Summary
Although stable intronic sequence RNAs (sisRNAs) are conserved in plants and animals, their functional significance is still unclear. We identify a pool of polyadenylated maternally deposited sisRNAs in Drosophila melanogaster. These sisRNAs can be generated by independent transcription from the cognate introns. The ovary-specific poly(A) polymerase Wispy mediates the polyadenylation of maternal sisRNAs and confers their stability as maternal transcripts. A developmentally regulated sisRNA sisR-3 represses the expression of a long noncoding RNA CR44148 and is required during development. Our results expand the pool of sisRNAs and suggest that sisRNAs perform regulatory functions during development in Drosophila.
Subject Areas: Molecular Biology, Molecular Mechanism of Gene Regulation, Developmental Biology, Transcriptomics
Graphical Abstract
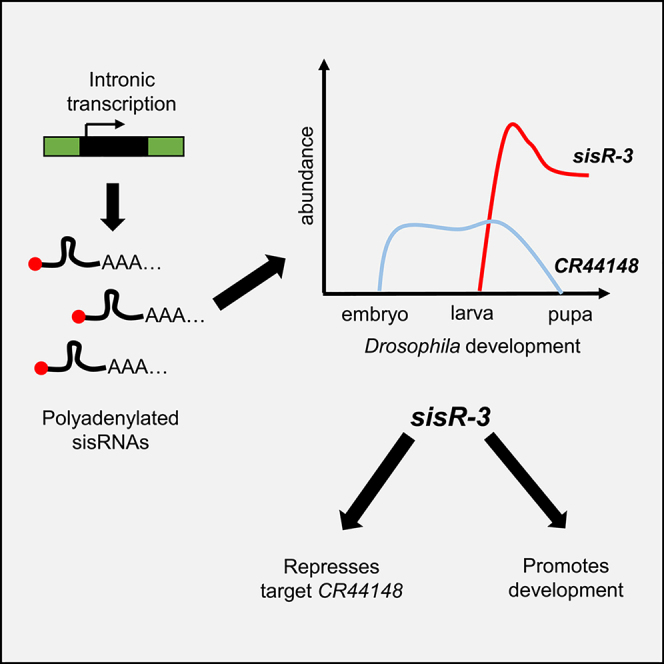
Highlights
-
•
Identification of polyadenylated sisRNAs
-
•
sisRNAs can be produced from independent transcription
-
•
sisR-3 regulates a long noncoding RNA
-
•
sisR-3 is required during development
Molecular Biology; Molecular Mechanism of Gene Regulation; Developmental Biology; Transcriptomics
Introduction
Noncoding RNAs (ncRNAs) have a profound impact on gene expression (Cech and Steitz, 2014, Ghildiyal and Zamore, 2009, Kung et al., 2013, Lee, 2012, Matera et al., 2007, Rinn and Chang, 2012). The identification of stable intronic sequence RNAs (sisRNAs) in the oocyte nucleus of Xenopus tropicalis raised the important question on the biological functions of this class of ncRNAs (Gardner et al., 2012). Although sisRNAs had been discovered in humans, mice, Xenopus, Drosophila, yeasts, and viruses, very little is known about their importance during development (Gardner et al., 2012, Lu et al., 2015, Moss and Steitz, 2013, Osman et al., 2016, Pek, 2018, Pek and Okamura, 2015, Pek et al., 2015, Talhouarne and Gall, 2014, Yin et al., 2012, Zhang et al., 2013, Zheng et al., 2015). Drosophila sisR-1 has been shown to repress a long ncRNA ASTR during embryonic development and regulate stem cell homeostasis (Pek et al., 2015, Wong et al., 2017). In addition, maternally deposited circular sisR-4 is important for embryonic development by promoting its parental gene transcription in Drosophila (Tay and Pek, 2017).
In our continuing effort to identify and characterize more sisRNAs, we performed deep sequencing of polyadenylated and non-polyadenylated maternally deposited sisRNAs in the unfertilized eggs of Drosophila. Unexpectedly, we found abundant polyadenylated sisRNAs that are produced by independent transcription from the introns, suggesting an alternative pathway for sisRNA biogenesis. Further analyses on a sisRNA sisR-3 revealed that sisR-3 represses a long ncRNA CR44148 and is essential for proper development.
