Abstract
Background
Prematurity and low birth weight are significant predictors of perinatal morbidity and mortality and are influenced by the overall health and socioeconomic status of the pregnant mother. Although Cyprus is characterized by the highest prematurity rate in Europe (13.1% in 2014), the relationship between maternal health and socioeconomic characteristics with prematurity and low birth weight has never been investigated. We aimed to investigate the association of maternal demographic, clinical and socioeconomic characteristics with premature delivery and low neonatal birth weight in Cyprus.
Methods
In a case-control design, questionnaire data were collected from 348 women who gave birth prematurely (cases) and 349 women who gave birth at term (controls). Information was obtained on gestation duration and birth weight as well as maternal demographic, socioeconomic and clinical profiles, including parameters such as smoking, body mass index, alcohol consumption, presence of gestational diabetes and mental health factors.
Results
Premature delivery was associated with greater maternal age (OR: 1.12, 95% CI: 1.06–1.18), absence of gestational diabetes (OR: 0.53, 95% CI: 0.30–0.97), long working hours (OR: 3.77, 95% CI: 2.08–6.84) and emotional stress (OR: 8.5, 95% CI: 3.03–23.89). Within the cases group, emotional stress was also associated with lower birth-weight (β: -323.68 (95% CI: -570.36, − 77.00).
Conclusions
The findings of this study demonstrate the positive association of maternal psychological factors, working conditions as well as maternal age with prematurity and low birth weight in Cyprus. Additional, prospective, studies are needed in the country to further investigate these associations and inform public health intervention measures.
Electronic supplementary material
The online version of this article (10.1186/s12978-018-0603-7) contains supplementary material, which is available to authorized users.
Keywords: Preterm birth, Low birth weight, Maternal risk factors, Socioeconomic factors
Plain english summary
Prematurity and low birth weight are important determinants of neonatal health and are influenced by the overall health and socioeconomic conditions of the pregnant mother. While prematurity rates vary significantly across the world, Cyprus is characterized by the highest prematurity rate in Europe. However, the factors that are associated with prematurity and low birth weight in Cyprus have never been studied.
This study was designed to understand the influence of demographic, mental health and socioeconomic factors on premature delivery and low birth weight in Cyprus. We identified 348 mothers with premature deliveries at the island’s largest maternity unit during a one year period and we also enrolled 349 mothers with term pregnancies as controls. Through the use of a specifically designed questionnaire we obtained data on socioeconomic characteristics from both groups of mothers. Basic information about clinical and demographic characteristics was available from medical charts.
Comparison between mothers with preterm pregnancies and mothers with term pregnancies demonstrated that maternal age at birth, long working hours and emotional stress were associated with a higher risk for prematurity while emotional stress was also associated with lower birth weight among premature neonates. Combinations of two or more factors were associated with a sharp increase of prematurity risk, demonstrating a cumulative effect.
Healthcare professionals should take into account the overall socioeconomic status of pregnant women during observation, towards identifying high risk pregnancies for prematurity and implementation of appropriate clinical management. Furthermore, healthcare policy makers should aim for the development of public health intervention measures targeting high risk pregnant women.
Background
Optimal fetal development is widely recognized (World Health Organization – WHO, 2006) as an important factor of infant’s survival and subsequent social development. In particular, birth weight, neonatal viability and gestational age, are considered as important health determinants throughout lifetime [1]. Furthermore, the good health and the favorable socioeconomic environment of the pregnant mother are also considered essential prerequisites for the mental and physical well-being of the infant [2].
Preterm labor is the leading cause of perinatal morbidity and mortality in developed countries, where the majority of deaths occur in neonates with a gestational age of less than 32 weeks [3–5]. In recent years, the care provided in Neonate Intensive Care Units (NICU) settings increased the survival of premature infants but at the same time increased duration of hospitalization and costs. As a result, the care of premature neonates currently accounts for a large proportion of the total in-hospital costs worldwide [6]. In total, fifteen million premature births are reported annually worldwide [4] and although the frequency of preterm labor varies considerably between countries, almost 90% of these premature births occur in developing countries in Africa and Asia [7]. In 2014, the rate of preterm births was 10% in the US [8], while in Europe in 2010, preterm birth rates varied markedly from 5 to 10.6% among live births [9]. Cyprus is characterized by the highest premature birth rate in Europe, reaching 10.6% and 13.1% in 2010 and 2014 respectively [10, 11] partly due to increase of multiple pregnancies following in-vitro fertilization [12].
Furthermore, low birth weight (LBW) is associated with increased mortality as well as acute and long-term health problems [13–15]. In Europe the percentage of LBW ranged between 4 and 9% in 2010 [10]. LBW infants also face significant neurodevelopmental problems, whereas in adulthood they are at higher risk to develop type 2 diabetes mellitus, hypertension and coronary artery disease [16].
Several risk factors have been found to be associated with premature birth and LBW, including maternal age, ethnicity, lifestyle maternal characteristics such as smoking and alcohol consumption, education level, working conditions, access to obstetric observation, diabetes mellitus, mental stress and depression, body mass index (BMI) before pregnancy and additional weight-gain during pregnancy [5]. Despite the increasing incidence of premature births in Cyprus, there is lack of knowledge regarding the prevalent maternal risk factors for premature birth and LBW, which is a necessary prerequisite for development of prevention strategies by the national health system and awareness initiatives among the public and health professionals. We aimed to assess the relation of demographic and maternal socioeconomic and lifestyle characteristics with the risk of premature birth among Cypriot pregnant women and investigate the contribution of the same characteristics to LBW risk among premature infants.
