cardiopulmonary exercise test. Int J Cardiol. 2016;220:564–8. doi: 10.1016/j.ijcard.2016.06.209. [DOI] [PubMed] [Google Scholar]
- 16.Khairy P, Fernandes SM, Mayer JE, Jr, Triedman JK, Walsh EP, Lock JE, Landzberg MJ. Long-term survival, modes of death, and predictors of mortality in patients with Fontan surgery. Circulation. 2008;117:85–92. doi: 10.1161/CIRCULATIONAHA.107.738559. [DOI] [PubMed] [Google Scholar]
- 17.Pundi KN, Johnson JN, Dearani JA, Pundi KN, Li Z, Hinck CA, Dahl SH, Cannon BC, O’Leary PW, Driscoll DJ, Cetta F. 40-Year Follow-Up After the Fontan Operation: Long-Term Outcomes of 1,052 Patients. J Am Coll Cardiol. 2015;66:1700–10. doi: 10.1016/j.jacc.2015.07.065. [DOI] [PubMed] [Google Scholar]
- 18.Iyengar AJ, Winlaw DS, Galati JC, Wheaton GR, Gentles TL, Grigg LE, Justo RN, Radford DJ, Weintraub RG, Bullock A, Celermajer DS, d’Udekem Y Australia and New Zealand Fontan R. The extracardiac conduit Fontan procedure in Australia and New Zealand: hypoplastic left heart syndrome predicts worse early and late outcomes. Eur J Cardiothorac Surg. 2014;46:465–73. doi: 10.1093/ejcts/ezu015. discussion 473. [DOI] [PubMed] [Google Scholar]
- 19.Alsaied T, Bokma JP, Engel ME, Kuijpers JM, Hanke SP, Zuhlke L, Zhang B, Veldtman GR. Factors associated with long-term mortality after Fontan procedures: a systematic review. Heart. 2017;103:104–110. doi: 10.1136/heartjnl-2016-310108. [DOI] [PubMed] [Google Scholar]
- 20.Ohuchi H, Yasuda K, Miyazaki A, Kitano M, Sakaguchi H, Yazaki S, Tsuda E, Yamada O. Haemodynamic characteristics before and after the onset of protein losing enteropathy in patients after the Fontan operation. Eur J Cardiothorac Surg. 2013;43:e49–57. doi: 10.1093/ejcts/ezs714. [DOI] [PubMed] [Google Scholar]
- 21.Agnoletti G, Ferraro G, Bordese R, Marini D, Gala S, Bergamasco L, Ferroni F, Calvo PL, Barletti C, Cisaro F, Longo F, Pace Napoleone C. Fontan circulation causes early, severe liver damage. Should we offer patients a tailored strategy? Int J Cardiol. 2016;209:60–5. doi: 10.1016/j.ijcard.2016.02.041. [DOI] [PubMed] [Google Scholar]
- 22.Mori H, Park IS, Yamagishi H, Nakamura M, Ishikawa S, Takigiku K, Yasukochi S, Nakayama T, Saji T, Nakanishi T. Sildenafil reduces pulmonary vascular resistance in single ventricular physiology. Int J Cardiol. 2016;221:122–7. doi: 10.1016/j.ijcard.2016.06.322. [DOI] [PubMed] [Google Scholar]
- 23.Khambadkone S, Li J, de Leval MR, Cullen S, Deanfield JE, Redington AN. Basal pulmonary vascular resistance and nitric oxide responsiveness late after Fontan-type operation. Circulation. 2003;107:3204–8. doi: 10.1161/01.CIR.0000074210.49434.40. [DOI] [PubMed] [Google Scholar]
- 24.Giardini A, Balducci A, Specchia S, Gargiulo G, Bonvicini M, Picchio FM. Effect of sildenafil on haemodynamic response to exercise and exercise capacity in Fontan patients. Eur Heart J. 2008;29:1681–7. doi: 10.1093/eurheartj/ehn215. [DOI] [PubMed] [Google Scholar]
- 25.Hebert A, Mikkelsen UR, Thilen U, Idorn L, Jensen AS, Nagy E, Hanseus K, Sorensen KE, Sondergaard L. Bosentan improves exercise capacity in adolescents and adults after Fontan operation: the TEMPO (Treatment With Endothelin Receptor Antagonist in Fontan Patients, a Randomized, Placebo-Controlled, Double-Blind Study Measuring Peak Oxygen Consumption) study. Circulation. 2014;130:2021–30. doi: 10.1161/CIRCULATIONAHA.113.008441. [DOI] [PubMed] [Google Scholar]
- 26.van Empel VP, Kaye DM, Borlaug BA. Effects of healthy aging on the cardiopulmonary hemodynamic response to exercise. Am J Cardiol. 2014;114:131–5. doi: 10.1016/j.amjcard.2014.04.011. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.