Abstract
Background
Myocardial dysfunction has been implicated in gradual heart failure in transposition of the great arteries (TGA) with a systemic right ventricle (RV). Fibrosis can be assessed using the extracellular volume fraction (ECV). Our aim was to measure ECV and determine its associations with clinical findings and outcomes.
Methods
We prospectively measured ECV in systemic RV subjects (either D-loop after atrial switch or L-loop) and healthy controls. T1 measurements for a single mid-ventricular short-axis plane before and 3, 7, and 15 minutes after gadolinium contrast was used to quantify systemic ventricular ECV. Individuals with elevated ECV were compared to those without.
Results
In 53 TGA subjects (age 34.6±10.3 years, 41% female) the mean ECV for the systemic RV (28.7±4.4%) was significantly higher than the left ventricle in 22 controls (26.1±2.8%, P=0.0104). Those with an elevated ECV (n=15, 28.3%) had a higher b-type natriuretic peptide (BNP) (P<0.011) and a longer 6-minute walk distance (P=0.021), but did not differ by age, arrhythmia history, ventricular volume, function, or circulating collagen byproducts. At followup (median 4.2±1.9 years), those experiencing major cardiovascular endpoints (new arrhythmia, arrhythmia device, heart failure hospitalization, listing for transplantation, mechanical support, or cardiovascular death, n=14) had a higher ECV. ECV, age, and BNP were independent predictors of cardiac events in Cox-proportional hazard models.
Conclusions
Myocardial fibrosis is common in the systemic RV and associated with a higher BNP. Elevated ECV was associated with adverse clinical outcome. The findings suggest a role of diffuse myocardial fibrosis in clinical deterioration of the systemic RV.
Keywords: congenital heart disease, myocardial fibrosis, ventricular dysfunction, transposition of the great arteries, systemic right ventricle, cardiac magnetic resonance
1. Introduction
There is growing recognition that myocardial preservation, not just anatomic correction, is paramount to long-term survival in adults with congenital heart disease (ACHD). Specifically, patients with transposition of the great arteries (TGA) who have a systemic right ventricle (RV), either in the setting of D-loop TGA after an atrial switch palliation, or L-loop TGA (congenitally corrected TGA), are both expected to develop myocardial dysfunction over time with important clinical consequences.
Myocardial fibrosis, as demonstrated by cardiovascular magnetic resonance (CMR), has been described in the systemic RV and is associated with age, ventricular size and function, and prior atrial arrhythmia.[1, 2] Newer methods measuring diffuse fibrosis using T1 measurements to calculate the extracellular volume fraction (ECV) have also shown changes in a number of different pathologic states as well as in congenital heart disease,[3–9] detecting fibrosis beyond that appreciable by LGE alone.[3]
To study the relationship between diffuse fibrosis and heart failure manifestations and outcomes, we sought to measure ECV prospectively in a larger cohort of systemic RV patients. Despite recognized differences between D and L-loop transposition, because both share the problems of a vulnerable systemic RV we included both groups in our study. We hypothesized that ECV would be higher in TGA compared to healthy individuals, correlate with circulating collagen peptide fragments, exercise capacity, and arrhythmia history, and be associated with adverse clinical outcomes.
2. Methods
2.1 Patient Selection
Outpatients age ≥18 years with TGA and a systemic RV (L- or D-loop TGA) were prospectively recruited. Patients with an implanted pacemaker or cardioverter-defibrillator (ICD), creatinine > 1.5 mg/dl, or severe claustrophobia were excluded. Healthy control subjects (non-smokers without known heart disease, diabetes, or hypertension) were also studied, and their data have been reported previously.[10] The study protocol was approved by the institutional review board at both participating institutions, and all subjects gave written, informed consent.
2.2 Study Protocol
An intravenous cannula was placed in each subject and blood samples were drawn for measurement of blood count, blood chemistries and b-type natriuretic peptide (BNP). Additional blood samples were spun, and serum placed in frozen storage for additional biomarker assays. Surgical history and medications were obtained from medical records. CMR was then performed, followed by a six-minute walk test (6MWT) in which distance, heart rate, and oxygen saturation were recorded immediately upon completion.
2.3 CMR Protocol
CMR was performed on a 1.5 Tesla scanner (Philips 1.5 Achieva or Integra). Standard cine acquisitions included a short-axis stack (30 phases, 7 mm thickness with 3 mm gap) for RV and left ventricle (LV) volume and function. Thereafter, a single mid-ventricle short-axis plane Look-Locker sequence was prescribed 3 cm apically from the atrioventricular valves viewed in 4 chamber view (8 mm thickness, 16 – 21 phases, TR/TE 8/2.5 ms, temporal resolution 40 ms). The Look-Locker method allowed for analysis of each phase to provide better scrutiny of avoiding trabecular pooling and makes no assumptions about interphase registration. Following gadolinium contrast administration (0.15 mmol/kg), the sequence was repeated at 3, 7, and 15 minutes post injection. To allow for full relaxation between inversion pulses, the expected heart rate was set to 20 – 30 bpm on the scanner, and the repetition time for inversion was set to ~4 seconds for pre-contrast T1 measurements and ~2 seconds post-contrast accounting for shorter T1 times. The inversion time increments in the Look-Locker read-out were approximately 120 ms for pre-contrast and 90 ms for post-contrast sequences, changed by adjustment of the segmentation factor. Late gadolinium enhancement imaging followed the final Look-Locker acquisition.
2.4 CMR Analysis
CMR studies were analyzed using QMass software (Medis, Leiden, The Netherlands) for all patients at a single institution. End-diastolic volume (EDV), end-systolic volume (ESV), and mass, each indexed to body surface area, and EF were measured for both the RV and LV by contouring short-axis sequences. Trabeculations were included as part of the myocardium. Wall stress was calculated as systolic blood pressure x end-systolic volume/mass.
For ECV quantification, each Look-Locker phase was used to trace the systemic ventricle along endo- and epicardial borders, divided into 6 equal segments (2 for septum, 4 for free wall). Care was taken to exclude non-compacted myocardium or trabeculations to avoid blood pooling, as well as epicardial fat. A region of interest was also placed in the RV lumen avoiding trabeculations or papillary muscles. All contours were manually traced and reviewed by two physicians. Signal intensity vs. time curves for myocardium and blood were plotted to quantify T1 through exponential fitting, and its reciprocal, R1. This was done for each acquisition pre and post contrast. The slope of the linear relationship between R1 for myocardium vs. blood before and after gadolinium administration (4 data points) defined the partition coefficient for gadolinium (lambda).[11] Values for all six myocardial segments were averaged in each subject. The partition coefficient was multiplied by (1-hematocrit/100) to obtain the extracellular volume of distribution of gadolinium, also referred to as ECV.