Methods
Study population
The source population were all mothers that gave birth between March 2015 and April 2016 at “Archbishop Makarios III” hospital in Nicosia, Cyprus. “Archibishop Makarios III” hospital is the main tertiary maternal and pediatric hospital in Cyprus which hosts the only tertiary NICU on the island. Mothers who gave birth to premature neonates (gestation < 37 weeks) were included in the study as cases and mothers that gave birth to term neonates (gestation > 37 weeks) were included in the study as controls. Data collection commenced in March 2015 and was completed in April 2016. Mothers with multiple pregnancies or mothers that underwent infertility treatment as well as mothers of stillborn neonates and mothers of neonates with chromosomal abnormalities were excluded from the study. Sample size calculation was performed using OpenEpi [17] assuming a 1:1 ratio of cases to controls, 95% confidence level, 80% power and a least extreme Odds Ratio (OR) to be detected equal to 1.55. The calculated sample size was 333 cases and 333 controls. All participants provided written informed consent and the study protocol was approved by the Cyprus National Bioethics Committee (EEBK EΠ 2015.01.25) and the Cyprus Ministry of Health (Protocol approval: 0282/2015).
Data collection and data processing
Information on duration of gestation period and birth weight was obtained from hospital records while all other information was collected through a structured self-administered questionnaire. The questionnaire (Additional file 1) was adopted from similar previous studies [18, 19] and consisted of questions on basic demographic (age, ethnicity) and socioeconomic factors such as marital status, socioeconomic status (income, education level, profession, working conditions (manual labour, standing, > 8 h shifts). Data on anthropometric indices and other potential maternal risk factors such as gestational diabetes, stress, depression, anti-depressants consumption, alcohol consumption, and smoking before and during pregnancy were also obtained through the questionnaire and the responses were crosschecked with available information in the mothers’ medical records. Anthropometric and clinical parameters such as weight, height, gestational diabetes and depression were derived from clinical assessments while experience of emotional stress and use of antidepressants or anxiolytics during pregnancy were obtained from self-assessment by the participants using binary (YES or NO) questions. Composite scores for variables describing working conditions and socioeconomic deprivation were developed with individual factors carrying the same weight.
Statistical analysis
All variables used in the analysis were checked for normality with the use of histograms. Summary statistics for participant characteristics were calculated separately for cases and controls and categorical variables are reported as absolute and relative (%) frequencies, while continuous variables are reported as mean estimates and standard deviation. In unadjusted analyses, differences in participant characteristics between cases and controls were investigated using chi square test in the case of categorical variables and independent t-test in the case of continuous variables. A multivariate logistic regression including all variables that were found to be significantly associated with prematurity (pvalue < 0.10) in the unadjusted analysis was used in order to calculate the OR and 95% Confidence Intervals (95% CI) for pre-term birth.
Among cases, the relationship between different characteristics and gestational age as well as birth weight were examined using the Pearson correlation coefficient and adjusted analyses were performed using a multivariate linear regression model. The linear regression models included all variables that were significantly correlated with birth weight (pvalue < 0.10).
To further illuminate the role of clustering of risk factors for prematurity, a cumulative individual risk score was calculated for each mother by summing the values (0 for negative or 1 for positive) of each significant risk factor. Lastly, to demonstrate the extent to which different significant risk factors overlap, a Venn diagram was produced using Venny software [20]. The OR of combinations of risk factors with significant overlap was calculated using binary logistic regression.
For the multivariate logistic, binary logistic and linear regressions a two tailed p-value < 0.05 was applied to demonstrate statistical significance. All statistical analyses were performed using STATA (Version 12, StataCorp, College Station, TX).
Results
A total of 697 mothers were included in the study (348 cases and 349 controls). Only one mother did not complete the questionnaire resulting in a final response rate of 99.8% for cases and 100% for controls. Cases were older than controls (30.7, 95% CI: 19.4–41.9 vs 28.8, 95% CI: 18.8–38.7, pvalue < 0.001) and more frequently reported practicing a manual labour profession (33.3% vs 19.8%, pvalue = 0.001) or working > 8 h per day (34.9% vs 11.6%, pvalue < 0.001). Birth weight was significantly lower in cases (mean: 2173 g, 95% CI: 829–3277 among cases Vs mean: 3225 g, 95% CI: 2433–4148 among controls, pvalue < 0.001). The distribution of birth weight in the two groups according to the WHO categorisation is displayed in Fig. 1.
Fig. 1.
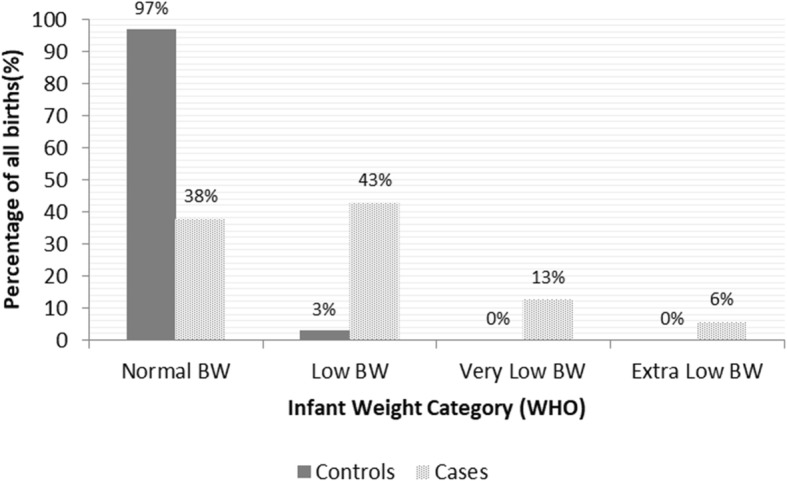
Distribution of infant weight in births from cases and controls. Distribution of infant weight (according to WHO categorization) among births from cases (preterm pregnancies) and controls (term pregnancies). BW: Birth Weight
Table 1 presents basic demographic and clinical characteristics of cases and controls. Between the two groups, there were statistically significant differences in the distributions of maternal age, family status, manual labour, long working hours, additional weight gain during pregnancy, pre-gestation BMI, gestational diabetes, depression and stress during pregnancy. Multivariate analysis demonstrated that significant maternal risk factors for prematurity were age (OR: 1.12,95% CI: 1.06–1.18, pvalue < 0.001), gestational diabetes (OR: 0.53,95% CI: 0.30–0.97, pvalue = 0.038), stress (OR: 8.5, 95% CI: 3.03–23.89, pvalue < 0.001) and long working hours (OR: 3.77, 95% CI: 2.08–6.84, pvalue < 0.001). The results of multivariate analysis for prematurity are summarized in Table 2.