Results
Deep Sequencing Identifies Polyadenylated sisRNAs
In Xenopus and Drosophila, maternally deposited sisRNAs are thought to be by-products of splicing (Gardner et al., 2012, Osman et al., 2016, Pek, 2018, Pek et al., 2015, Talhouarne and Gall, 2014, Tay and Pek, 2017). It is unknown how linear sisRNAs are conferred with unusual stability in the oocytes (Gardner et al., 2012, Osman et al., 2016, Pek, 2018, Pek et al., 2015). To identify more sisRNAs, we examined Drosophila melanogaster unfertilized eggs, which contain a maternal pool of stable and mature RNAs with no contamination from zygotic transcription (Pek et al., 2015). We performed strand-specific deep sequencing of (1) ribosomal RNA (rRNA)-depleted total RNA, (2) poly(A)+ RNA, and (3) rRNA-depleted poly(A)− RNA (Figure 1A). As a positive control for poly(A)− RNA, we detected the U85 small Cajal body-specific RNA (scaRNA) in the poly(A)− fraction but not in the poly(A)+ fraction (Figure S1A). Conversely, we detected exonic sequences from the messenger RNAs (mRNAs) in the poly(A)+ fraction but not in the poly(A)− sequences (Figures 1B, S1B, and S1C). These observations confirmed the validity of our poly(A)+ and poly(A)− sequencing experiments.
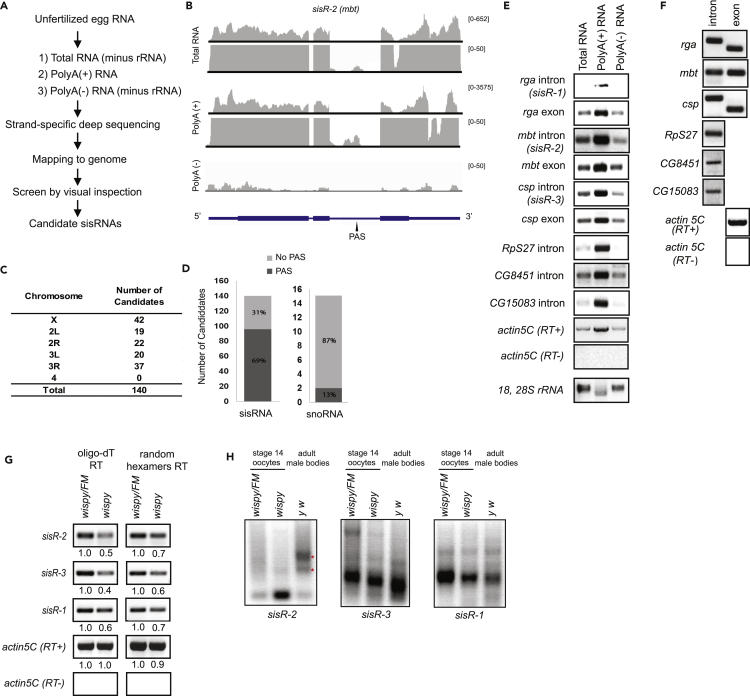
Figure 1.
Deep Sequencing Identifies a Pool of Polyadenylated sisRNAs in Unfertilized Eggs
(A) A schematic for identifying candidate sisRNAs in unfertilized eggs.
(B) Genome browser views of sisR-2 (mbt) gene locus. RNA sequencing results for total RNA, poly(A)+ RNA, and poly(A)− RNA from unfertilized eggs are shown. PAS, polyadenylation signal near the predicted 3′ ends of the sisRNAs.
(C) Table showing the number of candidate polyadenylated sisRNAs distributed over the chromosomes in Drosophila.
(D) Charts showing the numbers and percentages of candidate sisRNAs and snoRNAs with and without PAS.
(E) RT-PCR showing the presence of various intronic and exonic sequences in total, poly(A)+, and poly(A)− RNAs from unfertilized eggs.
(F) RT-PCR showing the presence of intronic and exonic sequences reverse transcribed by oligo-dT in unfertilized eggs.
(G) Semi-quantitative RT-PCR showing the abundance of sisR-1, sisR-2, and sisR-3 in stage 14 oocytes of wispy/FM versus wispy homozygous mutants using oligo-dT or random hexamers during reverse transcription. Numbers below indicate the relative band intensities of respective sisRNAs quantified using ImageJ software.
(H) RACE-PAT assay showing the relative lengths of sisRNA poly(A) tails in wispy/FM, wispy homozygous mutant stage 14 oocytes, and wild-type adult male body long poly(A) tails of sisR-2, red asterisks.
We previously identified three sisRNAs, sisR-1, sisR-2, and sisR-3, from the regena (rga), mushroom body tiny (mbt), and cysteine string protein (csp) loci, respectively, by northern blotting (Pek et al., 2015). Unexpectedly, we found that these three sisRNAs were present in the poly(A)+ fraction instead of the poly(A)− fraction (Figures 1B, S1B, and S1C), suggesting that some sisRNAs may be polyadenylated. Cleavage and polyadenylation requires the polyadenylation signal (PAS) (AUUAAA, AAUAAA, AUAAAA), which functions to recruit the cleavage and polyadenylation specificity factor (CPSF) to mediate the addition of poly(A) tails (Shi and Manley, 2015). PAS sequences were present upstream of the predicted 3′ ends of these sisRNAs (Figures 1B, S1B, and S1C). By visual inspection of the sequencing data on the genome browser, we identified a total of 140 candidate polyadenylated sisRNAs that mapped to chromosomes 1–3 (Figure 1C and Table S1). Of the 140 candidate sisRNAs, 96 (∼69%) have at least a PAS sequence, compared with 13% of the non-polyadenylated small nucleolar RNAs (snoRNAs) (Figure 1D and Table S1).