2.5 Collagen Peptide Fragments and Related Enzymes
Stored serum samples were thawed for batched analysis of collagen byproducts and other protein assays relevant to collagen turnover and myocardial fibrogenesis including pro-collagen 1 N-terminal peptide (PC1NP) and pro-collagen 3 N-terminal peptide (PC3NP) using commercially available assays (Orion Diagnostica Oy, Espoo, Finland). Additional assays included aldosterone (Alpco, Salem, NH), matrix metalloproteinase-2 (MMP-2) and tissue inhibitor of matrix metalloproteinase-1 (TIMP-1, R and D Systems, Minneapolis, MN). Each assay was run with appropriate quality controls by personnel blinded to any other imaging or clinical data.
2.6 Follow-Up
Clinical follow-up was obtained through clinic visits or review of available electronic medical records by an experienced ACHD provider blinded to all other variables. Patients who did not have recent follow-up were contacted by phone. Major clinical events were those related to either arrhythmia or congestive heart failure (CHF). Arrhythmia events included new atrial arrhythmia (found clinically or during 48-hour ambulatory ECG monitoring ordered for symptom evaluation, or requiring intervention such as cardioversion or ablation) in those without prior arrhythmia, ventricular arrhythmia, or new indications for pacemaker or ICD implantation given their potential relationship with fibrosis. CHF events included heart failure hospitalization (non-elective admission for intravenous diuretics), ventricular assist device (VAD) placement, listing for heart transplantation, or cardiovascular death. Those with events were compared to those without.
2.7 Statistical Analysis
Study data were collected and managed using the REDCap electronic data capture tools.[12] Elevated ECV was defined as >1.96 SD above the mean for healthy controls according to gender to account for gender differences in healthy controls. [10, 13, 14] [10, 13, 14] [10, 13, 14] [10, 13, 14] 10, 13, 14 10 Appropriate comparisons were made using a two-tailed Student’s t-test, Mann-Whitney U test, chi-squared or Fisher’s exact test as appropriate, with P<0.05 considered statistically significant. Univariate correlation of ECV with other parameters was done using Pearson’s correlation coefficient. Natural log transformation of BNP was used to account for its non-normal distribution.
Kaplan-Meier survival curves were used to estimate event-free survival distributions based on a normal vs. elevated ECV, and distributions were compared using the Log-Rank test. Time zero was defined as the date of CMR study. For patients with multiple outcome events, the time to first event was used. Cox proportional hazards analyses were also conducted to test for interaction between ECV and other variables that differed between those with and without events. Because of the limited sample size, only bivariate analyses were used. Beta coefficients with 95% confidence intervals and p values were calculated for each significant variable. For all statistical tests, two-tailed p values <0.05 were considered statistically significant. Analyses were done using SPSS (version 22.0, IBM, Armonk, NY). Results are presented as mean±SD, median [interquartile range] for non-normal variables, or N (%) for categorical variables.
3. Results
A total of 53 TGA subjects were studied (age 34.6±10.3 years, 41% female, 10 L-loop TGA). Of all TGA subjects, 14 (26%) had moderate or severe tricuspid valve regurgitation, 18 (34%) had a history of prior atrial arrhythmia, one had diabetes and one had been treated for hypertension. There were 16 former smokers and 6 current smokers. None had known coronary atherosclerosis or prior myocardial infarction. Eleven were taking a beta-blocker, 15 an angiotensin converting enzyme inhibitor or angiotensin receptor blocker, and 14 a loop diuretic. In addition, 22 healthy controls (40.2±11.9 years, 41% female) were enrolled.
3.1 TGA vs. Control Subjects
Comparisons between TGA and controls are shown (Table 1). TGA subjects were younger (34.6±10.3 vs. 40.2±11.3 years, P=0.042) but did not differ by gender, heart rate, blood pressure, or oxygen saturation. Systemic ventricular EF was lower in TGA than controls (51.1±10.8% vs. 68.6±6.4%, P<0.001).
Table 1.
Transposition patients compared to healthy controls
| TGA (N=53) | Controls (N=22) | P | |
|---|---|---|---|
| Age (years) | 34.6 ± 10.3 | 40.2 ± 11.3 | 0.0422 |
| Female | 22 (42%) | 9 (41%) | 0.94 |
| Height (in) | 67.3 ± 4.1 | 66.8 ± 4.3 | 0.65 |
| Weight (kg) | 85.8 ± 17.0 | 72.5 ± 14.3 | 0.002 |
| BSA (m2) | 1.97 ± 0.21 | 1.83 ± 0.22 | 0.011 |
| Hemoglobin (g/dl) | 14.5 ± 1.16 | 14.1 ± 1.2 | 0.21 |
| Hematocrit (%) | 42.2 ± 3.1 | 41.4 ± 3.4 | 0.13 |
| Sodium (mmol/l) | 139 ± 2 | 139 ± 2 | 0.30 |
| BUN (mg/dl) | 14.2 ± 4.0 | 12.8 ± 4.8 | 0.19 |
| Creatinine (mg/dl) | 0.82 ± 0.16 | 0.90 ± 0.11 | 0.068 |
| 6-minute walk distance (m) | 480 ± 117 | 639 ± 79 | <0.001 |
| CMR Findings | |||
| Systemic EDVi (ml/m2) | 102 ± 27 | 80 ± 25 | <0.001 |
| Systemic ESVi (ml/m2) | 52 ± 25 | 25 ± 6 | <0.001 |
| Pulmonic EDVi (ml/m2) | 81 ± 22 | 88 ± 14 | 0.16 |
| Pulmonic ESVi (ml/m2) | 34 ± 16 | 34 ± 8 | 0.99 |
| RV ejection fraction (%) | 52% ± 11% | 69% ± 6% | <0.001 |
| LV ejection fraction (%) | 59% ± 9% | 62 ± 56 | <0.001 |
| 15 min post contrast T1 (ms) | 397 ± 110 | 508 ± 45 | <0.001 |
| Lambda | 0.495 ± 0.070 | 0.445 ± 0.041 | 0.002 |
| ECV (%) | 28.7% ± 4.5% | 26.1% ± 2.8% | 0.010 |
| Biomarker Analysis | |||
| b-type natriuretic peptide (pg/ml) | 94 [162] | 19.5 [18] | 0.012 |
| ln BNP | 4.69 ± 1.17 | 2.92 ± 0.61 | <0.001 |
| Aldosterone (pg/ml) | 179 ± 95 | 181 ± 67 | 0.94 |
| PC1NP (pg/l) | 53.6 ± 23.7 | 44.7 ± 16.6 | 0.11 |
| PC3NP (pg/l) | 4.4 ± 2.1 | 2.8 ± 0.6 | 0.001 |
| TIMP-1(ng/ml) | 146 ± 31 | 130 ± 27 | 0.033 |
| MMP-2 (ng/ml) | 244 ± 53 | 182 ± 28 | <0.001 |
| MMP2/TIMP1 ratio | 1.71 ± 0.35 | 1.48 ± 0.42 | 0.021 |
Values are given as N (%), mean±SD, or median [interquartile range]. LV = left ventricular, RV = right ventricular, ECV = extracellular volume fraction. Lambda is the partition coefficient of gadolinium. PC1NP = procollagen 1 N terminal peptide, PC3NP = procollagen 3 N terminal peptide, MMP-2 = matrix metalloproteinase 2, TIMP-1 = tissue inhibitor of matrix metalloproteinase-1.