Table 1.
Distribution of demographic characteristics and maternal risk factors for prematurity among mothers with term (controls) and mother with pre-term deliveries (cases)
| Maternal characteristic | Controls N (%) | Cases N (%) | Statistical Significance† |
|---|---|---|---|
| Demographic | |||
| Age at childbirtha | 28.8 (18.8–38.7) | 30.7 (19.4–41.9) | < 0.001‡ |
| Family status | |||
| Married | 339/341 (98.3%) | 319/341 (93.6%) | |
| Divorced | 0/341 (0%) | 4/341 (1.2%) | 0.001 |
| Single | 4/341 (1.2%) | 18/341 (5.28%) | P trend: < 0.001 |
| Education level | |||
| Primary | 35/343 (10.2%) | 36/341 (10.6%) | |
| Secondary | 107/343 (31.2%) | 110/341 (32.3%) | |
| Post-secondary | 70/343 (20.4%) | 73/341 (21.4%) | 0.02 |
| Tertiary | 116/343 (33.8%) | 87/341 (25.5%) | |
| Post-graduate | 15/343 (4.4%) | 35/341 (10.3%) | P trend: 0.85 |
| Working Conditions | |||
| Unemployed | 77/307 (25.1%) | 89/320 (27.8%) | 0.44 |
| Partner unemployed | 64/338 (18.9%)` | 54/321 (16.8%) | 0.48 |
| Manual labour | 46/232 (19.8%) | 76/228 (33.3%) | 0.001 |
| Prolonged standing at work | 103/232(44.4%) | 112/229 (48.9%) | 0.33 |
| Working > 8 h per day | 27/232 (11.6%) | 80/229 (34.9%) | < 0.001 |
| Life-Style | |||
| Smoking before pregnancy | 92/340 (27.1%) | 97/339 (28.6%) | 0.65 |
| Smoking during pregnancy | 35/341 (10.3%) | 38/339 (11.2%) | 0.69 |
| Clinical | |||
| Additional weight gain during pregnancya | 12.23 (0.3–23.7) | 10.96 (3–24) | 0.003‡ |
| Body mass index | |||
| BMI < 20 | 56/334 (16.8%) | 65/279 (23.3%) | |
| 20 < BMI < 25 | 166/334 (49.7%) | 132/279 (47.3%) | |
| 25 < BMI < 30 | 66/334 (19.8%) | 55/279 (19.7%) | 0.23 |
| 30 < BMI < 35 | 31/334 (9.3%) | 19/279 (6.8%) | |
| BMI > 35 | 15/334 (4.5%) | 8/279 (2.9%) | Ptrend: 0.04 |
| Gestational diabetes | 74/341 (21.7%) | 44/338 (13.0%) | 0.003 |
| Clinically diagnosed depression | 5/339 (1.5%) | 12/339 (3.5%) | 0.09 |
| Stress during gestation | 12/341 (3.5%) | 64/337 (19.0%) | < 0.001 |
aMean and 95% Confidence Interval
†Independent Sample T test ‡ Pearson Chi Square
Table 2.
Association between maternal risk factors and prematurity in multivariate analysis
| Risk Factor | Contrast | Odds Ratio (95% CI) | P-value |
|---|---|---|---|
| Age at child birth | Continuous | 1.12 (1.06, 1.18) | < 0.001 |
| Pre-gestation BMI | Continuous | 0.96 (0.92, 1.01) | 0.11 |
| Gestational diabetes | Categorical | 0.53 (0.30, 0.97) | 0.04 |
| Depression | Categorical | 1.38 (0.25, 7.61) | 0.71 |
| Stress | Categorical | 8.5 (3.03, 23.89) | < 0.001 |
| Family status | Categorical | 1.11 (0.57, 2.15) | 0.77 |
| Manual labour | Categorical | 1.54 (0.90, 2.65) | 0.11 |
| Long working hours | Categorical | 3.77 (2.08, 6.84) | < 0.001 |
Among the significant maternal risk factors for prematurity, clustering of two or more factors was associated with a sharp increase of prematurity risk as displayed in Table 3. Compared to baseline (no risk factor), the OR for the presence of any one risk factor was 2.37 (95% CI: 1.69–3.32, pvalue < 0.001), the OR for the presence of any two risk factors was 6.13 (95% CI: 3.48–10.80, pvalue < 0.001) and the OR for the presence for any three risk factors was 25.70 (95% CI: 3.31–199.70, pvalue = 0.002). Trend test calculation was statistically significant (pvalue for trend< 0.001). Figure 2, presents the different combinations of significant maternal risk factors in a 3-way Venn diagram. The most frequent combination was advanced maternal age and long working hours (OR: 5.17, 95% CI: 2.60–10.30, pvalue < 0.001) followed by the combination of advanced maternal age and stress (OR: 9.89, 95% CI:3.66–26.72, pvalue < 0.001).