To verify whether sisRNAs are polyadenylated, we obtained RNA from unfertilized eggs and isolated poly(A)+ and poly(A)− fractions using oligo-dT beads and performed reverse-transcriptase polymerase chain reaction (RT-PCR). Consistent with our deep sequencing data, we detected an enrichment of sisRNAs in the poly(A)+ fraction compared with the poly(A)− fraction (Figure 1E). We finally confirmed the presence of polyadenylated sisRNAs in unfertilized eggs by performing RT-PCR using oligo-dT as the primer for reverse transcription (Figure 1F).
In Drosophila, the ovary-specific poly(A) polymerase (PAP) encoded by the wispy locus has been shown to polyadenylate maternal mRNAs and adenylate maternal microRNAs (miRNAs) in the cytoplasm during late oogenesis (Benoit et al., 2008, Lee et al., 2014). We asked whether wispy is required for polyadenylation and stability of maternal sisRNAs in the oocytes. Females homozygous for wispy12-3147 laid very few eggs, so we examined RNAs from the stage 14 oocytes, which also store mature maternal RNAs. By performing reverse transcription using oligo-dT followed by PCR, we found down-regulation of poly(A)-tail-containing sisRNAs in wispy mutants compared with controls (Figure 1G), suggesting that wispy is required for the polyadenylation of maternal sisRNAs. We next examined the entire population of sisRNAs by doing reverse transcription using random hexamers followed by PCR. We also observed a decrease in sisRNA abundance in wispy mutants (Figure 1G), indicating that polyadenylation promotes the stability of maternal sisRNAs.
We further examined the lengths of poly(A) tails of sisR-1, sisR-2, and sisR-3 by performing Rapid amplification of cDNA ends-PCR poly(A) test (RACE-PAT) assay. We examined RNA from stage 14 oocytes in wispy/FM and wispy mutants. In addition, RNA from adult male bodies (which do not express Wispy) was also included as a comparison for polyadenylation in somatic cells. In control stage 14 oocytes, the poly(A) tails of sisR-2 were long and heterogeneous, but they were dramatically short in wispy mutants (Figure 1H). Interestingly, in adult male bodies, the poly(A) tails of sisR-2 were long (Figure 1H, red asterisks), suggesting wispy-independent polyadenylation in somatic cells. For sisR-3 and sisR-1, the lengths of poly(A) tails were also generally shorter in wispy mutants (Figure 1H). Unlike sisR-2, the poly(A) tails of these two sisRNAs remained short in adult male bodies (Figure 1H). Taken together, our data suggest that wispy is required for the cytoplasmic polyadenylation of maternal sisRNAs to confer their stability. We do not exclude the possibility that sisRNAs are also polyadenylated by a nuclear PAP (Juge et al., 2002).
Production of sisRNAs by Independent Transcription
To examine the nature of these sisRNAs in detail, in addition to previously cloned sisR-1, we cloned two previously identified sisRNAs sisR-2 and sisR-3 from the mbt and csp loci, respectively (Pek et al., 2015). By performing 5′ and 3′ RACE analyses, we obtained full-length sequences of sisR-2 and sisR-3 in the unfertilized eggs. As predicted from the deep sequencing data, the PAS sequences are near the 3′ ends of all the three sisRNAs (Figures 1B, 2A, S1B, S1C, and S2, data not shown). Furthermore, the 5′ ends of the sisRNAs lie close to the 5′ splice sites of the introns (Figures 2A and S2, data not shown). These observations prompted us to examine the possibility that sisRNAs may be transcribed independently from the cognate introns. We first tested if sisRNAs possess an m7G cap, a 5′ end modification common to RNA polymerase II transcripts. Using an antibody that recognizes the m7G cap, we were able to immunoprecipitate sisRNAs from unfertilized eggs (Figure 2B), indicating that sisRNAs have 5′ m7G caps. As a positive control, actin5C mRNA was enriched in the immunoprecipitates but not for U85 intronic scaRNA.