ECV was 28.7±4.4% in the TGA subjects, and 26.1±2.8% in controls (P=0.0104). T1 values 15 minutes after contrast was shorter in TGA (397±110 vs. 508±45 ms, P<0.001). ECV was higher in women than men in controls (28.1± .3 vs. 24.0±1.62%, P<0.001) but not in TGA (28.9±2.8 vs. 28.4±5.2% respectively). ECV values by segment ranged from 29.4±4.8% in the anterior septum to 26.6±4.5% in the anterolateral free wall. There was no statistical difference between segments, including specifically no difference between the septum and free wall.
BNP was higher in TGA than controls (P<0.001), and correlated with ECV (R=0.44 for ln BNP, P<0.001). PC3NP was higher in TGA (4.4±2.1 vs. 2.8±0.6 μg/l, P=0.0015) but PC1NP was not statistically different (53.6±23.7 vs. 44.7±16.6 μg/l, P=0.11). MMP-2 was higher (243±54 vs. 182±28 ng/ml, P<0.001) as was TIMP-1 (146±31 vs. 130±27 ng/ml, P=0.033). The MMP/TIMP ratio was also higher than controls (1.71±0.35 in TGA vs. 1.48±0.42, P=0.021). Aldosterone did not differ.
3.2 Normal vs. Elevated ECV
Among the TGA subjects, 15 of 53 (28.3%, 95% CI 18% – 42%) had an elevated ECV value based on gender-specific cutoffs for the healthy controls (>28.9% for males and >31.4% for females). By univariate analysis for TGA subjects, ECV was associated with lnBNP (r=0.423 P<0.001) and weakly associated with systolic blood pressure (r=−0.302, P=0.025) but not associated with age, RV volumes, RVEF, RV mass index, mass/volume ratio, 6MWT distance, or collagen byproducts.
Comparisons of those with an elevated ECV to those with normal ECV are shown (Table 2). Those with elevated ECV did not differ by height, weight, smoking history, or resting heart rate. Those with an elevated ECV tended to be older though not significantly (38.1±5.4 vs. 33.3±11.5 years, P=0.13). There was no difference in RV volume or EF. BNP was higher in the elevated ECV group, which also showed a longer 6MWT distance (546±89 vs. 457±118 m, P=0.021). Frequency of medication use, smoking, and arrhythmia history were similar in both groups. Procollagen fragments, TIMP-1, MMP-2, and aldosterone were not different. Because others have previously reported difficulty measuring ECV in the systemic RV free wall,[5] we repeated our analysis with septum only, and found no change in the statistical significance of any comparison.
Table 2.
Comparisons between those with or without elevated ECV.
| Normal ECV (N=38) |
Elevated ECV (N=15) |
P | |
|---|---|---|---|
| Age (years) | 33.2 ± 11.5 | 38.1 ± 5.4 | 0.13 |
| Female gender | 19 (50%) | 3 (20%) | 0.046 |
| Current smoker | 2 (5%) | 3 (20%) | 0.09 |
| Smoker ever | 11 (29%) | 4 (27%) | 0.94 |
| Prior atrial arrhythmia | 11 (29%) | 7 (47%) | 0.22 |
| Prior heart surgery | 31 (82%) | 15 (100%) | 0.074 |
| Beta blocker | 8 (21%) | 3 (20%) | 0.93 |
| ACE/ARB | 10 (26%) | 5 (33%) | 0.32 |
| Spironolactone | 1 (3%) | 1 (7%) | 0.13 |
| Diuretic | 10 (26%) | 3 (20%) | 0.98 |
| QRS duration (ms) | 103 ± 30 | 93 ± 48 | 0.73 |
| L-loop TGA | 9 (24%) | 1 (7%) | 0.15 |
| Height (in) | 67.4 ± 4.6 | 68.0 ± 2.2 | 0.49 |
| Weight (kg) | 85.8 ± 17.9 | 85.1 ± 18.0 | 0.99 |
| BSA (m2) | 2.0 ± 0.2 | 2.0 ± 0.2 | 0.76 |
| Heart rate (bpm) | 70.2 ± 14.3 | 73.9 ± 15.1 | 0.41 |
| Systolic blood pressure (mmHg) | 123 ± 12 | 116.3 ± 7.9 | 0.022 |
| Diastolic blood pressure (mmHg) | 69 ± 10 | 66.5 ± 10.7 | 0.27 |
| Oxygen saturation (%) | 96.2 ± 2.3 | 96.6 ± 2.1 | 0.42 |
| Hemoglobin (g/dl) | 14.6 ± 1.1 | 14.2 ± 1.2 | 0.27 |
| Hematocrit (%) | 42.6 ± 3.2 | 40.9 ± 2.8 | 0.14 |
| Sodium (mmol/l) | 139 ± 2.3 | 137 ± 1.8 | 0.026 |
| BUN (mg/dl) | 13.8 ± 4.3 | 15.1 ± 3.0 | 0.29 |
| Creatinine (mg/dl) | 0.8 ± 0.2 | 0.8 ± 0.1 | 0.77 |
| 6-minute walk distance (m) | 457 ± 117 | 546 ± 89 | 0.021 |
| NYHA class | 1.7 ± 0.7 | 1.4 ± 0.7 | 0.93 |
| CMR Findings | |||
| RVEDVi (ml/m2) | 101 ± 27 | 106 ± 30 | 0.58 |
| RVESVi (ml/m2) | 51 ± 22 | 59 ± 90 | 0.34 |
| LVEDVi (ml/m2) | 78 ± 21 | 91 ± 26 | 0.12 |
| RV mass index (g/m2) | 89 ± 21 | 90 ± 14 | 0.69 |
| Mass/volume ratio | 0.91 ± 0.26 | 0.91 ± 0.17 | 0.95 |
| RVSV (ml/m2) | 49.2 ± 11.6 | 45.5 ± 9.8 | 0.29 |
| LVSV (ml/m2) | 47.9 ± 9.1 | 46.5 ± 11.6 | 0.64 |
| RV ejection fraction (%) | 52 ± 10 | 53 ± 15 | 0.38 |
| LV ejection fraction (%) | 62 ± 8 | 53 ± 9 | 0.006 |
| Tricuspid Regurg (mod-sev) | 7 (18%) | 7 (47%) | 0.036 |
| Pre contrast T1 (ms) | 949 ± 197 | 1001 ± 179 | 0.37 |
| 15 min post contrast T1 (ms) | 410 ± 112 | 365 ± 102 | 0.19 |
| lambda | 0.463 ± 0.045 | 0.575 ± 0.058 | <0.001 |
| ECV (%) | 26.6 ± 2.8 | 33.9 ± 3.2 | <0.001 |
| Biomarker Analysis | |||
| b-type natriuretic peptide (pg/ml) | 151.3 [173.7] | 456.5 [650.4] | 0.0 09 |
| ln BNP | 4.5 ± 1.1 | 5.3 ± 1.3 | 0.024 |
| Aldosterone (pg/ml) | 178 ± 94 | 181 ± 99 | 0.94 |
| PC1NP (pg/l) | 54.8 ± 21.6 | 50.9 ± 29.1 | 0.60 |
| PC3NP (pg/l) | 4.5 ± 1.1 | 4.1 ± 3.3 | 0.59 |
| TIMP-1(ng/ml) | 143 ± 31 | 152 ± 30 | 0.38 |
| MMP-2 (ng/ml) | 238 ± 51 | 258 ± 59 | 0.24 |
| MMP2/TIMP1 ratio | 1.7 ± 0.4 | 1.7 ± 0.3 | 0.94 |
Values are given as N (%), mean±SD, or median [interquartile range]. SBP = systolic blood pressure. DBP= diastolic blood pressure. LV = left ventricular, RV = right ventricular, ECV = extracellular volume fraction. SV=stroke volume. Lambda is the partition coefficient of gadolinium. PC1NP = procollagen 1 N terminal peptide, PC3NP = procollagen 3 N terminal peptide, MMP-2 = matrix metalloproteinase 2, TIMP-1 = tissue inhibitor of matrix metalloproteinase-1.