Table 3.
Combined score and associated risk for prematurity
| Risk Scorea | Total Number | Controls (N = 343) | Cases (N = 342) | OR (95% CI) | P-value |
|---|---|---|---|---|---|
| 0 | 259 | 172 | 87 | – | – |
| 1 | 330 | 150 | 180 | 2.37 (1.69–3.32) | < 0.001 |
| 2 | 82 | 20 | 62 | 6.13 (3.48–10.80) | < 0.001 |
| 3 | 14 | 1 | 13 | 25.70 (3.31–199.70) | 0.002 |
aThe cumulative individual risk score for each mother was calculated as the sum of values (0 for negative or 1 for positive) for each significant risk factor (long working hours, stress and maternal age). Maternal age was classified as 1 for values ≥30 years old and as 0 for values < 30 years old (30 years was the median value for mother age at childbirth in our dataset)
Fig. 2.
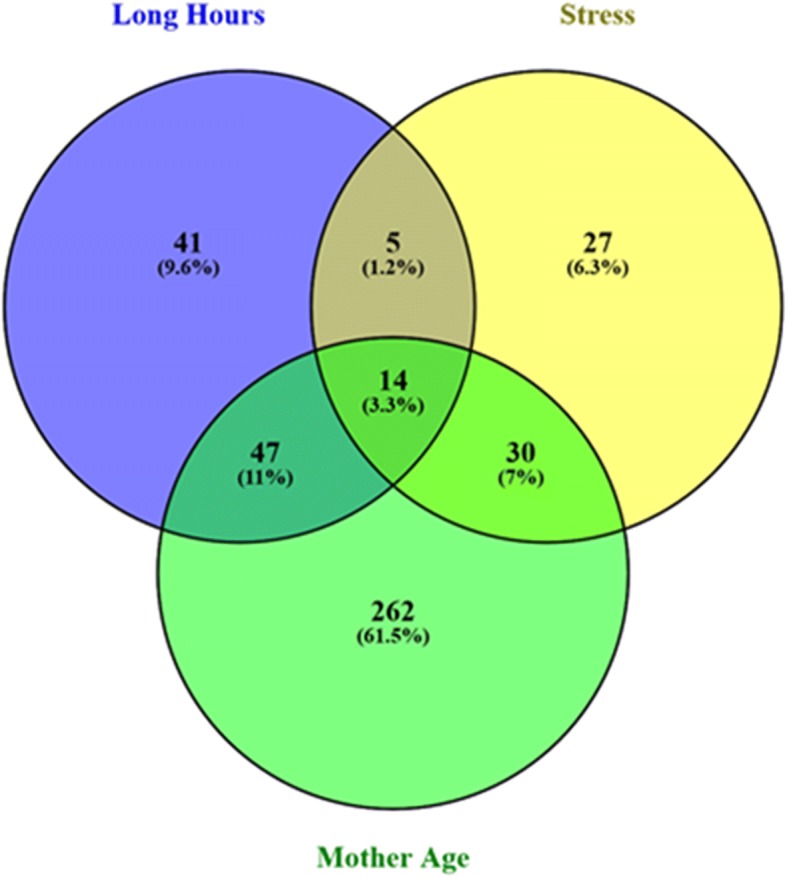
Overlap of significant risk factors for prematurity. Overlap of risk factors that were found to be significant predictors of prematurity (stress, long working hours, mother age > 30 years)
Multivariate analysis within the group of preterm infants, demonstrated that stress was the only parameter that was significantly associated with birth weight (β: -323.68, 95% CI: -570.36, − 77.00, pvalue = 0.010). The remaining parameters were not found to significant predictors of birth weight among preterm infants. Table 4 summarizes the results of multivariate analyses for gestational age and infant birth weight within the cases.
Table 4.
Association between maternal risk factors and birth weight within premature infants group
| Risk Factor | Contrast | Coefficient (95% CI) | P-value |
|---|---|---|---|
| Age at child birth | Continuous | −16.00 (−33.52, 1.54) | 0.07 |
| Pre-gestation BMI | Continuous | 13.37 (−8.43, 35.16) | 0.23 |
| Gestational diabetes | Categorical | 201.13 (−69.37, 471.64) | 0.14 |
| Depression | Categorical | −34.81 (− 647.09, 577.46) | 0.91 |
| Stress | Categorical | −323.68 (−570.36, −77.00) | 0.01 |
| Family status | Categorical | 145.16 (−66.15, 356.46) | 0.18 |
| Manual labour | Categorical | − 154.636 (− 362.05, 52.77) | 0.14 |
| Long working hours | Categorical | 123.25 (−80.72, 327.22) | 0.24 |
Discussion
This is the first study conducted in Cyprus, which investigates socioeconomic risk factors for spontaneous premature labor and low birth weight. Our results indicate that advanced maternal age during childbirth, maternal stress and working conditions, are important predictors for preterm delivery and low birth weight.
During the past three decades, an increase in the average childbearing age has been observed among women in high income countries. In the European Union, the average childbearing age was 29.8 years in 2009, compared to 29.3 in 2003 [21] while a similar trend was observed in Australia [22], Canada [23] and the United Kingdom [24, 25]. In 2016, the mean age of mothers at the first childbirth varied between the European Union Member States. The lowest mean age for the first childbirth was recorded in Bulgaria (26.0 years), followed by Romania (26.4), Latvia (26.8), Slovakia (27.0), Poland (27.2) and Lithuania (27.3). In contrast, the mother’s age for the first childbirth was above 30 in Italy (31.0 years), Spain (30.8), Luxembourg (30.5), Greece (30.3) and Ireland (30.1) [26]. In Cyprus, the average childbearing age has increased from 28 to 30.2 years between 1995 and 2015 [23]. Our results are in line with previous findings demonstrating that advanced childbearing age, is a risk factor for premature birth, low birth weight and for adverse pregnancy outcomes such as fetal distress and emergency caesarean section [22, 27, 28]. The increased risk is partly explained by the co-existence of conditions such as diabetes and hypertension [22] and the natural ageing of reproductive tissues which may result in reduced fetal intake of necessary nutrients for intrauterine growth [29].