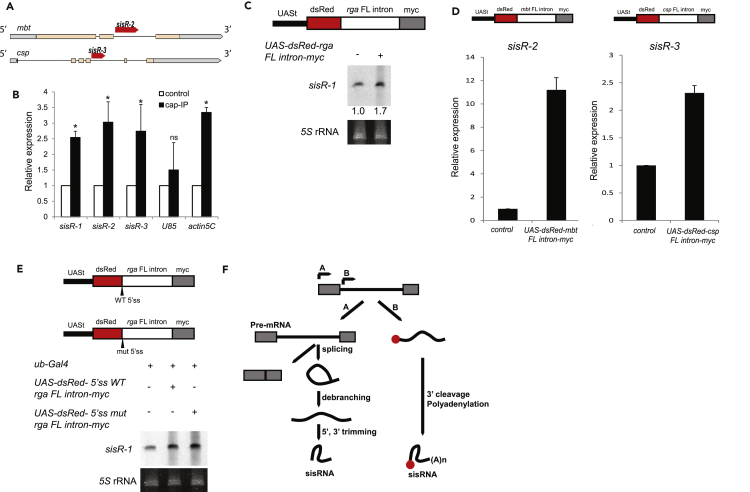
Figure 2.
sisRNAs Are Capped, Independently Transcribed, and Polyadenylated by Wispy
(A) The mbt and csp loci and the locations of sisR-2 and sisR-3 in the introns. The mbt and csp loci are not in the same scale.
(B) qRT-PCT showing enrichment of sisRNAs in m7G antibody immunoprecipitates in unfertilized eggs. *p < 0.01; ns, not significant, p > 0.05. N = 3. Two-tailed t test. Data are represented as mean ± SD. SD, standard deviation.
(C) Northern blot showing the expression of sisR-1 in S2 cells before and after transfection of UAS-dsRed-rga FL intron-myc. Numbers below indicate the relative band intensities of sisR-1 normalized to rRNA quantified using ImageJ software.
(D) qRT-PCR showing the relative expression of sisR-2 and sisR-3 in male bodies of y w controls (parental strain) and transgenic flies harboring UAS-dsRed-mbt/csp FL intron-myc transgenes. *p < 0.01, two-tailed t test. N = 3. Data are represented as mean ± SD.
(E) Northern blot showing the expression of sisR-1 in S2 cells transfected with plasmids containing wild-type or mutated 5′ splice site of rga intron.
(F) A proposed model of biogenesis of sisRNAs via splicing-dependent and splicing-independent transcription pathways.
See also Figures S2 and S3.
The following observations indicate that the rga, mbt, and csp introns contain sequences that can drive independent transcription of sisRNAs First, transfection of UAS-dsRed-intron-myc plasmids containing rga full-length intron (without Gal4 induction) into S2 cells led to an increase in sisR-1 levels as assayed by northern blotting (Figure 2C). Second, flies harboring extra copies of the UAS-dsRed-intron-myc transgenes containing mbt or csp full-length introns (without Gal4 induction) also expressed higher levels of sisR-2 or sisR-3, respectively, than the parental strains as assayed by quantitative RT-PCR (qRT-PCR) (Figure 2D). The UAS promoter was not leaky as we observed non-significant expression of dsRed (Figure S3). Finally, mutation of the 5′ splice site of the rga intron in the UAS-dsRed-intron-myc plasmid did not perturb the expression of sisR-1 in S2 cells (Figure 2E), suggesting that sisRNAs can also be processed in a splicing-independent manner. These observations are consistent with an alternative pathway for sisRNA biogenesis via direct transcription from the introns (Figure 2F).
sisR-3 Regulates Long Noncoding RNA CR44148
To understand the functional significance of these sisRNAs, we focused on sisR-3. Previously, sisR-1 was shown to repress the expression of ASTR ncRNA in vivo, possibly via base-pairing of its 3′ tail with the target (Pek et al., 2015). We asked if sisR-3 also shows similar sisRNA-target relationship properties in vivo. We examined the predicted secondary structure of sisR-3 using the Vienna RNAfold software. Interestingly, sisR-3 was predicted to form a secondary structure that has an exposed 3′ end (Figure 3A). The characteristic free 3′ end feature of sisR-3 is reminiscent of the one for sisR-1 (Pek et al., 2015), suggesting that they belong to a family of structurally related sisRNAs.
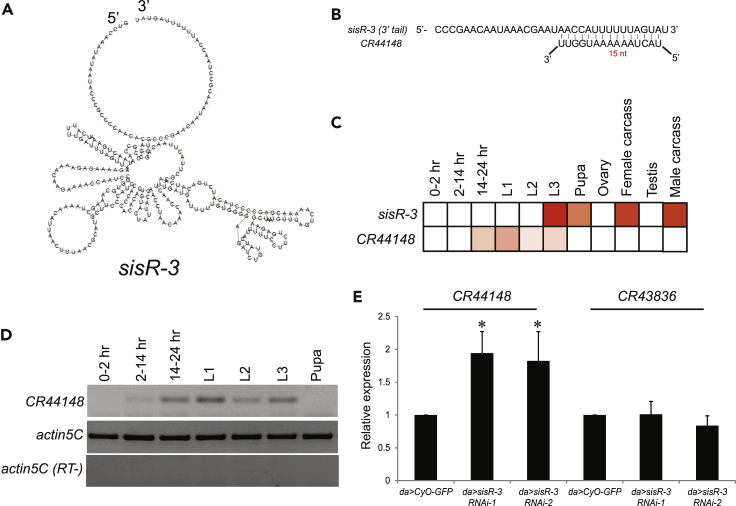
Figure 3.
sisR-3 Represses CR44148
(A) Predicted secondary structures of Drosophila sisR-3.