LGE anywhere in the heart was evident in 8 patients (15%, 2 with abnormal ECV and 6 with normal ECV, two with L-loop TGA, one of which had prior valve replacement), all in small quantities. No large transmural enhancement was seen. The presence of LGE did not differ in those with elevated ECV vs. normal ECV, nor correlate with ECV.
3.3 Follow-up Events
Median follow up time was 4.4 years, range 1.0 – 8.1 years. All but one subject was accounted for at least one year following the baseline visit. Fourteen patients had a major event, 6 (16%) in those with a normal ECV and 8 (53%) in those with an elevated ECV (P=0.006). Events in those with a normal ECV included 5 subjects with new arrhythmia event (three cardioversions, one with isolated symptomatic supraventricular tachycardia found during a 48-hour ambulatory electrocardiogram, and one required a pacemaker). One subject developed worsening heart failure leading to VAD placement followed by heart transplant. There were two non-cardiovascular deaths in this group which were not included as adverse events.
Among subjects with an elevated ECV, two experienced a new arrhythmia event alone (ablation for new atrial flutter in one and NSVT found on a Holter monitor leading to ICD implant in another). Six had a CHF event, all but two of whom also had a new atrial arrhythmia. These six included two subjects with multiple hospital admissions leading eventually to death from heart failure, two who have been referred and listed for transplant, one who has received a VAD and now awaits transplant, and one who was hospitalized for CHF necessitating diuresis. CHF events alone were more prevalent in the elevated ECV group (40% vs. 5%, p=0.002), while arrhythmia events alone were not statistically significant (33% vs. 14%, p=0.10).
Comparisons amongst those with vs. without events are shown (Table 3). Those with events differed by age, ECV, systolic blood pressure, RVESVi, RVEF, oxygen saturation after 6MWT, serum sodium, and ln BNP. Lambda and post contrast T1 were also different. Other variables did not differ. Outcome data did not differ when device implant was omitted as an outcome variable. By Kaplan-Meier analysis (Figure 1) using time to first event with abnormal ECV as the comparison factor, ECV was discriminatory (mean event-free survival 4.2±0.7 years vs. 6.5±0.5 years, median survival 3.7 vs. 5.9 years, log-rank statistic p=0.002).
Table 3.
Comparison of baseline variables between subject with or without a major outcome event.
| Variable | No Event N=38 |
Event N=14 |
P |
|---|---|---|---|
| N=38 | |||
| Age (years) | 32.7 ± 9.8 | 39.4 ± 10.7 | 0.038 |
| Female | 17 (45%) | 4 (29%) | 0.29 |
| ECV (%) | 27.6 ± 3.5 | 31.8 ± 5.3 | 0.002 |
| Elevated ECV | 7 (18%) | 8 (57%) | 0.005 |
| Lambda | 0.479 ± 0.056 | 0.543 ± 0.085 | 0.003 |
| Native T1 (ms) | 952 ± 183 | 984 ± 235 | 0.61 |
| Post contrast T1 (ms) | 425 ± 110 | 316 ± 67 | 0.001 |
| Hematocrit (%) | 42.3 ± 3.2 | 41.6 ± 3.1 | 0.49 |
| Congenitally-corrected TGA | 6 (16%) | 3 (21%) | 0.63 |
| Prior atrial arrhythmia | 11 (29%) | 6 (43%) | 0.34 |
| Beta blocker use | 9 (24%) | 2 (14%) | 0.46 |
| ACEi/ARB use | 9 (24%) | 6 (43%) | 0.18 |
| Aldosterone inhibitor use | 1 (3%) | 2 (14%) | 0.11 |
| Loop diuretic use | 8 (21%) | 5 (36%) | 0.28 |
| QRS duration (ms) | 99 ± 40 | 108 ± 11 | 0.57 |
| Baseline heart rate (bpm) | 69 ± 14 | 76 ± 16 | 0.12 |
| Baseline O2 saturation (%) | 97 ± 2 | 96 ± 3 | 0.29 |
| Systolic blood pressure (mmHg) | 122 ± 11 | 115 ± 10 | 0.051 |
| 6MWT post heart rate (bpm) | 105 ± 26 | 104 ± 22 | 0.89 |
| 6MWT post O2 saturation (%) | 96 ± 4 | 91 ± 5 | 0.007 |
| 6MWT distance (m) | 484 ± 120 | 471 ± 111 | 0.76 |
| Body surface area (m2) | 1.97 ± 0.22 | 1.96 ± 0.23 | 0.73 |
| RVEDVi (ml/m2) | 99 ± 25 | 112 ± 31 | 0.18 |
| RVESVi (ml/m2) | 47 ± 20 | 66 ± 32 | 0.013 |
| RVEF (%) | 54 ± 9 | 43 ± 11 | 0.002 |
| Moderate-severe TR | 9 (24%) | 5 (36%) | 0.39 |
| RV mass index (g/m2) | 88.1 ± 19.7 | 91.5 ± 19.6 | 0.62 |
| LGE present | 6 (16%) | 1 (7%) | 0.43 |
| Sodium (mmol/l) | 139 ± 2 | 137 ± 2 | 0.002 |
| Creatinine (mg/dl) | 0.83 ± 0.16 | 0.85 ± 0.13 | 0.53 |
| ln BNP | 4.48 ± 1.07 | 5.23 ± 1.34 | 0.047 |
Values are given as N (%), mean±SD, or median [interquartile range]. TGA = transposition of the great arteries. ECV = extracellular volume fraction. Lambda is the partition coefficient of gadolinium. ACEi= angiotensin converting enzyme inhibitor. ARB = angiotensin receptor blocker. 6MWT = six-minute walk test. RVEDVi = RV end-diastolic volume index, RVESVi = RV end-systolic volume index. BNP = b-type natriuretic peptide.
Figure 1.