Unfavorable working conditions, characterised by manual labour and long working hours in particular, were associated with preterm birth among Cypriot women in this study. Previous findings have also demonstrated a positive association of physical exertion related work, long hours and/or shift work with poor pregnancy outcomes [30, 31]. These effects can be attributed to muscles physical stress at work and increased release of catecholamines and arteriol constriction, which causes redistribution of blood flow in the pregnant woman and reduced blood flow to the placenta, as well as hormonal disturbances and nutritional deficits that can also adversely affect fetal growth [32]. Overall, although there is considerable inhomogeneity in the employment settings among the published studies that explore the relationship between working conditions and pregnancy outcomes [33], it is now well documented that long working hours constitute a significant risk factor for premature birth and low birth weight [34, 35].
Maternal stress during pregnancy was also a statistically significant predictor in the occurrence of both preterm birth and low birth weight in our study. Despite the heterogeneity of previous studies’ design and approaches to measuring stress, published literature indicates a strong association between stress during pregnancy and risks for premature birth and low birth weight [36, 37]. In a recent Swedish study more than 50% of pregnant women reporting stress during pregnancy experienced a premature labor [37], while other studies besides preterm birth associated stress with low birth weight and low head circumference [38, 39]. As a consequence, it is necessary for pregnant women to be monitored regularly to detect development of stress and other psychological problems [40]. According to the guidelines of the American College of Obstetricians and Gynaecologists, it is recommended that pregnant women are screened at least once every trimester during pregnancy for their psychological condition, irrespectively of the social and educational level, race and ethnicity, and referred for specialised treatment if applicable [40, 41].
In general, gestational diabetes mellitus has been found to be associated with medically indicated premature labor and lower gestational age [42, 43]. However, in our study, we found that the frequency of gestational diabetes was lower in mothers who had premature birth compared to controls. Similar negative associations between pregnancy outcomes and gestational diabetes have been also reported by few recent studies [44, 45]. These discrepancies can be attributed to the possibility of good glycemic control of women with gestational diabetes in these studies through good obstetric monitoring, balanced diet and insulin treatment, factors which have not been specifically assessed in our study [46, 47]. A recent study demonstrated that although presence of uncontrolled gestational diabetes and obesity during pregnancy is associated with negative prognosis, their effects can be counterbalanced by the application of glycaemic control combined with controlled weight gain [48]. Furthermore, comparison with previous studies is inherently difficult as the effect of gestational diabetes on perinatal outcomes is influenced by racial factors [49], the different diagnostic criteria for gestational diabetes that are used in each country, the heterogeneity of study populations and differences in the detection programs that are applied in each country, which eventually result in a wide range of gestational diabetes frequency from less than 1% to above 10% across the world [46].
This study benefited largely by including data from consecutive births in Cyprus, accurate assessment of study outcomes, a very high response rate and a questionnaire that captured responses on a wide array of socioeconomic factors. However, our study has several limitations, primarily originating from its retrospective nature which precluded the acquisition of participants’ detailed clinical data such as obstetric complications [50, 51], use of corticosteroids [52] and other medications that may affect occurrence of premature birth [28] and the type and quality of the provided obstetric care [53]. Like any questionnaire study, this study might have been influenced by subjectivity and recall bias, although factors like family status, working conditions and smoking habits during pregnancy are usually easily recollected characteristics by mothers. Furthermore, assessment of factors, such as emotional stress with the use of a one-time questionnaire during the postpartum period that was not specifically designed to address psychological parameters, might have also introduced some bias in our results [54]. Future studies, need to further explore the findings of the present study in the Cyprus population with a prospective study design and the use of validated instruments for measurement of mental health attributes [55–57]. Furthermore, our study excluded women with multiple pregnancies or infertile women who underwent infertility treatment. As a result, the generalizability of our findings is limited to women with singleton pregnancies following natural conception.
Conclusions
In summary, stress, prolonged working hours and advanced maternal age at childbirth, are associated with increased odds of preterm delivery and low birth weight in Cyprus, while the combination of adverse socioeconomic risk factors appears to have a cumulative effect on the odds of prematurity. Further, prospective, studies should further investigate risk factors for adverse pregnancy outcomes and eventually inform local public health authorities towards the development of evidence-based management protocols to limit premature births and subsequent neonatal complications and related healthcare costs.
Additional file
Study Questionnaire in English language. (DOCX 24 kb)
Acknowledgements
We would like to thank the study participants and the nursing staff of “Archbishop Makarios III” hospital for their cooperation.
Availability of data and materials
The datasets used and analysed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- BMI
Body Mass Index
- CI
Confidence Intervals
- LBW
Low Birth Weight
- NICU
Neonatal Intensive Care Unit
- OR
Odds Ratio
- WHO
World Health Organisation
Authors’ contributions
PSR conceived the hypothesis for this manuscript. PSR and TP collected patient data and performed the field work of the study. PK and PK performed the statistical analysis under the guidance of PKY. AR and PKY contributed to the interpretation of the findings. MT contributed towards the study design, supervised collection of data and contributed to the interpretation of the findings. All authors assisted in the interpretation of findings and critically revised the manuscript. All authors have read and approved the final version of the manuscript.