(B) Complementary base-pairing between the 3′ end of sisR-3 and CR44148.
(C) Heatmap showing the relative expression of sisR-3 and its predicted target CR44148 during development and in adults. Red, high expression; white, low or undetectable expression.
(D) Strand-specific RT-PCR showing the expression of CR44148 during development. Actin5C was used as a loading control.
(E) qPCR showing the relative abundance of CR44148, sisR-2, and CR43836 in the indicated genotypes. Actin5C was used as a loading control. *p < 0.05, two-tailed t test. N = 3. Data are represented as mean ± SD. SD, standard deviation.
See also Figure S4.
We predicted the target of sisR-3 by performing a BLAST search using the sequences of the exposed 3′ ends. The 3′ end of sisR-3 is predicted to target a long ncRNA CR44148 (Figure 3B). We next asked if sisR-3 and its predicted target CR44148 have reciprocal expression patterns. The modENCODE temporal and tissue expression data in FlyBase showed that CR44148 exhibited mutually exclusive temporal and spatial expression patterns to sisR-3. sisR-3 expression is highly expressed in the third-instar larvae, pupae, and adult somatic tissues, whereas CR44148 is abundantly expressed in the embryos (Figures 3C and 3D). These observations are consistent with a model that sisR-3 modulates robustness in gene expression by negatively regulating CR44148.
To test whether sisR-3 regulates its predicted target in vivo, we knocked down the expression of sisR-3 by two independent short hairpin RNAs (shRNAs) and then examined if there was any up-regulation of CR44148 (Figure S4A). The knockdown efficiency was tested in the ovaries by quantitative PCR (qPCR). Expression of sisR-3, but not its cognate csp mRNA, was down-regulated by driving sisR-3 shRNAs using act-Gal4 driver (Figure S4B). Since sisR-3 and CR44148 are co-expressed in the third-instar larvae, we examined the effect of sisR-3 knockdown on target expression during this stage of development. Knockdown of sisR-3 in the third-instar larvae using da-Gal4 resulted in up-regulation of CR44148, but not another non-targeted ncRNA CR43836 (Figure 3E), verifying that sisR-3 specifically represses CR44184.
sisR-3 Is Required during Development
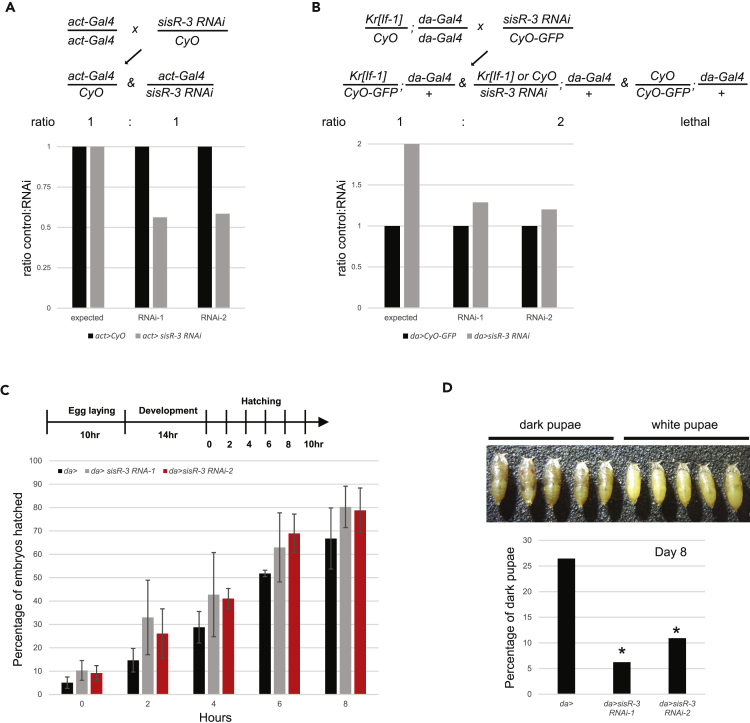
To investigate if sisR-3 is important for development, we ubiquitously knocked down sisR-3. Knockdown of sisR-3 using act-Gal4 revealed a semi-lethality phenotype. Based on the crossing scheme, we expected the ratio of eclosed act>CyO:act>sisR-3 RNAi adults to be 1:1 (Figure 4A). Instead, we observed that the ratio was 1:∼0.6 for both RNA interference (RNAi) lines (Figure 4A). This result implied that sisR-3 knockdown may affect development. We used an alternative crossing scheme to check for third-instar larvae development (Figure 4B). In this cross, we expected a ratio of 1:2 for da>CyO-GFP:da>sisR-3 RNAi; however, we observed a ratio of 1:∼1.2 for both RNAi lines (Figure 4B). This result suggests that sisR-3 knockdown might have an effect on larvae development. To confirm our results, we next directly examined the developmental effects of sisR-3 knockdown. Knockdown of sisR-3 in the embryos did not affect embryogenesis. We observed that da >sisR-3 RNAi embryos developed normally and had hatching rates similar to da-Gal4 controls (Figure 4C). We then compared the development of da-Gal4 controls and da >sisR-3 RNAi pupae. At day 8 of development, whereas ∼28% of da-Gal4 control pupae had begun to mature forming dark pupae, only 8%–11% of da>sisR-3 RNAi pupae were mature (Figure 4D, p < 0.01, chi-square test), indicating that sisR-3 is required for proper development. Taken together, our results demonstrate that sisR-3 is required for proper larva and pupa development.