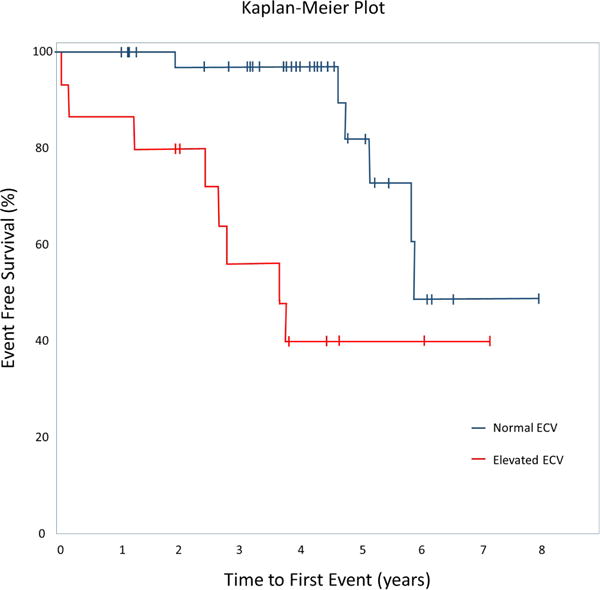
Kaplan-Meier Plot showing event-free survival curves for patients with a normal ECV vs. elevated ECV. Vertical bars indicate time of last follow up. P=0.001 log-rank statistic.
By Cox-Regression analysis (forward stepwise conditional) using only two comparators in each model, only ECV remained a significant predictor in comparisons with 6MWT post-walk saturation, systolic blood pressure, RVESVi and RVEF. In bivariate models with ECV and either age or lnBNP, both variables remained significant. Of these, ECV was the only variable that remained independently predictive in a trivariate model together with age and lnBNP (Beta coefficient ECV 1.4 (95%CI 1.16 – 1.68, p=0.001).
3.4 L-loop vs. D-loop TGA
ECV was not statistically different in L-loop TGA (N=10) vs. D-loop TGA (N=43). Of the L-loop subjects, five had prior surgeries; three for tricuspid valve replacement, one for VSD closure, and one with severe PS requiring an aortopulmonary shunt followed by VSD closure and an LV-PA conduit. The later subject had an elevated ECV, others did not. All D-TGA subjects had undergone an atrial switch palliation. In addition, one had also undergone a surgical septostomy, and one had undergone tricuspid valve replacement later in life, both of whom had a normal ECV.
To explore the impact of our decision to include L-loop transposition in the cohort, we repeated the analysis using only the 43 D-loop patients. Our findings were essentially unchanged. Significant variables in D-loop included higher ECV, longer 6MWT distance, larger RV volumes, lower RVEF, higher BNP and other biomarkers than controls. Significant variables in those with an elevated ECV included a higher 6MWT distance, higher BNP, and lower LVEF as well as a higher rate of events in follow up. D-loop subjects who experienced events (N=11) still had a higher ECV, higher NYHA class, lower 6MWT oxygen saturation, lower RVEF and RVESVi, and lower sodium.
4. Discussion
Elevated ECV, a measure of diffuse myocardial fibrosis, is associated with an increased risk of CHF and arrhythmia in TGA patients with a systemic RV. Despite a lack of association with other indicators of cardiovascular function, ECV appears to discriminate event-free survival in this cohort. Events in those with elevated ECV tended to occur early, and be more related to CHF, whereas events in the normal ECV group occurred later and were most often atrial arrhythmias. ECV was associated with serum BNP, another important predictor of health outcomes in ACHD.
The relationship of ECV to cardiovascular outcome is important since the mechanistic links between myocardial dysfunction and clinical heart failure in TGA have been elusive in prior research. Proposed predictors of outcome have included severe TR,[15, 16] atrial arrhythmia,[17] and BNP,[18] though not all studies concur. The largest long-term atrial switch follow-up study (60% of 468 patients survived after 30 years) found no outcome differences related to age, comorbid conditions, or arrhythmia.[19] There is lack of congruity between functional decline and change in EF or BNP.[20] In fact, self-assessment has been more predictive of clinical deterioration than objective parameters.[21]
Hence, although our findings need confirmation with larger populations, they are an important step towards understanding deterioration of myocardial function and clinical heart failure. The data are generally in harmony with others who reported an association between LGE and a composite clinical endpoint (mainly atrial arrhythmia).[22] Comparisons of the two studies are worth consideration. Their cohort similarly consisted of 55 subjects, though slightly younger (age 29 years), with a slightly lower RVESVi, (39 ml/m2), higher RVEF (59%), fewer atrial arrhythmias (N=12), and less moderate or severe TR (N=2). They were followed for a longer period (median 7.8 years), finding 19 atrial arrhythmias, 3 ventricular arrhythmias, 1 transplant and 1 cardiovascular death. Both studies show a link between myocardial fibrosis and adverse outcome. As well, both studies show the importance of atrial arrhythmia as a frequent antecedent event to CHF. LGE was found in 31 subjects (56%), compared to ECV elevation in 15 subjects (29%) in our study. This discrepancy could be due to the fact that LGE assessment typically covers the entire myocardium whereas ECV here was from a single short axis plane. Since ECV acquisitions took priority during post-contrast imaging in our study, LGE acquisitions lacked the uniformity and completeness needed for a comparison of LGE vs. ECV as event predictors. Still, the message of both studies is consistent; myocardial fibrosis is an important harbinger of adverse cardiovascular outcome in the systemic RV.
Considering ECV as a discriminator of those with future events, as our study suggests, it follows that pharmacotherapy targeted at myocardial fibrosis would be beneficial. Yet there is much uncertainty on the role of CHF pharmacotherapy for the systemic RV. Studies, though small, have repeatedly not shown favorable differences from standard CHF pharmacotherapy.[23–26] Beta-blockers may be protective of appropriate ICD shock in those with devices,[27] but there is as yet no demonstrated improvement in outcome from ICD implants,[27–30] despite being used in those with severely reduced systolic function who go on to eventual transplantation.[31] The use of ECV as an objective means to both detect and quantify fibrosis may be an important surrogate in studying this process further.
We found diffuse fibrosis in 28% of systemic RV patients and abnormal patterns of collagen turnover, consistent with other studies.[5, 23],[32] Yet this percentage of affected individuals is lower than that suggested by LGE studies. Some, but not all, previous studies in comparably-sized cohorts have shown LGE in 40-60% of systemic RV patients.[1, 2, 22, 33] Diffuse fibrosis is typically more abundant than that suggested by LGE.[3, 32] LGE in TGA has been found to be associated with age, QRS duration, EF, prior arrhythmia and/or syncope,[1] reduced exercise capacity,[2] and ventricular dyssychrony.[33] However such correlations with ECV were not found.
Several possibilities for the lower fibrosis prevalence could be considered. Foremost, we studied a single representative myocardial plane, not the entire myocardium. Most LGE studies evaluate the entire heart, giving additional sensitivity, which would have been impractical with our methods; the Look-Locker sequences yield more phases but are time consuming to analyze. Yet LGE findings vary considerably between publications; some show LGE in only 0-5% of systemic RVs despite poor systolic function,[5, 34, 35] in deference to 40-60% in others. [1],[2],[33] The range could reflect either methodologic or cohort differences between publications. Also, since histologically fibrosis has been shown to be diffuse in the systemic RV,[32] our ECV findings may not have differed if additional planes were included. Cohorts may differ in severity. For example, in our previous study the 12 TGA patients referred for CMR had a lower RVEF (46±11% compared to 54±11% in the present study, P=0.036) despite a comparable age distribution.[3] Yet the present cohort had a significant number of adverse events including death/transplant in 4 years of follow up, so it is hard to explain our lower prevalence based on inclusion of a healthier cohort alone.