Ethics approval and consent to participate
All participants provided written informed consent and the study protocol was approved by the Cyprus National Bioethics Committee (EEBK EΠ 2015.01.25) and the Cyprus Ministry of Health (Protocol approval: 0282/2015).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Paraskevi Stylianou-Riga, Email: skeviriga@yahoo.comm.
Panayiotis Kouis, Email: kouis.panayiotis@ucy.ac.cy.
Paraskevi Kinni, Email: kinni.paraskevi@ucy.ac.cy.
Angelos Rigas, Email: angerigas@yahoo.gr.
Thalia Papadouri, Email: th.papadouri@gmail.com.
Panayiotis K. Yiallouros, Email: pyiall01@ucy.ac.cy
Mamas Theodorou, Email: m.theodorou@ouc.ac.cy.
References
- 1.Barker DJ, Eriksson JG, Forsén T, Osmond C. Fetal origins of adult disease: strength of effects and biological basis. Int J Epidemiol. 2002;31(6):1235–1239. doi: 10.1093/ije/31.6.1235. [DOI] [PubMed] [Google Scholar]
- 2.World Health Organisation, Promoting optimal fetal development: report of a technical consultation, 2006 [http://apps.who.int/iris/handle/10665/43409].
- 3.Offiah I, O’Donoghue K, Kenny L. Preterm Birth-Mother and Child. InTech. 2012. Clinical risk factors for preterm birth. [Google Scholar]
- 4.Blencowe H, Cousens S, Chou D, Oestergaard M, Say L, Moller A, Kinney M, Lawn J. Born too soon: the global epidemiology of 15 million preterm births. Reprod Health. 2013;10(1):S2. doi: 10.1186/1742-4755-10-S1-S2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goldenberg RL, Culhane JF, Iams JD, Romero R. Epidemiology and causes of preterm birth. Lancet. 2008;371(9606):75–84. doi: 10.1016/S0140-6736(08)60074-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Comert S, Agzikuru T, Akin Y, Telatar B, Tan PD, Ergen SG, Dervisoglu P. The cost analysis of preterm infants from a NICU of a state Hospital in Istanbul. Iran J Pediatr. 2012;22(2):185–190. [PMC free article] [PubMed] [Google Scholar]
- 7.Beck Stacey, Wojdyla Daniel, Say Lale, Pilar Bertran Ana, Meraldi Mario, Harris Requejo Jennifer, Rubens Craig, Menon Ramkumar, Van Look Paul. The worldwide incidence of preterm birth: a systematic review of maternal mortality and morbidity. Bulletin of the World Health Organization. 2010;88(1):31–38. doi: 10.2471/BLT.08.062554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Center for Disease Control and Prevention, Preterm Birth, 2018, [https://www.cdc.gov/reproductivehealth/maternalinfanthealth/pretermbirth.htm].
- 9.Delnord M, Blondel B, Zeitlin J. What contributes to disparities in the preterm birth rate in European countries? Curr Opin Obstet Gynecol. 2015;27(2):133–142. doi: 10.1097/GCO.0000000000000156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Macfarlane A, Dattani N: European Perinatal Health Report Health and care of pregnant women and babies in Europe in 2010.
- 11.Cyprus Health Monitoring Unit, Perinatal health indicators for the year 2014, 2014, [https://www.moh.gov.cy/Moh/MOH.nsf/All/8DC461429CBC4DE7C22579CE002EF07D?OpenDocument].
- 12.Zeitlin J, Szamotulska K, Drewniak N, Mohangoo A, Chalmers J, Sakkeus L, Irgens L, Gatt M, Gissler M, Blondel B. Preterm birth time trends in Europe: a study of 19 countries. BJOG Int J Obstet Gynaecol. 2013;120(11):1356–1365. doi: 10.1111/1471-0528.12281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shah MK, Gee RE, Theall KP. Partner support and impact on birth outcomes among teen pregnancies in the United States. J Pediatr Adolesc Gynecol. 2014;27(1):14–19. doi: 10.1016/j.jpag.2013.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lawn JE, Cousens S, Zupan J. Lancet neonatal survival steering team: 4 million neonatal deaths: when? Where? Why? Lancet. 2005;365(9462):891–900. doi: 10.1016/S0140-6736(05)71048-5. [DOI] [PubMed] [Google Scholar]
- 15.OECD/WHO, Health at a Glance Asia/Pacific 2012, OECD Publishing [https://www.oecd-ilibrary.org/social-issues-migration-health/health-at-a-glance-asia-pacific-2012_9789264183902-en].