Figure 4.
sisR-3 Is Required for Proper Development
(A and B) Crossing schemes and charts showing the expected and observed ratios of the indicated genotypes.
(C) Chart showing the hatching rates of embryos of the indicated genotypes at different time points.
(D) Chart showing the percentage of pupae that are dark (see photograph) for the indicated genotypes on day 8 of development at 25°C. *p < 0.01, chi-square test. N > 100 pupae in three independent experiments.
Discussion
In this study, we identified an abundant pool of polyadenylated maternal sisRNAs in Drosophila. These sisRNAs can be generated by independent transcription from the cognate introns and require Wispy for their stabilization as maternal transcripts. Further characterization of a sisRNA sisR-3 demonstrates that sisR-3 represses its target long ncRNA CR44148 and is required for proper development. Together with our previous study on sisR-1, sisRNAs appear to function as regulators of other long ncRNAs, suggesting a robust mechanism to clear off unwanted long ncRNAs (Pek et al., 2015).
Our data suggest an alternative pathway for maternal sisRNA biogenesis (Figure 2F)— sisRNAs are transcribed from intronic promoters and are cleaved and polyadenylated in the nucleus after the PAS sequences in the introns. Maternal sisRNAs are further polyadenylated in the cytoplasm by Wispy to confer stability. Previous work had suggested that splicing and debranching of the intronic transcript is required for sisR-1 production during development (Pek et al., 2015). Therefore, we propose that there are two non-mutually exclusive pathways generating sisRNAs—a host pre-mRNA splicing-dependent pathway and an independent transcription pathway (Figure 2F), similar to what was reported for snoRNAs (Villa et al., 1998). The relative contribution of each pathway may differ in various tissues or developmental contexts.
Drosophila sisR-1 and sisR-3 are predicted to form related secondary structures that have exposed 3′ ends. Interestingly, the Epstein-Barr virus (EBV) ebv-sisRNA-1 was also predicted to adopt a similar secondary structure (Moss et al., 2014, Moss and Steitz, 2013). It was proposed that the 3′ tail of the ebv-sisRNA-1 may bind to RNAs by complementary base-pairing and possibly regulate RNA activity or abundance (Moss et al., 2014, Moss and Steitz, 2013). It implies that the 3′ tail is an important element for a sisRNA to function. The 3′ end may provide specificity to the targets by complementary base-pairing, thus providing a new paradigm for sisRNA-mediated gene regulation. This principle of ncRNA-target recognition has been seen in various ncRNAs such as snoRNAs, miRNAs, small nuclear RNAs (snRNAs), and CRISPR RNAs (Cech and Steitz, 2014).
Recent studies have uncovered a role for poly(A) tails in promoting the stability of viral and endogenous long ncRNAs such as MALAT and PAN RNA (Brown et al., 2012, Mitton-Fry et al., 2010, Tycowski et al., 2012, Tycowski et al., 2016, Wilusz et al., 2012). It is conceivable that the poly(A) tails of sisRNAs may form intra- or inter-molecular interactions with other nucleic acids or proteins, to protect them from 3′ exoribonucleases. Polyadenylation of intronic sequences was first described in sea urchin eggs more than 30 years ago (Calzone et al., 1988, Costantini et al., 1980, Ruzdijic and Pederson, 1987). In the Drosophila larvae, the delta locus was also shown to give rise to multiple polyadenylated intronic sequences more than 20 years ago (Kopczynski and Muskavitch, 1992). More recently, a study identified polyadenylated sisRNAs from the EBV (Cao et al., 2015). The identification of abundant polyadenylated maternal sisRNAs in Drosophila suggests that this paradigm may be more widely conserved than previously thought.
Methods
All methods can be found in the accompanying Transparent Methods supplemental file.
Acknowledgments
We thank Joseph Gall and the Bloomington Stock Center for reagents, Steven Ching and Allison Pinder for help in deep sequencing and bioinformatics, Amanda Ng for assistance in qPCR analyses, and members of the Pek laboratory for discussion. The GEO accession number for deep sequencing is GSE77294. The authors are supported by the Temasek Life Sciences Laboratory.