Our findings harmonize well with the first investigation of ECV in TGA. [5] Using different methods in 14 TGA adults, these authors reported that ECV of the septum was higher than controls and correlated with BNP but not with RVEF or RV volumes, which our data confirm. Also similar, none of the subjects had demonstrable LGE. In light of their findings, we reanalyzed our data using only ECV of the septum, and our results were unchanged.
Considering the bulk of evidence thus far reported, fibrosis consistently appears to be a plausible indicator of a failing systemic RV, despite the lack of association between ECV and ventricular size or function. The later may simply indicate that fibrosis in this context evolves independently of ventricular remodeling. Furthermore, measuring the RV can be a challenge, even with CMR, which may explain why systemic RV EF has been insensitive as a marker of clinical deterioration in this and other studies. Global longitudinal strain (GLS)[36, 37] may be more sensitive to differences in those with fibrosis, but we did not measure this in our cohort. Although prior cardiopulmonary bypass at the time of surgery has been theorized as a potential cause of myocardial damage, prior surgeries, in either our D- or L-loop subjects, did not appear to have a relationship with detectable fibrosis.
Limitations
It is also difficult to define “normal” for a systemic RV. By necessity we used healthy controls with a systemic LV to define cut points for ECV elevation, but acknowledge the inherent limitations of this application. Our necessary exclusion of pacemakers may be relevant, as pacing is associated with poorer long-term survival.[19, 38] There are unique features of L-loop TGA vs. D-loop TGA[39] that we did not explore given the cohort size, though we did not find significant differences in our analysis even when L-loop subjects were excluded. Different methodologies for T1 quantification exist,[40, 41] yet our T1 values were similar to other published data both in patients and healthy controls.[8, 42, 43] We used identical methodology in tetralogy of Fallot with significant associations.[10] We acknowledge this is a CMR-derived measure of fibrosis. Histologic validation was impractical in our study, though has been done by others.[44]
As is common in TGA, our sample size limits statistical power; a larger cohort may be able to unmask differences that we could not demonstrate. T1 may be altered in acute necrosis or inflammatory states,[45] but such states are not likely in these chronic patients. We paradoxically found a higher 6MWT in those with higher ECV, even after excluding outliers. Given the limited dataset, it is difficult to speculate on the significance of this finding; whether it represents a sampling error or whether there could be some functional advantage to a degree of RV fibrosis. We did not perform cardiopulmonary exercise testing, which may have been more sensitive than 6MWT to a relationship between fibrosis and functional capacity.[22] Finally, our use of bivariate regression, mandated by our sample size, is less robust than multivariate models that would be performed in a larger cohort. Therefore our data provide less certainty that ECV is independent.
Conclusions
TGA patients with a systemic RV showed CMR evidence of diffuse myocardial fibrosis and altered collagen metabolism. ECV identified patients at high risk for arrhythmia or heart failure. This confirms the role of fibrosis in the gradual development of clinical heart failure, presenting a potential target for therapeutic study.
Highlights.
Patients with a systemic right ventricle show CMR evidence of diffuse myocardial fibrosis.
Fibrosis as shown by CMR is not associated with other findings associated with myocardial dysfunction.
Systemic RV patients with CMR evidence of fibrosis have more cardiovascular events in 4 years.
Acknowledgments
The study was funded by a K23 Career-Development Grant from the National Heart, Lung, and Blood Institute (K23HL093024), and in part by a grant from the American Heart Association (Western States Affiliate), and the Oregon Clinical and Translational Research Institute (OCTRI) funded by the National Center for Advancing Translational Sciences (NCAT) through a Clinical and Translational Science Award (UL1TR000128). Special thanks to Sarah Egan, Carrie Farrar, Lissy Powell, Kelsey Hickey and Rebecca Duby for their work in research coordination and study administration.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
Authors have no relevant financial relationships to disclose regarding this work. All authors assume responsibility for the data integrity and have reviewed and agree to the manuscript as written.
References
- 1.Babu-Narayan SV, Goktekin O, Moon JC, Broberg CS, Pantely GA, Pennell DJ, et al. Late gadolinium enhancement cardiovascular magnetic resonance of the systemic right ventricle in adults with previous atrial redirection surgery for transposition of the great arteries. Circulation. 2005;111:2091–8. doi: 10.1161/01.CIR.0000162463.61626.3B. [DOI] [PubMed] [Google Scholar]
- 2.Giardini A, Lovato L, Donti A, Formigari R, Oppido G, Gargiulo G, et al. Relation between right ventricular structural alterations and markers of adverse clinical outcome in adults with systemic right ventricle and either congenital complete (after Senning operation) or congenitally corrected transposition of the great arteries. Am J Cardiol. 2006;98:1277–82. doi: 10.1016/j.amjcard.2006.05.062. [DOI] [PubMed] [Google Scholar]
- 3.Broberg CS, Chugh SS, Conklin C, Sahn DJ, Jerosch-Herold M. Quantification of diffuse myocardial fibrosis and its association with myocardial dysfunction in congenital heart disease. Circ Cardiovasc Imaging. 2010;3:727–34. doi: 10.1161/CIRCIMAGING.108.842096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dusenbery SM, Jerosch-Herold M, Rickers C, Colan SD, Geva T, Newburger JW, et al. Myocardial extracellular remodeling is associated with ventricular diastolic dysfunction in children and young adults with congenital aortic stenosis. J Am Coll Cardiol. 2014;63:1778–85. doi: 10.1016/j.jacc.2013.11.066. [DOI] [PubMed] [Google Scholar]
- 5.Plymen CM, Sado DM, Taylor AM, Bolger AP, Lambiase PD, Hughes M, et al. Diffuse myocardial fibrosis in the systemic right ventricle of patients late after Mustard or Senning surgery: an equilibrium contrast cardiovascular magnetic resonance study. European heart journal cardiovascular Imaging. 2013;14:963–8. doi: 10.1093/ehjci/jet014. [DOI] [PubMed] [Google Scholar]