- 16.Bian Y, Zhang Z, Liu Q, Wu D, Wang S. Maternal risk factors for low birth weight for term births in a developed region in China: a hospital-based study of 55,633 pregnancies. J Biomed Res. 2013;27(1):14–22. doi: 10.7555/JBR.27.20120046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sullivan KM, Dean A, Soe MM. On academics: OpenEpi: a web-based epidemiologic and statistical calculator for public health. Public Health Rep. 2009;124(3):471–474. doi: 10.1177/003335490912400320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sebayang SK, Dibley MJ, Kelly PJ, Shankar AV, Shankar AH, SUMMIT Study Group Determinants of low birthweight, small-for-gestational-age and preterm birth in Lombok, Indonesia: analyses of the birthweight cohort of the SUMMIT trial. Tropical Med Int Health. 2012;17(8):938–950. doi: 10.1111/j.1365-3156.2012.03039.x. [DOI] [PubMed] [Google Scholar]
- 19.Alijahan R, Hazrati S, Mirzarahimi M, Pourfarzi F, Ahmadi Hadi P. Prevalence and risk factors associated with preterm birth in Ardabil. Iran Iran J Reprod Med. 2014;12(1):47–56. [PMC free article] [PubMed] [Google Scholar]
- 20.Heberle H, Meirelles GV, da Silva FR, Telles GP, Minghim R: InteractiVenn: a web-based tool for the analysis of sets through Venn diagrams. BMC Bioinformatics 2015, 16(1):169. [DOI] [PMC free article] [PubMed]
- 21.Kenny LC, Lavender T, McNamee R, O’Neill SM, Mills T, Khashan AS. Advanced maternal age and adverse pregnancy outcome: evidence from a large contemporary cohort. PLoS One. 2013;8(2):e56583. doi: 10.1371/journal.pone.0056583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ludford I, Scheil W, Tucker G, Grivell R. Pregnancy outcomes for nulliparous women of advanced maternal age in South Australia, 1998–2008. Aust N Z J Obstet Gynaecol. 2012;52(3):235–241. doi: 10.1111/j.1479-828X.2012.01442.x. [DOI] [PubMed] [Google Scholar]
- 23.Adema W, del Carmen Huerta M, Panzera A, Thevenon O, Pearson M. The OECD family database: developing a cross-national tool for assessing family policies and outcomes. Child Indic Res. 2009;2(4):437. doi: 10.1007/s12187-009-9044-8. [DOI] [Google Scholar]
- 24.Eurostat, LUCAS micro data 2015 [http://ec.europa.eu/eurostat/web/lucas/data/primary-data/2015].
- 25.Public Health England, United Kingdom National Statistics, [https://www.ons.gov.uk/peoplepopulationandcommunity/birthsdeathsandmarriages/livebirths/bulletins/birthsbyparentscharacteristicsinenglandandwales/2016].
- 26.Eurostat, Births and fertility in 2016, 2018, [http://ec.europa.eu/eurostat/documents/2995521/8774296/3-28032018-AP-EN.pdf].
- 27.Khoshnood B, De Vigan C, Vodovar V, Goujard J, Lhomme A, Bonnet D, Goffinet F. Trends in prenatal diagnosis, pregnancy termination, and perinatal mortality of newborns with congenital heart disease in France, 1983-2000: a population-based evaluation. Pediatrics. 2005;115(1):95–101. doi: 10.1542/peds.2004-0516. [DOI] [PubMed] [Google Scholar]
- 28.Fuchs F, Monet B, Ducruet T, Chaillet N, Audibert F. Effect of maternal age on the risk of preterm birth: a large cohort study. PLoS One. 2018;13(1):e0191002. doi: 10.1371/journal.pone.0191002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Aras RY. Is maternal age risk factor for low birth weight? Archives of Medicine and Health Sciences. 2013;1(1):33. doi: 10.4103/2321-4848.113558. [DOI] [Google Scholar]
- 30.Mozurkewich EL, Luke B, Avni M, Wolf FM. Working conditions and adverse pregnancy outcome: a meta-analysis. Obstet Gynecol. 2000;95(4):623–635. doi: 10.1016/s0029-7844(99)00598-0. [DOI] [PubMed] [Google Scholar]
- 31.Bonzini M, Coggon D, Palmer KT. Risk of prematurity, low birthweight and pre-eclampsia in relation to working hours and physical activities: a systematic review. Occup Environ Med. 2007;64(4):228–243. doi: 10.1136/oem.2006.026872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mahmoodi Z, Karimlou M, Sajjadi H, Dejman M, Vameghi M, Dolatian M, Mahmoodi A. Association of maternal working condition with low birth weight: the social determinants of health approach. Annals of medical and health sciences research. 2015;5(6):385–391. doi: 10.4103/2141-9248.177982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Saurel-Cubizolles MJ, Zeitlin J, Lelong N, Papiernik E, Di Renzo GC, Breart G. Europop group: employment, working conditions, and preterm birth: results from the Europop case-control survey. J Epidemiol Community Health. 2004;58(5):395–401. doi: 10.1136/jech.2003.008029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Niedhammer I, O’Mahony D, Daly S, Morrison J, Kelleher C. Occupational predictors of pregnancy outcomes in Irish working women in the Lifeways cohort. BJOG Int J Obstet Gynaecol. 2009;116(7):943–952. doi: 10.1111/j.1471-0528.2009.02160.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.van Melick M. J. G. J., van Beukering M. D. M., Mol B. W., Frings-Dresen M. H. W., Hulshof C. T. J. Shift work, long working hours and preterm birth: a systematic review and meta-analysis. International Archives of Occupational and Environmental Health. 2014;87(8):835–849. doi: 10.1007/s00420-014-0934-9. [DOI] [PubMed] [Google Scholar]
- 36.Wadhwa PD, Entringer S, Buss C, Lu MC. The contribution of maternal stress to preterm birth: issues and considerations. Clin Perinatol. 2011;38(3):351–384. doi: 10.1016/j.clp.2011.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lilliecreutz C, Larén J, Sydsjö G, Josefsson A. Effect of maternal stress during pregnancy on the risk for preterm birth. BMC pregnancy and childbirth. 2016;16(1):5. doi: 10.1186/s12884-015-0775-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Su Q, Zhang H, Zhang Y, Zhang H, Ding D, Zeng J, Zhu Z, Li H. Maternal stress in gestation: birth outcomes and stress-related hormone response of the neonates. Pediatrics & Neonatology. 2015;56(6):376–381. doi: 10.1016/j.pedneo.2015.02.002. [DOI] [PubMed] [Google Scholar]