Author Contributions
S.S.J.N. and R.T.Z. performed the experiments. I.O. performed the experiments and wrote the paper. J.W.P. conceived the project, performed the experiments, and wrote the paper.
Declaration of Interests
The authors declare no competing interests.
Published: June 29, 2018
Footnotes
Supplemental Information includes Transparent Methods, four figures, and two tables and can be found with this article online at https://doi.org/10.1016/j.isci.2018.05.010.
Supplemental Information
References
- Benoit P., Papin C., Kwak J.E., Wickens M., Simonelig M. PAP- and GLD-2-type poly(A) polymerases are required sequentially in cytoplasmic polyadenylation and oogenesis in Drosophila. Development. 2008;135:1969–1979. doi: 10.1242/dev.021444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown J.A., Valenstein M.L., Yario T.A., Tycowski K.T., Steitz J.A. Formation of triple-helical structures by the 3'-end sequences of MALAT1 and MENbeta noncoding RNAs. Proc. Natl. Acad. Sci. USA. 2012;109:19202–19207. doi: 10.1073/pnas.1217338109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calzone F.J., Lee J.J., Le N., Britten R.J., Davidson E.H. A long, nontranslatable poly(A) RNA stored in the egg of the sea urchin Strongylocentrotus purpuratus. Genes Dev. 1988;2:305–318. doi: 10.1101/gad.2.3.305. [DOI] [PubMed] [Google Scholar]
- Cao S., Strong M.J., Wang X., Moss W.N., Concha M., Lin Z., O'Grady T., Baddoo M., Fewell C., Renne R. High-throughput RNA sequencing-based virome analysis of 50 lymphoma cell lines from the Cancer Cell Line Encyclopedia project. J. Virol. 2015;89:713–729. doi: 10.1128/JVI.02570-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cech T.R., Steitz J.A. The noncoding RNA revolution-trashing old rules to forge new ones. Cell. 2014;157:77–94. doi: 10.1016/j.cell.2014.03.008. [DOI] [PubMed] [Google Scholar]
- Costantini F.D., Britten R.J., Davidson E.H. Message sequences and short repetitive sequences are interspersed in sea urchin egg poly(A)+ RNAs. Nature. 1980;287:111–117. doi: 10.1038/287111a0. [DOI] [PubMed] [Google Scholar]
- Gardner E.J., Nizami Z.F., Talbot C.C., Jr., Gall J.G. Stable intronic sequence RNA (sisRNA), a new class of noncoding RNA from the oocyte nucleus of Xenopus tropicalis. Genes Dev. 2012;26:2550–2559. doi: 10.1101/gad.202184.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghildiyal M., Zamore P.D. Small silencing RNAs: an expanding universe. Nat. Rev. Genet. 2009;10:94–108. doi: 10.1038/nrg2504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juge F., Zaessinger S., Temme C., Wahle E., Simonelig M. Control of poly(A) polymerase level is essential to cytoplasmic polyadenylation and early development in Drosophila. EMBO J. 2002;21:6603–6613. doi: 10.1093/emboj/cdf633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopczynski C.C., Muskavitch M.A. Introns excised from the Delta primary transcript are localized near sites of Delta transcription. J. Cell Biol. 1992;119:503–512. doi: 10.1083/jcb.119.3.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kung J.T., Colognori D., Lee J.T. Long noncoding RNAs: past, present, and future. Genetics. 2013;193:651–669. doi: 10.1534/genetics.112.146704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J.T. Epigenetic regulation by long noncoding RNAs. Science. 2012;338:1435–1439. doi: 10.1126/science.1231776. [DOI] [PubMed] [Google Scholar]
- Lee M., Choi Y., Kim K., Jin H., Lim J., Nguyen T.A., Yang J., Jeong M., Giraldez A.J., Yang H. Adenylation of maternally inherited microRNAs by Wispy. Mol. Cell. 2014;56:696–707. doi: 10.1016/j.molcel.2014.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Z., Filonov G.S., Noto J.J., Schmidt C.A., Hatkevich T.L., Wen Y., Jaffrey S.R., Matera A.G. Metazoan tRNA introns generate stable circular RNAs in vivo. RNA. 2015;21:1554–1565. doi: 10.1261/rna.052944.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matera A.G., Terns R.M., Terns M.P. Non-coding RNAs: lessons from the small nuclear and small nucleolar RNAs. Nat. Rev. Mol. Cell Biol. 2007;8:209–220. doi: 10.1038/nrm2124. [DOI] [PubMed] [Google Scholar]