- 6.Chen CA, Dusenbery SM, Valente AM, Powell AJ, Geva T. Myocardial ECV Fraction Assessed by CMR Is Associated With Type of Hemodynamic Load and Arrhythmia in Repaired Tetralogy of Fallot. JACC Cardiovascular imaging. 2016;9:1–10. doi: 10.1016/j.jcmg.2015.09.011. [DOI] [PubMed] [Google Scholar]
- 7.Riesenkampff E, Messroghli DR, Redington AN, Grosse-Wortmann L. Myocardial t1 mapping in pediatric and congenital heart disease. Circ Cardiovasc Imaging. 2015;8:e002504. doi: 10.1161/CIRCIMAGING.114.002504. [DOI] [PubMed] [Google Scholar]
- 8.Kozak MF, Redington A, Yoo SJ, Seed M, Greiser A, Grosse-Wortmann L. Diffuse myocardial fibrosis following tetralogy of Fallot repair: a T1 mapping cardiac magnetic resonance study. Pediatric radiology. 2014;44:403–9. doi: 10.1007/s00247-013-2840-9. [DOI] [PubMed] [Google Scholar]
- 9.Riesenkampff E, Luining W, Seed M, Chungsomprasong P, Manlhiot C, Elders B, et al. Increased left ventricular myocardial extracellular volume is associated with longer cardiopulmonary bypass times, biventricular enlargement and reduced exercise tolerance in children after repair of Tetralogy of Fallot. J Cardiovasc Magn Reson. 2016;18:75. doi: 10.1186/s12968-016-0290-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Broberg CS, Huang J, Hogberg I, McLarry J, Woods P, Burchill LJ, et al. Diffuse LV Myocardial Fibrosis and its Clinical Associations in Adults With Repaired Tetralogy of Fallot. JACC Cardiovascular imaging. 2016;9:86–7. doi: 10.1016/j.jcmg.2015.10.006. [DOI] [PubMed] [Google Scholar]
- 11.Flacke SJ, Fischer SE, Lorenz CH. Measurement of the gadopentetate dimeglumine partition coefficient in human myocardium in vivo: normal distribution and elevation in acute and chronic infarction. Radiology. 2001;218:703–10. doi: 10.1148/radiology.218.3.r01fe18703. [DOI] [PubMed] [Google Scholar]
- 12.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)–a metadata-driven methodology and workflow process for providing translational research informatics support. Journal of biomedical informatics. 2009;42:377–81. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sarikouch S, Koerperich H, Dubowy KO, Boethig D, Boettler P, Mir TS, et al. Impact of gender and age on cardiovascular function late after repair of tetralogy of Fallot: percentiles based on cardiac magnetic resonance. Circ Cardiovasc Imaging. 2011;4:703–11. doi: 10.1161/CIRCIMAGING.111.963637. [DOI] [PubMed] [Google Scholar]
- 14.Sado DM, Flett AS, Banypersad SM, White SK, Maestrini V, Quarta G, et al. Cardiovascular magnetic resonance measurement of myocardial extracellular volume in health and disease. Heart. 2012;98:1436–41. doi: 10.1136/heartjnl-2012-302346. [DOI] [PubMed] [Google Scholar]
- 15.Carrel T, Pfammatter JP. Complete transposition of the great arteries: surgical concepts for patients with systemic right ventricular failure following intraatrial repair. Thorac Cardiovasc Surg. 2000;48:224–7. doi: 10.1055/s-2000-6894. [DOI] [PubMed] [Google Scholar]
- 16.Graham TP, Jr, Bernard YD, Mellen BG, Celermajer D, Baumgartner H, Cetta F, et al. Long-term outcome in congenitally corrected transposition of the great arteries: a multi-institutional study. J Am Coll Cardiol. 2000;36:255–61. doi: 10.1016/s0735-1097(00)00682-3. [DOI] [PubMed] [Google Scholar]
- 17.Wheeler M, Grigg L, Zentner D. Can we predict sudden cardiac death in long-term survivors of atrial switch surgery for transposition of the great arteries? Congenit Heart Dis. 2014;9:326–32. doi: 10.1111/chd.12145. [DOI] [PubMed] [Google Scholar]
- 18.Haberger S, Hauser M, Braun SL, Schuster T, Ewert P, Nagdyman N, et al. Prognostic Value of Plasma B-Type Natriuretic Peptide in the Long-Term Follow-up of Patients With Transposition of the Great Arteries With Morphologic Right Systemic Ventricle After Atrial Switch Operation. Circ J. 2015;79:2677–81. doi: 10.1253/circj.CJ-15-0348. [DOI] [PubMed] [Google Scholar]
- 19.Vejlstrup N, Sorensen K, Mattsson E, Thilen U, Kvidal P, Johansson B, et al. Long-Term Outcome of Mustard/Senning Correction for Transposition of the Great Arteries in Sweden and Denmark. Circulation. 2015;132:633–8. doi: 10.1161/CIRCULATIONAHA.114.010770. [DOI] [PubMed] [Google Scholar]
- 20.Roentgen P, Kaan M, Tutarel O, Meyer GP, Westhoff-Bleck M. Declining cardiopulmonary exercise capacity is not associated with worsening systolic systemic ventricular dysfunction in adults with transposition of great arteries after atrial switch operation. Congenit Heart Dis. 2014;9:259–65. doi: 10.1111/chd.12137. [DOI] [PubMed] [Google Scholar]
- 21.Book W, McConnell M, Oster M, Lyle T, Kogon B. Predicting functional capacity in patients with a systemic right ventricle: subjective patient self-assessment is better than B-type natriuretic peptide levels and right ventricular systolic function. Congenit Heart Dis. 2013;8:550–5. doi: 10.1111/chd.12039. [DOI] [PubMed] [Google Scholar]
- 22.Rydman R, Gatzoulis MA, Ho SY, Ernst S, Swan L, Li W, et al. Systemic right ventricular fibrosis detected by cardiovascular magnetic resonance is associated with clinical outcome, mainly new-onset atrial arrhythmia, in patients after atrial redirection surgery for transposition of the great arteries. Circ Cardiovasc Imaging. 2015;8 doi: 10.1161/CIRCIMAGING.114.002628. [DOI] [PubMed] [Google Scholar]
- 23.Dos L, Pujadas S, Estruch M, Mas A, Ferreira-Gonzalez I, Pijuan A, et al. Eplerenone in systemic right ventricle: double blind randomized clinical trial. The evedes study. Int J Cardiol. 2013;168:5167–73. doi: 10.1016/j.ijcard.2013.07.163. [DOI] [PubMed] [Google Scholar]
- 24.Doughan AR, McConnell ME, Book WM. Effect of beta blockers (carvedilol or metoprolol XL) in patients with transposition of great arteries and dysfunction of the systemic right ventricle. Am J Cardiol. 2007;99:704–6. doi: 10.1016/j.amjcard.2006.10.025. [DOI] [PubMed] [Google Scholar]
- 25.Dore A, Houde C, Chan KL, Ducharme A, Khairy P, Juneau M, et al. Angiotensin receptor blockade and exercise capacity in adults with systemic right ventricles: a multicenter, randomized, placebo-controlled clinical trial. Circulation. 2005;112:2411–6. doi: 10.1161/CIRCULATIONAHA.105.543470. [DOI] [PubMed] [Google Scholar]