- 39.Henrichs J, Schenk J, Roza S, Van den Berg M, Schmidt H, Steegers E, Hofman A, Jaddoe V, Verhulst FC, Tiemeier H. Maternal psychological distress and fetal growth trajectories: the generation R study. Psychol Med. 2010;40(4):633–643. doi: 10.1017/S0033291709990894. [DOI] [PubMed] [Google Scholar]
- 40.American College of Obstetricians and Gynecologists Psychosocial risk factors: perinatal screening and intervention. ACOG Committee opinion number 343. ObstetrGynecol. 2006;108:469–477. doi: 10.1097/00006250-200608000-00046. [DOI] [PubMed] [Google Scholar]
- 41.Jomeen J. The importance of assessing psychological status during pregnancy, childbirth and the postnatal period as a multidimensional construct: a literature review. Clin Eff Nurs. 2004;8(3–4):143–155. doi: 10.1016/j.cein.2005.02.001. [DOI] [Google Scholar]
- 42.Gasim T. Gestational diabetes mellitus: maternal and perinatal outcomes in 220 saudi women. Oman Med J. 2012;27(2):140–144. doi: 10.5001/omj.2012.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Köck K, Köck F, Klein K, Bancher-Todesca D, Helmer H. Diabetes mellitus and the risk of preterm birth with regard to the risk of spontaneous preterm birth. J Matern Fetal Neonatal Med. 2010;23(9):1004–1008. doi: 10.3109/14767050903551392. [DOI] [PubMed] [Google Scholar]
- 44.Mahalakshmi MM, Bhavadharini B, Maheswari K, Anjana RM, Jebarani S, Ninov L, Kayal A, Malanda B, Belton A, Uma R, Mohan V, Unnikrishnan R. Current practices in the diagnosis and management of gestational diabetes mellitus in India (WINGS-5) Indian J Endocrinol Metab. 2016;20(3):364–368. doi: 10.4103/2230-8210.180001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Aviram A, Guy L, Ashwal E, Hiersch L, Yogev Y, Hadar E. Pregnancy outcome in pregnancies complicated with gestational diabetes mellitus and late preterm birth. Diabetes Res Clin Pract. 2016;113:198–203. doi: 10.1016/j.diabres.2015.12.018. [DOI] [PubMed] [Google Scholar]
- 46.Xiong X, Saunders L, Wang F, Demianczuk N. Gestational diabetes mellitus: prevalence, risk factors, maternal and infant outcomes. Int J Gynecol Obstet. 2001;75(3):221–228. doi: 10.1016/S0020-7292(01)00496-9. [DOI] [PubMed] [Google Scholar]
- 47.Au CPY, Raynes-Greenow CH, Turner RM, Carberry AE, Jeffery HE. Antenatal management of gestational diabetes mellitus can improve neonatal outcomes. Midwifery. 2016;34:66–71. doi: 10.1016/j.midw.2016.01.001. [DOI] [PubMed] [Google Scholar]
- 48.Benhalima K, Robyns K, Van Crombrugge P, Deprez N, Seynhave B, Devlieger R, Verhaeghe J, Mathieu C, Nobels F: Differences in pregnancy outcomes and characteristics between insulin-and diet-treated women with gestational diabetes. BMC pregnancy and childbirth 2015, 15(1):271. [DOI] [PMC free article] [PubMed]
- 49.Silva JK, Kaholokula JK, Ratner R, Mau M. Ethnic differences in perinatal outcome of gestational diabetes mellitus. Diabetes Care. 2006;29(9):2058–2063. doi: 10.2337/dc06-0458. [DOI] [PubMed] [Google Scholar]
- 50.Zhang Y, Liu X, Gao S, Wang J, Gu Y, Zhang J, Zhou X, Li Q. Risk factors for preterm birth in five maternal and child health hospitals in Beijing. PLoS One. 2012;7(12):e52780. doi: 10.1371/journal.pone.0052780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Temu TB, Masenga G, Obure J, Mosha D, Mahande MJ. Maternal and obstetric risk factors associated with preterm delivery at a referral hospital in northern-eastern Tanzania. Asian Pacific Journal of Reproduction. 2016;5(5):365–370. doi: 10.1016/j.apjr.2016.07.009. [DOI] [Google Scholar]
- 52.Bonanno C, Wapner RJ. Antenatal corticosteroids in the management of preterm birth: are we back where we started? Obstet Gynecol Clin N Am. 2012;39(1):47–63. doi: 10.1016/j.ogc.2011.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Beeckman K, Louckx F, Downe S, Putman K. The relationship between antenatal care and preterm birth: the importance of content of care. The European Journal of Public Health. 2012;23(3):366–371. doi: 10.1093/eurpub/cks123. [DOI] [PubMed] [Google Scholar]
- 54.Witt WP, Litzelman K, Cheng ER, Wakeel F, Barker ES. Measuring stress before and during pregnancy: a review of population-based studies of obstetric outcomes. Matern Child Health J. 2014;18(1):52–63. doi: 10.1007/s10995-013-1233-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav. 1983;24(4):385–96. doi: 10.2307/2136404. [DOI] [PubMed] [Google Scholar]
- 56.Gunning M, Denison F, Stockley C, Ho S, Sandhu H, Reynolds R. Assessing maternal anxiety in pregnancy with the state-trait anxiety inventory (STAI): issues of validity, location and participation. Journal of Reproductive and Infant Psychology. 2010;28(3):266–273. doi: 10.1080/02646830903487300. [DOI] [Google Scholar]
- 57.Sidebottom AC, Harrison PA, Godecker A, Kim H. Validation of the patient health questionnaire (PHQ)-9 for prenatal depression screening. Archives of women's mental health. 2012;15(5):367–374. doi: 10.1007/s00737-012-0295-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Study Questionnaire in English language. (DOCX 24 kb)
Data Availability Statement
The datasets used and analysed during the current study are available from the corresponding author on reasonable request.