- Mitton-Fry R.M., DeGregorio S.J., Wang J., Steitz T.A., Steitz J.A. Poly(A) tail recognition by a viral RNA element through assembly of a triple helix. Science. 2010;330:1244–1247. doi: 10.1126/science.1195858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss W.N., Lee N., Pimienta G., Steitz J.A. RNA families in Epstein-Barr virus. RNA Biol. 2014;11:10–17. doi: 10.4161/rna.27488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss W.N., Steitz J.A. Genome-wide analyses of Epstein-Barr virus reveal conserved RNA structures and a novel stable intronic sequence RNA. BMC Genomics. 2013;14:543. doi: 10.1186/1471-2164-14-543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osman I., Tay M.L., Pek J.W. Stable intronic sequence RNAs (sisRNAs): a new layer of gene regulation. Cell Mol. Life Sci. 2016;73:3507–3519. doi: 10.1007/s00018-016-2256-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pek J.W. Stable intronic sequence RNAs engage in feedback loops. Trends Genet. 2018;34:330–332. doi: 10.1016/j.tig.2018.01.006. [DOI] [PubMed] [Google Scholar]
- Pek J.W., Okamura K. Regulatory RNAs discovered in unexpected places. Wiley Interdiscip. Rev. RNA. 2015;6:671–686. doi: 10.1002/wrna.1309. [DOI] [PubMed] [Google Scholar]
- Pek J.W., Osman I., Tay M.L., Zheng R.T. Stable intronic sequence RNAs have possible regulatory roles in Drosophila melanogaster. J. Cell Biol. 2015;211:243–251. doi: 10.1083/jcb.201507065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinn J.L., Chang H.Y. Genome regulation by long noncoding RNAs. Annu. Rev. Biochem. 2012;81:145–166. doi: 10.1146/annurev-biochem-051410-092902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruzdijic S., Pederson T. Evidence for an association between U1 RNA and interspersed repeat single-copy RNAs in the cytoplasm of sea urchin eggs. Development. 1987;101:107–116. [PubMed] [Google Scholar]
- Shi Y., Manley J.L. The end of the message: multiple protein-RNA interactions define the mRNA polyadenylation site. Genes Dev. 2015;29:889–897. doi: 10.1101/gad.261974.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talhouarne G.J., Gall J.G. Lariat intronic RNAs in the cytoplasm of Xenopus tropicalis oocytes. RNA. 2014;20:1476–1487. doi: 10.1261/rna.045781.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tay M.L., Pek J.W. Maternally inherited stable intronic sequence RNA triggers a self-reinforcing feedback loop during development. Curr. Biol. 2017;27:1062–1067. doi: 10.1016/j.cub.2017.02.040. [DOI] [PubMed] [Google Scholar]
- Tycowski K.T., Shu M.D., Borah S., Shi M., Steitz J.A. Conservation of a triple-helix-forming RNA stability element in noncoding and genomic RNAs of diverse viruses. Cell Rep. 2012;2:26–32. doi: 10.1016/j.celrep.2012.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tycowski K.T., Shu M.D., Steitz J.A. Myriad triple-helix-forming structures in the transposable element RNAs of plants and fungi. Cell Rep. 2016;15:1266–1276. doi: 10.1016/j.celrep.2016.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villa T., Ceradini F., Presutti C., Bozzoni I. Processing of the intron-encoded U18 small nucleolar RNA in the yeast Saccharomyces cerevisiae relies on both exo- and endonucleolytic activities. Mol. Cell. Biol. 1998;18:3376–3383. doi: 10.1128/mcb.18.6.3376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilusz J.E., JnBaptiste C.K., Lu L.Y., Kuhn C.D., Joshua-Tor L., Sharp P.A. A triple helix stabilizes the 3' ends of long noncoding RNAs that lack poly(A) tails. Genes Dev. 2012;26:2392–2407. doi: 10.1101/gad.204438.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong J.T., Akhbar F., Ng A.Y.E., Tay M.L., Loi G.J.E., Pek J.W. DIP1 modulates stem cell homeostasis in Drosophila through regulation of sisR-1. Nat. Commun. 2017;8:759. doi: 10.1038/s41467-017-00684-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin Q.F., Yang L., Zhang Y., Xiang J.F., Wu Y.W., Carmichael G.G., Chen L.L. Long noncoding RNAs with snoRNA ends. Mol. Cell. 2012;48:219–230. doi: 10.1016/j.molcel.2012.07.033. [DOI] [PubMed] [Google Scholar]
- Zhang Y., Zhang X.O., Chen T., Xiang J.F., Yin Q.F., Xing Y.H., Zhu S., Yang L., Chen L.L. Circular intronic long noncoding RNAs. Mol. Cell. 2013;51:792–806. doi: 10.1016/j.molcel.2013.08.017. [DOI] [PubMed] [Google Scholar]
- Zheng S., Vuong B.Q., Vaidyanathan B., Lin J.Y., Huang F.T., Chaudhuri J. Non-coding RNA generated following lariat debranching mediates targeting of AID to DNA. Cell. 2015;161:762–773. doi: 10.1016/j.cell.2015.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.