- 26.van der Bom T, Winter MM, Bouma BJ, Groenink M, Vliegen HW, Pieper PG, et al. Effect of valsartan on systemic right ventricular function: a double-blind, randomized, placebo-controlled pilot trial. Circulation. 2013;127:322–30. doi: 10.1161/CIRCULATIONAHA.112.135392. [DOI] [PubMed] [Google Scholar]
- 27.Khairy P, Harris L, Landzberg MJ, Fernandes SM, Barlow A, Mercier LA, et al. Sudden death and defibrillators in transposition of the great arteries with intra-atrial baffles: a multicenter study. Circulation Arrhythmia and electrophysiology. 2008;1:250–7. doi: 10.1161/CIRCEP.108.776120. [DOI] [PubMed] [Google Scholar]
- 28.Buber J, Ackley TJ, Daniels CJ, Roble SL, Mah ML, Kamp AN, et al. Outcomes following the implantation of cardioverter-defibrillator for primary prevention in transposition of the great arteries after intra-atrial baffle repair: a single-centre experience. Europace. 2016;18:1016–22. doi: 10.1093/europace/euv297. [DOI] [PubMed] [Google Scholar]
- 29.Backhoff D, Kerst G, Peters A, Ludemann M, Frische C, Horndasch M, et al. Internal Cardioverter Defibrillator Indications and Therapies after Atrial Baffle Procedure for d-Transposition of the Great Arteries: A Multicenter Analysis. Pacing Clin Electrophysiol. 2016;39:1070–6. doi: 10.1111/pace.12933. [DOI] [PubMed] [Google Scholar]
- 30.Backhoff D, Muller M, Ruschewski W, Paul T, Krause U. ICD therapy for primary prevention of sudden cardiac death after Mustard repair for d-transposition of the great arteries. Clinical research in cardiology : official journal of the German Cardiac Society. 2014;103:894–901. doi: 10.1007/s00392-014-0727-x. [DOI] [PubMed] [Google Scholar]
- 31.Bouzeman A, Marijon E, de Guillebon M, Ladouceur M, Duthoit G, Amet D, et al. Implantable cardiac defibrillator among adults with transposition of the great arteries and atrial switch operation: case series and review of literature. Int J Cardiol. 2014;177:301–6. doi: 10.1016/j.ijcard.2014.09.015. [DOI] [PubMed] [Google Scholar]
- 32.Ladouceur M, Bruneval P, Mousseaux E. Cardiovascular flashlight. Magnetic resonance assessment of fibrosis in systemic right ventricle after atrial switch procedure. Eur Heart J. 2009;30:2613. doi: 10.1093/eurheartj/ehp336. [DOI] [PubMed] [Google Scholar]
- 33.Babu-Narayan SV, Prati D, Rydman R, Dimopoulos K, Diller GP, Uebing A, et al. Dyssynchrony and electromechanical delay are associated with focal fibrosis in the systemic right ventricle – Insights from echocardiography. Int J Cardiol. 2016;220:382–8. doi: 10.1016/j.ijcard.2016.06.090. [DOI] [PubMed] [Google Scholar]
- 34.Preim U, Hoffmann J, Lehmkuhl L, Kehrmann J, Riese F, Daehnert I, et al. Systemic right ventricles rarely show myocardial scars in cardiac magnetic resonance delayed-enhancement imaging. Clinical research in cardiology : official journal of the German Cardiac Society. 2013;102:337–44. doi: 10.1007/s00392-013-0539-4. [DOI] [PubMed] [Google Scholar]
- 35.Fratz S, Hauser M, Bengel FM, Hager A, Kaemmerer H, Schwaiger M, et al. Myocardial scars determined by delayed-enhancement magnetic resonance imaging and positron emission tomography are not common in right ventricles with systemic function in long-term follow up. Heart. 2006;92:1673–7. doi: 10.1136/hrt.2005.086579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tutarel O, Orwat S, Radke RM, Westhoff-Bleck M, Vossler C, Schulke C, et al. Assessment of myocardial function using MRI-based feature tracking in adults after atrial repair of transposition of the great arteries: Reference values and clinical utility. Int J Cardiol. 2016;220:246–50. doi: 10.1016/j.ijcard.2016.06.108. [DOI] [PubMed] [Google Scholar]
- 37.Thattaliyath BD, Forsha DE, Stewart C, Barker PC, Campbell MJ. Evaluation of Right Ventricular Myocardial Mechanics Using Velocity Vector Imaging of Cardiac MRI Cine Images in Transposition of the Great Arteries Following Atrial and Arterial Switch Operations. Congenit Heart Dis. 2015;10:371–9. doi: 10.1111/chd.12250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hofferberth SC, Alexander ME, Mah DY, Bautista-Hernandez V, del Nido PJ, Fynn-Thompson F. Impact of pacing on systemic ventricular function in L-transposition of the great arteries. J Thorac Cardiovasc Surg. 2016;151:131–8. doi: 10.1016/j.jtcvs.2015.08.064. [DOI] [PubMed] [Google Scholar]
- 39.Grothoff M, Fleischer A, Abdul-Khaliq H, Hoffmann J, Lehmkuhl L, Luecke C, et al. The systemic right ventricle in congenitally corrected transposition of the great arteries is different from the right ventricle in dextro-transposition after atrial switch: a cardiac magnetic resonance study. Cardiology in the young. 2013;23:239–47. doi: 10.1017/S1047951112000790. [DOI] [PubMed] [Google Scholar]
- 40.Kellman P, Wilson JR, Xue H, Ugander M, Arai AE. Extracellular volume fraction mapping in the myocardium, part 1: evaluation of an automated method. J Cardiovasc Magn Reson. 2012;14:63. doi: 10.1186/1532-429X-14-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Flett AS, Hayward MP, Ashworth MT, Hansen MS, Taylor AM, Elliott PM, et al. Equilibrium contrast cardiovascular magnetic resonance for the measurement of diffuse myocardial fibrosis: preliminary validation in humans. Circulation. 2010;122:138–44. doi: 10.1161/CIRCULATIONAHA.109.930636. [DOI] [PubMed] [Google Scholar]
- 42.Wong TC, Piehler K, Meier CG, Testa SM, Klock AM, Aneizi AA, et al. Association between extracellular matrix expansion quantified by cardiovascular magnetic resonance and short-term mortality. Circulation. 2012;126:1206–16. doi: 10.1161/CIRCULATIONAHA.111.089409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lee JJ, Liu S, Nacif MS, Ugander M, Han J, Kawel N, et al. Myocardial T1 and extracellular volume fraction mapping at 3 tesla. J Cardiovasc Magn Reson. 2011;13:75. doi: 10.1186/1532-429X-13-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.de Meester de Ravenstein C, Bouzin C, Lazam S, Boulif J, Amzulescu M, Melchior J, et al. Histological Validation of measurement of diffuse interstitial myocardial fibrosis by myocardial extravascular volume fraction from Modified Look-Locker imaging (MOLLI) T1 mapping at 3 T. J Cardiovasc Magn Reson. 2015;17:015–0150. doi: 10.1186/s12968-015-0150-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lurz JA, Luecke C, Lang D, Besler C, Rommel KP, Klingel K, et al. CMR-Derived Extracellular Volume Fraction as a Marker for Myocardial Fibrosis: The Importance of Coexisting Myocardial Inflammation. JACC Cardiovascular imaging. 2017 doi: 10.1016/j.jcmg.2017.01.025. [DOI] [PubMed] [Google Scholar]


